Abstract
Embryonic stem cell-derived cardiomyocytes (ESC-CMs) hold great interest in many fields of research including clinical applications such as stem cell and gene therapy for cardiac repair or regeneration. ESC-CMs are also used as a platform tool for pharmacological tests or for investigations of cardiac remodeling. ESC-CMs have many different aspects of morphology, electrophysiology, calcium handling, and bioenergetics compared with adult cardiomyocytes. They are immature in morphology, similar to sinus nodal-like in the electrophysiology, higher contribution of trans-sarcolemmal Ca2+ influx to Ca2+ handling, and higher dependence on anaerobic glycolysis. Here, I review a detailed electrophysiology and Ca2+ handling features of ESC-CMs during differentiation into adult cardiomyocytes to gain insights into how all the developmental changes are related to each other to display cardinal features of developing cardiomyocytes.
Keywords: calcium handling, cardiomyocytes, electrophysiology, embryonic stem cell, ion channels
1. Introduction
Embryonic stem cell-derived cardiomyocytes (ESC-CMs) are greatly promising for stem cell therapy against cardiovascular diseases such as myocardial infarction and potentially life-threatening arrhythmias because of their ability to differentiate into sinus–nodal, atrial, or ventricular-type of cardiomyocytes.1, 2 ESC-CMs also have a potential to be a novel pharmacological tool in, for example, high-throughput screening tests of newly developed drug for cardiotoxicity.3 In addition, ESC-CMs could be a powerful new model system to study mechanisms of inherited cardiomyopathies.4
ESC-CMs are immature in both morphological and functional aspects. They have underdeveloped contractile machinery and lack transverse-tubular (T-tubular) system.5, 6 They display diastolic potentials similar to those of adult sinus–nodal cells and slower upstroke velocity.1, 2 They have fewer mitochondria5 and are predominantly glycolytic.7 Ca2+ handling is also significantly different from that of their adult counterpart displaying slower kinetics and smaller amplitude.8 This review will primarily focus on the electrophysiological properties and Ca2+ handling features of ESC-CMs during differentiation into adult cardiomyocytes.
2. Electrophysiological properties of ESC-CMs
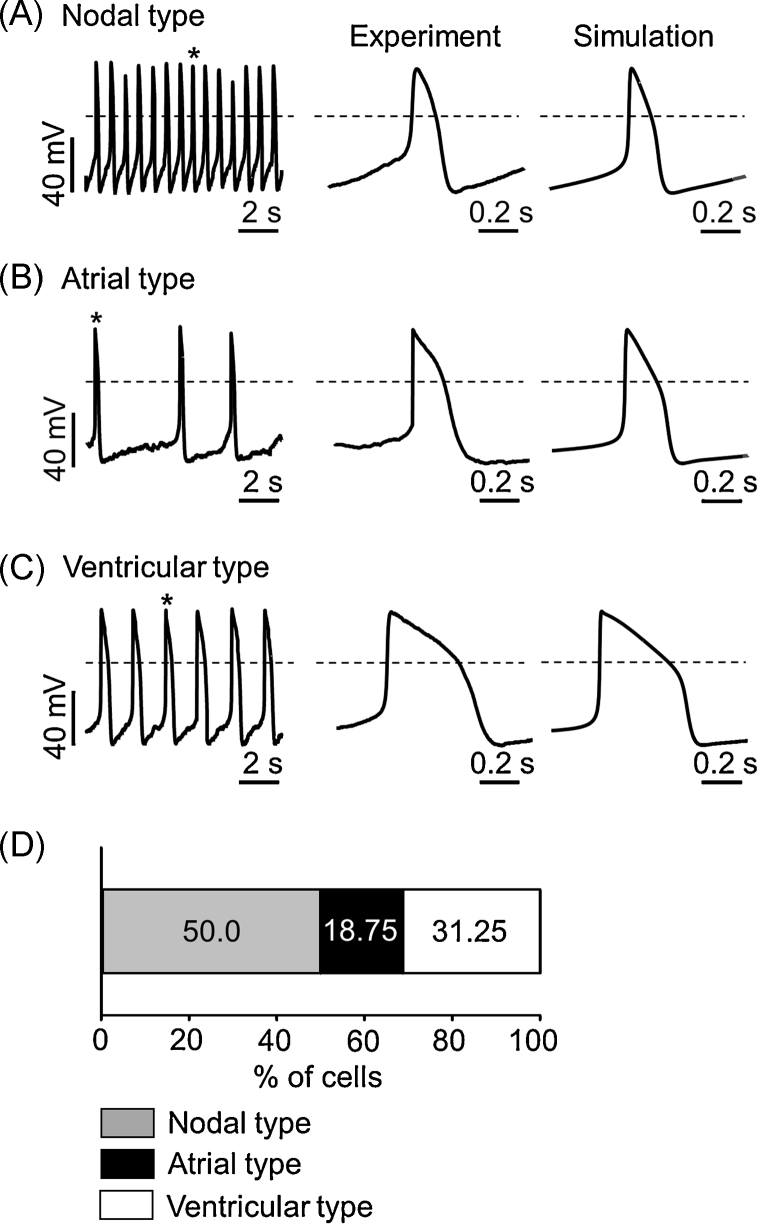
Early studies on the differentiation of pluripotent mouse embryonic stem cells (ESCs) into adult cardiomyocytes revealed that the rhythmic action potentials (APs) of early differentiated cardiomyocytes are very similar to those of sinus–node cells.1, 2 Terminally differentiated cardiomyocytes generated APs similar to those of adult sinus–nodal, atrial, and ventricular myocytes, suggesting that there should be developmental changes in cardiac ion channels and calcium handling properties along with differentiation into adult cardiomyocytes. Representative traces of nodal-like, atrial-like, and ventricular-like APs from patch–clamp recording and mathematical modelling are demonstrated in Fig. 1. The mathematical model was based on the Kharche's model9 and modified parameters are summarized in Table 1. In the following, functional changes in electrophysiological properties during differentiation are described in detail.
