Abstract
Background:
Improving islet graft revascularization has become a crucial task for prolonging islet graft survival. Endothelial cells (ECs) are the basis of new microvessels in an isolated islet, and EC coating has been demonstrated to improve the vascularization and survival of an islet. However, the traditional method of EC coating of islets has low efficiency in vitro. This study was conducted to evaluate the effect of a polyglycolic acid (PGA) scaffold on the efficiency of islet coating by ECs and the angiogenesis in the coated islet graft.
Methods:
A PGA fibrous scaffold was used for EC coating of islet culture and was evaluated for its efficiency of EC coating on islets and islet graft angiogenesis.
Results:
In in vitro experiments, we found that apoptosis index of ECs-coating islet in PGA group (27% ± 8%) was significantly lower than that in control group (83% ± 20%, P < 0.05) after 7 days culture. Stimulation index was significantly greater in the PGA group than in the control group at day 7 after ECs-coating (2.07 ± 0.31 vs. 1.80 ± 0.23, P < 0.05). vascular endothelial growth factor (VEGF) level in the PGA group was significantly higher than the coating in the control group after 7 days culture (52.10 ± 13.50 ng/ml vs. 16.30 ± 8.10 ng/ml, P < 0.05). Because of a tight, circumvallated, adhesive and three-dimensional growth microenvironment, islet cultured in a PGA scaffold had higher coating efficiency showing stronger staining intensity of enzyme than those in the control group after 14 days of culture following ECs-coating. For in vivo study, PGA scaffold significantly prolonged the average survival time of EC-coated islet graft after transplantation compared with control group (15.30 ± 5.60 days vs. 8.30 ± 2.45 days, P < 0.05). The angiogenesis and area of survived grafts were more in the PGA group compared with the control group by measuring the mean microvessel density (8.60 ± 1.21/mm2 vs. 5.20 ± 0.87/mm2, P < 0.05). In addition, expression of VEGF and tyrosin-protein kinase receptor (Tie-2) gene increased in PGA scaffold group than that in control group by real-time reverse transcription-polymerase chain reaction analysis.
Conclusions:
These results demonstrate that the efficiency of EC coating of islets was successfully increased by culturing ECs on a PGA scaffold. This method enhances the function, survival, and vascularization of isolated islets in vitro and in vivo.
Keywords: Endothelial Cells, Islet, Polyglycolic Acid Fibrous, Revascularization
Introduction
Type 1 diabetes mellitus can be alleviated through islet transplantation (ITx).[1,2,3] However, islet cell death at the early period after transplantation remains an obstacle to a successful ITx. The primary reason for islet death is the lack of a vascular network that can effectively support the newly transplanted islet. The vascular system in the transplanted islet is often destroyed during islet isolation and purification, threatening the survival of β-cells inside the islet.[4] Isolated islets are typically avascular and their vascular network is found to regenerate within 14 days after transplantation.[5] During this period, transplanted islets may suffer from hypoxia, resulting in early islet graft death. A study revealed that approximately 60% of islets can engraft within 3 days after intraportal ITx.[6] Therefore, at the early stage of transplantation, the survival and function of transplanted islets depend on the timing and degree of revascularization.[7]
The reconstruction of microcirculation in islets is delayed due to loss of endothelial cells (ECs).[8] In a previous study, a novel technique to coat islets with ECs has been developed to generate a biologically active surface on the islets to inhibit instant blood-mediated inflammatory reaction.[9] Since ECs on the surface of the islet are involved in multiple steps during angiogenesis, our previous study has demonstrated that revascularization in islets can be improved by coating with ECs, as well as decreasing the apoptosis of β-cells inside the islets under hypoxia stress.[10] However, since the revascularization in the early stage of ITx relies on the coating of islets with ECs, the previous coating method is unable to completely coat the islet and thus affects revascularization.[9,11] During in vitro culture, islet cells and ECs have huge differences in biological characteristics. ECs growing adherently are difficult to effectively coat islet cells growing in suspension in a two-dimensional culture system.[10] ECs may drop off from the islet later if they are not firmly attached to the islet, even if the initial coating was successful, resulting in low coating efficiency. Therefore, it is important to investigate how a culture system that enables the full attachment of ECs to the islet surface can be established for better coating efficiency.
In recent years, with the development of tissue engineering, excellent biodegradable and biocompatible polymers have become available for use in biomedicine and medicine.[12] For example, polyglycolic acid (PGA) has been widely used in bionic biomedical studies. It can be prepared as a cell scaffold to support and protect cells and can be used to provide a three-dimensional (3D) environment for in vitro cell cultures.[13] Studies have shown that islets have significantly better survival and functionality when cultured in a PGA scaffold, compared with traditional culture.[14] If EC-coated islets are cultured in the PGA scaffold, the 3D culture environment would shorten the distance between islets and ECs, providing better support for ECs to attach to the islet cells during the early culture period. This would reduce the dropping-off of ECs and improve their proliferation for better vascularization. In addition, the PGA scaffold can be used as a nonantigenic implant, which would not have a rejection reaction once implanted. After the islet is vascularized, the scaffold would be degraded; and therefore, it is very safe to use. In the present study, we evaluated the effect of a PGA scaffold on the efficiency of islet coating by ECs and the angiogenesis in the coated islet graft.
