Abstract
Research in the Visual Development Unit on “dorsal stream vulnerability' (DSV) arose from research in two somewhat different areas. In the first, using cortical milestones for local and global processing from our neurobiological model, we identified cerebral visual impairment in infants in the first year of life. In the second, using photo/videorefraction in population refractive screening programs, we showed that infant spectacle wear could reduce the incidence of strabismus and amblyopia, but many preschool children, who had been significantly hyperopic earlier, showed visuo-motor and attentional deficits. This led us to compare developing dorsal and ventral streams, using sensitivity to global motion and form as signatures, finding deficits in motion sensitivity relative to form in children with Williams syndrome, or perinatal brain injury in hemiplegia or preterm birth. Later research showed that this “DSV” was common across many disorders, both genetic and acquired, from autism to amblyopia. Here, we extend DSV to be a cluster of problems, common to many disorders, including poor motion sensitivity, visuo-motor spatial integration for planning actions, attention, and number skills. In current research, we find that individual differences in motion coherence sensitivity in typically developing children are correlated with MRI measures of area variations in parietal lobe, fractional anisotropy (from TBSS) of the superior longitudinal fasciculus, and performance on tasks of mathematics and visuo-motor integration. These findings suggest that individual differences in motion sensitivity reflect decision making and attentional control rather than integration in MT/V5 or V3A. Its neural underpinnings may be related to Duncan's “multiple-demand” (MD) system.
Keywords: infant vision, visual brain development, dorsal stream, motion coherence, attention
Introduction
Emerging cortical function and infant cortical impairment
The work discussed at the beginning of this review arose out of the first twenty years of my research with Oliver Braddick and our team in the Visual Development Unit in Cambridge, particularly John Wattam-Bell and Shirley Anker (Atkinson, 2000). We began by devising new methods, both behavioral (automated forced-choice preferential looking) and electrophysiological (steady-state VEP/VERP—Visual Evoked Potential/Visual Event Related Potential) to measure the normal visual capacities of infants such as acuity and contrast sensitivity, over the first years of life (Atkinson, Braddick, & Braddick, 1974; Harris, Atkinson, & Braddick, 1976; Atkinson, Braddick, & Moar, 1977a, 1977b, 1977c; Atkinson, Braddick, & French, 1979; Atkinson, French, & Braddick, 1981). We then looked at the timescale of development of basic visual cortical functions: binocularity (e.g., Braddick et al., 1980), orientation (e.g., Braddick, Wattam-Bell & Atkinson, 1986) and direction selectivity (e.g., Wattam-Bell, 1991, 1992), symmetry of monocular optokinetic nystagmus (Atkinson, 1979; Atkinson & Braddick, 1981) and control of visual attention (Atkinson, Hood, Wattam-Bell, Anker, & Braddick, 1988; Atkinson, Hood, Wattam-Bell, & Braddick, 1992). This research led to a neurobiological model (of which an updated version appears as Figure 1) of the course of visual cortical brain development, in which an initially subcortical system came under the control of progressively emerging cortical functions (Atkinson, 1984, 2000). The milestones of this sequence provided the basis for identifying cortical visual impairment in infants with early brain injury (e.g., Hood & Atkinson, 1990; Atkinson & van Hof-van Duin, 1993). This was a starting point for extended collaborations with pediatric neurologists including extensive studies of the visuocognitive consequences of perinatal brain injury in infants identified with hypoxic ischemic encephalopathy (HIE) and in infants with very preterm birth (Mercuri et al., 1996; Mercuri, Atkinson, Braddick, Anker, Cowan, et al., 1997; Mercuri, Atkinson, Braddick, Anker, Nokes, et al., 1997; Mercuri et al., 1999; Atkinson, Anker, Rae, & Weeks, 2002; Atkinson & Braddick, 2007).
Figure 1.

Model of the development of visual brain systems, and the behavior they control, on the timeline (left) from birth to one year of age. The early connections through the pulvinar, shown in orange, have been suggested (e.g., Johnson, 2005; Warner, Kwan, & Bourne, 2012), although such links have not been demonstrated in human development. These links, colored in orange, are additions to earlier published versions of this figure (Atkinson, 2000; Atkinson & Braddick, 2012b).
Infant refractive screening
A second strand of clinical application was in pediatric ophthalmology and optometry. It came out of our work, in collaboration with the ophthalmologists in Cambridge, initially identifying amblyopia in children with a range of paediatric visual disorders such as congenital cataract and strabismus (e.g., Atkinson, Braddick, & Pimm-Smith, 1982; Atkinson & Braddick, 1982, 1986; Atkinson et al., 1988) and analyzing early binocularity (Wattam-Bell, Braddick, Atkinson, & Day, 1987; Smith, Atkinson, Anker, & Moore, 1991). We devised with Howard Howland isotropic photorefraction to study infants' development of accommodation and refraction, including early astigmatism and its reduction (Howland, Atkinson, Braddick, & French, 1978; Braddick, Atkinson, French, & Howland, 1979; Atkinson & French, 1979; Atkinson, Braddick, & French, 1980). Using photo- and video-refraction we devised and led large-scale population screening programs to identify strabismus and refractive errors in typically developing infants at 9 months of age, screening over 8000 infants (Atkinson, Braddick, Durden, Watson, & Atkinson, 1984; Atkinson, 1993). Our controlled trial of spectacle correction of hyperopic infants (including hyperopic anisometropia and/or astigmatism) showed that refractive correction could reduce the subsequent incidence of strabismus (21% to 6%) and amblyopia (68% to 29%) (Atkinson et al., 1996; Atkinson, Braddick, Nardini, & Anker, 2007; Anker, Atkinson, Braddick, Nardini, & Ehrlich, 2004). However, in the extensive follow-up in these programs, we found that many of these children (including those who had worn glasses in infancy) showed subtle deficits in visuo-motor control and in measures of early attention (Atkinson, Anker, Nardini et al., 2002; Atkinson et al., 2005; Atkinson et al., 2007).
Thus these two strands of work converged in findings about the vulnerability of the developing visual brain in different disorders—neurological in one case and ophthalmological in the other. Through this route, and from studies of Williams syndrome discussed below, we arrived at the idea that this vulnerability in developmental disorders was dominated by the dorsal cortical stream—the topic explored in the rest of this review.
Infant motion sensitivity
Processing motion is one of the most fundamental ways in which the sense of vision informs us about our environment and our own actions, and one of the key functions of the visual cortex. Human infants, while they have only very limited, mostly reflexive, responses to visual motion at birth (Kremenitzer, Vaughan, Kurtzberg, Dowling, 1979; Atkinson & Braddick, 1981; Mason, Braddick, & Wattam-Bell, 2003), can be shown to discriminate directional motion from around 6–8 weeks of age, using visual evoked potential and behavioral measures (Wattam-Bell, 1991, 1992, 1994, 1996a, 1996b, 1996c; Braddick, 1993), and also show activation of a network of brain areas specifically by motion stimuli (Biagi, Crespi, Tosetti, & Morrone, 2015). Only a few weeks later, by around 3–4 months of age, they can exploit this ability in a wide variety of complex perceptual discriminations. For example, Arterberry and Yonas (1988, 2000) showed discrimination of 3-D structure from motion (e.g., the presence of an interior corner on a cube, represented by random dot kinematograms). Kellman and Spelke (1983) and Johnson and Aslin (1996) showed that infants link the parts of a partially occluded object by their common motion, and Johnson et al. (2003) showed that they can predict the trajectory of a moving object that passes behind an occluder. Infants can discriminate the simulated direction of heading in optic flow displays (Gilmore, Baker, & Grobman, 2004) and have also shown sensitivity to the patterns of point-light motion that characterize biological motions (Fox & McDaniel, 1982; Bertenthal, Proffitt, & Cutting, 1984; Bertenthal, Proffitt, Spetner, & Thomas, 1985; Booth, Pinto, & Bertenthal, 2002). This ability to make these complex inferences requires something beyond detecting the simple, local direction of motion; it requires global motion processing of directional signals, with integration over time and space of these signals, to allow analysis of the global structure of the pattern of motions.
Dorsal and ventral streams in infancy
Responses to directional motion within a local receptive field occur in area V1 (Hubel & Wiesel, 1968), and in both macaques and humans these are integrated to provide responses to global motion in area V5/MT (Zeki, 1974; Movshon, Adelson, Gizzi, & Newsome, 1986; Mikami, Newsome, & Wurtz, 1986; Maunsell & Newsome, 1987; Newsome & Paré, 1988; Britten, Shadlen, Newsome, & Movshon, 1992; Watson et al, 1993; Tootell, Dale, Sereno, & Malach, 1996; Morrone et al., 2000). Adult functional magnetic resonance imaging (fMRI) studies show that the network of areas processing global motion goes beyond V5 to include other extrastriate areas, including V3A and V6 (Sunaert, Van Hecke, Marchal, & Orban, 1999; Braddick et al., 2000; Braddick et al., 2001; Pitzalis et al., 2010; Helfrich, Becker, & Haarmeier, 2013). In our adult fMRI study, contrasting globally coherent with incoherent patterns for both form and motion, we identified independent separate networks for motion and form, each running from occipital extrastriate areas to the intraparietal sulcus and to ventral areas (Braddick, O'Brien, Wattam-Bell, Atkinson, & Turner, 2000). The motion areas V5/MT, V3A, V6, and intraparietal sulcus (IPS) have been identified as part of the dorsal cortical stream (Felleman & Van Essen, 1991), projecting to parietal cortex and serving to use visual information for understanding movement and spatial layout, and translating these into the control of action (Mishkin, Ungerleider, & Macko, 1983; Milner & Goodale, 1995). Global form information, in contrast, is required for object recognition subserved by the ventral stream. Areas that integrate local contours for global shape processing are less well understood than their counterparts for motion, but include V4, an extrastriate area occupying a similar hierarchical position in the ventral stream to that of V5/MT in the dorsal stream. V4 responds to global configurations such as concentrically arranged contours (Gallant, Braun, & Van Essen, 1993; Gallant, Connor, Rakshit, Lewis, & Van Essen, 1996). Measures of sensitivity to global form and global motion can therefore serve as comparable indicators of extrastriate ventral and dorsal stream function, respectively.
To measure the early development of dorsal and ventral streams in infants, we designed a novel steady-state VERP paradigm which compared responses to global motion with those to concentrically organized global form. Stimulus sequences alternated between random and coherent global structure of either form or motion, in different blocks of trials (Braddick & Atkinson, 2007; Wattam-Bell et al., 2010). We demonstrated that infants show a neural response to coherent motion by around 3 months of age, with sensitivity to global motion developing earlier than sensitivity to static form. We concluded that the development of the extrastriate dorsal stream at this early stage preceded that of the ventral stream (Braddick & Atkinson, 2007). Later, using a montage with a high density EEG (electroencephalographic) sensor array (“net”), we confirmed that global sensitivity for motion was more advanced at 4–5 months of age than for global static form (Wattam-Bell et al., 2010). Analogous results have been found for infant monkeys' behavioral sensitivity to global form and motion (Kiorpes, Price, Hall-Haro, & Movshon, 2012). Our EEG data showed distinct topographic patterns of activation for form and motion stimuli, confirming that distinct neural systems were activated both in adults and in infants. However, these patterns were quite different in infants from those in adults, implying a major reorganization of the networks processing global motion and form sensitivity between infancy and adulthood (Wattam-Bell et al., 2010; Wattam-Bell, Chiu, & Kulke, 2012).
A further finding was that in comparing term-born infants with infants born before 33 weeks gestation, the preterm-born infants showed similar activation to term-born for the simple VEP (Visual Evoked Potential) pattern-reversal stimulus and for global static form. However, there was a striking difference in global motion responses. The term-born infants and preterm-born infants, with very mild or normal neonatal MRI findings, showed a greater activation in this VERP response to global motion than that seen for the group of preterm infants with brain injury categorized as “severe” from neonatal MRI (Braddick, Atkinson, & Wattam-Bell, 2011). In addition, the preterm infants categorized as having “mild” or “moderate” perinatal brain injury appeared to show a more immature configuration, compared to term-born infants, in terms of lateralization of the activation, which changes from infancy to adulthood (Wattam-Bell et al., 2010). This implies that in preterm infants, even those without major brain injury, the development of the dorsal stream underpinning motion coherence sensitivity is already delayed compared to term-born infants (Birtles, Braddick, Wattam-Bell, Wilkinson, & Atkinson, 2007; Braddick et al., 2011).
Development of global form and motion sensitivity in childhood
To compare global thresholds for form and motion sensitivity in preschool and school age children, we first devised two new behavioral tasks, especially suitable for children as young as 4 years of age, but appropriate up to adulthood. These were the “Road in the Snowstorm” for measuring transverse motion coherence thresholds and “the Ball in the Grass” for detecting a circular array of static arc line segments. These ventral and dorsal stream tasks, described in detail in Atkinson et al. (1997) and Gunn et al. (2002), both used adaptive threshold procedures to assess relative sensitivity, and were very similar in their cognitive demands. To make the geometry of the two displays as directly comparable as possible, we went on to design a dynamic (motion) version of the “Ball in the Grass” test (Atkinson & Braddick, 2005), a display of limited-lifetime random dots moving in concentric circular arcs in one region of the field, with coherence manipulated by varying the proportion of randomly oriented arcs (Figure 2). For both form and motion displays, the circular “ball” was presented either right or left of center of the laptop screen, and the child indicated on which side it appeared on each trial. Using these comparable tasks, we found that adults generally showed similar coherence thresholds for both form and motion (around 10%–15% coherence) and that, in a number of studies, although coherence sensitivity could be demonstrated in early infancy for patterns with 100% coherence, improvements in sensitivity to global coherence extended over many years of childhood (Gunn et al., 2002; Atkinson & Braddick, 2005; Braddick, Atkinson, Newman et al., 2016). Adult levels of form coherence sensitivity were reached for most typically developing children around 7–10 years, but global motion sensitivity showed a slower developmental course, being considerably poorer in 4–5 year olds and not reaching adult levels till around 8–12 years of age. However, the exact age at which adult global sensitivity is found depends critically on the comparability of the stimuli used to compare form with motion, and the spatial and temporal parameters of the stimuli used in particular studies (Meier & Giaschi, 2014; Hadad, Schwartz, Maurer, & Lewis, 2015).
