Summary
Background & aims
Mortality resulting from influenza (flu) virus infections occurs primarily in the elderly through declining immunity. Studies in mice have suggested beneficial effects of selenium (Se) supplementation on immunity to flu but similar evidence is lacking in humans. A dietary intervention study was therefore designed to test the effects of Se-supplementation on a variety of parameters of anti-flu immunity in healthy subjects aged 50–64 years.
Methods
A 12-week randomized, double-blinded, placebo-controlled clinical trial (ClinicalTrials.govNCT00279812) was undertaken in six groups of individuals with plasma Se levels <110 ng/mL. Four groups were given daily capsules of yeast enriched with 0 μg Se/day (SeY-0/d; n = 20), 50 μg Se/d (SeY-50/d; n = 18), 100 μg Se/d (SeY-100/d; n = 21) or 200 μg Se/d (SeY-200/d; n = 23). Two groups were given onion-containing meals with either <1 μg Se/d (SeO-0/d; n = 17) or 50 μg Se/d (SeO-50/d; n = 18). Flu vaccine was administrated at week 10 and immune parameters were assessed until week 12.
Results
Primary study endpoints were changes in cellular and humoral immune responses. Supplementation with SeY and SeO affected different aspects of cellular immunity. SeY increased Tctx-ADCC cell counts in blood (214%, SeY-100/d) before flu vaccination and a dose-dependent increase in T cell proliferation (500%, SeY-50/100/200/d), IL-8 (169%, SeY-100/d) and IL-10 (317%, SeY-200/d) secretion after in vivo flu challenge. Positive effects were contrasted by lower granzyme B content of CD8 cells (55%, SeY-200/d). SeO (Se 50 μg/d) also enhanced T cell proliferation after vaccination (650%), IFN-γ (289%), and IL-8 secretion (139%), granzyme (209%) and perforin (190%) content of CD8 cells but inhibited TNF-α synthesis (42%). Onion on its own reduced the number of NKT cells in blood (38%). These effects were determined by comparison to group-specific baseline yeast or onion control groups. Mucosal flu-specific antibody responses were unaffected by Se-supplementation.
Conclusion
Se-supplementation in healthy human adults with marginal Se status resulted in both beneficial and detrimental effects on cellular immunity to flu that was affected by the form of Se, supplemental dose and delivery matrix. These observations call for a thorough evaluation of the risks and benefits associated with Se-supplementation.
Keywords: Selenium, Influenza, Food supplements, Cellular immunity, Humoral immunity
Abbreviations: APC, allophycocyanin; ECD, energy coupled dye; flu, influenza; IFN-γ, interferon gamma; Ig, immunoglobulin; IL, interleukin; LAK, lymphokine activated killer; LGL, large granular lymphocytes; MNC, mononuclear cells; NK, natural killer; Se, selenium; SeMet, selenomethionine; SeMSC, γ-glutamyl-methylselenocysteine; SeY, selenium-yeast; SeO, selenium-onion; TNF-α, tumor necrosis factor alpha
1. Introduction
Selenium status is known to influence ability of the immune system to respond to infections [1]. Evidence for the importance of adequate Se intakes is provided by the impact that Se deficiency has on the immune system, reportedly reducing proliferation of T cells [2], lymphocyte-mediated toxicity and NK cell activity [3], all of which are important for antiviral immunity. While the mechanisms by which Se exerts its antiviral effects are unknown, Se/selenoproteins regulate cellular redox balance and it is known that the establishment and progression of viral infections are influenced by the redox state of the host cell [4]. Dietary Se is mainly present in organic complexes, such as selenomethionine (SeMet) found in meat, cereals and plant foods, and Se-methylselenocysteine (SeMSC) in Se-accumulator plants such as onions and brassica vegetables, with smaller quantities of inorganic Se derived from dietary supplements. One of the proposed consequences of marginal Se status is impaired immune function. With regard to anti-viral response the benefits of higher Se intake have been demonstrated solely in the recall responses to polio virus vaccination of healthy volunteers with marginal selenium status [5]. This prompted us to evaluate the effects of Se supplementation on a set of immunological parameters that pertain to both cellular and humoral immunity to a different type of virus, such as the influenza (flu) virus. The reason for this investigation is twofold. First, the polio virus is an enteric pathogen and does not undergo the rapid antigenic drifts seen in flu virus; as such, the benefits in recall responses to attenuated polio vaccination cannot therefore be extrapolated to flu virus infections. Secondly, flu infection has very high socio-economic cost worldwide [6] and to identify food supplements with the ability to potentiate immunity in at risk populations, such as the elderly and chronically ill may have an important impact on overstretched healthcare systems worldwide. Indeed, in annual flu epidemics 5–15% of the population is affected with upper respiratory tract infections that result in three to five million cases of severe illness and between 250,000 and 500,000 deaths every year around the world [6]. The aims of this study were to measure both cellular and humoral immune responses to flu vaccine in healthy older (50–64 years) individuals with marginal Se status after Se supplementation.
