Abstract
Background
Cadmium (Cd), a nonessential heavy metal, is a major environmental and public health concern. Oxidative stress plays an important role in Cd-induced kidney dysfunction. Tinospora cordifolia, a medicinal plant rich in phytochemicals, possesses antioxidant activity. The objective of the present study was to assess the protective effect of Tinospora cordifolia-stem methanolic extract (TCE) on Cd-induced nephrotoxicity in Wistar rats.
Methods
Male Wistar rats were administered ∼5 mg/kg body weight Cd orally and 100 mg/kg body weight TCE for 28 days. At the end of Cd and TCE treatment, biochemical assays were performed in serum and tissue homogenate.
Results
Cd-induced oxidative stress in the kidney resulted in increased levels of lipid peroxidation and protein carbonyl content with a significant decrease in cellular antioxidants, such as reduced GSH, SOD, CAT, GPX, and GST. Cd-induced nephrotoxicity was further confirmed by marked changes in the histology of the kidney and increased levels of kidney markers. Additionally, Cd-treated rats showed alterations in membrane-bound ATPase activity and decreased levels of tissue glycoproteins. Cotreatment with TCE considerably reduced the biochemical alterations in serum and renal tissue induced by Cd, and also restored ATPase activity and glycoproteins to near normal levels.
Conclusion
Our results suggested that TCE with its antioxidant effect offered cytoprotection against Cd-induced toxicity in kidneys by restoring the altered cellular antioxidants and renal markers. TCE treatment for 28 days reversed ATPase activity and tissue glycoprotein levels. These results revealed the protective effect of TCE on Cd-induced toxicity in kidneys and oxidative stress.
Keywords: antioxidants, Cd, kidney, oxidative stress, Tinospora cordifolia
1. Introduction
Cadmium (Cd) is one of the most abundant toxic metals in the environment, and is known to cause adverse effects in humans.1 Cd is widely used in industrial processes as anticorrosive agents, stabilizers in PVC plastic products, pigments, neutron absorbers in nuclear power plants, in the fabrication of nickel-Cd batteries, and is found in soil through the use of fertilizers for agriculture.2 Humans are generally exposed to Cd via drinking water, food, and by the inhalation of cigarette smoke. The International Agency for Research on Cancer classified Cd as a known human carcinogen in 1993, and it is ranked as the seventh toxicant in the priority list of hazardous substances on the Agency for Toxic Substances and Disease Registry.3 Cd stress causes typical changes in seed germination, growth, enzymatic and nonenzymatic antioxidants, and phytochelating synthase gene expression in plants.4 Cd has a long biological half-life in humans (> 10 years) and accumulates in vital organs, such as the kidney, liver, lungs, heart, and spleen.5, 6 Cd mediates its toxic effects via oxidative damage to cellular organelles through indirect generation of reactive oxygen species (ROS) by Fenton chemistry.1, 7 Cd-induced renal toxicity is mediated through a small cysteine-rich metal-binding protein metallothionein (MT), which carries Cd to the kidney as a Cd-MT complex by glomerular filtration. The Cd-MT complex is then taken up by renal tubular cells, followed by the catabolism of MT in lysosomes, which leads to the release of free Cd ions that cause membrane damage.8 Another important mechanism of Cd toxicity is the depletion of glutathione and protein-bound sulfhydryl groups, resulting in enhanced ROS production. This causes increased lipid peroxidation, enhanced excretion of urinary lipid metabolites, modulation of intracellular oxidized states, DNA damage, membrane damage, altered gene expression, and apoptosis.9 When the kidney MT defense and detoxification systems are overwhelmed, free Cd damages the renal tubules.10
In recent years, ayurvedic medicine became popular due to its advantages. In India, medicinal plants are used for various therapeutic purposes in Ayurvedic and folk medicines.11 Plants are the ideal source of drugs, which are currently available. The herb Tinospora cordifolia (Menispermaceae) belongs to a group of medicinal plants known as guduchi that grow in the tropical and subtropical regions of India. It is a large, glabrous, succulent, deciduous climbing shrub.12 The plant has been used in ayurveda as rasayanas for the treatment of diabetes, rheumatoid arthritis, gout, and infections. The stem is a bitter, stomachic, diuretic that stimulates bile secretion, enriches the blood, cures jaundice, and has been considered an indigenous source of medicine.13, 14 A variety of compounds isolated from this plant belongs to different classes, such as alkaloids, diterpenoid lactones, glycosides, steroids, phenolics, and polysaccharides.15 The plant is well known for its hepatoprotective, immunostimulatory, antidiabetic, radioprotective, anti-inflammatory, anticancer, and free-radical scavenging activity.12, 13 Reports indicated the antioxidant potential of T. cordifolia-stem extracts in vitro16 and in vivo in diabetic rat models.12 Since Cd-induced renal damage is mediated through free radical generation, the antioxidant potential of T. cordifolia-stem methanolic extract (TCE) might help in combating against the oxidative stress induced by Cd. Thus, the aim of the present study was to evaluate the protective role of TCE on Cd-induced nephrotoxicity in Wistar rats.
