Abstract
Background:
Hyperglycemia on admission has been found to elevate risk for mortality and adverse clinical events after acute myocardial infarction (AMI), but there are evidences that the relationship of blood glucose and mortality may differ between diabetic and nondiabetic patients. Prior studies in China have provided mixed results and are limited by statistical power. Here, we used data from a large, nationally representative sample of patients hospitalized with AMI in China in 2001, 2006, and 2011 to assess if admission glucose is of prognostic value in China and if this relationship differs depending on the presence or absence of diabetes.
Methods:
Using a nationally representative sample of patients with AMI in China in 2001, 2006, and 2011, we categorized patients according to their glucose levels at admission (< 3.9, 3.9–7.7, 7.8–11.0, and ≥11.1 mmol/L) and compared in-hospital mortality across these admission glucose categories, stratified by diabetes status. Among diabetic and nondiabetic patients, separately, we employed logistic regression to assess the differences in outcomes across admission glucose levels while adjusting for the same covariates.
Results:
Compared to patients with euglycemia (5.8%), patients with moderate hyperglycemia (13.1%, odds ratio [OR] = 2.44, 95% confidence interval [CI, 2.08–2.86]), severe hyperglycemia (21.5%, OR = 4.42, 95% CI [3.78–5.18]), and hypoglycemia (13.8%, OR = 2.59, 95% CI [1.68–4.00]), all had higher crude in-hospital mortality after AMI regardless of the presence of recognized diabetes mellitus. After adjustment for patients’ characteristics and clinical status, however, the relationship between admission glucose and in-hospital mortality was different for diabetic and nondiabetic patients (P for interaction = 0.045). Among diabetic patients, hypoglycemia (OR = 3.02, 95% CI [1.20–7.63]), moderate hyperglycemia (OR = 1.75, 95% CI [1.04–2.92]), and severe hyperglycemia (OR = 2.97, 95% CI [1.87–4.71]) remained associated with elevated risk for mortality, but among nondiabetic patients, only patients with moderate hyperglycemia (OR = 2.34, 95% CI [1.93–2.84]) and severe hyperglycemia (OR = 3.92, 95% CI [3.04–5.04]) were at elevated mortality risk and not hypoglycemia (OR = 1.12, 95% CI [0.60–2.08]). This relationship was consistent across different study years (P for interaction = 0.900).
Conclusions:
The relationship between admission glucose and in-hospital mortality differs for diabetic and nondiabetic patients. Hypoglycemia was a bad prognostic marker among diabetic patients alone. The study results could be used to guide risk assessment among AMI patients using admission glucose.
Trial Registration:
www.clinicaltrials.gov, NCT01624883; https://clinicaltrials.gov/ct2/show/NCT01624883
Keywords: Acute Myocardial Infarction, Blood Glucose, Diabetes Mellitus, Mortality
Introduction
Studies in Western populations have shown that hyperglycemia on admission is associated with mortality and adverse clinical events after acute myocardial infarction (AMI).[1,2,3,4] However, there are evidences that the relationship between blood glucose and mortality may differ between diabetic and nondiabetic patients.[2,5] For example, among nondiabetic patients, hyperglycemia at admission is associated with a graded increase in mortality risk. This is in contrast to patients with diabetes, where the elevated risk of mortality is appreciable only at higher levels of hyperglycemia. In addition, some studies have shown that hypoglycemia, particularly in diabetic patients, is an equally potent risk factor for death.[6,7] However, these studies only assessed the influence of hypoglycemia on short-term mortality after AMI due to insufficient statistical power.
There remains a need for a comprehensive understanding of the prognostic value of admission glucose over the whole spectrum of glucose values. In addition, it is unknown if these relationships are population-specific. It is widely appreciated that there are important differences in the pathophysiology and epidemiology of diseases between Western and other populations.[8,9] China is in the midst of an epidemic of AMI and it is important to validate findings from Western populations to accurately inform practice in China. Prior studies evaluating the relationship of blood glucose and outcomes after AMI in China have provided mixed results and have methodological limitations.[10,11,12,13]
Accordingly, we used data from the China Patient-centered Evaluative Assessment of Cardiac Events-Retrospective AMI (China PEACE-Retrospective AMI) study – a large, nationally representative sample of patients hospitalized with AMI in China in 2001, 2006, and 2011 – which provides a unique opportunity to assess if admission glucose is of prognostic value in China and whether this relationship has changed over the past decade. We also assessed if this relationship differs depending on the presence or absence of diabetes.
Methods
China Patient-centered Evaluative Assessment of Cardiac Events-Retrospective acute myocardial infarction study
The design of the China PEACE-Retrospective AMI study has been published previously,[14] and detailed information are shown in the Supplementary Text (188.3KB, pdf) . In brief, we created a nationally representative sample of patients hospitalized for AMI during 2001, 2006, and 2011, using two-stage random sampling [Figure 1]. In the first stage, we identified hospitals using a simple random sampling procedure within each of the five study strata: Eastern-rural, Central-rural, Western-rural, Eastern-urban, and Central/Western-urban regions, since hospital size and clinical capacities differ between urban and rural areas as well among the three official economic-geographic regions (Eastern, Central, and Western) of Mainland China. In the second stage, from the 2010 rural hospitals and 833 urban hospitals, we randomly selected 162 hospitals to include in the study. Subsequently, we randomly sampled patients admitted to these hospitals with a definite discharge diagnosis of AMI. We excluded patients who were discharged alive within 24 h and patients who were transferred in from another facility because the detailed clinical status and treatments at their initial presentation could not be ascertained as well as those who were transferred out to other hospitals because their admissions were truncated [Figure 1].
Figure 1.
