Highlights
-
•
We numerically investigated the physiological relationship between the severity of regurgitation and the effect of a left ventricular assist device (LVAD) on cardiovascular system responses.
-
•
Under conditions of mitral regurgitation, the effects of both pulsatile and continuous LVAD treatment on ventricular unloading were significant.
-
•
Under conditions of aortic regurgitation (AR), the effects of the LVADs on ventricular unloading were not significant. The effects of LVAD treatment decreased according to the severity of AR.
Keywords: aortic regurgitation, left ventricular assist device, mitral regurgitation, regurgitation severity, Windkessel model
Abstract
Background
A left ventricular assist device (LVAD) is normally contraindicated in significant aortic regurgitation (AR) and requires intraoperative valve repair or exclusion. Nevertheless, AR can coexist with an LVAD, so a valid question when asked might still be of clinical significance. The purpose of this study is to analyze the effects of valve regurgitation on the pumping efficacy of continuous and pulsatile LVADs with a computational method.
Methods
A cardiovascular model was developed based on the Windkessel model, which reflects the hemodynamic flow resistance and the blood wall elasticity. Using the Windkessel model, important cardiovascular components, such as the right atrium, right ventricle, pulmonary artery, pulmonary vein, left atrium (LA), left ventricle (LV), aorta, and branching blood vessels, were expressed.
Results
In the case of AR, continuous and pulsatile LVADs improved cardiac output and reduced mechanical load slightly. In the case of mitral regurgitation, the LVADs improved cardiac output (cardiac outputs were about 5 L/min regardless of the severity of regurgitation) and reduced afterload significantly.
Conclusion
AR reduced both continuous and pulsatile LVAD function significantly while mitral regurgitation did not affect their pumping efficacy.
1. Introduction
A left ventricular assist device (LVAD) is an electromechanical device used to replace part of the function of a failed heart, by helping to circulate blood through the body. Recently, LVADs have been used as a bridge to transplantation1 or destination therapy.2, 3 Valve regurgitation, i.e., backward flow in the heart when a cardiac valve does not completely close, has been found to have a significant effect on cardiac function.4 Heart valves are located between the atria and the ventricles (the mitral and tricuspid valves), and between the ventricles and the aortas (the aortic and pulmonary aortic valves). Cardiac responses, such as cardiac output and blood pressure, vary according to which valve is affected and the severity of regurgitation. Valve regurgitation also affects the LVAD pumping efficacy differently following LVAD therapy.5, 6 LVAD is normally contraindicated in significant aortic regurgitation (AR) and requires intraoperative valve repair or exclusion.7, 8 Furthermore, LVAD aggravates preexisting AR.9, 10 Nevertheless, AR can coexist with LVAD,11 so a valid question when asked might still be of clinical significance.
While much research has focused on determining regurgitation volume using medical imaging techniques, and also predicting the effects of regurgitant volume on ventricular mechanical function,12, 13 no study has focused on predicting the effects of regurgitation severity in specific valvular regurgitation on LVAD function in the failed ventricle following LVAD therapy.
The pumping efficacy of LVADs according to the severity of valvular insufficiency can be investigated in animal and clinical studies. However, experimental methods to document and evaluate cardiac responses in detail, such as the ventricular unloading effect of LVAD, are hampered by low spatiotemporal resolution. As an alternative, computational methods can be applied. We have performed several studies comparing ventricular unloading effects under different pumping types14, 15 and cannulation sites16 using computational methods.
We used a previously developed computational model of the cardiovascular system to analyze the effects of aortic and mitral valve regurgitation on the pumping efficacy of continuous and pulsatile LVADs.
2. Methods
2.1. Cardiovascular model
As shown in Fig. 1, the cardiovascular model was developed based on the Windkessel model, which reflects the hemodynamic flow resistance and the blood wall elasticity.17 Dynamic parameters, such as flow resistance and blood wall elasticity, were expressed as the electric resistance and the electric compliance components, which are linear electric circuit elements. Using the Windkessel model, important cardiovascular components, such as the right atrium, right ventricle, pulmonary artery, pulmonary vein, left atrium (LA), left ventricle (LV), aorta, and branching blood vessels, were expressed.