2. Methods
2.1. Chemicals
Cd chloride was obtained from Sigma Aldrich Pvt. Ltd. (Bangalore, India). T. cordifolia was collected from the Western Ghats near Palaghat, Kerala, India, during the season of November to January. Plant material was authenticated by a taxonomist and submitted in herbarium collections at Bharathiar University, Coimbatore, India for reference. Methanolic extract of T. cordifolia was prepared by the soxhlet-extraction method. Dried and powdered stem of T. Cordifolia was extracted first with petroleum ether to remove lipids and fats, and then the residue was extracted again with methanol. The extract solution was evaporated under reduced pressure at 40 °C, concentrated using a rotary vapor evaporator, and used for further assays and experiments. All other chemicals used were of analytical grade and were purchased from HiMedia Laboratories (Mumbai, India).
2.2. Animals
Male albino Wistar rats were purchased from Small Animal Breeding Station, College of Veterinary and Animal Sciences, Mannuthy, Thrissur, Kerala, India. Adult male Wistar rats aged 100–120-days and weighing 200 ± 20 g were used for the study. After acclimatization, animals were grouped six rats per cage and maintained at a temperature of 25 ± 2 °C with a normal 12-hour light/dark cycle. The animals were fed with commercially available pelleted rat chow (SaiDurga Pvt. Ltd., Bangalore, India) and water ad libitum. The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, and the study was approved by the Institutional Animals Ethics Committee at Bharathiar University, Coimbatore, India.
2.3. Treatment schedule
Cd chloride (5 mg/kg) dissolved in water and TCE (100 mg/kg) dissolved in 0.3% carboxymethyl cellulose (CMC), were administered to rats orally for 28 days. This dosage was chosen based on previous reports and pilot studies (results not shown).1
2.4. Experimental procedure
The animals were divided into four groups with a minimum of six rats in each group: Group I, Control; Group II, treated with Cd chloride [Cd; 5 mg/kg body weight (BW)]; Group III, treated with TCE (100 mg/kg BW); and Group IV, treated with Cd chloride (5 mg/kg BW) and TCE (100 mg/kg BW0; Cd + TCE.
After 28-days treatment, the animals were deprived of food overnight, anesthetized by exposure to diethyl ether, and then sacrificed by cervical decapitation. Blood was collected and serum was separated and used for kidney marker assays. The kidney tissue was dissected, washed in ice-cold saline, patted dry, and weighed. A small portion of the tissue was stored in 10% formalin for histopathological examination. From the remaining tissue, 100 mg was weighed and homogenized in chilled 0.1 M Tris-HCl buffer in a Potter-Elvejhem Teflon homogenizer (REMI, Mumbai, India). The homogenate was used for biochemical investigation.
2.5. Determination of lipid peroxidation and protein carbonyl content
Lipid peroxidation and total protein carbonyl content in kidney tissue homogenate were estimated according to the method of Ohkawa et al17 and Levine et al,18 respectively.
2.6. Determination of cellular antioxidant status
Oxidative stress was measured by estimating the enzymatic and nonenzymatic antioxidant concentrations, such as reduced glutathione (GSH),19 superoxide dismutase (SOD),20 catalase (CAT),21 glutathione peroxidase (GPX),22 and glutathione-s-transferase (GST)23 in kidney tissue homogenate.
2.7. Serum kidney marker analysis
Blood samples were allowed to clot at room temperature and then centrifuged at 1200 g for 15 minutes. The clear serum was separated and used for biochemical assays. Renal function was assessed by measurement of serum urea24 and creatinine levels.25
2.8. Histopathological examination
A small portion of kidney tissue from the experimental animals was fixed in 10% neutral buffered formalin. The formalin-stored tissue was processed using standard procedures of paraffin embedding, and ∼5-μm sections were cut and stained with hematoxylin and eosin dye. The histological changes were studied by light microscopy.
2.9. Determination of ATPase activity and tissue glycoproteins
Membrane-bound ATPase enzyme activities, namely Na+K+ATPase,26 Ca2+ATPase,27 and Mg2+ATPase,28 and tissue glycoproteins, such as hexose,29 hexosamine,30 fucose,31 and sialic acid,32 were measured in the tissue homogenate.
2.10. Statistical analysis
Biochemical data were analyzed using one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test with the aid of the SPSS version 17 statistical package program (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effect of TCE on Cd-induced decrease in organ and body weight
During the treatment period, the BW of the animals was measured intermittently. A significant decrease in the BW of Cd-treated rats was observed when compared to controls. However, TCE cotreatment with Cd showed a significant increase in BW when compared to Cd-alone-treated rats. Organ weight of the Cd-treated animals was also found to be significantly decreased when compared to control animals, whereas in Cd and TCE cotreated groups, the organ weight was found to be increased when compared to Cd-alone-treated rats (Table 1).