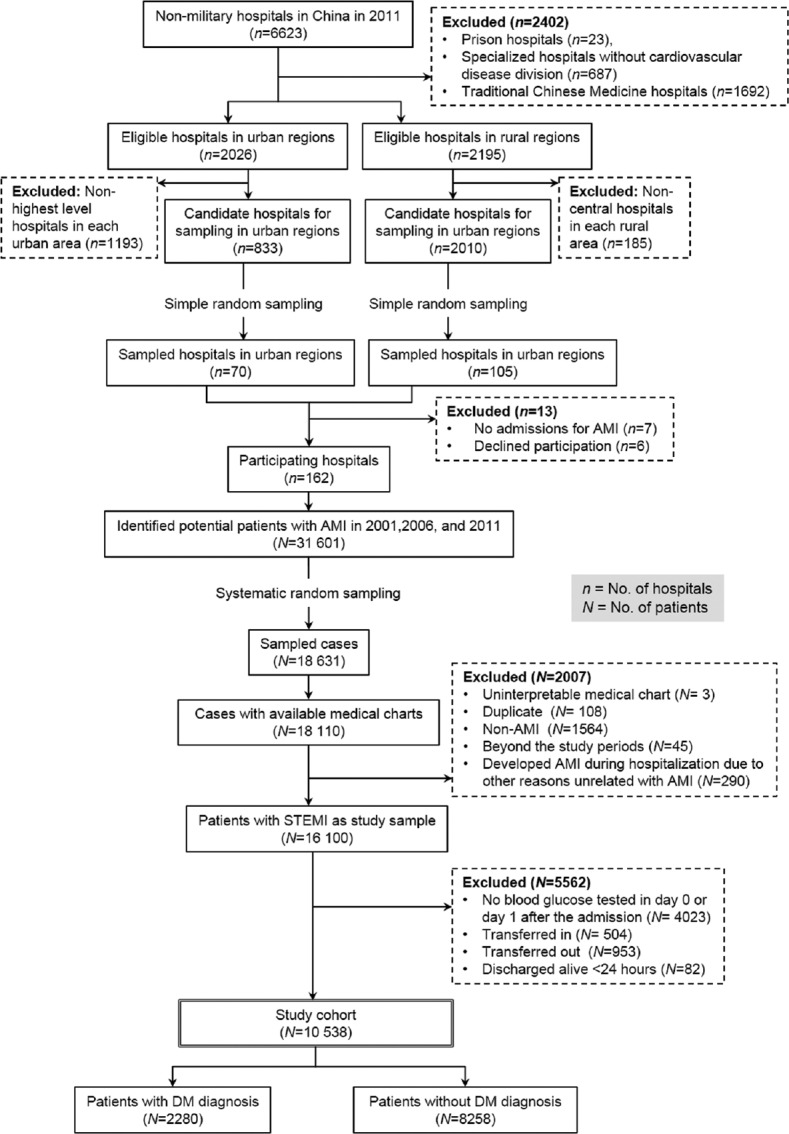
Patient flowchart in a large, nationally representative sample of patients hospitalized with AMI in China in 2001, 2006, and 2011. AMI: Acute myocardial infarction; STEMI: ST-segment elevation myocardial infarction; DM: Diabetes mellitus.
All data were collected by abstracting clinical information documented in the medical chart using standardized data definitions.[14] AMI hospitalizations were identified according to the International Classification of Diseases Clinical Modification codes, including versions 9 (410.xx) and 10 (I21.xx) when available, or through principal diagnosis terms noted at discharge, such as AMI, non-ST-segment elevation myocardial infarction, and inferior myocardial infarction. We conducted rigorous monitoring at each stage to ensure data quality. Data abstraction quality was monitored by randomly auditing 5% of the medical records, with overall variable accuracy exceeding 98%.
The Central Ethics Committee at the China National Center for Cardiovascular Diseases approved the China PEACE-Retrospective AMI study. All collaborating hospitals accepted the central ethics approval except for five hospitals, which obtained local approval by the Internal Ethics Committees.
Data collection
Variables collected included age, sex, cardiovascular risk factors, medical history, and clinical characteristics at admission, tests, treatments, procedures, and in-hospital outcomes. Comorbidities, including cardiogenic shock, acute stroke, in-hospital infection, hypertension, and dyslipidemia, were defined as documented history in the admission notes, discharge diagnosis, or positive laboratory test. The clinical features on admission that were assessed included chest discomfort, delayed time to admission, length of stay in-hospital, presence of ST-segment elevation, admission systolic blood pressure, respiratory rate, heart rate, estimated glomerular filtration rate (eGFR), and left ventricular ejection fraction (LVEF).
Admission glucose and diabetes status
For this analysis, the sample was restricted to those patients with glucose levels tested at admission for AMI. Admission glucose was defined as the earliest plasma glucose value on day 0 or day 1 of hospitalization. Admission glucose levels were categorized into four groups, namely, hypoglycemia (admission glucose <3.9 mmol/L), euglycemia (3.9–7.7 mmol/L), moderate hyperglycemia (7.8–11.0 mmol/L), and severe hyperglycemia (≥11.1 mmol/L), according to the diabetes mellitus (DM) guidelines.[15] We classified patients as diabetic if they had a documented history of diabetes in the medical charts.
In-hospital mortality
In-hospital mortality was defined as a composite of death occurring during hospitalization or withdrawal from the treatment due to a terminal status at discharge. Withdrawal from the treatment is common in China due to the reluctance of many people to die in the hospital. The Chinese government uses in-hospital death or withdrawal from the treatment as a composite quality measure for hospitals.[16]
Statistical analysis
We used percentages to describe categorical variables and medians (interquartile range [IQR]) to describe continuous variables. Pearson's Chi-square tests or Mann-Whitney tests were used to assess differences in demographic and clinical characteristics, treatments, tests, and in-hospital outcomes across admission glucose levels. Among diabetic and nondiabetic patients, separately, we employed logistic regression to assess the differences in outcomes across admission glucose levels, while adjusting for the same covariates, which include sociodemographic factors (age, sex); medical history and risk factors (current smoker, prior coronary heart disease, prior percutaneous coronary intervention [PCI], prior ischemic stroke, dyslipidemia, hypertension); clinical features at admission (chest discomfort lasting for more than 10 min, delay before admission, systolic blood pressure, respiratory rate, heart rate, type of AMI [ST- or non-ST-segment elevation], presence of anterior infarct, cardiogenic shock, acute stroke, LVEF). We repeated the analyses using time to event analyses with multivariable Cox regression analyses. We evaluated temporal changes in the association between admission glucose and in-hospital mortality by including a time-admission glucose level interaction into the logistic models. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported. All comparisons were two-sided, with a P < 0.05 considered statistically significant. Statistical analysis was performed using SAS software (version 9.2, SAS Institute, Cary, NC, USA) and SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Study sample
We identified 16,100 patients hospitalized for AMI from 162 hospitals across China. After excluding patients with undocumented admission glucose values (n = 4023), those transferred in from other facilities (n = 504), transferred out to other facilities (n = 953), or discharged alive within 24 h of admission (n = 82), the remaining 10,538 patients were included in our analysis [Figure 1].
