Introduction
In 2002, Cherqui et al. reported the first purely laparoscopic living-donor left lateral sectionectomy (LDLLS).[1] This technique has now become a standardized procedure and has significantly shortened donor hospitalization.[2] The da Vinci robotic surgical system was introduced into the field of liver surgery more than 10 years ago. Its flexible mechanical “wrist” and stable three-dimensional (3D) visual field help minimize risks from complicated procedures. However, robotic LDLLS has not yet been reported. Recently, we performed such a case of robotic LDLLS.
Case Report
A 7-month-old male patient was admitted to our hospital for congenital biliary atresia. His jaundice did not dissipate after the Kasai procedure and was eligible for liver transplantation. Preoperative computed tomography (CT) did not reveal his common bile duct but did reveal extensive dilation of the intrahepatic biliary tree and severe ascites. His serum total bilirubin was 343.3 μmol/L, direct bilirubin was 284.2 μmol/L, albumin was 29.6 g/L, and prothrombin time was extended by 6 s.
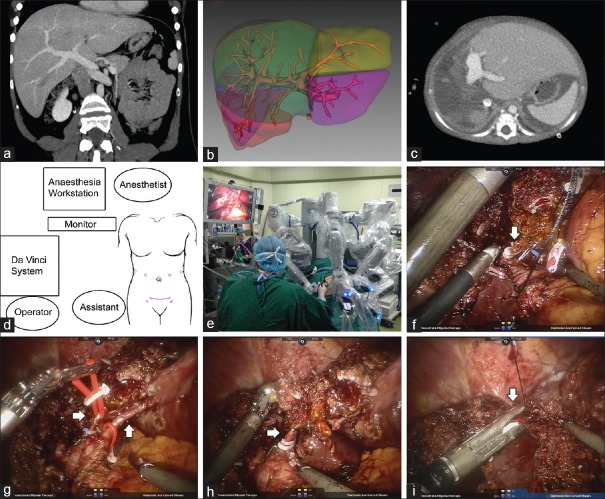
His 27-year-old mother (height: 168 cm; weight: 68 kg; blood type: A) volunteered to donate her liver's left lateral lobe to him. Preoperative evaluation, including triphasic contrast-enhanced CT with 3D reconstruction and magnetic resonance cholangiopancreatography, showed normal hepatic structures [Figure 1a]. Several gallstones were identified under ultrasonography. The estimated total liver volume was 1545 ml, while the left lateral lobe's volume was estimated as 285 ml [Figure 1b]. The calculated graft-versus-body weight ratio of the recipient was 3.2% (285 ml/9.0 kg).
Figure 1.
(a) Preoperative CT scan of donor; (b) Preoperative estimation of liver volumes; (c) CT scan of the recipient on postoperative day 15; (d) Position of the trocars and the incision; (e) Position of the mechanical arms during robotic surgery; (f) (arrow) The stump of the left bile duct; (g) (arrows) The left hepatic arteries; (h) (arrow) The left portal vein; (i) (arrow) The left hepatic vein. CT: Computed tomography.
Our center has great experience with purely laparoscopic living-donor hemihepatectomy. The possibility of using minimally invasive robotic LDLLS was discussed during the process of informed consent. All technical aspects and safety issues were explained, emphasizing the fact that robotic LDLLS had never been reported. Later, the Ethics Committee of the West China Hospital, Sichuan University, evaluated the donor in accordance with the previously published standard protocol. The donor agreed to undergo the first robotic LDLLS in our center.
The donor lay in a supine position and the reverse Trendelenburg position was applied with a slight left tilt. A laparoscopic trocar was placed above the umbilicus, and the other four trocars were placed on the curve toward the surgical region, with at least 10 cm between each [Figure 1d]; then, the da Vinci robotic surgical system (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was installed [Figure 1e]. The gallbladder was removed first, and then, the liver was carefully scanned under laparoscopic ultrasonography. The left lateral branches of the artery, portal vein, and hepatic vein were located; then, the liver hilum was dissected to isolate the respective vessels. Thereafter, the left lateral lobe was split with a robotic harmonic scalpel; vessels >5 mm were closed with Hem-o-lok clips. While transecting the liver parenchyma, the left bile duct, hepatic artery, and proximal branch of the left portal vein were dissected [Figure 1f-1h]. Finally, the left hepatic vein was transected with an Echelon stapler [Figure 1i]. There was no inflow occlusion during the procedure. The left lateral lobe was placed in a bag and harvested from a 7-cm bikini incision on the lower abdomen. The donor's procedure lasted 6.5 h with blood loss of 400 ml.