2. Subjects and methods
2.1. Subjects and study design
A randomized, double-blind, placebo-controlled study was undertaken in adults aged 50–64 years with suboptimal Se status (plasma Se < 110 ng/mL), to determine the effects of Se supplementation on immune responses to flu vaccine. The study was approved by the Norfolk Research Ethics Committee (ref 05/Q0101/32) and registered as a clinical trial (ClinicalTrials.gov NCT00279812). Volunteers were excluded if they had abnormal hematology, blood chemistry, blood pressure measurements, or BMI <18.5 or >35 kg/m2. They had to be non-smokers, not on prescription medication for a chronic ailment or taking any immunosuppressive drugs, antacids or laxatives. Any dietary or herbal supplements being used had to be forfeited from at least one month prior to the start of the study and for its duration. People that had donated blood within 16 weeks of the first study sample and/or were due to do so less than 16 weeks after the last study sample were excluded. Neither immunizations during the study period nor antibiotic use from within four weeks prior to starting the study until the study end were permitted. Concurrent participation in another research project was discouraged. Anyone who needed more than 2 weeks' absence from the study were also excluded. Allergy to eggs or egg products, chicken protein, the antibiotic gentamicin or history of Guillain-Barré syndrome were also exclusion criteria. More detailed information can be found in Hurst et al. [7]. Briefly, each subject was randomly assigned to one of six groups by the study scientists and given tablets containing SeMet in yeast matrix (SeY) at a daily dose of either 0 (SeY-0/d; n = 20), 50 (SeY-50/d; n = 18), 100 (SeY-100/d; n = 21) or 200 (SeY-200/d; n = 23) μg Se or test meals three times a week made with Se-enriched onions (SeO) containing SeMSC with the equivalent of 50 μg Se/day (SeO-50/d; n = 18) or unenriched onions (SeO-0/d; n = 17), for a period of 12 weeks. Se-containing yeast tablets were prepared and Se-enriched onions were grown as described elsewhere [7]. A computerized random number generator was used for the allocation of volunteers. The double-blind coding was not revealed until the completion of final data analyses.
2.2. Flu vaccination process and monitoring of pre- and post-vaccination responses
At week 10 participants were vaccinated with a trivalent influenza vaccine (Solvay Pharmaceuticals, Weesp, The Netherlands) developed according to World Health Organization (WHO) guidelines. Vaccines were administered intramuscularly in the deltoid region of the arm by the study nurses. The flu vaccine strains used were determined according to annual recommendations by the WHO. Vaccines contained the following: influenza A strain subtype H1N1/New Caledonia (years 1 and 2), Solomon Islands (year 3); subtype H3N2/California (year 1), Wisconsin (years 2 and 3); Influenza strain B subtype Jiangsu (year 1), Malaysia (years 2 and 3). Strains of H1N1 (A), H3N2 (A) and B viruses were administered in equal amounts (15 μg of each in 0.5 mL). Fasting blood samples (65 mL) were drawn at weeks 0 (baseline), 10 (Se-intervention, pre-vaccination), 11 (Se-intervention, post vaccination week 1) and 12 (Se-intervention, post vaccination week 2). Enrollment and all study procedures involving human volunteers were performed at the Human Nutrition Unit at the Institute of Food Research, Norwich, UK.
2.3. Volunteer randomization and dietary intervention
Volunteers were randomly allocated to one of six study groups. A computerized random number generator was used (URL: http://www.randomizer.org/form.htm) to generate 3 digit numbers with the group codes A, B, C, D, E or F to facilitate allocation of volunteers. This was done by the study statistician. Yeast supplements were supplied in coded form as either A, B, C, or D. The supplements were taken with a meal on a daily basis. Onions for the study meals were grown at the University of Nottingham, Sutton Bonington, Leicestershire, UK. Their Se content was analyzed by inductively coupled mass spectroscopy. Study meals were also blinded in that the onions supplied by the University of Nottingham were labeled simply as white or yellow onions. Meals made with the yellow onions were designated group E and those made with white onion as group F. Meals were prepared to contain the right weight of onions to achieve 117 μg Se/meal and three meals were consumed per week by each participant in group E or F as an average of 50 μg Se/d. Although volunteers in the onion meal groups could not be blinded, the type of onion in their meal was blinded. The identities of intervention codes used were not known to the researchers, volunteers, statistician or study nurses. Randomization data were kept confidential until study end and completion of data analyses.
2.4. Dietary intervention with Se-enriched yeast or onion
The Se doses used in our study were within the safe limits suggested by the European Food Safety Agency [8]. Yeast supplements and onions used to prepare test meals were supplied in coded form to ensure that the trial remained double-blind [7]. Se-yeast supplements containing 60% SeMet were manufactured, packed and supplied by Pharma Nord (Vojens, Denmark) [9]. Since the normal diet of all participants enlisted to the study was clearly low in selenium (as evidenced by their low selenium status), meals were not specified in the group given selenium-enriched yeast or unenriched yeast tablets.
2.5. Isolation and culture of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (MNC) with a viability >95% were prepared and cultured as described previously [10] in the absence or presence of 0.2 μg/mL of H1N1 flu antigens appropriate for each year of the study (Solway Healthcare, Southampton, UK). All cultures were placed at 37 °C in a humidified atmosphere of 5% CO2 in air. Cells were harvested for analyses on days 3 or 5. Culture supernatants were removed at day 5 and frozen at −80 °C.
2.6. Flu vaccine-induced T cell proliferation
Cells cultured for 5 days, as described above, were stained with CD3-FITC (Becton Dickinson, UK), washed in PBS (Sigma) and the pellets resuspended in 75% (v/v) ethanol in distilled water and stored at −20 °C. Prior to data acquisition, samples were centrifuged, washed once with PBS and resuspended in PBS before staining for 45 min with a propidium iodide reagent (DNA prep reagent kit, Beckman Coulter, High Wycombe, UK). Data were acquired and analyzed on a Beckman Coulter FC500 MPL flow cytometer using MXP software. CD3+ T cells were identified after doublet exclusion which was effected using a PI peak versus integral plot. All phases of the cell cycle were put together to give a combined S, G2 and M value with a dual doublet discriminant and CD3 gate. % CD3+ PI+ cells in unstimulated cultures were subtracted from values derived for cells stimulated with flu antigens.
2.7. Enumeration of cytotoxic cell subsets in whole blood
Heparinized whole blood was stained with an antibody cocktail comprising CD8-APC (Invitrogen, UK), CD16-FITC (Becton–Dickinson, Oxford, UK), CD56-PE (Becton–Dickinson) and CD3-energy coupled dye (ECD) (Beckman Coulter, High Wycombe UK). In parallel, blood was stained with appropriate isotype control antibodies. Erythrocytes were lysed with FACSLysing solution (Becton Dickinson) and washed once. Stained cells were kept at 4 °C and to enable subset enumeration 50 μL of Flow Count Fluorospheres (Beckman Coulter) were added to each sample just before same-day data acquisition on a Beckman Coulter FC500 MPL flow cytometer.