Admission glucose values
The median glucose value on admission was 7.0 (IQR 5.7–9.3) mmol/L; 39.2% had either moderate or severe hyperglycemia, while 1.7% was hypoglycemic. Overall, 21.6% of patients had known DM. The admission glucose of diabetic patients was higher compared with nondiabetic patients (10.6 [IQR 7.7–14.5] mmol/L vs. 6.4 [IQR 5.3–8.0] mmol/L, P < 0.001). The distribution of admission glucose values in patients with or without known diabetes is shown in the Supplementary Figure 1 (320.9KB, tif) . Moderate or severe hyperglycemia was present in 76.0% of diabetic patients and 29.0% of nondiabetic patients. Among patients with severe hyperglycemia at admission, 34.9% did not have known DM [Table 1].
Table 1.
Baseline characteristics of patients with different admission glucose levels in the China PEACE-Retrospective AMI study
| Description | Overall (n = 10,538) | Admission glucose (mmol/L) | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| <3.9 (n = 181) | 3.9–7.7 (n = 6227) | 7.8–11.0 (n = 2423) | ≥11.1 (n = 1707) | ||||
| Demographics | |||||||
| Age (years) | 67 (57, 75) | 67 (57, 74) | 66 (56, 74) | 67 (58, 75) | 68 (58, 75) | 48.98 | <0.001 |
| Female | 3210 (30) | 61 (33) | 1590 (25) | 825 (34) | 734 (42) | 213.68 | <0.001 |
| Medical history/comorbidities | |||||||
| Diabetes mellitus | 2280 (22) | 39 (22) | 508 (8) | 622 (26) | 1111 (65) | 317.83 | <0.001 |
| Current smoker | 3646 (34) | 49 (27) | 2358 (37) | 786 (32) | 453 (26) | 87.94 | <0.001 |
| CHD history | |||||||
| None | 8145 (77) | 140 (77) | 4864 (78) | 1881 (77) | 1260 (73) | 17.43 | 0.080 |
| CHD, but no MI | 1270 (12) | 23 (12) | 709 (11) | 305 (12) | 233 (13) | ||
| MI | 1123 (10) | 18 (9) | 654 (10) | 237 (9) | 214 (12) | ||
| Ischemic stroke history | 1136 (10) | 15 (8) | 604 (10) | 292 (12) | 225 (13) | 23.03 | <0.001 |
| Dyslipidemia history | 6724 (63) | 92 (50) | 3938 (63) | 1573 (64) | 1121 (65) | 17.93 | <0.001 |
| Hypertension history | 5528 (52) | 89 (49) | 3104 (49) | 1343 (55) | 992 (58) | 48.26 | <0.001 |
| Cardiogenic shock | 544 (5) | 17 (9) | 186 (2) | 152 (6) | 189 (11) | 272.02 | <0.001 |
| Clinical features | |||||||
| Time delay to admission (h) | 14 (3, 72) | 24 (4, 72) | 20 (4, 72) | 8 (3, 48) | 10 (3, 48) | 135.98 | <0.001 |
| Length of stay (days) | 11 (7, 15) | 10 (5, 14) | 11 (7, 15) | 11 (7, 16) | 11 (5, 15) | 32.47 | <0.001 |
| STEMI | 8944 (84) | 144 (79) | 5278 (84) | 2081 (85) | 1441 (84) | 6.26 | 0.100 |
| SBP at admission (mmHg) | 130 (110, 149) | 120 (103, 142) | 130 (110, 148) | 130 (110, 150) | 130 (110, 150) | 9.61 | 0.022 |
| RR at admission (beats/min) | 20 (18, 20) | 20 (19, 22) | 20 (18, 20) | 20 (18, 21) | 20 (18, 22) | 64.74 | <0.001 |
| HR at admission (beats/min) | 78 (66, 90) | 78 (64, 92) | 76 (65, 88) | 78 (66, 90) | 82 (70, 100) | 187.46 | <0.001 |
| eGFR | |||||||
| <30 ml/min | 399 (3) | 24 (13) | 158 (2) | 106 (4) | 111 (6) | 297.90 | <0.001 |
| 30–59 ml/min | 2067 (19) | 39 (21) | 1001 (16) | 533 (21) | 494 (28) | ||
| ≥60 ml/min | 7269 (68) | 97 (53) | 4538 (72) | 1637 (67) | 997 (58) | ||
| Unmeasured | 803 (7) | 21 (11) | 530 (8) | 147 (6) | 105 (6) | ||
| LVEF | |||||||
| <40% | 615 (5) | 8 (4) | 320 (5) | 169 (6) | 118 (6) | 35.45 | <0.001 |
| ≥40% | 4789 (45) | 62 (34) | 2928 (47) | 1076 (44) | 723 (42) | ||
| Unmeasured | 5134 (48) | 111 (61) | 2979 (47) | 1178 (48) | 866 (50) | ||
Data are presented as n (%) or median (inter quartile range). P<0.05, which means distributions of the characteristic in groups of different admission glucose level were not totally the same statistically. PEACE: Patient-centered Evaluative Assessment of Cardiac Events; CHD: Coronary heart disease; MI: Myocardial infarction; STEMI: ST-segment elevation myocardial infarction; DM: Diabetes mellitus; PCI: Percutaneous coronary intervention; SBP: Systolic blood pressure; HR: Heart rate; RR: Respiratory rate; eGFR: Estimated glomerular filtration rate; LVEF: Left ventricular ejection fraction; 1 mmHg = 0.133 kPa.