Immediately, histidine-tryptophan-ketoglutarate solution was perfused through the portal vein branch, and the hepatic artery and bile duct were also rinsed. The warm ischemia time was 15 min. The left lobe was reshaped according to the size of the recipient's abdominal cavity. The final weight of the graft lobe was 198 g, and the graft-to-recipient weight ratio was 2.2% (198 ml/9.0 kg). The graft was implanted with a cold ischemia time of 185 min. The graft's left hepatic vein was anastomosed to the recipient inferior vena cava's lateral wall; bile duct to intestinal anastomosis and Roux-en-Y reconstruction were performed. Graft implantation was succeeded with no event.
On the 1st postoperative day, the donor returned to the ward from the intensive care unit and returned to oral feeding on the 3rd postoperative day. She was discharged on the 8th postoperative day without any complications. One month after discharge, she was confirmed to have normal hepatic function without special discomfort. The recipient experienced mild pulmonary infection but recovered and was discharged on the 20th postoperative day [Figure 1c]. No transplant-related complication has occurred to the recipient to date.
Discussion
While enabling operation through 1–2 cm incisions, the da Vinci Surgical System can rotate in all directions with 90° articulation and 7° of freedom, which would overwhelm the human hand. This ability allows manipulation and suturing in small spaces, such as second liver hilum or retrohepatic spaces, at angles not possible with rigid instruments. The improved 3D visualization and dexterity allow surgeons to perform fine movements similar to that with open surgery but much more precise and minimally invasive and facilitate curved transection lines for more complex resections.
Compared with purely laparoscopic left lateral sectionectomy, the learning curve to perform complex surgeries is shorter with robotic assistance, and surgeons do not need abundant laparoscopic experience to perform surgical robotic techniques.[3] However, it was somewhat more difficult to maintain the distance and angle between the mechanical arms from occlusions, especially when dissecting the left triangular ligament, falciform ligament, or suprahepatic vena cava. Although the mechanical arms can rotate 360°, the camera cannot visualize some distal sites directly during operation. Additional instruments should be designed for these situations.
An obvious limitation of the robot is the surgical instruments. At present, only ultrasonic scalpels, Hem-o-lock clips, and staplers such as Echelon can be used during robotic liver surgery. Even the ultrasonic probe was laparoscopic, the head of the probe could not bend. It should be stressed that the CUSA system, the most useful instrument in laparoscopic liver surgery, cannot be applied in robotic surgeries.[4,5] In addition, medical insurance does not cover the relatively high expense, which limits the development of robotic surgeries.
In summary, we reported the first LDLLS performed on the da Vinci robotic system, but new techniques and specialized equipment are still needed for these complicated procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
References
- 1.Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392–6. doi: 10.1016/S0140-6736(02)07598-0. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 2.Scatton O, Katsanos G, Boillot O, Goumard C, Bernard D, Stenard F, et al. Pure laparoscopic left lateral sectionectomy in living donors: From innovation to development in France. Ann Surg. 2015;261:506–12. doi: 10.1097/SLA.0000000000000642. doi: 10.1097/SLA.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 3.Salloum C, Lim C, Azoulay D. Robot-assisted laparoscopic left lateral sectionectomy for benign and malignant liver tumors. J Visc Surg. 2015;152:373–8. doi: 10.1016/j.jviscsurg.2015.09.007. doi: 10.1016/j.jviscsurg.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Coelho FF, Kruger JA, Fonseca GM, Araújo RL, Jeismann VB, Perini MV, et al. Laparoscopic liver resection: Experience based guidelines. World J Gastrointest Surg. 2016;8:5–26. doi: 10.4240/wjgs.v8.i1.5. doi: 10.4240/wjgs.v8.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calin ML, Sadiq A, Arevalo G, Fuentes R, Flanders VL, Gupta N, et al. The first case report of robotic multivisceral resection for synchronous liver metastasis from pancreatic neuroendocrine tumor: A case report and literature review. J Laparoendosc Adv Surg Tech A. 2016;26:816–24. doi: 10.1089/lap.2016.0342. doi: 10.1089/lap.2016.0342. [DOI] [PubMed] [Google Scholar]