Abstract
Epidemiological studies support a protective role of lycopene against stroke occurrence or mortality, but the results have been conflicting. We conducted a meta-analysis to assess the relationship between dietary or circulating lycopene and stroke risk (including stroke occurrence or mortality). Relevant papers were collected by screening the PubMed database through October 2013. Only prospective studies providing relative risk estimates with 95% confidence intervals for the association between lycopene and stroke were included. A random-effects model was used to calculate the pooled estimate. Subgroup analysis was conducted to investigate the effects of various factors on the final results. The pooled analysis of seven prospective studies, with 116,127 participants and 1,989 cases, demonstrated that lycopene decreased stroke risk by 19.3% (RR = 0.807, 95% CI = 0.680–0.957) after adjusting for confounding factors. No heterogeneity was observed (p = 0.234, I2 = 25.5%). Circulating lycopene, not dietary lycopene, was associated with a statistically significant decrease in stroke risk (RR = 0.693, 95% CI = 0.503–0.954). Lycopene could protect European, or males against stroke risk. Duration of follow-up had no effect on the final results. There was no evidence of publication bias. Lycopene, especially circulating lycopene, is negatively associated with stroke risk.
There is considerable evidence for a causal connection between oxidative stress and stroke1. Thus, antioxidants such as carotenoids may have beneficial effects against the occurrence or mortality of stroke. Accumulating epidemiological findings have demonstrated that high consumption of fruits and vegetables is inversely and significantly associated with stroke occurrence and/or mortality2,3,4,5,6, which may be attributable to fruits and vegetables being rich in carotenoids. Accordingly, various epidemiological studies have shown that high concentrations of carotenoids in the serum are associated with decreased stroke occurrence7,8,9,10 and/or stroke mortality11,12.
Lycopene is a dietary carotenoid found in some plant foods, especially tomatoes and tomato products. Lycopene may reduce the risk of stroke more than other carotenoid antioxidants, such as α-carotene and β-carotene, because of its significant antioxidant activity and it being the most effective quencher of free radicals in vitro13,14. However, epidemiological studies have generated conflicting results regarding lycopene and stroke. For example, an inverse association between circulating lycopene and stroke risk has been reported in some prospective studies8,9,15, whereas other studies that focused on dietary lycopene intake have found inconsistent associations with stroke risk; some have shown negative associations16,17,18 and some have reported positive ones19. In view of this uncertainty, we conducted a meta-analysis to address the relationships between the intake of lycopene or tomato-based products, as well as circulating lycopene, and the occurrence or mortality of stroke, which is defined as risk of stroke in our present study.
Results
Literature collection
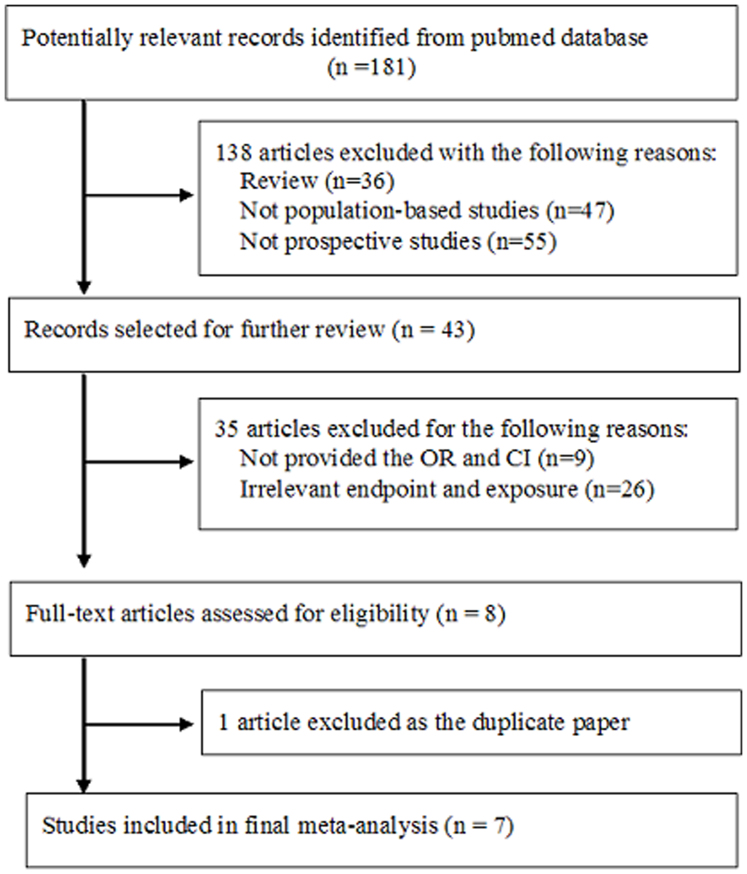
As shown in Figure 1, 181 papers were initially collected; 138 were excluded based on publication type (e.g., were reviews), for not being population-based trials, or not being prospective studies. Of 43 potentially relevant articles, 7 studies were finally included in the meta-analysis8,9,15,16,17,18,19 (exclusions: 26 articles with irrelevant exposure or end-points, 9 that did not provide RRs or CIs, and 1 duplicate report).