Methods
Ethics statement
Sprague-Dawley and Wistar rats were purchased from the Experimental Animal Center of Medical College, Xi’an Jiaotong University (Shaanxi, China). This study was conducted in accordance with the Guidelines on the Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research and the Guidelines of Animal Care. All animal protocols were approved by the Institutional Animal Care and Use Committees of Xi’an Jiaotong University. All efforts were made to minimize the animals’ suffering and the number of animals used.
Isolation of islets and endothelial cells
Pancreatic islets and ECs were isolated from inbred Lewis rats (weight, 200–250 g). Pancreatic islets were isolated using collagenase P digestion (Roche Diagnostics, Germany) and discontinuous Ficoll gradient centrifugation (Type 400DL, Sigma, St. Louis, MO, USA).[15] Aortic ECs were isolated by incubation with collagenase II (1 mg/ml, Sigma, St. Louis, MO, USA), followed by centrifugation at 150 × g for 10 min. After washing with phosphate-buffered saline (PBS), aortic ECs were resuspended in RPMI 1640 culture medium (Gibco Laboratories, Grand Island, NY, USA), containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA).[10]
Polyglycolic acid scaffold preparation
The porous PGA scaffold (Synthecon, USA) was constructed with 15 μm-diameter fibers. Ten mg of unwoven PGA fibers were compressed into a 5-mm diameter and 2-mm thick cylinder. The diameter of the pores in the scaffold (2-mm thick) was 100–150 μm, and porosity was 97%. The scaffold was dipped in 75% ethanol solution for 30 min, washed three times with PBS, dried on a sterilized bench, and sterilized by exposing to ultraviolet rays for 1 h. Then, the scaffold was modified with 10 mg/ml of poly-L-lysine (PLYS) for 30 min and used in cell culture.
Coating of endothelial cells on islets
ECs were harvested using ×1 trypsin-ethylenediaminetetraacetic acid (Life Technologies, USA), washed with endothelial growth medium-2 (EGM-2) medium, centrifuged at 180 ×g for 5 min in a 14-ml Falcon tube (Becton-Dickinson Labware, USA), and counted in a Burker chamber. After washing, ECs were suspended in EGM-2 medium at 3 × 106 cells/ml. Islets of 1000 islet equivalent (IEQ) numbers were mixed with 3.0 × 106 ECs in 500 μl of culture medium. The mixtures were co-cultured in a culture tube for 2 h. After incubation, the mixtures were resuspended in RPMI-1640 medium supplemented with 10% FBS (control group) or in the same medium containing the PGA scaffolds (PGA group) in low adsorbent-treated Petri dishes (15 cm2), and cultured in an incubator at 37°C with 5% CO2. The culture media were changed every day.
Islet cell survival
Islet cells were stained with acridine orange-propidium iodide (AO-PI) fluorochrome (Sigma Chemical, USA) and assayed for survival rate.[15] Green and red fluorescence were indicative of living and dead cells, respectively. The apoptosis index (AI) of islets (×40) was calculated in five randomly selected fields as the ratio of red fluorescent cells/total cells (red and green) ×100%. Experiments were repeated three times.
In vitro function test
A glucose-stimulated insulin secretion test was performed on days 1, 3, 5, and 7 after EC coating. Islet-specific functions were evaluated in a dynamic perfusion system.[15] One hundred islets were challenged with two concentrations of glucose (first at 1.67 mmol/L and second at 16.7 mmol/L). Fractions were collected and analyzed for insulin content using a commercial insulin enzyme-linked immunosorbant assay (ELISA) kit (Mercodia, Stockholm, Sweden). Insulin release stimulation index (SI) was calculated based on yields in high/low glucose. Experiments were repeated three times.
Cytokine detection by enzyme-linked immunosorbant assay
Culture supernatants were assayed for vascular endothelial growth factor (VEGF) on days 1, 3, 5, and 7 after EC coating using a commercial ELISA kit (Bender MedSystems, Vienna, Austria). Experiments were repeated three times.
Efficiency of endothelial cell-coating of islets
An inverted phase contrast microscope was used for real-time monitoring of EC-coating efficiency by comparing the number of ECs on the surface of the islet after staining with dithizone (DTZ; Sigma, St. Louis, USA). EC-coated islets were collected after 7 days of culture, fixed in 10% phosphate-buffered formalin overnight, and stained with tetraethyl rhodamine isothiocyanate (TRITC)-labeled rabbit anti-rat von Willebrand factor (vWF) antibody (1:200 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The slides were examined using a fluorescence microscope (IX71; Olympus, Tokyo, Japan), and relative fluorescence intensities were compared by Image Plus 6 software (Media Cybernetics, USA) in five randomly selected fields. Samples were fixed in glutaraldehyde (3%), and the clusters of islet cells and PGA scaffold were observed under a scanning electron microscope (SEM, S-34000N; Hitachi Inc., Tokyo, Japan). Experiments were repeated three times.
