Abstract
Background:
Obstructive sleep apnea syndrome (OSAS) has been shown to generate hypertension and endothelial dysfunction. Retinal vessel is the only vessel that can be observed directly and noninvasively; retinal vascular abnormalities can serve as a predictive marker for the occurrence, clinical course, and prognosis of cardiovascular and cerebrovascular diseases. The objective of this study was to identify the effect of OSAS severity on the morphological changes of retinal vessels.
Methods:
Adult patients complained of snoring were included in this study. The patients’ general information, polysomnography, and fundus photography parameters including central retinal artery equivalent (CRAE), central retinal vein equivalent (CRVE), and arteriole-to-venule ratio (AVR) were collected. Patients were divided into four groups according to their apnea-hypopnea index (AHI) results: Group I, AHI ≤5/h; Group II, 5/h < AHI ≤30/h; Group III, 30/h < AHI ≤60/h; and Group IV, AHI >60/h.
Results:
A total of 133 patients were included in this study with 111 males (83.5%) and 22 females (16.5%). Mean age was 41.6 ± 9.9 years, and the mean body mass index was 28.1 ± 4.0 kg/m2. AHI ranged between 0 and 130.8/h with a mean of 39.1 ± 30.7/h. There were 24, 34, 35, and 40 patients in Group I, Group II, Group III, and Group IV, respectively. Significant differences were found for AHI (F = 388.368, P < 0.001), minimal pulse oxygen saturation (F = 91.902, P < 0.001), and arousal index (F = 31.014, P < 0.001) among four groups; no significant differences were found for CRAE (F = 0.460, P = 0.599) and CRVE (F = 0.404, P = 0.586) among groups; there were significant differences for AVR between Group I and Group IV (63.6 ± 5.1% vs. 67.2 ± 5.5%, P = 0.010) Group II and Group IV (64.5 ± 6.0% vs. 67.2 ± 5.5%, P = 0.030), and Group III and Group IV (64.7 ± 4.1% vs. 67.2 ± 5.5%, P = 0.043). A main group-by-AHI effect was found on the AVR: patients with higher AHI showed higher AVR results (r = 0.225, P = 0.009). Multivariate logistic regression analysis was used for multi-variable factors. A group-by-age effect was found on the AVR: younger patients showed higher AVR results (β = −0.001, P = 0.020).
Conclusions:
This study indicated that increased AVR of retinal vessel can be observed in extremely severe OSAS patients. For patients with OSAS, retinal vascular abnormalities may become an early indication for further cardiovascular abnormalities.
Keywords: Fundus Photography, Obstructive Sleep Apnea Syndrome, Retina, Vascular
Introduction
The prevalence of obstructive sleep apnea syndrome (OSAS) in population aged between 30 and 60 years is 2–4%.[1,2] Patients with OSAS present with repetitive collapse and construction in upper respiratory tract, resulting in characteristic chronic intermittent hypoxia and sleep fragmentation as well as recurrent hypoxemia and hypercapnia, which are also complicated with damage of major organs, such as hypertension and coronary heart disease. Now, OSAS has been considered as an independent risk factor for hypertension. The study by Parati et al.[3] has reported that 35–80% of patients with OSAS were complicated with hypertension. Conversely, 40% of patients with hypertension were diagnosed as having OSAS.[4,5]
However, whether OSAS is an independent risk factor for cardiovascular disease is still controversial. Some studies have shown that OSAS is an independent risk factor for obesity and hypertension, promoting the remodeling of heart, predicting atrial fibrillation and heart failure.[6] Moreover, severe OSAS increases the chance of fatal and nonfatal cardiovascular events.[7] Retinal vessel, which is the only vessel that can be observed directly and noninvasively, has similar anatomy, physiology, and developmental characteristics with coronary and cerebrovascular system. Retinal vascular abnormalities can occur prior to cardiovascular and cerebrovascular diseases and serve as a predictive marker for the occurrence, clinical course, and prognosis of cardiovascular and cerebrovascular diseases.[8,9] Therefore, this study assumed that OSAS can cause retinal vascular damage. The morphological changes of retinal vessels in OSAS patients will be observed and analyzed directly, and retinal vascular markers of OSAS will be investigated, which will be used to evaluate the damage of cardiovascular and cerebrovascular diseases objectively.