2.8. Evaluation of perforin and granzyme expression
MNC cultured for 3 days in the presence of flu antigens were fixed and permeabilized (Fix and Perm; Invitrogen) according to the manufacturer's instructions before staining with either a ‘positive’ or a ‘negative’ antibody cocktail. The ‘positive’ mixture comprised CD3-ECD (Beckman Coulter), CD8-allophycocyanin (APC) (Invitrogen), anti-perforin-PE and anti-granzyme-FITC (both from Becton–Dickinson). The ‘negative’ mixture contained the same antibodies except for isotype controls (Becton Dickinson) as substitutes for perforin and granzyme antibodies. Cells were analyzed by flow cytometry (Beckman Coulter FC500 MPL).
2.9. Cytokine quantification
Cytokines were measured in 5-day supernatants collected from cells cultured in the presence of flu antigens as described above, using a 4-plex Luminex assay for interleukin-8 (IL-8), interleukin 10 (IL-10), interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) (Biorad, Hertfordshire, UK). The assay was performed according to the manufacturer's instructions. Data were acquired and analyzed on a Luminex 200 System (Applied Cytometry Systems, Sheffield, UK).
2.10. Measurement of immunoglobulins (Ig) G1 and G3 in serum
Serum flu-specific antibody titers were measured by standard enzyme-linked immunosorbent assay (ELISA) using 96-well microtiter plates coated with 1 μg/mL flu antigens (Solvay Pharmaceuticals, Netherlands). Sera were diluted prior to use. Dilutions of serum were incubated for 2 h on coated and blocked ELISA plates. Horseradish peroxidase labeled detection reagents anti-human IgG1 and anti-human IgG3 antibodies were purchased from Cambridge Bioscience (Cambridge, UK). Absorbance values were determined at 450 nm using a Benchmark Plus plate reader (Bio-Rad, Hemel Hempstead, Herts., UK). Inter-plate variability was monitored by including the same selected serum samples on each plate.
2.11. Measurement of IgA in saliva
Volunteers were asked not to clean their teeth immediately before collection of saliva samples which were stored at −80 °C. Before use samples were thawed, vortexed and centrifuged at 800 × g for 10 min. The ELISA procedure used was similar to the one described above except for the use of biotin-labeled anti-human sIgA (Stratech Scientific, Newmarket, Suffolk, UK) instead of anti-human IgG, followed by extravidin-peroxidase (Sigma, UK) as detection reagents. Fold change in titers was calculated simply as the ratio of final value to the initial value.
2.12. Statistics
Primary endpoints were changes in cellular and humoral immune responses; secondary endpoints were changes in selenium status, Se-protein levels and Se-biomarkers (ClinicalTrials.gov NCT00279812). This work focuses on the effects of Se on immune function. Sample size was determined from previous studies [11], [12] and designed to give 80% power. In order to be able to detect a range of 1 standard deviation between the groups with a power of 80%, a sample size of 144 (or 24 in each group) was considered to be sufficient. This allowed a drop-out rate of 25%, in which 18 volunteers needed to complete the study in each group. A repeated measures ANOVA was used to compare the means of matched groups. Post-hoc multiple comparisons were made using Dunnett's test, where comparisons were made with relevant group controls. Inter-group base-line comparisons were made using one-way ANOVA followed by post-hoc Tukey test. While meaningful linear or polynomial (quadratic) regression data could not be derived with only four data points, trend lines were constructed to visualize treatment dose effects. Values in the text are means ± standard errors of the mean (SEMs) unless otherwise stated. A test was considered significant at P < 0.05.
3. Results
3.1. Patient recruitment and retention
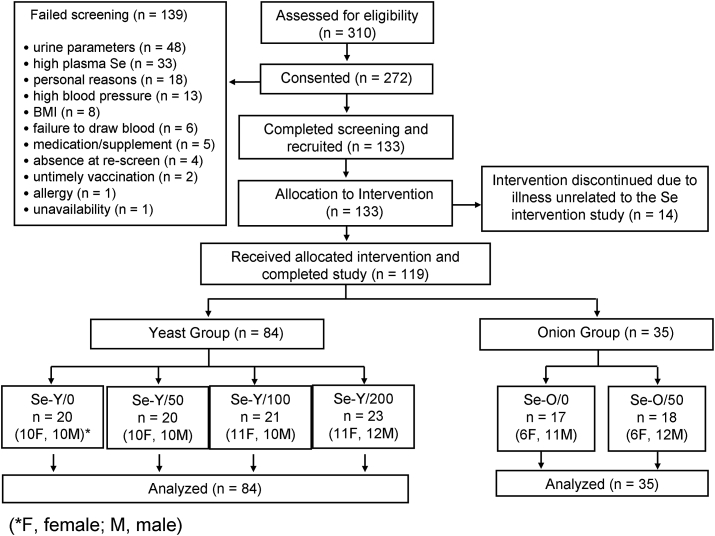
In total, 310 study interviews were conducted and, 272 screenings were carried out. In total 139 volunteers were excluded at the screening stage, most failing as a result of blood or urine parameters falling outside the accepted range (n = 48). The next largest cause of exclusion was high plasma Se (n = 33). Other reasons included personal situations (n = 18), high blood pressure (n = 13), BMI > 35 (n = 7), inability to withdraw blood (n = 6), incompatible medication or supplement (n = 5), not returning for re-screen (n = 4), untimely vaccinations (n = 2), BMI < 18 (n = 1), allergy (n = 1) and unavailability (n = 1). Finally, 133 volunteers began the study and were randomly assigned into each of 6 study groups (Fig. 1) [7], From these 14 participants discontinued their intervention due to illness unrelated to the Se intervention study (n = 14). Finally, 119 (54 men and 65 women) were retained until study completion.
Fig. 1.