Distribution of admission glucose levels in diabetic and non-diabetic patients with acute myocardial infarction.
Patients’ characteristics
Compared with euglycemic patients, those with either moderate or severe hyperglycemia were older, more often female, more commonly reported an antecedent history of ischemic stroke, dyslipidemia, and hypertension, had higher heart rates at admission, and had a greater prevalence of cardiogenic shock, acute stroke, low eGFR (< 30 ml/min), and depressed LVEF (< 40%). On the other hand, they had less time delay before admission [Table 1] and were more likely to receive primary PCI, clopidogrel, angiotensin-converting enzyme inhibitors, and statins [Table 2].
Table 2.
In-hospital treatments of patients with different admission glucose levels
| Description | Overall (n = 10,538) | Admission glucose (mmol/L) | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| <3.9 (n = 181) | 3.9–7.7 (n = 6227) | 7.8–11.0 (n = 2423) | ≥11.1 (n = 1707) | ||||
| Fibrinolytic therapy | 2035 (19) | 30 (16) | 1129 (18) | 545 (22) | 331 (19) | 22.19 | <0.001 |
| Primary PCI | 1243 (11) | 12 (6) | 659 (10) | 361 (14) | 211 (12) | 36.40 | <0.001 |
| Aspirin within 24 h | 9220 (87) | 152 (83) | 5500 (88) | 2131 (87) | 1437 (84) | 23.54 | <0.001 |
| Clopidogrel within 24 h | 6341 (60) | 90 (49) | 3725 (59) | 1515 (62) | 1011 (59) | 14.81 | 0.002 |
| Statin | 8378 (79) | 126 (69) | 4942 (79) | 1957 (80) | 1353 (79) | 13.38 | 0.004 |
| ACEI/ARB | 6860 (65) | 110 (60) | 4032 (64) | 1628 (67) | 1090 (63) | 7.65 | 0.054 |
| Beta-blocker within 24h | 5054 (47) | 86 (47) | 3069 (49) | 1143 (47) | 756 (44) | 14.22 | 0.003 |
| TCM within 24 h | 6034 (57) | 108 (59) | 3606 (57) | 1391 (57) | 929 (54) | 7.14 | 0.068 |
| TCM injection within 24 h | 5530 (52) | 99 (54) | 3279 (52) | 1287 (53) | 865 (50) | 3.06 | 0.382 |
| MgSO4 | 1996 (18) | 38 (20) | 1147 (18) | 497 (21) | 314 (18) | 5.82 | 0.120 |
Data are presented as n (%). P<0.05, which means treatment rates in groups of different admission glucose level were not totally the same statistically. PCI: Percutaneous coronary intervention; ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; TCM: Traditional Chinese medicine.
Patients with admission hypoglycemia compared with euglycemic patients were more often female, less commonly reported antecedent history of ischemic stroke, dyslipidemia, and hypertension, less time delay before admission, lower admission systolic blood pressure, higher heart rate at admission, and had a greater prevalence of cardiogenic shock, acute stroke, low eGFR (< 30 ml/min); all above P < 0.001 [Table 1].
Relationship between admission glucose and in-hospital mortality
Overall, euglycemic patients had higher in-hospital mortality in the unadjusted model (5.8%), compared with patients with moderate hyperglycemia (13.1%, OR 2.44, 95% CI [2.08–2.86]), severe hyperglycemia (21.5%, OR 4.42, 95% CI [3.78–5.18]), and hypoglycemia (13.8%, OR 2.59, 95% CI [1.68–4.00]). After multivariable adjustment for demographic characteristics, medical history, and clinical characteristics at admission, patients with moderate hyperglycemia (OR 2.11, 95% CI [1.76–2.52]) and severe hyperglycemia (OR 3.06, 95% CI [2.53–3.69]) were at elevated risk for in-hospital mortality but not those with hypoglycemia (OR 1.32, 95% CI [0.80–2.19]).
In the unadjusted model, the relationship of admission glucose with in-hospital mortality for both patients with and without recognized DM was similar to the overall cohort, when compared to patients with euglycemia, patients with moderate hyperglycemia, severe hyperglycemia, and hypoglycemia were all at elevated risk for in-hospital mortality [Table 2]. However, on multivariable adjustment for demographic characteristics, medical history, and clinical characteristics at admission, the relationship between admission glucose and in-hospital mortality was different for diabetic and nondiabetic patients (P for interaction = 0.045). Among diabetic patients, hypoglycemia (OR = 3.02, 95% CI [1.20–7.63]), moderate hyperglycemia (OR 1.75, 95% CI[1.04–2.92]), and severe hyperglycemia (OR 2.97, 95% CI [1.87–4.71]) remained associated with elevated risk for mortality, but among nondiabetic patients, only moderate hyperglycemia (OR 2.34, 95% CI [1.93–2.84]) and severe hyperglycemia (OR 3.92, 95% CI [3.04–5.04]) elevated risk for mortality, but not hypoglycemia (OR 1.12, 95% CI [0.60–2.08], [Figure 2] and [Table 3]). Survival curves for diabetic and nondiabetic patients by groups of admission glucose levels in the multivariable Cox regression analyses are shown in Figure 3.
Figure 2.
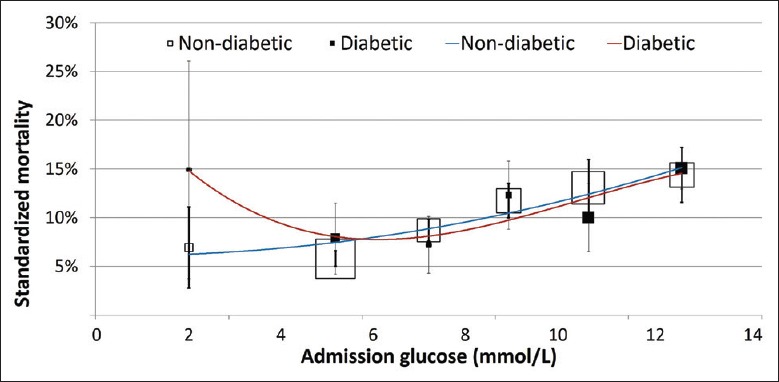
In-hospital mortality associated with admission glucose in diabetic and nondiabetic patients with AMI. Adjusted for patient characteristics, risk factors, medical history, and clinical features at admission. AMI: Acute myocardial infarction.