Methods
Participants
Between May 2014 and April 2016, the adult patients complained of snoring were monitored by polysomnography (PSG) within 7 hours of sleep and received fundus photography in Beijing Tongren Hospital, which were performed separately and blindly with time interval <1 week. Patients who have received treatment for OSAS or who have been diagnosed with coronary heart disease, heart failure, arrhythmia, renal dysfunction, diabetes mellitus, hypothyroidism, acromegaly, chronic obstructive pulmonary disease, neoplasm, severe neurological disorder, psychiatric disorder, glaucoma, cataract, eye injury, uncontrolled hypertension (>140/90 mmHg despite more than two antihypertensive drugs), or who are taking vasodilator, β- or β-agonist or antagonist, nitric acid derivatives, glucocorticoids, theophylline, sildenafil, immunosuppressor, antipsychotic drugs, and hypnotics were excluded from this study. The patients’ age, gender, occupation, height, body weight, neck circumference, and disease history including hypertension, coronary heart disease, and eye disease were documented. Questionnaire about sleeping and Epworth Sleepiness Scale were completed. This study was approved by the Ethics Committee of Beijing Tongren Hospital, and all patients provided written informed consent.
Polysomnography
According to the standard protocol recommended by the American Academy of Sleep Medicine:[10,11] the electroencephalogram of frontal area, parietal region, and occipital region was recorded simultaneously; air flow through mouth and nose, thoracic-abdominal respiration, electro-oculogram, saturation of pulse oximetry, jaw electromyography, pretibial electromyography, and electrocardiogram were also recorded. All data were analyzed manually. Sleep apnea is defined as signal of thermal airflow decreased by more than 90% for more than 10 s during sleeping. Hypopnea is scored when the peak signal excursions drop by ≥30% of pre-event baseline using nasal pressure for more than 10 s combined with more than 3% decrease in pulse oxygen saturation or arousal. Apnea-hypopnea index (AHI) was defined as the sum of events of sleep apnea and hypoventilation in 1 h of sleeping. Patients were divided into four groups according to their AHI results: Group I, AHI ≤5/h; Group II, 5/h < AHI ≤30/h; Group III, 30/h < AHI ≤60/h; and Group IV, AHI >60/h.
Fundus photography
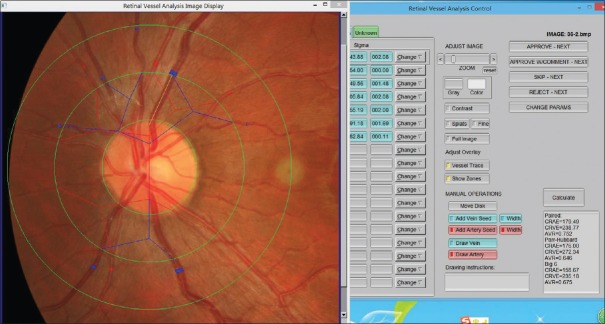
The fundus photography was performed with Canon CR-DGi nonmydriatic digital fundus camera (Canon U.S.A. Inc., USA) in the dark environment, under natural pupil size. According to the criteria of risk factors for atherosclerosis, the retinal vascular abnormalities in the fundus photographs were evaluated, including central retinal artery equivalent (CRAE), central retinal vein equivalent (CRVE), and arteriole-to-venule ratio (AVR) to represent diffuse retinal vascular changes. The retinal arteriovenous diameter was measured using computer-aided software (IVAN, University of Melbourne, Australia), the parameters of CRAE, CRVE, and AVR were calculated using formula. Then, the diameters of six big branches of retinal artery and vein within the range of 1/2–1 optic disc diameter away from the edge of optic disc were measured using the formula proposed by Knudtson et al. (modified Parr-Hubbard formula),[12,13] which reflects the relationship between diameter of main branches of retinal vessels [Figure 1].