Fig. 1.
Schematic drawing of the cardiovascular system model that consists of eight compartments. See Lim et al15 for the detailed parameter information.
SF, scale factor for the leakage resistance.
The heart spontaneously contracts and relaxes repeatedly through electrical signals, action potentials, to change its elasticity, which is a dynamic material property. Considering these features, the time-varying compliance suggested by Suga and Sagawa17 was used in this study, unlike other linear capacitors (Fig. 2). The LVAD was assumed to be a flow generator that continuously ejects blood, and was connected in parallel to the LV and the aorta, as shown in Fig. 1. The cardiovascular model is composed of eight Windkessel compartments, each of which is expressed as Eq. (1), Eq. (2), and Eq. (3) based on Poiseuille's law, mass balance law, and Hooke's law, respectively.
| (1) |
| (2) |
| (3) |
Fig. 2.
Time-varying compliance of the right and left ventricles.
HF, heart failure; NORM, normal condition.
Here, i represents the compartment index, V the blood volume, Qin the blood inflow rate, Qout the blood flow rate, P the internal blood vessel pressure, Pex the external blood vessel pressure, C the blood vessel or heart wall compliance, and Vun the volume when the pressure in the blood vessels differs from that outside.
2.2. Heart failure and valvular insufficiency condition
To mimic spontaneous pumping of the heart, time-varying compliance was used for the model in this study. This is an equation for the internal pressure and volume of the pulsating heart. Several previous cardiovascular hemodynamic studies simulated the heart using this time-varying compliance concept.15, 18, 19 Fig. 2 shows the ventricular time-varying compliance of a normal individual and a patient with suggested heart failure. A heart failure model was implemented by increasing the end-systolic compliance value to 2.5× normal. This produced hemodynamic results that were consistent with data reported for systolic heart failure patients in previous clinical studies.14, 20
To simulate the valve regurgitation caused by mitral and aortic valve insufficiency, one further route was added to the aortic and mitral valve compartment for regurgitation (see forward diodes and back diodes next to RPV and RLV in Fig. 1). There were forward and reverse diodes, which had different resistance components. The hemodynamic phenomena with the mitral and aortic valve insufficiency are expressed as follows:
| (4) |
| (5) |
Here, QMI represents the left mitral valve inflow rate, PLA the LA pressure, PLV the LV pressure, RMI the forward flow resistance through the mitral valve, RMI.Leak the leakage flow resistance through the mitral valve, QAO the forward flow through the aortic valve, PAO the aortic blood pressure, RAO the forward flow resistance through the aortic valve, and RAO.Leak the leakage flow resistance through the aortic valve. SF is a scale factor that expresses the severity of regurgitation through the valve. SF was increased from 0% to 10% in 2% increments. SF = 0% indicates that the valve is completely closed when PLV > PLA, or when PLV < PAO, and SF = 10% indicates that the valve is not completely closed, so ∼10% of the flow, which is estimated by Poiseuille's law with a given pressure difference, is regurgitated. According to our previous research,21 regurgitant volumes were saturated from SF 10%. That is why we set the range of the SF value from 0% to 10%.