Islet transplantation into diabetic rats
Inbred Lewis rats (weight, 200–250 g) were rendered diabetic by an intraperitoneal injection of 220 mg/kg of streptozotocin (STZ; Sigma Chemical Co., USA). In this study, twenty mice were randomly divided into two groups, each receiving 2000 IEQ/kg islets alone or with PGA scaffolds in renal capsules, as previously described.[16] Blood glucose levels and insulin concentrations were monitored using Glucomètre (Bayer Inc., Germany) and radioimmunoassay kit (Mercodia, Sweden) to assess the function of islet graft. Experiments were repeated three times.
Graft survival
A blood sugar level <200 mg/dL for 2 consecutive days was defined as a successful islet function, while a blood sugar level >200 mg/dL for 2 consecutive days was defined as graft loss. Graft survival at 30 days post-ITx was recorded in the two groups.
Observation of vascularization
Animals were sacrificed by CO2 inhalation 14 days after ITx, and islet grafts were retrieved. Photographs of fresh organs were taken to assess the new vessels that formed around the islet graft. The islets were fixed in 10% phosphate-buffered formalin overnight, embedded in paraffin, sectioned at 4.5-μm thickness, and stained with hematoxylin-eosin (H and E) using standard protocols. Islet cells and ECs were detected by double immunofluorescence staining with TRITC-labeled rabbit anti-rat vWF antibody and FITC-labeled rabbit anti-rat insulin antibody (1:200 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Coated slides were examined by light microscopy (IX71; Olympus Corporation, Tokyo, Japan), and mean microvessel density (MVD) was measured, as described.[17] Experiments were repeated three times.
Reverse transcription-polymerase chain reaction analysis
Total RNA was purified from approximately 30 mg of tissue samples collected at the islet graft site 14 days after ITx using the RNAqueous-4 PCR kit (Ambion Applied Biosystems, Austin, TX, USA), and complementary DNA was synthesized from 0.2 μg of total RNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, California, USA). Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed using primers that amplify VEGF, tyrosin-protein kinase receptor (Tie-2), and β-actin genes, as previously described.[15] Experiments were repeated three times.
Statistical analysis
SPSS 13.0 software (SPSS Inc., USA) was used for statistical analysis. Statistical significance was determined using Student's t-test or one-way analysis of variance (ANOVA) with post hoc Bonferroni's test, as appropriate. Islet graft survival curves were analyzed with Mantel-Cox log-rank test. The statistical significance level was set as P < 0.05.
Results
Polyglycolic acid scaffold improved the survival of endothelial cell-coated islet in vitro
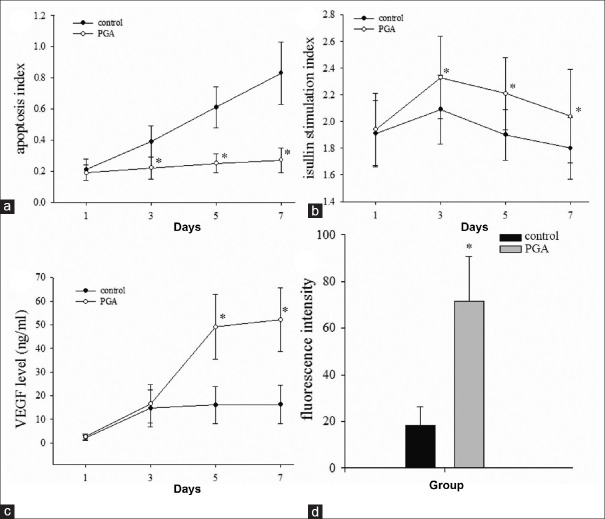
We measured the AI of the islet graft by AO-PI fluorescence staining. After 1 day of culture of ECs-coated islets, AIs were 21% ± 7% and 19% ± 5% in the control and PGA groups, respectively. There was no significant difference in AI between the two groups. In the control group, AIs increased from 39% ± 10% on day 3 to 83% ± 20% on day 7. AIs in the PGA group increased from 22% ± 7% on day 3 to approximately 27% ± 8% on day 7. Islet AI on day 3 was significantly higher in the control group than in the PGA group (P < 0.05). Differences in islet AI between the control and PGA groups were found to increase on days 3, 5, and 7 [Figure 1a].
Figure 1.
Polyglycolic acid scaffold improves the function and efficiency of endothelial cell-coating islets and vascular endothelial growth factor secretion in vitro. (a and b) The polyglycolic acid scaffold effectively decreased apoptosis and increased the insulin release stimulation index of endothelial cell-coating islet after 1, 3, 5, and 7 days of culture. (c) Vascular endothelial growth factor level in culture media of endothelial cell-coating islets in the polyglycolic acid group was higher than in the control group. (d) Fluorescence intensity of von Willebrand factor-positive endothelial cells was stronger in the polyglycolic acid group than in the control group. The results are representative of at least three independent samples, *P < 0.05 versus control group. PGA: Polyglycolic acid; VEGF: Vascular endothelial growth factor.
Polyglycolic acid scaffold increased insulin secretion from endothelial cell-coated islet in vitro
In the control group, the SI of islets decreased dramatically from 2.09 ± 0.26 on day 3 to 1.80 ± 0.23 on day 7 after ECs coating, while both groups exhibited a gradual decrease in SI, in which the PGA group had better SIs across all time points [Figure 1b]. In addition, SI was significantly greater in the PGA group than in the control group from day 3 to 7 after ECs coating (day 3: 2.33 ± 0.31, day 7: 2.07 ± 0.31; P < 0.05).
