Abstract
It has been demonstrated that tumour necrosis factor receptor (TNFR) homologues encoded by viruses are usually involved in virus immune evasion by regulating the host immune response or mediating apoptotic cell death. Here, a novel TNFR-like protein encoded by Singapore grouper iridovirus (SGIV VP51) was cloned and characterized. Amino acid analysis showed that VP51 contained three cysteine-rich domains (CRDs) and a transmembrane domain at its C terminus. The expression of VP51 in vitro enhanced cell proliferation, and affected cell cycle progression via altering the G1/S transition. Furthermore, VP51 overexpression improved cell viability during SGIV infection via inhibiting virus-induced apoptosis, evidenced by the reduction of apoptotic bodies and the decrease of caspase-3 activation. In addition, overexpression of VP51 increased viral titre and the expression of viral structural protein gene MCP and cell proliferation promoting gene ICP-18. In contrast, the expression of the viral apoptosis inducing gene, LITAF, was significantly decreased. Although all three CRDs were essential for the action of VP51, CRD2 and CRD3 exerted more crucial roles on virus-induced apoptosis, viral gene transcription and virus production, while CRD1 was more crucial for cell proliferation. Together, SGIV TNFR-like products not only affected cell cycle progression and enhanced cell growth by increasing the expression of the virus encoded cell proliferation gene, but also inhibited virus-induced apoptotic cell death by decreasing the expression of the viral apoptosis inducing gene. Our results provided new insights into understanding the underlying mechanism by which iridovirus regulated the apoptotic pathway to complete its life cycle.
Introduction
Apoptosis plays a significant role in the immune response by eliminating cells that might be harmful to the host, and several functional viral genes are employed to regulate apoptosis in the host to enhance the production of progeny (Hong et al., 2002; Roulston et al., 1999). Increasing evidence has revealed that viruses encode several tumour necrosis factor receptor (TNFR) homologues to evade the host immune system (Rahman & McFadden, 2006). The TNFR superfamily members can be subdivided into three major groups: death domain (DD)-containing receptors that can activate apoptosis, TNF receptor-associated factor (TRAF) binding receptors that can activate transcription factors such as NF-κB and decoy receptors (Aggarwal, 2003; Benedict et al., 2003; Pontejo et al., 2013). It has already been reported that the T2 protein encoded by the myxoma virus (M-T2) is a TNFR-like protein that has two distinct activities: secreted M-T2 binds and inhibits rabbit tumour necrosis factor alpha (TNF-α), while intracellular M-T2 blocks virus-induced lymphocyte apoptosis (Sedger et al., 2006). To our knowledge, the function of viral TNFR homologues in lower vertebrate viruses remains largely unknown.
Iridoviruses are large dsDNA viruses that infect three classes of ectothermic vertebrates: amphibians, bony fish and reptiles (Gray et al., 2009; Zhang & Gui, 2015). The lymphocystis disease viruses (LDVs), LDVICp016, LDVICp95 and LDVICp167 have been characterized as TNFR homologues that do not have a TNFR-like function (Pontejo et al., 2013). Singapore grouper iridovirus (SGIV) is a novel Ranavirus isolated from diseased grouper (Qin et al., 2001), and evidence showed that SGIV infection induced typical apoptosis in fathead minnow (FHM) cells (Huang et al., 2011a, b). It has been confirmed that SGIV encodes several functional products that might be associated with apoptosis, including a lipopolysaccharide-induced TNF-α factor (SGIV LITAF) homologue and three TNFR homologues: SGIV VP50 (ORF050), SGIV VP51 (ORF051) and SGIV VP96 (ORF096) (Song et al., 2004). Previous studies revealed that overexpression of SGIV LITAF induced cell apoptosis, while overexpression of VP96 reduced virus-induced apoptosis in FHM cells (Huang et al., 2008, 2013). The functions of the other two TNFR homologues encoded by SGIV remain unknown.
In this study, we characterized another SGIV-encoded TNFR homologue, VP51, as an immediate early (IE) gene and demonstrated that it could increase cell proliferation and viral production. Overexpression of VP51 inhibited SGIV-induced apoptosis in FHM cells. Furthermore, deletion of the cysteine-rich domains (CRDs) had different effects on VP51, and we confirmed that CRD2 was the major functional component of VP51 that regulates viral proliferation. These results provided new insights into the function of a TNFR homologue from iridovirus, and increased our understanding of SGIV pathogenesis as well.
Results
SGIV VP51 encoded a TNFR-like protein
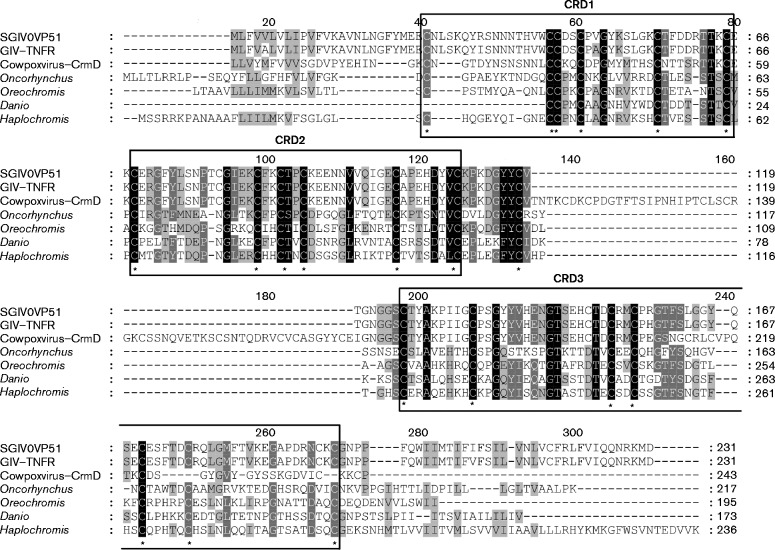
Sequence analysis of SGIV ORF051L (GenBank accession number YP_164146) suggested that it encodes a predicted 231 aa protein with a molecular mass of 26.1 kDa, and it shares 34 %, 32 % and 31 % identity to TNFR members from Oreochromis niloticus (GenBank accession number XP_005451163), Haplochromis burtoni (GenBank accession number XP_005918349) and Danio rerio (GenBank accession number XP_004575223), respectively. Sequence analysis revealed that the deduced amino acid sequence of VP51 contained a transmembrane domain at its C terminus and three extracellular CRDs with six, six and seven conserved cysteine residues, respectively (Fig. 1). The different numbers of CRDs in VP51 and VP96 indicated that VP51 was a novel viral TNFR-like protein encoded by SGIV (Huang et al., 2013).