Table 1.
Effect of Tinospora cordifolia extract on body weight and organ weight of Cd-treated rats
| Treatment group | Body weight (g) | Organ weight (g) |
|---|---|---|
| Group I | 240.83 ± 9.34 | 1.59 ± 0.03 |
| Group II | 217.50 ± 4.95* | 1.41 ± 0.03* |
| Group III | 249.16 ± 8.40† | 1.65 ± 0.01† |
| Group IV | 242.50 ± 9.50‡ | 1.57 ± 0.07‡ |
Data are presented as mean ± standard error (n = 6).
p < 0.001, compared to control.
Not significant as compared to control.
p < 0.001, compared to Cd-treated group.
Cd, cadmium; Group I, Control; Group II, treated with Cd chloride [5 mg/kg body weight (BW)]; Group III, treated with Tinospora cordifolia-stem methanolic extract (TCE; 100 mg/kg BW); Group IV; treated with Cd chloride (5 mg/kg BW) and TCE (100 mg/kg BW); Cd + TCE.
3.2. TCE lowers Cd-induced oxidative stress
Results of the present study showed a significant increase in the lipid peroxidation and protein carbonyl content, with significant decreases in the levels of cellular antioxidants, such as GSH, SOD, CAT, GPX, and GST, in rats treated with Cd when compared to the control group. Cotreatment of TCE with Cd significantly decreased the lipid peroxidation and protein carbonyl contents, whereas the antioxidant status was found to be significantly increased when compared to Cd-treated rats (Table 2).
Table 2.
Effect of Tinospora cordifolia extract treatment on lipid peroxidation, protein carbonyl content, endogenous antioxidant levels, and the activities of antioxidant enzymes in Cd-induced oxidative stress in rats
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| LPO | 44.93 ± 1.93 | 61.81 ± 3.34* | 45.32 ± 0.72‡ | 47.07 ± 2.35† |
| PCC | 1.22 ± 0.02 | 1.5 ± 0.03* | 1.25 ± 0.01‡ | 1.25 ± 0.02† |
| GSH | 366.47 ± 0.26 | 359.99 ± 0.09* | 365.97 ± 0.54‡ | 363.98 ± 0.26† |
| SOD | 100 ± 0.34 | 85.95 ± 0.58* | 99.7 ± 0.64‡ | 93.15 ± 0.91† |
| CAT | 100 ± 1.46 | 78.62 ± 1.92* | 99.13 ± 2.87‡ | 86.2 ± 1.98† |
| GST | 100 ± 1.8 | 80.22 ± 0.92* | 97.91 ± 1.09‡ | 89.82 ± 2.1† |
| GPX | 100 ± 1.04 | 90.89 ± 0.62* | 100.85 ± 1.14‡ | 96.22 ± 0.84† |
Data are presented as mean ± standard error (n = 6). Units are expressed as follows: LPO, μmoles of TBA reactants/mg of protein and PCC, μmoles of pcc/mg of protein. The specific activities of the enzymes were calculated as units/mg of protein, and expressed as % relative activity by comparing with control.
p < 0.001, compared to control.
p < 0.001, compared to the Cd-treated group.
Not significant as compared to the control.
CAT, catalase; Cd, cadmium; Group I, Control; Group II, treated with Cd chloride [5 mg/kg body weight (BW)]; Group III, treated with TCE (100 mg/kg BW); Group IV; treated with Cd chloride (5 mg/kg BW) and TCE (100 mg/kg BW); Cd + TCE; GSH, glutathione; GSX, glutathione-s-transferase; GPX, glutathione peroxidase; LPO, lipid peroxidation; PCC, propionyl-CoA carboxylase; SOD, superoxide dismutase; TCE, Tinospora cordifolia-stem methanolic extract.