Fig. 1.
Representative action potential (AP) morphologies from patch clamp recordings and mathematical modelling. (A) Nodal type, (B) atrial type, and (C) ventricular type APs recorded from different embryonic stem cell-derived cardiomyocytes in a current–clamp mode. Each AP trace in the middle panel corresponds to an expanded trace of single AP denoted with an asterisk in the left panel. Each AP trace in the right panel corresponds to a simulated AP trace. Dotted lines indicate zero voltage level. (D) Percentile distribution of three different types of APs in embryonic stem cell-derived cardiomyocytes. See Table 1 for model parameters used in the mathematical model.
Note. Fig. 1D is from “Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells,” by S.W. Cho, J.S. Park, H.J. Heo, S.W. Park, S. Song, I. Kim et al., 2014, J Am Heart Assoc, 3, e000693. Copyright 2014 The Authors. Reprinted with permission.
Table 1.
Model parameter values in mathematical model*
| Model parameters | Nodal type (AP) |
Atrial type (AP) |
Ventricular type (AP) |
|---|---|---|---|
| ECaL, mV | 47 | 47 | 47 |
| gCaL, nS | 11.0140 | 12.5874 | 12.5874 |
| k1 | 0.9 | 1.1 | 1.8 |
| k2 | 1.0 | 1.3 | 2.2 |
| gHCN,Na, nS | 0.0529 | 0.0529 | 0.0529 |
| gHCN,K, nS | 0.0851 | 0.0851 | 0.0851 |
| gto,UF, nS | 0.3346 | 1.3382 | 0.6691 |
| gto,F, nS | 0.1338 | 0.5352 | 0.2676 |
| gto,S, nS | 0.2091 | 0.8364 | 0.4182 |
| gKs, nS | 0.0411 | 0.2057 | 0.4114 |
| gb,Ca, nS | 0 | 0 | 0 |
| gb,Na, nS | 0.170 | 0.122 | 0.122 |
| gb,K, nS | 0.035 | 0.025 | 0.025 |
| gCaT, nS | 0 | 0 | 0 |
| gKl, nS | 0 | 0.6075 | 2.2275 |
| gKr, nS | 1.5319 | 0.6383 | 0.6383 |
| gNa,1.5, nS | 0.0059 | 0.0237 | 0.0296 |
| gNa,1.1, nS | 0.0059 | 0.0237 | 0.0296 |
| gst, nS | 0 | 0 | 0 |
| gsus, nS | 0 | 0 | 0 |
See glossary of Kharche et al's9 model for detailed explanation of each channel.
Note. Adapted from “Dual modulation of the mitochondrial permeability transition pore and redox signaling synergistically promotes cardiomyocyte differentiation from pluripotent stem cells,” by S.W. Cho, J.S. Park, H.J. Heo, S.W. Park, S. Song, I. Kim et al., 2014, J Am Heart Assoc, 3, e000693. Copyright 2014 The Authors. Reprinted with permission.
AP; action potential.
2.1. Capacitance
In one early study on mouse ESC-CMs, membrane capacitance was found to steadily increase from 24.5 pF to 50.0 pF during differentiation.10 As the specific membrane capacitance was calculated to be 0.85–0.86 μF/cm2, it was concluded that the T-tubular system apparently was not developed during differentiation.10 T-tubule formation was not observed at the ultrastructural level in human pluripotent stem cell-derived cardiomyocytes, when electron microscopy or membrane staining by Di-8-ANNEPPS were used.6
2.2. L-type Ca2+ current
L-type Ca2+ current (ICaL) is the most prominent inward current at the early stage of differentiation in mouse ESC-CMs and is found throughout the entire differentiation period.10 The maximum current density (pA/pF) occurred near 0 mV and were apparently 13 pA/pF at early stage and 25 pA/pF at terminally differentiated stage, respectively. ICaL is thought to synchronize multiple local Ca2+ releases (LCRs) to induce global Ca2+ release.11
The mRNA of CACNA1C, a gene encoding one subtype of L-type Ca2+ channel, was detected in undifferentiated human ESCs and at all stages of differentiation by using reverse transcription–polymerase chain reaction (RT-PCR) analysis.12 ICaL was recorded in both human ESCs and ESC-CMs at all stages of differentiation. Maximum current densities were 0.9 pA/pF in human ESCs and 5.8 pA/pF in human ESC-CMs, respectively.12
As for the pharmacology, there is a report13 that human ESC-CMs have an altered pharmacology for activators of ICaL while the pharmacology for antagonist is preserved.
2.3. T-type Ca2+ current
An inward current permeable for Ca2+ and Ba2+ with lower threshold for activation than that of ICaL was detected in an early study on differentiation of pluripotent ESCs of the mouse.10 As it was insensitive to 1,4-dihydropyridines but was almost completely blocked by 50 μM Ni2+, the T-type Ca2+ channel was suggested to be the candidate channel to allow the inward current.