Patient recruitment and retention is described (M, Male; F, Female).
3.2. Subject characteristics at baseline
Volunteers within each of the 6 study groups had similar demographic and baseline characteristics (Table 1) [7]. Inter-group variations at baseline for T cell proliferation, cytolytic subsets, cytokines or immunoglobulins were not statistically significant.
Table 1.
Characteristics of study participants at base-line.a
| Yeast groups |
Onion groups |
|||||
|---|---|---|---|---|---|---|
| SeY-0/d (n = 20) |
SeY-50/d (n = 20) |
SeY-100/d (n = 21) |
SeY-200/d (n = 23) |
SeO-0/d (n = 17) |
SeO-50/d (n = 18) |
|
| Age (years) | 55.8 ± 3.9 (50–62) |
56.5 ± 4.6 (50–64) |
58.4 ± 3.9 (52–64) |
56.1 ± 3.9 (50–64) |
58.2 ± 5.1 (50–64) |
57.7 ± 4.2 (52–65) |
| Sex (men/women) | 10/10 | 9/9 | 11/10 | 11/12 | 6/11 | 6/12 |
| bBMI (kg/m2) | 25.0 ± 2.7 (22.0–33.0) |
26.1 ± 3.0 (19.5–33.0) |
26.3 ± 3.9 (20.0–33.5) |
25.9 ± 3.8 (20.5–34.5) |
26.6 ± 4.1 (20.0–21.5) |
26.1 ± 2.4 (21.5–30.5) |
No significant differences were noted between the groups at baseline.
BMI values are means ± SEMs.
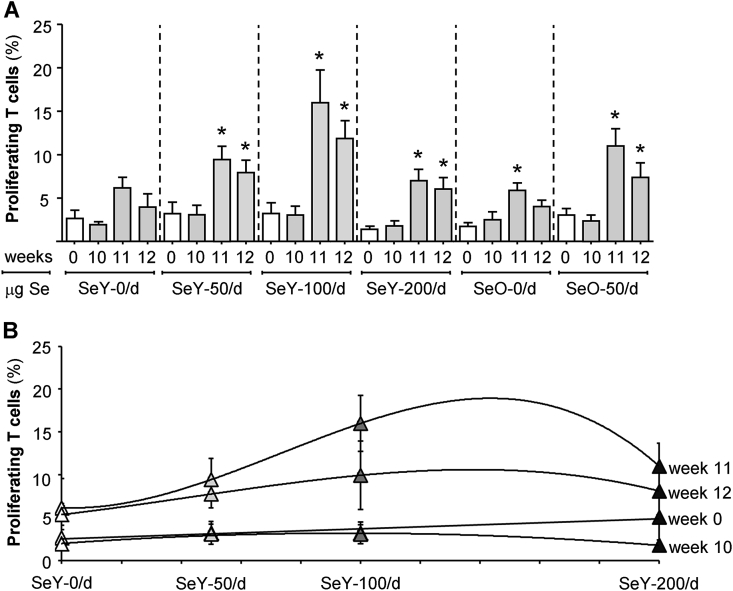
3.3. Se dose-dependent increases in T cell proliferation to flu challenge
At week 10 of supplementation, there were no group-specific differences in the percentages of proliferating T cells within cultures of MNC challenged in vitro with flu antigens (Fig. 2A). However, under similar culture conditions, both yeast and onion Se-supplemented groups had significantly higher T cell proliferation following flu vaccination with peak proliferation occurring at week 11 (P < 0.05). In order to visualize any Se dose effects on T cell proliferation above those induced by flu vaccination, linear or polynomial trend lines were constructed for data derived from the SeY groups (Fig. 2B). It was not possible to construct similar curves for the SeO groups as only one dose of Se was used. These clearly show a trend towards higher proliferation within the SeY-100 group at week 11 than that of SeY-50 or SeY-200 groups. These data demonstrate that the ability of Se to enhance T cell recall responses to flu is influenced by the supplemental dose.
Fig. 2.
Proliferating T cells in isolated mononuclear cells cultured for 5 days with flu antigens. Weeks 0 and 10 samples were respectively collected before Se supplementation or flu vaccination. Panel A shows group specific differences throughout the study period. Panel B shows the same data as Se dose-associated trends by linear or polynomial fit. Values are means ± SEMs; *different from relevant control group (P < 0.05). SeY-0/d, n = 10; SeY-50/d, n = 9; SeY-100/d, n = 28; SeY-200/d, n = 15; SeO-0/d, n = 12; SeO-50/d, n = 14. SeO, selenium onion; SeY, selenium yeast.
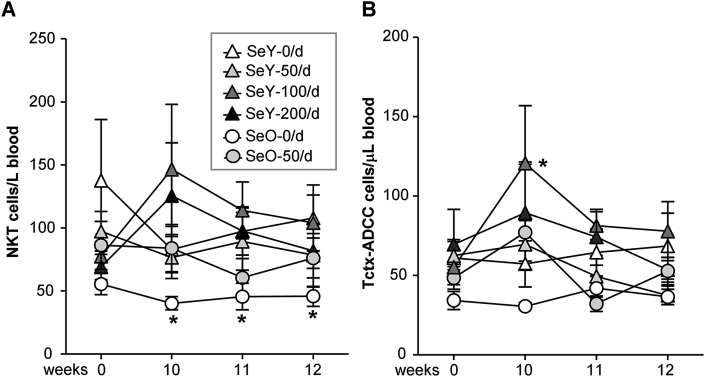
3.4. Se supplementation and delivery matrix affect blood cytolytic cells
Initially we monitored the effects of Se intake on whole blood absolute counts of a variety of large granular lymphocyte (LGL) subsets (Supplementary Table 1). Numbers of NKT cells at baseline did not differ between yeast and onion study groups, but at week 10 the SeO-0/d unsupplemented onion group had significantly fewer NKT cells/μL blood than at baseline (P < 0.05) (Fig. 3A). Immunization with flu vaccine had no influence on this matrix effect. At the same time period, before flu vaccination there were higher numbers of Tctx-ADCC cells/μL blood in the SeY-100/d group. Supplementation with Se, irrespective of its form and supplemental dose, did not have any effect on the number of any of the additional cytotoxic cell subsets included in our investigation. These remained unchanged throughout the study period in all groups (Supplementary Fig. 1).