Table 3.
In-hospital mortality in patients with different admission glucose levels
| Admission glucose groups (mmol/L) | Mortality | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Nondiabetic | |||||
| <3.9 | 14 (9.9) | 1.77 (1.01–3.11) | 0.047 | 1.12 (0.60–2.08) | 0.727 |
| 3.9–7.7 | 333 (5.8) | 1 | – | 1 | – |
| 7.8–11.0 | 259 (14.4) | 2.72 (2.29–3.23) | <0.001 | 2.34 (1.93–2.84) | <0.001 |
| ≥11.1 | 175 (29.4) | 6.72 (5.46–8.28) | <0.001 | 3.92 (3.04–5.04) | <0.001 |
| Diabetic | |||||
| <3.9 | 11 (28.2) | 6.26 (2.84–13.78) | <0.001 | 3.02 (1.20–7.63) | 0.019 |
| 3.9–7.7 | 30 (5.9) | 1 | – | 1 | – |
| 7.8–11.0 | 59 (9.5) | 1.67 (1.06–2.64) | 0.028 | 1.75 (1.04–2.92) | 0.032 |
| ≥11.1 | 192 (17.3) | 3.33 (2.23–4.97) | <0.001 | 2.97 (1.87–4.71) | <0.001 |
Data are presented as n (%) or median (inter quartile range). P<0.05, which means mortality in this group was different statistically with in the euglycemic group (3.9–7.7mmol/L). OR: Odds ratio; CI: Confidence interval; –: No data.
Figure 3.
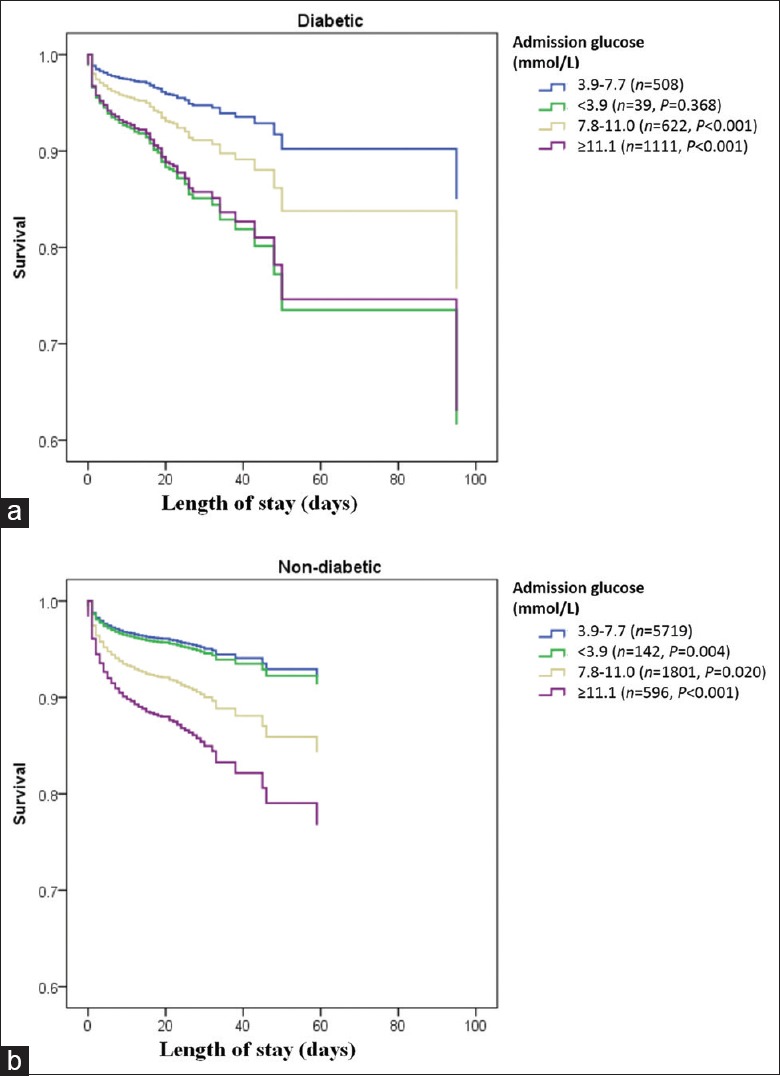
Survival curves by different admission glucose levels in diabetic and nondiabetic patients with AMI. (a) Compared with the euglycemia group, both the hypoglycemia and hyperglycemia groups were associated with lower survival rates in patients with DM (P < 0.05). (b) Only the hyperglycemia groups had lower survival rates in patients without DM (P < 0.001). AMI: Acute myocardial infarction; DM: Diabetes mellitus.
Trend over time
There was an increase in the prevalence of DM among patients hospitalized for AMI over the study period (17.5%, 20.1%, and 23.1% in 2001, 2006, and 2011, respectively, P < 0.001). The median (IQR) admission glucose level among patients without diabetes changed across years but remained similar in patients with diabetes. In-hospital mortality among patients presenting with hyperglycemia on admission increased from 17.2% in 2001 to 21.2% in 2011, but this was not statistically significant (P = 0.200 for trend). The relationship between admission glucose and in-hospital mortality risk in patients with or without recognized DM did not change across different study years (P for interaction = 0.900).