2.4. Voltage-gated Na+ current
The density of voltage-gated Na+ current (INa) in mouse ESC-CMs was 95 pA/pF at the early differentiation stage and increased to above 300 pA/pF at the terminal differentiation stage.10 INa was predominantly observed in atrial-like and ventricular-like cells but to a lesser degree in sinus-nodal-like cells. The density of INa in human ESC-CMs was 244 pA/pF at mid-stage (20–35 days postplating).14 As the spontaneous electrical activity from cell clusters was completely abolished by perfusion of tetrodotoxin, a specific blocker of INa, it was suggested that the INa is crucial for initiation of spontaneous excitation in cell clusters at mid-stage development. Interestingly, an AP clamp using the morphology of APs from human ESC-CMs elicited a larger early and late INa than that using the morphology of APs from either the canine epicardial or endocardial ventricular myocytes in some voltage-gated Na+ channel (NaV1.5) variants.15 The proposed mechanism was that the AP from human ESC-CMs displays a relatively slower upstroke velocity than those from canine ventricular myocytes, which induces increased open probability and driving force of INa.15
2.5. Voltage-gated K+ current
A transient outward current (Ito) was found from the very early stage of differentiation in mouse ESC-CMs.10 The density of Ito was 14.9 pA/pF at the early differentiation stage and 41.5 pA/pF at the terminal differentiation stage, respectively. The 4-aminopyridine sensitive transient outward current (Ito) was also observed in the other early stage murine cardiomyocytes where the current density was 10.3 pA/pF.16 In human ESC-CMs, expression of two isoforms, Kv1.4 and Kv4.3, was detected by RT-PCR.12 The two isoforms were not detected in undifferentiated human ESC. A 4-aminopyridine sensitive transient outward current was also recorded in the presence of intracellular EGTA, a chelator of Ca2+, indicating that it is the Ca2+-independent transient outward K+ current (Ito1). Its peak density increased during development from 4.2 pA/pF to 7.7 pA/pF.
The delayed rectifier K+ current was not detectable at the early stage of differentiation but was seen only at the intermediate and terminal differentiation stages.10 The density of delayed rectifier K+ current was 3.5 pA/pF at the intermediate differentiation stage and 9.4 pA/pF at the terminal differentiation stage.
Gryshchenko et al16 identified two components of rapidly activating, outwardly rectifying current in early and late stage cardiomyocytes, which were characterized by slow (IKsus) or no inactivation (Ires), respectively, during depolarizing test pulses. Ires was 4-aminopyridine resistant and was observed in 75% of early stage cardiomyocytes. The maximum current density was increased from 3.6 pA/pF to 8 pA/pF during differentiation into late stage cardiomyocytes. Meanwhile, IKsus was observed in only 25% of early stage cardiomyocytes and its maximum density was increased from 3.5 pA/pF to 8.3 pA/pF during differentiation into late stage cardiomyocytes.
AP durations from beating embryoid bodies cultured for 40–95 days were significantly prolonged by addition of E4031, a specific blocker of rapidly activating delayed rectifier K+ current (IKr), indicating the ESC-CMs express HERG channels.17 Even early after-depolarizations appeared after treatment with E4031. In both undifferentiated human ESCs and ESC-CMs, the mRNA of HERG was detected by using RT-PCR analysis.12 E4031-sensitive outward currents were also recorded upon depolarizing test pulses from a holding potential of –40 mV, indicating both undifferentiated human ESCs and ESC-CMs functionally express HERG channels.
An E4031-resistant but chromanol 293B-sensitive K+ current was observed in human ESC-CMs.18 Current-voltage relationship, activation kinetics, and pharmacological response all demonstrated that it is the slowly activating delayed rectifier K+ current (IKs). The maximum current density was apparently 0.7 pA/pF as measured at the end of 2 second depolarization test pulse to 40 mV.
2.6. Inward rectifier K+ current
Inward rectifier K+ current (IK1) was observed predominantly in atrial-like and ventricular-like cells at the terminal differentiation stage.10 The maximum density of IK1 in ventricular-like cells was slightly increased from 4.9 pA/pF to 5.4 pA/pF during differentiation into terminal stage. In a study of transgenic mouse ESCs expressing enhanced green fluorescent protein (EGFP), both the early sinus–nodal- and atrial-like cardiomyocytes expressed IK1 but the percentage of IK1 expressing sinus-nodal-like cardiomyocytes was very small compared with that of IK1 expressing atrial-like cardiomyocytes.19 The maximum density was also lower in sinus-nodal-like cardiomyocytes (1.6 pA/pF vs. 3.6 pA/pF). The mRNA of Kir2.1, a molecular identity of IK1, was detected in both undifferentiated human ESCs and ESC-CMs by using RT-PCR analysis.12 Ba2+-sensitive background K+ currents were recorded at all stages of differentiation indicating that human ESC-CMs functionally express IK1.
2.7. ATP-dependent K+ current
Some cardiomyocytes in early differentiation were found to display a bursting behavior of APs, characterized by alternating periods of burst firing and silence.20 The positive chronotropy by glibencamide, a blocker of ATP-dependent K+ current (IK,ATP) and negative chronotropy by dinitrophenol, a known activator of IK,ATP, suggest that the ATP-dependent K+ channels are present and play a role in bursting behavior of early stage cardiomyocytes. Perforated patch-clamp recordings in the voltage-clamp mode were conducted to isolate IK,ATP, and the maximum current densities were 45.1 pA/pF in early stage cells and 90.9 pA/pF in late stage cells, respectively. The IK,ATP was suggested to mirror the metabolic oscillations and serve as a cardioprotective role under conditions of low O2 saturation in the embryo.