Fig. 3.
Effect of Se supplementation on (A) NKT and (B) Tctx-ADCC cell counts/μL blood. Weeks 0 and 10 samples were respectively collected before Se supplementation or flu vaccination. Values are means ± SEMs; *different from relevant control group (P < 0.05). SeY-0/d, n = 20; SeY-50/d, n = 20; SeY-100/d, n = 21; SeY-200/d, n = 23; SeO-0/d, n = 17; SeO-50/d, n = 18. NKT, natural killer T.; Tctx-ADCC, T cells that mediate antibody-dependent cell cytotoxicity, SeO, selenium onion; SeY, selenium yeast.
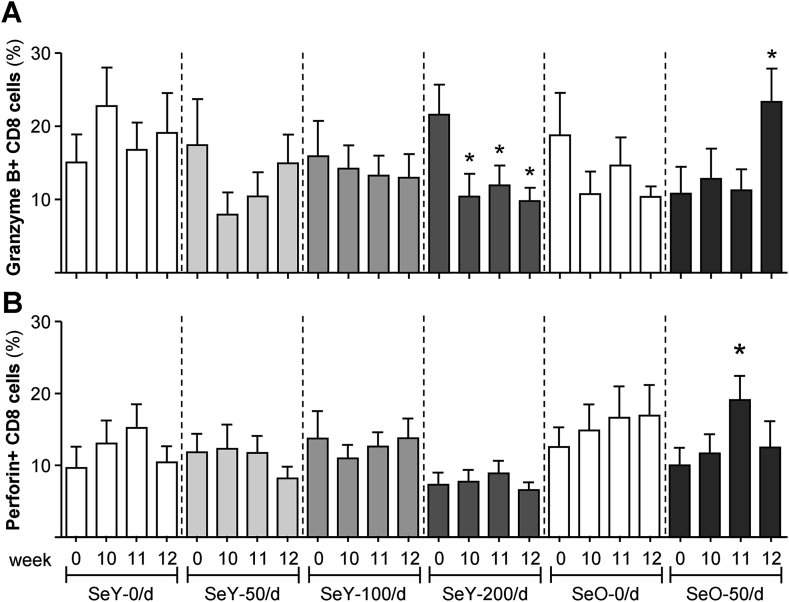
3.5. Effects of Se on cytolytic granules
We have evaluated the influence of Se on granzyme B and perforin present within cytolytic granules on individual specialized CD8+ cytolytic subsets. In comparison with the SeY-0/d control group, we observed lower granzyme content within CD8 cells from the SeY-200/d group at week 10 (Fig. 4A). This effect can be considered to be due to Se-supplemental dose since it was observed before flu vaccination and apparent only at a supplemental dose of 200 μg/day. Vaccination did not reverse this decline. In contrast, CD8 cells from the SeO-50/d group had significantly more granzyme B at week 12 (Fig. 4A) and more perforin at week 11 (Fig. 4B) than the control group. Taken together, these data suggest that beneficial or detrimental effects on the production of cytolytic enzymes, which are important effector molecules involved in anti-viral defense, depends on dosage and form of Se intake.
Fig. 4.
Granzyme B+ (A) and perforin+ (B) CD8 subsets within isolated mononuclear cells cultured for 3 days with flu antigens. Weeks 0 and 10 samples were respectively collected before Se supplementation or flu vaccination. Values are means ± SEMs; *different from relevant control group (P < 0.05). SeY-0/d, n = 15; SeY-50/d, n = 12; SeY-100/d, n = 16; SeY-200/d, n = 19. SeO-0/d, n = 12, SeO-50/d, n = 16. SeO, selenium onion, SeY, selenium yeast.
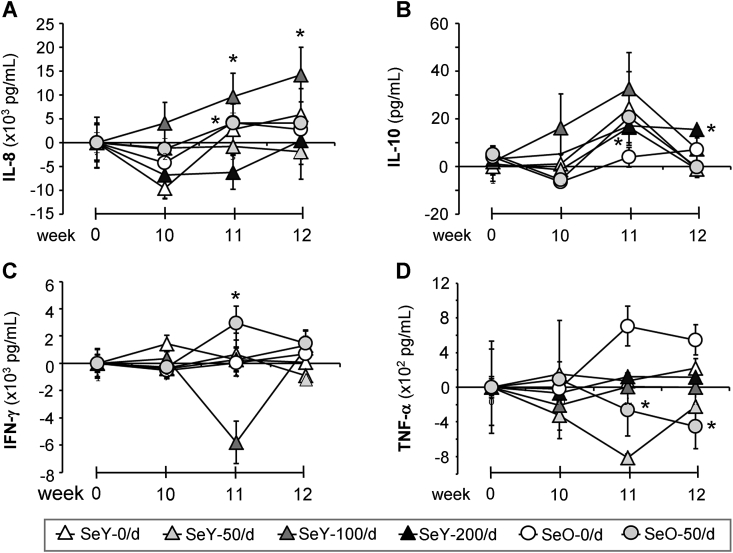
3.6. The dose and form of Se intake affect cytokine secretion in vitro
Cytokine secretion patterns are shown in Fig. 5. To remove differences between groups at baseline (week 0), these values have been subtracted from those derived at subsequent time points. Negative values seen are relative to baseline. Post-vaccination at week 11, both SeY-100/d and SeO-50/d supplemented groups had produced significantly more IL-8 than their control groups (Fig. 5A). While no further increases were seen within the SeO-50/d group, rises in IL-8 production were sustained within the SeY-100/d group up to week 12 (Fig. 5A). Although a clear dose-dependent trend is seen in IL-10 production within the SeY-100/d group from weeks 10–11, there was a high degree of variability within the group (Fig. 5B). Significantly larger amounts of this cytokine were noted after flu vaccination in the SeY-200/d group at week 11 and levels were maintained up to week 12. Only the Se-O-50/d group showed higher IFN-γ synthesis after vaccination at week 11 and this was followed by a decline by week 12 (Fig. 5C). In contrast, the SeO-50/d group showed reduced levels of TNF-α secretion compared to its control group (Fig. 5D).