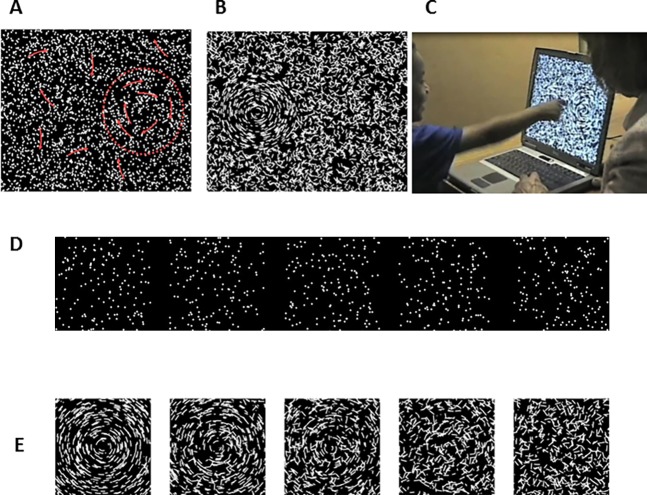
Figure 2.
The “Ball in the Grass” test for measuring children's thresholds for global coherence of motion and form. (A) motion test pattern, with the target on the right. Trajectories of dots are indicated by red arrows. The boundary of the “ball,” shown by red dashed line, was not present on the screen. (B) form test pattern, with target (100% coherence) on the left. (C) child performing the form coherence test on a laptop screen. (D) movie showing a graded series of global motion patterns, with coherence values (from left) of 100%, 75%, 50%, 25%, and 0%. (E) graded series of global form patterns, with the same coherence values as in (D).
Global motion and global form in Williams syndrome
We used these coherence measures of dorsal versus ventral stream function to study children with developmental disorders. This work began with young children with Williams syndrome (WS). Children with this developmental disorder, related to a specific deletion on chromosome 7, were known to show a very characteristic cognitive profile, with relatively strong expressive language abilities, although there are some differences in WS to normal language development combined with unusual semantic knowledge and “hyper-social” behavior. Within the visual domain, children with Williams syndrome showed relatively good ability to recognize faces (although not necessarily using the same strategies as typically developing children (e.g., Deruelle, Mancini, Livet, Casse-Perrot, & De Schonen, 1999; D'Souza et al., 2015), but marked spatial difficulties (e.g., Bellugi, Lichtenberger, Mills, Galaburda, & Korenberg, 1999; Atkinson et al., 2001). We studied a large group of WS children, between 30 and 100, aged 3 to 15 years, with the number in each task depending on the feasibility of the test for the mental age of the individual child. We found that, while over 50% had basic visual problems, including refractive errors (usually hyperopic), reduced acuity, stereo deficiency, and strabismus, these sensory problems did not account for their poor performance on relatively simple spatial tasks (Atkinson et al., 2001).
We also tested sensitivity to form and motion coherence, using the “Ball in the Grass” and “Road in the Snowstorm” tests (Atkinson et al., 1997). We found that WS children showed persistent deficits in global motion coherence sensitivity, relative to static form coherence, compared to typically developing children. We suggested that this deficit was related to poor dorsal stream development, relative to the ventral stream (Atkinson et al., 1997), a view which was supported by the same children's poor performance in posting a card through an angled slot (discussed below). The deficit in global motion sensitivity was not just a developmental delay, but persisted in adults with WS (Atkinson et al, 2006). The idea of a dorsal stream anomaly was supported by results from our structural MRI study of two very young WS children (∼2.5 years of age), who showed anomalous fiber tract development within the centrum semiovale, together with abnormal cerebellar structure (Mercuri, Atkinson, Braddick, Rutherford et al., 1997). Later MRI neuroimaging work with WS adults also showed dorsal stream abnormalities (e.g., Meyer-Lindenberg et al., 2004).
Dorsal-stream vulnerability: A widespread feature of developmental disorders
However, it soon became apparent that a deficit in global motion, compared to global form sensitivity, was not a special characteristic seen only in children with Williams syndrome. Work from our group and others found that this pattern, of elevated thresholds for motion compared to form, characterized children's performance in many different forms of developmental disorder and disruption, including hemiplegia (Gunn et al., 2002), fragile-X syndrome (Kogan et al., 2004), developmental dyslexia (e.g., Cornelissen, Richardson, Mason, Fowler, & Stein, 1995; Hansen, Stein, Orde, Winter, & Talcott, 2001; Ridder, Borsting, & Banton, 2001), children with very preterm birth (e.g., Atkinson & Braddick, 2007; Taylor, Jakobson, Maurer, & Lewis, 2009), and young children with developmental coordination disorder (Corbett, Atkinson, & Braddick, 2016). Motion coherence deficits in autism spectrum disorder (ASD) have been widely reported, (e.g., Spencer et al., 2000; Koldewyn, Whitney, & Rivera, 2010; Robertson et al., 2014), with the relationship between local and global motion processing in ASD and the role played by related cognitive biases being widely debated (see Dakin & Frith, 2005; Manning, Tibber, Charman, Dakin, & Pellicano, 2015). Early visual deprivation due to congenital cataract reduces global motion sensitivity by a factor of 4.9 compared to only 1.6 for global form (comparing Ellemberg, Lewis, Maurer, Brar, & Brent, 2002, with the same patients in Lewis et al., 2002). Adult strabismic amblyopes also show a greater reduction in global motion sensitivity than for static form (e.g., Simmers, Ledgeway, Hess, & McGraw, 2003; Ho et al., 2005; Simmers, Ledgeway, & Hess, 2005; see also the review by Hamm, Black, Dai, & Thompson, 2014). Several results suggest that both local motion processing and global integration are impaired in both amblyopic and fellow eyes in amblyopia (Aaen-Stockdale & Hess, 2008; Hou, Pettet, & Norcia, 2008; Knox, Ledgeway, & Simmers, 2013; Levi, 2013).
Thus, there appears to be broad “dorsal stream vulnerability” (Braddick, Atkinson & Wattam-Bell, 2003; Atkinson & Braddick, 2011; Braddick & Atkinson, 2011) in both genetic and acquired developmental disorders. As this motion coherence deficit appears to be a common feature across a wide variety of pediatric disorders with very different aetiologies and neurodevelopmental profiles, we initially suggested that it represented an early vulnerability in neural systems processing motion information of a very basic nature. In the case of dyslexia, this has been described as the “magnocellular hypothesis” (Stein, Talcott, & Walsh, 2000), although the relationship between the magnocellular system, as defined at the geniculate level, and the motion processing areas of the cortex, is not straightforward.
Broader dorsal stream functions
The dorsal stream is an extensive network in the brain. In considering the pathways by which the dorsal stream provides input for the visual control of actions, we realized that many cortical dorsal stream areas overlapped with those which have been shown in adults and nonhuman primates to be involved in the control of attention. Figure 3 (see Atkinson, 2000; Atkinson & Braddick, 2011) shows a schematic of the dorsal stream pathways which feed specific visuomotor modules for the control of manual and oculomotor actions, and highlights those areas within these pathways which have been shown to have functions related to attention (although we have not included all the networks for attentional control in adults).
Figure 3.

Schematic summary of dorsal stream connections of visuo-motor modules for control of four behaviors—arm movements for reaching, hand movements for grasping, saccadic eye movements, and smooth pursuit eye movements. Brain areas in white or green are dorsal-stream, V4, TEO, STS, and IT are ventral-stream, and black are subcortical areas. Areas which have been shown to be involved in spatial direction of attention are highlighted in green. Networks shown are based on primate studies and human neuropsychology data reviewed by Jeannerod (1988), Milner and Goodale (1995), and Rizzolatti, Fogassi and Gallese (1997). Redrawn from Atkinson & Braddick (2011). Key to abbreviations for brain areas: V1-5 = visual areas 1–5; PO = parietal-occipital; MDP = medial dorsal parietal; MIP = medial intraparietal; AIP = anterior intraparietal; VIP = ventral intraparietal; LIP = lateral intraparietal; BA6 = Brodmann area 6 (F4, F5, etc., are fields within BA6); FEF = frontal eye fields; NOT = nucleus of the optic tract; sup coll = superior colliculus; TEO = a posterior region of inferotemporal cortex; STS = superior temporal sulcus; IT = inferotemporal.
In 2011, Kravitz, Saleem, Baker, & Mishkin, drawing evidence from studies on nonhuman primates, human neuropsychology, and adult neuroimaging, reviewed the multiple branches and target areas for the dorsal stream, and showed that their functions go beyond visuo-motor control. Their evidence shows that the dorsal stream has three distinct target branches (Kravitz et al., 2011) schematized in our diagram of Figure 4. These branches of the extended dorsal stream are (a) one connecting through parietal areas to premotor cortex, including the visuomotor modules for the guidance of action, as exemplified for reaching and grasping in Figure 3; (b) the second branch connecting to the frontal eye fields, including the saccadic and pursuit systems, with reciprocal connections to prefrontal areas, a branch which also underpins spatial memory and attention; and (c) the third pathway running from parietal areas via cingulate cortex to the medial temporal lobe and hippocampus, which is involved in delivering spatial information, and integrating it with information from the ventral stream for navigation and topographic cognition.
Figure 4.
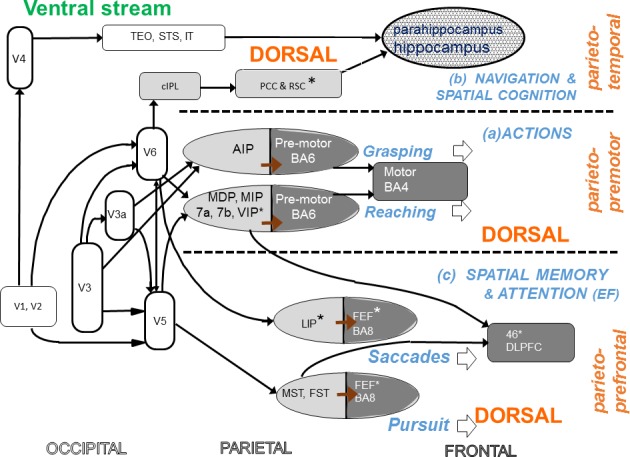
The scheme proposed by Kravitz et al. (2011) for three networks of the dorsal stream with distinct functional roles, shown as three horizontal bands separated by dashed horizontals. The scheme has been drawn to allow direct comparison with the visuo-motor modules suggested in Figure 3. In Kravitz's scheme, the modules for manual actions come within the “parieto-premotor circuit” (a, middle strip of the figure) subserving action control, and those for oculomotor actions within the “parieto-prefrontal circuit” (b, lower strip) involved in spatial memory and attentional control. The third “parieto-medial temporal” pathway (c) involves interaction with the ventral stream in the parahippocampal cortex and hippocampus and is proposed to be involved in spatial navigation. However this diagram is not intended as a comprehensive chart of the connections of the areas shown—for example it omits connections with the superior temporal sulcus where motion information interacts with the ventral stream in biological motion—a function not discussed in this review. Abbreviations as for Figure 3. Additional abbreviation: EF = executive functions.
Visuomotor control and spatial cognition in Williams syndrome
Children with Williams syndrome are commonly described as clumsy and as having specific difficulties with some areas of motor behaviour such as stepping down kerbs or stairs (Chapman, du Plessis, & Pober, 1996; Withers, 1996; Atkinson et al., 2001; Hocking, McGinley, Moss, Bradshaw, & Rinehart, 2010; Hocking, Rinehart, McGinley, Moss, & Bradshaw, 2011; Cowie, Braddick, & Atkinson, 2012). Given the key role of dorsal stream circuits in visuo-motor control, these problems fit the account of WS as involving a specific dorsal stream deficit. Our assessments of WS children included the ABCDEFV battery (Atkinson Battery of Child Development for Examining Functional Vision), a set of functional visual subtests of sensory, perceptual, and cognitive vision which we had normalized for typically developing children from birth to 5 years of age (Atkinson, Anker, Rae, Hughes, & Braddick, 2002). The battery includes block construction copying and shape-fitting subtests, suitable for typically developing children between 2 and 5 years of age. Fluent performance in these tasks requires coordinated sequences of actions, using visual information for both action selection (e.g., the next block to be positioned in a block construction) and on-line control (e.g., positioning and rotating a shape to fit into its slot). In our study of a large cohort of children with WS (50–100 WS children, depending on the mental age appropriateness of specific tests) we found that all the children showed deficits on these early spatial subtasks of the ABCDEFV, but only around 50% of the same WS children showed poor acuity, strabismus, and/or marked refractive errors. We also found that the extent of individual deficit on the spatial tasks did not correlate well with whether that child had acuity deficits, refractive errors and poor accommodation, or strabismus (Atkinson et al., 2001). This suggests that these spatial problems are more consistent with deficits in higher levels of the dorsal stream networks rather than lower level deficits of vision, and that there is no simple causal link between early visual problems, such as strabismus, and more complex spatial problems at a later age.