2.3. Simulation method
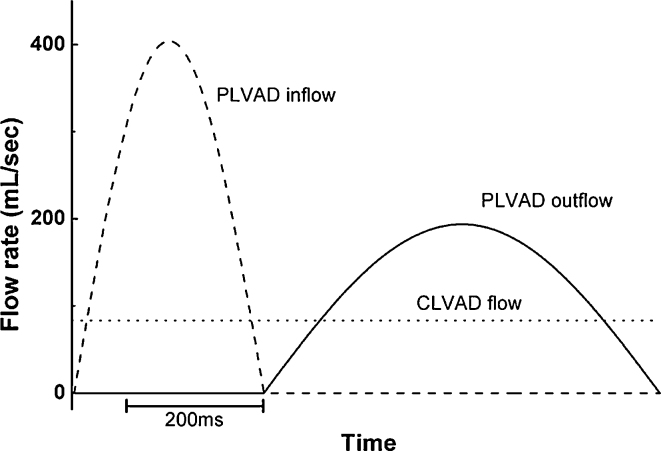
Simulations were conducted for the case without valvular insufficiency and the five different mitral valvular and aortic valvular insufficiency conditions with use of the continuous LVAD (CLVAD) and the pulsatile LVAD (PLVAD). To mimic the universal LVAD flow, the mean flow rate (5 L/min) was set for the CLVAD, and the cosine wave function that generated a mean flow rate identical to that of the CLVAD was used for the PLVAD. Fig. 3 shows the rates of inlet flow to the LVAD and the outlet flow from the LVAD. In the PLVAD, the counter-pulsation method4, 5, which was shown to be the optimal pulsation condition for improving the ventricular afterload, decreased and the coronary circulation was used. The LVAD intubation was connected to the aorta in parallel to the LV. The regurgitation levels, indicated as SF in Eqs. (4), (5), in the aortic and mitral valves, were set at 0%, 2%, 4%, 6%, 8%, and 10%, and simulations were conducted using the two types of LVAD according to the regurgitation level.
Fig. 3.
Left ventricular assist device (LVAD) flow rate for one pumping cycle.
Dashed line, pulsatile LVAD (PLVAD) inflow; dotted line, continuous LVAD (CLVAD) inflow and outflow; solid line, PLVAD outflow.
3. Results
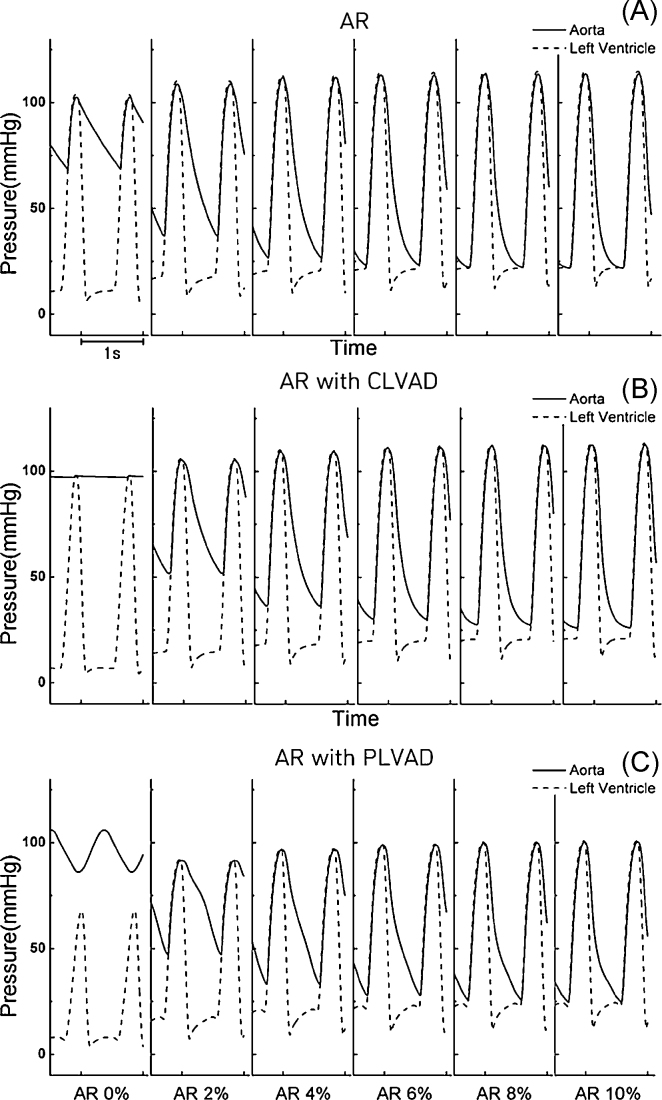
Fig. 4 shows the LV and aortic blood pressures in the case of aortic valvular insufficiency without LVAD treatment (Fig. 4A) and with CLVAD (Fig. 4B) and PLVAD (Fig. 4C) treatment. Without LVAD treatment, more severe aortic valvular regurgitation resulted in increases in ventricular and aortic systolic blood pressures, and a decrease in the aortic diastolic pressure. Without LVAD treatment, when aortic valvular insufficiency developed, the amount of blood from the LA to the LV was normal, but the blood ejected to the aorta flowed back to the LV. Consequently, the systolic LV and aortic blood pressures increased with increases in severity of regurgitation, the mean arterial blood pressure decreased (48 mmHg under 10% AR condition), and the difference between the mean LV blood pressure and the mean aortic blood pressure decreased (Fig. 4A). As the LVAD helped the LV pump blood, the mean arterial blood pressure remained higher than without LVAD treatment, and the LV systolic blood pressure decreased (ventricular pressure unloading). In