Abstract
Background:
Recombinant human-erythropoietin (rh-EPO) has therapeutic efficacy for premature infants with brain damage during the active rehabilitation and anti-inflammation. In the present study, we found that the rh-EPO was related to the promotion of neovascularization. Our aim was to investigate whether rh-EPO augments neovascularization in the neonatal rat model of premature brain damage through the phosphatidylinositol 3 kinase (PI3K)/protein kinase B (Akt) signaling pathway.
Methods:
Postnatal day 5 (PD5), rats underwent permanent ligation of the right common carotid artery and were exposed to hypoxia for 2 h. All the rat pups were randomized into five groups as follows: (1) control group; (2) hypoxia-ischemic (HI) group; (3) HI + LY294002 group; (4) HI + rh-EPO group; and (5) HI + rh-EPO + LY294002 group. The phospho-Akt protein was tested 90 min after the whole operation, and CD34, vascular endothelial growth factor receptor 2 (VEGFR2), and vascular endothelial growth factor (VEGF) were also tested 2 days after the whole operation.
Results:
In the hypoxic and ischemic zone of the premature rat brain, the rh-EPO induced CD34+ cells to immigrate to the HI brain zone (P < 0.05) and also upregulated the VEGFR2 protein expression (P < 0.05) and VEGF mRNA level (P < 0.05) through the PI3K/Akt (P < 0.05) signaling pathway when compared with other groups.
Conclusions:
The rh-EPO treatment augments neovascularization responses in the neonatal rat model of premature brain damage through the PI3K/Akt signaling pathway. Besides, the endogenous EPO may exist in the HI zone of rat brain and also has neovascularization function through the PI3K/Akt signaling pathway.
Keywords: Erythropoietin, Hypoxia-ischemia, Neovascularization, Premature Brain
Introduction
Incidence and mortality of neonatal-acquired brain damage, especially hypoxic-ischemic brain damage (HIBD), have an increasing trend.[1] Brain damage of prematurity is the predominant form of the acquired brain damage in neonates who undergo neurological morbidity.[2] There are only limited therapies to improve the outcome from the HIBD.[3] Administration of the recombinant human-erythropoietin (rh-EPO) into extremely preterm infants at Neonatal Intensive Care Unit improved neurodevelopmental outcomes.[4] However, the underlying mechanisms that support the neuroprotective effects of the rh-EPO following premature brain damage remain unclear. The present study therefore used an animal model to investigate the role of rh-EPO in enhanced neurological recovery, which was thought to be related to the promotion of angiogenesis.[2,5]
Some in vitro experiments suggest that EPO enhances vascular endothelial growth factor (VEGF) secretion in neural progenitor cells through the activation of phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway. The neural progenitor cells, treated with the rh-EPO, upregulate vascular endothelial growth factor receptor 2 (VEGFR2) expression in the cerebral endothelial cells (ECs).[6] In addition, the PI3K/Akt signaling also mediates angiogenesis and VEGF expression in the ECs.[7,8,9] Wang et al.[10] found that the rh-EPO increased the mobilization of endothelial progenitor cells (EPCs) and subsequent promotion of angiogenesis.
Based on these studies, we hypothesized that: (1) rh-EPO may increase the amount of EPCs or ECs in the hypoxia-ischemic (HI) region of the neonatal rat model of premature brain damage through the PI3K/Akt signaling pathway by promoting neovascularization; (2) rh-EPO may induce the secretion of VEGF in the HI region in the neonatal rat model of premature brain damage through the PI3K/Akt signaling pathway and then promote neovascularization through the VEGF/VEGFR system. To verify our hypothesis, we tested the phospho-Akt, CD34, VEGFR2 proteins (the surface markers for ECs and EPCs), and VEGF mRNA after administration of the rh-EPO.
Methods
Reagents
The rh-EPO injection was obtained from Zhongda Hospital Affiliated to Southeast University (China). LY294002 was obtained from Cell Signaling Technology (USA). Antibodies were obtained from the following sources: phospho-Akt (Ser 473, D9E) XP rabbit mAb from CST (USA); rat CD34 antibody from R and D (USA); and anti-VEGF receptor 2 antibody from Abcam (UK).
Experimental animals
Pregnant Sprague-Dawley rats were obtained from Nanjing Medical University of China and were allowed to deliver. All 5-day-old Sprague-Dawley rat pups were used for experiments. The animals were double housed with food and water supply ad libitum at a temperature- and light-controlled environment (12-h light/dark cycle, daily humidity, and temperature monitoring). All the protocols were approved by the Institutional Animal Care and Use Committee of Southeast University.
