Abstract
Background: Mortality in patients with stages 4 and 5 chronic kidney disease (CKD) is higher than in the general population. Body composition predicts mortality. Our objective was to evaluate the effect of body composition on mortality in patients with stages 4 and 5 non-dialysis CKD.
Methods: We performed a prospective study of 356 patients with stages 4 and 5 non-dialysis CKD. At baseline, we recorded general characteristics, history of cardiovascular events, body composition, serum inflammatory markers, nutrition and cardiac biomarkers. Body composition was analysed using bioimpedance spectroscopy. We recorded the lean tissue index (LTI), fat tissue index (FTI) and overhydration (OH). During a median (range) follow-up of 22 (3–49) months, we recorded mortality, cardiovascular events and progress to renal replacement therapy.
Results: At baseline, mean (± standard deviation) age was 67 ± 13 years (men 64%; diabetes 36%). Mean body mass index was 28.2 ± 12.8 kg/m2, the FTI was 12.3 ± 5.6 kg/m2, the LTI was 15.7 ± 3.4 kg/m2 and median (interquartile range) OH was 0.6 (−0.4 to 1.5) L. Sixty-four (18%) patients died during follow-up. The univariate Cox analysis showed an association between mortality and age, low LTI, high Charlson comorbidity index, previous cardiovascular events, OH, low albumin and prealbumin levels, and high C-reactive protein levels. Kaplan–Meier analysis revealed higher survival in patients with a higher LTI (log-rank, 9.47; P = 0.002). The multivariate Cox analysis confirmed an association between mortality and low LTI (P = 0.031), previous cardiovascular events (P = 0.003) and high Charlson comorbidity index (P = 0.01). We did not find any association between body composition and cardiovascular events or renal replacement therapy.
Conclusions: A low LTI is an independent factor for mortality in patients with stages 4 and 5 CKD.
Keywords: bioimpedance, body mass index, chronic renal failure, nutrition, survival
Introduction
Mortality in patients with advanced chronic kidney disease (CKD) is higher than in the general population [1, 2]. CKD is currently accepted as an independent risk factor for cardiovascular disease and death [3]. Several risk factors for death have been studied in patients with CKD, and those related to uraemia as a consequence of bone mineral disorders and vascular calcifications have been well analysed [4].
During recent years, mortality-related factors, such as body composition, have been studied in patients with CKD [5, 6]. The association between body mass index (BMI) and mortality remains unclear in advanced CKD. The relationship between BMI and all-cause mortality has been confirmed in the general population, however this association has not always been displayed in CKD and results remains confusing [7, 8]. As body composition depends on both lean tissue and fat tissue mass, results may vary depending on the proportion of each relative to the other.
Current techniques for measuring fat tissue mass, lean tissue mass and overhydration (OH) have improved our understanding and interpretation not only of BMI, but also of body composition and its association with survival. High BMI is associated with mortality in the general population and in patients with CKD. However, when haemodialysis is included in the analysis, the patient’s profile improves [9, 10]. Lean tissue mass is a marker of good health and is associated with a lower risk of death in non-CKD patients, but this association has not yet been shown in patients with CKD [11].
The objective of the present study was to evaluate the effect of body composition on mortality in a cohort of patients with stages 4 and 5 non-dialysis CKD.
Materials and methods
Design
We performed a prospective observational study of all patients with stages 4 and 5 CKD who were not on dialysis in our reduced renal function outpatients clinic. All of the patients fulfilled the inclusion criteria. We analysed risk factors related to mortality.
Patients
The study population comprised 373 patients with stages 4 and 5 non-dialysis CKD from a single centre (Hospital General Universitario Gregorio Marañón, Madrid, Spain). CKD was defined and staged according to KDOQI guidelines using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12]. The inclusion criteria were stable clinical condition with no recent hospitalization (previous 3 months), outpatient follow-up for more than 3 months, age over 18 years and written informed consent. The exclusion criteria were inability to understand the study, contraindication to bioimpedance spectroscopy (BIS), loss of laboratory parameters or loss to follow-up. Seventeen patients were excluded.