2.8. Acetylcholine-dependent K+ current
As the carbachol activated inwardly rectifying K+ currents at the terminal differentiation stage, which was almost completely blocked by atropine, the presence of acetylcholine-dependent K+ current was suggested.10 In a study of transgenic mouse ESC-CMs expressing EGFP, five out of nine sinus-nodal-like cardiomyocytes completely stopped spontaneous electrical activity in response to carbachol indicating that acetylcholine-dependent K+ current exists.19 Meanwhile, only one out of 10 atrial-like cardiomyocytes completely stopped spontaneous electrical activity by addition of carbachol.
2.9. Hyperpolarization-activated nonselective cation current
Hyperpolarization-activated nonselective cation current (If) was first observed at the intermediate differentiation stage of mouse ESC-CMs.10 Peak amplitudes of If were between 50 pA and 500 pA at the intermediate differentiation stage and ranged between 500 pA and 2000 pA at the terminal differentiation stage. Abi-Gerges et al21 found that the percentage of cardiomyocytes expressing If slightly decreased from 65% to 45% throughout differentiation. Meanwhile, peak If densities increased from 11.4 pA/pF to 15.5 pA/pF throughout differentiation. They also demonstrated that the activation of If during early and late stages of differentiation is not mediated by direct cAMP binding but by phosphorylation via protein kinase A. The automaticity seen in atrial-like and ventricular-like cardiomyocytes during differentiation is in part due to their relatively high densities of If compared with those of adult atrial and ventricular cardiomyocytes. In the study of transgenic mouse ESC-CMs expressing EGFP, both the early sinus–nodal-like and atrial-like cardiomyocytes expressed If and peak densities were about 11 pA/pF and 8 pA/pF, respectively.19 The expression of HCN, a molecular identity of If, were detected in mouse ESC-CMs and were significantly decreased at Day 23.5 of cell culture compared with that at Day 9.5.22 Functional expression of If was also observed in human ESC-CMs.12, 14 The mRNA level for HCN1 isoform, which has the fastest activation kinetics among four isoforms,23 was not detected in adult heart but was detected in undifferentiated human ESCs.12 The expression of HCN1 was then significantly reduced during differentiation and maturation. By contrast, the mRNA level for HCN2 isoform with slower kinetics was persistent throughout the differentiation process.
2.10. Ca2+ activated K+ current
In a study on the pacemaking mechanism of human ESC-CMs,24 a previously unrecognized Ca2+-activated K+ channel (KCa3.1, SK4, or IKCa channel) was identified by using the patch–clamp technique, real-time PCR, western blotting, and immunocytochemistry. Pharmacological inhibition of IKCa by using clotrimazole or 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole resulted in the depolarization of maximum diastolic potential and slowing of AP beating rate. It was suggested that IKCa contributes to shaping of diastolic potential and pacemaker activity via fine tuning the balance between If and Na+/Ca2+ exchanger current (INCX).
2.11. Background current
Background currents with a linear current–voltage relationship and an apparent reversal potential near 0 mV were observed.10 The canonical transient receptor potential isoform 3 (TRPC3) channel, one possible molecular identity of the background inward current,25 was recently identified in mouse ESC-CMs based on immunocytochemistry and patch clamp recordings.26 Immunocytochemistry revealed that TRPC3 is located at the T-tubules. A Pyr3-sensitive TRPC3 current was recorded by whole-cell patch clamp technique. The block of TRPC3 decreased frequency and amplitude of spontaneous Ca2+ transients. The most prominent change in spontaneous AP by Pyr3 was seen in Phase 4 diastolic depolarization which was significantly delayed. As TRPC3 permeates both Ca2+ and Na+, activation of TRPC3 current is considered to depolarize the membrane.27 In addition, Ca2+ influx through TRPC3 could induce LCRs, which subsequently activates the forward mode of Na+/Ca2+ exchanger (NCX).28
2.12. INCX
RT-PCR analyses of transcripts were conducted on mouse ESC-CMs and NCX1 was clearly detectable from preplating stage.29 The role of NCX was evaluated by using Na+ free solution, which inhibits NCX resulting in a decreased removal of Ca2+ from cytosol. In early stage mouse ESC-CMs, basal level of [Ca2+]i was found to be regulated by NCX, whereas spontaneous Ca2+ transients were not. In late stage ESC-CMs, both basal level of [Ca2+]i and amplitude of Ca2+ transients were affected by NCX. In human ESC-CMs, NCX was expressed at a higher level than the adult counterpart. The Ni2+-sensitive inward current was recorded in ventricular-like human ESC-CMs.30 INCX density was 3.6 pA/pF at 60 mV in 40-day ventricular-like ESC-CMs and increased to 7.9 pA/pF in 90-day. NCX was suggested to translate a sporadic generation of LCRs into the diastolic depolarization, which in turn activates L-type Ca2+ currents to generate APs in late-stage mouse ESC-CMs.11
3. Calcium handling
3.1. [Ca2+]i oscillation drives spontaneous contraction in early stage ESC-CMs
Based on the finding that the early stage ESC-CMs continue beating in high extracellular K+ solution, not transmembrane ion currents but [Ca2+]i oscillations were suggested as the mechanism to drive spontaneous contractions of cardiomyocytes in the early stage of differentiation into adult cardiomyocytes.31 Interestingly, many small fluctuations of membrane potential were superimposed with fewer number of APs in the early stage cardiomyocytes. 50 nM nisoldipine, a blocker of ICaL, halted APs but not the membrane potential fluctuations and accompanying spontaneous contractions. The small fluctuations of membrane potential were completely blocked by the addition of thapsigargin, a sarcoplasmic Ca2+-ATPase inhibitor. Therefore, it was concluded that the [Ca2+]i oscillations primarily drive small fluctuations of membrane potential and that if the fluctuations are big enough to activate L-type Ca2+ channels, the APs are generated. Recently, NCX was suggested to translate spontaneous Ca2+ oscillations into fluctuations of membrane potential.32 NCX was also suggested to translate spontaneous Ca2+ oscillations into spontaneous inward currents in some voltage clamp conditions.33 In addition, the [Ca2+]i oscillations in early stage embryonic stem cell-derived cardiomyocytes are thought to be crucial for structural maturation of the cardiomyocytes.34 The appearance of spontaneous contractions under high extracellular K+ solution was not observed in terminally differentiated cardiomyocytes indicating that calcium handling properties change with maturation (Fig. 2). In the following, Ca2+ handling in mouse and human ESC-CMs are described in more detail.