Neonatal rat model of premature brain damage and drug administration
Five-day-old (PD5) postnatal rats underwent permanent ligation of the right common carotid artery (CCA). The infant rats were then exposed to hypoxia (94% N2, 6% O2) for 2 h as described by Back et al.[11] All the rat pups were randomized into five groups as follows: (1) control group (I): without HI, rh-EPO, and LY294002 (C19H17 NO3; a PI3K inhibitor); (2) HI group (II): underwent permanent ligation of the right CCA and exposed to hypoxia, but without rh-EPO and LY294002; (3) HI + LY294002 group (III): underwent permanent ligation of the right CCA; exposed to hypoxia; and given LY294002, without rh-EPO; (4) HI + rh-EPO group (IV): underwent permanent ligation of the right CCA, exposed to hypoxia, and given rh-EPO, without LY294002; (5) HI + rh-EPO + LY294002 group (V): underwent permanent ligation of the right CCA; exposed to hypoxia; given rh-EPO and LY294002. Each group was further divided into nine subgroups. The rats were then sacrificed 90 min and 2 days after the whole operation.
Immunofluorescence experiments
Coronal sections (10 μm) from the brains were cut using a freezing microtome (Leica CM3050; Leica Instruments, Germany). The slides were then baked for 1 h at 50°C. After repairing antigen of the slides, the slides were then closed by 10% donkey serum for 60 min at 37°C. The slides were then incubated overnight at 4°C with the primary antibody: anti-CD34 (10 μg/ml), and then followed by treatment with appropriate secondary antibody for CD34 for 1 h at room temperature. Each of the aforementioned steps was followed by 5-min rinses in PBS for three times. The slides were then mounted with glycerin for immunofluorescence analysis. The nuclear staining procedures using DAPI (Abcam, UK) were the same as above. The CD34 protein (red) and nucleus (blue) were examined with an epifluorescence microscope (Nikon, Japan). Three sections from each rat were taken from the right white matter region and the cells in the white matter were counted (per ×400) in three areas by two blinded independent observers.
Western blot assays
Protein concentration was determined by the BCA protein assay kit (KeyGEN BioTECH, China). Homogenate protein (30 μl) was heated for 5 min at 99°C and then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel. After electrophoresis, the proteins were electroblotted on the nitrocellulose (NC) membrane and blocked with 5% skim milk. The immobilized proteins were exposed to commercially available antibodies such as phospho-Akt (1:2000), CD34 (0.1 μg/ml), and VEGFR2 (0.2 μg/ml). Specific proteins were detected with secondary antibody and visualized by High-sig ECL reagents (Tanon, China). Western blotting for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; Abcam, UK) was performed as loading control, and band densities were semi-quantified using Image-J software (National Institutes of Health, Bethesda, USA).
Quantitative real-time-polymerase chain reaction
Total RNA was extracted from the right brains of rats using Trizol (Invitrogen, California, USA), and cDNA was synthesized using a kit (Invitrogen). Polymerase chain reaction (PCR) was performed using the SYBR Green OPCR Mix kit (Invitrogen), and β-actin was used as an internal control. The reverse transcription conditions were 10 min at 25°C, 30 min at 42°C, and 5 min at 85°C. The amplification program consisted of activation at 94°C for 30 s, followed by 45 amplification cycles, each consisting of 94°C for 10 s, 60°C for 12 s, and 72°C for 30 s. Primer pairs used for amplification were as follows: VEGF, forward 5′-AACGTCACTATGCAGATCATGC-3′, reverse 5′-CTCCGCTCTGAACAAGGCT-3′; β-actin, forward 5’-CTGAACCCTAAGGCCAACC-3′, and reverse 5′-AGCGCGTAACCCTCATAGAT-3′. Data were analyzed using Gel-pro32 analyzer (Thermo Fisher Scientific, USA). The relative value of mRNA = The absorbance value of objective fragment/the absorbance value of β-actin.
Data analysis
Multiple comparisons were conducted using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test. A statistically significant difference between means was considered when P < 0.05. Data in the figures and text are expressed as means ± standard deviation (SD).