Fig. 1.
Amino acid sequence alignment of SGIV VP51 with other TNFR homologues from fish and viruses. The black shaded regions indicate completely conserved residues, while the grey shaded regions are partially conserved residues with greater than 80 % conservation. The numerical labeling on the residues indicated the total number of the amino acids at the corresponding position. The conserved cysteines (C) are indicated by asterisks. Boxes above the sequences indicate the putative CRDs. Accession numbers of the sequences used for the above analysis are as follows: SGIV VP51, YP_164146; Grouper iridovirus (GIV) TNFR, AAV91053; cowpox virus CrmD, AGZ01143; Oncorhynchus mykiss, NP_001158656; Oreochromis niloticus, XP_005453111; Danio rerio, XP_009302223; Haplochromis burtoni, XP_005951647.
SGIV VP51 was an IE viral gene
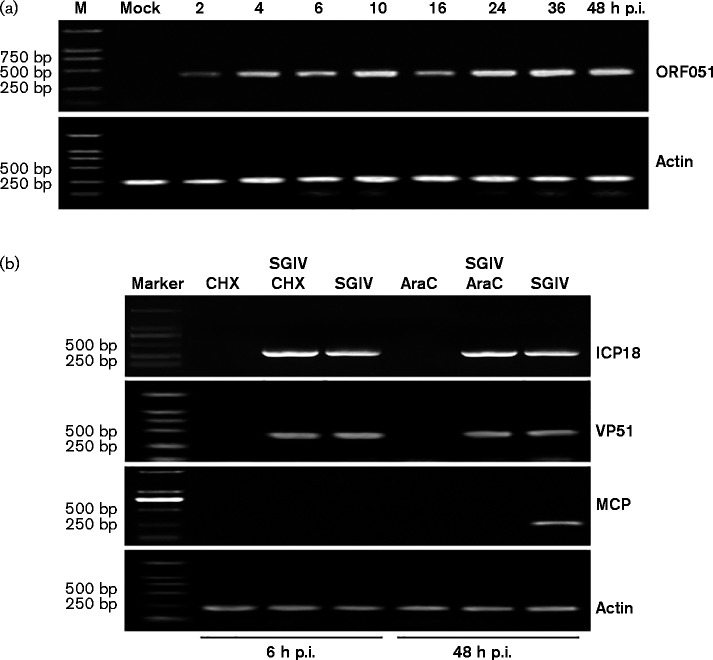
To detect the temporal transcription pattern of VP51, its transcriptional kinetics were examined at different time points after SGIV infection. As shown in Fig. 2(a), VP51 mRNA was detected at 2 h post-infection (p.i.). With infection time, VP51 transcription gradually increased and its transcription stayed at a high level until 48 h p.i., suggesting that VP51 was transcribed at an early stage of SGIV infection (Fig. 2a).
Fig. 2.
SGIV VP51 is an IE viral gene during SGIV infection. (a) RT-PCR detection of the VP51 transcript at different time points. The transcription of VP51 was detected as early as 2 h p.i. and a high level of transcription continued until 48 h p.i. β-actin was detected as an internal control. (b) Reverse transcription (RT)-PCR examination of VP51 transcripts under drug treatment. The expressions of VP51, ICP18 and MCP were evaluated in SGIV-infected cells either in the absence or presence of CHX and AraC. β-actin was detected as an internal control.
In order to determine whether VP51 was an IE, early (E) or late (L) gene, cyclohexane (CHX) and arabinoside cytosine (AraC) were used in drug inhibition assays. CHX and AraC can inhibit protein or DNA synthesis, respectively, and they were used to classify the transcripts of iridovirus genes as IE, E or L genes (Huang et al., 2008). As shown in Fig. 2(b), the transcription of SGIV ICP-18 (an IE gene) was not affected by CHX or AraC, while the transcription of SGIV MCP (an L gene) was inhibited significantly in the presence of the drugs. The VP51 transcription pattern was similar to that of SGIV ICP-18, and the drug treatment had no effect on its transcription, indicating that VP51 was a viral IE gene (Fig. 2b).
SGIV VP51 localized in the cytoplasm
The subcellular localization of VP51 was investigated by transfection of different GFP recombinant plasmids and observation under fluorescence microscopy. As a control, cells were transfected with an empty pEGFP-N3 vector, and green fluorescence was observed in both the nucleus and cytoplasm in fish cells (Fig. 3). In pEGFP-VP51 transfected cells, the green fluorescence was mostly observed in the cytoplasm. In contrast, in pEGFP-ΔCRD3 transfected cells, the green fluorescence signal was observed in both the cytoplasm and nucleus like in the control vector cells. Compared to the cells transfected with pEGFP-VP51, no obvious changes were observed in the cells transfected with pEGFP-ΔTM, pEGFP-ΔCRD1 or pEGFP-ΔCRD2 (Fig. 3).
Fig. 3.
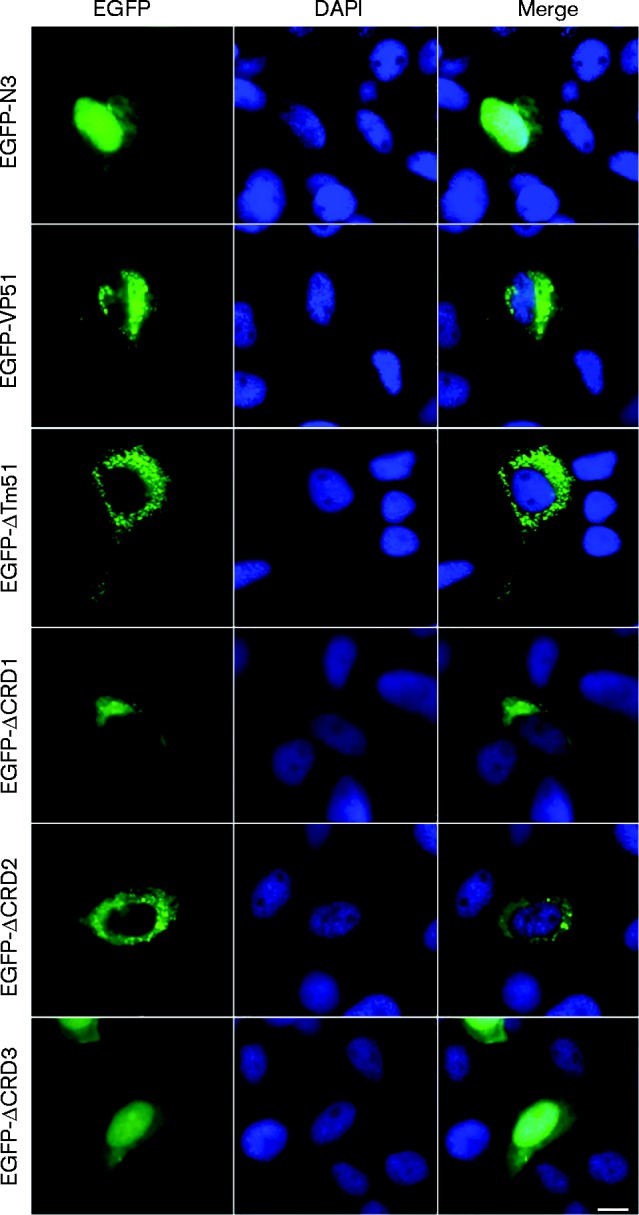
SGIV VP51 localizes in the cytoplasm. The plasmids pEGFP-N3, pEGFP-VP51 and different VP51 mutants were transfected into FHM cells and stained with DAPI. Samples were detected under a fluorescence microscope. Bar, 10 μm.