Fig. 5.
Secreted protein concentrations in supernatants from isolated mononuclear cells cultured for 5 days with or without flu antigens. Weeks 0 and 10 samples were respectively collected before Se supplementation or flu vaccination. In order to account for different baseline (week 0) values these have been subtracted from subsequent time points. Values are means ± SEMs; *different from relevant control group (P < 0.05). SeY-0/d, n = 17; SeY-50/d, n = 15; SeY-100/d, n = 18; SeY-200/d, n = 19; SeO-0/d, n = 13; SeO-50/d, n = 16. SeO, selenium onion; SeY, selenium yeast.
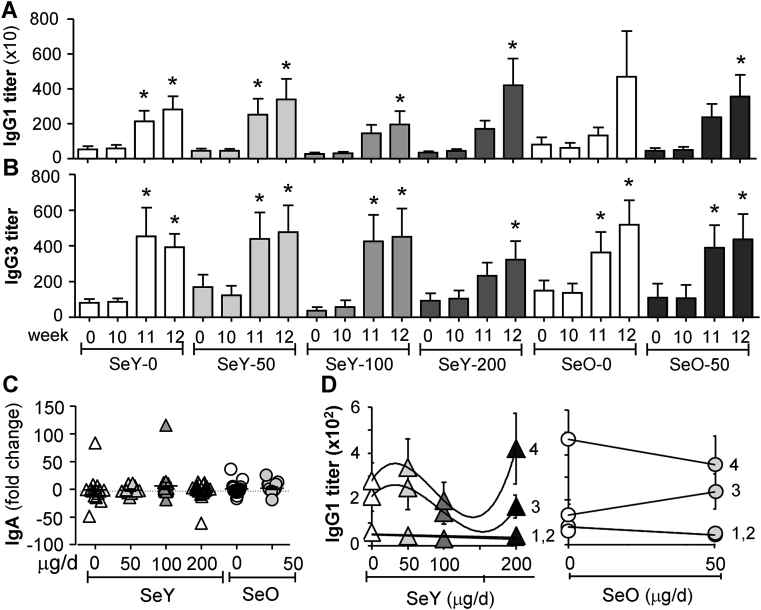
3.7. Se did not affect in vivo antibody responses to flu vaccine
Flu-specific antibody titers of systemic IgG1 (Fig. 6A) and IgG3 (Fig. 6B) and mucosal (salivary) IgA (Fig. 6C) measured by ELISA showed a great inter-individual variability. Significant changes observed in serum IgG1 and IgG3 levels could be ascribed only to in vivo stimulation by flu vaccination and not Se supplementation. This is more evident in the trend lines shown for serum IgG1 (Fig. 6D). Similar trends were seen for serum IgG3 production (data not shown). There was no change in salivary IgA measured as fold change from baseline.
Fig. 6.
Titers of flu-specific serum IgG1 (A,D), serum IgG3 (B) and fold change in salivary IgA (C). Weeks 0 and 10, samples were respectively taken before Se supplementation or flu vaccination. 1, week 0; 2, week 10; 3, week 11; 4, week 12. Values are means ± SEMs for serum IgG (A,B); *different from relevant control group (P < 0.05). SeY-0/d, n = 17; SeY-50/d, n = 16; SeY-100/d, n = 19; SeY-200/d, n = 21; SeO-0/d, n = 15; SeO-50/d, n = 17. SeO, selenium onion; SeY, selenium yeast.
4. Discussion
This study was undertaken to assess the effects of Se-supplementation on a comprehensive set of parameters of both cellular and humoral immunity to influenza in older subjects. Their baseline plasma Se levels between 92 and 98 ng/mL were deemed to reflect marginal Se status despite being within stated normal range because plasma Se concentration is subject to large effects of dietary Se species. Therefore, it cannot be considered a direct marker of the functional Se body pool. Instead, the level of selenoprotein P is more informative as it is responsive to different forms of Se intake [13] and the point at which it reaches a plateau in response to increasing doses of Se is considered indicative of an adequate supply of Se to all tissues [14]. Selenoprotein P levels reach saturation in plasma at a Se concentration of 120 ng/mL [7] which is higher than the plasma Se levels of any volunteer recruited onto our study. Details of the dose–response relations for different forms of selenium with regard to plasma Se levels, platelet glutathione peroxidase activity and selenoprotein P status are described elsewhere [7].
The main findings of this work are that Se-supplementation may have both beneficial and detrimental effects on cellular immunity and that these effects are largely dependent on the form of Se and the supplemental dose. As Se up-regulates the expression of many cell cycle-related genes to promote cell proliferation [15], we tested proliferative responses to in vitro challenge with flu antigens. While we examined total T cell proliferation, it is known that proliferative responses to flu vaccine are generally higher within CD4 than CD8 T cell subsets [16], [17]. At the pre-vaccination 10-week period Se supplementation did not enhance T cell proliferation above that seen in cultures stimulated at baseline. However, these responses were boosted through flu vaccination, with significantly higher T cell proliferation in the Se-Y/100/d group. As a possible explanation for the increase in cell proliferation at this particular dose of Se-yeast, in hepatocytes it has been shown that at this dose Se induces a greater amount of protein per cell and an increase in cellular GSSG-Rd (glutathione reductase) activity than 50 μg/d Se-yeast. Se affects all “synthetic” stages of the cell cycle and elevated GSSG or the GSSG:GSH (oxidized:reduced glutathione) ratio may explain the anti-proliferative effects of higher doses of Se on cells [16], [18]. These data nevertheless show that at certain supplemental doses, Se-yeast can enhance T cell proliferative responses to viral challenge.