3.3. Effect of TCE on kidney serum markers and Cd-induced histopathological alterations
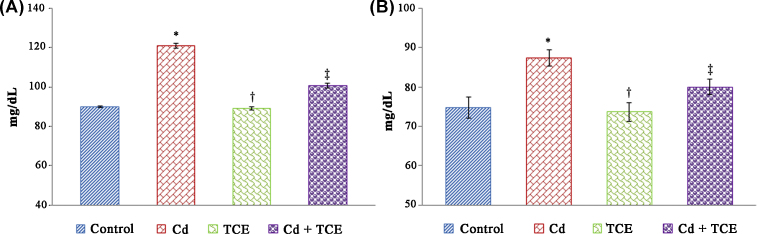
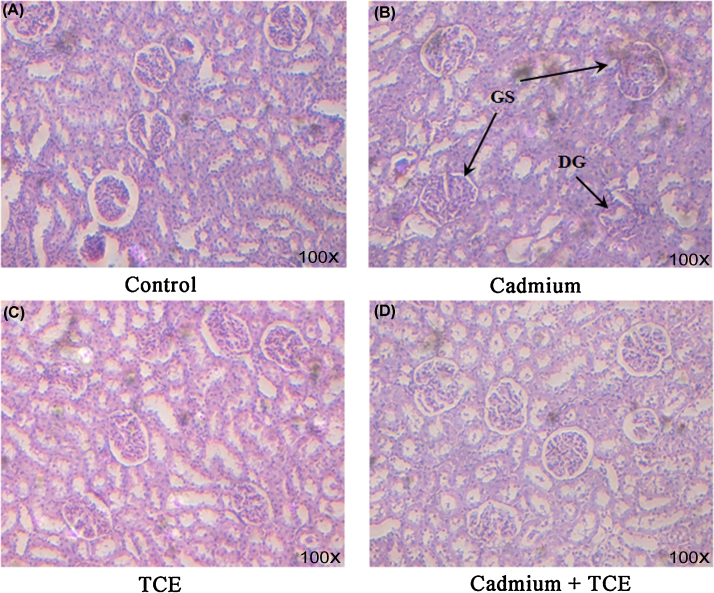
A significant increase in the levels of urea and creatinine in serum was observed in the Cd-treated rats when compared to the controls. Cotreatment with TCE along and Cd restored the levels of urea and creatinine to near normal levels when compared to rats treated with Cd alone (Fig. 1). Histopathological examination of kidney tissue of rats treated with Cd showed marked alterations compared to control rats. Degeneration of glomeruli, glomerular swelling, and thickening of the mesangium of Bowman's capsule were observed in Cd-treated rats, whereas TCE and Cd cotreated rats showed normal tissue architecture with minimal alterations when compared to Cd-alone-treated rats (Fig. 2).
Fig. 1.
Protective effect of Tinospora cordifolia extract on serum markers in Cd-treated rats. (A) Serum urea. (B) Serum creatinine.
Each bar represents the mean ± standard error of the mean for each group.
* p < 0.001, compared to control.
‡p < 0.001, compared to the Cd-treated group.
† Not significant as compared to the control (one way analysis of variance, followed by Tukey's multiple comparison).
Cd, cadmium.
Fig. 2.
Histology of kidney tissue. (A) Control, (B) Cd, (C) TCE, (D) Cd + TCE.
Magnification: 100×.
Cd, cadmium; DG, degeneration of glomeruli, GS, glomerular swelling; TCE, Tinospora cordifolia-stem methanolic extract.
3.4. Effect of TCE and Cd on membrane-bound ATPase activities
The animals treated with Cd showed a significant decrease in both Na+ and K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase activity when compared to control rats. Cotreatment with TCE and Cd resulted in a significant increase in Na+ K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase activities when compared to the Cd-alone-treated group (Table 3).
Table 3.
Effect of Tinospora cordifolia extract on the activities of membrane-bound ATPases in Cd-induced nephrotoxicity
| Membrane-bound ATPases | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Na+ K+ ATPase | 9.03 ± 0.3 | 8.38 ± 0.06* | 9.22 ± 0.61‡ | 8.76 ± 0.09† |
| Ca2+ ATPase | 7.48 ± 0.64 | 5.38 ± 0.25* | 7.55 ± 0.42‡ | 6.94 ± 0.31† |
| Mg2+ ATPase | 7.91 ± 0.15 | 5.9 ± 0.04* | 7.97 ± 0.52‡ | 7.53 ± 1.20† |
Data are presented as mean ± standard error (n = 6) and nmols of pi-liberated/mg of protein.
p < 0.001, compared to control.
p < 0.001, compared to the Cd-treated group.
Not significant as compared to control.
Cd, cadmium; Group I, Control; Group II, treated with Cd chloride [5 mg/kg body weight (BW)]; Group III, treated with Tinospora cordifolia-stem methanolic extract (TCE; 100 mg/kg BW); Group IV; treated with Cd chloride (5 mg/kg BW) and TCE (100 mg/kg BW); Cd + TCE.
3.5. Effect of TCE and Cd on tissue glycoproteins levels
The results of our study showed a significant decrease in tissue glycoproteins, such as hexose, hexosamine, fucose, and sialic acid in rats that received Cd when compared to control rats. However, cotreatment with TCE and Cd significantly increased the levels of tissue glycoproteins when compared to Cd-alone-treated rats (Table 4).
Table 4.