Figure 1.
Window interface of retinal vessel diameter measurement software (IVAN). The fundus is divided into four areas (the optic disc zone, A zone, B zone, and the perimeter zone from inside to outside) by three rings (disc diameter ring, 1/2 optic disc diameter away from the edge of the optic disc ring, and 1 optic disc diameter away from the edge of the optic disc ring from inside to outside), and the diameter of artery and vein was measured in different regions.
Formula for calculating the diameter of the artery:
D0 = 0.88 × (D12 + D22)
[0.88=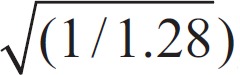 ]
]
Formula for calculating the diameter of the vein:
D0 = 0.95 × (D12 + D22)
[0.95 = 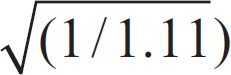 ]
]
D1 represents the diameter of branch vessel 1, D2 represents the diameter of branch vessel 2, and D0 represents the diameter of main vessel. The main parameters of CRAE, CRVE, and AVR were calculated.
Statistical analysis
Data analysis was conducted with SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard error (SE). Normality was assessed using skewness and kurtosis tests. One-way analysis of variance (ANOVA) was used to evaluate each parameter among all groups. The qualitative data were compared using Chi-square test. Pearson's correlation coefficients were used to analyze the correlation of different factors. Multivariate logistic regression analysis was used for multi-variable factors. Statistical significance was set at P < 0.05.
Results
Patient characteristics
Finally, 133 patients were included in this study including 111 males (83.5%) and 22 females (16.5%), with a mean age of 41.6 ± 9.9 years (range: 20–64 years). The body mass index (BMI) ranged between 20.0 kg/m2 and 42.6 kg/m2 with a mean value of 28.1 ± 4.0 kg/m2. The incidence of controlled hypertension was 33.8%. AHI ranged between 0 and 130.8/h with a mean of 39.1 ± 30.7/h. There were 24, 34, 35, and 40 patients in Group I, Group II, Group III, and Group IV, respectively.
Baseline characteristics are summarized in Table 1. There were significant differences in gender, age, and BMI, but there was no significant difference in the incidence of controlled hypertension among four groups. Significant differences in BMI were found between Group I (25.5 ± 2.9 kg/m2) and Group III (28.7 ± 3.2 kg/m2, P = 0.005), Group IV (29.7 ± 3.8 kg/m2, P < 0.001), and between Group II and Group IV (27.1 ± 4.7 kg/m2 vs. 29.7 ± 3.8 kg/m2, P = 0.010); and Group I had the lowest BMI. There were significant differences in age between Group II and Group III (38.5 ± 11.5 years vs. 46.4 ± 10.0 years, P = 0.006), Group III and Group IV (46.4 ± 10.0 years vs. 40.6 ± 9.0 years, P = 0.023), and Group II had the lowest age.
Table 1.
Baseline characteristics of all patients in this study
| Characteristics | Group I (n = 24) | Group II (n = 34) | Group III (n = 35) | Group IV (n = 40) | Statistical values | P |
|---|---|---|---|---|---|---|
| Male/female (n) | 20/4 | 23/11 | 33/2 | 36/4 | 9.008* | 0.029 |
| Age (years), mean ± SE | 41.7 ± 8.1 | 38.5 ± 11.5 | 46.4 ± 10.0 | 40.6 ± 9.0 | 2.863† | 0.040 |
| Controlled hypertension, n (%) | 8 (33.3) | 11 (32.4) | 11 (31.4) | 15 (37.5) | 1.642† | 0.650 |
| BMI (kg/m2), mean ± SE | 25.5 ± 2.9 | 27.1 ± 4.7 | 28.7 ± 3.2 | 29.7 ± 3.8 | 6.979† | <0.001 |
*Chi-square value; †F value. BMI: Body mass index; SE: Standard error.