Results
Recombinant human erythropoietin promoted phosphorylation on Akt
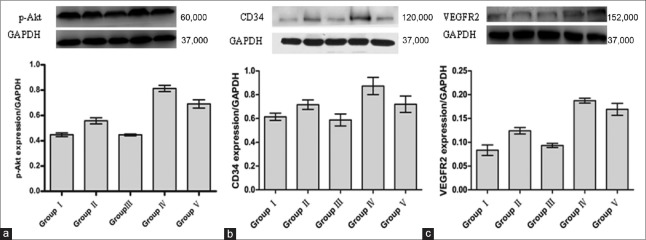
The content of phospho-Akt in the right white matter of premature brains was determined by Western blot assays. Nineteen minutes after the whole operation, the results revealed a significant increase in phospho-Akt in Group IV compared with the other groups [P < 0.05; Figure 1a]. Group III was less than Groups IV, V, and II [P < 0.05; Figure 1a]. However, there was no significant statistical significance when we compared Group I and Group III (P > 0.05). Group II was higher than Group I [P < 0.05; Figure 1a].
Figure 1.
Expressions of p-Akt, CD34, and VEGFR2 in the right white matter of premature brain were analyzed by Western blot analysis. (a) Representative bands and bar graph showing expression of p-Akt 90 min after the whole operation. (b) Representative bands and bar graph showing expression of CD34 2 days after the whole operation. (c) Representative bands and bar graph showing expression of VEGFR2 2 days after the whole operation. Group IV showing significant difference from other groups (P < 0.05); Group III showing significant difference from Groups IV, V, and II (P < 0.05); Group II showing significant difference from Group I (P < 0.05). VEGFR2: Vascular endothelial growth factor receptor 2.
Recombinant human erythropoietin increased the expression of CD34 and vascular endothelial growth factor receptor 2 through phosphatidylinositol 3 kinase/Akt pathway
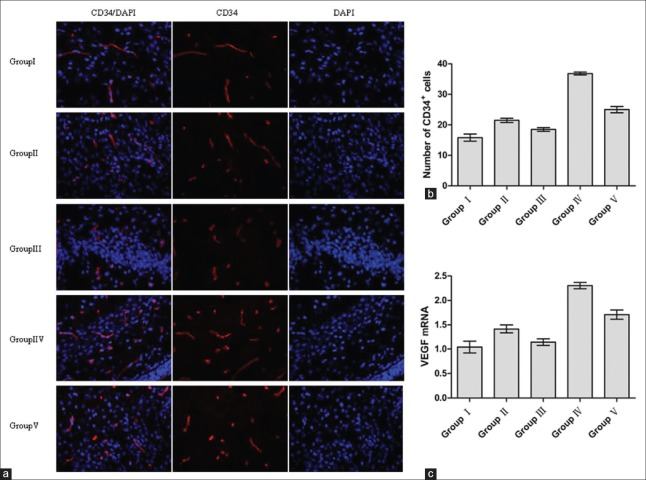
The content of CD34 and VEGFR2 proteins in the right white matter of premature brain was also determined by Western blot assays. Two days after the whole operation, the amount of CD34 and VEGFR2 proteins in Group IV was higher than in the other groups [P < 0.05; Figure 1b and 1c]. Group V was lower than Group IV [P < 0.05; Figure 1b and 1c] but higher than Group III [P < 0.05; Figure 1b and 1c]. Group III was lower than Group II [P < 0.05; Figure 1b and 1c] but had no significant statistical significance when compared with Group I [P > 0.05; Figure 1b and 1c]. Group II was a little higher than Group I [P < 0.05; Figure 1b and 1c]. The trends of the CD34 and VEGFR2 proteins in the five groups were consistent with the amount of phospho-Akt in the right white matter of premature brains. Two days after the whole operation, the trends of CD34+ cells’ count in the five groups after the immunofluorescence experiments were consistent with results for the CD34 protein in the Western blot assays [Figure 2a and 2b]. At the same time, Group II was a little higher than Group I [P < 0.05; Figure 2a and 2b] and Group III [P < 0.05; Figure 2a and 2b].
Figure 2.
Expressions of CD34+ cells and VEGF in the right white matter of premature brain 2 days after the whole operation. (a) Immunofluorescence staining for CD34+ cells (red: CD34; blue: nucleus). (b) A histogram showing quantification difference in the number of CD34+ cells. (c) VEGF mRNA level was analyzed by qRT-PCR. Group IV showing significant difference from other groups (P < 0.05); Group III showing significant difference from Groups IV, V, and II (P < 0.05); Group II showing significant difference from Group I (P < 0.05). GAPDH: glyceraldehyde-3-phosphate dehydrogenase; VEGF: Vascular endothelial growth factor; qRT-PCR: Quantitative real-time-polymerase chain reaction.