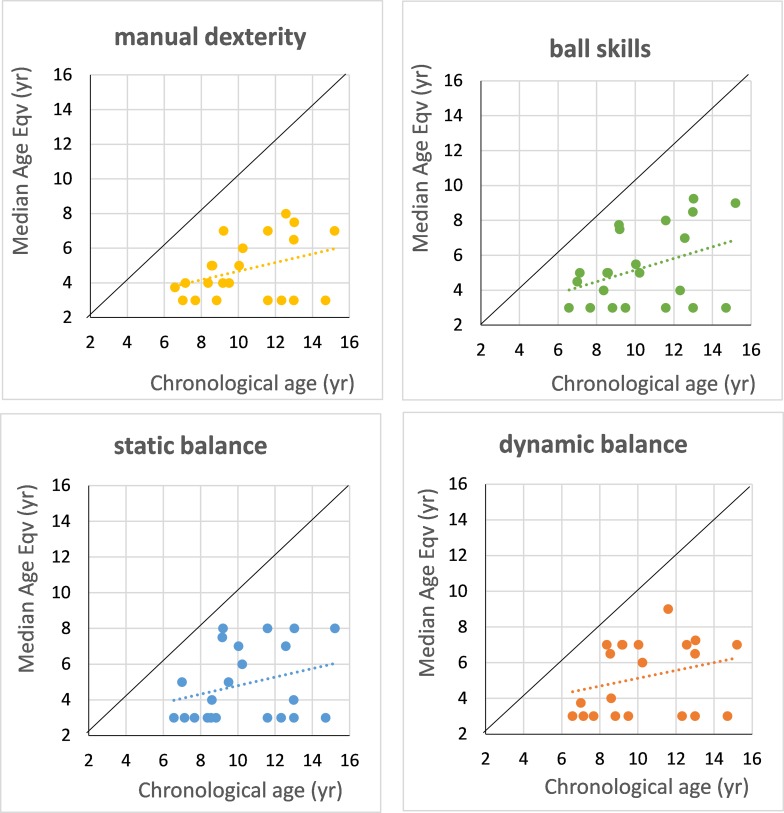
In older children with WS, we found wide-ranging visuomotor deficits. Figure 5 illustrates results from a study of a group of WS children aged 6–15 years using the standardized Movement Assessment Battery for Children (Movement ABC—Henderson & Sugden, 1992). In all four components of this battery—manual dexterity, ball skills, static balance, and dynamic balance—the development of all the WS children fell well below their age norms and in many cases did not attain the minimal 4-year-old equivalent for the tests, even when entering adolescence.
Figure 5.
Performance (norm age-equivalents) of a group of 24 Williams syndrome children and adolescents (chronological ages 6.5–15 years), shown as median scores across tests within the four subscales of the Movement ABC battery (Henderson & Sugden, 1992). Points plotted at 3 years on the age-equivalence scale are children who were unable to perform at the minimum (4-year-old equivalent) level for the test. The diagonal line indicates age-equivalence = chronological age.
For a direct comparison of dorsal and ventral stream function using the same visual information, we adapted the “mailbox task” of Perenin and Vighetto (1988) and Milner and Goodale (1995) as a child-friendly test. For assessing the dorsal stream control of action, the angle of a card is measured as the child moves it towards the slot of the “mailbox,” whose orientation is varied from trial to trial. In the ventral-stream version, the child rotates the card in a fixed location to match the slot orientation. The WS children showed much greater impairment compared to typical controls in performing a fluent posting action than they did in the matching task, supporting the idea of specific dorsal stream impairment (Atkinson et al., 1997).
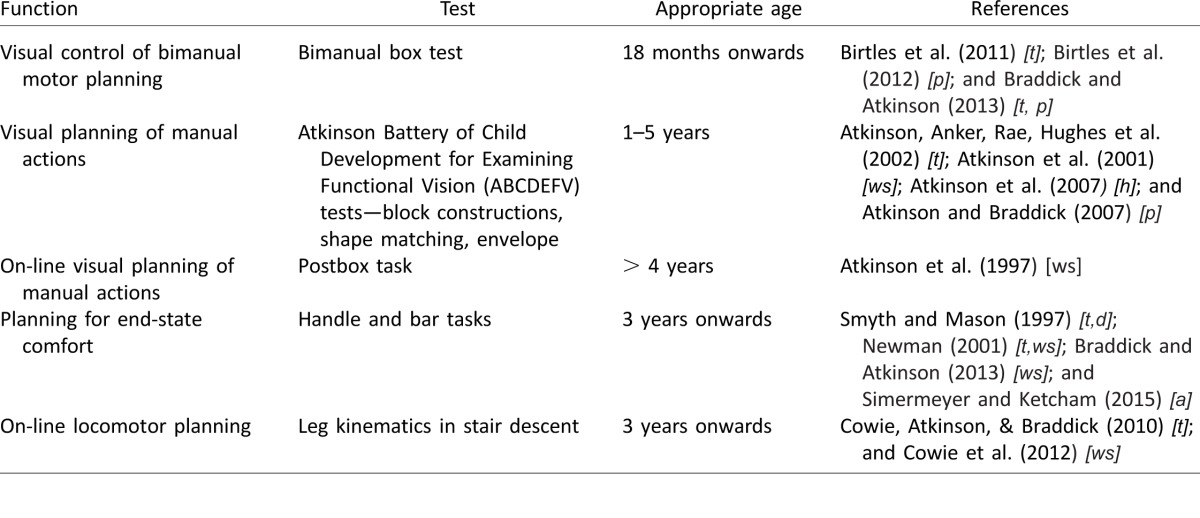
Visuo-motor control involves using visual information both for the on-line control of the motion trajectory, and in selecting an appropriate motor program to plan an action, or a sequence of actions, in advance. Both aspects are impaired in Williams syndrome. Table 1 lists a number of measures of motor planning that we have used with young children, both typically developing and with developmental disorders. WS children showed deficits in the “handle task”, which requires them to select a grip before rotating a handle, where the selected grip will lead to either a comfortable or an uncomfortable end-state posture of the wrist (Smyth & Mason, 1997; Newman, 2001; Braddick & Atkinson, 2013).
Table 1.
Tests of motor planning used in studies of developmental disorders. Notes: Reference codes for developmental groups: [t] = typically developing; [p] = preterm born; [f] = full-term-born with brain injury; [ws] = Williams syndrome; [a] = autism; [d] = developmental coordination disorder; [h] = children following infant hyperopia.
Locomotion is a further domain where visual information is required to prepare safe and accurate motor responses. Cowie, Braddick, & Atkinson (2008) and Cowie et al. (2010) showed that the action sequence of the leg while descending stairs is determined by advance visual information about the step height, in children as young as 3 years old, but that this linkage is seriously disrupted in WS (Cowie et al., 2012).
Spatial cognition and navigation are the domain of Kravitz et al.'s (2011) third branch of the dorsal stream. Nardini, Atkinson, Braddick, & Burgess (2008) showed that WS children showed a poor and anomalous pattern in using different frames of reference to recall the location of a hidden object. Similar anomalies have been found in locating objects in larger-scale space (Mandolesi et al., 2009) and in the spatial reference frames and strategies used by WS children for navigation (e.g., Broadbent, Farran, & Tolmie, 2014; Farran, Formby, Daniyal, Holmes, & Van Herwegen, 2016).
Visuomotor and spatial deficits in other developmental disorders
Deficits and delays in visuomotor development and action planning are seen not only in children with WS; Table 1 includes a range of tasks we have used to study a number of neurodevelopmental disorders. We found deficits based on the visuo-motor and spatial tasks from the ABCDEFV not only in children with WS, but also in typically developing children who had had significant hyperopic refractive errors in infancy (some with continuing amblyopia) (Atkinson, Anker, Nardini et al., 2002; Atkinson, Braddick, Nardini, & Anker, 2007), and in children who had been born very prematurely (Atkinson & Braddick, 2007).
Action planning of a specific kind is needed in tasks which require a coordinated sequence of operations by the two hands. Birtles et al. (2011) describe a test requiring young children to use one hand to lift and hold the lid of a transparent box, so that the other hand can pick up a toy seen inside. Smooth coordination is typically achieved at around 18–24 months, although the timing and synchrony of the two hands continues to be refined through later childhood. In preterm-born children, the initial development is sensitive to the degree of brain injury; children with white matter problems show an immature timing pattern even if they achieve successful use of the two hands (Birtles et al., 2011, 2012; Braddick & Atkinson, 2013).
Looking more broadly at the pattern of development in children born very prematurely, we see a general pattern of impairment in a number of functions served by the dorsal stream. Figure 6 shows a sample of 67 children born < 33 weeks gestation and tested at 6–7 years (Atkinson & Braddick, 2007). The tests in which a significant proportion of children performed worse than the 5th centile of typically developing children are indicated by an asterisk (*). As well as motion coherence and visuomotor skills (Movement ABC), many preterm children performed poorly on a spatial memory task requiring different frames of reference (Nardini et al., 2006), and on block copying, and attention tests (whose relation to the dorsal stream is discussed below). Studies from other research groups of preterm-born and very low birthweight children have found similar visuomotor deficits (e.g., de Kieviet, Piek, Aarnoudse-Moens, & Oosterlaan, 2009; O'Connor, Birch, & Spencer, 2009; Bos, Van Braeckel, Hitzert, Tanis, & Roze, 2013) and visuospatial problems (Geldof, van Wassenaer, de Kieviet, Kok, & Oosterlaan, 2012). Both Autistic Spectrum Disorder—ASD (e.g., Fournier, Hass, Naik, Lodha, & Cauraugh, 2010; Whyatt & Craig, 2012; Liu, 2013; Simermeyer & Ketcham, 2015; Paquet, Olliac, Bouvard, Golse, & Vaivre-Douret, 2016; Purpura, Fulceri, Puglisi, Masoni, & Contaldo, 2016) and dyslexia (e.g., Haslum & Miles, 2007) have been found to be associated with poor visuo-motor control. However, in dyslexia this has often been manifested in poor balance control which has been considered as reflecting a cerebellar problem (but see Stoodley & Stein, 2013 for discussion of this). In addition, abnormalities in hand-eye coordination in reaching and grasping for objects has been found in children with amblyopia and deficits in binocularity (e.g., Grant, Suttle, Melmoth, Conway, & Sloper, 2014), with these deficits being reduced in children following binocularity training.
Figure 6.
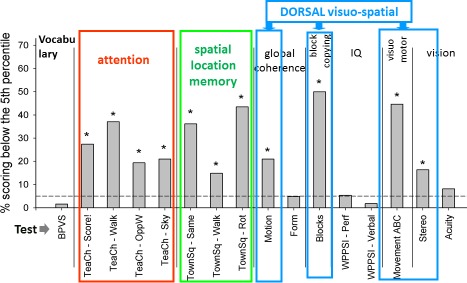
Performance of a group of 67 children born before 33 weeks gestational age on a range of cognitive and visuomotor tasks at 6–7 years. Tests related to dorsal stream function are outlined in color. Attention = four subtests of the TEA-Ch (Manly et al., 2001). Spatial location memory = the “Town Square” test of Nardini, Burgess, Breckenridge, and Atkinson (2006) and Nardini et al. (2008). Motion = global motion “Ball in the Grass” test. Block copying from the ABCDEFV battery (Atkinson et al., 2002a). Movement ABC refers to battery of Henderson & Sugden (1992). Stereo = TNO test (Cooper, Feldman, & Medlin, 1979). The horizontal dashed line indicates the 5th percentile of age norms for these tests; * indicates tests where the proportion of the preterm group performing at or below this level was significantly greater than 5% (p < 0.05; redrawn from Atkinson & Braddick, 2007).
Thus the pattern of deficits in visuomotor and visuospatial cognition is widely distributed in developmental disorders, consistent with the concept of a broad “dorsal stream vulnerability.” A further aspect of dorsal stream function is in the control of attention, which is discussed in the next section.
Attention in developmental disorders
Attention can be broadly defined as the ability to deploy the resources of the brain, so as to optimize performance towards behavioral goals. We have summarized much of our research on the development of attention in a recent review (Atkinson & Braddick, 2012a). This research began with the Fixation Shift test (FS). In this test the infant's ability to initiate a saccade from a centrally fixated target to a newly appearing peripheral target is measured using an adapted forced choice preferential looking (FPL) method, where the observer is unaware (“blind”) as to whether the peripheral target appears on the left or right of the initial centrally fixated target, and has to guess the side of the appearing peripheral target from the infant's head and eye movements. There are two conditions: In the “noncompetition” condition the central target disappears at the same time as the peripheral target appears, whereas in the “competition” condition the central target remains visible when the peripheral target appears (Atkinson, Hood, Wattam-Bell, & Braddick, 1988, 1992; Hood & Atkinson, 1990, 1993; Kulke, Atkinson, & Braddick, 2015). We found that typically developing infants were very slow to make shifts under competition in the first 2–3 months of life and sometimes did not shift their attention at all, showing “sticky fixation.” In addition, we studied two very young infants who had undergone hemispherectomy surgery to relieve intractable epilepsy generated in one hemisphere. Following surgery, we found that these infants did not make significant shifts of attention under competition to peripheral targets in their “bad” half-field (i.e., the side opposite to the impaired and removed hemisphere), but they could make shifts to both sides under noncompetition (Braddick et al., 1992). These results supported the idea that shifts under competition required development of cortical mechanisms which controlled disengagement from the currently fixated target.
