Abstract
Transient receptor potential (TRP) proteins are a family of ion channels, which are responsible for a wide array of cellular functions. In particular, TRP melastatin type (TRPM) 7 is expressed everywhere and permeable to divalent cations such as Mg2+ and Ca2+. It contains a channel and a kinase domain. Recent studies indicate that activation of TRPM7 plays an important role in the growth and survival of gastric cancer cells. In this review, we describe and discuss the findings of recent studies that have provided novel insights of the relation between TRPM7 and gastric cancer.
Keywords: gastric cancer, ion channel, proliferation, transient receptor potential melastatin 7, TRPM7
1. Introduction
Gastric cancer is responsible for almost 1 million deaths worldwide per year, and thus is an important global healthcare issue. Although the age-adjusted mortality of gastric cancer has decreased over the past few decades, it remains the third leading cause of cancer-related mortality.1 Cytosolic free Ca2+ concentration ([Ca2+]i) changes represent a ubiquitous signaling mechanism, which integrates with other signal-transduction cascades and controls a variety of cellular processes.2 Alterations of calcium signaling and homeostasis have wide ranging consequences, and it is not surprising that some Ca2+-mediated signaling pathways are implicated in tumorigenesis and tumor progression.3, 4, 5 Accordingly, Ca2+ channels are crucial for a wide variety of biological processes, including tumor development and cancer growth.6, 7 Transient receptor potential (TRP) proteins are a family of ion channels and are responsible for many cellular functions. They were initially identified in Drosophila, in which spontaneously occurring mutations in the trp and trpl genes selectively abolish the delayed, light-sensitive, sustained depolarization caused by Na+ and Ca2+ influx into photoreceptors.8 As a consequence, TRP Drosophila mutants exhibit transient rather than sustained light sensitive depolarization and receptor potentials, which are used to designate TRP channels.9 These channels have been shown to be gated by a variety of physical and chemical stimuli, such as stretch, temperature changes, and a large number of endogenous (e.g., diacylglycerol and Ca2+) and exogenous ligands. Furthermore, some of these channels have been reported to be activated by intracellular Ca2+-store depletion.8, 9, 10
Research over the years has added considerably to our knowledge of the expressions and functional aspects of TRP melastatin 7 (TRPM7) channels. TRPM7 currents have an outward-rectifying cation current with reversal potential of 0 mV. However, in the absence of external divalent cations, TRPM7 outward rectification is largely abolished.11 Also, TRPM7 has a property of more permeability with trace metal ions. Monteilh-Zoller et al11 published a permeation profile order for TRPM7, as follows: Zn2+ ≈ Ni2+ ≫ Ba2+ > Co2+ > Mg2+.
TRPM7 currents are evoked with pipette solutions lacking magnesium or magnesium adenosine triphosphate and activated constitutively in whole-cell configuration patch clamp experiments.12 TRPM7 has the same properties as TRPM6. First, they Exhibit 49% primary amino acid sequence identity. In addition, they are Mg2+ and Ca2+ permeable ion channels with a channel kinase (atypical α-kinases) and show Mg2+ and Ca2+ permeable ion channels.13, 14, 15 Recent studies have demonstrated that the TRPM7 channels can be modulated by 2-aminoethoxydiphenyl borate (2-APB). 2-APB can be used to differentiate between TRPM7 and TRPM6. 2-APB inhibits TRPM7 currents but potentiates TRPM6 at micromolar concentrations.15, 16 TRPM7 is also inhibited by carvacrol,17 5-lipoxygenase (LOX) inhibitors (NDGA, AA861, and MK886),18 nafamostat mesylate,19 Ca2+-activated small conductance K+ channel blocker NS8593,20 waixenicin A,21 sphingosine, and FTY720.22 TRPM7 channel activity is regulated by extracellular pH,23 and although the effects of protons on TRPM7 currents remain controversial,24, 25, 26 it is known that TRPM7 can be regulated by acidic conditions, which is associated with ischemic stroke.27, 28
TRPM7 channel is ubiquitously expressed in almost all tissues,29, 30 and several research groups31, 32, 33, 34 have suggested that it is closely associated with cellular growth and development under physiological conditions. Furthermore, the overexpression of TRPM7 in HEK-293 cells has been reported to lead to cell rounding and reduced adhesion and m-calpain activation,35, 36 which suggest that TRPM7 also plays a role in cell adhesion. In sympathetic neurons, synaptic vesicles have TRPM7 proteins, and in synaptic vesicles, synaptic vesicular synapsin 1, synaptotagmin 1, and snapin form complexes with TRPM7.37 In addition, TRPM7 channel has a characteristic to alter acetylcholine release in neurons.37 TRPM7 also has an important role in cardiac pathophysiology. TRPM7 is critical for myocardial proliferation during early cardiogenesis,38 and impaired automaticity in Trpm7-deleted sinoatrial node cells induces abnormal diastolic depolarization, associated with a slowed diastolic Ca2+ rise and reduced pacemaker current If (encoded by Hcn4 expression).39 In the gastrointestinal tract, TRPM7 protein is essentially required for the pacemaking activities of interstitial cells of Cajal.28, 40
Recently, many research studies have been conducted about the relationship between TRPM7 channel and cancer, such as the regulation of tumor proliferation, differentiation, apoptosis, angiogenesis, migration, and invasion.30, 31 Moreover, TRPM7 overexpression has been found in head and neck carcinoma,41 retinoblastoma,33 breast cancer,42 and in gastric cancer.43 Thus, TRPM7 channel is an important diagnostic and/or prognostic marker, and is a recognized target for pharmaceutical intervention. Nevertheless, further investigations are required to improve our understanding of the role of TRPM7 channel in cancer.28, 41 In this review, we discuss the findings of recent studies and provide novel insights of relations between TRPM7 and gastric cancer.
