Abstract
Background:
A relationship between hyperthyroidism and insulin secretion in type 2 diabetes mellitus (T2DM) has been reported. Therefore, this study explored the use of first-phase insulin secretion in the differential diagnosis of thyroid diabetes (TDM) and T2DM.
Methods:
In total, 101 patients with hyperthyroidism were divided into hyperthyroidism with normal glucose tolerance (TNGT), hyperthyroidism with impaired glucose regulation (TIGR), and diabetes (TDM) groups. Furthermore, 96 patients without hyperthyroidism were recruited as control groups (normal glucose tolerance [NGT], impaired glucose regulation [IGR], and T2DM). The following parameters were evaluated: homeostasis model assessment (HOMA)-IR, HOMA-β, modified β-cell function index (MBCI), peak insulin/fasting insulin (IP/I0), AUCins-OGTT, and AUCins-OGTT/AUCglu-OGTT from the oral glucose tolerance test (OGTT) insulin release test were utilized to assess the second-phase insulin secretion, while the IP/I0, AIR0′~10′, and AUCins-IVGTT from the intravenous glucose tolerance test (IVGTT) insulin release test were used to assess the first-phase insulin secretion.
Results:
In the OGTT, the HOMA-β values of the TNGT and TDM groups were higher than those of the NGT and T2DM groups (all P < 0.05). In the hyperthyroidism groups, the MBCI of the TDM group was lower than that of the TNGT and TIGR groups (all P < 0.05). Among the control groups, the MBCI values of the IGR and T2DM groups were lower than that of the normal glucose tolerance (NGT) group (all P < 0.05). In the IVGTT, insulin secretion peaked for all groups at 2–4 min, except for the T2DM group, which showed a low plateau and no secretion peak. The IP values of the TNGT, TIGR, and TDM groups were higher than those of the NGT, IGR, and T2DM groups (all P < 0.05). The Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the TDM group were higher than those of the T2DM group but were lower than those of the TNGT, TIGR, NGR, and IGR groups (all P < 0.05). Compared with the other five groups, the Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the T2DM group were significantly decreased (all P < 0.05). The Ip/I0 and AUCins-IVGTT values of the TNGT group were higher than those of the NGT group (all P < 0.05).
Conclusions:
β-cell function in TDM patients is superior to that in T2DM patients. First-phase insulin secretion could be used as an early diagnostic marker to differentiate TDM and T2DM.
Keywords: Diabetes Mellitus, First-phase Insulin Secretion, Intravenous Glucose, Oral Glucose, Thyroid Diabetes
Introduction
High thyroid hormone levels in hyperthyroidism can perturb glucose metabolism and result in diabetes mellitus, which denoted as thyroid diabetes (TDM).[1] Clinically, TDM is difficult to be distinguished from type 2 diabetes mellitus (T2DM). Because there is a need to screen for complications resulting from T2DM after diagnosis, and because effective treatment of TDM could normalize blood glucose levels, early differentiation of these diseases could reduce treatment costs by reducing unnecessary screening. The mechanism of TDM involves thyroid hormone-induced insulin resistance, while T2DM is mainly caused by pancreatic islet β-cell dysfunction. Theoretically, β-cell function, particularly first-phase insulin secretion, in TDM is better than in T2DM. However, there have been few reports concerning differentiating between TDM and T2DM. In the present study, clinical data of hyperthyroidism patients with either normal glucose tolerance (TNGT), hyperthyroidism with impaired glucose regulation (TIGR), or diabetes (TDM) were collected. Potential differences in insulin secretion between TDM and T2DM raise the possibility that hyperthyroidism patients with normal first-phase insulin secretion may have TDM. Herein, we have clinically validated this and provided new insights regarding the early diagnosis of TDM.
Methods
Participants
In total, 101 patients with hyperthyroidism, including inpatients and outpatients, were enrolled from the Department of Endocrinology of the First Affiliated Hospital of Guangxi Medical University from January 2010 to September 2015. Patients included 47 males (46.53%) and 54 females (53.47%), with an average age of 48.15 ± 13.22 years and an average body mass index (BMI) of 22.20 ± 4.81 kg/m2. Based on oral glucose tolerance test (OGTT) results, hyperthyroidism patients were further divided into TNGT, TIGR, and TDM groups. Meanwhile, 96 patients with NGT, impaired glucose regulation (IGR), and T2DM were recruited as control groups, including 63 males (65.62%) and 33 females (34.38%), with an average age of 49.44 ± 14.08 years and an average BMI of 25.68 ± 4.70 kg/m2. Hyperthyroidism was diagnosed based on the 2011 guidelines by the American Association of Clinical Endocrinologists and the American Thyroid Association. The diagnosis and classification of diabetes were performed according to the 1999 guidelines of the World Health Organization. The hyperthyroidism group inclusion criteria were: (i) the diagnostic criteria for hyperthyroidism were met; (ii) patients had not received anti-hyperthyroidism treatment for the prior 3 months. The hyperthyroidism group exclusion criteria were: (i) patients with nonhyperthyroidism-induced thyrotoxicosis; (ii) patients with infections, operative trauma, and other stresses; (iii) type 1 diabetes mellitus, T2DM, gestational diabetes mellitus. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki. Informed consent was obtained from all patients for being included in the study.