Effect of Tinospora cordifolia extract on the levels of tissue glycoproteins in Cd-induced nephrotoxicity
| Glycoprotein | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Hexose | 477.09 ± 14.98 | 392.71 ± 22.57* | 477.09 ± 13.70‡ | 471.79 ± 17.98† |
| Hexoseamine | 212.33 ± 15.28 | 153.68 ± 9.36* | 210.64 ± 9.36‡ | 186.32 ± 18.72† |
| Fucose | 65.71 ± 2.34 | 52.74 ± 2.78* | 67.74 ± 3.83‡ | 61.64 ± 1.91† |
| Sialic acid | 190.58 ± 13.36 | 158.22 ± 10.95* | 192.66 ± 12.2‡ | 173.2 ± 7.26† |
Data are presented as mean ± standard error (n = 6) and as μg/g of tissue.
p < 0.001, compared to control.
p < 0.001, compared to the Cd-treated group.
Not significant as compared to the control.
Cd, cadmium; Group I, Control; Group II, treated with Cd chloride [5 mg/kg body weight (BW)]; Group III, treated with Tinospora cordifolia-stem methanolic extract (TCE; 100 mg/kg BW); Group IV; treated with Cd chloride (5 mg/kg BW) and TCE (100 mg/kg BW); Cd + TCE.
4. Discussion
Cd has been recognized as one of the most toxic environmental and industrial pollutants. Chronic exposure of Cd causes damage to numerous organs and systems primarily the kidneys.33 Cd induces oxidative damage by disturbing the antioxidant balance in tissues.34 Cd-induced renal toxicity was reported earlier by Ahn et al.35 Neutralizing heavy-metal-induced toxicity by use of plant-derived components or extracts with antioxidant potential is of great interest in recent research. T. cordifolia has a long history of use in ayurvedic medicine due to its immense medicinal properties.36 The presence of polyphenols and flavonoids with antioxidant potential in TCE was reported by Sivakumar et al.37 The present study assessed the protective role of TCE on Cd-induced nephrotoxicity in experimental rats. We observed a significant decrease in BW of Cd-treated rats. TCE cotreatment resulted in a significant increase in the BW of rats. This may be due to the presence of nutritional compounds, such as flavonoids and phenolic compounds, present in TCE extract. The decrease in BW upon Cd treatment and antioxidant-mediated restoration of weight gain had been reported previously by Milton Prabu et al.38
Cd intoxication causes oxidative stress in the cells through the generation of free radicals, such as hydroxyl radical, superoxide radical, and nitrogen species, such as peroxy nitrite and nitric oxide, which, in turn, lead to destabilization and disintegration of the cell membrane as a result of lipidperoxidation.9, 39 Cd-treated rats showed an increased level of lipid peroxidation and protein carbonyl content, and cotreatment with TCE and Cd resulted in decreased levels of lipid peroxidation and protein carbonyl content. Cd toxicity causes modifications to many enzymes as a result of increased levels of protein carbonylation and decreased levels of protein thiol groups.40 Reddy et al12 reported the protective effect of TCE in reducing lipid peroxidation and protein carbonyl content; our findings were in line with these reports.
ROS generated in tissues are normally scavenged by cellular enzymatic and nonenzymatic antioxidants, which protect cells from oxidative stress. GSH is an important nonenzymatic antioxidant that plays a major role in antioxidant defense by direct scavenging of free radicals through its thiol groups. The level of GSH is used to assess oxidative stress and chemo preventive ability.41 GSH levels were found to be decreased in Cd-treated rats, possibly due to the binding of Cd to the GSH thiol group. GSH depletion leads to enhanced lipid peroxidation, which, in turn, consumes more GSH.42 In the present study, TCE cotreatment resulted in a significant increase in GSH levels. This might be due to the free-radical scavenging and glutathione-sparing action of TCE during Cd-induced lipid peroxidation. This result was consistent with previous findings published by Hamas and Kutten,41 where TCE enhanced GSH content against cyclophosphamide-induced urotoxicity.
Our results indicated a significant decrease in the activities of enzymatic antioxidants, such as SOD, CAT, GPX, and GST, in the kidneys of rats exposed to Cd treatment. GPX, GST, and CAT, which act as preventive antioxidants, and SOD, a chain-breaking antioxidant, play an key role in protection against the harmful effects of lipid peroxidation.43 GSH unavailability reduces the activities of GPX and GST, as noted from a previous report.44 Our result supported the previous report of Renugadevi and Prabu.39 However, TCE cotreatment with Cd resulted in a significant increase in these enzymatic antioxidants. This may be due to the presence of natural antioxidants in TCE, which help improve the antioxidant status of TCE and CD cotreated rats. Similar results were reported by Sangeetha et al45 and Agrawal et al46 in a diabetic rat model.
High blood levels of urea and creatinine indicate an inability of the kidney to excrete urea and creatinine, resulting in renal disease.47 Cd-treated rats showed increased levels of urea and creatinine in serum. The results of serum kidney markers revealed that Cd induced severe damage to the kidney tissues. These results agreed with the previous report of Renugadevi and Prabu,39 in which urea and creatinine levels were reduced in the urine of Cd-intoxicated rats. Serum urea and creatinine levels were decreased in Cd and TCE cotreated groups. Our results were in agreement with the earlier report of Hamsa and Kuttan,41 who reported reduced blood urea nitrogen levels in cyclophosphamide-induced urotoxicity in Swiss albino mice models after TCE treatment.