Recombinant human erythropoietin upregulated the expression of vascular endothelial growth factor through phosphatidylinositol 3 kinase/Akt pathway
The VEGF mRNA level was assessed by quantitative real-time PCR during HI in the right white matter of premature brains. Two days after the whole operation, the VEGF mRNA level in Group IV was significantly higher than in the other four groups [P < 0.05; Figure 2c]. Group III was significantly lower than in the other four groups [P < 0.05; Figure 2c]. The exogenous rh-EPO could have upregulated the expression of VEGF through the PI3K/Akt signaling pathway during the HI in the white matter of premature brains. Group II was a little higher than Group I [P < 0.05; Figure 2c] and Group III [P < 0.05; Figure 2c].
Discussion
Administration of the rh-EPO after ischemia, either peripherally or centrally injected, suggested beneficial effects on brain edema by delaying the neuronal death and resulted in functional improvement of neurons in neonatal animal models.[12] The injection of rh-EPO centrally is not a practical approach in clinical settings, and indeed, systemic delivery of the rh-EPO has advantages in that it is universally available to the capillary endothelium and thus potentially present everywhere in the brain.[7,13] In addition, the receptors of EPO are extensively expressed in the mature brain, including hippocampus.[14] A study showed that the peak time of administrating EPO peripherally was 3 h in the brain tissue of rodents.[15] Based on this finding, in our experiments, we administered the rh-EPO by intraperitoneal injection before HI (2 h).
Akt was originally identified by Staal in 1987,[16] which was also known as PKB. The Akt is the downstream protein for the PI3K and can be activated by growth factors and other extracellular stimulators.[17] LY294002 can inactive the sites (Ser473/Thr308) of the Akt to inhibit its phosphorylation. In this study, the rh-EPO increased the number of CD34+ cells in the HI zone of rat brain through the PI3K/Akt signaling pathway. The CD34 is the common surface marker for the ECs and EPCs.[18] Moreover, the rh-EPO induces EPCs’ immigration into the HI zone and then the EPCs’ proliferation and differentiation to form the new blood vessels. This physiological process is called vasculogenesis, whereby the new blood vessels are formed where no preexisting vessels were present.[19] On the other hand, the ECs from the capillaries in the HI zone of brain proliferate and form the new blood vessels. This physiological process is called angiogenesis, which involves the growth and development of new blood vessels from preexisting vessels.[19] The rh-EPO may therefore regulate blood vessels’ growth through two mechanisms: vasculogenesis and angiogenesis.
VEGFR2 can combine with the VEGF and can be activated to induce neovascularization. In this study, the expression of VEGFR2 protein and VEGF mRNA levels were significantly upregulated by the rh-EPO through the PI3K/Akt pathway in the HI zone of the brain. First, this result indicated that the rh-EPO could activate the VEGF/VEGFR2 signaling pathway through the PI3K/Akt pathway. Second, the increased expression of VEGFR2 could combine with more VEGF and then enhance neovascularization of the VEGF. Third, the VEGFR2 is 100% expressed in the mature ECs but not in the EPCs.[18] Our study has showed that the rh-EPO could significantly stimulate the ECs’ proliferation by the PI3K/Akt pathway in the HI brain.
In our study, the protein levels of p-Akt, CD34, VEGFR2, and mRNA level of VEGF in the HI group were a little higher than in the same group. Hence, we hypothesized that these changes were induced by the endogenous EPO which was induced by hypoxia. Fandrey had proved that hypoxia could induce the expression of EPO gene.[20] The expressions of p-Akt, CD34, VEGFR2, and VEGF in Group V were all between Group III and Group IV in our study. This shows that the PI3K/Akt signaling pathway was partly inhibited by LY294002. There may also be some other signaling pathways which were activated by the rh-EPO that could not be inhibited by LY294002. Moderate neovascularization is beneficial to the HI zone of premature brain[8] which can provide enough oxygen and energy to the surrounding cells. This information offers hope for the potential neovascularization by these molecules for clinical benefits.
In conclusion, our study has demonstrated that the PI3K/Akt signaling pathway is one of the essential mechanisms for neovascularization of rh-EPO. The rh-EPO induces CD34+ cell immigration into the hypoxic and ischemic zone of premature rat brain and also upregulates the expression of VEGFR2 protein and VEGF mRNA level through the PI3K/Akt signaling pathway.