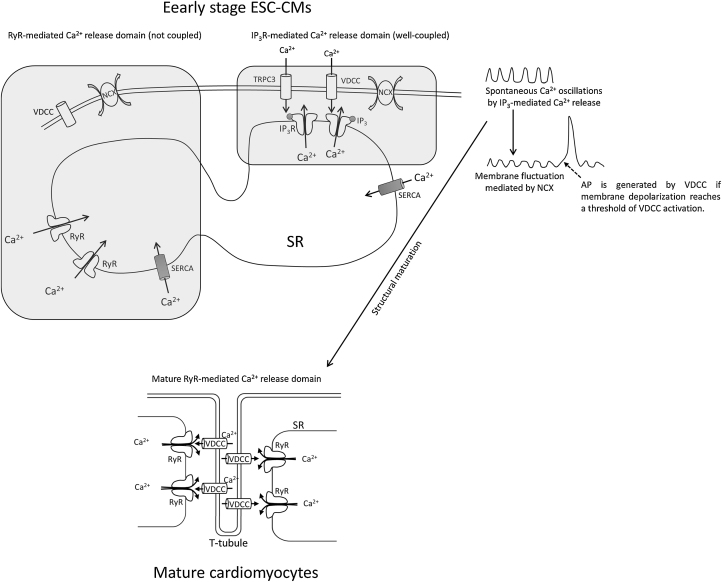
Fig. 2.
Schematic model of Ca2+ handling and AP generation in early stage ESC-CMs and mature cardiomyocytes. In early stage ESC-CMs, T-tubules are not developed and RyRs are poorly coupled to VDCC. Instead, IP3R is well coupled to NCX and the IP3R-mediated Ca2+ release predominantly drives spontaneous Ca2+ oscillations which subsequently cause spontaneous beating even under high extracellular K+ condition.31 Trans-sarcolemmal pathways such as TRPC3 and VDCC also significantly contribute to IP3R-mediated Ca2+ release. Ca2+ removal depends on both NCX and SERCA in the SR. NCX is believed to translate the spontaneous Ca2+ oscillations into membrane potential fluctuations.32 If the fluctuation of membrane depolarization reaches a threshold for VDCC, an AP is generated. Ca2+ oscillation is essential for maturation of T-tubule and RyR-VDCC coupling.34 Therefore, the RyR-mediated Ca2+ release is dominant in mature cardiomyocytes.
AP, action potential; ESC-CMs, embryonic stem cell-derived cardiomyocytes; IP3, inositol-1,4,5-trisphosphate; IP3R, inositol-1,4,5-trisphosphate receptor; NCX, Na+/Ca2+ exchanger; RyRs, ryanodine receptor channels; SERCA, sarcoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum; T-tubule, transverse tubule; VDCC, voltage-dependent L-type Ca2+ channel.