Figure 1. Flow chart for literature search.
Of the seven selected studies, one17 reported total risk estimates of stroke and subtype-stratified risks, and only data on total strokes were collected for analysis. Another study19 reported the association between lycopene and stroke using repeated measurements of lycopene intake, and only data for the longer follow-up were extracted. The same study also presented risk estimates stratified by total lycopene intake and tomato intake, and we only collected data regarding lycopene and stroke because tomato is only one source of lycopene. In summary, seven data sets from the seven selected papers were extracted for the meta-analysis.
Characteristics of the selected studies
Characteristics of the papers included in the final meta-analysis are shown in Table 1. They were published from 1999 to 2012. Four studies8,16,18,19 were conducted in the United States, two15,17 were European, and one9 was Asian. Four studies16,17,18,19 assessed dietary lycopene and three8,9,15 analyzed circulating lycopene. The dose of dietary lycopene, assessed using a validated food-frequency questionnaire (FFQ), ranged from 0.59 to 18.798 mg/d. Except for one study16, the studies assessed lycopene intake using only one baseline measurement. The circulating concentration of lycopene, determined by HPLC, ranged between 0.03 and 1.13 μmol/L. The end-point was the first occurrence of stroke, except in one study that reported stroke mortality9. The mean duration of follow-up varied from 6.1 to 13 years (median, 9.6 years). Two studies9,19 included both female and male subjects, one18 selected only postmenopausal females, and four8,15,16,17 included only males, among which one study17 included only male smokers and one16 involved male subjects taking a multivitamin supplement. Ascertainment of stroke case was by a variety of sources, case number ranged from 37 to 914, with a total number of 1,989. The sample sizes of the selected studies varied between 594 and 43,738, and the total number of subjects was 116,127. The major adjusting confounding factors included age, gender, SBP, total cholesterol, ratio of total cholesterol/HDL, BMI, smoking (number of packs/day), hypertension treatment, diabetes, intake of saturated fat, energy, and other carotenoids.
Table 1. Characteristics of the included studies.