Variables collected at baseline
The data collected at baseline were as follows: demographic and clinical data, including age, sex, aetiology of CKD (identified by clinical features and confirmed mostly by biopsy); presence of diabetes mellitus; hypertension (defined based on the Report of the Eighth Joint National Committee [13]); dyslipidaemia (defined using ATP III guidelines [14]); Charlson comorbidity index [15]; history of heart disease including congestive heart failure and myocardial infarction; peripheral vascular disease; and stroke.
Body composition was assessed at baseline using BIS (Body Composition Monitor, BCM®, Fresenius Medical Care, Bad Homburg, Germany). Measurements were taken after resting for 10 min in the supine position. The hydration parameters were total body water (in litres), extracellular water (in litres), intracellular water (in litres) and fluid overload (in litres), which is defined as water not included in lean tissue or fat tissue and considered excess water. Body composition parameters included the lean tissue index (LTI, kg/m2) and fat tissue index (FTI, kg/m2). We tested whether FTI and LTI measurements were normalized for age and sex using the BCM algorithm. We excluded patients under the 10th percentile for both measurements (4% of patients for LTI and 3% for FTI).
The laboratory variables recorded included creatinine and glomerular filtration rate (CKD-EPI), proteinuria, N-terminal prohormone of brain natriuretic peptide (Nt-proBNP), nutritional and inflammatory parameters (prealbumin, albumin) and C-reactive protein (CRP).
Follow-up
During follow-up [median (range), 22 (3–49) months], mortality, cardiovascular events and the date of the start of renal replacement therapy were recorded.
The cause of mortality was defined as cardiovascular, tumour-related, infectious, resulting from conservative treatment (i.e. treatment not involving haemodialysis defined as mortality due to uraemic syndrome in patients not candidates for renal replacement therapy) and other.
Cardiovascular events were defined as ischaemic or haemorrhagic cerebrovascular accident (diagnosed using computed tomography), myocardial infarction (diagnosed based on elevated cardiac marker levels and electrocardiography and confirmed by coronary angiography), congestive heart failure (defined as the presence of acute pulmonary oedema and echocardiography findings of ventricular systolic dysfunction and left ventricular ejection fraction <45%) and peripheral vascular events (defined as stenosis of major arteries or arteries in the lower limbs confirmed by arteriography and/or need for amputation). We also distinguished between acute events (cerebrovascular accident, myocardial infarction, acute pulmonary oedema and acute peripheral vascular events) and chronic events (congestive heart failure and limb artery stenosis)
Statistical analysis
The distribution of the variables was analysed using the Kolmogorov–Smirnov test. Values are given as mean (standard deviation) or median (interquartile range). Categorical data were compared using the chi-square test, while continuous variables were compared using the t-test or Mann–Whitney test. Receiver operating characteristic (ROC) curves were used to determine the cut-off of LTI and FTI. Linear regression analysis was used to assess factors associated with higher LTI and FTI. Cardiovascular events, mortality and need for renal replacement therapy were analysed, and associations were analysed using Cox regression. A multivariate Cox regression analysis was performed for each outcome in order to establish independent predictors of cardiovascular events, mortality and progression to renal replacement therapy. The models included factors showing a significant association or those considered confounding factors. Outcomes were analysed using Cox plots and Kaplan–Meier plots, and survival curves were compared using the log-rank test. All statistical analyses were performed with PASW Statistics for Windows, Version 18.0® (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant. All statistical tests were performed by a statistician.
Results
General characteristics
We included 356 patients with stages 4 and 5 non-dialysis CKD. The baseline characteristics are shown in Table 1. Men accounted for 64% of the sample, and mean age was 67 ± 13 years.
Table 1.