3.2. Ca2+ handling in mouse ESC-CMs
Ca2+ sparks, which represent Ca2+ release from the opening of clustered ryanodine receptor channels (RyRs) within restricted sarcoplasmic reticulum (SR) junctional regions,35 were not observed in very early developmental stage.36 By contrast, global [Ca2+]i fluctuations occurred from the very early developmental stage. Both the Ca2+ sparks and global [Ca2+]i fluctuations were markedly increased in amplitude and frequency during differentiation into late stage cardiomyocytes. Perfusion of either 10 mM caffeine or high extracellular K+ (140 mM) solution to the cardiomyocytes in very early developmental stage resulted in Ca2+ transients indicating that RyRs and L-type Ca2+ channels are present and work from the beginning of differentiation. The presence of RyRs in early stage cardiomyocytes was further confirmed by the finding that the heart rate was markedly depressed in mouse ESC-CMs with a functional knockout of the RyR2.37, 38 The absence of Ca2+ spark in spite of functional RyRs and L-type Ca2+ channels in the very early developmental stage indicates that they are not yet in close apposition in the very early stage of differentiation. Indeed, it was found that there is no colocalization of L-type Ca2+ channels and RyRs in mouse and human ESC-CMs.39
Although Ca2+ sparks were not observed in RyR2 knockout cells, spontaneous Ca2+ transients were still observable but with significantly slower kinetics.37 The residual source of Ca2+ increase in RyR2 KO cells is possibly reverse mode NCX, L-type Ca2+ channels, or inositol-1,4,5-trisphosphate receptor (IP3R) channels. Indeed, 2-aminoethoxydipheylborate, an inhibitor of store-operated channels or inositol-1,4,5-trisphosphate (IP3)-induced Ca2+ release,40 significantly reduced amplitude of Ca2+ transients. The inhibitory effect of 2-aminoethoxydipheylborate was significantly attenuated at late stage of differentiation indicating the contribution of IP3Rs to total SR Ca2+ release is downregulated during differentiation.37, 41 Furthermore, there is strong evidence that IP3R and NCX are spatially coupled to turn Ca2+ oscillations into membrane potential fluctuations in early stage of differentiation.32
Nifedipine, a blocker of L-type Ca2+ channels, also reduced amplitude of Ca2+ transients in electrically stimulated cardiomyocytes.29 It was suggested that the L-type Ca2+ channel is the predominant contributor to spontaneous Ca2+ transients in early differentiation and then Ca2+-induced Ca2+ release takes over the role of major contributor to Ca2+ transients in late stage differentiation.41 Frequency and amplitude of Ca2+ transients were both significantly increased from early to late stage differentiation.38
As the exposure of mouse ESC-CMs to caffeine elicited a bigger size of Ca2+ transients during differentiation, the effects of differentiation on SR were proposed to be increasing its volume and Ca2+ content.38
As for the Ca2+-removal mechanism, NCX was suggested to be the main contributor in the early stage of differentiation whereas sarcoplasmic reticulum Ca2+-ATPase (SERCA) takes over the main role in the late stage of differentiation.41 In a later study,42 however, SERCA was evaluated to be responsible for ∼76% of total Ca2+ removal, while NCX was responsible for only ∼21% at early differentiation stage of mouse ESC-CMs. At late differentiation stage, the contribution of SERCA to Ca2+ removal was further increased to ∼88% while that of NCX was decreased to ∼10%, indicating that the trend of chronological changes in contribution of two Ca2+ removal mechanisms is the same between these two studies.
3.3. Ca2+ handling in human ESC-CMs
In a study of human ESC-CMs, ryanodine, thapsigargin, and caffeine did not affect Ca2+ transients and contraction indicating that SR Ca2+ release does not significantly contribute to Ca2+ handling in early stage of differentiation.43 Instead, trans-sarcolemmal Ca2+ influx was suggested to contribute predominantly to Ca2+ handling in human ESC-CMs based on the finding that verapamil, a blocker of L-type Ca2+ channel, completely blocked contraction. Later studies, however, revealed that there are caffeine-responsive human ESC-CMs as well as caffeine-insensitive human ESC-CMs.44, 45 Furthermore, the presence of SR Ca2+ release channels such as RyR2 and IP3R was also demonstrated by immunostaining studies.45 Ca2+ sparks were also identified by confocal Ca2+ imaging.45, 46, 47 The involvement of IP3-dependent Ca2+ release in intracellular Ca2+ transient was demonstrated in human ESC-CMs by using angiotensin II (AT-II) and endothelin 1 (ET-1), which activate the IP3R-mediated Ca2+ release.48 As Ca2+ exclusion pathways, SERCA2 and NCX were expressed at levels comparable to those of the adult porcine myocardium. However, other Ca2+-regulatory proteins such as calsequestrin, triadin, junction, and phospholamban were not detectable in human ESC-CMs.43, 44 The absence of calsequestrin and phospholamban is possibly the cause for the dysfunctional SR seen in Ca2+-handling of human ESC-CMs. Indeed, a somatic gene-transfer of calsequestrin into human ESC-CMs rendered their Ca2+ handling more adult-like, as demonstrated by augmented size of Ca2+-transient and accelerated kinetics.49
4. Conclusion and future directions
Electrophysiological properties and Ca2+ handling features are significantly different between early stage ESC-CMs and mature cardiomyocytes. The most prominent difference lies in the Ca2+ recruitment mechanism. Immature cardiomyocytes rely on trans-sarcolemmal Ca2+ influx and IP3R-mediated Ca2+ release while mature cardiomyocytes rely on RyR-mediated Ca2+ release with well-developed T-tubular system and coupling between RyR and L-type Ca2+ channels. However, the role of TRPC3 in spontaneous Ca2+ oscillation in early stage ESC-CMs is not fully resolved. Studies on the role of IKCa in diastolic depolarization of pacemaker potential is also in very primitive stage. It is essential to integrate all the aspects of electrophysiology and Ca2+ handling into a comprehensive mathematical model.
Conflicts of interest
No competing financial interests exist.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A01056864).