Polysomnography and fundus photography results
Table 2 shows the results of PSG and fundus photography among four groups. Significant differences were found for AHI (F = 388.368, P < 0.001), minimal pulse oxygen saturation (F = 91.902, P < 0.001), and arousal index (F = 31.014, P < 0.001) among four groups; no significant differences were found for CRAE (F = 0.460, P = 0.599) and CRVE (F = 0.404, P = 0.586) among groups; there were significant differences for AVR between Group I (63.6 ± 5.1% vs. 67.2 ± 5.5%, P = 0.010) and Group IV, Group II and Group IV (64.5 ± 6.0% vs. 67.2 ± 5.5%, P = 0.030), Group III and Group IV (64.7 ± 4.1% vs. 67.2 ± 5.5%, P = 0.043).
Table 2.
The parameters of polysomnographic and fundus photography among four groups
| Parameters | Group I (n = 24) | Group II (n = 34) | Group III (n = 35) | Group IV (n = 40) | F | P |
|---|---|---|---|---|---|---|
| AHI (/h) | 1.5 ± 1.4 | 16.2 ± 6.4 | 42.9 ± 9.4 | 77.7 ± 14.3 | 388.368 | <0.001 |
| MSpO2 (%) | 93.5 ± 2.9 | 84.3 ± 5.3 | 76.5 ± 8.6 | 63.0 ± 9.6 | 91.902 | <0.001 |
| Arousal index (/h) | 2.7 ± 2.6 | 11.3 ± 7.1 | 28.4 ± 12.1 | 44.1 ± 26.1 | 31.014 | <0.001 |
| CRAE | 156.6 ± 21.2 | 149.4 ± 26.4 | 145.5 ± 16.2 | 150.3 ± 18.8 | 0.460 | 0.599 |
| CRVE | 241.4 ± 42.7 | 230.3 ± 35.7 | 224.8 ± 25.2 | 225.0 ± 33.9 | 0.404 | 0.586 |
| AVR (%) | 63.6 ± 5.1 | 64.5 ± 6.0 | 64.7 ± 4.1 | 67.2 ± 5.5 | 2.938 | 0.036 |
The data are shown as mean ± SE. AHI: Apnea-hypopnea index; MSpO2: Minimal pulse oxygen saturation; CRAE: Central retinal artery equivalent; CRVE: Central retinal vein equivalent; AVR: Arteriole-to-venular ratio; SE: Standard error.
The relationship between polysomnography parameters and arteriole-to-venule ratio
Pearson's correlation coefficients were used to analyze the correlation of AVR and PSG parameters. A main group-by-AHI effect was found on the AVR: patients with higher AHI showed higher AVR results (r = 0.225, P = 0.009). Multilogistic analysis was used for multi-variable factors. A group-by-age effect was found on the AVR: younger patients showed higher AVR results (β = −0.001, P = 0.020).
Discussion
We used an automatic noninvasive technique to detect the morphologic changes of retinal vessels. The results showed that compared with Group I, Group II, and Group III, AVR was significantly higher in Group IV. Similar results were obtained when laser Doppler flowmetry was used to assess the response of choroid vascular blood flow to hypoxia and high concentration of CO2 in patients with simple OSAS and healthy controls.[14] In contrast, capillary density in forearm and perineal area, calculated by capillaroscopy, in patients with severe OSAS complicated with mild untreated hypertension, was significantly decreased compared with healthy controls.[15]
Considering that the factor of hypertension has the damage on retinal vessel, the patients with uncontrolled hypertension were excluded from this study, so no significant difference was observed among different groups for incidence of hypertension. However, we could not rule out the possibility that morphologic changes of retinal vessel occurred at early stage in patients with OSAS complicated with uncontrolled hypertension. Hypertension might play a more critical role in damaging the vessels within the development of OSAS. Moreover, AVR value is influenced by many factors. The association of narrowed retinal arterioles and higher blood pressure was stronger in younger persons; retinal venular diameters narrowed with increasing age but not with increasing blood pressure.[16] In this study, the incidence of controlled hypertension was 33.8%, and significant differences in age were found among four groups with youngest age in Group II, therefore patients with higher AHI showed higher AVR results. Through multilogistic analysis, younger patients showed higher AVR results (P < 0.05).