Overexpressing SGIV VP51 enhanced cell proliferation and affected cell cycle progression
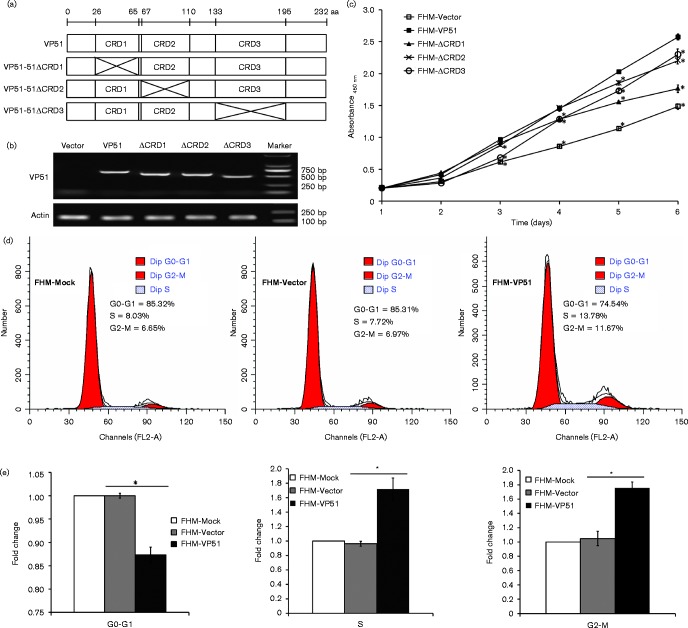
To evaluate the detailed roles of VP51 in SGIV replication in vitro, cells stably expressing the full-length and different CRD mutants of VP51 were also established (Fig. 4a). The expression of the full-length VP51 and its mutants were confirmed by reverse-transcription PCR (RT-PCR) (Fig. 4b). To uncover the cellular function of VP51 in vitro, we firstly examined the effect of VP51 on cell proliferation by determining the cell growth curves. As shown in Fig. 4(c), the VP51 overexpressing cells were exceptional from the third day, and the absorbance of it was about twofold compared with that of only vector transfected cells on the sixth day, suggesting that overexpression of VP51 enhanced fish cell proliferation in vitro. The deletion of each CRD reduced the effect of VP51 on promoting cell proliferation to various degrees. CRD1 deletion had the most impact, as shown in Fig. 4(c).
Fig. 4.
Overexpression of SGIV VP51 enhanced cell proliferation and affected cell cycle progression. (a) Diagram of VP51 and its CRD mutants VP51-ΔCRD1, -ΔCRD2, and -ΔCRD3. (b) FHM cells stably expressing VP51 or its mutants were confirmed by RT-PCR. (c) Overexpression of VP51 or its mutants enhances cell proliferation. WST-1 assay was carried out daily for six days, and the absorbance was measured in a multi-label plate reader at 450 nm. (d) Cell cycle profiles of FHM cells that were mock transfected (FHM-Mock), transfected with the empty vector (FHM-Vector) or VP51 (FHM-VP51) were analysed by flow cytometry after culture for 36 h. (e) Quantification was expressed as the ratio of FHM-VP51 cells in different phases of the cell cycle to mock-transfected cells. n = 3; error bars represent the mean ± sd; *P < 0.05 compared with the control group.
We further assessed the effects of VP51 on cell cycle progression. The results showed that overexpression of VP51 accelerated the G1/S transition, compared with the control vector cells (Fig. 4d). Statistical analysis showed that FHM-Mock cells and FHM-Vector cells shared similar percentages of cells in G1 S and G2 M phases, but ectopic expression of VP51 significantly increased the percentages of cells both in S and G2 M phases, suggesting that VP51 promoted cell cycle progression into S phase and enhanced cell mitosis.
SGIV VP51 improved cell viability and inhibited SGIV-induced apoptosis
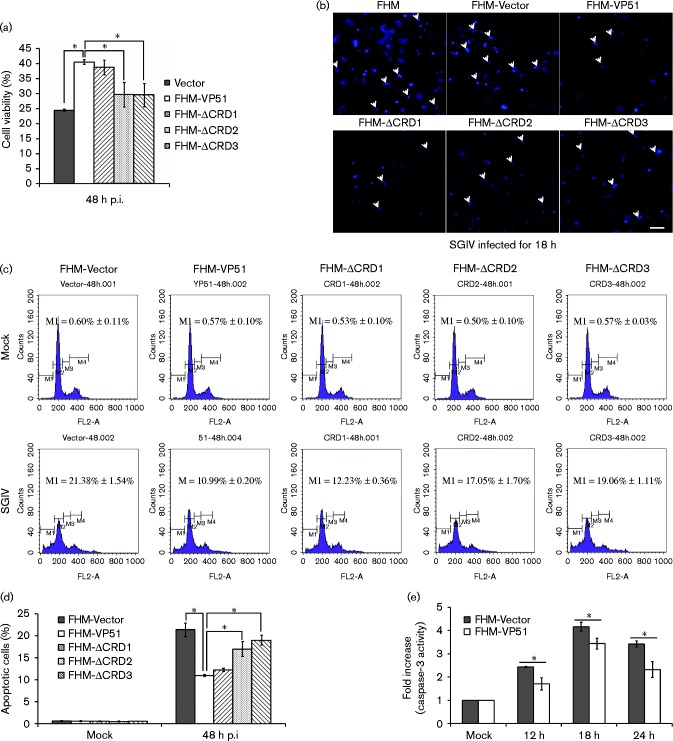
To explore the impact of VP51 on cell fate during SGIV infection, a trypan blue exclusion assay was carried out at 48 h p.i. The percentage of viable cells was calculated and the data are shown in Fig. 5(a). The number of VP51 overexpressing cells was about 1.6-fold higher than that of vector alone cells at 48 h p.i., and about 1.4-fold higher than FHM-ΔCRD2 or FHM-ΔCRD3. However, the FHM-ΔCRD1 group stayed at a similar level to the VP51 overexpressing cells (Fig. 5a). Given that our previous studies showed that SGIV induced apoptosis in FHM cells (Huang et al., 2011a), we stained SGIV infected cells with Hoechst 33342 and observed them under fluorescence microscopy. As shown in Fig. 5(b), after SGIV infection, the number of apoptotic bodies in VP51 and VP51-ΔCRD1 overexpressing cells clearly decreased compared with vector control cells.