Financial support and sponsorship
This work was supported by a grant from the National Natural Science Foundation of China (No. 81370739).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Lee HS, Song J, Min K, Choi YS, Kim SM, Cho SR, et al. Short-term effects of erythropoietin on neurodevelopment in infants with cerebral palsy: A pilot study. Brain Dev. 2014;36:764–9. doi: 10.1016/j.braindev.2013.11.002. doi: 10.1016/j.braindev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhu L, Bai X, Wang S, Hu Y, Wang T, Qian L, et al. Recombinant human erythropoietin augments angiogenic responses in a neonatal rat model of cerebral unilateral hypoxia-ischemia. Neonatology. 2014;106:143–8. doi: 10.1159/000362262. doi: 10.1159/000362262. [DOI] [PubMed] [Google Scholar]
- 3.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67:657–66. doi: 10.1002/ana.21977. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 5.Diao YP, Cui FK, Yan S, Chen ZG, Lian LS, Guo LL, et al. Nerve growth factor promotes angiogenesis and skeletal muscle fiber remodeling in a murine model of hindlimb ischemia. Chin Med J. 2016;129:313–9. doi: 10.4103/0366-6999.174496. doi: 10.4103/0366-6999.174496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Chopp M, Gregg SR, Zhang RL, Teng H, Jiang A, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–8. doi: 10.1038/jcbfm.2008.32. doi: 10.1038/jcbfm.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–53. doi: 10.1073/pnas.040560897. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imazuru T, Matsushita S, Hyodo K, Tokunaga C, Kanemoto S, Enomoto Y, et al. Erythropoietin enhances arterioles more significantly than it does capillaries in an infarcted rat heart model. Int Heart J. 2009;50:801–10. doi: 10.1536/ihj.50.801. doi: 10.1536/ihj.50.801. [DOI] [PubMed] [Google Scholar]
- 9.Kawachi K, Iso Y, Sato T, Wakabayashi K, Kobayashi Y, Takeyama Y, et al. Effects of erythropoietin on angiogenesis after myocardial infarction in porcine. Heart Vessels. 2012;27:79–88. doi: 10.1007/s00380-011-0197-2. doi: 10.1007/s00380-011-0197-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Wang X, Su H, Han Z, Yu H, Wang D, et al. Recombinant human erythropoietin improves the neurofunctional recovery of rats following traumatic brain injury via an increase in circulating endothelial progenitor cells. Transl Stroke Res. 2015;6:50–9. doi: 10.1007/s12975-014-0362-x. doi: 10.1007/s12975-014-0362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–63. doi: 10.1523/JNEUROSCI.22-02-00455.2002. doi: 0270-6474/02/210455-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, et al. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–56. doi: 10.1016/s0014-2999(00)00466-0. doi: 10.1016/S0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 13.Liu K, Wang XJ, Li YN, Li B, Qi JS, Zhang J, et al. Tongxinluo reverses the hypoxia-suppressed claudin-9 in cardiac microvascular endothelial cells. Chin Med J. 2016;129:442–7. doi: 10.4103/0366-6999.176076. doi: 10.4103/0366-6999.176076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: Can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25:577–83. doi: 10.1016/j.tips.2004.09.006. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Digicaylioglu M. Erythropoietin in stroke: Quo vadis. Expert Opin Biol Ther. 2010;10:937–49. doi: 10.1517/14712598.2010.481435. doi: 10.1517/14712598.2010.481435. [DOI] [PubMed] [Google Scholar]
- 16.Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–7. doi: 10.1073/pnas.84.14.5034. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27. doi: 10.1016/j.cellsig.2011.05.004. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Fan CL, Li Y, Gao PJ, Liu JJ, Zhang XJ, Zhu DL. Differentiation of endothelial progenitor cells from human umbilical cord blood CD 34 cells in vitro. Acta Pharmacol Sin. 2003;24:212–8. [PubMed] [Google Scholar]
- 19.Ribatti D. Erythropoietin and tumor angiogenesis. Stem Cells Dev. 2010;19:1–4. doi: 10.1089/scd.2009.0402. doi: 10.1089/scd.2009.0402. [DOI] [PubMed] [Google Scholar]
- 20.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–88. doi: 10.1152/ajpregu.00577.2003. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]