Fig. 4B and 4C, the mean arterial blood pressure decreased with increasing severity of regurgitation in the case of aortic valvular insufficiency with CLVAD or PLVAD treatments. The aortic blood pressure was slightly lower than that in the absence of LVAD treatment (Fig. 4B and 4C). Accordingly, the increase in arterial blood pressure, which is a major function of LVAD, was inhibited (Fig. 4A–C).
Fig. 4.
Left ventricular and aortic pressure profiles under the aortic valve regurgitation. (A) Without left ventricular assist device (LVAD) treatment. (B) With continuous LVAD (CLVAD) treatment. (C) With pulsatile LVAD (PLVAD) treatment.
AR, aortic regurgitation.
Fig. 5 shows the LV and aortic blood pressures in the case of mitral valvular insufficiency without LVAD treatment (Fig. 5A) and with CLVAD (Fig. 5B) or PLVAD (Fig. 5C) treatment. The mitral valve regurgitation resulted in decreases in LV and aortic systolic blood pressures with increases in severity of regurgitation (72 mmHg of mean arterial pressure under 10% mitral regurgitation (MR) severity). In the case of mitral valvular insufficiency without LVAD treatment, the blood from the LA to the LV flowed back to the LA. With more severe regurgitation, the flow of blood ejected to the aorta decreased, which also decreased the aortic blood pressure (Fig. 5A). With CLVAD and PLVAD treatments, the arterial mean blood pressure remained high regardless of the severity of regurgitation (Fig. 5B and 5C). Mean arterial pressure was 98 mmHg with CLVAD treatment and 97 mmHg with PLVAD treatment. Accordingly, it was confirmed that the arterial blood pressure increased and the ventricular load decreased with LVAD treatment (Fig. 5A–C). With PLVAD treatment, the ventricular systolic blood pressure decreased with more severe valvular regurgitation, and became lower than that with CLVAD treatment (Fig. 5C). LV peak pressure (LVPP) was 48 mmHg with CLVAD and 30 mmHg with PLVAD. Accordingly, PLVAD treatment was more effective than CLVAD treatment under conditions of mitral regurgitation in terms of the ventricular pressure unloading effect.
Fig. 5.
Left ventricular and aortic pressure profiles under the mitral valve regurgitation. (A) Without left ventricular assist device (LVAD) treatment. (B) With continuous LVAD (CLVAD) treatment. (C) With pulsatile LVAD (PLVAD) treatment.
MR, mitral regurgitation.