Polyglycolic acid scaffold increased the concentrations of vascular endothelial growth factor in culture supernatants
Medium levels of VEGF, an important angiogenesis cytokine, were measured by ELISA. At day 1 after ECs coating, VEGF was detected at very low concentrations with no significant difference between the control and PGA groups (2.17 ± 1.01 ng/ml vs. 2.66 ± 1.21 ng/ml). VEGF levels increased in both the control and PGA groups but were not significantly different from each other 3 days after ECs coating (14.70 ± 7.80 ng/ml vs.16.50 ± 8.10 ng/ml). VEGF levels in the PGA group (day 5: 49.10 ± 13.70 ng/ml, and day 7: 52.10 ± 13.50 ng/ml) were significantly higher than the coating in the control group (day 5: 16.10 ± 7.90 ng/ml and day 7, 16.30 ± 8.10 ng/ml) from day 5 after ECs coating [P < 0.05, Figure 1c].
Polyglycolic acid scaffold improved the efficiency of endothelial cell-coating on islet in vitro
After 14 days of culture following coating, ECs adhered to the surface of DTZ-stained scarlet islets in both the control and PGA groups. There were a large number of ECs on the islet surface or ECs that adhered to the scaffold, in which a small amount of ECs were dropped off in the PGA group [Supplementary Figure 1b (3.6MB, tif) ]. In contrast, there were many free ECs around, but not on the islet surface [Supplementary Figure 1a (3.6MB, tif) ]. vWF-stained ECs appeared as red fluorescence. Although the staining intensity of enzyme revealed that ECs could be detected around the islet at 14 days after coating in the control group [Supplementary Figure 1d (3.6MB, tif) ], islets in the PGA group had more vWF-positive ECs around it, showing stronger staining intensity of enzyme than those in the control group [Figure 1d and Supplementary Figure 1c (3.6MB, tif) , P < 0.05].
Polyglycolic acid scaffold improves the efficiency of endothelial cell-coating islet in vitro. After 14 days of culture (a and b), the polyglycolic acid scaffold increased the number of endothelial cells that adhered on the islet, as observed by inverted phase contrast microscopy (×1000). (c and d) The enzyme-staining intensity of von Willebrand factor-positive endothelial cells (blue) around insulin-positive islet (brown) was stronger in the polyglycolic acid group than in the control group (×200). (e and f) Islet and endothelial cells on polyglycolic acid scaffolds formed cell clusters that exhibited a tight, circumvallated, adhesive, and three-dimensional growth under scanning electron microscope observation (×1000).
SEM observation of the 14 day-old culture after EC coating revealed that the islets and ECs on the PGA scaffolds formed cell clusters that exhibited a tight, circumvallated, adhesive, and 3D growth [Supplementary Figure 1f (3.6MB, tif) ]. On the other hand, the islets and ECs were separated and not connected to each other in the control group [Supplementary Figure 1e (3.6MB, tif) ].
Polyglycolic acid scaffold improved the survival of endothelial cell-coated islet graft after transplantation
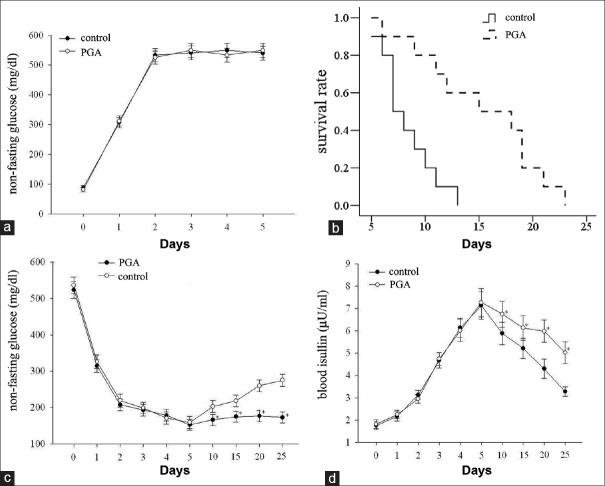
The nonfasting glucose levels increased from day 6 after STZ injection and there was no significant difference in nonfasting glucose levels between PGA and control groups [P > 0.05, Figure 2a]. After transplantation, compared with the control group, the average survival time of EC-coated islet graft in the PGA group was greater (15.30 ± 5.60 days vs. 8.30 ± 2.45 days, P < 0.05). On day 10 after ITx, only 30% of the grafts in the control group survived, while 70% of the graft survived in the PGA group. There was a significant difference in graft survival between the two groups by log-rank test [P = 0.015, Figure 2b]. Rats in PGA group had significantly lower nonfasting glucose levels from day 10 after transplantation, compared with the rats in control group [P < 0.05, Figure 2c]. In addition, the change of blood insulin concentration level was inversely proportional to glucose level. Recipient rats in PGA group had higher insulin than those in control group between 10 and 25 days posttransplantation [P < 0.05, Figure 2d].
Figure 2.