Fig. 5.
Overexpression of SGIV VP51 inhibits SGIV-induced apoptosis in FHM cells. (a) Overexpression of VP51 increases cell viability after SGIV infection. Cell viability was evaluated using a trypan blue exclusion assay. n = 3; error bars represent the mean ± sd; *P < 0.05 compared with the FHM-VP51. (b) Nuclear morphology after SGIV infection in WT cells (FHM), vector control cells (FHM-Vector), VP51-expressing cells (FHM-VP51) and the mutant-expressing cells (FHM-ΔCRD1, FHM-ΔCRD2 and FHM-ΔCRD3). Cells were infected with SGIV at an m.o.i. of 2 and stained with Hoechst 33342 at 18 h p.i. Bar, 25 μm. (c) Flow cytometric analysis of apoptosis in FHM-Vector cells, FHM-VP51 cells and the mutant-expressing cells infected with SGIV at 48 h p.i. (d) The percentage of subG0/G1 phase cells was calculated and indicated on the histogram. n = 3; error bars represent the mean ± sd; *P < 0.05 compared with the FHM-VP51. (e) Overexpression of VP51 inhibited caspase-3 activation induced by SGIV in FHM cells. n = 3; error bars represent the mean ± sd; *P < 0.05 compared with the control.
We further quantitatively analysed the effect of VP51 overexpression on SGIV-induced apoptosis using flow cytometry. As shown in Fig. 5(d), the percentage of the cells in SubG1 phase was reduced significantly in VP51 and VP51-ΔCRD1 expressing cells, while the percentage of apoptotic cells in VP51-ΔCRD2 or VP51-ΔCRD3 expressing cells were at a higher level compared with the full-length expressing cell line (Fig. 5d). In addition, caspase-3 activity assay showed that caspase-3 was activated in SGIV-infected control cells at 6 h p.i. and reached a peak at 18 h p.i. In VP51 overexpressing cells, caspase-3 activity was clearly reduced at each time point compared with control cells (Fig. 5e). Together, overexpression of VP51 improved cell viability via inhibition of SGIV-induced apoptosis in FHM cells, and the CRD2 and CRD3 domains were essential for these functions.
Overexpression of SGIV VP51 increased virus replication
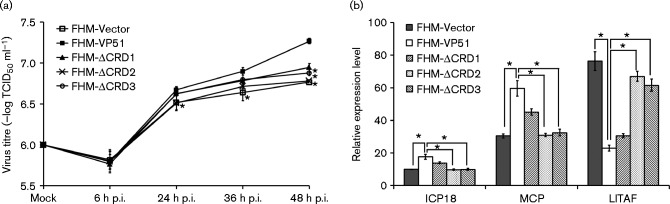
To evaluate the effect of VP51 on virus replication, virus production in VP51 or mutant overexpressing cells was determined at indicated time points. As shown in Fig. 6(a), the virus titre in VP51 overexpressing cells increased from 24 h p.i., up to 3.16-fold at 48 h p.i., compared with control vector cells. Interestingly, compared with full-length VP51, the deletion of CRD2 and CRD3 both significantly decreased the ability of VP51 on virus production. In addition, we also evaluated the effects of VP51 on transcription of different viral genes during SGIV infection using RT-qPCR. As shown in Fig. 6(b), the expression of the cell proliferation promoting gene SGIV ICP-18 and the viral structural gene SGIV MCP were increased significantly in VP51 overexpressing cells compared with the control vector cells. In contrast, the transcription of virus immune regulatory protein gene LITAF, which could induce apoptosis, was significantly decreased in VP51 overexpressing cells. Consistently, the deletion of CRD2 and CRD3 also clearly impaired the regulatory effects on viral gene transcriptions. Together, VP51 was able to regulate viral gene transcription differentially during virus replication.
Fig. 6.
Effect of SGIV VP51 overexpression on virus replication. (a) Virus production of SGIV infected VP51 overexpressing cells. Stable FHM cells infected with SGIV at different time points (0, 6, 24, 36 and 48 h p.i., respectively) were collected, and the viral titres were determined using the TCID50 method. (b) Relative mRNA levels of SGIV ICP-18, MCP and LITAF in different stable FHM lines after SGIV infection at 48 h p.i. were assessed by a qPCR assay. n = 3; error bars represent the mean ± sd; *P < 0.05 compared with FHM cells expressing VP51 (FHM-VP51).
Discussion
Apoptosis is a significant host modulation mechanism that limits viral replication by killing virus-infected cells, while some viruses utilize it to promote cell killing and viral spread (Roulston et al., 1999). Large DNA viruses usually encode certain viral products to suppress or delay apoptosis of infected cells until abundant progeny viruses are produced (Cuconati and White, 2002; Meseda et al., 2000). Increasing reports showed that viral TNFR homologues were capable of affecting the cellular TNF/TNFR pathway by regulating the host apoptotic response (Benedict et al., 2003). For poxviruses, a set of secreted viral TNFRs (vTNFRs) can bind to and inhibit the signalling induced by distinct host TNF super family members (Alejo et al., 2011; Epperson et al., 2012). Moreover, vaccinia virus encoded cytokine response modifier E (CrmE) was demonstrated to protect infected cells from apoptosis induced by TNF-α (Graham et al., 2007).