Baseline characteristics of the study population
| N: 356 | |
|---|---|
| General characteristics | |
| Sex (male, %) | 64 |
| Age (years) | 67 ± 13 |
| Charlson comorbidity index | 7.2 ± 2.7 |
| Diabetes (%) | 36 |
| Hypertension (%) | 87 |
| Dyslipidaemia (%) | 72 |
| CKD aetiology (%) | |
| Glomerular | 23 |
| Diabetes | 19 |
| Vascular | 28 |
| Interstitial | 13 |
| Polycystic | 10 |
| Other | 7 |
| Previous cardiovascular events (%) | |
| Myocardial infarction (%) | 28 |
| CHF (%) | 27 |
| Stroke (%) | 15 |
| PVD (%) | 12 |
| Laboratory parameters | |
| Creatinine (mg/dL) | 3.5 ± 1.5 |
| CKD-EPI (mL/min/1.73 m²) | 16 ± 5.5 |
| Urine protein (g/24 h)a | 0.5 (0.2–1.5) |
| Albumin (g/dL) | 4.1 ± 0.4 |
| Nt-proBNP (ng/dL)a | 84 (37–181) |
| CRP (mg/dL)a | 0.3 (0.1–0.7) |
| Prealbumin (mg/dL)a | 32 (27–38) |
| Hydration status and body composition | |
| BMI (kg/m²) | 28.0 ± 5.2 |
| FTI (kg/m²) | 12.3 ± 5.6 |
| LTI (kg/m²) | 15.7 ± 3.4 |
| OH (L)a | 0.6 (−0.4 to 1.5) |
| ECW (L) | 17.0 ± 3.5 |
| ICW (L) | 19.7 ± 4.7 |
| ECW/ICW | 0.8 ± 0.1 |
| OH/ECW (%) | 2.3 ± 0.8 |
Median and interquartile range.
CKD, chronic kidney disease. CHF, congestive heart failure. PVD, Peripheral vascular disease; CKD-EPI, Chronic Disease Epidemiology equation to estimate glomerular filtration rate; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; CRP, C-reactive protein; BMI, body mass index; FTI, fat tissue index; LTI, lean tissue index; OH, overhydration; ECW, extracellular water; ICW, intracellular water.
Characteristics related to low LTI
Mean LTI was 15.7 ± 3.4 kg/m2. We divided patients into two groups using a ROC curve to determine the cut-off. With an area of 0.687 [95% confidence interval (95% CI) (0.604–0.769); P = 0.001], the LTI cut-off was 14.05 kg/m2. Patients with a low LTI were older than those with a high LTI, had a higher Charlson comorbidity index, lower BMI, higher FTI, lower extracellular water, lower intracellular water, lower serum creatinine levels and lower urine protein levels (Table 2).
Table 2.
Comparison of LTI between groups with higher or lower mean values (LTI cut-off value, 14.05 kg/m²)
| LTI |
|||
|---|---|---|---|
| <14.05 kg/m² | >14.05 kg/m² | P | |
| Age (years) | 72 ± 11 | 62 ± 10 | 0.001 |
| Charlson comorbidity index | 7.7 ± 2.1 | 6.8 ± 3.0 | 0.011 |
| BMI (kg/m²) | 27.3 ± 5.1 | 28.7 ± 5.0 | 0.015 |
| FTI (kg/m²) | 14.6 ± 5.4 | 10.5 ± 5.2 | 0.001 |
| ECW (L) | 14.9 ± 2.9 | 18.3 ± 3.2 | 0.001 |
| ICW (L) | 16.6 ± 12.2 | 23.2 ± 10.7 | 0.001 |
| Creatinine (mg/dL) | 3.0 ± 0.9 | 3.8 ± 1.8 | 0.001 |
| Urine protein (mg/24 h) | 848.9 ± 127.8 | 1239 ± 539.7 | 0.001 |
LTI, lean tissue index; BMI, body mass index; OH, overhydration; ICW, intracellular water; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; CRP, C-reactive protein.