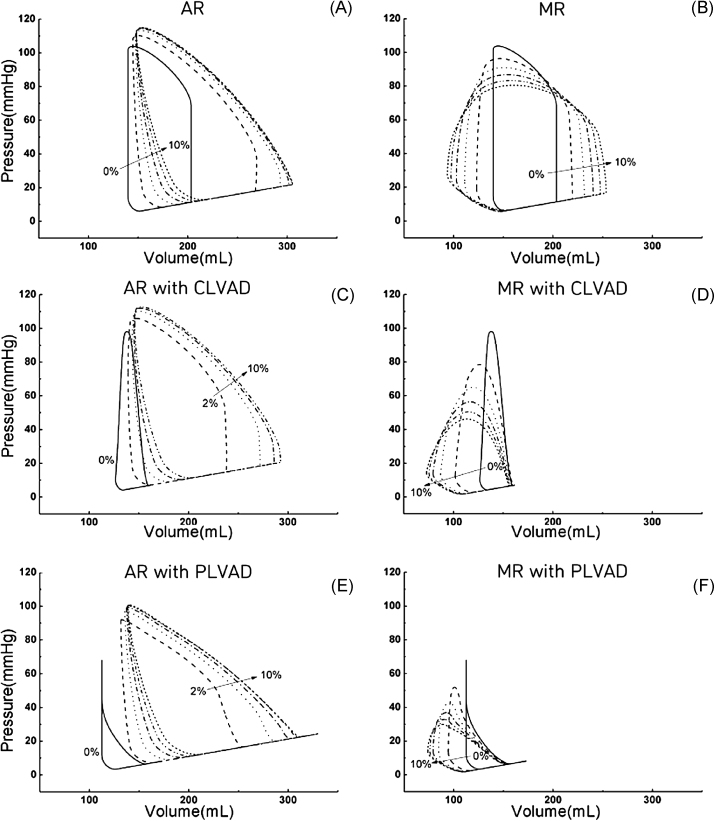
Fig. 6 shows the LV pressure-volume graph according to the severity of aortic valvular insufficiency and mitral valvular insufficiency under conditions of CLVAD and PLVAD treatment. Without LVAD treatment, the end-diastolic volume (EDV) and the end-systolic volume (ESV) increased with more severe aortic valvular regurgitation, so the graph tends to lean toward the right (Fig. 6A). In mitral valve regurgitation, the EDV increased but the ESV decreased with more severe valvular regurgitation, so the graph tended to spread to the right and left (Fig. 6B). When CLVAD treatment was performed in the case of aortic valvular insufficiency, the EDV and ESV increased with more severe regurgitation (Fig. 6C). In the case of mitral valvular insufficiency, the EDV was unaffected by valvular insufficiency, but the ESV tended to decrease with more severe valvular insufficiency (Fig. 6D).
Fig. 6.
Pressure-volume loops under the aortic and mitral valve regurgitation conditions (A and B) without left ventricular assist device (LVAD) treatment, (C and D) with continuous LVAD (CLVAD) treatment, and (E and F) with pulsatile LVAD (PLVAD) treatment.
AR, aortic regurgitation; MR, mitral regurgitation.
With PLVAD treatment, the levels fluctuated in a manner similar to those with CLVAD treatment (Fig. 6E and 6F). The internal area of the LV volume-pressure graph indicates stroke work (SW), which is the amount of work performed in one ventricular pulsation. Fig. 7C shows the SW in the cases of aortic and mitral valvular insufficiency. In Fig. 6, in the case of aortic valvular insufficiency, the internal area of the LV pressure-volume graph has a high value regardless of LVAD type (Fig. 6A, 6C, and 6E). By contrast, in the case of mitral valvular insufficiency, the internal area of the LV pressure-volume graph decreased with LVAD treatment (Fig. 6B, 6D, and 6F). Accordingly, in the case of aortic valvular insufficiency, the left ventricle SW (LVSW) did not decrease with LVAD treatment, whereas in the case of mitral valvular insufficiency, the LVSW decreased with LVAD treatment.
Fig. 7.