Previous studies displayed the conclusions that patients with higher severity and longer history of OSAS had higher risks of cardiovascular disease.[17,18] Nguyen et al.[19] believed that following CPAP treatment for 3 months, myocardial perfusion reserve and flow-mediated dilation (FMD) could be significantly increased; meanwhile, the damage in FMD of brachial artery in OSAS patients was detected by ultrasound;[20] pulse-wave velocity (PWV) and transthoracic echocardiography were also performed in recruited patients, which showed that OSAS patients with normal blood pressure and patients with simple hypertension presented with increase in left atrial diameter, interventricular septal thickness, left ventricular posterior wall thickness, left ventricular mass index, and incidence of left ventricular hypertrophy, compared with healthy controls without OSAS and hypertension.[21] The PWV, left ventricular mass index, and percentage of left ventricular hypertrophy had a further significant increase in patients with OSAS and hypertension.[22] The change in intima-media thickening (IMT) usually preceded the early stage of atherosclerosis. Doppler ultrasonography was performed in all patients and the results showed that common carotid artery of patients with severe OSAS was significantly thicker compared with that of patients with mild or moderate OSAS. In addition, patients with severe OSAS also had significantly higher IMT ratio relative to patients with mild or moderate OSAS.[23] Patients who have been demonstrated with severe cardiovascular abnormalities by other ways were ruled out from this study. However, some of the patients with severe OSAS already showed significant vascular damage (increase of AVR in this case), which indicated that, compared with traditional noninvasive tests of cardiovascular diseases, tests of retinal vascular may offer higher sensitivity.
Currently, correlation between severity of OSAS and heart diseases and major vessels is drilled deeply, but concentrating on minor vessels is rare. Whereas Doppler guidewire during percutaneous coronary interventions to measure coronary flow velocity in case of myocardial infarction in patients with OSAS is an invasive examination,[24] retinal vessels can be observed directly through noninvasive method, making it possible to observe systemic vascular lesions through fundus photography, which is widely used in grading of diabetic retinopathy. Vascular disease is one of the most important complications of diabetes and has definite fundus characteristics. Grading of diabetic retinopathy can reflect the course of diabetes and guide the treatment of retinal vascular disease. Comparatively, the relationship between other disease and retinopathy has not been confirmed, such as hypertension. The traditional fundus examination method, direct ophthalmoscopy, is characterized by obvious subjective evaluation and poor comparison during follow-up period. Therefore, digital fundus photography and image processing technology in this study were firstly used to observe and analyze the retinal vascular disease of OSAS patients. Digital fundus photography and image processing technology are simple and fast, which makes it possible to carry out relevant research in large scale. Some well-known epidemiological studies on cardiovascular disease and eye disease, such as Atherosclerosis Risk in Communities Study and Blue Mountains Eye Study, showed that retinal vascular abnormalities were related to the occurrence and severity of hypertension, stroke, and coronary heart disease, which might be associated with increased cardiovascular mortality.[25,26] Prospective studies have indicated that retinal vascular abnormalities might occur before the onset of cardiovascular disease, such as hypertension, and might become an independent predictor of systemic disease.