References
- 1.Wobus A.M., Wallukat G., Hescheler J. Pluripotent mouse embryonic stem cells are able to differentiate into cardiomyocytes expressing chronotropic responses to adrenergic and cholinergic agents and Ca2+ channel blockers. Differentiation. 1991;48:173–182. doi: 10.1111/j.1432-0436.1991.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 2.Maltsev V.A., Rohwedel J., Hescheler J., Wobus A.M. Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev. 1993;44:41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 3.Barbuti A., Robinson R.B. Stem cell-derived nodal-like cardiomyocytes as a novel pharmacologic tool: insights from sinoatrial node development and function. Pharmacol Rev. 2015;67:368–388. doi: 10.1124/pr.114.009597. [DOI] [PubMed] [Google Scholar]
- 4.Jung G., Bernstein D. hiPSC modeling of inherited cardiomyopathies. Curr Treat Options Cardiovasc Med. 2014;16:320. doi: 10.1007/s11936-014-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gherghiceanu M., Barad L., Novak A., Reiter I., Itskovitz-Eldor J., Binah O. Cardiomyocytes derived from human embryonic and induced pluripotent stem cells: comparative ultrastructure. J Cell Mol Med. 2011;15:2539–2551. doi: 10.1111/j.1582-4934.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundy S.D., Zhu W.Z., Regnier M., Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCain M.L., Agarwal A., Nesmith H.W., Nesmith A.P., Parker K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials. 2014;35:5462–5471. doi: 10.1016/j.biomaterials.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S., Chen G., Li R.A. Calcium signalling of human pluripotent stem cell-derived cardiomyocytes. J Physiol. 2013;591:5279–5290. doi: 10.1113/jphysiol.2013.256495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kharche S., Yu J., Lei M., Zhang H. A mathematical model of action potentials of mouse sinoatrial node cells with molecular bases. Am J Physiol Heart Circ Physiol. 2011;301:H945–H963. doi: 10.1152/ajpheart.00143.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maltsev V.A., Wobus A.M., Rohwedel J., Bader M., Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Zahanich I., Sirenko S.G., Maltseva L.A., Tarasova Y.S., Spurgeon H.A., Boheler K.R. Rhythmic beating of stem cell-derived cardiac cells requires dynamic coupling of electrophysiology and Ca cycling. J Mol Cell Cardiol. 2011;50:66–76. doi: 10.1016/j.yjmcc.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartiani L., Bettiol E., Stillitano F., Mugelli A., Cerbai E., Jaconi M.E. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 13.Kang J., Chen X.L., Ji J., Lei Q., Rampe D. Ca2+ channel activators reveal differential L-type Ca2+ channel pharmacology between native and stem cell-derived cardiomyocytes. J Pharmacol Exp Ther. 2012;341:510–517. doi: 10.1124/jpet.112.192609. [DOI] [PubMed] [Google Scholar]
- 14.Satin J., Kehat I., Caspi O., Huber I., Arbel G., Itzhaki I. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magyar J., Kiper C.E., Dumaine R., Burgess D.E., Banyasz T., Satin J. Divergent action potential morphologies reveal nonequilibrium properties of human cardiac Na channels. Cardiovasc Res. 2004;64:477–487. doi: 10.1016/j.cardiores.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Gryschenko O., Lu Z.J., Fleischmann B.K., Hescheler J. Outwards currents in embryonic stem cell-derived cardiomyocytes. Pflugers Arch. 2000;439:798–807. doi: 10.1007/s004249900196. [DOI] [PubMed] [Google Scholar]
- 17.He J.Q., Ma Y., Lee Y., Thomson J.A., Kamp T.J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 18.Wang K., Terrenoire C., Sampson K.J., Iyer V., Osteen J.D., Lu J. Biophysical properties of slow potassium channels in human embryonic stem cell derived cardiomyocytes implicate subunit stoichiometry. J Physiol. 2011;589:6093–6104. doi: 10.1113/jphysiol.2011.220863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolossov E., Lu Z., Drobinskaya I., Gassanov N., Duan Y., Sauer H. Identification and characterization of embryonic stem cell-derived pacemaker and atrial cardiomyocytes. FASEB J. 2005;19:577–579. doi: 10.1096/fj.03-1451fje. [DOI] [PubMed] [Google Scholar]
- 20.Gryshchenko O., Fischer I.R., Dittrich M., Viatchenko-Karpinski S., Soest J., Böhm-Pinger M.M. Role of ATP-dependent K+ channels in the electrical excitability of early embryonic stem cell-derived cardiomyocytes. J Cell Sci. 1999;112:2903–2912. doi: 10.1242/jcs.112.17.2903. [DOI] [PubMed] [Google Scholar]
- 21.Abi-Gerges N., Ji G.J., Lu Z.J., Fischmeister R., Hescheler J., Fleischmann B.K. Functional expression and regulation of the hyperpolarization activated non-selective cation current in embryonic stem cell-derived cardiomyocytes. J Physiol. 2000;523:377–389. doi: 10.1111/j.1469-7793.2000.t01-2-00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagi K., Takano M., Narazaki G., Uosaki H., Hoshino T., Ishii T. Hyperpolarization-activated cyclic nucleotide-gated channels and T-type calcium channels confer automaticity of embryonic stem cell-derived cardiomyocytes. Stem Cells. 2007;25:2712–2719. doi: 10.1634/stemcells.2006-0388. [DOI] [PubMed] [Google Scholar]