The difference between competition and noncompetition has been supported in our current studies. These have measured the latency of the fixation shift with an eye tracker, combined with a multielectrode geodesic net to carry out simultaneous EEG recording. This allows for an accurate measure of the latency of the saccadic shift of attention, and a simultaneous EEG recording from areas of cortex activated in making the attention shift before the eye movement itself (Kulke, Atkinson, & Braddick, 2017).
The Fixation Shift (FS) test therefore provides a probe for atypical development of the cortical attention mechanisms. We found delays and deficits in the ability to shift fixation, especially in the competition condition (Atkinson & Braddick, 2012a) in many term-born infants with perinatal brain injury (focal lesions or hypoxic-ischemic encephalopathy; Hood & Atkinson, 1990; Mercuri et al., 1996; Mercuri, Atkinson, Braddick, Anker, Cowan et al., 1997), nearly all infants born very preterm, below 33 weeks gestational age (even many with normal or mild structural brain anomalies on neonatal MRI; Atkinson & Braddick, 2007; Atkinson et al., 2008), and many young children with WS (age between 1 and 7 years; Atkinson, Braddick, Anker, Curran, & Andrew, 2003). In addition to the effects of cortical damage, there appear to be areas within the basal ganglia which play a significant role in development of these early attention systems (Mercuri, Atkinson, Braddick, Anker, Nokes et al., 1997). The large size of the peripheral targets in these studies (paired high contrast black and white bars over 20° × 5°, phase reversing at 3 Hz) ensured that the problem these infants and young children had in switching attention was not related to any acuity losses they might have had. We have also found that these measures of early attention shifts using the FS test, when used as early surrogate outcome measures, predicted levels of later cognitive development at 2–3 years of age (Mercuri et al., 1999; Atkinson et al., 2008).
Attention is a multidimensional concept. Based on neuropsychological and neuroimaging studies, in normal adults and adult patients, Posner and his colleagues have proposed that this broad ability can be subdivided into neural systems for selective attention, sustained attention, and the executive control of attention (Posner & Petersen, 1990; Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). One key aspect of executive function is the ability to inhibit a prepotent response (Diamond, 2013) which we tested in children using the pointing/counterpointing test, with the same stimulus configuration as the fixation shift test. This can be considered a child-appropriate version of the “antisaccade” test, in which participants are required to make a saccade in the opposite direction to an appearing target (Hallett, 1978), and which requires control by prefrontal areas (Pierrot-Deseilligny, Rivaud, Gaymard, & Agid, 1991). In the pointing/counterpointing task, the child has to first point towards the peripheral target as soon as it appears, and then in counterpointing trials point to the opposite side of the screen to that containing the target. Typically developing children over the age of 4 years can rapidly point and counterpoint, without making any errors. WS children between the ages of 4 years and 15 years could point to the target rapidly when it appeared, but in the counterpointing condition their responses were slowed, and they frequently pointed initially to the target rather than the other side of the screen (Atkinson et al., 2003). We used two further tests of executive function, the “Detour Box” (after Hughes & Russell, 1993) and the “Day-Night verbal opposites test” (devised by Gerstadt, Hong, & Diamond, 1994), finding once again that WS children had great difficulty inhibiting a prepotent spatial response. However, in line with their relatively preserved verbal abilities, they showed better ability to overcome the prepotent verbal response in the Day-Night task (Atkinson et al., 2003). Thus the WS deficit here seems to affect specifically the inhibition of spatial responses, presumably reflecting a frontal executive mechanism modulating dorsal-stream visuo-spatial mechanisms (networks [a] and [b] in Figure 4).
Using the spatial executive function tests, such as our “pointing/counterpointing” test and the “Detour Box” with a group of children born very prematurely, we found similar deficits to those in children with WS (Atkinson & Braddick, 2007). The children in this study had neonatal structural MRI measures which were graded in terms of severity of brain abnormality. There was an association between the grade of severity on MRI and scores on the executive function tests. However, even many of those with normal or minor signs of brain injury on MRI failed the executive function tests (Atkinson & Braddick, 2007). We went on to test 6–7-year-old children born preterm (gestational age under 33 weeks) across a wide range of cognitive domains discussed above, including different components of attention. None of the children entered in the study had a diagnosis of cerebral palsy, and most were in mainstream school. These children, previously graded on neonatal structural MRI in terms of severity of brain injury, were in either the “mild/normal” grade or the “moderate” grade. The components of attention were analysed by comparison with the norms for typically developing children on a standardized battery called the TEA-Ch (Test of Everyday Attention in Children; Manly et al., 2001), based on Posner's three-component model of attention. We found that on the TEA-Ch battery there were a significant number of these preterm-born children who fell below the 5th percentile norms (Figure 6; Atkinson & Braddick, 2007). In contrast, performance IQ and verbal IQ, and vocabulary scores, did not differ from the norms. Tinelli et al. (2015) have also reported an attention deficit among 29 preterm born children (who were without identified brain lesions on ultrasound) in a motion-related task, the multiple-object tracking test.
The TEA-Ch battery is only appropriate for children over 6 years mental age, and many of even the older children in clinical groups are unable to perform at the 6 year-old level on any tests in the TEA-Ch, even those appropriate for the bottom of the age range (Breckenridge, Atkinson, & Braddick, 2012). The Early Childhood Attention Battery (ECAB) devised by Kate Breckenridge in our group uses the principles of the TEA-Ch in a format adapted for preschool children (age 3–6 years) and individuals with developmental delays or abnormalities which put them in this mental age range (Atkinson & Braddick, 2012a; Breckenridge, Braddick & Atkinson, 2013). Work with Williams and Down's syndrome groups showed that there were distinctive attention profiles for these developmental disorders, with sustained attention being an area of relatively greater strength in both groups relative to mental age, and WS children showing a particular marked impairment in visual search and executive control related to spatially directed attention (Breckenridge, Braddick, Anker, Woodhouse, & Atkinson, 2013). Thus, in both disorders attention is a vulnerable aspect of brain function beyond any general intellectual disability, and in WS this vulnerability is most evident in the spatial functions most likely to have strong dorsal-stream involvement.
Recently we used some of the ECAB subtests to test a group of children who had reached 4.5–7 years, from the Oxford longitudinal study of infants who had been identified with perinatal brain injury, making them at “high risk” of cerebral palsy. Preliminary results from the ECAB subtests showed that this group of 37 children showed significant correlations between ECAB scores and motion coherence sensitivity using the Ball in the Grass test (Atkinson et al., 2016). There was also a significant correlation between these children's early measures of attention (fixation shifts at 4–7 months of age) with their scores on the ECAB subtests. Over half of these children had difficulty with the ECAB visual sustained attention subtest and in understanding and completing the spatial-counterpointing subtest and flanker subtests (both these visual subtests measuring aspects of executive function including a spatial component). All these ECAB scores were expressed relative to each child's mental age rather than chronological age, to dissociate these measures of attention from any overall intellectual disability. In our previous study testing a group of older children with WS, whose mental age equivalence spanned the 3–6 years age range, we had found that WS children did relatively well on the test of sustained attention, when compared to their mental age, but had poor ECAB scores on the counterpointing spatial subtest measuring inhibition of prepotent responses, an executive function test. This shows some differences, together with some similarities between the components of attention in which WS children and children with relatively severe perinatal brain damage show marked attentional difficulties.
In many other studies, varying attentional deficits have been found as a particular, long-lasting effect of very preterm birth and/or very low birth weight (VLBW; e.g., van de Weijer-Bergsma, Wijnroks, & Jongmans, 2008; Mulder, Pitchford, Hagger, & Marlow, 2009; Anderson et al., 2011; Lindström, Lindblad, & Hjern, 2011; Hitzert, Van Braeckel, Bos, Hunnius, & Geuze, 2014; Geldof et al., 2016; Johnson et al., 2016; Johnson & Marlow, 2017), although not all of these studies have assessed deficits of attention independently from the general level of intellectual disability found in many of these children. In contrast, there is at least one study (Hunnius, Geuze, Zweens, & Bos, 2008) testing healthy preterm-born infants, showing that the development of the ability to disengage and switch attention is accelerated in development compared to term-born infants. Hunnius et al. suggest that this accelerated development may be a consequence of their extra visual experience following premature birth. Interestingly in an unpublished study (Atkinson et al., 1990) we found a similar shortened latency to make a saccadic shift of attention, in both noncompetition and competition conditions of the Fixation Shift test, in a group of healthy premature infants (with no identified structural perinatal brain damage on ultrasound) tested at 6 posterm weeks of age. This acceleration in prematures' motor control may reflect extra experience in control of head and eye movements under gravity rather than in utero.
Attention disorders have been found across many other childhood developmental disorders, including dyslexia (e.g., Sexton, Gelhorn, Bell, & Classi, 2012; Lukasova, Silva, & Macedo, 2016) and autism (e.g., Craig et al., 2016) although again, many of these studies do not take into account the level of attention performance expected, considering the overall level of intellectual ability in the children concerned.
Overall, it is clear that, from infancy through childhood, quite diverse neurodevelopmental disorders are associated with deficits of attention mechanisms. The overlap between attention-related structures and the dorsal stream (Figure 3), and the association of attention scores with motion coherence sensitivity in our perinatal brain injury group, suggest that these deficits may have a basis in the vulnerability of the dorsal stream. However, it is quite possible that there are major differences between specific disorders, both in the type of attentional deficit and in the severity of consequent attentional problems. This remains a question for future research.
Brain structure associated with global motion performance in typical development
The striking deficits in global motion sensitivity in many developmental disorders encouraged us to look at the variations seen in typically developing children. Particularly between age 4–7 years, when average motion coherence thresholds are improving steeply, there are marked individual differences in children's motion coherence thresholds—more striking than those seen in the more slowly changing form coherence thresholds (Gunn et al., 2002; Atkinson & Braddick, 2005; Braddick, Atkinson, Newman et al., 2016). The Pediatric Longitudinal Imaging, Neurocognition, and Genetics (PLING) study at the University of California, San Diego (Jernigan et al., 2016), which has tracked cognitive performance and brain structure in a cohort of children from 5 years onwards, provided the opportunity to examine these individual differences in a wider context.
At the cognitive level, high sensitivity to motion coherence was associated in these children with good visuo-motor integration as assessed by Beery's Visuo-Motor Integration (VMI) test (copying geometrical figures), as might be predicted for a dorsal-stream-based function (Braddick, Atkinson, Newman, et al., 2016). It was also associated with relatively better numerosity judgments (Panamath test) and mathematical achievement (Woodcock-Johnson tests). The children's form coherence thresholds showed none of these correlations, even though form and motion coherence were well correlated, implying that it is the variance unique to motion processing which is linked to mathematical and visuomotor performance. Furthermore, reading skills showed no correlation with motion thresholds, implying that the relationship to mathematical performance did not simply reflect a broader association with level of scholastic attainment.
There is extensive evidence for a link between numerical cognition and processing in the parietal lobe (e.g., Dehaene, Piazza, Pinel, & Cohen, 2003; Price, Holloway, Räsänen, Vesterinen, & Ansari, 2007; Butterworth, Varma, & Laurillard, 2011; Ranpura et al., 2013), and this may be the key to its relation with motion processing. Local cortical surface area was measured in the PLING cohort, and motion performance was positively related to individual structural differences in the area of the parietal lobe (allowing for effects of age and gender), and negatively to area of the occipital lobe (Braddick, Atkinson, Newman et al., 2016). More detailed exploration of the effect size of these relations across the cortical surface highlighted the region of the intraparietal sulcus (IPS), particularly its inferior bank. A number of occipital areas—V5/MT, V3a—are known to have populations of neurons sensitive to motion coherence (Newsome & Paré, 1988; Britten et al., 1992; Sunaert et al., 1999; Braddick et al., 2001; Orban et al., 2003; Helfrich et al., 2013). While these areas are too small, and perhaps too variable, to be specifically identified in the map of association, the overall negative correlation with the occipital lobe suggests that these areas, which are presumed to carry out the initial integration of coherent motion information, are not the source of children's individual differences in global motion processing. Single unit studies in macaque have shown that neurons in the IPS receive signals from V5/MT and accumulate the evidence that is used in the animals' decisions on coherent motion (Shadlen & Newsome, 2001; Huk & Shadlen, 2005). Our findings, then, support the idea that the bottleneck which determines the individual child's performance may be at this stage of using perceptual evidence for decision-making, rather than the earlier stages where global motion signals are generated. None of the measures correlate with global form coherence sensitivity, so the variations are not in a general decision-making process, but in a process specific to the handling of motion information in the dorsal stream (Braddick, Atkinson, Newman et al., 2016).
A further step in the arguments comes from analyzing fiber tracts in the PLING cohort of children (Braddick, Atkinson, Akshoomoff et al., 2016). Our analysis focused on the superior longitudinal fasciculus (SLF), the major pathway carrying two-way information between the parietal and frontal lobes, and one associated with functions of visuo-spatial attention (e.g., Bennett, Motes, Rao, & Rypma, 2012; Mayer & Vuong, 2014; Chechlacz, Gillebert, Vangkilde, Petersen, & Humphreys, 2015). Fractional anisotropy (FA)—a measure of how far the tract is organized so that water molecules diffuse most easily along the direction of the tract—revealed a statistical relationship with the children's motion coherence sensitivity. This relationship showed a striking asymmetry (Braddick, Atkinson, Akshoomoff et al., 2016). For the right SLF, FA was high in the children with high global motion sensitivity, but on the left, the reverse relationship was found. Again, no statistical associations with global form performance were apparent.