The function of endothelial cell-coating islet grafts in the control and polyglycolic acid groups in vivo. (a) Nonfasting glucose levels in rats of control and polyglycolic acid groups before and 5 days after streptozotocin injection, (b) survival analysis of islet graft after transplantation (n = 10). Polyglycolic acid significantly prolonged the survival of the endothelial cell-coated islet grafts compared with the control group (P < 0.05), nonfasting glucose levels (c) and blood insulin levels (d) in rats of control and polyglycolic acid groups before and after endothelial cell-coated islet transplantation at different time points. The results are representative of at least three independent samples, *P < 0.05 versus control group. PGA: polyglycolic acid.
Polyglycolic acid scaffold promoted vascularization of endothelial cell-coated islet graft in vivo
Photographs of the excised kidney taken 14 days after ITx revealed that angiogenesis appeared more prominent in the PGA group relative to the control group [Figure 3a and 3b]. Fourteen days after ITx, mature vasculatures were observed in the PGA group. Furthermore, H and E staining revealed that there were more ECs in tissues adjacent to the islet graft that formed microvessels in the PGA group, compared with the control group [Figure 3c and 3d]. Double immunofluorescence staining revealed that there were more vWF-positive (red) ECs around the islet in the PGA group than in the control group. In addition, the islet in the PGA group had a higher insulin expression than in the control group [Figure 3e and 3f]. The angiogenesis and area of survived grafts were more in the PGA group compared with the control group by measuring the mean MVD (8.60 ± 1.21/mm2 vs. 5.20 ± 0.87/mm2) [Figure 3g, P < 0.05].
Figure 3.
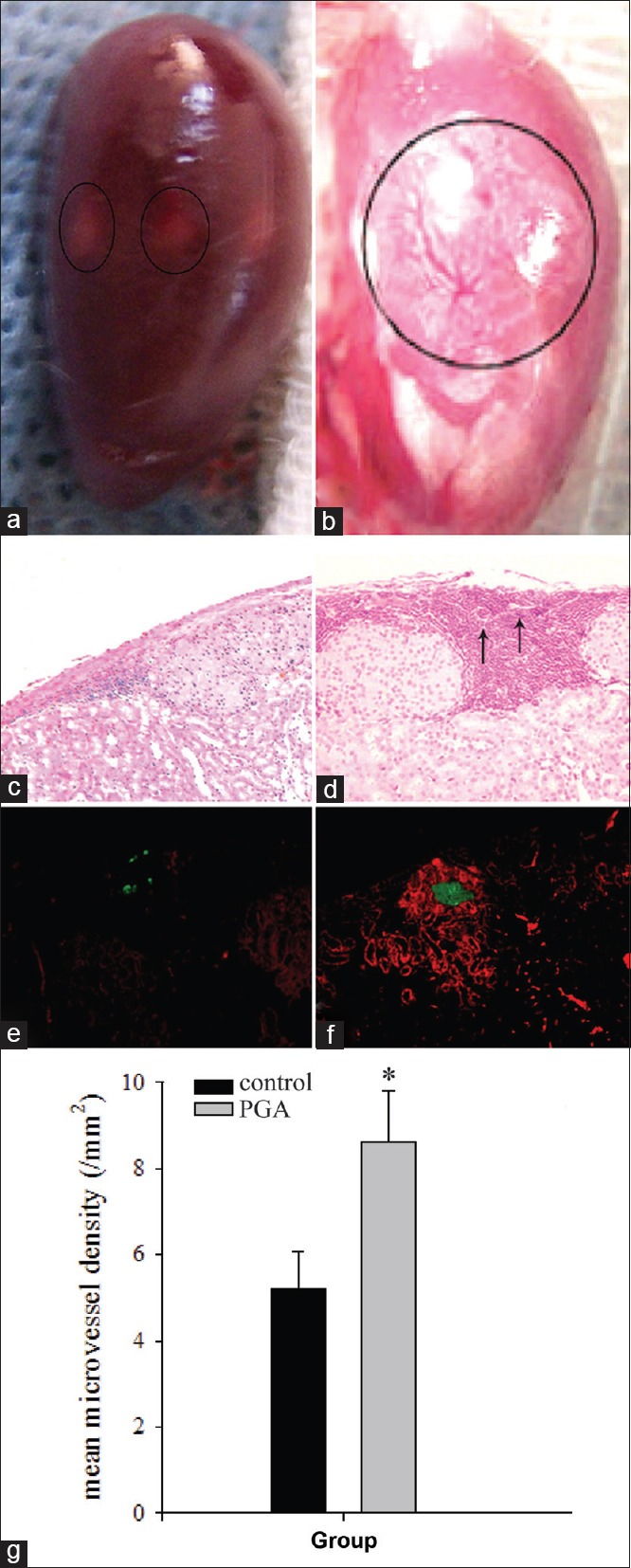
The vascularization analysis of endothelial cell-coating islet grafts in vivo. Fourteen days of culture after endothelial cell-coated islet transplantation. (a and b) Photomicrographs of fresh kidneys recovered from rats in the control and polyglycolic acid groups. New vessels were detected in the polyglycolic acid group (b), which could not be detected in the control group (a). (c and d) H and E staining reveals new microvessels (arrows) at the islet graft site, and many endothelial cells formed vessels in the islet graft site in the polyglycolic acid group (d) than in the control group (c) (original magnification ×100). (e and f) Double immunofluorescence staining to detect islets and endothelial cells in the graft site, there are more von Willebrand factor-positive endothelial cells around the islet in the polyglycolic acid group (f) than that in the control group (e) (original magnification ×200). (g) Microvessel density in the graft was significantly higher in the polyglycolic acid group than in the control group at 14 days after transplantation. The results are representative of at least three independent samples, *P < 0.05 versus control group.