All participants were asked about their clinical history and underwent a physical examination. Patient information, including age, sex, height, and weight, were collected. Moreover, the levels of triiodothyronine (T3), thyroxine (T4), free T3 (FT3), free T4 (FT4), and thyroid-stimulating hormone (TSH) in all participants were measured.
Oral glucose tolerance test and insulin release test
Blood plasma glucose and specific insulin were measured after 1, 2, and 3 h postoral consumption of 75 g anhydrous glucose after overnight fasting. The following parameters were evaluated: (i) the homeostasis model assessment of insulin resistance (HOMA-IR);[2,3] (ii) the homeostasis model assessment of β-cell function (HOMA-β);[4] (iii) the area under the insulin curve (AUCins-OGTT); (iv) the area under the glucose curve (AUCglu-OGTT). HOMA-IR was used to assess insulin resistance. HOMA-β and the modified β-cell function index (MBCI) were used to evaluate the β-cell secretory function. Peak insulin/fasting insulin (IP/I0),[5] AUCins-OGTT, and AUCins-OGTT/AUCglu-OGTT were used to evaluate second-phase insulin secretion. These parameters were calculated as follows: (i) HOMA-IR = (G0 × I0)/22.5, (ii) HOMA-β = 20 × I0/(G0 − 3.5), (iii) AUCins-OGTT[6] = 0.5I0 + I1 + I2+ 0.5I3, (iv) AUCglu-OGTT[7] = 0.5G0 + G1 + G2+0.5G3, ans (v) MBCI[8] = (G0 × I0)/(G2 + G1 − 2G0), where G0, G1, G2, and G3 denote blood glucose levels (mmol/L) at 0, 1, 2, and 3 h, respectively, and I0, I1, I2, and I3 denote insulin levels (μU/ml) at 0, 1, 2, and 3 h, respectively.
Intravenous glucose tolerance test and insulin release test
Glucose (25 g; 50 ml of 50% glucose) was rapidly injected intravenously within 1 min. Using the injection time as the start time, blood was taken at 0, 1, 2, 4, 6, and 10 min to measure blood glucose and specific insulin. After glucose stimulation, the acute insulin secretary response (AIR0′~10′)[9], IP/I0,[5] and the area under the glucose curve of intravenous glucose tolerance test (AUCins-IVGTT)[10] were adopted to assess the first-phase insulin secretion. These parameters were calculated as follows: (i) AIR0′~10′= (I1′+ I2′+ I4′+ I6′+ I10)/5 − I0′, (ii) AUCins-IVGTT = 0.5I0′+ I1′+ 1.5I2′+ 2I4′+ 3I6′+ 2I10′, where I0, I1, I2, I4, I6, and I10 denote insulin levels at 0, 1, 2, 4, 6, and 10 min, respectively.
Blood glucose was measured using the 7600 automatic biochemical analyzer (Hitachi, Tokyo, Japan). Thyroid function was measured using a Beckman DXI800 analyzer (Beckman Coulter, Inc., California Pasadena, USA). Specific serum insulin was measured using a Roche E170 ECL instrument (Roche Diagnostics GmbH, Switzerland, Germany).
Statistical analysis
Data were processed using SPSS version 18.0. (SPSS Inc., USA). Normally distributed data are expressed as mean ± standard deviation (SD) while nonnormally distributed data were converted to logarithm (Ln) values and then expressed as mean ± SD. Ln values that were nonnormally distributed are presented as median (Q1, Q3). Based on the distribution characteristics of the clinical data of each group, t-tests were performed to compare data showing a normal distribution and homogeneity of between-group variance, while analysis of variance (ANOVA) was used to compare data from multiple groups. The homogeneity of variance was calculated using Fisher's least significant difference, while the heterogeneity of variance was calculated using the Games-Howell method. Ln values for nonnormally distributed data were assessed using either t-test or ANOVA. A two-tailed P < 0.05 was considered statistically significant.
Results
Clinical data of participants
The BMI and TSH values of the hyperthyroidism group were lower than those of the control groups, whereas T3, T4, FT3, and FT4 levels were higher than those of the control groups. The ages of the TDM and T2DM groups were higher than those of the TNGT and NGT groups. These differences were statistically significant (P < 0.05). The duration of T2DM group was significantly higher than that of the other five groups, the difference was statistically significant [P < 0.05; Table 1].
Table 1.