The importance of using an appropriate Se dose was further highlighted by analysis of cytolytic enzymes. Following supplementation with Se-yeast at 200 μg/day, fewer granzyme+ CD8 cells were noted only within this group suggesting that a higher supplemental dose may not be beneficial. Meanwhile, at a dose of 50 μg/day Se-onion increased both granzyme and perforin content of CD8 cells. So, both dose and form of Se used in supplementation can have different effects. Since cytotoxic granule-mediated killing by CTL occurs within hours of target cell recognition [19] and perforin up-regulation occurs within hours of activation [20], it is unlikely that this was an immediate consequence of loss or gain through cytolytic activity.
There is no doubt that Se plays an important role in nutritional sufficiency and immunological integrity and it has been found that although plasma Se levels remained relatively unchanged following a daily intake of Se-onion in meals [7], supplementation with Se-onion up-regulated the expression of several selenoprotein genes, including selenoprotein S [21] that is involved in several aspects of immune responses [22]. Importantly, the latter observation lent support for the relationship between Se status and immune function [21]. However, our study signals caution when attributing changes in expression of selenoprotein genes with Se supplementation alone. Through adverse effects of onions alone on NKT cell numbers, it is possible that the matrix used for delivery of Se can somehow interfere with its immunoregulatory properties, either to enhance or otherwise. For example, the presence of phytochemicals in onions should be considered. These include organosulfur compounds, flavonoids and pigments that are believed to mediate effects through different modes of action [23], [24], [25], [26]. Overall, onions have anti-inflammatory activity through inhibition of the proinflammatory cyclooxygenase and lipoxygenase enzymes, alongside inhibition of eicosanoid (e.g. prostaglandins) biosynthesis [25] thus affecting various immunological parameters. Inhibition of TNF-α secretion by Se-onion could be a combined anti-inflammatory effect of onion plus Se. The flavonoids in onions also inhibit both cytosolic and membrane tyrosine kinases that are involved in a variety of functions including signal transduction [27]. While these effects of onions may give them their anti-cancer properties, they may have some unwanted effects on immune cells. With regard to yeast as a matrix for Se delivery, Saccharomyces cerevisiae in the Pharma Nord products used in our study is a potential source of beta 1,3/1,6 glucans, which can prime the innate immune system. Our data do suggest that the matrix used for delivery of Se should be carefully selected. Furthermore, a lack of data on the effects of Se on Ab responses to flu virus in humans prompted us to assess both mucosal and systemic Ab response following Se supplementation. In agreement with previous animal study that showed no changes in levels of flu-specific Abs between Se-deficient and -adequate mice [28], we did not observe any Se-associated increase in flu-specific Ab response. This would confirm that Se affects cell-mediated immunity to a greater extent than humoral anti-influenza responses.
Overall, our study demonstrated that the effect of Se supplementation is context dependent. For example, a dose of 200 μg/day Se-yeast both enhanced IL-10 secretion and reduced the numbers of granzyme+ CD8 cells in blood; thus it is possible that these two effects act together to inhibit anti-viral responses. Similarly, Se-onion both increased the numbers of granzyme+ and perforin+ CD8 cells but reduced the numbers of NKT-cells in blood. Thus, our evidence does not support the simple assumption that a Se concentration that is beneficial for one immune parameter will be beneficial for all. It also showed that two important effector functions of anti-viral immunity, that is antigen-specific T cell proliferation and T cell content of cytotoxic granules, are differentially regulated by Se-supplementation. The findings that when used as a supplement, Se has a narrow effective range and might even be detrimental raises questions about dietary recommendation. While Se deficiency is known to have consequences on the health status, our study shows that higher levels of se supplementation may also have a negative impact on health. That is not to undermine the importance of host Se status; indeed in mice lower baseline levels of Se have been shown to contribute to the emergence of new flu strains through avirulence to virulence transformation [5], [29]. We do not know what role the immune response plays in these changes or how adequate Se status would have affected the immune parameters examined in our study. In countries like the United Kingdom, the generally low selenium status has been used as an argument to promote supplementation practice; however, our study suggests that it may be prudent to thoroughly evaluate the risks and benefits associated with Se supplementation especially in segments of the population particularly vulnerable to influenza infection, such as the elderly and chronically ill.
Statement of authorship
Claudio Nicoletti and Kamal Ivory designed the study; Kamal Ivory, Elena Prieto, Andrew J Goldson, Caroline Spinks and Charlotte N Armah conducted the research and analyzed data; Kamal Ivory and Claudio Nicoletti wrote the paper. Kamal Ivory had final responsibility for content.
Conflict of interest
All authors read and approved the final manuscript. K Ivory, E Prieto, C Spinks, CN Armagh, AJ Goldson, JR Dainty and C Nicoletti have no conflicts of interest to declare.
Funding sources
This work was supported by the Food Standard Agency, UK, Project Grant [N05059] and the Biotechnology and Biological Sciences Research Council (BBSRC), UK, Institute Strategic Programme Grant (BB/J00452945/1: Food and Health-ISP).
Acknowledgments
We thank C. Furniss and B. Teucher for technical help, M Broadley for the Se-enriched onions, E. Bassity, C. Pin and M. Muller for helpful discussions. Pharma Nord (Denmark) donated the placebo and the selenium-enriched yeast supplement tablets.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.clnu.2015.12.003.
Contributor Information
Kamal Ivory, Email: kamal.ivory@ifr.ac.uk.
Elena Prieto, Email: elenaprieto0102@gmail.com.
Caroline Spinks, Email: caroline.spinks@ifr.ac.uk.
Charlotte N. Armah, Email: charlotte.armah@ifr.ac.uk.
Andrew J. Goldson, Email: Andrew.Goldson@ifr.ac.uk.