As large DNA viruses, iridoviruses also encoded one or more TNFR homologues, like poxviruses and herpesviruses (Song et al., 2004; Zhang et al., 2004). In the complete genome of SGIV, three putative TNFR-like genes were annotated, including SGIV VP50, SGIV VP51 and SGIV VP96 (Song et al., 2004). Among them, VP96 was identified as an E gene which was capable of enhancing cell proliferation and inhibiting SGIV-induced cell apoptosis (Huang et al., 2013). Here, the structural features and cellular function of VP51 were investigated. The sequence analysis indicated that VP51 contained three extracellular CRDs and a transmembrane domain, which is different from VP96. Notably, the subcellular localization of VP51 was also different from that of VP96 and other DNA virus-encoded TNFR receptors (Cheung et al., 2005; Poole et al., 2006; Huang et al., 2013), suggesting that VP51 might exert further different functions during SGIV infection. In addition, VP51 and VP96 were classified as an ‘IE’ and ‘E’ gene, respectively, suggesting that they might play different roles at various stages of virus infection. For poxvirus, CrmB is expressed at the early stage of infection and it binds to both mouse and human TNF and lymphotoxin (LT-α), while CrmC is expressed during the late stage and cannot bind to mouse or human LT-α (Saraiva et al., 2002; Smith et al., 1996).
Increasing evidence demonstrates that viruses have developed different strategies to regulate the cell cycle for efficient viral replication (Emmett et al., 2005; Nascimento et al., 2012). Moreover, viruses usually encode certain genes to affect cell proliferation to promote efficient viral replication (Guo et al., 2010). Human cytomegalovirus utilizes the US27 gene product to enhance cell proliferation and alters cellular gene expression during virus infection (Lares et al., 2013). In our previous study, we also found that SGIV-encoded ICP-18, VP96 and IGF all promoted cell proliferation during virus infection (Huang et al., 2013; Xia et al., 2009; Yan et al., 2013). Here, we also found that VP51 could increase cell proliferation and accelerated the G1/S transition (promoting cell cycle progression into S phase). Additionally, all of the three CRDs were proven to be essential for cell proliferation, with CRD1 having the largest impact. Moreover, ectopic expression of VP51 enhanced the transcription of SGIV ICP-18 during SGIV infection. Whether VP51 could regulate the expression of other cell proliferation related genes, originating from virus or cell, still remains unknown. However, we also speculate that the IE gene VP51 is another important cell proliferation-promoting gene for SGIV to complete replication efficiently.
During co-evolution, viruses have evolved multiple anti-apoptotic strategies to prevent or delay host antiviral responses (Gelgor et al., 2015; Pontejo et al., 2015a). Viral homologues of anti-apoptotic Bcl-2 family proteins have been identified in poxviruses, herpesviruses, adenoviruses and iridovirus (Lin et al., 2008; Ring et al., 2013). Moreover, they were demonstrated to protect the infected cells from apoptosis (Gelgor et al., 2015; Okamoto et al., 2012). Viruses also encode cellular TNFR homologues to prevent the virus infected cells from apoptotic cell death (Huang et al., 2013; Pontejo et al., 2015b; Saraiva & Alcami, 2001). In our study, we found that VP51 overexpression inhibited SGIV infection induced apoptosis, evidenced by reduced apoptotic bodies and decreased caspase-3 activity. Additionally, CRD2 and CRD3 played significant roles in reducing cell apoptosis induced by SGIV infection. Notably, our results also indicated that the ectopic expression significantly decreased the transcription of SGIV LITAF which was capable of inducing apoptosis in fish cells (Huang et al., 2008). The effect of VP51 on cell proliferation and apoptosis ultimately contributed to its impact on virus replication. Moreover, the three typical CRDs were all essential for the action of VP51 on virus production. Together, we speculate that VP51 might be exploited by SGIV to regulate host cell proliferation and cell apoptotic fate for more progeny production.
In summary, a novel vTNFR-like gene, VP51, encoded by SGIV, was identified in this study. VP51 was identified as an IE gene and encoded a cytoplasmic protein. The ectopic expression of VP51 was capable of affecting cell cycle progression and enhancing cell proliferation. Moreover, overexpression of VP51 inhibited virus-induced apoptosis via decreasing the expression of a viral apoptosis inducing gene, and finally enhanced the virus replication. The three typical CRD domains were all essential for the action of VP51 on virus replication, but CRD2 exerts more crucial roles. Our present study provided new insights into the function of vTNFR homologues encoded by iridovirus, and contributed greatly to better understanding of iridovirus pathogenesis.
Methods
Cells and virus
Grouper spleen (GS) cells from red-spotted grouper and FHM cells were cultured in Leibovitz's L-15 and M199 medium containing 10 % FBS at 25 °C (Huang et al., 2009). SGIV was propagated in GS cells, and the isolation of SGIV genomic DNA was performed as described previously (Qin et al., 2003).
Computer-assisted analysis and plasmid construction
Homology analyses of SGIV ORF051 were conducted using the Basic Local Alignment Tool Search Tool (blast) program from the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast). Conserved domains and motifs were predicted using the Simple Modular Architecture Research Tool (smart) search program (http://smart.embl-heidelberg.de/). Multiple sequence alignment of SGIV ORF051 amino acid sequences was performed using ClustalX version 2.1 (http://www.clustal.org/clustal2/) and edited with the GeneDoc 2.6 program.
The amplification of the full-length SGIV ORF051L gene, as well as a transmembrane domain deletion, was performed by PCR from SGIV genomic DNA using two pairs of primers: P1/P2 and P1/P3 (Table 1), respectively. The fragments were cloned into the eukaryotic expression vector pEGFP-N3 to yield the recombinant plasmids pEGFP-VP51 and pEGFP-ΔTm51. Primers P1/P5/P6/P2, P1/P8/P9/P2 and P1/P10/P11/P2 were used to amplify CRD mutant fragments by the overlapping PCR method, and the fragments were cloned into pEGFP-N3. To detect the function of VP51 and the roles of its CRDs in vitro, different sets of primers, P4/P5/P6/P7, P4/P8/P9/P7 and P4/P10/P11/P7, were used to clone the gene into the pcDNA3.1 vector. The overlapping PCR method was used to amplify the pcDNA3.1-ΔCRD1, pcDNA3.1-ΔCRD2 and pcDNA3.1-ΔCRD3 mutants, which were confirmed by DNA sequencing.