Histopathological studies revealed alterations in the kidney architecture of Cd-treated rats. The alterations of damage included degeneration of glomeruli, glomerular swelling, and thickening of the mesangium of Bowman's capsule. Similar results were reported earlier by Tripathi and Srivastav,6 where cotreatment with TCE offered significant protection against Cd-induced histological changes and maintained normal tissue architecture. Agrawal et al46 also reported that TCE restored the normal histological structure in diabetes-induced damage in rat kidneys.
Glycoproteins are carbohydrate-linked protein macromolecules found on the cell surface, and form the principle component of cell membrane and membranes of subcellular organelles.48 Oxidation of protein is a common phenomenon mediated by high ROS levels, and oxidized proteins, in turn, induce oxidative stress, a potential mediator of pathogenic conditions.42 Matte et al49 reported a significant decrease in the tissue glycoprotein content of liver upon homocysteine-induced oxidative stress in rats. In the present study, a significant reduction in the content of tissue glycoproteins, such as hexose, hexosamine, fucose, and sialic acid, was observed in Cd-treated rats, which is in line with previously mentioned reports. The decline in tissue glycoprotein content might be due to inhibition of glycoprotein synthesis. The TCE cotreatment along with Cd significantly increased the tissue glycoprotein content, which might be attributed to the antioxidant activity of TCE and reduced oxidative stress in this group.
Na+ K+ ATPase, Ca2+ ATPase, and Mg2+ ATPase are membrane-bound enzymes that play a major role in maintaining the electrolyte balance in cells. In the kidney, Na+ K+ ATPase catalyses active transport of Na+ in exchange for K+, maintains intracellular ion balance and membrane potential, and is also responsible for maintaining the Na+ gradient. In proximal tubules, glucose and amino acid reabsorption is achieved due to the Na+ K+ ATPase-generated Na+ gradient. Thus, alterations in Na+ K+ ATPase functions are related to development of several pathological conditions.50 Lipid peroxidation of the biological membrane results in changes in its fluidity and activities of membrane-bound enzymes.51 Previous studies reported that Cd could induce lipid peroxidation and subsequently decrease the activity of membrane-bound Na+ K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase.38, 52 The decreased activity of Na+ K+ ATPase might be due to proteolysis of Na+ K+ ATPase through the proteasome system and lysosomal proteases.52 Our results also showed decreased activity of Na+ K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase enzymes upon Cd treatment, and are in line with the above reports. Combined treatment with Cd and TCE resulted in a significant increase in Na+ K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase enzyme activities. Treatment with TCE alone did not result any significant effect on these enzymes. Maintenance of the levels of Na+ K+ ATPase, Mg2+ ATPase, and Ca2+ ATPase enzyme activities by TCE treatment was consistent with the results of Bafna and Balaraman.53
To conclude, our results revealed that cotreatment with TCE offered protection against Cd-induced renal toxicity in rats, as evidenced by serum markers and histopathological analysis. The results indicated that toxicity induced by Cd leads to kidney dysfunction by increasing lipid peroxidation and altering its cellular antioxidant system. TCE offered protection to the kidney by inhibiting lipid peroxidation, reducing the protein carbonyl content, strengthening the cellular antioxidant pool, and maintaining membrane ATPase activities. Our results revealed that TCE efficiently protects the kidney from oxidative damage caused by Cd through minimizing cell membrane disturbances and tissue damage.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
R.B. acknowledges the Director, DRDO BU CLS, Coimbatore, and DIPAS, New Delhi, for providing financial assistance. All authors thank S. Kalaiselvi for her help in collection of the T. cordifolia plant and extraction. The authors acknowledge the financial support of UGC (SAP) and DST (FIST) to the Department of Biotechnology, Bharathiar University.