| Study | Subjects | Study design and duration (y) | Cases/subjects | Lycopene source and ascertainment (dose) | Stroke type | Stroke ascertainment | Adjustments |
|---|---|---|---|---|---|---|---|
| Jacques, 2012(19) | Male and female,mean aged 54 y in Framingham | Follow-up study11 | 99/2667 | By a validated SFFQ, considering food sources and multivitamin supplements(7.9 mg/d) | total stroke including transient ischemic attack | Hospitalization records, physician office visit records reviewed by a panel of two neurologists, with obvious criteria | Age, sex, SBP, TC, ration of TC/HDL, BMI, smoking, number of packs/d, hypertension treatment, diabetes, intake of saturated fat, energy, β-carotene, flavonol, vitamin C and vitamin E |
| Karppi, 2012(15) | Male,aged 46–65 y,27.5% are current smoker in Kuopio | Cohort study12.1 | 67/1031 | Serum lycopene,Assessed by HPLC,(<0.03 ~ >0.22 umol/L) | any stroke and ischemic stroke | The FINMONICA stroke register data that annually rechecked and Finnish national hospital discharge registry and death certificate registers. | age, examination year, BMI, SBP, smoking, serum LDL cholesterol, diabetes, and history of stroke |
| Ito, 2006(9) | Male and female,aged 39–80 y, 52.4% male, 11.1% female are smokers in Hokkaido | Population-based follow-up study11.9 | 37/3059* | Serum lycopene,Assessed by HPLC,(0.3346 umol/L) | total stroke | Using mortality records from Agency of general affairs and the ministry of health and welfare | sex, age, smoking status, alcohol consumption, BMI, SBP, serum total cholesterol, serum value of TG, ALT activity |
| Hak, 2004(8) | Male, aged 45–70 y in Boston | prospective, nested case-control analysis13 | 297/594 | Plasma lycopene, considering dietary intakes using validated SFFQ,Assessed by HPLC | ischemic stroke | Medical records reviewed by neurologist | BMI, TC, HDL cholesterol, history of hypertension, DM, parental history of MI < 60 y, frequency of vigorous exercise, alcohol consumption, multivitamin use, assignment to aspirin or β-carotene treatment or placebo. |
| Sesso, 2003(18) | postmenopausal female,aged 53.9 ± 7 y in United states | prospective cohort study7.2 | 247/38445 | By a 131-item validated SFFQ,(16741 ~ 3326 ug/d) | total stroke | Defined as a typical neurological deficit, sudden or rapid in onset, lasting > 24 h. | age, randomized aspirin, randomized vitamin E, and randomized β-carotene, BMI, exercise, smoking, PHU, parental history of MI < 60 y, diabetes, hypertension, high cholesterol, intake of fruit and vegetables, alcohol, fiber, folate, non supplemental vitamin E and saturated fat. |
| Hirvonen, 2000(17) | male smokers,aged 50–69 y | Cohort study6.1 | 914/26 593 | By self-administered, validated, modified diet history method.(0·59 mg/d) | cerebral infarctions, subarachnoid hemorrhages, and intracerebralhemorrhages | Identified from national registers by using the unique personal identification number. The validity of the stroke diagnoses has beenevaluated. | age, supplementation group, SBP, DBP, serum TC, HDL cholesterol, BMI, height, smoking-years, number of cigarettes daily, history of diabetes or coronary heart disease, alcohol intake, and education. |
| Ascherio, 1999(16) | Male,aged 40–75 y,With multivitamin supplement | Prospective observational study8 | 328/43738 | By repeated and validated FFQ, lycopene intake was updated using cumulative averages during the follow-up(3442 ~ 18798 ug/d) | total stroke, ischemic,hemorrhagic, and unclassified stroke | By participant's medical records, with a typical neurologic defect of sudden or rapid onset that lasted at least 24 hours | Age, calendar time, total energy intake, smoking, alcohol consumption, history of hypertension, parental history of MI < 65 y, profession, and quintiles of BMI and PA. |
SFFQ: semiquantitative food-frequency questionnaire.
FFQ: food-frequency questionnaire.
HPLC: high-performance liquid chromatography.
FINMONICA: Finnish part of Monitoring of Trends and Determinants in Cardiovascular Diseases.
BMI: body mass index.
SBP: systolic blood pressures.
DBP: diastolic blood pressures.
TC: total cholesterol.
TG: triglyceride.
ALT: alanine transamininase.
DM: diabetes mellitus.
PHU: postmenopausal hormone use.
MI: myocardial infarction.
PA: physical activity.
*: End point is stroke mortality.