Cardiac responses. (A) Cardiac output (CO). (B) Left ventricular stroke work (LV SW). (C) Left ventricular stroke work (LV SW). (D) left ventricular ejection fraction (LV EF). (E) left ventricular peak pressure (LV PP). (F) Under-valve regurgitation by the CLVAD and the PLVAD.
AR, aortic regurgitation; CLVAD, continuous left ventricular assist device, MR, mitral regurgitation; PLVAD, pulsatile left ventricular assist device.
Fig. 7 shows the LV cardiac output, SW, and LVPP without LVAD treatment and with CLVAD or PLVAD treatment, according to the severity of aortic and mitral valve insufficiency. In the case of aortic valvular insufficiency, the cardiac output decreased with more severe regurgitation when LVAD treatment was not performed. In addition, the LVAD blood flow rate, which contributed to the cardiac output, decreased with more severe aortic valve regurgitation (Fig. 7A). The SW increased with more severe valvular regurgitation up to a severity of 6%, and decreased thereafter, regardless of the LVAD type, and was lowest with PLVAD treatment (Fig. 7C). LVPP increased with increasing valvular regurgitation severity. At valvular regurgitation levels of 6–10%, LVPP varied only slightly according to the severity of regurgitation (Fig. 7E).
In summary, in the case of aortic valvular insufficiency, continuous and pulsatile LVADs improved cardiac output and reduced mechanical load slightly (Fig. 7A, 7C, and 7E). In the case of mitral valvular insufficiency, the cardiac output decreased with more severe valvular regurgitation without LVAD treatment (3.3 L/min under 10% AR severity), whereas when CLVAD or PLVAD treatment was performed, a constant value of ∼5 L/min was observed, regardless of the severity of regurgitation (Fig. 7 B). The SW increased without LVAD treatment, and decreased with CLVAD or PLVAD treatment (Fig. 7D). LVPP decreased in all cases with increasing severity of regurgitation. The mean value was highest in the absence of LVAD treatment and lowest in its presence (Fig. 7F).
4. Discussion
In this study, the effects of valvular insufficiency on LVAD functions were numerically investigated by estimating the CLVAD and PLVAD pumping efficiency according to the severity of aortic and mitral valvular insufficiency using an eight compartment lumped parameter model. The pumping efficiency was estimated by predicting the hemodynamic responses, such as LV and aortic blood pressures, LV pressure-volume curves, cardiac output, LVSW, and LVPP.
When aortic valvular insufficiency developed during the LVAD treatment period, the mean arterial blood pressure and the cardiac output decreased with increasing regurgitation severity; but when mitral valvular insufficiency developed, the mean arterial blood pressure and the cardiac output remained constant, regardless of the regurgitation severity. The anatomical position of the regurgitant valves and cannulation position of LVAD affect the hemodynamic responses. As the LVAD helps eject the blood from the LV to the aorta, it is normally intubated from the LV to the aorta. Aortic valvular insufficiency results in the regurgitation of blood from the aorta to the LV. The blood ejected to the aorta by the LVAD flows back to the LV and the total cardiac output and blood pressure decrease. Mitral valvular insufficiency results in the regurgitation of blood from the LV to the LA, so the functions of the LVAD, which sends blood from the LV to the aorta, are not markedly affected.
In the case of aortic valvular insufficiency, the LV load did not decrease with LVAD treatment. In the case of mitral valvular insufficiency, however, the LV load decreased with LVAD treatment. This was due to the LVAD cannulation location compared to valve location where the regurgitation developed. In the case of aortic valvular insufficiency, the blood from the LA and the blood regurgitated from the aorta were mixed to increase the LV blood volume. In addition, the end-systolic pressure increases to eject the blood (Frank-Starling Law; the LV end-systolic pressure is shown in Fig. 7E). The LV load did not significantly decrease with LVAD treatment due to aortic valve regurgitation. Accordingly, in the case of aortic valve insufficiency, the LVAD functions deteriorated in terms of ventricular unloading. In the case of mitral valvular insufficiency, the blood flowed in from the LV and then flowed out to the LA due to regurgitation. A greater volume of blood flowed into the LA from the LV due to the regurgitated blood, and the amount of blood in the LA increased abruptly. Accordingly, the work output also increased. Fig. 6 shows the process. From the viewpoint of energy, this implied that the ventricular pumping load increased with valvular insufficiency. With LVAD treatment, the aortic ejection of blood was facilitated, and the LV load decreased. Eventually, in the case of mitral valvular insufficiency, the LVAD could function properly in terms of ventricular load reduction.