Right hemisphere lateralization is a clear property of the brain network subserving spatial attention, which includes a greater volume for the right SLF (e.g., Thiebaut de Schotten et al., 2011). Although it is not obvious why the motion task should make greater demands on the attention network than the form task, we must consider seriously the role of attention as a factor in individual differences in motion processing. fMRI evidence has indicated that parietal activity is associated with attentional modulation of the transmission of motion information between V1/V2 and V5 (Friston & Büchel, 2000). Attentional effects are presumed to have their origins in the frontal lobes, so a possible picture emerges in which the SLF transmits attentional signals to the IPS, which may both send signals to control motion processing in extrastriate areas, and receive motion signals which are integrated in decision processing.
The functioning of the dorsal stream, and its impairment in neurodevelopmental disorders, may then reflect a close and specific association between motion processing, evaluation of sensory evidence for decisions, and top-down attentional control. The apparently simple task of detecting global motion may provide a specific index of this complex nexus of bottom-up and top-down interaction.
A further speculation arises from the close anatomical relation between the IPS and the targets of the SLF within the “multiple demand network,” proposed by Duncan (2010). From a combination of single neuron recordings in nonhuman primates and fMRI data from human adults, the multiple-demand (MD) system is identified as a pattern of frontal and parietal activation which is associated with diverse cognitive demands, and with performing standard tests of fluid intelligence (Duncan, 2010; Mitchell et al., 2016). Figure 7 illustrates the similarity between the parietal area in Duncan's MD network and the area whose expansion is related to global motion performance in Braddick, Atkinson, Newman et al. (2016); it also shows how the SLF is well placed to communicate between this area and the frontal parts of the MD network.
Figure 7.

(A) Effect size map of the association between children's global motion sensitivity and relative local cortical expansion in the left hemisphere (Braddick et al., 2016). Red and yellow indicate increasing t values for positive associations, especially around the intraparietal sulcus (IPS); blue indicates negative associations, especially in the occipital lobe. (B) The “multiple demand” network, indicated by BOLD measures of activity in tasks with diverse cognitive demands (from figure 1 in Duncan, 2010). (C) The superior longitudinal fasciculus (SLF), traced from figure 4b in Wakana, Jiang, Nagae-Poetscher, van Zijl, and Mori (2004), superimposed on Figure 7A. (D) The SLF as in (C), superimposed on Figure 7B to show potential connectivity between foci of the “multiple demand” system in the intraparietal sulcus and frontal cortex. Key to abbreviations: IPS = intraparietal sulcus; IFS = inferior frontal sulcus; AI= anterior insula; FO = frontal operculum.
Duncan proposes that the MD network controls the subtasks which make up intelligent behavior, focusing on attending to the specific content of a current cognitive operation, and modulating goal-directed programs in tasks demanding fluid intelligence. The anatomical parallels with our findings on global motion sensitivity suggest the possibility that global motion sensitivity is a signature of these wider cognitive processes, with their vulnerability in atypical development arising through many different genetic and acquired aetiological pathways.
Overview
I have ranged widely through evidence for the particular vulnerability of the dorsal cortical stream in development, and the value of relative sensitivity to global motion and form as a measure of this vulnerability and of variations even in typical development. However, given the very different cognitive and social-behavioral profiles in the phenotypes of different developmental disorders and the commonality of deficits in global motion processing, there must be much debate as to whether the deficit originates across all disorders at the same neural level of visual processing, within the complex dynamic cascade from retina to cortex and within the cortex itself—whether we are talking about top-down constraints or bottom-up, or both. There is the added problem of identifying whether a deficit in early life at a relatively low level of processing has a knock-on effect at later ages at “higher” levels in the visual and visuo-cognitive systems. Lack of space prevents these issues from being fully explored here. However, from our own work with typically and atypically developing infants and children, we would argue that there is unlikely to be a single limiting constraint at one level in processing global motion across all disorders, unless it is a very early developing visual attentional deficit, such as infant “disengagement” in selective attention tasks. When added to the different perceptual biases (or priors) across different disorders, this leads to an apparently common deficit in global motion sensitivity and to other “higher level” deficits in spatial, mathematical, and attentional cognition.
So we are left with many unanswered questions including the following:
Is “dorsal vulnerability” determined by the shared anatomy and neural processing of motion, attention, visuo-motor control, numerical cognition—or are there developmental cascades between these functions?
Does “dorsal stream vulnerability” have the same underpinning in terms of faulty neural networks across all disorders in which it has been demonstrated? Or can this vulnerability occur in different areas and branches of the network, from the magnocellular system in the geniculostriate pathway, to target areas in frontal lobe for top-down decision making—or even in separate pathways through MT/V5 avoiding V1 via the pulvinar or other routes?
Why are the attention/decision processes that appear to be vulnerable specific to global motion, and not shared with global form? Is this vulnerability really one of dorsal/ventral stream integration in the “motion task”?
Are there differences at a very early stage of development of motion processing which fundamentally change the course of complex development, underpinning later intelligent behavior?
How do genetic and environmental differences interplay in determining brain structure?
Do we know how a system with the properties of Duncan's adult multiple demand system develops in childhood?
These questions, and many more, need to be answered by interdisciplinary teams in developmental vision from psychology, cognitive neuroscience, genetics, pediatric ophthalmology and optometry, pediatric neurology, developmental computational science, and education, especially if we are to go on to find child-friendly, reliable interventions to reduce and overcome these visually related problems which may affect many aspects of everyday life. The exploration of global motion sensitivity as a signature of some of these wider cognitive processes, and their vulnerability in atypical development, provides goals for future research.
Supplementary Material
Acknowledgments
The research discussed in this article was mainly supported by program (five years) and project (three years) grants from the UK Medical Research Council, with additional grants from Economic & Social Research Council, National Institutes of Health, Williams Syndrome Foundation, Wellcome Trust, and the Leverhulme Trust. I thank the University of Cambridge, University College London and the University of Oxford for supporting the facilities of the Visual Development Units in each location.
I thank all my colleagues and collaborators (both in the Visual Development Unit and elsewhere) who contributed to this work, often over many years, and whose names appear as my coauthors in the cited references. But, primarily, I thank Oliver Braddick, without whose collaboration and support none of this would have been possible.
Commercial relationships: none.
Corresponding author: Janette Atkinson.
Email: j.atkinson@ucl.ac.uk.
Address: University College London, London, UK.
Web page: https://iris.ucl.ac.uk/iris/browse/profile?upi=JATKI15.
References
- Aaen-Stockdale, C., & Hess, R. F.. (2008). The amblyopic deficit for global motion is spatial scale invariant. Vision Research 48, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Anderson, P. J.,, De Luca, C. R.,, Hutchinson, E.,, Spencer-Smith, M. M.,, Roberts, G.,, Doyle L. W., Victorian Infant Collaborative Study Group (2011). Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Developmental Neuropsychology, 36, 57–73. [DOI] [PubMed] [Google Scholar]
- Anker, S., Atkinson, J., Braddick, O., Nardini, M., & Ehrlich, D.. (2004). Non-cycloplegic refractive screening can identify infants whose visual outcome at 4 years is improved by spectacle correction. Strabismus, 12, 227–245. [DOI] [PubMed] [Google Scholar]
- Arterberry, M. E., & Yonas, A.. (1988). Infant's sensitivity to kinetic information for 3-dimensional object shape. Perception & Psychophysics, 44, 1–6. [DOI] [PubMed] [Google Scholar]
- Arterberry, M. E., & Yonas, A.. (2000). Perception of three-dimensional shape specified by optic flow by 8-week-old infants. Perception and Psychophysics, 62, 550–556. [DOI] [PubMed] [Google Scholar]
- Atkinson, J. (1979). Development of optokinetic nystagmus in the human infant and monkey infant: An analogue to development in kittens. Freeman R. D. (Ed.), NATO advanced study institute series: Developmental neurobiology of vision (pp 277–287). New York: Plenum Press. [Google Scholar]
- Atkinson, J. (1984). Human visual development over the first six months of life: A review and a hypothesis. Human Neurobiology, 3, 61–74. [PubMed] [Google Scholar]
- Atkinson, J. (1993). Infant vision screening: Prediction and prevention of strabismus and amblyopia from refractive screening in the Cambridge photorefraction programme. Simons K. (Ed.), Early visual development: Normal and abnormal (pp 355–347). New York, NY: Oxford University Press. [Google Scholar]
- Atkinson, J. (2000). The developing visual brain. Oxford, UK: Oxford University Press. [Google Scholar]
- Atkinson, J., Anker, S., Evans, C., Hall, R., & Pimm-Smith, E.. (1988). Visual acuity testing of young children with the Cambridge Crowding Cards at 3 and 6 metres. Acta Ophthalmologica, 66, 505–508. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Anker, S., Braddick, O., Nokes, L., Mason, A., & Braddick, F.. (2001). Visual and visuo-spatial development in young Williams Syndrome children. Developmental Medicine and Child Neurology, 43, 330–337. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Anker, S., Nardini, M., Braddick, O., Hughes, C., Rae, S.,… Atkinson, S.. (2002). Infant vision screening predicts failures on motor and cognitive tests up to school age. Strabismus, 10, 187–198. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Anker, S., Rae, S., Hughes, C., & Braddick, O.. (2002). A test battery of child development for examining functional vision (ABCDEFV). Strabismus, 10, 245–269. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Anker, S., Rae, S., Weeks, F., Braddick, O., & Rennie, J.. (2002). Cortical visual evoked potentials in very low birthweight premature infants. Archives of Disease in Childhood Fetal & Neonatal Edition. 86, F28–F31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, J., & Braddick, O. J.. (1981). Development of optokinetic nystagmus in infants: An indicator of cortical binocularity? Fisher, D. F. Monty, R. A. & Senders J. W.. (Eds.), Eye movements: Cognition and visual perception (pp 53–64). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Atkinson, J., & Braddick, O. J.. (1982). Assessment of visual acuity in infancy and early childhood. Acta Ophthalmologica (Copenhagen) 157 Suppl., 18–26. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., & Braddick, O. J.. (1986). Population vision screening and individual visual assessment. Documenta Ophthalmologica Proceedings Series 45, 376–391. [Google Scholar]
- Atkinson, J., & Braddick, O.. (2005). Dorsal stream vulnerability and autistic disorders: The importance of comparative studies of form and motion coherence in typically developing children and children with developmental disorders. Cahiers de psychologie cognitive [Current Psychology of Cognition] 23, 49–58. [Google Scholar]
- Atkinson, J., & Braddick, O.. (2007). Visual and visuocognitive development in children born very prematurely. Progress in Brain Research, 164, 123–149. Amsterdam, the Netherlands: Elsevier. [DOI] [PubMed] [Google Scholar]
- Atkinson J., & Braddick, O.. (2011). Linked brain development for vision, visual attention and visual cognition in typical development and developmental disorders. Riva D. (Ed.), Mariani foundation paediatric neurology series 25: Brain lesion localization and developmental function: Frontal lobes, limbic system, visuo-cognitive system (pp 247–270). Montrouge, France: Libbey Eurotext Ltd. [Google Scholar]
- Atkinson, J., & Braddick, O.. (2012a). Visual attention in the first years: Typical development and developmental disorders. Developmental Medicine and Child Neurology, 54, 589–595 [DOI] [PubMed] [Google Scholar]
- Atkinson, J., & Braddick, O.. (2012b). Visual development. Zelazo P. D. (Ed.), Oxford handbook of developmental psychology (pp 271–309). New York, NY: Oxford University Press. [Google Scholar]