The mechanisms responsible for these changes include the following: hyperactivity of sympathetic-catecholamine system, hypersecretion of catecholamine, high plasma concentration of catecholamine due to chronic intermittent hypoxia in patients with OSAS;[27,28] chronic intermittent hypoxia results in dramatic increase in neutrophils and monocytes, subsequently leading to systemic inflammation; increase in the production of inflammatory cytokines and levels of interleukin-6, tumor necrosis factor-alpha, and C-reactive protein (CRP; CRP could induce cardiovascular dysfunction and disorder by inhibiting nitric oxide synthase and increase the expression of cell adhesion molecules);[29,30] release of large amount of pressor substance associated with hypoxemia, hypercapnia, and apnea; stimulates the release of vasoactive substance and damages vascular endothelial function, leading to the increase in endothelin, continuous constriction of vessels, and other pathological changes in cardiovascular system.[31,32]
There were several limitations in this study. Patients included in this study were mostly patients with severe OSAS and heavy snoring and predominantly men, and therefore the results could not be extrapolated to women. A balanced distribution of severity of OSAS, gender distribution, and age may decrease subject bias. Moreover, the present study did not include the changes of retinal vessel following a period of effective CPAP. The analysis of length of incurred disease and blood serum chemicals was also excluded from this study. In the future, large and well-designed studies are needed to further confirm the change of retinal vascular morphology in OSAS patients.
In this study, the digital fundus photography and image processing technology were applied to the quantitative measurement of retinal vessel in OSAS patients. In the exclusion of uncontrolled hypertension, diabetes, eye diseases, and other interfering factors, this study has identified that increased AVR of retinal vessel can be observed in extremely severe OSAS patients. For patients with OSAS, retinal vascular abnormalities may become an early indication for further cardiovascular abnormalities.
Financial support and sponsorship
This study was supported by a grant from the Priming Scientific Research Foundation for the Senior Researcher in Beijing Tongren Hospital, Capital Medical University (No. 2016-YJJ-GGL-002).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Zhang YL, Chen R, Wang Y, Xiong KP, Huang JY, et al. Elevated serum liver enzymes in patients with obstructive sleep apnea-hypopnea syndrome. Chin Med J. 2015;128:2983–7. doi: 10.4103/0366-6999.168943. doi: 10.4103/0366-6999.168943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parati G, Lombardi C, Hedner J, Bonsignore MR, Grote L, Tkacova R, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41:523–38. doi: 10.1183/09031936.00226711. doi: 10.1183/09031936.00226711. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA. Obstructive sleep apnea and hypertension. Curr Hypertens Rep. 2010;12:189–95. doi: 10.1007/s11906-010-0112-8. doi: 10.1007/s11906-010-0112-8. [DOI] [PubMed] [Google Scholar]
- 5.Luo M, Zheng HY, Zhang Y, Feng Y, Li DQ, Li XL, et al. Anomogram for predicting the likelihood of obstructive sleep apnea to reduce the unnecessary polysomnography examinations. Chin Med J. 2015;128:2134–40. doi: 10.4103/0366-6999.162514. doi: 10.4103/0366-6999.162514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otto ME, Belohlavek M, Romero-Corral A, Gami AS, Gilman G, Svatikova A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–302. doi: 10.1016/j.amjcard.2006.12.052. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. doi: 10.1016/s0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR, Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: Multi-ethnic study of atherosclerosis. Hypertension. 2008;51:119–26. doi: 10.1161/HYPERTENSIONAHA.107.098343. doi: 10.1161/HYPERTENSIONAHA.107.098343. [DOI] [PubMed] [Google Scholar]