References
- 1.Renugadevi J., Prabu S.M. Cd-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Godt J., Scheidig F., Grosse-Siestrup C., Esche V., Brandenburg P., Reich A. The toxicity of Cd and resulting hazards for human health. J Occup Med Toxicol. 2006;1:22. doi: 10.1186/1745-6673-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pari L., Shagirtha K. Hesperetin protects against oxidative stress-related hepatic dysfunction by Cd in rats. Exp Toxicol Pathol. 2012;64:513–520. doi: 10.1016/j.etp.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Shanmugaraj B.M., Chandra H.M., Srinivasan B., Ramalingam S. Cd-induced physio-biochemical and molecular response in Brassica juncea. Int J Phytoremediation. 2013;15:206–218. doi: 10.1080/15226514.2012.687020. [DOI] [PubMed] [Google Scholar]
- 5.Morales A.I., Vicente-Sanchez C., Sandoval J.M., Egido J., Mayoral P., Arevalo M.A. Protective effect ofquercetin on experimental chronic Cd nephrotoxicity in rats is based on its antioxidantproperties. Food Chem Toxicol. 2006;44:2092–2100. doi: 10.1016/j.fct.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi S., Srivastav A.K. Cytoarchitectural alterations in kidney of Wistar rats after oral exposure to Cd chloride. Tissue cell. 2011;43:131–136. doi: 10.1016/j.tice.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe M., Henmi K., Ogawa Ki, Suzuki T. Cd-dependent generation of reactive oxygen species and mitochondrial DNA breaks in photosynthetic and non-photosyntheticstrains of Euglena gracilis. Comp Biochem Physiol C Toxicol Pharmacol. 2003;134:227–234. doi: 10.1016/s1532-0456(02)00253-3. [DOI] [PubMed] [Google Scholar]
- 8.Nordberg G.F. Cd and health in the 21st century–historical remarks and trends for the future. Biometals. 2004;17:485–489. doi: 10.1023/b:biom.0000045726.75367.85. [DOI] [PubMed] [Google Scholar]
- 9.Stohs S.J., Bagchi D., Hassoun E., Bagchi M. Oxidative mechanisms in the toxicity of chromium and Cd ions. J Environ Pathol Toxicol Oncol. 2000;19:201–213. [PubMed] [Google Scholar]
- 10.Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and Cd toxicity. Altern Med Rev. 2003;8:106–128. [PubMed] [Google Scholar]
- 11.Grover J.K., Vats V., Rathi S.S., Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76:233–238. doi: 10.1016/s0378-8741(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 12.Reddy S.S., Ramatholisamma P., Karuna R., Saralakumari D. Preventive effect of Tinospora cordifolia against high-fructose diet-induced insulin resistance and oxidative stress in male Wistar rats. Food Chem Toxicol. 2009;47:2224–2229. doi: 10.1016/j.fct.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Joladarashi D., Chilkunda N., Salimath P. Glucose uptake-stimulatory activity of Tinospora cordifolia-stem extracts in Ehrlich ascites tumor cell model system. J Food Sci Technol. 2014;51:178–182. doi: 10.1007/s13197-011-0480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S.S., Pandey S.C., Srivastava S., Gupta V.S., Patro B., Ghosh A.C. Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J Pharmacol. 2003;35:83–91. [Google Scholar]
- 15.Patel M.B., Mishra S. Isoquinoline Alkaloids from Tinospora cordifolia inhibit rat lens aldose reductase. Phytother Res. 2012;26:1342–1347. doi: 10.1002/ptr.3721. [DOI] [PubMed] [Google Scholar]
- 16.Pushp P., Sharma N., Joseph G.S., Singh R.P. Antioxidant activity and detection of (−)epicatechin in the methanolic extract of stem of Tinospora cordifolia. J Food Sci Technol. 2013;50:567–572. doi: 10.1007/s13197-011-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 19.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 20.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 21.Takahara S., Hamilton H.B., Neel J.V., Kobara T.Y., Ogura Y., Nishimura E.T. Hypocatalasemia: a new genetic carrier state. J Clin Invest. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 23.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic stepin mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 24.Natelson S., Scott M.L., Beffa C. A rapid method for the estimation of urea in biologic fluids. Am J Clin Pathol. 1951;21:275–281. doi: 10.1093/ajcp/21.3_ts.275. [DOI] [PubMed] [Google Scholar]
- 25.Brod J., Sirota J.H. The renal clearance of endogenous “creatinine” in man. J Clin Invest. 1948;27:645–654. doi: 10.1172/JCI102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bontings S.L. Sodium-potassium activated adenosine triphosphatase and cation transport. In: Bittar E.E., editor. Membranes and ion transport. Wiley-Interscience; London: 1970. pp. 257–263. [Google Scholar]
- 27.Hjerten S., Pan H. Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi T., Suzuki T., Suzuki Y., Ozawa K. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating, and malignant cells. Biochim Biophys Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- 29.DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 30.Wagner W.D. A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem. 1979;94:394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 31.Dische Z., Shettles L.B. A specific color reaction of methylpentoses and aspectrophotometric micromethod for their determination. J Biol Chem. 1948;175:595–603. [PubMed] [Google Scholar]