Claudio Nicoletti, Email: claudio.nicoletti@ifr.ac.uk.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Spallholz J.E., Boylan L.M., Larsen H.S. Advances in understanding selenium's role in the immune system. Ann N Y Acad Sci. 1990;587:123–139. doi: 10.1111/j.1749-6632.1990.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 2.Gill H., Walker G. Selenium, immune function and resistance to viral infections. Nut Diet. 2008;65:S41–S47. [Google Scholar]
- 3.Jackson M.J., Broome C.S., McArdle F. Marginal dietary selenium intakes in the UK: are there functional consequences? J Nutr. 2003;133:1557S–1559S. doi: 10.1093/jn/133.5.1557S. [DOI] [PubMed] [Google Scholar]
- 4.Nencioni L., Sgarbanti R., Amatore D., Checconi P., Celestino I., Limongi D. Intracellular redox signaling as therapeutic target for novel antiviral strategy. Curr Pharm Des. 2011;17:3898–3904. doi: 10.2174/138161211798357728. [DOI] [PubMed] [Google Scholar]
- 5.Broome C.S., McArdle F., Kyle J.A.M., Andrews F., Lowe N.M., Hart C.A. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80:154–162. doi: 10.1093/ajcn/80.1.154. [DOI] [PubMed] [Google Scholar]
- 6.WHO Influenza (Seasonal) Fact sheet N°211 March 2014.
- 7.Hurst R., Armah C.N., Dainty J.R., Hart D.J., Teucher B., Goldson A.J. Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2010;91:923–931. doi: 10.3945/ajcn.2009.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Food Safety Authority . 2006. Tolerable upper intake levels for vitamins and minerals. [Google Scholar]
- 9.Larsen E.H., Hansen M., Paulin H., Moesgaard S., Reid M., Rayman M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J AOAC Int. 2004 Jan–Feb;87(1):225–232. [PubMed] [Google Scholar]
- 10.Ivory K., Chambers S.J., Pin C., Prieto E., Arques J.L., Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-specific immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38:1282–1289. doi: 10.1111/j.1365-2222.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 11.Cox R.J., Mykkeltveldt E., Sjursen H., Haaheim L.R. The effect of zanamir on the early immune response to influenza vaccination. Vaccine. 1999;19:4743–4749. doi: 10.1016/s0264-410x(01)00219-5. [DOI] [PubMed] [Google Scholar]
- 12.Shortt C.T., Duthie G.G., Robertso J.D., Morrice P.C., Nicol F., Arthur J. Selenium status of a group of Scottish adults. EJCN. 1997;51:400–404. doi: 10.1038/sj.ejcn.1600421. [DOI] [PubMed] [Google Scholar]
- 13.Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- 14.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific opinion on dietary reference values for selenium. EFSA J. 2014;12(10) 3846, 67 pp. [Google Scholar]
- 15.Zeng H. Selenium as an essential micronutrient: roles in cell cycle and apoptosis. Molecules. 2009;14:1263–1278. doi: 10.3390/molecules14031263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera M.T., Gonzalez Y., Juárez E., Hernández-Sánchez F., Carranza C., Sarabia C. Humoral and cellular responses to a non-adjuvanted monovalent H1N1 pandemic influenza vaccine in hospital employees. BMC Infect Dis. 2013;13:544. doi: 10.1186/1471-2334-13-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman T.J., Castrucci M.R., Padrick R.C., Bradley L.M., Topham D.J. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:2296–2306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 18.LeBoeuf R.A., Laishes B.A., Hoekstra W.G. Effects of selenium on cell proliferation in rat liver and mammalian cells as indicated by cytokinetic and biochemical analysis. Cancer Res. 1985;45:5496–5504. [PubMed] [Google Scholar]
- 19.Sanderson C.J. The mechanism of T cell mediated cytotoxicity: I. The release of different cell components. Proc R Soc Lond B Biol Sci. 1976;192:221–239. doi: 10.1098/rspb.1976.0010. [DOI] [PubMed] [Google Scholar]
- 20.Hersperger A.R., Makedonas G., Betts M.R. Flow cytometric detection of perforin upregulation in human CD8 T cells. Cytometry. 2008;73A:1050–1057. doi: 10.1002/cyto.a.20596. [DOI] [PubMed] [Google Scholar]
- 21.Goldson A.J., Fairweather Tait S.J., Armah C.N., Bao Y., Broadley M.R., Dainty J.R. Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomized controlled trial. PLoS One. 2011;6:e14771. doi: 10.1371/journal.pone.0014771. [PMCID: PMC3061857] [PubMed: 21445287] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoedter M., Renko K., Hög A., Schomburg L. Selenium controls the sex-specific immune response and selenoprotein expression during the acute-phase response in mice. Biochem J. 2010;429:43–51. doi: 10.1042/BJ20091868. [DOI] [PubMed] [Google Scholar]
- 23.Dorsch W., Wagner H. New antiasthmatic drugs from traditional medicine? Int Arch Allergy Appl Immunol. 1991;94:262–265. doi: 10.1159/000235378. [DOI] [PubMed] [Google Scholar]
- 24.Goldman I., Kopelberg M., Devaene J., Schwartz B. Antiplatelet activity in onion is sulfur dependent. Thromb Haemost. 1996;76:450–452. [PubMed] [Google Scholar]
- 25.Dorant E., Van Din Brandt P., Goldbohm R. A prospective cohort study on allium vegetable consumption, garlic supplement use, and the risk of lung carcinoma in the Netherlands. Cancer Res. 1994;54:6148–6153. [PubMed] [Google Scholar]
- 26.Fitzpatrick D., Hirschfield S., Coeffey R. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am Physiol Soc. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- 27.Nijveldt R.J., van Nood E., Danny van Hoorn D.E.C., Boelens P.G., van Norren K., van Leeuwen P.A.M. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 28.Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J Nutr. 2003;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 29.Nelson H.K., Shi Q., van Dael P., Schiffrin E.J., Blum S., Barclay D. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–1848. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.