2. Gastric cancer
Gastric cancer is responsible for considerable morbidity and mortality, and is the third leading cause of cancer-related death in men and women.1 Clinically, the symptoms of gastric cancer tend to emerge late during disease development, and thus treatment options are often limited. However, the search to find an optimal treatment continues amid improvements in our understanding of key aspects of its pathogenesis, which undoubtedly improve the likelihood of our identifying potential therapeutic targets. The majority of gastric cancers are adenocarcinomas, and traditionally gastric cancer is classified as intestinal or diffuse, as described by Lauren.44 In general, gastric cancer arises from a gastric change, such as atrophic gastritis, which then develops into intestinal metaplasia and dysplasia. Moreover, Helicobacter pylori infection often induced gastric cancer through a chronic inflammation.45 By contrast, diffuse gastric cancer is associated with pathological characteristics, such as loss of cell cohesion and signet ring cells, and (usually) negative H. pylori conditions.45 In the past several years, many advances in science and technology have provided greater opportunity for molecular treatments of gastric cancer.46
3. TRPM7 channels and gastric cancer
The TRPM7 cation channel supports multiple cellular and physiological functions, including cell death. Jiang et al25 suggested that TRPM7 channel inhibits the growth and proliferation of FaDu and SCC25 cells, two common human head and neck squamous carcinoma cell lines. Furthermore, suppression of TRPM7 channels by Gd3+, 2-APB, or small interfering RNA (siRNA) about TRPM7 inhibited the growth and proliferation of these cells.25 TRPM7 is required for pancreatic adenocarcinoma cell proliferation and metastasis via the mitogen-activated protein kinase (MAPK) pathway.47, 48 In the BxPC-3 cell line, dialyzing cytoplasm during patch clamp whole-cell recordings with a Mg2+ free pipette solution activated a nonselective cation current with strong outward rectification, and this cation current was inhibited by intracellular Mg2+ depletion and by TRPM7 silencing.47 In another study, TRPM7-deficient cells underwent replicative senescence after exhibiting p16 (CDKN2A) and WRN mRNA upregulation, and in the same study anti-TRPM7 siRNA and gemcitabine in combination were found to be more toxic to cells than gemcitabine alone.48 TRPM7 levels in human breast cancer indicate that its overexpression may be a characteristic of higher-grade, highly proliferative breast cancer,49 which is in line with its calcium-independent kinase activity (MAPK pathway activation and regulation of cellular adhesion).50, 51 In addition, it has been proposed TRPM7 may play a role in the proliferation of MCF-7 breast cancer cells. TRPM7 was also found to facilitate 5-8F cell migration by enabling Ca2+ influx, and thus it was suggested as a potential therapeutic target for nasopharyngeal carcinoma.52 TRPM7 also plays an important role in the epidermal growth factor (EGF)-induced migration of A549 cells and thus is also viewed as a potential therapeutic target in lung cancer53; in fact, TRPM7 is expressed in lung carcinoma tissues. TRPM7 is regulated at the mRNA and protein levels by EGF, which activated cell migration in this lung cancer model. In another study, the role of TRPM7 in migration was evaluated by siRNA knockdown and by nonspecific pharmacological inhibition, and its inhibition was found to reduce cell migration under basal or EGF-activated conditions.53 Sun et al54 proposed that the cholesterol-induced activation of TRPM7 regulates human prostate cell proliferation, migration, and viability. Cholesterol increases Ca2+ entry via the TRPM7 channel, which promotes the proliferation of prostate cells by inducing the activations of the AKT and/or ERK pathways.54 TRPM7 also regulates the mobility of PCa cells via cholesterol-dependent calcium entry, and high levels of circulating cholesterol have been shown to increase the risk aggressiveness of these cells.55 Cholesterol activates TRPM7 channel, which in turn increases basal intracellular calcium concentrations and stimulates calpain (a calcium dependent protein), which in turn decreases E-cadherin expression, and suggests a possible link between TRPM7 and migration.54 Mizuno et al56 reported that TRPM7-overexpressing MBT-2 cells proliferated more slowly than mock-transfected cells, and suggested that TRPM7 serves as a negative regulator of bladder cancer cell proliferation.56 Wang et al57 concluded that TRPM7 is required for ovarian cancer cell growth, migration, and invasion, and their mechanistic investigation revealed that silencing of TRPM7 decreased the phosphorylations of Akt, Src, and p38 and increased filamentous actin levels in ovarian cancer cells and their focal adhesion.57 TRPM7 is also involved in tumor progression; for example, it has been reported to contribute to neuroblastoma progression by maintaining progenitor-like features through a developmental transcriptional program involving the transcription factor SNAI2.58 Accordingly, it appears TRPM7 plays an important role in the regulation of cancer cell death.