Patient characteristics
| Variables | Hyperthyroidism group | Control groups | F | P | ||||
|---|---|---|---|---|---|---|---|---|
| TNGT | TIGR | TDM | NGT | IGR | T2DM | |||
| n (male/female) | 32 (11/21) | 35 (18/17) | 34 (18/16) | 33 (22/11) | 28 (19/9) | 35 (22/13) | ||
| Age (years) | 41.48± 11.71‡,¶ | 48.00± 12.27 | 54.71± 12.71*,§ | 45.26± 15.43‡,¶ | 48.29± 12.98 | 54.31± 12.40*,§ | 4.093 | 0.002 |
| BMI (kg/m2) | 22.21± 4.45§,|| | 21.67± 4.83§,|| | 22.79± 5.45§,|| | 26.80± 4.97*,†,‡,¶ | 26.32± 5.05*,†,‡ | 24.31± 3.98§ | 9.416 | 0.000 |
| Diabetes duration (months) | 0¶ | 0¶ | 0¶ | 0¶ | 0.27 (0.10± 3.25)¶ | 38.00 (15.00± 61.00)*,†,‡,§,|| | 15.555 | 0.000 |
| T3 (nmol/L) | 4.09± 1.88§,||,¶ | 4.04± 1.74§,||,¶ | 3.08± 1.73||,¶ | 2.27± 1.60*,† | 1.93± 1.34*,†,‡ | 1.73± 1.31*,†,‡ | 11.278 | 0.000 |
| T4 (nmol/L) | 172.90± 1.94§,||,¶ | 218.59± 1.51§,||,¶ | 156.27± 1.61§,||,¶ | 100.08± 1.85*,†,‡ | 111.81± 1.26*,†,‡ | 114.33± 1.24*,†,‡ | 9.167 | 0.000 |
| FT3 (pmol/L) | 12.42± 2.74§,||,¶ | 13.21± 1.97§,||,¶ | 9.33± 2.37§,||,¶ | 5.30± 1.63*,†,‡ | 5.14± 1.53*,†,‡ | 4.43± 1.12*,†,‡ | 12.848 | 0.000 |
| FT4 (pmol/L) | 26.25± 3.16§,||,¶ | 33.82± 1.89§,||,¶ | 23.76± 2.78§,||,¶ | 11.34± 1.21*,†,‡ | 12.01± 1.39*,†,‡ | 12.17± 1.17*,†,‡ | 11.028 | 0.000 |
| TSH (mU/L) | 0.02 (0.01, 0.07)§,||,¶ | 0.01 (0.01, 004)§,||,¶ | 0.06 (0.01, 0.24)§,||,¶ | 1.97 (1.01, 2.63)*,†,‡ | 1.44 (0.81, 2.51)*,†,‡ | 1.79 (0.94, 2.48)*,†,‡ | 32.067 | 0.000 |
Data are expressed as mean ± standard deviation or median (Q1, Q3). *P<0.05 by one-way ANOVA (vs. TNGT); †P<0.05 by one-way ANOVA (vs. TIGR); ‡P<0.05 by one-way ANOVA (vs. TDM); §P<0.05 by one-way ANOVA (vs. NGT); ||P<0.05 by one-way ANOVA (vs. IGR); ¶P<0.05 by one-way ANOVA (vs. T2DM). TNGT: Hyperthyroidism with normal glucose tolerance; TIGR: Hyperthyroidism with impaired glucose regulation; TDM: Hyperthyroidism with diabetes mellitus; NGT: Normal glucose tolerance control group; IGR: Impaired glucose regulation control group; T2DM: Type 2 diabetes mellitus control group; BMI: Body mass index; T3: Triiodothyronine; T4: Thyroxine; FT3: Free T3; FT4: Free T4; TSH: Thyroid-stimulating hormone; ANOVA: Analysis of variance.
Comparison between oral glucose tolerance test and insulin release test
Comparison between peak values and peak decline of oral glucose tolerance test insulin secretion
Ratios of insulin peaks, declines, and delayed peaks of the TNGT, TIGR, and TDM groups were 15.6%, 45.7%, and 67.6%, respectively, while the ratios of the NGT, IGR, and T2DM groups were 18.2%, 35.7%, and 91.4%, respectively. Compared with the T2DM group, the IP of the TDM group was increased, although the difference was not significant [P > 0.05; [Table 2].
Table 2.
Comparison of peak decline or delayed peak of insulin secretion during the oral glucose tolerance test
| Group | Peak decline or delayed peak | LnIp |
|---|---|---|
| TNGT | 5 (15.6) | 4.56 ± 0.51 |
| TIGR | 16 (45.7) | 4.69 ± 0.48 |
| TDM | 23 (67.6) | 4.03 ± 1.14 |
| NGT | 6 (18.2) | 4.63 ± 0.49 |
| IGR | 10 (35.7) | 4.67 ± 0.64 |
| T2DM | 32 (91.4) | 3.93 ± 0.60 |
Data are expressed as n (%) and mean ± standard deviation. Peak decline was defined as Ip/I0 <5, and delayed peaks denoted the appearance of insulin peaks after 1 h; Ip was presented as mean ± standard deviation after conversion into normally distributed Ln values. Ln: Logarithm; TNGT: Hyperthyroidism with normal glucose tolerance; TIGR: Hyperthyroidism with impaired glucose regulation; TDM: Hyperthyroidism with diabetes mellitus; NGT: Normal glucose tolerance control group; IGR: Impaired glucose regulation control group; T2DM: Type 2 diabetes mellitus control group; IP/I0: Peak insulin/fasting insulin.