- 32.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 33.Jin T., Nordberg M., Frech W., Dumont X., Bernard A., Ye Tt. Cd biomonitoring and renal dysfunction among a population environmentally exposed to Cd from smelting in China (ChinaCad) Biometals. 2002;15:397–410. doi: 10.1023/a:1020229923095. [DOI] [PubMed] [Google Scholar]
- 34.Ognjanovic B.I., Markovic S.D., Pavlovic S.Z., Zikic R.V., Stajn A.S., Saicic Z.S. Effect of chronic Cd exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res. 2008;57:403–411. doi: 10.33549/physiolres.931197. [DOI] [PubMed] [Google Scholar]
- 35.Ahn D.W., Kim Y.M., Kim K.R., Park Y.S. Cd binding and sodium-dependent solute transport in renal brush-border membrane vesicles. Toxicol Appl Pharmacol. 1999;154:212–218. doi: 10.1006/taap.1998.8581. [DOI] [PubMed] [Google Scholar]
- 36.Rajalakshmi M., Eliza J., Priya C.E., Nirmala A., Daisy P. Antidiabetic properties of Tinospora cordifolia-stem extracts on streptozotocin- induced diabetic rats. Afr J Pharm Pharmacol. 2009;3:171–180. [Google Scholar]
- 37.Sivakumar V., Rajan M.S., Riyazullah M.S. Preliminary phytochemical screening and evaluation of free radical scavenging activity of Tinospora cordifolia. Int J Pharm Pharm Sci. 2010;2 [Google Scholar]
- 38.Milton Prabu S., Muthumani M., Shagirtha K. Quercetin potentially attenuates Cd induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Eur Rev Med Pharmacol Sci. 2013;17:582–595. [PubMed] [Google Scholar]
- 39.Renugadevi J., Prabu S.M. Quercetin protects against oxidative stress-related renal dysfunction by Cd in rats. Exp Toxicol Pathol. 2010;62:471–481. doi: 10.1016/j.etp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Moskovitz J., Yim M.B., Chock P.B. Free radicals and disease. Arch Biochem Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 41.Hamsa T.P., Kuttan G. Tinospora cordifolia ameliorates urotoxic effect of cyclophosphamide by modulating GSH and cytokine levels. Exp Toxicol Pathol. 2012;64:307–314. doi: 10.1016/j.etp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Ganesan B., Buddhan S., Anandan R., Sivakumar R., Anbin Ezhilan R. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep. 2010;37:1319–1327. doi: 10.1007/s11033-009-9508-4. [DOI] [PubMed] [Google Scholar]
- 43.Ray G., Husain S.A. Oxidants, antioxidants, and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- 44.Padma V.V., Devi C.S., Ramkumar K.M. Modulatory effect of fish oil on the myocardial antioxidant defense system in isoproterenol-induced myocardial infarction. J Basic Clin Physiol Pharmacol. 2006;17:1–15. doi: 10.1515/jbcpp.2006.17.1.1. [DOI] [PubMed] [Google Scholar]
- 45.Sangeetha M.K., Balaji Raghavendran H.R., Gayathri V., Vasanthi H.R. Tinospora cordifolia attenuates oxidative stress and distorted carbohydrate metabolism in experimentally induced type 2 diabetes in rats. J Nat Med. 2011;65:544–550. doi: 10.1007/s11418-011-0538-6. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal S.S., Naqvi S., Gupta S.K., Srivastava S. Prevention and management of diabetic retinopathy in STZ diabetic rats by Tinospora cordifolia and its molecular mechanisms. Food Chem Toxicol. 2012;50:3126–3132. doi: 10.1016/j.fct.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 47.Lieberman M., Marks A.D. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2008. Marks basic medical biochemistry: a clinical approach. [Google Scholar]
- 48.Zachariah B., Basu D. Surface carbohydrates in cell biology. Indian J Biochem Biol. 1993;30:422–425. [PubMed] [Google Scholar]
- 49.Matte C., Stefanello F.M., Mackedanz V., Pederzolli C.D., Lamers M.L., Dutra-Filho C.S. Homocysteine induces oxidative stress, inflammatory infiltration, fibrosis, and reduces glycogen/glycoprotein content in liver of rats. Int J Dev Neurosci. 2009;27:337–344. doi: 10.1016/j.ijdevneu.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Silva E., Pinto V., Simao S., Serrao M.P., Afonso J., Amaral J. Renal aging in WKY rats: changes in Na+, K+ -ATPase function andoxidative stress. Exp Gerontol. 2010;45:977–983. doi: 10.1016/j.exger.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Senthil S., Sridevi M., Pugalendi K.V. Cardioprotective effect of oleanolic acid onisoproterenol-induced myocardial ischemia in rats. Toxicol Pathol. 2007;35:418–423. doi: 10.1080/01926230701230312. [DOI] [PubMed] [Google Scholar]
- 52.Pari L., Murugavel P. Diallyl tetrasulfide improves Cd induced alterations of acetylcholinesterase, ATPases, and oxidative stress in brain of rats. Toxicology. 2007;234:44–50. doi: 10.1016/j.tox.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 53.Bafna P.A., Balaraman R. Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J Ethnopharmacol. 2004;90:123–127. doi: 10.1016/j.jep.2003.09.036. [DOI] [PubMed] [Google Scholar]