Table 1. Sequences of primers used in this study.
| Name | Sequence (5′–3′) |
|---|---|
| VP51-P1 | GGAAGCTTCGATGCTGTTTGTAGTGCTCGTCTTG |
| VP51-P2 | CGGGGTACCCCGATCCATTTTGCGGTTCTGTTG |
| VP51-P3 | CGGGGTACCCCGGTTCGCTGGTTCCGTTCT |
| VP51-P4 | CCAAGCTTGGATGCTGTTTGTAGTGCTCGTCTTG |
| VP51-P5 | GTAAAAACCTCTTTCACATTTTTCCTCTTCCATATAGAAACCGTTG |
| VP51-P6 | CTCTTCCATATAGAAACCGTTGGAAAAATGTGAAAGAGGTT |
| VP51-P7 | CCGCTCGAGATCCATTTTGCGGTTCTGTTG |
| VP51-P8 | GTAATAGCCGTCCTTTGGTTTTTCGCATTTGGTTGTTCTGTC |
| VP51-P9 | GATGACAGAACAACCAAATGCGAAAAACCAAAGGACGGCTATTAC |
| VP51-P10 | CCATTGAAAGGGTGGGTTTCCAATTATTGGTTTGGCGTAC |
| VP51-P11 | GTACGTACGCCAAACCAATAATTGGAAACCCACCCTTTCAATG |
| VP51-P12 | ATGCTGTTTGTAGTGCTCGTCTTG |
| VP51-P13 | AGTGTTCGCTGGTTCCGTTC |
| VP51-P14 | ATCCATTTTGCGGTTCTGTTG |
| MCP-PF-P15 | ACTCGTAAGATCGCCACGGAAGATT |
| MCP-PR-P16 | ACGTTTCTCAAATGCATGTCTGCCAC |
| ICP18-PF-P17 | ATCGGATCTACGTGGTTGG |
| ICP18-PR-P18 | CCGTCGTCGGTGTCTATTC |
| LITAF-PF-P19 | GATGCTGCCGTGTGAACTG |
| LITAF-PR-P20 | GCACATCCTTGGTGGTGTTG |
| FHM-Actin-PF-P21 | TCTTCCAGCCATCCTTCCTTGG |
| FHM-Actin-PR-P22 | CTGCATACGGTCAGCAATGCC |
| GS-Actin-PF-P23 | TACGAGCTGCCTGACGGACA |
| GS-Actin-PR-P24 | GGCTGTGATCTCCTTCTGCA |
Temporal transcription analysis and drug inhibition assays
To determine the SGIV VP51 transcriptional profile during SGIV infection in vitro, cells were seeded in 24-well plates, and total RNA was extracted from mock- and virus-infected GS cells at 2, 4, 6, 10, 16, 24, 36 and 48 h p.i. using an SV Total RNA Isolation kit (Promega), followed by reverse transcription reactions using a ReverTra Ace qPCR RT kit (TOYOBO) (Huang et al., 2011a, b). PCR amplification of VP51 was conducted using a pair of gene-specific primers P12/P13, and β-actin mRNA was amplified as an internal control using the primers P23/P24 (Table 1).
To determine the temporal kinetic class of the VP51 transcript during SGIV infection, cyclohexane (CHX) and arabinoside cytosine (AraC) were used in drug inhibition assays. After culturing for 18 h, GS cells were pretreated with 50 μg CHX ml− 1 or 100 μg AraC ml− 1 for 1 h before SGIV infection. Cells were harvested at 6 and 48 h p.i., and total RNA was extracted for RT-PCR. The previously characterized SGIV genes SGIV ICP-18 (P17/P18) and MCP (P15/P16) were used as controls.
Cell transfection and establishment of stable cell lines
To determine the roles of VP51 in vitro, FHM cells were transfected with pcDNA3.1, pcDNA3.1-VP51, pcDNA3.1-ΔCRD1, pcDNA3.1-ΔCRD2 or pcDNA3.1-ΔCRD3. The transfected cells were cultured in complete medium containing 400 μg ml− 1 G418 (Sigma-Aldrich), selected for 6 weeks, and the stable transfectants were confirmed by RT-PCR using primers P12/P14 (Table 1).
Fluorescence microscopy
FHM cells were seeded in 24-well plates for 18 h, and then transfected with pEGFP-N3, pEGFP-VP51, pEGFP-ΔTm51, pEGFP-ΔCRD1, pEGFP-ΔCRD2 or pEGFP-ΔCRD3 using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. After 24 h, the cells were fixed with 4 % paraformaldehyde for 1 h at 4 °C, and then stained with DAPI (Sigma-Aldrich) for 5 min, and finally observed under a fluorescence microscope (Leica).
Cell proliferation and cell viability
To examine the impact of VP51 on cell proliferation, FHM cells stably expressing VP51, the empty vector or the VP51 mutants were cultured into 96-well plates at an initial density of 5 × 103 cells per well at 25 °C. A WST-1 assay was carried out daily for 6 days. In brief, after adding 10 μl of cell proliferation reagent WST-1 (Roche) into each well and incubation at 37 °C for 4 h, the absorbance was measured in a multi-label plate reader (PerkinElmer Life and Analytical Science) at 450/655 nm.
To investigate the effect of VP51 on cell viability during SGIV infection in vitro, FHM cells stably expressing the empty vector or VP51 as well as its mutants were seeded in 24-well plates for 18 h and then infected with SGIV at an m.o.i. of 2 for 48 h. The SGIV-infected cells were harvested at the indicated time point by trypsinization and stained with 0.2 % trypan blue. Cell viability was presented as the proportion of viable cells among the total number of cells, which were counted by three independent haemocytometer counts each time.
The WT FHM cells, as well as the transfected cells, were infected with SGIV for 18 h. After staining with Hoechst 33342, the apoptotic bodies were observed under a fluorescence microscope (Leica).
Viral replication kinetics assay
A virus titre assay was conducted to determine the effect of VP51 on SGIV infection. The stable cell lines were seeded in 24-well plates and infected with SGIV at an m.o.i. of approximately 0.1. The virus-infected cells were harvested at 0, 6, 24, 36 and 48 h p.i., lysed by three freeze–thaw cycles, and viral titres were determined using the 50 % TCID50 assay (Reed & Muench, 1938). The cytopathic effect was observed daily under a light microscope (Leica).
A real-time qPCR assay was performed to examine the expression profiles of SGIV MCP (P15/P16), SGIV ICP-18 (P17/P18) and SGIV LITAF (P19/P20) in FHM cells stably expressing the empty vector, VP51 or its mutants after SGIV infection using β-actin (P21/P22) as a reference gene.