Comparisons of homeostasis model assessment of insulin resistance, homeostasis model assessment-β, modified β-cell function index, Ip/I0, AUCins-OGTT, and AUCins-OGTT/AUCglu-OGTT values
The HOMA-β values of the TNGT and TDM groups were higher than those of the NGT and T2DM groups (P < 0.05). In the hyperthyroidism groups, the MBCI of the TDM group was lower than those of the TNGT and TIGR groups (P < 0.05). In the control groups, the MBCI values of the IGR and T2DM groups were lower than that of the NGT group and differences were statistically significant [P < 0.05; Table 3].
Table 3.
Comparison of oral glucose tolerance-related indexes among all groups
| Group | HOMA-IR | LnHOMA-β | LnMBCI | LnIp/I0 | AUCins-OGTT | AUCins-OGTT/AUCglu-OGTT |
|---|---|---|---|---|---|---|
| Hyperthyroidism groups | ||||||
| TNGT | 1.47 ± 0.58 | 5.41 ± 1.08†,‡,§,||,¶ | 1.70 ± 0.43‡,¶ | 2.68 ± 0.40¶ | 139.82 ± 83.75†,|| | 7.17 ± 3.83‡,¶ |
| TIGR | 1.56 ± 0.56 | 4.88 ± 1.11*,¶ | 1.20 ± 0.78‡,§ | 2.65 ± 0.64¶ | 214.02 ± 105.07*,‡,¶ | 8.47 ± 3.85†,‡,¶ |
| TDM | 1.67 ± 0.72 | 4.59 ± 1.06*,¶ | 0.59 ± 0.21*,†,§,|| | 2.32 ± 0.51 | 137.61 ± 57.18†,|| | 4.67 ± 2.06*,§,|| |
| Control groups | ||||||
| NGT | 1.41 ± 0.52 | 4.79 ± 0.72*,¶ | 1.99 ± 0.90†,‡,||,¶ | 2.41 ± 0.52¶ | 178.37 ± 77.80¶ | 8.68 ± 3.70‡,¶ |
| IGR | 1.50 ± 0.47 | 4.71 ± 0.92*,¶ | 1.31 ± 0.79‡,§,¶ | 2.68 ± 0.67¶ | 199.66 ± 107.14*,‡,¶ | 8.38 ± 4.23‡,¶ |
| T2DM | 1.57 ± 0.63 | 3.75 ± 0.81*,†,‡,§,|| | 0.73 ± 0.39*,§,|| | 2.11 ± 0.57*,†,§,|| | 102.88 ± 43.66†,§,|| | 2.86 ± 1.92*,†,§,|| |
| F | 1.472 | 9.249 | 8.231 | 4.729 | 5.978 | 11.260 |
| P | 0.203 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Data are presented as mean ± standard deviation. HOMA-β, MBCI, and Ip/I0 are presented as mean ± standard deviation, after conversion into normally distributed Ln values. *P<0.05 by one-way ANOVA (vs. TNGT); †P<0.05 by one-way ANOVA (vs. TIGR); ‡P<0.05 by one-way ANOVA (vs. TDM); §P<0.05 by one-way ANOVA (vs. NGT); ||P<0.05 by one-way ANOVA (vs. IGR); ¶P<0.05 by one-way ANOVA (vs. T2DM). Ln: Logarithm; HOMA-IR: Homeostatic assessment model of insulin resistance; HOMA-β: Homeostatic assessment model of β-cell function; MBCI: Modified β-cell function index; AUC: Area under the curve; TNGT: Hyperthyroidism with normal glucose tolerance; TIGR: Hyperthyroidism with impaired glucose regulation; TDM: Hyperthyroidism with diabetes mellitus; NGT: Normal glucose tolerance control group; IGR: Impaired glucose regulation control group; T2DM: Type 2 diabetes mellitus control group; ANOVA: Analysis of variance; IP/I0: Peak insulin/fasting insulin.
Comparison between intravenous glucose tolerance test and insulin release test
Comparison between peak values and peak decline of intravenous glucose tolerance test insulin secretion
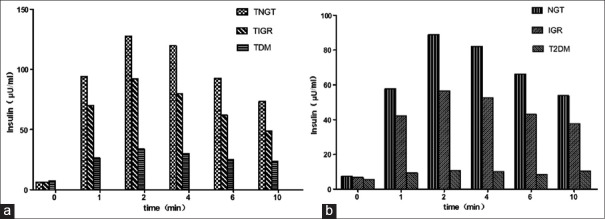
All groups showed insulin secretion peaks at 2–4 min, except for the T2DM group, which showed a low plateau and an absence of insulin peaks [Figure 1]. The ratios of peak values, declines, and delayed peaks for the TNGT, TIGR, and TDM groups were 6.3%, 11.4%, and 55.9%, respectively, while the ratios for the NGT, IGR, and T2DM groups were 9.1%, 32.1%, and 80.0%, respectively. The IP of the hyperthyroidism groups (TNGT, TIGR, and TDM) were higher compared with the corresponding control groups (NGT, IGR, and T2DM) [P < 0.05; Table 4].