- 23.Moosmang S., Stieber J., Zong X., Biel M., Hofmann F., Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- 24.Weisbrod D., Peretz A., Ziskind A., Menaker N., Oz S., Barad L. SK4 Ca2+ activated K+ channel is a critical player in cardiac pacemaker derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:E1685–E1694. doi: 10.1073/pnas.1221022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kucka M., Kretschmannova K., Stojilkovic S.S., Zemkova H., Tomic M. Dependence of spontaneous electrical activity and basal prolactin release on nonselective cation channels in pituitary lactotrophs. Physiol Res. 2012;61:267–275. doi: 10.33549/physiolres.932301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi Z., Wong C.K., Suen C.H., Wang J., Long C., Sauer H. TRPC3 regulates the automaticity of embryonic stem cell-derived cardiomyocytes. Int J Cardiol. 2015;203:169–181. doi: 10.1016/j.ijcard.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Owsianik G., Talavera K., Voets T., Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 28.Doleschal B., Primessnig U., Wolkart G., Wolf S., Schernthaner M., Lichtenegger M. TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc Res. 2015;106:163–173. doi: 10.1093/cvr/cvv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J.D., Yu H.M., Wang R., Liang J., Yang H.T. Developmental regulation of intracellular calcium transients during cardiomyocyte differentiation of mouse embryonic stem cells. Acta Pharmacol Sin. 2006;27:901–910. doi: 10.1111/j.1745-7254.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 30.Fu J.D., Jiang P., Rushing S., Liu J., Chiamvimonvat N., Li R.A. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev. 2010;19:773–782. doi: 10.1089/scd.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viatchenko-Karpinski S., Fleischmann B.K., Liu Q., Sauer H., Gryshchenko O., Ji G.J. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc Natl Acad Sci U S A. 1999;96:8259–8264. doi: 10.1073/pnas.96.14.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapoor N., Maxwell J.T., Mignery G.A., Will D., Blatter L.A., Banach K. Spatially defined InsP3-mediated signaling in embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2014;9:e83715. doi: 10.1371/journal.pone.0083715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S.W., Lee H.A., Moon S.H., Park S.J., Kim H.J., Kim K.S. Spontaneous inward currents reflecting oscillatory activation of Na/Ca exchangers in human embryonic stem cell-derived cardiomyocytes. Pflugers Arch. 2015 Dec 21 doi: 10.1007/s00424-015-1769-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Fu J.D., Yang H.T. Developmental regulation of intracellular calcium homeostasis in early cardiac myocytes. Sheng Li Xue Bao. 2006;58:95–103. [PubMed] [Google Scholar]
- 35.Blatter L.A., Huser J., Rios E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks in cardiac muscle. Proc Natl Acad Sci U S A. 1997;94:4176–4181. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer H., Theben T., Hescheler J., Lindner M., Brandt M.C., Wartenberg M. Characteristics of calcium sparks in cardiomyocytes derived from embryonic stem cells. Am J Physiol Heart Circ Physiol. 2001;281:H411–H421. doi: 10.1152/ajpheart.2001.281.1.H411. [DOI] [PubMed] [Google Scholar]
- 37.Yang H.T., Tweedie D., Wang S., Guia A., Vinogradova T., Bogdanov K. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A. 2002;99:9225–9230. doi: 10.1073/pnas.142651999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu J.D., Li J., Tweedie D., Yu H.M., Chen L., Wang R. Crucial role of the sarcoplasmic reticulum in the developmental regulation of Ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB J. 2006;20:181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 39.Lieu D.K., Liu J., Siu C.W., McNerney G.P., Tse H.F., Abu-Khalil A. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bootman M.D., Collins T.J., Mackenzie L., Roderick H.L., Berridge M.J., Peppiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 41.Kapur N., Banach K. Inositol-1,4,5-trisphosphate-mediated spontaneous activity in mouse embryonic stem cell-derived cardiomyocytes. J Physiol. 2007;581:1113–1127. doi: 10.1113/jphysiol.2006.125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo A., Yang H.T. Ca2+ removal mechanisms in mouse embryonic stem cell-derived cardiomyocytes. Am J Physiol Cell Physiol. 2009;297:C732–C741. doi: 10.1152/ajpcell.00025.2009. [DOI] [PubMed] [Google Scholar]
- 43.Dolnikov K., Shilkrut M., Zeevi-Levin N., Gerecht-Nir S., Amit M., Danon A. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Fu J.D., Siu C.W., Li R.A. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 45.Satin J., Itzhaki I., Rapoport S., Schroder E.A., Izu L., Arbel G. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 46.Li S., Cheng H., Tomaselli G.F., Li R.A. Mechanistic basis of excitation-contraction coupling in human pluripotent stem cell-derived ventricular cardiomyocytes revealed by Ca2+ spark characteristics: direct evidence of functional Ca2+-induced Ca2+ release. Heart Rhythm. 2014;11:133–140. doi: 10.1016/j.hrthm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Zhu W.Z., Santana L.F., Laflamme M.A. Local control of excitation-contraction coupling in human embryonic stem cell-derived cardiomyocytes. PLoS ONE. 2009;4:e5407. doi: 10.1371/journal.pone.0005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedan O., Dolnikov K., Zeevi-Levin N., Fleishmann N., Spiegel I., Berdichevski S. Human embryonic stem cell-derived cardiomyocytes can mobilize 1,4,5-inositol trisphosphate-operated [Ca2+]i stores: the functionality of angiotensin-II/endothelin-1 signaling pathways. Ann N Y Acad Sci. 2010;1188:68–77. doi: 10.1111/j.1749-6632.2009.05085.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Lieu D.K., Siu C.W., Fu J.D., Tse H.F., Li R.A. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol. 2009;297:C152–C159. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]