Flow cytometric analysis
To test the impact of VP51 on the cell cycle, the FHM cells overexpressing VP51 or the empty vector, as well as mock-transfected cells, were seeded in six-well plates. The cells were harvested after culturing for 36 h and fixed in 70 % ice-cold ethanol overnight at − 20 °C. After washing with PBS, the cells were centrifuged at 500 g, and stained in PBS containing propidium iodide (PI, 50 μg ml− 1, Sigma-Aldrich) and RNase A (100 μg ml− 1, Sigma-Aldrich). The PI fluorescence was measured with a FACScan flow cytometer (Becton Dickinson); 1 × 104 cells were analysed for each sample and the percentage of apoptotic cells was determined. All samples were examined in triplicate, and the data were represented as means ± sd. The cell cycle analysis of FHM cells was conducted by flow cytometry as previously described (Huang et al., 2011a, b; Yu et al., 1993).
To detect the effect of VP51 and the CRDs on SGIV-induced apoptosis, FHM cells transfected with the empty vector or cells stably expressing VP51 as well as its mutants were seeded in six-well plates and infected with SGIV. The cytometric analysis was conducted as described previously.
Caspase-3 activity analysis
Caspase-3 activity was tested using a Fluorometric Protease Assay kit (BioVision) according to the manufacturer's instructions. Briefly, VP51-expressing FHM cells or FHM cells transfected with the empty vector were seeded in 24-well plates for 18 h and inoculated with SGIV. Cells were harvested by trypsinization at the indicated time points (0, 12, 18 and 24 h p.i.). The reactions were measured on a multi-label plate reader (PerkinElmer Life and Analytical Science) at 405 nm. Each sample was analysed in triplicate, and the relative caspase-3 activity was described as the ratio between the absorbance of p-nitroaniline (pNA) from the treated sample and that from mock-infected cells.
Statistical analysis
Results were expressed as means ± sd. Statistical comparisons were conducted using the Student's t-test, and a P value of less than 0.05 was considered to be statistically significant.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31330082, 30700616, 31372566), the National Basic Research Program of China (973) (2012CB114402; 2012CB114406) and the National High Technology Development Program of China (863) (2014AA093507). We would like to thank Mr J. L. Zhang from the Scientific Equipment Service Center of South China Sea Institute of Oceanology for flow cytometry technical assistance.
References
- Aggarwal B. B. (2003). Signalling pathways of the TNF superfamily: a double-edged sword Nat Rev Immunol 3745–756, 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Alejo A., Pontejo S. M., Alcami A. (2011). Poxviral TNFRs: properties and role in viral pathogenesis Adv Exp Med Biol 691203–210 10.1007/978-1-4419-6612-4_21. [DOI] [PubMed] [Google Scholar]
- Benedict C. A., Banks T. A., Ware C. F. (2003). Death and survival: viral regulation of TNF signaling pathways Curr Opin Immunol 1559–65, 10.1016/S0952-7915(02)00018-3. [DOI] [PubMed] [Google Scholar]
- Cheung T. C., Humphreys I. R., Potter K. G., Norris P. S., Shumway H. M., Tran B. R., Patterson G., Jean-Jacques R., Yoon M., other authors (2005). Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway Proc Natl Acad Sci U S A 10213218–13223, 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A., White E. (2002). Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection Genes Dev 162465–2478, 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Emmett S. R., Dove B., Mahoney L., Wurm T., Hiscox J. A. (2005). The cell cycle and virus infection Methods Mol Biol 296197–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson M. L., Lee C. A., Fremont D. H. (2012). Subversion of cytokine networks by virally encoded decoy receptors Immunol Rev 250199–215, 10.1111/imr.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelgor A., Kalt I., Bergson S., Brulois K. F., Jung J. U., Sarid R. (2015). Viral Bcl-2 encoded by the Kaposi's sarcoma-associated herpesvirus is vital for virus reactivation J Virol 895298–5307, 10.1128/JVI.00098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. C., Bahar M. W., Abrescia N. G., Smith G. L., Stuart D. I., Grimes J. M. (2007). Structure of CrmE, a virus-encoded tumour necrosis factor receptor J Mol Biol 372660–671, 10.1016/j.jmb.2007.06.082. [DOI] [PubMed] [Google Scholar]
- Gray M. J., Miller D. L., Hoverman J. T. (2009). Ecology and pathology of amphibian ranaviruses Dis Aquat Organ 87243–266, 10.3354/dao02138. [DOI] [PubMed] [Google Scholar]
- Guo Q., Qian L., Guo L., Shi M., Chen C., Lv X., Yu M., Hu M., Jiang G., Guo N. (2010). Transactivators Zta and Rta of Epstein-Barr virus promote G0/G1 to S transition in Raji cells: a novel relationship between lytic virus and cell cycle Mol Immunol 471783–1792, 10.1016/j.molimm.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Hong J. R., Gong H. Y., Wu J. L. (2002). IPNV VP5, a novel anti-apoptosis gene of the Bcl-2 family, regulates Mcl-1 and viral protein expression Virology 295217–229, 10.1006/viro.2001.1336. [DOI] [PubMed] [Google Scholar]
- Huang X., Huang Y., Gong J., Yan Y., Qin Q. (2008). Identification and characterization of a putative lipopolysaccharide-induced TNF-alpha factor (LITAF) homolog from Singapore grouper iridovirus Biochem Biophys Res Commun 373140–145, 10.1016/j.bbrc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Huang X. H., Huang Y. H., Sun J. J., Han X., Qin Q. W. (2009). Characterization of two grouper Epinephelus akaara cell lines: application to studies of Singapore grouper iridovirus (SGIV) propagation and virus–host interaction Aquaculture 292172–179, 10.1016/j.aquaculture.2009.04.019. [DOI] [Google Scholar]
- Huang X., Huang Y., Ouyang Z., Xu L., Yan Y., Cui H., Han X., Qin Q. (2011a). Singapore grouper iridovirus, a large DNA virus, induces nonapoptotic cell death by a cell type dependent fashion and evokes ERK signaling Apoptosis 16831–845, 10.1007/s10495-011-0616-y. [DOI] [PubMed] [Google Scholar]
- Huang Y., Huang X., Cai J., Ye F., Qin Q. (2011b). Involvement of the mitogen-activated protein kinase pathway in soft-shelled turtle iridovirus-induced apoptosis Apoptosis 16581–593, 10.1007/s10495-011-0595-z. [DOI] [PubMed] [Google Scholar]