Figure 1.
Intravenous glucose tolerance test-specific insulin concentrations at various time points. a: TNGT, hyperthyroidism with normal glucose tolerance; TIGR, hyperthyroidism with impaired glucose regulation; TDM, hyperthyroidism with diabetes mellitus. b: NGT, normal glucose tolerance control group; IGR, impaired glucose regulation control group; T2DM, type 2 diabetes mellitus control group.
Table 4.
Comparison of peak declines and delayed peaks of insulin secretion during the intravenous glucose tolerance test
| Group | Peak decline or delayed peak | LnIp |
|---|---|---|
| TNGT | 2 (6.3) | 4.97 ± 0.53†,‡,§,||,¶ |
| TIGR | 4 (11.4) | 4.53 ± 0.65*,‡,||,¶ |
| TDM | 19 (55.9) | 3.60 ± 1.01*,†,§,||,¶ |
| NGT | 3 (9.1) | 4.60 ± 0.67*,‡,||,¶ |
| IGR | 9 (32.1) | 4.11 ± 0.75*,†,‡,§,¶ |
| T2DM | 28 (80.0) | 2.55 ± 0.62*,†,‡,§,|| |
Data are expressed as n (%) or mean ± standard deviation. Peak decline was defined as Ip/I0 <5, and delayed peaks denoted the appearance of insulin peaks after 4 min; Ip was presented as mean ± standard deviation, after conversion into normally distributed Ln values; *P<0.05 by one-way ANOVA (vs. TNGT); †P<0.05 by one-way ANOVA (vs. TIGR); ‡P<0.05 by one-way ANOVA (vs. TDM); §P<0.05 by one-way ANOVA (vs. NGT); ||P<0.05 by one-way ANOVA (vs. IGR); ¶P<0.05 by one-way ANOVA (vs. T2DM). TNGT: Hyperthyroidism with normal glucose tolerance; TIGR: Hyperthyroidism with impaired glucose regulation; TDM: Hyperthyroidism with diabetes mellitus; NGT: Normal glucose tolerance control group; IGR: Impaired glucose regulation control group; T2DM: Type 2 diabetes mellitus control group; ANOVA: Analysis of variance; IP/I0: Peak insulin/fasting insulin.
Comparisons of Ip/I0, AIR0′~10′, and AUCins-IVGTT
The Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the TDM group were higher than those of the T2DM group but were lower than those of the TNGT, TIGR, NGT, and IGR groups. Compared with the other five groups, the Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the T2DM group were decreased significantly (P < 0.05). The Ip/I0 and AUCins-IVGTT values of the TNGT group were higher than those of the NGT group [P < 0.05; Table 5].
Table 5.
Comparison of intravenous glucose tolerance test-related indexes among all groups
| Group | LnIp/I0 | Ln AIR0′~10′ | LnAUCins-IVGTT |
|---|---|---|---|
| Hyperthyroidism groups | |||
| TNGT | 3.11 ± 0.78†,‡,§,||,¶ | 4.59 ± 0.61‡,||,¶ | 6.91 ± 0.57†,‡,§,||,¶ |
| TIGR | 2.67 ± 0.88*,‡,¶ | 4.15 ± 0.75‡,¶ | 6.50 ± 0.71*,‡,¶ |
| TDM | 1.50 ± 0.96*,†,§,||,¶ | 2.78 ± 1.47*,†,§,||,¶ | 5.61 ± 0.97*,†,§,||,¶ |
| Control groups | |||
| NGT | 2.60 ± 0.66*,‡,¶ | 4.16 ± 0.75‡,¶ | 6.53 ± 0.70*,‡,||,¶ |
| IGR | 2.26 ± 0.83*,‡,¶ | 3.63 ± 0.88*,‡,¶ | 6.11 ± 0.74*,‡,§,¶ |
| DM | 0.84 ± 0.56*,†,‡,§,|| | 1.03 ± 0.72*,†,‡,§,|| | 4.58 ± 0.64*,†,‡,§,|| |
| F | 34.390 | 44.564 | 42.017 |
| P | 0.000 | 0.000 | 0.000 |
Data are expressed as mean ± standard deviation. Note: Ip/I0, AIR0′~10′, and AUCins-IVGTT were presented as mean ± standard deviation, after conversion into normally distributed Ln values. *P<0.05 by one-way ANOVA (vs. TNGT); †P<0.05 by one-way ANOVA (vs. TIGR); ‡P<0.05 by one-way ANOVA (vs. TDM); §P<0.05 by one-way ANOVA (vs. NGT); ||P<0.05 by one-way ANOVA (vs. IGR); ¶P<0.05 by one-way ANOVA (vs. T2DM). AIR: Acute insulin secretary response; AUC: Area under the curve; TNGT: Hyperthyroidism with normal glucose tolerance; TIGR: Hyperthyroidism with impaired glucose regulation; TDM: Hyperthyroidism with diabetes mellitus; NGT: Normal glucose tolerance control group; IGR: Impaired glucose regulation control group; T2DM: Type 2 diabetes mellitus control group; ANOVA: Analysis of variance; IP/I0: Peak insulin/fasting insulin.