In the LVAD treatment method, the pumping efficiency can be predicted in terms of the decrease in ventricular load and the maintenance of normal arterial blood pressure. In the case of mitral valvular insufficiency in the simulation, the ventricular load decreased and the arterial blood pressure increased due to the CLVAD or PLVAD, so the LVAD treatment method was effective. In the case of aortic valvular insufficiency, the ventricular load increased due to CLVAD or PLVAD treatment, and the arterial blood pressure decreased, which showed that the LVAD treatment method was ineffective. In the case of mitral valvular insufficiency, which responded well to LVAD treatment, the ventricular load decreased more significantly with PLVAD treatment than with CLVAD treatment, which showed that the PLVAD had better pumping efficiency than the CLVAD.
This study had several limitations. We used a lumped parameter model of the cardiovascular system based on the Windkessel element and the heart was modeled using the concept of time-varying compliance.17 In order to reduce modeling complexity, we did not implement the coronary circulation and nervous short-term regulation of the cardiovascular system which are implemented in our previous paper.15 That is because the only short-term blood pressure regulation by the nervous system without other long-term regulation would not give us significant insight. To simulate the valve regurgitation caused by mitral and aortic valve insufficiency, only one further route was added to the aortic and mitral valve compartment for regurgitation, while regurgitation is also influenced by peripheral parameters. However, these limitations are not expected to greatly alter the main findings of this study.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was partially supported by the Ministry of Science, ICT and Future Planning, Korea, under the Convergence Information Technology Research Center support program (IITP-2015-H8601-15-1011) supervised by the Institute for Information & communications Technology Promotion and National Research Foundation (NRF-2011-0009335) and Quality of Working Life (QWL; N0000842) Program funded by the Ministry of Trade, Industry and Energy.
References
- 1.Casarotto D., Bottio T., Gambino A., Testolin L., Gerosa G. The last to die is hope: prolonged mechanical circulatory support with a Novacor left ventricular assist device as a bridge to transplantation. J Thorac Cardiovasc Surg. 2003;125:417–418. doi: 10.1067/mtc.2003.131. [DOI] [PubMed] [Google Scholar]
- 2.John R., Kamdar F., Liao K., Colvin-Adams M., Boyle A., Joyce L. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–1234. doi: 10.1016/j.athoracsur.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Daneshmand M.A., Rajagopal K., Lima B., Khorram N., Blue L.J., Lodge A.J. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg. 2010;89:1205–1209. doi: 10.1016/j.athoracsur.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 4.Uretsky S., Supariwala A., Nidadovolu P., Khokhar S.S., Comeau C., Shubayev O. Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson. 2010;12 doi: 10.1186/1532-429X-12-32. http://jcmr-online.biomedcentral.com/articles/10.1186/1532-429X-12-32, Article number 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant A.S., Holman W.L., Nanda N.C., Vengala S., Blood M.S., Pamboukian S.V. Native aortic valve insufficiency in patients with left ventricular assist devices. Ann Thorac Surg. 2006;81:6–8. doi: 10.1016/j.athoracsur.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 6.Holman W.L., Bourge R.C., Fan P., Kirklin J.K., Pacifico A.D., Nanda N.C. Influence of longer term left ventricular assist device support on valvular regurgitation. ASAIO J. 1994;40:454–459. doi: 10.1097/00002480-199407000-00041. [DOI] [PubMed] [Google Scholar]