Results of the meta-analysis
Lycopene and stroke
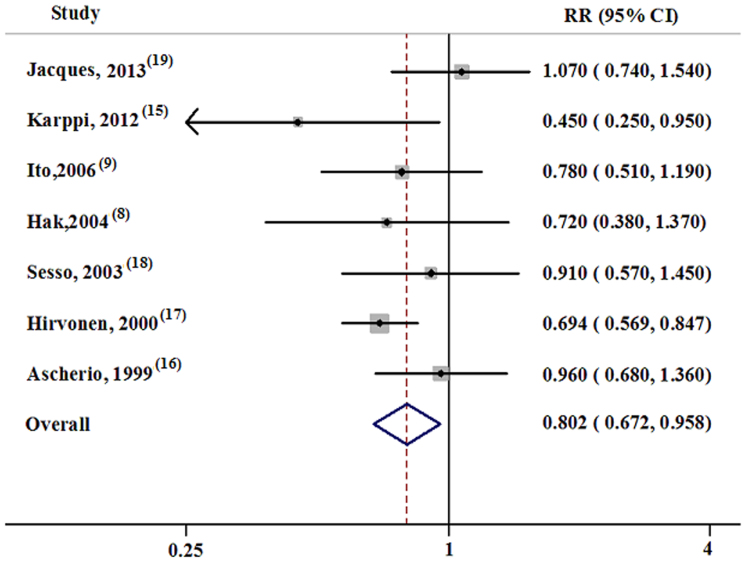
The individual RRs, adjusted by multiple variables with the corresponding 95% CIs and the pooled RR, are presented in Figure 2. Among the seven studies included, one19 showed an insignificant positive association between lycopene and stroke, and the other six showed negative associations, two of which15,17 showed statistical significance. Specifically, lycopene decreased stroke risk by 19.3% (RR = 0.807, 95% CI = 0.680–0.957), and no heterogeneity was observed (p = 0.234, I2 = 25.5%).
Figure 2. Forest plot about the association between lycopene and stroke.
Subgroup analyses
Subgroup analyses were conducted using a random-effects model, and the results are shown in Table 2. The pooled RR for stroke was 0.655 (95% CI = 0.491–0.874; n = 2) for studies conducted in Europe, and 0.917 (95% CI, 0.760–1.107; n = 5) outside of Europe. Lycopene significantly decreased the risk of stroke among male subjects (RR = 0.738, 95% CI = 0.585–0.931; n = 4) but not significantly among females (RR = 0.910, 95% CI = 0.571–1.451) or combined subjects (RR = 0.931, 95% CI = 0.684–1.266). Circulating lycopene effectively decreased stroke risk (RR = 0.693, 95% CI = 0.503–0.954, n = 3), but dietary lycopene did not show the same effect (RR = 0.862, 95% CI, 0.688–1.080, n = 4). Duration of follow-up had no substantial effect on the relationship between lycopene and stroke. Stratifying the results by number of cases showed that excluding studies with fewer than 100 cases resulted in a significant difference (RR = 0.771, 95% CI = 0.654–0.909). No heterogeneity was observed across the subgroup analysis.
Table 2. Results of subgroup analyses.
| Group | Total data included | RR (95% CI) | P | P for heterogeneity | I2, % |
|---|---|---|---|---|---|
| All | 7 | 0.807 (0.680, 0.957) | 0.014 | 0.234 | 25.5 |
| Source of lycopene | |||||
| Dietary lycopene | 4 | 0.862 (0.688, 1.080) | 0.198 | 0.127 | 47.3 |
| Circulating lycopene | 3 | 0.693 (0.503, 0.954) | 0.024 | 0.456 | 0 |
| Location | |||||
| Europe | 2 | 0.655 (0.491, 0.874) | 0.004 | 0.277 | 15.5 |
| Non Europe | 5 | 0.917 (0.760, 1.107) | 0.367 | 0.762 | 0.0 |
| Sex | |||||
| Female | 1 | 0.910 (0.571, 1.451) | 0.647 | ||
| Male | 4 | 0.738 (0.585, 0.931) | 0.010 | 0.238 | 29.0 |
| Mix | 2 | 0.931 (0.684, 1.266) | 0.692 | 0.269 | 18.3 |
| Number of case | 0.796 0.526 1.205 | ||||
| <100 | 3 | 0.796 (0.526, 1,205) | 0.281 | 0.111 | 54.5 |
| >100 | 4 | 0.771 (0.654, 0.909) | 0.002 | 0.371 | 4.3 |
| Duration of follow-up | |||||
| >9.6 y | 4 | 0.798 (0.582, 1.095) | 0.163 | 0.200 | 35.4 |
| <9.6 y | 3 | 0.801 (0.639, 1.004) | 0.055 | 0.213 | 35.4 |
Results of sensitivity analyses
In the sensitivity analyses, omitting the trial of Hirvonen et al17 resulted in an insignificant reduction in stroke risk (RR = 0.860, 95% CI = 0.704–1.051), while the other studies had no substantial effect on the pooled results. The resulting estimates varied from 0.754 (95% CI = 0.644–0.884) to 0.820 (95% CI = 0.701–0.958).