Discussion
In this nationally representative study of patients hospitalized with AMI in China, we found that after adjustment for patient characteristics, both moderate and severe hyperglycemia on admission were associated with an elevated risk for mortality among both nondiabetic and diabetic patients, especially so with severe hyperglycemia when the blood glucose is more than 11.1 mmol/L. This is consistent with what has been observed in Western populations. On the other hand, with hypoglycemia (< 3.9 mmol/L), only diabetics had an increased risk for in-hospital mortality. This relationship between admission glucose and in-hospital mortality has not changed over the past decade.
Prior studies in China have attempted to characterize the relationship of admission serum glucose level to outcomes.[10,11,12,13] Zhang et al. in 2007[13] showed that the cumulative 1-year major adverse cardiac event-free survival rate was significantly lower in diabetic than that in nondiabetic group. Liu et al.[10] in 2009 indicated that nondiabetic patients with an admission glucose of 7.8–11.0 mmol/L, 11.1–13.0 mmol/L, and >13.0 mmol/L had a higher risk for 30-day mortality than those with an admission glucose <6.1 mmol/L. However, among diabetic patients, only those with an admission glucose >13.0 mmol/L had a higher risk for mortality. Li et al.[11] in 2011 showed nondiabetics had higher crude rate of in-hospital mortality at glucose levels of 9–11 mmol/L and >11 mmol/L, but among diabetics, the mortality rate was significantly higher only among patients with admission glucose >11 mmol/L. The regression analysis in this study, however, was conducted on the entire study sample and not stratified by diabetes, which precludes meaningful interpretation of these results. Another study by Liu et al.[12] in 2012 showed a graded increase in 7- and 30-day mortality risk with increasing glucose levels, but this study was limited to nondiabetics. Liang et al.[17] found a J-curve relationship between fasting glucose and mortality in diabetic patients with AMI, but with limited study size to show statistical significance. Moreover, a single site study in Russian by Karetnikova et al.[18] showed that hypoglycemia in diabetic patients with AMI was related with poor long-term outcomes.
These prior studies, however, were limited by sample size and thus unable to elicit the relationship of serum glucose with mortality over different degrees of hyperglycemia and how this relationship varied between diabetics and nondiabetics. In addition, these studies did not evaluate the relationship between hypoglycemia and mortality and how it varied with the presence or absence of diabetes. Further, these studies did not originate from nationally representative data, which limit their generalizability. The China PEACE study, with its nationally representative design and large population, overcomes these shortcomings and provides an ideal opportunity to study the relationship of admission glucose with mortality among Chinese patients.
At a physiologic level, several perturbations in glucose metabolism occur during an acute event like myocardial infarction. Hyperglycemia may be a marker or a mediator of adverse outcomes in patients with AMI. AMI induces a stress reaction; the more severe the infarction, the stronger the stress reaction can be. This stress reaction promotes hepatic glycogen decomposition and secretion of glucagon, resulting in the inhibition of insulin secretion. In addition, the stress reaction causes activation of the neuroendocrine system increasing catecholamine levels and levels of cortisol and growth hormone, which together lead to stress-induced hyperglycemia.[19] However, this hyperglycemia could be harmful for ischemic myocardium. In patients with AMI, elevated glucose levels are associated with higher free fatty acid concentrations, insulin resistance, pro-oxidative/pro-inflammatory state, and impaired myocardial glucose use,[20,21] which in turn increase oxygen consumption and decrease ventricular contractility, potentially worsening ischemia.[22] Hyperglycemia also enhances the inflammatory reaction of the endothelium by promoting the expression of intercellular adhesion molecules, which increases the adhesion and aggregation of platelets, causing further endothelial injury and microvascular dysfunction. Further, elevated blood glucose levels decrease the ischemic preconditioning of myocardial cells, making them vulnerable to ischemia and hypoxic injury.[23,24,25] Hyperglycemia mediates activation of nonoxidative glucose pathways (NOGPs) (i.e., the polyol pathway, formation of advanced glycation end products [AGEs], the hexosamine biosynthetic pathway, and activation of protein kinase C). There is a unique interplay between NOGPs and a downstream convergence of detrimental effects that especially affect cardiac endothelial cells, thereby contributing to contractile dysfunction. In this process, the AGE pathway acts as a crucial mediator of hyperglycemia-mediated detrimental effects. In addition, a vicious metabolic cycle is established whereby hyperglycemia-induced NOGPs further fuel their own activation by generating even more oxidative stress, thereby exacerbating damaging effects on cardiac function.[26]
The study showed that the in-hospital mortality risk with AMI increased when admission glucose levels were lower than 3.9 mmol/L for diabetic patients, but the same was not observed among nondiabetic patients. A possible explanation for this phenomenon is that the mechanism of hypoglycemia differs among diabetic patients and the dysregulated physiological responses to hypoglycemia may confer the increased mortality risk with hypoglycemia in diabetic patients with AMI. For instance, first, diabetic patients have a defect in glucagon secretion because of pancreatic β-cell dysfunction, leading to frequent hypoglycemia episodes in the first place. Second, the high magnitude of blood glucose variation drives further hypoglycemia, which is a pathophysiological characteristic of diabetes. Monnier et al.[27] showed that oxidative stress is closely correlated with the mean amplitude of glycemic excursions, but not with mean glucose or HbA1c. Third, diabetic patients often develop functional disturbances of autonomic nervous system which attenuates the stress response system and thus they are unable to correct hypoglycemia. Fourth, spontaneous hypoglycemia could be the marker of other underlying disease states which elevate mortality risk, such as renal insufficiency, liver disease, sepsis, malnutrition, secondary adrenocortical insufficiency, and energy and metabolism dysfunction.[28,29] Fifth, insufficient dietary intake could also play a role in inducing hypoglycemia.