Linear regression analysis confirmed an independent association between low LTI and lower BMI, higher FTI and lower extracellular water (Table 3).
Table 3.
Lineal regression analysis of LTI and significant variables (mean LTI cut-off value, 14.05 kg/m²)
| Exp (β) | 95% CI | P | |
|---|---|---|---|
| Higher age (years) | 1.05 | 0.97, 1.16 | 0.325 |
| Charlson comorbidity index | −0.01 | −0.09, 0.04 | 0.572 |
| Creatinine (mg/dL) | −0.01 | −0.013, 0.007 | 0.531 |
| FTI kg/m²) | −1.396 | −0.896, −0.875 | 0.001 |
| BMI (kg/m²) | 1.388 | 0.939, 0.961 | 0.001 |
| ECW (L) | 0.246 | 0.228, 0.278 | 0.001 |
| Urine protein (mg/24 h) | 0.02 | 0.01, 0.01 | 0.703 |
LTI, lean tissue index; BMI, body mass index; OH, overhydration; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; CRP, C-reactive protein.
Characteristics related to low FTI
Mean FTI was 12.2 ± 5.6 kg/m2. We divided patients into two groups using a ROC curve to determine the cut-off. With an area of 0.558 [95% CI (0.354–0.531); P = 0.001], the FTI cut-off was 11.9 kg/m2. Patients with a low FTI had a lower BMI than those with a high FTI (25.3 ± 3.5 versus 31.7 ± 4.2 kg/m2; P = 0.001) and higher intracellular water levels (20.4 ± 4.9 versus 18.5 ± 3.8 kg/m2; P = 0.001) and prealbumin levels (33.4 ± 10.5 versus 30.8 ± 7.8 mg/dL; P = 0.03). Linear regression analysis confirmed an independent association between lower FTI and lower BMI [Exp β 1.2; 95% CI (0.9–3.0); P = 0.001] and higher intracellular water [Exp β 0.42; 95% CI (0.3–0.5); P = 0.001].
Development of cardiovascular events during follow-up
Ninety-two (26%) patients experienced a cardiovascular event during follow-up. The most common was chronic congestive heart failure (45%). The distribution of the rest of cardiovascular events was as follows: 35% myocardial infarction, 12% acute peripheral vascular disease and 8% cerebrovascular accident.
We found an independent association between development of cardiovascular events and previous myocardial infarction, heart disease, and low levels of albumin and urine protein. We did not find an association with body composition parameters. We did not find differences between acute and chronic events.
Mortality during follow-up
Sixty-four (18%) patients died. The causes of death were cardiovascular events (10%), cancer (3%), infections (2%), uraemia (1%) and other (2%).
In the univariate analysis, all-cause mortality was associated with higher age, a higher Charlson comorbidity index, diabetes, previous cardiovascular events, low albumin and prealbumin levels, high CRP, low LTI, OH and low intracellular water. The Kaplan–Meier analysis confirmed better survival in patients with a high LTI (log-rank, 9.47; P = 0.002) (Figure 1).
Fig. 1.
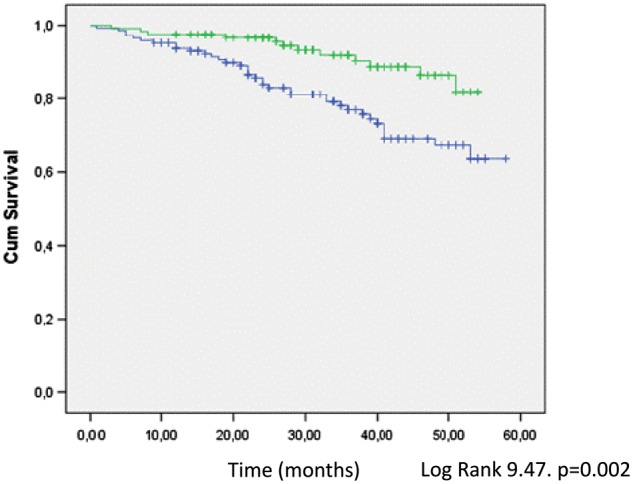
Comparative survival between patients with high (green line) versus low (blue line) LTI (Kaplan–Meier plot).