- Atkinson, J., Braddick, O., Anker, S., Curran, W., & Andrew, R.. (2003). Neurobiological models of visuo-spatial cognition in young Williams Syndrome children: Measures of dorsal-stream and frontal function. Developmental Neuropsychology, 23, 141–174. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., Anker, S., Hood, B., Wattam-Bell, J., Weeks, F.,… Coughtrey, H.. (1990). Visual development in the VLBW infant. Transactions of the IVth European Conference on Developmental Psychology, University of Stirling: 193. [Google Scholar]
- Atkinson, J., Braddick, O., Anker, S., Nardini, M., Birtles, D., Rutherford, M.,… Cowan, F.. (2008). Cortical vision, MRI and developmental outcome in preterm infants. Archives of Disease in Childhood. Fetal Neonatal Edition, 93, F292–F297. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., Bobier, W., Anker, S., Ehrlich, D., King J.,… Moore, A. T.. (1996). Two infant vision screening programmes: Prediction and prevention of strabismus and amblyopia from photo- and video-refractive screening. Eye, 10, 189–198. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & Braddick, F.. (1974). Acuity and contrast sensitivity of infant vision. Nature, 247, 403–404 [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., Durden, K., Watson, P. G., & Atkinson, S.. (1984). Screening for refractive errors in 6–9 month old infants by photorefraction. British Journal of Ophthalmology, 68, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & French, J.. (1979). Contrast sensitivity of the human neonate measured by the visual evoked potential. Investigative Ophthalmology and Visual Science, 18, 210–213. [PubMed] [Article] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & French, J.. (1980). Infant astigmatism: Its disappearance with age. Vision Research 20, 891–893. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & Moar, K.. (1977a). Development of contrast sensitivity over the first three months of life in the human infant. Vision Research, 17, 1037–1044. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & Moar, K.. (1977b). Contrast sensitivity of the infant for moving and static patterns. Vision Research, 17, 1045–1048. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & Moar, K.. (1977c). Infants' detection of image defocus. Vision Research, 17, 1125–1126. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O., Montague-Johnson, C., Andrew, M., Baker, B., Parr, J., & Sullivan, P.. (2016). Specific vulnerability of components of visual attention and global motion following perinatal brain injury. Journal of Vision, 16 12: 1124, doi: 10.1167/16.12.1124. [Abstract] [Google Scholar]
- Atkinson, J., Braddick, O., Nardini, M., & Anker, S.. (2007). Infant hyperopia: Detection, distribution, changes and correlates—outcomes from the Cambridge Infant Screening Programs. Optometry & Vision Science 84, 84–96. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O. J., & Pimm-Smith, E.. (1982). Monocular and binocular assessment of infant acuity by preferential looking. British Journal of Ophthalmology, 66, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, J., Braddick, O., Rose, F. E., Searcy, Y. M., Wattam-Bell, J., & Bellugi, U.. (2006). Dorsal-stream motion processing deficits persist into adulthood in Williams Syndrome. Neuropsychologia, 44, 828–833. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., & French, J.. (1979). Astigmatism and orientation preference in human infants. Vision Research 19, 1315–1317. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., French, J., & Braddick, O. J.. (1981). Contrast sensitivity function of preschool children. British Journal of Ophthalmology 65, 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, J., Hood, B., Wattam-Bell, J., Anker, S., & Tricklebank, J.. (1988). Development of orientation discrimination in infancy. Perception, 17, 587–595. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Hood, B., Wattam-Bell, J., & Braddick, O.. (1988). Infants' control of fixation shifts with single and competing targets: Mechanisms of shifting attention. Perception, 17, 367. [Google Scholar]
- Atkinson, J., Hood, B., Wattam-Bell, J., & Braddick, O.. (1992). Changes in infants' ability to switch visual attention in the first three months of life. Perception, 21, 643–653. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., King, J., Braddick, O., Nokes, L., Anker, S., & Braddick, F.. (1997). A specific deficit of dorsal stream function in Williams' syndrome. NeuroReport 8, 1919–1922. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., Nardini, M., Anker, S., Braddick, O., Hughes, C., & Rae, S.. (2005). Refractive errors in infancy predict reduced performance on the Movement Assessment Battery for Children at 3.5 and 5.5 years. Developmental Medicine & Child Neurology, 47, 243–251. [DOI] [PubMed] [Google Scholar]
- Atkinson, J., & van Hof-van-Duin, J.. (1993). Assessment of normal and abnormal vision during the first years of life. Fielder A. & Bax M.. (Eds.), Management of visual handicap in childhood (pp 9–29). London, UK: Mac Keith Press. [Google Scholar]
- Bellugi, U., Lichtenberger, L., Mills, D., Galaburda, A., & Korenberg, J. R.. (1999). Bridging cognition, the brain, and molecular genetics: Evidence from Williams syndrome. Trends in Neurosciences, 22, 197–207. [DOI] [PubMed] [Google Scholar]
- Bennett, I. J., Motes, M. A., Rao, N. K., & Rypma, B.. (2012). White matter tract integrity predicts visual search performance in young and older adults. Neurobiology of Aging, 33 433, e21–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenthal, B. I., Proffitt, D. R., & Cutting, J. E.. (1984). Infant sensitivity to figural coherence in biomechanical motions. Journal of Experimental Child Psychology, 37, 213–230. [DOI] [PubMed] [Google Scholar]
- Bertenthal, B. I., Proffitt, D. R., Spetner, N. B., & Thomas, M. A.. (1985). The development of infant sensitivity to biomechanical motions. Child Development, 56, 531–543. [PubMed] [Google Scholar]
- Biagi, L., Crespi, S. A., Tosetti, M., & Morrone, M. C.. (2015). BOLD response selective to flow-motion in very young infants. PLoS Biology, 13, e1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, A. E., Pinto, J., & Bertenthal, B. I.. (2002). Perception of the symmetrical patterning of human gait by infants. Developmental Psychology, 38, 554–563. [PubMed] [Google Scholar]
- Birtles, D. B.,, Anker, S.,, Atkinson, J.,, Shellens, R.,, Briscoe, A.,, Mahoney, M.,, Braddick, O. J. (2011). Bimanual strategies for object retrieval in infants and young children. Experimental Brain Research, 211, 207–218. [DOI] [PubMed] [Google Scholar]
- Birtles, D. B., Atkinson, J., Anker, S., Braddick, O. J., Ricci, D., Groppo, M.,… Rutherford, M.. (2012). Bimanual coordination in young children born preterm: Effects of white matter injury. Developmental Medicine and Child Neurology 54 S4, 162. [Google Scholar]
- Birtles, D., Braddick, O. J., Wattam-Bell, J., Wilkinson, A., & Atkinson, J.. (2007). Orientation and motion-specific visual cortex responses in infants born preterm. NeuroReport 18, 1975–1979. [DOI] [PubMed] [Google Scholar]
- Bos, A. F., Van Braeckel, K. N., Hitzert, M. M., Tanis, J. C., & Roze, E.. (2013). Development of fine motor skills in preterm infants. Developmental Medicine and Child Neurology, 55 Suppl. 4, 1–4. [DOI] [PubMed] [Google Scholar]
- Braddick, O. (1993). Orientation- and motion-selective mechanisms in infants. Simons K. (Ed.), Early visual development: Basic and clinical research (pp 163–177). New York, NY: Oxford University Press. [Google Scholar]
- Braddick, O. J., & Atkinson, J.. (2007). Development of brain mechanisms for visual global processing and object segmentation. Progress in Brain Research, 164, 151–168. [DOI] [PubMed] [Google Scholar]
- Braddick, O., & Atkinson, J.. (2011). Development of human visual function. Vision Research, 51, 1588–1609. [DOI] [PubMed] [Google Scholar]
- Braddick, O., & Atkinson, J.. (2013). Visual control of manual actions: Brain mechanisms in typical development and developmental disorders. Developmental Medicine & Child Neurology, 55 S4, 13–18. [DOI] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., Akshoomoff, N., Newman, E., Curley, L. B., Gonzalez, M. R.,… Jernigan, T.. (2016). Individual differences in children's global motion sensitivity correlate with TBSS-based measures of the superior longitudinal fasciculus. Vision Research, Advance online publication, doi.org/10.1016/j.visres.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., French, J., & Howland, H. C.. (1979). A photorefractive study of infant accommodation. Vision Research, 19, 319–330. [DOI] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., Hood, B., Harkness, W., Jackson, G., & Vargha-Khadem, F.. (1992). Possible blindsight in babies lacking one cerebral hemisphere. Nature, 360, 461–463. [DOI] [PubMed] [Google Scholar]
- Braddick, O. J., Atkinson, J., Julesz, B., Kropfl, W., Bodis-Wollner, I., & Raab, E.. (1980). Cortical binocularity in infants. Nature, 288, 363–365. [DOI] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., Newman, E., Akshoomoff, N., Kuperman, J. M., Bartsch, H.,… Jernigan, T. L.. (2016). Global visual motion sensitivity: Associations with parietal area and children's mathematical cognition. Journal of Cognitive Neuroscience, 78, 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., & Wattam-Bell, J.. (2003). Normal and anomalous development of visual motion processing: Motion coherence and “dorsal stream vulnerability.” Neuropsychologia, 41, 1769–1784. [DOI] [PubMed] [Google Scholar]
- Braddick, O., Atkinson, J., & Wattam-Bell, J.. (2011). VERP and brain imaging for identifying levels of visual dorsal and ventral stream function in typical and preterm infants. Progress in Brain Research. 189, 95–111. [DOI] [PubMed] [Google Scholar]
- Braddick, O. J., O'Brien, J. M. D., Wattam-Bell, J., Atkinson, J., Hartley, T., & Turner, R.. (2001). Brain areas sensitive to coherent visual motion. Perception, 30, 61–72. [DOI] [PubMed] [Google Scholar]
- Braddick, O. J., O'Brien, J. M. D., Wattam-Bell, J., Atkinson, J., & Turner, R.. (2000). Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Current Biology, 10, 731–734. [DOI] [PubMed] [Google Scholar]
- Braddick, O. J., Wattam-Bell, J., & Atkinson, J.. (1986). Orientation-specific cortical responses develop in early infancy. Nature, 320, 617–619. [DOI] [PubMed] [Google Scholar]
- Breckenridge, K., Atkinson, J., & Braddick, O.. (2012). Attention. Farran E. & Karmiloff-Smith A.. (Eds.), Neurodevelopmental disorders across the lifespan (pp 119–134). Oxford, UK: University Press. [Google Scholar]
- Breckenridge, K., Braddick, O., Anker, S., Woodhouse, M., & Atkinson, J.. (2013). Attention in Williams syndrome and Down's syndrome: Performance on the new Early Childhood Attention Battery (ECAB). British Journal of Developmental Psychology, 31, 257–269. [DOI] [PubMed] [Google Scholar]
- Breckenridge, K., Braddick, O., & Atkinson, J.. (2013). The organisation of attention in typical development: A new preschool attention test battery. British Journal of Developmental Psychology, 31, 271–288. [DOI] [PubMed] [Google Scholar]
- Broadbent, H. J., Farran, E. K., & Tolmie, A.. (2014). Egocentric and allocentric navigation strategies in Williams syndrome and typical development. Developmental Science, 17, 920–934. [DOI] [PubMed] [Google Scholar]
- Britten, K. H., Shadlen, M. N., Newsome, W. T., & Movshon, J. A.. (1992). The analysis of visual motion: A comparison of neuronal and psychophysical performance. Journal of Neuroscience, 12, 4745–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth, B., Varma, S., & Laurillard, D.. (2011). Dyscalculia: From brain to education. Science, 332, 1049–1053. [DOI] [PubMed] [Google Scholar]
- Chapman, C. A., du Plessis, P. A., & Pober, B. R.. (1996). Neurologic findings in children and adults with Williams syndrome. Journal of Child Neurology, 11, 63–65. [DOI] [PubMed] [Google Scholar]
- Chechlacz, M., Gillebert, C. R., Vangkilde, S. A., Petersen, A., & Humphreys, G. W.. (2015). Structural variability within frontoparietal networks and individual differences in attentional functions: An approach using the theory of visual attention. Journal of Neuroscience, 35, 10647–10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J., Feldman, J., & Medlin, D.. (1979). Comparing stereoscopic performance of children using the Titmus, TNO, and Randot stereo tests. Journal of the American Optometric Association. 50, 821–825. [PubMed] [Google Scholar]
- Corbett, F., Atkinson, J., & Braddick, O.. (2016). Impaired visual form and motion sensitivity in children with DCD. Sixth UK Biennial Academic and Practitioner Conference on Developmental Coordination Disorder, University of Leeds, July 2016 (p. 29). [Google Scholar]
- Cornelissen, P., Richardson, A., Mason, A., Fowler, S., & Stein, J.. (1995). Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Research, 35, 1483–1494. [DOI] [PubMed] [Google Scholar]
- Cowie, D., Atkinson, J., & Braddick, O.. (2010). Development of visual control in stepping down. Experimental Brain Research, 202, 181–188. [DOI] [PubMed] [Google Scholar]
- Cowie, D., Braddick, O., & Atkinson, J.. (2008). Visual control of action in step descent. Experimental Brain Research, 186, 343–348. [DOI] [PubMed] [Google Scholar]
- Cowie, D., Braddick, O., & Atkinson, J.. (2012). Visually guided step descent in children with Williams syndrome. Developmental Science, 15, 74–86. [DOI] [PubMed] [Google Scholar]
- Craig, F., Margari, F., Legrottaglie, A. R., Palumbi, R., de Giambattista, C., & Margari, L.. (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatric Disease & Treatment 12, 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin, S., & Frith, U.. (2005). Vagaries of visual perception in autism. Neuron, 48, 497–507 [DOI] [PubMed] [Google Scholar]
- de Kieviet, J. F., Piek, J. P., Aarnoudse-Moens, C. S., & Oosterlaan, J.. (2009). Motor development in very preterm and very low-birth-weight children from birth to adolescence: A meta-analysis. JAMA, 302, 2235–2242. [DOI] [PubMed] [Google Scholar]