In addition, hypoglycemia has been found to be to be associated with higher sympathetic activity and catecholamine levels, which promotes destabilization of atherosclerotic plaques.[29,30] In diabetic patients particularly, hypoglycemia induces an increase in platelet aggregation as well as leads to adverse hemodynamic changes which increase myocardial demand furthering cardiac ischemia.[31,32] In addition, Robinson et al.[33] found an increased frequency of ischemic electrocardiogram changes and a nocturnal increase in the corrected QT interval accompanied by rhythm disturbances among diabetics when hypoglycemia developed. Hypoglycemia causes QT prolongation and the risk of ventricular tachycardia by directly suppressing K+ currents activated during repolarization. Since diabetes, myocardial infarction, hypertrophy, autonomic neuropathy, and congestive heart failure also cause QT prolongation, the arrhythmogenic effect of hypoglycemia is likely to be greatest in patients with preexistent cardiac disease and diabetes. Furthermore, the catecholamine surge during hypoglycemia raises intracellular Ca2+, thereby increasing the risk of ventricular tachycardia and fibrillation by the mechanism that activated by sympathomimetic inotropic agents and digoxin.[34] This might in part explain why hypoglycemia is associated with a higher risk in diabetic patients. The tolerance for hypoglycemia among diabetic patients was much lower than that for hyperglycemia. Hypoglycemia in diabetic patients predicts a more severe outcome with a remarkable increase in acute cerebrovascular events and all-cause mortality rate.[30,35]
The study provides the most comprehensive assessment in China of the relationship of admission glucose and in-hospital mortality across the full spectrum of glucose values in patients with and without known diabetes. Given the high prevalence of blood glucose disturbances in diabetic patients with AMI,[36] appropriate risk stratification and intervention based on admission glucose values may be of substantial benefit. Going forward, the results of our study could inform risk assessment protocols using admission glucose.
The study had several limitations. First, the analysis was limited to admission glucose values. Therefore, we do not know how the in-hospital treatment of hyperglycemia and hypoglycemia and how the subsequent glucose levels during hospitalization affected mortality risk. Second, although our multivariable adjustment accounted for several key demographic and clinical factors, a possibility of residual confounding by unmeasured factors cannot be entirely excluded. Third, the associations identified in the current cross-sectional study might be affected by reverse causality; thus, the findings need to be validated in future mechanic researches and prospective studies before causal inference.
In conclusions, hyperglycemia at presentation is common among patients with AMI in China and associated with a similar increased risk of death in both diabetics and nondiabetics. However, hypoglycemia increased risk of death only in diabetic patients. Further study is needed to elucidate the reasons for this pattern in China.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81370942 and No. 31400916), the Research Special Fund for Public Welfare Industry of Health from the National Health and Family Planning Commission of China (No. 201502009), the National Key Technology R & D Program from the Ministry of Science and Technology of China (No. 2013BAI09B01, No. 2015BAI12B01, and No. 2015BAI12B02), the 111 Project from the Ministry of Education (No. B16005), and the Center for Cardiovascular Outcomes Research at Yale University (No. U01 HL105270).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet. 2000;355:773–8. doi: 10.1016/S0140-6736(99)08415-9. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 2.Kosiborod M, Rathore SS, Inzucchi SE, Masoudi FA, Wang Y, Havranek EP, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: Implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–86. doi: 10.1161/CIRCULATIONAHA.104.517839. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 3.Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. ICONS Investigators. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–54. doi: 10.1016/s0735-1097(02)02483-x. doi: 10.1016/S0735-1097(02)02483-X. [DOI] [PubMed] [Google Scholar]
- 4.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, et al. Glucometrics in patients hospitalized with acute myocardial infarction: Defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–27. doi: 10.1161/CIRCULATIONAHA.107.740498. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara M, Kojima S, Sakamoto T, Kimura K, Kosuge M, Asada Y, et al. Comparison of blood glucose values on admission for acute myocardial infarction in patients with versus without diabetes mellitus. Am J Cardiol. 2009;104:769–74. doi: 10.1016/j.amjcard.2009.04.055. doi: 10.1016/j.amjcard.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 6.Yang SW, Zhou YJ, Hu DY, Nie XM, Liu YY, Hua Q, et al. Association between admission hypoglycaemia and in-hospital and 3-year mortality in older patients with acute myocardial infarction. Heart. 2010;96:1444–50. doi: 10.1136/hrt.2009.189316. doi: 10.1136/hrt.2009.189316. [DOI] [PubMed] [Google Scholar]
- 7.Svensson AM, McGuire DK, Abrahamsson P, Dellborg M. Association between hyper- and hypoglycaemia and 2 year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J. 2005;26:1255–61. doi: 10.1093/eurheartj/ehi230. doi: 10.1093/eurheartj/ehi230. [DOI] [PubMed] [Google Scholar]
- 8.Haynes R, Jiang L, Hopewell JC, Li J, Chen F, Parish S, et al. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–91. doi: 10.1093/eurheartj/eht055. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–58. doi: 10.2165/00003088-200241120-00002. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Liu LS, et al. Impact of admission blood glucose on prognosis of ST-segment elevation myocardial infarction patients with or without known diabetes (In Chinese) Chin J Cardiol. 2009;37:590–4. doi: 10.3760/cma.j.issn.0253-3758.2009.07.006. [PubMed] [Google Scholar]
- 11.Li DB, Hua Q, Guo J, Li HW, Chen H, Zhao SM. Admission glucose level and in-hospital outcomes in diabetic and non-diabetic patients with ST-elevation acute myocardial infarction. Intern Med. 2011;50:2471–5. doi: 10.2169/internalmedicine.50.5750. doi: 10.2169/internalmedicine.50.5750. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Li JD. Haemoglobin A (1c), acute hyperglycaemia and short-term prognosis in patients without diabetes following acute ST-segment elevation myocardial infarction. Diabet Med. 2012;29:1493–500. doi: 10.1111/j.1464-5491.2012.03641.x. doi: 10.1111/j1464-5491.2012.03641.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Shen J, Zhang RY, Qiu JP, Lu JD, Zhang Y, et al. Outcomes after primary coronary intervention with drug-eluting stent implantation in diabetic patients with acute ST elevation myocardial infarction. Chin Med J. 2007;120:1862–7. [PubMed] [Google Scholar]
- 14.Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L. The China patient-centered evaluative assessment of cardiac events (China PEACE) retrospective study of acute myocardial infarction: Study design. Circ Cardiovasc Qual Outcomes. 2013;6:732–40. doi: 10.1161/CIRCOUTCOMES.113.000441. doi: 10.1161/CIRCOUTCOMES.113.000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health of the People's Republic of China. The Qualification Standard of Secondary General Hospital (Version 2012) 2012. [Last accessed on 2014 Mar 06]. Available from: http://www.nhfpc.gov.cn/yzygj/s3586q/201201/b8dda05b1d23413c94150b5c17b5cc6f.shtml .