Recently, we investigated the presence and potential roles of TRPM7 channels during the growth and survival of gastric cancer cells using mainly electrophysiological and molecular techniques. Reverse transcription polymerase chain reaction detected the presence of TRPM7 mRNA in human gastric adenocarcinoma cell lines (AGS, MKN-1, MKN-45, SNU-1, and SNU-484), and the expressions of TRPM7 proteins in these lines were further confirmed by Western blotting and immunostaining.43 In this study, we mainly used the AGS cell line, a commonly used human gastric adenocarcinoma cell line. Whole-cell voltage clamp recordings were used to investigate electrophysiological characteristics in AGS cells, and a voltage ramp from +100 mV to –100 mV was found to evoke small inward currents at negative potentials, whereas larger outward currents were evoked at positive potentials, which is indicative of outward-rectifying cation currents. In a divalent-free solution, however, large inward and outward currents were shown with little rectification. Furthermore, the amplitudes of TRPM7-like currents were larger at low Mg2+ concentrations, and the presence of 2 mM Mg2+ internally significantly inhibited these currents. In addition, the median inhibitory concentration value for free Mg2+ was found to be 560 μM in AGS cells.43 These features are similar to those of recently cloned TRPM6 and TRPM7 channels.59, 60 To differentiate TRPM6 and TRPM7, we used 2-APB.11 2-APB (at micromolar concentrations) enhanced TRPM6 currents but inhibited TRPM7 currents,11 demonstrating the involvement of TRPM7 channels and not of TRPM6 channels. To confirm that this TRPM7-like current was really mediated by TRPM7 channel activation, we transfected approximately 90% of AGS cells with siRNA. TRPM7 siRNA knocked down TRPM7 protein expression by 70–80% without reducing the expression of the internal control. To confirm if TRPM7 siRNA had reduced TRPM7 protein expression, cells were analyzed by electrophysiology, and it was subsequently found that the amplitudes of TRPM7-like currents were significantly reduced in TRPM7 siRNA transfected AGS cells. We then sought to determine whether the activities of TRPM7 channels influence cell survival, and the subsequent MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay showed significant apoptosis among AGS cells transfected with TRPM7siRNA. In addition, we found that Mg2+ was critical for the growth and survival of AGS cells.43
More recently, in a study on ginseng (the root of Panax ginseng C.A. Meyer and a well-known folk medicine and tonic), ginseng total saponin (GTS)61 and ginsenosides Rg362 and Rd63 were found to induce AGS cell death by blockading TRPM7 channel activity. Thus, these results suggested that TRPM7 channels are involved in GTS, ginsenoside Rg3, and Rd-induced gastric cancer cell apoptosis. Furthermore, zinc ions, like calcium, have been reported to play an vital role in cellular homeostasis in various physiological conditions.64, 65 Zinc has a function of a range of enzymes associated with transcription, translation, protein synthesis, and signal transductions.66 In fact, TRPM7 is the only known zinc-permeable channel in the TRP channels,8, 11, 67 and the zinc permeability of TRPM7 is fourfold higher than its Ca2+ permeability.11 We found that the activation of TRPM7 channel augmented the Zn2+-induced AGS cell apoptosis, and that the TRPM7 overexpressed HEK293 cells increased Zn2+-induced cell damage.62 Therefore, it appears that TRPM7 channel also represents a new target for physiological diseases in which Zn2+ toxicity plays an important role.