Test of publication bias
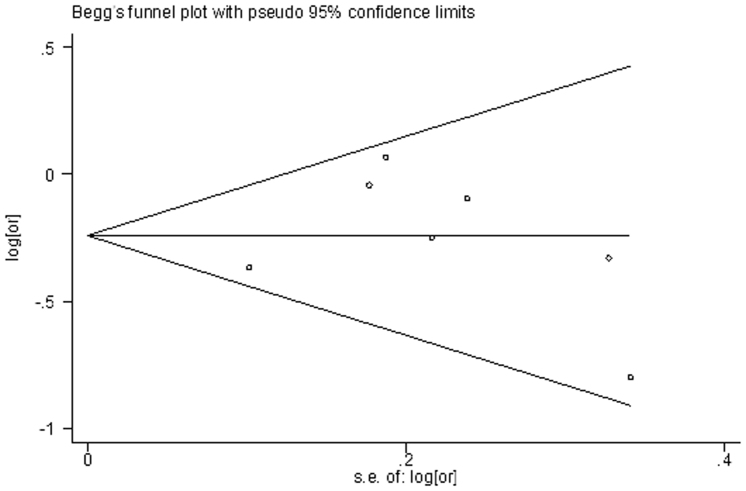
Testing publication bias using funnel plots (Fig. 3) and Egger's test did not produce statistically significant results (Begg, p = 0.293; Egger, p = 0.277).
Figure 3. Funnel plot of lycopene and stroke.
Discussion and Conclusions
Our present meta-analysis demonstrated a significant inverse association between lycopene and stroke risk. Indeed, lycopene may decrease the risk of stroke by 19.8% after adjusting for various confounding factors (RR = 0.807, 95% CI = 0.653–0.997). Our results support the opinion of a protective effect of lycopene against stroke risk. No heterogeneity was observed across our study (p = 0.234, I2 = 25.5%).
Results from subgroup analyses stratified by source of lycopene demonstrated that circulating lycopene, but not dietary lycopene, may effectively decrease stroke risk. Although the dietary source of lycopene, its geometric isomer configuration, food processing and cooking method, the nutrient composition of co-consumed foods, and variation in host genes and nutrient status could affect the absorption and tissue distribution of lycopene, blood is always the main tissue for lycopene distribution20. Results from carefully controlled human intervention trials have demonstrated that changes in dietary exposure can significantly impact serum and tissue concentrations20,21. Thus, circulating lycopene concentrations parallel dietary lycopene intake. The discrepancy between the effects of circulating lycopene and dietary lycopene on stroke risk may be partly due to measurement error or misclassification of exposure in assessing lycopene intake from a dietary questionnaire18,19. For example, Jacques's study19 did not consider ketchup and lycopene supplements in the questionnaire, although ketchup is a relatively concentrated source of lycopene. Furthermore, a lack of accurate food composition data22, low and variable bioavailability and absorption may result in greater misclassification of actual lycopene exposures based on dietary intake, making it more difficult to establish a relationship between lycopene intake and health outcomes23,24. When we performed the same subgroup analysis using the fixed-effects model, both dietary and circulating lycopene decreased the risk of stroke (data not shown), which suggested that the relatively smaller sample size might be another reason that deduced the discrepancy.