The multivariate Cox regression analysis confirmed an independent association between mortality and low LTI (P = 0.031), previous cardiovascular events (P = 0.03) and high Charlson comorbidity index (P = 0.01) (Table 4). The Cox plot confirmed an independent association between high LTI and survival (Figure 2).
Table 4.
Cox proportional hazards regression analysis for mortality
| HR (95% CI) univariate | P | HR (95% CI) multivariate | P | |
|---|---|---|---|---|
| Sex (male, %) | 1.35 (0.76–1.79) | 0.34 | ||
| Higher age (years) | 1.04 (1.02–1.05) | 0.001 | 1.02 (1.00–1.04) | 0.05 |
| Diabetes (%) | 1.89 (1.08–3.30) | 0.024 | 1.30 (0.62–1.87) | 0.32 |
| Hypertension (%) | 1.65 (0.33–1.98) | 0.76 | 1.21 (1.05–1.40) | |
| Dyslipidaemia (%) | 1.43 (0.54–1.87) | 0.45 | ||
| Charlson comorbidity index | 1.24 (1.14–1.36) | 0.02 | 3.59 (2.39–4.80) | 0.01 |
| AHT (diastolic) | 1.41 (0.98–1.74) | 0.37 | 1.49 (0.70–2.06) | |
| Previous global CVev | 3.12 (1.77–5.48) | 0.001 | 0.76 (0.34–1.33) | 0.003 |
| Previous MI | 2.29 (1.23–4.01) | 0.003 | 1.33 (0.65–2.01) | 0.44 |
| Previous CHF | 1.94 (1.10–3.94) | 0.021 | 0.50 | |
| Previous PVD | 2.55 (1.37–4.75) | 0.003 | 0.66 | |
| Previous Ictus | 1.32 (0.87–1.88) | 0.44 | ||
| Creatinine (mg/dL) | 1.02 (0.89–1.18) | 0.34 | 0.84 (0.68–1.12) | |
| Urine protein (g/24 h) | 1.001 (0.99–1.01) | 0.69 | 0.99 (0.89–1.23) | |
| Albumin (g/dL) | 0.41 (0.21–0.81) | 0.011 | 1.23 (0.92–1.43) | 0.54 |
| Prealbumin (mg/dL) | 0.95 (0.92–0.99) | 0.025 | 0.31 | |
| CRP (mg/dL) | 1.44 (1.18–1.74) | 0.001 | 0.75 | |
| Nt-proBNP (ng/dL) | 1.00 (0.99–1.01) | 0.677 | 1.10 (0.99–1.20) | |
| Cholesterol (mg/dL) | 0.99 (0.98–1.01) | 0.07 | ||
| OH (L) | 1.13 (1.01–1.27) | 0.028 | 1.01 (0.91–1.17) | 0.08 |
| ECW (L) | 0.96 (0.88–1.05) | 0.640 | 1.43 (0.95–2.29) | |
| ICW (L) | 0.88 (0.81–0.94) | 0.001 | 0.09 | |
| ECW/ICW | 2.45(2.01–3.62) | 0.001 | 0.22 | |
| BMI (kg/m²) | 0.98 (0.93–1.04) | 0.650 | 0.82 (0.69–0.98) | |
| FTI (kg/m²) | 1.04 (0.89–1.08) | 0.124 | ||
| LTI (kg/m²) | 0.79 (0.71–0.89) | 0.001 | 0.031 |
HR, hazard ratio; CVev, cardiovascular events; MI, myocardial infarction; CHF, congestive heart failure; PVD, peripheral vascular disease; CRP, C-reactive protein; Nt-ProBNP, N-terminal prohormone of brain natriuretic peptide; OH, overhydration; ECW, extracellular water; ICW, intracellular water; ECW/ICW, relation between extracellular and intracellular waterBMI, body mass index; FTI, fat tissue index; LTI, lean tissue index.