- 7.Rao V., Slater J.P., Edwards N.M., Naka Y., Oz M.C. Surgical management of valvular disease in patients requiring left ventricular assist device support. Ann Thorac Surg. 2001;71:1448–1453. doi: 10.1016/s0003-4975(01)02479-1. [DOI] [PubMed] [Google Scholar]
- 8.Haghi D., Suselbeck T., Saur J. Aortic regurgitation during left ventricular assist device support. J Heart Lung Transplant. 2007;26:1220–1221. doi: 10.1016/j.healun.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Frazier O.H. Unforeseen consequences of therapy with continuous-flow pumps. Circ Heart Fail. 2010;3:647–649. doi: 10.1161/CIRCHEARTFAILURE.110.959023. [DOI] [PubMed] [Google Scholar]
- 10.Cowger J., Pagani F.D., Haft J.W., Romano M.A., Aaronson K.D., Kolias T.J. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3:668–674. doi: 10.1161/CIRCHEARTFAILURE.109.917765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorde U.P., Uriel N., Nahumi N., Bejar D., Gonzalez-Costello J., Thomas S.S. Prevalence, significance, and management of aortic insufficiency in continuous flow left ventricular assist device recipients. Circ Heart Fail. 2014;7:310–319. doi: 10.1161/CIRCHEARTFAILURE.113.000878. [DOI] [PubMed] [Google Scholar]
- 12.Gaasch W.H., Meyer T.E. Left ventricular response to mitral regurgitation: implications for management. Circulation. 2008;118:2298–2303. doi: 10.1161/CIRCULATIONAHA.107.755942. [DOI] [PubMed] [Google Scholar]
- 13.Peter C.A., Jones R.H. Cardiac response to exercise in patients with chronic aortic regurgitation. Am Heart J. 1982;104:85–91. doi: 10.1016/0002-8703(82)90645-7. [DOI] [PubMed] [Google Scholar]
- 14.Monrad E.S., Baim D.S., Smith H.S., Lanoue A.S. Milrinone, dobutamine, and nitroprusside: comparative effects on hemodynamics and myocardial energetics in patients with severe congestive heart failure. Circulation. 1986;73:III168–III174. [PubMed] [Google Scholar]
- 15.Lim K.M., Kim I.S., Choi S.W., Min B.G., Won Y.S., Kim H.Y. Computational analysis of the effect of the type of LVAD flow on coronary perfusion and ventricular afterload. J Physiol Sci. 2009;59:307–316. doi: 10.1007/s12576-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deb B., Bradford K., Pearl R.G. Additive effects of inhaled nitric oxide and intravenous milrinone in experimental pulmonary hypertension. Crit Care Med. 2000;28:795–799. doi: 10.1097/00003246-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 17.Suga H., Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974;35:117–126. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 18.Lim K.M., Constantino J., Gurev V., Zhu R., Shim E.B., Trayanova N.A. Comparison of the effects of continuous and pulsatile left ventricular-assist devices on ventricular unloading using a cardiac electromechanics model. J Physiol Sci. 2012;62:11–19. doi: 10.1007/s12576-011-0180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim K.M., Lee J.S., Song J.H., Youn C.H., Choi J.S., Shim E.B. Theoretical estimation of cannulation methods for left ventricular assist device support as a bridge to recovery. J Korean Med Sci. 2011;26:1591–1598. doi: 10.3346/jkms.2011.26.12.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grose R., Strain J., Greenberg M., LeJemtel T.H. Systemic and coronary effects of intravenous milrinone and dobutamine in congestive heart failure. J Am Coll Cardiol. 1986;7:1107–1113. doi: 10.1016/s0735-1097(86)80231-5. [DOI] [PubMed] [Google Scholar]
- 21.Lim K.M., Hong S.B., Lee B.K., Shim E.B., Trayanova N.A. Computational analysis of the effect of valvular regurgitation on ventricular mechanics using a 3D electromechanics model. J Physiol Sci. 2015;65:159–164. doi: 10.1007/s12576-014-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]