Discussion
There has been a sharp increase in the prevalence of diabetes in China.[11] As T2DM progresses silently, 50% of patients display chronic complications upon diagnosis. Hence, local and international guidelines recommend immediate screening for complications upon diagnosis of T2DM, yet patients with TDM do not require such screening. Previous studies have found a strong correlation between hyperthyroidism and diabetes. Hyperthyroidism can exacerbate diabetic symptoms, induce ketoacidosis,[12] and disrupt glucose metabolism.[13] Approximately 50% of hyperthyroidism patients show impaired glucose tolerance and 2–3% develop diabetes. Clinically, TDM patients are not uncommon; thus, more attention should be paid to the differential diagnosis of TDM and T2DM, in order to ameliorate T2DM progression by allowing early screening for and delaying the development of complications, as well as preserving medical resources by preventing unnecessary screening for patients with TDM.
In the current study, the ratios of insulin peak declines and delayed peaks of the OGTT in the T2DM group were significantly higher than those in the TDM group. Moreover, the HOMA-β values of the TNGT and TDM groups were higher than those of the NGT and T2DM groups. In addition, as compared with the IGR group, the HOMA-β value of the TIGR group was increased. The HOMA-IR values of the NHGT, TIGR, and TDM groups were higher than those of the NGT, IGR, and T2DM groups, although the differences were not significant, probably due to the small sample size. These results indicate that β-cell function in TDM patients is superior to that in T2DM patients. It might be due to the mechanism underlying elevated blood glucose in T2DM patients stems from insulin resistance and defects in insulin secretion, of which pancreatic β-cell dysfunction is a central cause.[14] In addition, along with disease progression, the failure of β-cells to respond to insulin is a common mechanism underlying the pathogenesis of T2DM. In contrast, in hyperthyroidism patients, thyroid hormone increases intestinal glucose absorption through rapid stomach emptying and increased intestinal hexokinase and phosphokinase activity, directly influencing islet β-cells and increasing hepatic gluconeogenesis and glycogenolysis.[15] Furthermore, a reduction in peripheral glucose uptake among other effects leads to elevated blood glucose and insulin resistance,[16] resulting in diabetes. Accordingly, we speculate that, in the early stage of TDM, insulin resistance initiates a compensatory increase in insulin secretion in the absence of damage to β-cells.[17] It is worth noting that the duration of T2DM group was significantly higher than that of the other five groups, so we cannot neglect the effect of diabetes on insulin secretion.
Based on the inverse relationship between blood glucose, β-cell insulin secretion, and whole-body insulin sensitivity, Li et al. proposed the use of the MBCI.[8] In the current study, MBCI values sensitively reflected a difference (1.1 mmol glucose/L at 2 h) in β-cell function between the IGT and NGT groups. Furthermore, the MBCI became gradually lower in all groups. In the control groups, the MBCIs of the IGR and T2DM groups were lower compared with the NGT group, indicating that the MBCI reflects functional differences in β-cells between NGT and IGR patients, as well as between NGT and T2DM patients. However, in the hyperthyroidism groups, despite the lower MBCI in the TIGR group compared with the TNGT group, the difference was not statistically significant, indicating no difference in β-cell function between these groups. One possible explanation for this might be that, while TDM mainly stems from insulin resistance, insulin resistance and defects in insulin secretion, in which β-cell dysfunction is important, cause T2DM. Based on the formula for the MBCI, if blood glucose levels in the NGT, IGR, and T2DM groups at various stages were similar, the I0 more strongly influenced MBCI values. However, the MBCI was derived from the inverse relationship between blood glucose level and insulin secretion and sensitivity; thus, the use of MBCI in TDM patients is more biased than its use in T2DM. Consequently, the MBCI may not be recommended for use in TDM patients but could be used for T2DM patients as a rough indicator of β-cell function, especially for NGT and IGR patients.
Postprandial insulin secretion is divided into first-phase and second-phase secretion. First-phase insulin secretion reflects insulin reserve, while second-phase insulin secretion reflects insulin synthesis. In the OGTT, although the Ip/I0 values of the TNGT and TDM groups were higher than those of the NGT and T2DM groups, the AUCins-OGTT and AUCins-OGTT/AUCglu-OGTT values of the TIGR and TDM groups were higher than those of the IGR and T2DM groups, although the differences were not statistically significant. These results indicate that second-phase insulin secretion in TDM patients may be similar to that in T2DM patients. Therefore, second-phase insulin secretion is not an appropriate indicator to differentiate TDM and T2DM.