Polyglycolic acid scaffold upregulated the expression of vascularization-related genes in vivo
The expression of VEGF and Tie-2 messenger RNA (mRNA) in transplanted graft was detected in the two groups by RT-PCR. Our analysis demonstrated that the expression of VEGF and Tie-2 mRNA was significantly increased in the PGA group, compared with the control group [Figure 4, P < 0.05].
Figure 4.
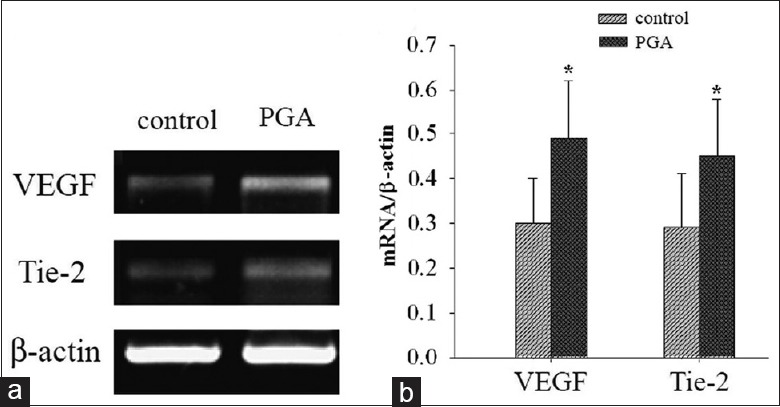
Effects of the polyglycolic acid scaffold on the expression of angiogenesis-related genes. (a) Expression of vascular endothelial growth factor and Tie-2 genes in grafts increases in the polyglycolic acid group compared to the control group, as detected by reverse transcription-polymerase chain reaction. (b) Comparison on the relative expression levels of vascular endothelial growth factor and Tie-2 genes between the two groups. The results are representative of at least three independent samples, *P < 0.05 versus control group. PGA: Polyglycolic acid; VEGF: Vascular endothelial growth factor; Tie-2: Tyrosin-protein kinase receptor.
Discussion
ECs from donor islets persist after transplantation and could become integrated to form new microvessels.[8] Since ECs are the basis of angiogenesis in islets, angiogenesis and revascularization of islets would be promoted with the addition of exogenous ECs. In our previous studies, we tried to improve islet survival through the co-culture and co-transplantation of ECs.[18,19] However, ECs used in those experiments for coating islets were distributed in the peripheral parts or on the surface of islets. The formed microvascular system was not able to extend into internal islet cells to provide oxygen for β-cells. A technique to coat isolated islets with ECs has been developed to improve vascularization in the islet graft in our previous study.[10] However, the efficiency of the coating achieved by the method differs greatly from the lowest of 50% to up to 90%.[9] For effective vascularization, the efficiency of the coating has to be stable and higher, enabling ECs to effectively attach to the surface of the islet to grow for better angiogenesis. Due to the difference in growth characteristics between suspending islet cells and adhesive ECs, a suitable microenvironment is needed to achieve the coexistence and close contact of the two types of cells. This would result in the improved attachment of ECs to the surface of the islet for coating. The PGA scaffold could improve the function of islets as reported.[14] Furthermore, ECs coating was described in detail in a previous report.[9] When islets were cocultured with ECs, ECs could attach on the islets’ surface. However, the coated ECs would become detached shortly after they have attached on the islets’ surface since there is no scaffold to support those cells. In addition, functional degeneration would occur in islets, leading to the failure of coating. Thus, the PGA scaffold was used in this study. The function of the scaffold was to supply a basic framework and supportive environment for islets coated by ECs. Moreover, the PGA scaffold can improve the function of islets and increase coating efficiency.
In the present study, the PGA scaffold was modified with a layer of PLYS to increase the growth approach and inner surface areas. This modification facilitates the intake of nutrients and excretion of cellular metabolites, providing a suitable microenvironment for the survival of islets and growth of adhesive ECs. The modified PGA scaffolds can prevent the adhesion of ECs to the well of Petri dishes and from being separated from islet suspension cells. In this way, there would be more ECs around the islet for better attachment to the islet's surface to support the coating. PLYS is polymerized by several amino acid components through van der Waals forces. Amino, carboxyl, hydroxyl, and phenols-amino all benefit the adhesive growth and coating of ECs on islets. This method may improve the surface activity and adherence of islets, which are required to improve the efficiency of EC coating.