- 17.Liang H, Guo YC, Chen LM, Li M, Han WZ, Zhang X, et al. Relationship between fasting glucose levels and in-hospital mortality in Chinese patients with acute myocardial infarction and diabetes mellitus: A retrospective cohort study. BMC Cardiovasc Disord. 2016;16:156. doi: 10.1186/s12872-016-0331-2. doi: 10.1186/s12872-016-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karetnikova V, Gruzdeva O, Uchasova E, Osokina A, Barbarash O. Glucose levels as a prognostic marker in patients with ST-segment elevation myocardial infarction: A case-control study. BMC Endocr Disord. 2016;16:31. doi: 10.1186/s12902-016-0108-8. doi: 10.1186/s12902-016-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishihara M, Kagawa E, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99:1674–9. doi: 10.1016/j.amjcard.2007.01.044. doi: 10.1016/j.amjcard.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112:305–11. doi: 10.1016/s0002-9343(01)01104-4. doi: 10.1016/S0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 21.Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet. 1983;2:419–22. doi: 10.1016/s0140-6736(83)90388-4. doi: 10.1016/S0140-6736(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Maitland A, Weisel RD, Li SH, Fedak PW, Pomroy NC, et al. Hyperglycemia exaggerates ischemia-reperfusion-induced cardiomyocyte injury: Reversal with endothelin antagonism. J Thorac Cardiovasc Surg. 2002;123:1120–4. doi: 10.1067/mtc.2002.121973. doi: 10.1067/mtc.2002.121973. [DOI] [PubMed] [Google Scholar]
- 23.Abebe W, Mozaffari M. Endothelial dysfunction in diabetes: Potential application of circulating markers as advanced diagnostic and prognostic tools. EPMA J. 2010;1:32–45. doi: 10.1007/s13167-010-0012-7. doi: 10.1007/s13167-010-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marfella R, Siniscalchi M, Esposito K, Sellitto A, De Fanis U, Romano C, et al. Effects of stress hyperglycemia on acute myocardial infarction: Role of inflammatory immune process in functional cardiac outcome. Diabetes Care. 2003;26:3129–35. doi: 10.2337/diacare.26.11.3129. doi: 10.2337/diacare.26.11.3129. [DOI] [PubMed] [Google Scholar]
- 25.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: General concepts. Circulation. 2002;105:1727–33. doi: 10.1161/01.cir.0000012466.50373.e8. doi: 10.1161/01.CIR.0000012466.50373.E8. [DOI] [PubMed] [Google Scholar]
- 26.Mapanga RF, Essop MF. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am J Physiol Heart Circ Physiol. 2016;310:H153–73. doi: 10.1152/ajpheart.00206.2015. doi: 10.1152/ajpheart.00206.2015. [DOI] [PubMed] [Google Scholar]
- 27.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. doi: 10.1001/jama.295.14.1681. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 28.Vriesendorp TM, van Santen S, DeVries JH, de Jonge E, Rosendaal FR, Schultz MJ, et al. Predisposing factors for hypoglycemia in the Intensive Care Unit. Crit Care Med. 2006;34:96–101. doi: 10.1097/01.ccm.0000194536.89694.06. doi: 10.1097/01.CCM.0000194536.89694.06. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Hendler R, Christensen N. Stimulation of counterregulatory hormonal responses in diabetic man by a fall in glucose concentration. Diabetes. 1980;29:125–31. doi: 10.2337/diab.29.2.125. doi: 10.2337/diab.29.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Hsu PF, Sung SH, Cheng HM, Yeh JS, Liu WL, Chan WL, et al. Association of clinical symptomatic hypoglycemia with cardiovascular events and total mortality in type 2 diabetes: A nationwide population-based study. Diabetes Care. 2013;36:894–900. doi: 10.2337/dc12-0916. doi: 10.2337/dc12-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutton RA, Mikhailidis D, Dormandy KM, Ginsburg J. Platelet aggregation studies during transient hypoglycaemia: A potential method for evaluating platelet function. J Clin Pathol. 1979;32:434–8. doi: 10.1136/jcp.32.5.434. doi: 10.1136/jcp.32.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frier BM, Corrall RJ, Davidson NM, Webber RG, Dewar A, French EB. Peripheral blood cell changes in response to acute hypoglycaemia in man. Eur J Clin Invest. 1983;13:33–9. doi: 10.1111/j.1365-2362.1983.tb00061.x. doi: 10.1111/j.1365-2362.1983.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 33.Robinson RT, Harris ND, Ireland RH, Macdonald IA, Heller SR. Changes in cardiac repolarization during clinical episodes of nocturnal hypoglycaemia in adults with type 1 diabetes. Diabetologia. 2004;47:312–5. doi: 10.1007/s00125-003-1292-4. doi: 10.1007/s00125-003-1292-4. [DOI] [PubMed] [Google Scholar]
- 34.Nordin C. The case for hypoglycaemia as a proarrhythmic event: Basic and clinical evidence. Diabetologia. 2010;53:1552–61. doi: 10.1007/s00125-010-1752-6. doi: 10.1007/s00125-010-1752-6. [DOI] [PubMed] [Google Scholar]
- 35.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolk RP, Pols HA, Lamberts SW, de Jong PT, Hofman A, Grobbee DE. Diabetes mellitus, impaired glucose tolerance, and hyperinsulinemia in an elderly population. The Rotterdam Study. Am J Epidemiol. 1997;145:24–32. doi: 10.1093/oxfordjournals.aje.a009028. doi: 10.1093/oxfordjournals.aje.a009028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of admission glucose levels in diabetic and non-diabetic patients with acute myocardial infarction.