There were two insulin secretion phases in the IVGTT. The insulin peak in the first secretion phase appeared 3–5 min after intravenous injection of glucose and remained for approximately 10 min. Insulin secreted during the first-phase is released by the secretory granules near the β-cell membrane,[5] whereas insulin secreted during the second-phase represents newly synthesized insulin that is released from the secretory granules in the inner cells. The insulin peak of the second-phase secretion appeared 30–60 min later as a response to extended high blood sugar levels. In patients with T2DM, first-phase insulin secretion is reduced or lost, while second-phase secretion is increased; however, insulin peaks become delayed and subsequently disappear, and basal secretion is eventually impaired. In patients with early-stage diabetes, first-phase insulin secretion may be impaired, but second-phase secretion remains normal.
For the IVGTT in the current study, all but the T2DM group showed insulin peaks at 2–4 min; the T2DM group showed a low insulin plateau and no insulin peaks. The ratio of peak disappearance and delayed peaks for the T2DM group was significantly higher than that of the TDM group. The IP values of the hyperthyroidism groups (TNGT, TIGR, and TDM) were significantly higher than those of the NGT, IGR, and T2DM groups, indicating that all but the T2DM group retained normal first-phase insulin secretion. Furthermore, the Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the TDM group were higher than those of the T2DM group, although the Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the T2DM group were lower than those of the other five groups, indicating that first-phase insulin secretion in TDM patients is likely better than that in T2DM patients. Moreover, the Ip/I0, AIR0′~10′, and AUCins-IVGTT values in both the hyperthyroidism and control groups were reduced from NGT to impaired glucose tolerance in the diabetic state. This is consistent with previous findings[18] and suggests that first-phase insulin secretion is gradually reduced from NGT to impaired glucose tolerance and finally to a diabetic state.
It is worth noting that, although the Ip, Ip/I0, AIR0′~10′, and AUCins-IVGTT values of the TDM group were higher than those of the T2DM group, they remained lower than those of the TNGT, TIGR, NGT, and IGR groups. This indicates that first-phase insulin secretion is present but reduced in TDM. It may be that the excessive thyroid hormone levels associated with hyperthyroidism causes damage to β-cells, further impairing glucose tolerance and promoting the development of TDM, without suppressing rapid insulin secretion within the first few minutes. It may also be that thyroid hormone-induced metabolic acceleration results in high levels of insulin secretion.[19]
Treatment for TDM and T2DM varies. Under normal circumstances, the timely treatment of hyperthyroidism helps control symptoms by normalizing serum thyroid hormone levels, which halts the diabetic state.[20,21] If hyperthyroidism is not treated in a timely manner, the continuous effects of high thyroid hormone levels irreversibly damage β-cells, leading to incurable diabetes.[19] Therefore, the early differentiation of TDM and T2DM in clinical practice is particularly important.
In summary, the current data strongly suggest that, while second-phase insulin secretion should not be used to differentiate between TDM and T2DM, first-phase insulin secretion may be a useful indicator for differentiating between TDM and T2DM. Furthermore, this study demonstrated that the IVGTT could be used to assess first-phase insulin secretion and β-cell function in TDM patients. If first-phase insulin secretion is detected in these patients, screening for diabetic complications can be temporarily suspended. Once the hyperthyroidism symptoms have improved and serum thyroid hormone levels have returned to normal, the IVGTT can be repeated to assess first-phase insulin secretion. If first-phase insulin secretion is improved compared with the previous measurement, the patient can be diagnosed with TDM. Conversely, if there are no significant changes in first-phase insulin secretion, or it has been reduced or even disappeared, T2DM should be suspected. In addition, this study showed that Ip/I0 and AIR0′~10′ may be used as alternate indicators to assess first-phase insulin secretion, since calculating AUCins-IVGTT is overly complicated.