Our study demonstrates that the PGA scaffold is a novel method for revascularization and better survival of islet grafts. After 3 days of culture, the number of islet cells in the control group markedly decreased. By day 7, this decrease was more significant. However, the survival rate of islets remained unchanged in the PGA group during the same period. Furthermore, islet function, as measured by high glucose-stimulated insulin release, improved compared with the control. Histological analysis revealed an increased efficiency of EC coating through the use of the PGA scaffold. Inverted phase contrast and fluorescence microscopy observations revealed that more ECs were adhered to the surface of islets in the PGA group, compared to the control group. Furthermore, in vivo evidence of difference in survival between the two groups also demonstrated that increased angiogenesis in islet grafts was achieved through the PGA scaffold. SEM demonstrated that the islet in the PGA scaffold modified by PLYS was surrounded by tightly adhered ECs. These findings suggest that PGA scaffolds could increase the adhesion surface of islets with 3D EC-coating capacity. Due to better nutrient uptake and metabolite excretion in PGA scaffolds, this 3D structure provides a suitable microenvironment for islets for improved survival.[14] ECs coating in the PGA group was shown to have higher coating efficiency, which is likely due to benefits of the PGA scaffolds.[20] Prolonged survival of the islet graft after ITx in the PGA group was due to the improvement of vascularization and absence of immunologic rejection in the syngeneic transplantation system. H and E and immunofluorescence staining analyses both revealed an increase in ECs and neo-vessels around the islet in the transplantation site using the PGA scaffold. In addition, the expression of insulin in the islet was higher, indicating better islet function.
VEGF is one of the best described angiogenesis-stimulating cytokines both in vitro and in vivo,[21] which mediates its angiogenic properties by specifically binding to the receptor tyrosine kinase KDR, promoting cell proliferation and inhibiting differentiation.[22,23] Tie-2 is involved in the formation and reconstruction of the vascular network, as well as in the VEGF-induced angiogenesis pathway.[24,25] A recent study has demonstrated the long-term formation and maturation of a capillary network in mice after the administration of VEGF, which did not express in the islets themselves.[26] This suggests that the improved islet graft function could be attributed to cytokines such as VEGF, which is possibly secreted by ECs’ culture in scaffold.[27] Therefore, the secretion of VEGF is dependent on the efficiency of islet coating, and the expression of Tie-2 is induced by VEGF. In our study, the expression levels of VEGF and Tie-2 were significantly higher in the PGA group than in the control group, both in vitro and in vivo, demonstrating that the PGA scaffold promotes VEGF secretion and Tie-2 expression through improving the efficacy of islet coating with ECs.
In summary, we have applied a PGA scaffold method to improve EC coating on islets and demonstrated that this method improves islet va, scularization in vitro and in vivo. Our findings indicate that the use of PGA scaffolds in the pretransplanting culture of islet cells and ECs can significantly improve the function of EC-coated islet graft by promoting vascularization.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (No. 81400677), the Science and Technology Project of Shaanxi (No. 2014KW20-03, 2016KW-023), the Fundamental Research Funds for the Central Universities (No. 08143014), and the Higher Specialized Research Fund for the Doctoral Program of the new class of teachers funded project (No. 20130201120081).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–9. doi: 10.2337/diabetes.54.7.2060. doi: 10.4103/0366-6999.193452. [DOI] [PubMed] [Google Scholar]
- 2.Hering BJ. Achieving and maintaining insulin independence in human islet transplant recipients. Transplantation. 2005;79:1296–7. doi: 10.1097/01.tp.0000157321.55375.86. doi: 10.1097/01.TP.0000157321.55375.86. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Emamaullee JA, Rajotte RV, Liston P, Korneluk RG, Lakey JR, Shapiro AM, et al. XIAP overexpression in human islets prevents early posttransplant apoptosis and reduces the islet mass needed to treat diabetes. Diabetes. 2005;54:2541–8. doi: 10.2337/diabetes.54.9.2541. doi: 10.2337/diabetes.54.9.2541. [DOI] [PubMed] [Google Scholar]
- 5.Menger MD, Yamauchi J, Vollmar B. Revascularization and microcirculation of freely grafted islets of Langerhans. World J Surg. 2001;25:509–15. doi: 10.1007/s002680020345. doi: 10.1007/s002680020345. [DOI] [PubMed] [Google Scholar]
- 6.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51:66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Fiorina P, Folli F, Maffi P, Placidi C, Venturini M, Finzi G, et al. Islet transplantation improves vascular diabetic complications in patients with diabetes who underwent kidney transplantation: A comparison between kidney-pancreas and kidney-alone transplantation. Transplantation. 2003;75:1296–301. doi: 10.1097/01.TP.0000061788.32639.D9. doi: 10.1097/01.TP.0000061788.32639.D9. [DOI] [PubMed] [Google Scholar]
- 8.Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17:881–3. doi: 10.1096/fj.02-0615fje. doi: 10.1096/fj.02-0615fje. [DOI] [PubMed] [Google Scholar]