- Huang X., Huang Y., Cai J., Wei S., Gao R., Qin Q. (2013). Identification and characterization of a tumor necrosis factor receptor like protein encoded by Singapore grouper iridovirus Virus Res 178340–348, 10.1016/j.virusres.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Lares A. P., Tu C. C., Spencer J. V. (2013). The human cytomegalovirus US27 gene product enhances cell proliferation and alters cellular gene expression Virus Res 176312–320, 10.1016/j.virusres.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. W., Huang Y. J., John J. A., Chang Y. N., Yuan C. H., Chen W. Y., Yeh C. H., Shen S. T., Lin F. P., other authors (2008). Iridovirus Bcl-2 protein inhibits apoptosis in the early stage of viral infection Apoptosis 13165–176, 10.1007/s10495-007-0152-y. [DOI] [PubMed] [Google Scholar]
- Meseda C. A., Arrand J. R., Mackett M. (2000). Herpesvirus papio encodes a functional homologue of the Epstein-Barr virus apoptosis suppressor, BHRF1 J Gen Virol 811801–1805, 10.1099/0022-1317-81-7-1801. [DOI] [PubMed] [Google Scholar]
- Nascimento R., Costa H., Parkhouse R. M. (2012). Virus manipulation of cell cycle Protoplasma 249519–528, 10.1007/s00709-011-0327-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Campbell S., Mehta N., Thibault J., Colman P. M., Barry M., Huang D. C., Kvansakul M. (2012). Sheeppox virus SPPV14 encodes a Bcl-2-like cell death inhibitor that counters a distinct set of mammalian proapoptotic proteins J Virol 8611501–11511, 10.1128/JVI.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontejo S. M., Alejo A., Alcami A. (2015a). Comparative biochemical and functional analysis of viral and human secreted tumor necrosis factor (TNF) decoy receptors J Biol Chem 29015973–15984, 10.1074/jbc.M115.650119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontejo S. M., Alejo A., Alcami A. (2015b). Poxvirus-encoded TNF decoy receptors inhibit the biological activity of transmembrane TNF J Gen Virol 963118–3123, 10.1099/jgv.0.000255. [DOI] [PubMed] [Google Scholar]
- Pontejo S. M., Sánchez C., Martín R., Mulero V., Alcami A., Alejo A. (2013). An orphan viral TNF receptor superfamily member identified in lymphocystis disease virus Virology Journal 10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole E., King C. A., Sinclair J. H., Alcami A. (2006). The UL144 gene product of human cytomegalovirus activates NFκB via a TRAF6-dependent mechanism EMBO J 254390–4399, 10.1038/sj.emboj.7601287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q. W., Lam T. J., Sin Y. M., Shen H., Chang S. F., Ngoh G. H., Chen C. L. (2001). Electron microscopic observations of a marine fish iridovirus isolated from brown-spotted grouper Epinephelus tauvina. J Virol Methods 9817–24, 10.1016/S0166-0934(01)00350-0. [DOI] [PubMed] [Google Scholar]
- Qin Q. W., Chang S. F., Ngoh-Lim G. H., Gibson-Kueh S., Shi C., Lam T. J. (2003). Characterization of a novel ranavirus isolated from grouper Epinephelus tauvina Dis Aquat Organ 531–9, 10.3354/dao053001. [DOI] [PubMed] [Google Scholar]
- Rahman M. M., McFadden G. (2006). Modulation of tumor necrosis factor by microbial pathogens PLoS Pathog 2e4. 10.1371/journal.ppat.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J., Muench H. (1938). A simple method of estimating 50 % endpoints Am J Epidemiol 27493–497. [Google Scholar]
- Ring B. A., Ferreira Lacerda A., Drummond D. J., Wangen C., Eaton H. E., Brunetti C. R. (2013). Frog virus 3 open reading frame 97R localizes to the endoplasmic reticulum and induces nuclear invaginations J Virol 879199–9207, 10.1128/JVI.00637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulston A., Marcellus R. C., Branton P. E. (1999). Viruses and apoptosis Annu Rev Microbiol 53577–628, 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- Saraiva M., Alcami A. (2001). CrmE, a novel soluble tumor necrosis factor receptor encoded by poxviruses J Virol 75226–233, 10.1128/JVI.75.1.226-233.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M., Smith P., Fallon P. G., Alcami A. (2002). Inhibition of type 1 cytokine-mediated inflammation by a soluble CD30 homologue encoded by ectromelia (mousepox) virus J Exp Med 196829–839, 10.1084/jem.20020319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedger L. M., Osvath S. R., Xu X. M., Li G., Chan F. K., Barrett J. W., McFadden G. (2006). Poxvirus tumor necrosis factor receptor (TNFR)-like T2 proteins contain a conserved preligand assembly domain that inhibits cellular TNFR1-induced cell death J Virol 809300–9309, 10.1128/JVI.02449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Hu F. Q., Smith T. D., Richards C. L., Smolak P., Goodwin R. G., Pickup D. J. (1996). Cowpox virus genome encodes a second soluble homologue of cellular TNF receptors, distinct from CrmB, that binds TNF but not LT alpha Virology 223132–147, 10.1006/viro.1996.0462. [DOI] [PubMed] [Google Scholar]
- Song W. J., Qin Q. W., Qiu J., Huang C. H., Wang F., Hew C. L. (2004). Functional genomics analysis of Singapore grouper iridovirus: complete sequence determination and proteomic analysis J Virol 7812576–12590, 10.1128/JVI.78.22.12576-12590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L., Cao J., Huang X., Qin Q. (2009). Characterization of Singapore grouper iridovirus (SGIV) ORF086R, a putative homolog of ICP18 involved in cell growth control and virus replication Arch Virol 1541409–1416, 10.1007/s00705-009-0457-y. [DOI] [PubMed] [Google Scholar]
- Yan Y., Cui H., Guo C., Li J., Huang X., Wei J., Qin Q. (2013). An insulin-like growth factor homologue of Singapore grouper iridovirus modulates cell proliferation, apoptosis and enhances viral replication J Gen Virol 942759–2770, 10.1099/vir.0.056135-0. [DOI] [PubMed] [Google Scholar]
- Yu H., Bauer B., Lipke G. K., Phillips R. L., Van Zant G. (1993). Apoptosis and hematopoiesis in murine fetal liver Blood 81373–384. [PubMed] [Google Scholar]
- Zhang Q., Gui J. F. (2015). Virus genomes and virus–host interactions in aquaculture animals Sci China Life Sci 58156–169, 10.1007/s11427-015-4802-y. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Y., Xiao F., Xie J., Li Z. Q., Gui J. F. (2004). Complete genome sequence of lymphocystis disease virus isolated from China J Virol 786982–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]