Fig. 2.
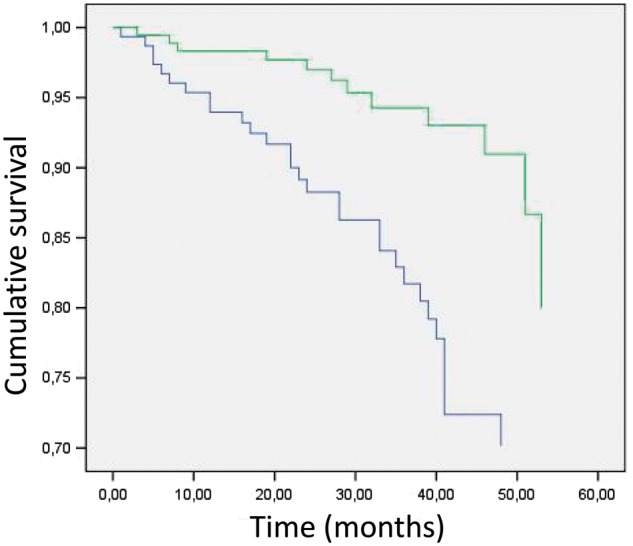
Comparative survival between patients with high (green line) versus low (blue line) LTI (Cox plot, Charlson comorbidity index and previous cardiovascular events have been included).
Cardiovascular mortality was also independently associated with low LTI, previous cardiovascular events and a high Charlson comorbidity index.
Progression to renal replacement therapy
Patients started renal replacement therapy when mean glomerular filtration rate was <9 ± 3 mL/min/1.73 m2. We found an independent association between progression to renal replacement therapy and proteinuria, low levels of albumin and arterial hypertension. We did not find an association with body composition.
Discussion
The present study provides data on the association between lean tissue mass and mortality in patients with advanced CKD. Our findings highlight the importance of an accurate analysis of body composition in these patients. The LTI and its relationship with mortality have been widely studied in haemodialysis patients, although data and consistent conclusions are lacking in CKD [16]. In a study of healthy patients and CKD patients, high LTI was the only variable associated with a lower risk of death in the healthy patients, although this association could not be confirmed in patients with kidney disease (any grade) [11]. However, in our study, the many potential causes of mortality analysed included serum inflammatory markers, diabetes, urine protein and OH, although the only factors independently related to mortality were low LTI, previous cardiovascular events, age and high Charlson comorbidity index.
It is important to accurately assess body composition in terms of both BMI and lean and fat tissue mass, since a low LTI is associated with mortality. This observation could explain why a high BMI is associated with better survival in some groups of haemodialysis patients [17]. When lean and fat tissue mass were taken into account in haemodialysis patients, survival was better when both LTI and FTI were within the 10th and 90th percentiles for a healthy population [18].
In view of our results, we suggest monitoring body composition with the same frequency as serum inflammatory markers to predict worsening of clinical status and death.
We found no association between mortality and the remaining parameters of body composition, such as fat tissue, OH and BMI. With respect to OH, the study patients had low levels of fluid overload (mean OH was only 0.6 L and the ratio of OH to extracellular water was 2.3%), as a result of frequent visits in our clinic to measure fluid intakes and diuresis and insistence on avoiding salt intake. We do not have a clear explanation for the BMI or fat tissue values recorded. Since BMI can be high owing to high fat tissue or lean tissue, the clinical situation of patients with the same BMI can vary. It is probable that the association between low FTI and mortality is closer in advanced stages of malnutrition. In addition, FTI could be a marker of mortality in patients with severe malnutrition disorders. Obesity and high BMI had previously been associated with survival in haemodialysis patients [9].