Other subgroup results also supported the beneficial role of lycopene in decreasing stroke risk, especially studies conducted in Europe, which may be attributable to the different genetic backgrounds and lifestyles between Europeans and non-Europeans.
Lycopene exhibited a clear protective effect in males but not females, which may be explained by the different bioavailability and absorption of lycopene between males and females. Moreover, hormonal factors may also affect circulating lycopene concentrations25,26.
Restricting the subgroup analysis to studies with more than 100 cases also supported the beneficial role of lycopene. Given studies with more subjects, stronger statistical power, and less possibility of research bias, these results may more authentically reflect the role of lycopene in decreasing stroke risk.
Duration of follow-up had no significant effect on stroke risk. However, given the relatively small number of events, the failure to observe any relationship with stroke might simply be a consequence of inadequate statistical power.
The mechanism by which lycopene protects against stroke may be multifactorial, but likely is mainly due to it being a very effective quencher of singlet oxygen. Furthermore, lycopene generally inhibits the production of reactive oxygen species, inflammation and platelet aggregation, reduces lipid peroxides and decreases total and LDL-cholesterol levels27. These effects may all play a role in decreasing the risk of stroke.
Sensitivity analysis demonstrated that, with one exception17, removing studies from analyses had no statistical effects on pooled estimations. The exception17 resulted in a marginally significant inverse association (RR = 0.860, 95% CI = 0.704–1.051). Hirvonen's study contained a large sample size (26,593 male smokers) and a relatively low dose of lycopene (median intake, 0.59 mg/day). In light of the strong inverse relationship between lycopene and stroke, combined with seeing no obvious heterogeneity across the studies, we believe that our results are robust, and that the observed decrease in stroke risk is due to a protective role of lycopene.
There are other issues that should also be considered. First, the relationship between lycopene and different stroke subtypes should be considered. Different subtypes have different patterns of etiopathogenesis and thus likely also have different associations with dietary antioxidants17. Second, the dose dependence of the effect between lycopene and stroke should be considered. Whether different doses of lycopene have different effects on stroke risk needs additional investigation. Finally, it may be important to further assess the effects of lycopene versus tomatoes on the risk of stroke. Although tomato is a major dietary source of lycopene, it also contains other bioactive substances. Whether lycopene itself and/or other components of tomatoes are responsible for the observed association between lycopene and stroke needs further research.
The current study has several limitations. First, only a small number of trials (n = 7) were included in the study. Second, the dose of dietary lycopene varied widely; due to this, combined with the small number of cases, we were unable to assess the dose dependency between the increase of lycopene dosage and the decrease in stroke risk. The effects of lycopene on different types of stroke on the pooled results was not assessed. Third, misclassification or measurement errors for dietary lycopene intake may have influenced the pooled results. Information on food and nutrient intake was not included during the follow-up, and analyses were based on a single baseline measurement, and thus may not reflect levels over a longer period of time. However, baseline measurements were reasonably correlated with levels measured 5 years later, and all adjusted RRs were estimated on the basis of the highest compared to the lowest dose of lycopene intake, in addition, the wide ranges of lycopene intake may reduce this bias.
Several advantages of our study should also be addressed. First, although only seven studies were included, the analyses provide relatively more statistical power and reliable estimates than individual studies in detecting an association between lycopene and stroke. Second, the trials included in our final meta-analysis were high -quality prospective studies, such as the Framingham Heart Study Offspring cohort19, KIHD15, PHS8, WHS18, ATBC Study17, and the Health Professionals Follow-up Study16, which greatly reduces any likelihood of recall bias, and provides stronger support for a causal association than observational studies. Furthermore, the relatively complete follow-up, the validity of the diagnoses of stroke, and the dietary assessment methods should also provide strong support for the pooled results. Third, no heterogeneity and publication bias were observed in our analyses, indicating that our results are robust.