- Dehaene, S., Piazza, M., Pinel, P., & Cohen, L.. (2003). Three parietal circuits for number processing. Cognitive Neuropsychology 20, 487–506. [DOI] [PubMed] [Google Scholar]
- Deruelle, C., Mancini, J., Livet, M. O., Casse-Perrot, C., & De Schonen, S.. (1999). Configural and local processing of faces in children with Williams syndrome. Brain & Cognition, 41, 276–298. [DOI] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, J. (2010). The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behavior. Trends in Cognitive Science, 14, 172–179. [DOI] [PubMed] [Google Scholar]
- D'Souza, D., Cole, V., Farran, E. K., Brown, J. H., Humphreys, K., Howard, J.,… Karmiloff-Smith, A.. (2015). Face processing in Williams syndrome is already atypical in infancy. Frontiers in Psychology, 6, 760, doi:10.3389/fpsyg.2015.07760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg, D., Lewis, T. L., Maurer, D., Brar, S., & Brent, H. P.. (2002). Better perception of global motion after monocular than after binocular deprivation. Vision Research, 42, 169–179. [DOI] [PubMed] [Google Scholar]
- Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., & Posner, M. I.. (2005). The activation of attentional networks. Neuroimage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Farran, E. K., Formby, S., Daniyal, F., Holmes, T., & Van Herwegen, J.. (2016). Route-learning strategies in typical and atypical development; eye tracking reveals atypical landmark selection in Williams syndrome. Journal of Intellectual Disabilities Research, 60, 933–44. [DOI] [PubMed] [Google Scholar]
- Felleman, D. J., & Van Essen, D. C.. (1991). Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex, 1, 1–47. [DOI] [PubMed] [Google Scholar]
- Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N., & Cauraugh, J. H.. (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism and Developmental Disorders, 40, 1227–1240. [DOI] [PubMed] [Google Scholar]
- Fox, R., & McDaniel, C.. (1982). The perception of biological motion by human infants. Science 218, 486–487. [DOI] [PubMed] [Google Scholar]
- Friston, K. J., & Büchel, C.. (2000). Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proceedings of the National Academy of Sciences, USA, 97, 7591–7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant, J. L., Braun, J., & Van Essen, D. C.. (1993). Selectivity for polar, hyperbolic, and Cartesian gratings in macaque visual cortex. Science, 259, 100–103. [DOI] [PubMed] [Google Scholar]
- Gallant, J. L., Connor, C. E., Rakshit, S., Lewis, J. W., & Van Essen, D. C.. (1996). Neural responses to polar, hyperbolic, and Cartesian gratings in area V4 of the macaque monkey. Journal of Neurophysiology 76, 2718–2739 [DOI] [PubMed] [Google Scholar]
- Geldof, C. J., van Hus, J. W., Jeukens-Visser, M., Nollet, F., Kok, J. H., Oosterlaan, J., & van Wassenaer-Leemhuis, A. G.. (2016). Deficits in vision and visual attention associated with motor performance of very preterm/very low birth weight children. Research in Developmental Disabilities, 53–54, 258–266. [DOI] [PubMed] [Google Scholar]
- Geldof, C. J., van Wassenaer, A. G., de Kieviet, J. F., Kok, J. H., & Oosterlaan J.. (2012). Visual perception and visual-motor integration in very preterm and/or very low birth weight children: A meta-analysis. Research in Developmental Disabilities, 33, 726–736. [DOI] [PubMed] [Google Scholar]
- Gerstadt, C. L., Hong, Y. J., & Diamond, A.. (1994). The relationship between cognition and action: Performance of children 3 1/2–7 years old on a Stroop-like day-night test. Cognition, 53, 129–153. [DOI] [PubMed] [Google Scholar]
- Gilmore, R. O., Baker, T. J., & Grobman, K. H.. (2004). Stability in young infants' discrimination of optic flow. Developmental Psychology, 40, 259–270. [DOI] [PubMed] [Google Scholar]
- Grant, S., Suttle, C., Melmoth, D. R., Conway, M. L., & Sloper, J. J.. (2014). Age- and stereovision-dependent eye-hand coordination deficits in children with amblyopia and abnormal binocularity. Investigative Ophthalmology & Visual Science, 55, 5687–5701. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn, A., Cory, E., Atkinson, J., Braddick, O. J., Wattam-Bell, J., Guzzetta, A., & Cioni, G.. (2002). Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport 13, 843–847. [DOI] [PubMed] [Google Scholar]
- Hadad, B. S., Schwartz, S., Maurer, D., & Lewis, T. L.. (2015). Motion perception: A review of developmental changes and the role of early visual experience. Frontiers in Integrative Neuroscience, 9, 49, doi:10.3389/fpsyg.2014.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett, P. E. (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18, 1279–1296. [DOI] [PubMed] [Google Scholar]
- Hamm, L. M., Black, J., Dai, S., & Thompson, B.. (2014). Global processing in amblyopia: A review. Frontiers in Psychology, 5, 583, doi: 10.3389/fpsyg.2014.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, P. C., Stein, J. F., Orde, S. R., Winter, J. L., & Talcott, J. B.. (2001). Are dyslexics' visual deficits limited to measures of dorsal stream function? NeuroReport 12, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Harris, L., Atkinson, J., & Braddick, O. J.. (1976). Visual contrast sensitivity of a 6-month infant measured by the evoked potential. Nature, 264, 570–571. [DOI] [PubMed] [Google Scholar]
- Haslum, M. N., & Miles, T. R.. (2007). Motor performance and dyslexia in a national cohort of 10-year-old children. Dyslexia, 13, 257–275. [DOI] [PubMed] [Google Scholar]
- Helfrich, R. F., Becker, H. G., & Haarmeier, T.. (2013). Processing of coherent visual motion in topographically organized visual areas in human cerebral cortex. Brain Topography 26, 247–263. [DOI] [PubMed] [Google Scholar]
- Henderson, S. E., & Sugden, D. A.. (1992). The movement ABC manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Hitzert, M. M., Van Braeckel, K. N., Bos, A. F., Hunnius, S., & Geuze, R. H.. (2014). Early visual attention in preterm and fullterm infants in relation to cognitive and motor outcomes at school age: An exploratory study. Frontiers in Pediatrics, 2, 106, doi:10.3389/fped.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, C. S., Giaschi, D. E., Boden, C., Dougherty, R., Cline, R., & Lyons, C.. (2005). Deficient motion perception in the fellow eye of amblyopic children. Vision Research, 45, 1615–1627. [DOI] [PubMed] [Google Scholar]
- Hocking, D. R., McGinley, J. L., Moss, S. A., Bradshaw, J. L., & Rinehart, N. J.. (2010). Effects of external and internal cues on gait function in Williams syndrome. Journal of the Neurological Sciences, 291, 57–63. [DOI] [PubMed] [Google Scholar]
- Hocking, D., Rinehart, N. J., McGinley, J. L., Moss, S. A., & Bradshaw, J. L.. (2011). A kinematic analysis of visually-guided movement in Williams syndrome. Journal of the Neurological Sciences, 301, 51–58. [DOI] [PubMed] [Google Scholar]
- Hood, B., & Atkinson, J.. (1990). Sensory visual loss and cognitive deficits in the selective attentional system of normal infants and neurologically impaired children. Developmental Medicine and Child Neurology, 32, 1067–1077. [DOI] [PubMed] [Google Scholar]
- Hood, B., & Atkinson, J.. (1993). Disengaging visual attention in the infant and adult. Infant Behaviour and Development, 16, 405–422. [Google Scholar]
- Hou, C., Pettet, M. W., & Norcia, A. M.. (2008). Abnormalities of coherent motion processing in strabismic amblyopia: Visual-evoked potential measurements. Journal of Vision, 8 4: 2, 1–12, doi:10.1167/8.4.2. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland, H. C., Atkinson, J., Braddick, O. J., & French, J.. (1978). Infant astigmatism measured by photorefraction. Science, 202, 331–333. [DOI] [PubMed] [Google Scholar]
- Hubel, D. H., & Wiesel, T. N.. (1968). Receptive fields and functional architecture of monkey striate cortex. Journal of Physiology, 195, 215–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnius, S., Geuze, R. H., Zweens, M. J., & Bos, A. F.. (2008). Effects of preterm experience on the developing visual system: A longitudinal study of shifts of attention and gaze in early infancy. Developmental Neuropsychology, 33, 521–535. [DOI] [PubMed] [Google Scholar]
- Hughes, C., & Russell, J.. (1993). Autistic children's difficulty with mental disengagement from an object: Its implications for theories of autism. Developmental Psychology, 29, 498–510. [Google Scholar]
- Huk, A. C., & Shadlen, M. N.. (2005). Neural activity in macaque parietal cortex reflects temporal integration of visual motion signals during perceptual decision making. Journal of Neuroscience, 25, 10420–10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod, M. (1997). The cognitive neuroscience of action. Oxford, UK: Blackwell. [Google Scholar]
- Jernigan, T. L., Brown, T. T., Hagler, D. J., Jr., Akshoomoff, N., Bartsch, H., Newman, E.,… Dale, A. M.. (2016). Pediatric imaging, neurocognition and genetics study. The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage, 124 Pt. B, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2005). Subcortical face processing. Nature Reviews Neuroscience, 6, 766–774. [DOI] [PubMed] [Google Scholar]
- Johnson, S., Kochhar, P., Hennessy, E., Marlow, N., Wolke, D., & Hollis, C.. (2016). Antecedents of attention-deficit/hyperactivity disorder symptoms in children born extremely preterm. Journal of Developmental and Behavioral Pediatrics, 37, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S., & Marlow, N.. (2017). Early and long-term outcome of infants born extremely preterm. Archives of Disease in Childhood, 102, 97–102. [DOI] [PubMed] [Google Scholar]
- Johnson, S. P., & Aslin, R. N.. (1996). Perception of object unity in young infants: The roles of motion, depth, and orientation. Cognitive Development, 11, 161–180 [Google Scholar]
- Johnson, S. P., Bremner, J. G., Slater, A., Mason, U., Foster, K., & Cheshire, A.. (2003). Infants' perception of object trajectories. Child Development, 74, 94–108 [DOI] [PubMed] [Google Scholar]
- Kellman, P. J., & Spelke, E. S.. (1983). Perception of partly occluded objects in infancy. Cognitive Psychology, 15, 483–524. [DOI] [PubMed] [Google Scholar]
- Kiorpes, L., Price, T., Hall-Haro, C., & Movshon, J. A.. (2012). Development of sensitivity to global form and motion in macaque monkeys (Macaca nemestrina). Vision Research, 63, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, P. J., Ledgeway, T., & Simmers A. J.. (2013). The effects of spatial offset, temporal offset and image speed on sensitivity to global motion in human amblyopia. Vision Research, 86, 59–65. [DOI] [PubMed] [Google Scholar]
- Kogan, C. S., Boutet, I., Cornish, K., Zangenehpour, S., Mullen, K. T., Holden, J. J. A.,… Chaudhuri, A.. (2004). Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain, 127, 591–601. [DOI] [PubMed] [Google Scholar]
- Koldewyn, K., Whitney, D., & Rivera, S. M.. (2010). The psychophysics of visual motion and global form processing in autism. Brain, 133, 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz, D. J., Saleem, K. S., Baker, C. I., & Mishkin, M.. (2011). A new neural framework for visuospatial processing. Nature Reviews Neuroscience, 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremenitzer, J. P., Vaughan, H. G., Kurtzberg, D., & Dowling, K.. (1979). Smooth pursuit eye movements in the new-born infant. Child Development, 50, 442–448. [PubMed] [Google Scholar]
- Kulke, L., Atkinson, J., & Braddick, O.. (2015). Automatic detection of attention shifts in infancy: Eye tracking in the fixation shift paradigm. PLoS One 10 12, e0142505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke, L., Atkinson, J., & Braddick, O.. (2017). Neural mechanisms of attention become more specialised during infancy: Insights from combined eye tracking and EEG. Developmental Psychobiology, 59, 250–260. [DOI] [PubMed] [Google Scholar]
- Levi, D. M. (2013). Linking assumptions in amblyopia. Visual Neuroscience, 30, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, T. L., Ellemberg, D., Maurer, D., Wilkinson, F., Wilson, H. R., Dirks, M., & Brent, H. P.. (2002). Sensitivity to global form in Glass patterns after early visual deprivation in humans. Vision Research, 42, 939–948. [DOI] [PubMed] [Google Scholar]
- Lindström, K., Lindblad, F., & Hjern, A.. (2011). Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics, 127, 858–865. [DOI] [PubMed] [Google Scholar]
- Liu, T. (2013). Sensory processing and motor skill performance in elementary school children with autism spectrum disorder. Perceptual & Motor Skills, 116, 197–209. [DOI] [PubMed] [Google Scholar]
- Lukasova, K., Silva, I. P., & Macedo, E. C.. (2016). Impaired oculomotor behavior of children with developmental dyslexia in antisaccades and predictive saccades tasks. Frontiers of Psychology, 7, 987, doi:10.3389/fpsyg.2016.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi, L., Addona, F., Foti, F., Menghini, D., Petrosini, L., & Vicari, S.. (2009). Spatial competences in Williams syndrome: A radial arm maze study. International Journal of Developmental Neuroscience. 27, 205–213. [DOI] [PubMed] [Google Scholar]
- Manly, T., Nimmo-Smith, I., Watson, P., Anderson, V., Turner, A., & Roberston, I. H.. (2001). The differential assessment of children's attention: The Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. Journal of Child Psychology and Psychiatry, 42, 1065–1081. [DOI] [PubMed] [Google Scholar]
- Manning, C., Tibber, M. S., Charman, T., Dakin, S. C., & Pellicano, E.. (2015). Enhanced integration of motion information in children with autism. Journal of Neuroscience, 35, 6979–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, A. J. S., Braddick, O. J., & Wattam-Bell, J.. (2003). Motion coherence thresholds in infants—different tasks identify at least two distinct motion systems. Vision Research, 43, 1149–1157. [DOI] [PubMed] [Google Scholar]