- 9.Johansson U, Elgue G, Nilsson B, Korsgren O. Composite islet-endothelial cell grafts: A novel approach to counteract innate immunity in islet transplantation. Am J Transplant. 2005;5:2632–9. doi: 10.1111/j.1600-6143.2005.01076.x. doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Xue W, Liu H, Fan P, Wang X, Ding X, et al. Combined strategy of endothelial cells coating, Sertoli cells coculture and infusion improves vascularization and rejection protection of islet graft. PLoS One. 2013;8:e56696. doi: 10.1371/journal.pone.0056696. doi: 10.1371/journal.pone.0056696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation. 1995;60:123–7. doi: 10.1097/00007890-199507000-00002. [PubMed] [Google Scholar]
- 12.Hu X, Lui W, Cui L, Wang M, Cao Y. Tissue engineering of nearly transparent corneal stroma. Tissue Eng. 2005;11:1710–7. doi: 10.1089/ten.2005.11.1710. doi: 10.1089/ten.2005.11.1710. [DOI] [PubMed] [Google Scholar]
- 13.Andrades P, Asiedu C, Rodriguez C, Goodwin KJ, McCarn J, Thomas JM. Subcutaneous pancreatic islet transplantation using fibrin glue as a carrier. Transplant Proc. 2007;39:191–2. doi: 10.1016/j.transproceed.2006.10.019. doi: 10.1016/j.transproceed.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Chun S, Huang Y, Xie WJ, Hou Y, Huang RP, Song YM, et al. Adhesive growth of pancreatic islet cells on a polyglycolic acid fibrous scaffold. Transplant Proc. 2008;40:1658–63. doi: 10.1016/j.transproceed.2008.02.088. doi: 10.1016/j.transproceed.2008.02.088. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Xue W, Tian X, Ding X, Tian P, Feng X, et al. Improved survival and function of rat cryopreserved islets by coculture with sertoli cells. Artif Organs. 2011;35:634–44. doi: 10.1111/j.1525-1594.2010.01155.x. doi: 10.1111/j.1525-1594.2010.01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang XH, Ding XM, Li Y, Liu HB, Xue WJ, Tian XH, et al. Simultaneous blockade of the CD40/CD40L and NF-kB pathways prolonged islet allograft survival. Transpl Int. 2012;25:118–26. doi: 10.1111/j.1432-2277.2011.01374.x. doi: 10.1111/j.1432-2277.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E. Bone marrow cell cotransplantation with islets improves their vascularization and function. Transplantation. 2010;89:686–93. doi: 10.1097/TP.0b013e3181cb3e8d. doi: 10.1097/TP.0b013e3181cb3e8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan X, Xue W, Li Y, Feng X, Tian X, Ding C. Islet graft survival and function: Concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92:1208–14. doi: 10.1097/TP.0b013e3182356ca7. doi: 10.1097/TP.0b013e3182356ca7. [DOI] [PubMed] [Google Scholar]
- 19.Song HJ, Xue WJ, Li Y, Tian XH, Ding XM, Feng XS, et al. Prolongation of islet graft survival using concomitant transplantation of islets and vascular endothelial cells in diabetic rats. Transplant Proc. 2010;42:2662–5. doi: 10.1016/j.transproceed.2010.06.003. doi: 10.1016/j.transproceed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–70. doi: 10.2337/diabetes.53.4.963. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 21.Diao YP, Cui FK, Yan S, Chen ZG, Lian LS, Guo LL, et al. Nerve growth factor promotes angiogenesis and skeletal muscle fiber remodeling in a murine model of hindlimb ischemia. Chin Med J. 2016;129:313–9. doi: 10.4103/0366-6999.174496. doi: 10.4103/0366-6999.174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juang JH, Kuo CH, Hsu BR. Effects of vascular endothelial growth factor on islet isografts. Transplant Proc. 2002;34:2690–2. doi: 10.1016/s0041-1345(02)03377-8. doi: 10.1016/S0041-1345(02)03377-8. [DOI] [PubMed] [Google Scholar]
- 23.Millauer B, Wizigmann-Voos S, Schnürch H, Martinez R, Møller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–46. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y, Komi Y, Ashino H, Yamashita J, Inoue J, Yoshiki A, et al. Retinoic acid controls blood vessel formation by modulating endothelial and mural cell interaction via suppression of Ti.e2 signaling in vascular progenitor cells. Blood. 2004;104:166–9. doi: 10.1182/blood-2003-09-3293. doi: 10.1182/blood-2003-09-3293. [DOI] [PubMed] [Google Scholar]
- 25.Chong AY, Caine GJ, Lip GY. Angiopoietin/tie-2 as mediators of angiogenesis: A role in congestive heart failure? Eur J Clin Invest. 2004;34:9–13. doi: 10.1111/j.1365-2362.2004.01284.x. doi: 10.1111/j.1365-2362.2004.01284.x. [DOI] [PubMed] [Google Scholar]
- 26.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Xu C, Ma BP, Zheng ZB, Li YL, Ma Q, et al. Three-dimensional printed scaffolds with gelatin and platelets enhance in vitro preosteoblast growth behavior and the sustained-release effect of growth factors. Chin Med J. 2016;129:2576–81. doi: 10.4103/0366-6999.192770. doi: 10.4103/0366-6999.192770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Polyglycolic acid scaffold improves the efficiency of endothelial cell-coating islet in vitro. After 14 days of culture (a and b), the polyglycolic acid scaffold increased the number of endothelial cells that adhered on the islet, as observed by inverted phase contrast microscopy (×1000). (c and d) The enzyme-staining intensity of von Willebrand factor-positive endothelial cells (blue) around insulin-positive islet (brown) was stronger in the polyglycolic acid group than in the control group (×200). (e and f) Islet and endothelial cells on polyglycolic acid scaffolds formed cell clusters that exhibited a tight, circumvallated, adhesive, and three-dimensional growth under scanning electron microscope observation (×1000).