- 9.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–48. doi: 10.1111/j.1469-7580.2005.00395.x. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 11.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–9. doi: 10.1076/ceyr.27.3.143.16049. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 13.Patton N, Aslam TM, MacGillivray T, Deary IJ, Dhillon B, Eikelboom RH, et al. Retinal image analysis: Concepts, applications and potential. Prog Retin Eye Res. 2006;25:99–127. doi: 10.1016/j.preteyeres.2005.07.001. doi: 10.1016/j.preteyeres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Tonini M, Khayi H, Pepin JL, Renard E, Baguet JP, Lévy P, et al. Choroidal blood-flow responses to hyperoxia and hypercapnia in men with obstructive sleep apnea. Sleep. 2010;33:811–8. doi: 10.1093/sleep/33.6.811. doi: 10.1093/sleep/33.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazzaro P, Schirosi G, Clemente R, Battista L, Serio G, Boniello E, et al. Severe obstructive sleep apnoea exacerbates the microvascular impairment in very mild hypertensives. Eur J Clin Invest. 2008;38:766–73. doi: 10.1111/j.1365-2362.2008.02011.x. doi: 10.1111/j.1365-2362.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Klein R, Klein BE, Meuer SM, Hubbard LD. Retinal vessel diameters and their associations with age and blood pressure. Invest Ophthalmol Vis Sci. 2003;44:4644–50. doi: 10.1167/iovs.03-0079. doi: 10.1167/iovs.03-0079. [DOI] [PubMed] [Google Scholar]
- 17.Phillips C, Hedner J, Berend N, Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28:604–9. doi: 10.1093/sleep/28.5.604. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- 18.Noda A, Nakata S, Fukatsu H, Yasuda Y, Miyao E, Miyata S, et al. Aortic pressure augmentation as a marker of cardiovascular risk in obstructive sleep apnea syndrome. Hypertens Res. 2008;31:1109–14. doi: 10.1291/hypres.31.1109. doi: 10.1291/hypres.31.1109. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen PK, Katikireddy CK, McConnell MV, Kushida C, Yang PC. Nasal continuous positive airway pressure improves myocardial perfusion reserve and endothelial-dependent vasodilation in patients with obstructive sleep apnea. J Cardiovasc Magn Reson. 2010;12:50. doi: 10.1186/1532-429X-12-50. doi: 10.1186/1532-429X-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yim-Yeh S, Rahangdale S, Nguyen AT, Stevenson KE, Novack V, Veves A, et al. Vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19:17–22. doi: 10.1038/oby.2010.116. doi: 10.1038/oby.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–536. doi: 10.1093/eurheartj/ehm236. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- 23.Salepci B, Fidan A, Ketenci SC, Parmaksiz ET, Comert SS, Kiral N, et al. The effect of obstructive sleep apnea syndrome and snoring severity to intima-media thickening of carotid artery. Sleep Breath. 2015;19:239–46. doi: 10.1007/s11325-014-1002-0. doi: 10.1007/s11325-014-1002-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima H, Muto S, Amenomori K, Shiraishi Y, Nunohiro T, Suzuki S. Impact of obstructive sleep apnea on myocardial tissue perfusion in patients with ST-segment elevation myocardial infarction. Circ J. 2011;75:890–6. doi: 10.1253/circj.cj-10-0768. doi: 10.1253/circj.CJ-10-0768. [DOI] [PubMed] [Google Scholar]
- 25.Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–80. doi: 10.1016/s0161-6420(99)90525-0. doi: 10.1016/S0161-6420(99)90525-0. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Moss SE. The relation of systemic hypertension to changes in the retinal vasculature: The Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 1997;95:329–48. doi: 10.1016/S0002-9394(99)80172-X. [PMC free article] [PubMed] [Google Scholar]
- 27.Marrone O, Salvaggio A, Bue AL, Bonanno A, Riccobono L, Insalaco G, et al. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: Relationship with nocturnal sympathetic activity. Clin Exp Hypertens. 2011;33:373–80. doi: 10.3109/10641963.2010.531853. doi: 10.3109/10641963.2010.531853. [DOI] [PubMed] [Google Scholar]
- 28.Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol. 2011;589(Pt 6):1463–76. doi: 10.1113/jphysiol.2010.200691. doi: 10.1113/jphysiol.2010.200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: An emerging risk factor for atherosclerosis. Chest. 2011;140:534–42. doi: 10.1378/chest.10-2223. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation. 2010;121:1014–21. doi: 10.1161/CIRCULATIONAHA.109.900357. doi: 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz EI, Puhan MA, Schlatzer C, Stradling JR, Kohler M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology. 2015;20:889–95. doi: 10.1111/resp.12573. doi: 10.1111/resp.12573. [DOI] [PubMed] [Google Scholar]