Financial support and sponsorship
The study was funded by grants from the Department of Education, Guangxi Zhuang Autonomous Region of China (No. YB2014077) and the Department of Health Self funded research projects, Guangxi Zhuang Autonomous Region of China (No. Z2010345).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Lenzen S, Bailey CJ. Thyroid hormones, gonadal and adrenocortical steroids and the function of the islets of Langerhans. Endocr Rev. 1984;5:411–34. doi: 10.1210/edrv-5-3-411. doi: 10.1210/edrv-5-3-411. [DOI] [PubMed] [Google Scholar]
- 2.Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico city diabetes study. Diabetes Care. 1996;19:1138–41. doi: 10.2337/diacare.19.10.1138. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- 3.Shi YL, Liu WJ, Zhang XF, Su WJ, Chen NN, Lu SH, et al. Effect of Chinese herbal medicine jinlida granule in treatment of patients with impaired glucose tolerance. Chin Med J. 2016;129:2281–6. doi: 10.4103/0366-6999.190676. doi: 10.4103/0366-6999.190676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang D, Liu ZH, Zhao Q, Luo Q. Effects of nasal continuous positive airway pressure treatment on insulin resistance and ghrelin levels in non-diabetic apnoeic patients with coronary heart disease. Chin Med J. 2013;126:3316–20. doi: 10.3760/cma.j.issn.0366-6999.20122769. [PubMed] [Google Scholar]
- 5.Li Q, Wang L, Xiao L, Wang Z, Wang F, Yu X, et al. Effect of intensive insulin therapy on first-phase insulin secretion in newly diagnosed type 2 diabetic patients with a family history of the disease. Exp Ther Med. 2015;9:612–618. doi: 10.3892/etm.2014.2114. doi: 10.3892/etm.2014.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia WP, Xiang KS. Assessment of islet b-cell function: From basic research to clinical practice (in Chinese) Chin J Endocrinol Metab. 2005;3:295–301. doi: 10.3760/j.issn:1000-6699.2005.03.003. [Google Scholar]
- 7.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Järvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care. 2000;23:295–301. doi: 10.2337/diacare.23.3.295. doi: 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 8.Li GW, Yang WY, Jiang YY. The possibility of (FINSXFPG)/(PG2h+PG1h-2FPG) being taken as an index of pancreatic b-cell insulin secretion in a population based study (in Chinese) Chin J Internal Med. 2000;4:17–21. doi: 10.3760/jissn:0578-1426.2000.04.005. [Google Scholar]
- 9.Yan L, He Y, Xue SN, Cheng H, Liu XR, Tang JY, et al. Comparison of various indexes for evaluating secretion of islet b-cell-clinical analysis of glucose tolerance test in 186 subjects with different glucose tolerance (in Chinese) Chin J Endocrinol Metab. 2005;6:503–6. doi: 10.3760/j.issn:1000-6699.2005.06.006. [Google Scholar]
- 10.Vila G, Krebs M, Riedl M, Baumgartner-Parzer SM, Clodi M, Maier C, et al. Acute effects of hydrocortisone on the metabolic response to a glucose load: Increase in the first-phase insulin secretion. Eur J Endocrinol. 2010;163:225–31. doi: 10.1530/EJE-10-0282. doi: 10.1530/EJE-10-0282. [DOI] [PubMed] [Google Scholar]
- 11.Society CD. Guidelines for prevention and treatment of type 2 diabetes in china (2013 edition) Chin J Diabetes. 2014;8:2–42. doi: 10.3760/cma.j.issn.1674-5809.2014.07.004. [Google Scholar]
- 12.Díez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119:201–7. doi: 10.1055/s-0031-1271691. doi: 10.1055/s-0031-1271691. [DOI] [PubMed] [Google Scholar]
- 13.Maratou E, Hadjidakis DJ, Peppa M, Alevizaki M, Tsegka K, Lambadiari V, et al. Studies of insulin resistance in patients with clinical and subclinical hyperthyroidism. Eur J Endocrinol. 2010;163:625–30. doi: 10.1530/EJE-10-0246. doi: 10.1530/EJE-10-0246. [DOI] [PubMed] [Google Scholar]
- 14.Li GW, Bennett PH. Possibility of evaluating islet b-cell function by oral glucose tolerance test in epidemiological study on diabetes mellitus – Analysis of euglycemic clamp study in 468 non-diabetic pima indians (in Chinese) Chin J Endocrinol Metab. 2003;1:12–6. doi: 10.3760/j.issn:1000-6699.2003.01.003. [Google Scholar]
- 15.Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011. 2011:439463. doi: 10.4061/2011/439463. doi: 10.4061/2011/439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res 2013. 2013:390534. doi: 10.1155/2013/390534. doi: 10.1155/2013/390534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai S, Kumar JA, PraJna K, Shetty SK, Rai T, Shrinidhi, et al. Thyroid function in type 2 diabetes mellitus and in diabetic nephropathy. J Clin Diagn Res. 2013;7:1583–5. doi: 10.7860/JCDR/2013/6216.3299. doi: 10.7860/JCDR/2013/6216.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratley RE, Weyer C. The role of impaired early insulin secretion in the pathogenesis of type II diabetes mellitus. Diabetologia. 2001;44:929–45. doi: 10.1007/s001250100580. doi: 10.1007/s001250100580. [DOI] [PubMed] [Google Scholar]
- 19.Gierach M, Gierach J, Junik R. Insulin resistance and thyroid disorders. Endokrynol Pol. 2014;65:70–6. doi: 10.5603/EP.2014.0010. doi: 10.5603/EP.2014.0010. [DOI] [PubMed] [Google Scholar]
- 20.Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol 2013. 2013:417920. doi: 10.1155/2013/417920. doi: 10.1155/2013/417920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang TT, Mu YM. The hyperglycemic management of secondary diabetes (in Chinese) Chin J Pract Internal Med. 2010;30:777–9. [Google Scholar]