- Maunsell, J. H. R., & Newsome, W. T.. (1987). Visual processing in monkey extrastriate cortex. Annual Review of Neuroscience, 10, 363–401. [DOI] [PubMed] [Google Scholar]
- Mayer, K. M., & Vuong, Q. C.. (2014). TBSS and probabilistic tractography reveal white matter connections for attention to object features. Brain Structure & Function, 219, 2159–2171. [DOI] [PubMed] [Google Scholar]
- Meier, K., & Giaschi, D.. (2014). The maturation of global motion perception depends on the spatial and temporal offsets of the stimulus. Vision Research, 95, 61–67. [DOI] [PubMed] [Google Scholar]
- Mercuri, E., Atkinson, J., Braddick, O., Anker, S., Cowan, F., Rutherford, M., … Dubowitz, L.. (1997). Visual function in full term infants with hypoxic-ischaemic encephalopathy. Neuropediatrics, 28, 155.–. [DOI] [PubMed] [Google Scholar]
- Mercuri, E., Atkinson, J., Braddick, O., Anker, S., Nokes, L., Cowan, F.,… Dubowitz, L.. (1996). Visual function and perinatal focal cerebral infarction. Archives of Disease in Childhood, 75, F76–F81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri, E., Atkinson, J., Braddick, O., Anker, S., Nokes, L., Cowan, F.,… Dubowitz, L.,. (1997). Basal ganglia damage in the newborn infant as a predictor of impaired visual function. Archives of Disease in Childhood, 77, F111–F114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri, E., Atkinson, J., Braddick, O., Rutherford, M., Cowan, F., Counsell, S.,… Bydder, G.. (1997). Chiari I malformation and white matter changes in asymptomatic young children with Williams syndrome: Clinical and MRI study. European Journal of Pediatric Neurology, 5/6, 177–181. [DOI] [PubMed] [Google Scholar]
- Mercuri, E., Haataja, L., Guzzetta, S., Anker, S., Cowan, F., Rutherford, M.,… Atkinson, J.. (1999). Visual function in term infants with hypoxic-ischaemic insults: Correlation with neurodevelopment at 2 years of age. Archives of Diseases in Childhood, Fetal Neonatal Ed, 80, F99–F104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg, A., Kohn, P., Mervis, C. B., Kippenhan, J. S., Olsen, R. K., Morris, C. A., & Berman, K. F.. (2004). Neural basis of genetically determined visuospatial construction deficit in Williams Syndrome. Neuron, 43, 623–631. [DOI] [PubMed] [Google Scholar]
- Mikami, A., Newsome, W. T., & Wurtz, R. H.. (1986). Motion selectivity in macaque visual cortex. I. Mechanisms of direction and speed selectivity in extrastriate area MT. Journal of Neurophysiology, 55, 1308–1327. [DOI] [PubMed] [Google Scholar]
- Milner A. D., & Goodale, M. A.. (1995). The visual brain in action. Oxford, UK: Oxford University Press. [Google Scholar]
- Mishkin, M., Ungerleider, L., & Macko, K. A.. (1983). Object vision and spatial vision: Two critical pathways. Trends in Neuroscience, 6, 414–417. [Google Scholar]
- Mitchell, D. J., Bell, A. H., Buckley, M. J., Mitchell, A. S., Sallet, J., & Duncan, J.. (2016). A putative multiple-demand system in the macaque brain. Journal of Neuroscience, 36, 8574–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrone, M. C., Tosetti, M., Montanaro, D., Fiorentini, A., Cioni, G., & Burr, D. C.. (2000). A cortical area that responds specifically to optic flow, revealed by fMRI. Nature Neuroscience, 3, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Movshon, J. A., Adelson, E. H., Gizzi, M. S., & Newsome, W. T.. (1986). The analysis of moving visual patterns. Experimental Brain Research, Supplementum 11, 117–151. [Google Scholar]
- Mulder, H., Pitchford, N. J., Hagger, M., & Marlow, N.. (2009). Development of executive function and attention in preterm children: A systematic review. Developmental Neuropsychology, 34, 393–421. [DOI] [PubMed] [Google Scholar]
- Nardini, M., Atkinson, J., Braddick, O., & Burgess N.. (2008). Developmental trajectories for spatial frames of reference in Williams syndrome. Developmental Science 11, 583–595. [DOI] [PubMed] [Google Scholar]
- Nardini, M., Burgess, N., Breckenridge, K., & Atkinson, J.. (2006). Differential developmental trajectories for egocentric, environmental and intrinsic frames of reference in spatial memory. Cognition, 101, 153–172. [DOI] [PubMed] [Google Scholar]
- Newman, C. (2001). The planning and control of action in normal infants and children with Williams Syndrome. London, UK: University of London Press. [Google Scholar]
- Newsome, W. T., & Paré, E. B.. (1988). A selective impairment of motion perception following lesions of the middle temporal visual area (MT). Journal of Neuroscience, 8, 2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, A. R., Birch, E. E., & Spencer, R.. (2009). Factors affecting development of motor skills in extremely low birth weight children. Strabismus, 17, 20–23. [DOI] [PubMed] [Google Scholar]
- Orban, G. A., Fize, D., Peuskens, H., Denys, K., Nelissen, K., Sunaert, S.,… & Vanduffel, W.. (2003). Similarities and differences in motion processing between the human and macaque brain: Evidence from fMRI. Neuropsychologia, 41, 1757–1768. [DOI] [PubMed] [Google Scholar]
- Paquet, A., Olliac, B., Bouvard, M.-P., Golse, B., & Vaivre-Douret, L.. (2016). The semiology of motor disorders in autism spectrum disorders as highlighted from a standardized neuro-psychomotor assessment. Frontiers in Psychology, 7, 1292, doi:10.3389/fpsyg.2016.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perenin, M. T., & Vighetto, A.. (1988). Optic ataxia: A specific disruption in visuomotor mechanisms. I. Different aspects of the deficit in reaching for objects. Brain, 111, 643–674. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny, C., Rivaud, S., Gaymard, B., & Agid, Y.. (1991). Cortical control of reflexive visually-guided saccades. Brain, 14, 1473–1485. [DOI] [PubMed] [Google Scholar]
- Pitzalis, S., Sereno, M. I., Committeri, G., Fattori, P., Galati, G., Patria, F., & Galletti, C.. (2010). Human V6: The medial motion area. Cerebral Cortex, 20, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner, M. I., & Petersen, S. E.. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. [DOI] [PubMed] [Google Scholar]
- Price, G. R., Holloway, I., Räsänen, P., Vesterinen, M., & Ansari, D.. (2007). Impaired parietal magnitude processing in developmental dyscalculia. Current Biology, 17, R1042–R1043. [DOI] [PubMed] [Google Scholar]
- Purpura, G., Fulceri, F., Puglisi, V., Masoni, P., & Contaldo, A.. (2016). Motor coordination impairment in children with autism spectrum disorder: A pilot study using Movement Assessment Battery for Children-2 Checklist. Minerva Pediatrics, Advance online publication, Oct 12, 2016. [DOI] [PubMed] [Google Scholar]
- Ranpura, A., Isaacs, E., Edmonds, E., Singhal, M. A., Clayden, J., Clark, C., & Butterworth, B.. (2013). Developmental trajectories of grey and white matter in dyscalculia. Trends in Neuroscience & Education, 2, 56–64. [Google Scholar]
- Ridder, W. H., Borsting, E., & Banton, T.. (2001). All developmental dyslexic subtypes display an elevated motion coherence threshold. Optometry and Vision Science, 78, 510–517. [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G., Fogassi, L., & Gallese, V.. (1997). Parietal cortex: From sight to action. Current Opinion in Neurobiology, 7, 562–567. [DOI] [PubMed] [Google Scholar]
- Robertson, C. E., Thomas, C., Kravitz, D. J., Wallace, G. L., Baron-Cohen, S., Martin, A., & Baker, C. I.. (2014). Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain, 137, 2588–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton, C. C., Gelhorn, H. L., Bell, J. A., & Classi, P. M.. (2012). The co-occurrence of reading disorder and ADHD: Epidemiology, treatment, psychosocial impact, and economic burden. Journal of Learning Disabilities, 45, 538–564. [DOI] [PubMed] [Google Scholar]
- Shadlen, M. N., & Newsome, W. T.. (2001). Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. Journal of Neurophysiology, 86, 1916–1936. [DOI] [PubMed] [Google Scholar]
- Simermeyer, J. L., & Ketcham, C. J.. (2015). Motor planning and end-state comfort in children with autism spectrum disorders. Autism Open Access, 5, 138, doi:10.4172/2165-7890.1000138. [Google Scholar]
- Simmers, A. J., Ledgeway, T., & Hess, R. F.. (2005). The influences of visibility and anomalous integration processes on the perception of global spatial form versus motion in human amblyopia. Vision Research, 45, 449–460. [DOI] [PubMed] [Google Scholar]
- Simmers, A. J., Ledgeway, T., Hess, R. F., & McGraw, P. V.. (2003). Deficits to global motion processing in human amblyopia. Vision Research, 43, 729–738. [DOI] [PubMed] [Google Scholar]
- Smith, J. C., Atkinson, J., Anker, S., & Moore, A. T.. (1991). A prospective study of binocularity and amblyopia in strabismic infants before and after corrective surgery: Implications for the human critical period. Clinical Vision Sciences, 6, 335–353. [Google Scholar]
- Smyth, M. M., & Mason, U. M.. (1997). Planning and execution of action in children with and without developmental co-ordination disorder. Journal of Child Psychology & Psychiatry, 38, 1023–1037. [DOI] [PubMed] [Google Scholar]
- Spencer, J., O'Brien, J., Riggs, K., Braddick, O., Atkinson, J., & Wattam-Bell, J.. (2000). Motion processing in autism: Evidence for a dorsal stream deficiency. NeuroReport, 11, 2765–2767. [DOI] [PubMed] [Google Scholar]
- Stein, J., Talcott, J., & Walsh, V.. (2000). Controversy about the visual magnocellular deficit in developmental dyslexics. Trends in Cognitive Science, 4, 209–211. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J., & Stein, J. F.. (2013). Cerebellar function in developmental dyslexia. Cerebellum, 12, 267–276. [DOI] [PubMed] [Google Scholar]
- Sunaert, S., Van Hecke, P., Marchal, G., & Orban, G. A.. (1999). Motion-responsive regions of the human brain. Experimental Brain Research, 127, 355–370. [DOI] [PubMed] [Google Scholar]
- Taylor, N. M., Jakobson, L. S., Maurer, D., & Lewis, T. L.. (2009). Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia, 47, 2766–2778. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten, M., Dell'Acqua, F., Forkel, S. J., Simmons, A., Vergani, F., Murphy, D. G., & Catani, M.. (2011). A lateralized brain network for visuospatial attention. Nature Neuroscience, 14, 1245–1246. [DOI] [PubMed] [Google Scholar]
- Tinelli, F.,, Anobile, G.,, Gori, M.,, Aagten-Murphy, D.,, Bartoli, M.,, Burr, D. C.,, Morrone, M. C. (2015). Time, number and attention in very low birth weight children. Neuropsychologia, 73, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell, R. B. H., Dale, A. M., Sereno, M. I., & Malach, R.. (1996). New images from human visual cortex. Trends in Neurosciences, 19, 481–489. [DOI] [PubMed] [Google Scholar]
- van de Weijer-Bergsma, E., Wijnroks, L., & Jongmans, M. J.. (2008). Attention development in infants and preschool children born preterm: A review. Infant Behavior & Development, 31, 333–351. [DOI] [PubMed] [Google Scholar]
- Wakana, S., Jiang, H., Nagae-Poetscher, L. M., van Zijl, P. C. M., & Mori, S.. (2004). Fiber tract–based atlas of human white matter anatomy. Radiology, 230, 77–87. [DOI] [PubMed] [Google Scholar]
- Warner, C. E., Kwan, W. C., & Bourne, J. A.. (2012). The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. Journal of Neuroscience, 32, 17073–17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. D. G., Myers, R., Frackowiak, R. S. J., Hajnal, J. V., Woods, R. P., Mazziotta, J. C.,… Zeki, S.. (1993). Area V5 of the human brain—evidence from a combined study using positron emission tomography and magnetic-resonance-imaging. Cerebral Cortex, 3, 79–94. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J. (1991). The development of motion-specific cortical responses in infants. Vision Research, 31, 287–297. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J. (1992). The development of maximum displacement limits for discrimination of motion direction in infancy. Vision Research, 32, 621–630. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J. (1994). Coherence thresholds for discrimination of motion direction in infants. Vision Research, 34, 877–883. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J. (1996a). The development of visual motion processing. Vital-Durand, F. Braddick, O. J. & Atkinson J.. (Eds.), Infant vision. Oxford, UK: Oxford University Press. [Google Scholar]
- Wattam-Bell, J. (1996b). Visual motion processing in one-month-old infants: Preferential looking experiments. Vision Research, 36, 1679–1685. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J. (1996c). Visual motion processing in one-month-old infants: Habituation experiments. Vision Research, 36, 1671–1677. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J., Birtles, D., Nystrom, P., von Hofsten, C., Rosander, K., Anker, S.,… Braddick, O.. (2010). Reorganization of global form and motion processing during human visual development. Current Biology, 20, 411–415. [DOI] [PubMed] [Google Scholar]
- Wattam-Bell, J., Braddick, O. J., Atkinson, J., & Day, J.. (1987). Measures of infant binocularity in a group at risk for strabismus. Clinical Vision Sciences 1, 327–336. [Google Scholar]
- Wattam-Bell, J., Chiu, M., & Kulke, L.. (2012). Developmental reorganisation of visual motion pathways. i-Perception 3, 230. [Google Scholar]
- Withers, S. (1996). A new clinical sign in Williams syndrome. Archives of Disease in Childhood, 75, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt, C. P., & Craig, C. M.. (2012). Motor skills in children aged 7–10 years, diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 1799–1809. [DOI] [PubMed] [Google Scholar]
- Zeki, S. M. (1974). Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. Journal of Physiology, 236, 549–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.