In conclusion, our meta-analysis confirmed the role of circulating lycopene in reducing the risk of stroke. With increasing incidence rates, stroke is becoming a severe burden for individuals and society. Our findings on the beneficial effects of lycopene for decreasing stroke risk offer an opportunity to reduce stroke risk with lycopene supplements. However, further studies of longer duration and with larger numbers of subjects are needed before recommending the use of lycopene or tomato extract for stroke prevention.
Methods
Literature search strategy
We screened the PubMed database for relevant papers through October 2013 using the keywords “lycopene”, “tomato”, “carotenoids”, and stroke, in combination with “prospective studies, cohort studies”, and “follow-up studies”, with restriction, to “Humans”. We also reviewed the reference lists of selected articles, reviews, and the PubMed option “related articles” to search for other relevant papers. We only selected publications where the full text was available. We did not contact authors for additional data.
Criteria for study selection
All studies included in the final meta-analysis satisfied the following criteria: (1) prospective trials, (2) the exposure factors were dietary or circulating lycopene, (3) the end-point was the first occurrence of stroke or stroke death, (4) the relationship between lycopene and stroke was evaluated, so that the RR and the corresponding 95% confidence intervals (CIs) were reported, and (5) participants were accepted without age limitations, but had to be free of stroke at baseline.
Data collection
According to guidelines of Cochrane Collaboration28, and Stroup DF et al29, the following information was extracted by X-L LI and J-H X independently: last name of the first author, publication year, location, duration of follow-up, characteristics of the participants, number of cases and participants, assessment of lycopene levels or doses, ascertainment of stroke incidence or death, risk estimate for the highest versus the lowest dietary lycopene intake or circulating lycopene level with the corresponding 95% CI, and adjustment for confounding factors. If the study reported total risk estimates of stroke and subtype-stratified risks, only data for total stroke were collected for the analysis. Any inconsistency in data collection was resolved by discussion.
Statistical methods
The Relative Risk (RR) was used to analyze the relationship between lycopene and stroke. One study17 presented final results stratified by stroke subtype; we combined the risk estimates using a random-effects model and extracted the pooled estimates in our meta-analysis.
Heterogeneity was tested using a chi-square -based Q-test at the p = 0.10 level. We also calculated the I2 statistic to describe the proportion of total variation caused by heterogeneity30, According to the Cochrane handbook (5.1), I2 < 40% may not be important heterogeneity. The random-effects model was used to calculate the pooled estimates if the heterogeneity test was statistically significant; otherwise, the fixed-effects model or random-effects model was used31.
Subgroup analysis was conducted to investigate the effects of various study characteristics on the pooled estimate. We performed a sensitivity analysis using both fixed- and random-effects models to evaluate the influence of any single study on the pooled risk estimates by dropping one study at a time from the analysis.
We assessed potential publication bias using Begg's funnel plots and Egger's test at the p < 0.10 level of significance32, An asymmetric plot suggests possible publication bias.
We used STATA (ver. 11.0; Stata Corp) for all analyses. A p value < 0.05 was considered to indicate statistical significance, unless specified otherwise.
The authors declare no competing financial interests.
Author Contributions J.H.X. and X.L.L. screened the literature, selected studies for exclusion and inclusion, and performed data extraction and meta-analysis. X.L.L. drafted the manuscript. All authors reviewed and approved the final manuscript.