The fact that we found no association between mortality and body composition, OH, FTI or BMI highlights the importance of analysing lean tissue mass. LTI could be considered an early marker of mortality, although this possibility should be confirmed in controlled studies.
Our study has several limitations. Only one BIS measurement was available for each patient, and no additional BIS measurements could be taken during follow-up or at the end of the study to evaluate any changes observed and their consequences. Median follow-up was 22 (3–49) months, which was too short to evaluate mortality in these patients. Therefore, during the follow-up we had patients who finished the study in a short period of time due to sudden death or quick progression to renal replacement therapy. This is the reason why, although median of follow-up was 22 months, the range was so wide (3–49 months). As our data are from a single centre, the results should be confirmed in a multicentre study. Our results should be extrapolated with caution. We did not record whether patients took exercise regularly or which kind of exercise they took. This is a major limitation, because mortality could be affected by exercise, and increased lean tissue mass could be the result of a healthy lifestyle.
In conclusion, body composition is associated with mortality in patients with advanced CKD. Low lean tissue is an independent risk factor for mortality. We did not find an association between mortality and OH or fat tissue.
Acknowledgement
The authors thank Thomas O’Boyle for proofreading the manuscript.
Conflict of interest statement
None declared.
References
- 1. Mariani L, Stengel B, Combe C. et al. The CKD outcomes and practice patterns study (CKDopps): rationale and methods. Am J Kidney Dis 2016; 68: 402–413 [DOI] [PubMed] [Google Scholar]
- 2. Sumida K, Molnar MZ, Potukuchi PK. et al. Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 2016; 91: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013; 3: S1–S150 [DOI] [PubMed] [Google Scholar]
- 4. Bover J, Ureña P, Ruiz-García C. et al. Clinical and practical use of calcimimetics in dialysis patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol 2016; 11: 161–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai YC, Chiu YW, Tsai JC. et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol 2015; 10: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan J, Watanabe M, Suliman M. et al. Serum hepatocyte growth factor is associated with truncal fat mass and increased mortality in chronic kidney disease stage 5 patients with protein-energy wasting. Nephrol Dial Transplant 2015; 30: 274–282 [DOI] [PubMed] [Google Scholar]
- 7. Zoccali C, Torino C, Tripepi G. et al. Assessment of obesity in chronic kidney disease: what is the best measure? Curr Opin Nephrol Hypertens 2012; 21: 641–646 [DOI] [PubMed] [Google Scholar]
- 8. Caetano C, Valente A, Oliveira T. et al. Body composition and mortality predictors in hemodialysis patients. J Ren Nutr 2016; 26: 81–86 [DOI] [PubMed] [Google Scholar]
- 9. Park J, Ahmadi SF, Streja E. et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis 2014; 56: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalantar-Zadeh K, Abbott KC, Salahudeen AK. et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr 2005; 81: 543–554 [DOI] [PubMed] [Google Scholar]
- 11. Navaneethan SD, Kirwan JP, Arrigain S. et al. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC Nephrol 2014; 15: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. James PA, Oparil S, Carter BL. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520 [DOI] [PubMed] [Google Scholar]
- 14. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421 [PubMed] [Google Scholar]
- 15. Charlson M, Szatrowski TP, Peterson J. et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47: 1245–1251 [DOI] [PubMed] [Google Scholar]
- 16. Rosenberger J, Kissova V, Majernikova M. et al. Body composition monitor assessing malnutrition in the hemodialysis population independently predicts mortality. J Ren Nutr 2014; 24: 172–176 [DOI] [PubMed] [Google Scholar]
- 17. Kalantar-Zadeh K,, Abbott KC, Salahudeen AK. et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr 2005; 81: 543–54 [DOI] [PubMed] [Google Scholar]
- 18. Marcelli D, Usvyat LA, Kotanko P. et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol 2015; 10: 1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]