08/14/2014
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- Pandya R. S. et al. Central nervous system agents for ischemic stroke: neuroprotection mechanisms. Cent Nerv Syst Agents Med Chem 11, 81–97 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshipura K. J. et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 282, 1233–9 (1999). [DOI] [PubMed] [Google Scholar]
- Johnsen S. P. et al. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr 78, 57–64 (2003). [DOI] [PubMed] [Google Scholar]
- Larsson S. C., Virtamo J. & Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: a prospective study. Atherosclerosis 227, 147–52 (2013). [DOI] [PubMed] [Google Scholar]
- Mahe G. et al. An unfavorable dietary pattern is associated with symptomatic ischemic stroke and carotid atherosclerosis. J Vasc Surg 52, 62–8 (2010). [DOI] [PubMed] [Google Scholar]
- Oude Griep L. M., Verschuren W. M., Kromhout D., Ocke M. C. & Geleijnse J. M. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in The Netherlands. Eur J Clin Nutr 65, 791–9 (2011). [DOI] [PubMed] [Google Scholar]
- Suter P. M. Effect of vitamin E, vitamin C, and beta-carotene on stroke risk. Nutr Rev 58, 184–7 (2000). [DOI] [PubMed] [Google Scholar]
- Hak A. E. et al. Prospective study of plasma carotenoids and tocopherols in relation to risk of ischemic stroke. Stroke 35, 1584–8 (2004). [DOI] [PubMed] [Google Scholar]
- Ito Y. et al. Cardiovascular disease mortality and serum carotenoid levels: a Japanese population-based follow-up study. J Epidemiol 16, 154–60 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. Y., Chen J. Y., Ke D. & Hu M. L. Plasma levels of lipophilic antioxidant vitamins in acute ischemic stroke patients: correlation with inflammation markers and neurological deficits. Nutrition 21, 987–3 (2005). [DOI] [PubMed] [Google Scholar]
- Ray A. L. et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the women's health and aging studies. J Nutr 136, 172–6 (2006). [DOI] [PubMed] [Google Scholar]
- Cherubini A. et al. Antioxidant profile and early outcome in stroke patients. Stroke 31, 2295–300 (2000). [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Kaiser S. & Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 274, 532–8 (1989). [DOI] [PubMed] [Google Scholar]
- Bohm F., Edge R., Burke M. & Truscott T. G. Dietary uptake of lycopene protects human cells from singlet oxygen and nitrogen dioxide: ROS components from cigarette smoke. J Photochem Photobiol B 64, 176–8 (2001). [DOI] [PubMed] [Google Scholar]
- Karppi J., Laukkanen J. A., Sivenius J., Ronkainen K. & Kurl S. Serum lycopene decreases the risk of stroke in men: a population-based follow-up study. Neurology 79, 1540–7 (2012). [DOI] [PubMed] [Google Scholar]
- Ascherio A. et al. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med 30, 963–70 (1999). [DOI] [PubMed] [Google Scholar]
- Hirvonen T., Virtamo J., Korhonen P., Albanes D. & Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 31, 2301–6 (2000). [DOI] [PubMed] [Google Scholar]
- Sesso H. D., Liu S., Gaziano J. M. & Buring J. E. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr 133, 2336–41 (2003). [DOI] [PubMed] [Google Scholar]
- Jacques P. F., Lyass A., Massaro J. M., Vasan R. S. & D'Agostino R. B. Sr. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br J Nutr 110, 545–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. E., Erdman J. W. Jr, & Clinton, S. K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys 539, 171–80 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C. M., Schwartz S. J., Craft N. E., Giovannucci E. L., De Groff V. L. & Clinton S. K. Changes in plasma and oral mucosal lycopene isomer concentrations in healthy adults consuming standard servings of processed tomato products. Nutr Cancer 47, 48–56 (2003). [DOI] [PubMed] [Google Scholar]
- Eskanich D. et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 93, 790–6 (1993). [DOI] [PubMed] [Google Scholar]
- Tang G. et al. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. J Nutr Biochem 16, 229–35 (2005). [DOI] [PubMed] [Google Scholar]
- Brown M. J. et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 80, 396–403 (2004). [DOI] [PubMed] [Google Scholar]
- Forman M. R. et al. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: a controlled diet study. Am J Clin Nutr 64, 559–65 (1996). [DOI] [PubMed] [Google Scholar]
- Michaud D. S. et al. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Biomarkers Prev 7, 283–90 (1998). [PubMed] [Google Scholar]
- Bohm V. Lycopene and heart health. Mol Nutr Food Res 56, 296–303 (2012). [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. T. & Green, S. editors. Cochrane handbook for systematic reviews of interventions Version 5.0.2.The Cochrane Collboration; www.cochrane-handbook.org (2009). Date of access: 01/03/2010.
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–12 (2000). [DOI] [PubMed] [Google Scholar]
- Jadad A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–88 (1986). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]