Regarding the effects of various TRPM7 blockers on gastric cancer cells, Chen et al18 suggested that the LOX inhibitors, nordihydroguaiaretic acid (NDGA), 3-[1-(p-chlorobenzyl)-5-(isopropyl)-3-tert-butylthioindol-2-yl]-2,2-dimethylpropanoic acid (MK886), and 2,3,5-trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861) potently block TRPM7 channel, and are capable of attenuating TRPM7 function, but found that zileuton (another 5-LOX specific inhibitor) was ineffective at suppressing TRPM7 channel activity. Zierler et al21 suggested that waixenicin A is also a potent inhibitor of TRPM7. In gastric cancer cells, NDGA, AA861, MK886, and waixenicin A all potently blocked TRPM7-like currents and induced cell death, but zileuton did not suppress TRPM7-like current activity or induce cell death.68, 69 To confirm the effects of TRPM7 channel blockers, we also used a pyrazole compound (Pyr3; a specific TRPC3 inhibitor)70 and 9-phenanthrol (a specific TRPM4 inhibitor).71 These specific inhibitors did not affect TRPM7-like currents or induce gastric cell death. Therefore, our findings suggested that the TRPM7-specific blockers, NDGA, MK886, AA861, and waixenicin A play an important role in the survival of gastric cancer cells.68, 69 Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is one of the most distributed flavonols found in many fruits, vegetables, leaves, and grains.72 Furthermore, quercetin has many (patho)physiological properties, such as anticancer, anti-inflammatory, antiatherogenic, and antihypertensive effects.72 Thus, we investigated the effects of quercetin in various gastric adenocarcinoma cell lines and explored the mechanisms responsible for its activities. Quercetin induced gastric cancer cell apoptosis, and this was inhibited by various MAPK inhibitors, such as p38 kinase inhibitor (SB203580), JNK inhibitor (SP600125), and ERK inhibitor (PD98059). In addition, quercetin inhibited TRPM7 currents. Moreover, quercetin-induced cell death was increased with upregulation of TRPM7 channels. These results suggest that quercetin induces gastric cancer cell apoptosis through both MAPK and TRPM7 channel inhibition.73 Accordingly, we suggest that quercetin might be a therapeutic drug for the treatment of gastric cancer.
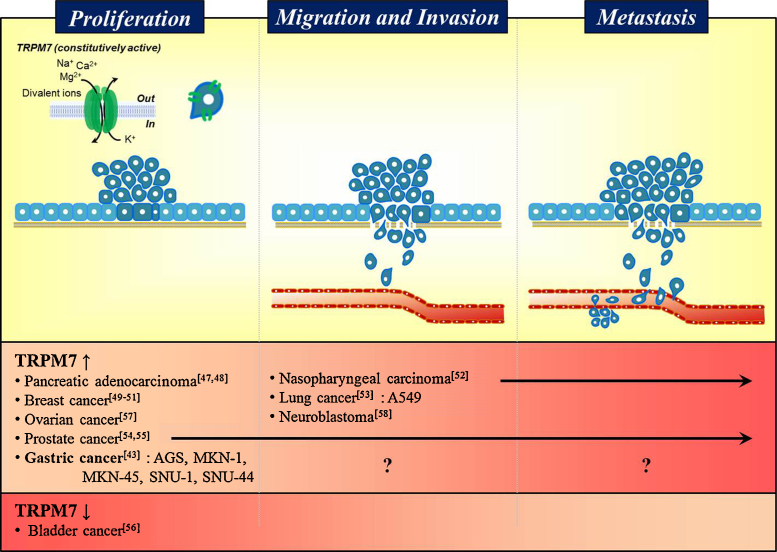
Traditional medicine is mainly based on the use of plants, and some of the many herbal medicines may be useful for the treatment of cancer.74, 75 These medicines usually contain many compounds that affect multiple targets,76, 77 and these combinations of multiple drugs are considered to maximize therapeutic efficacy by facilitating synergistic effects and preventing potential adverse effects, which complicates efforts to identify the mechanisms involved. Orostachys japonicus (OJ) is used in traditional medicine and has anti-inflammatory, antifebrile, hemostatic, antidote, and anticancer activities.78, 79 During our studies, we found that OJ extract inhibited the growth and survival of gastric cancer cells by blockading TRPM7 channel activity.80 Also, Sophorae Radix extract and Ulmi Pumilae Cortex extract showed the same mechanism in inhibition of gastric cancer cell survival with OJ extract.81, 82 However, Buxus microphylla var. Koreana Nakai extract (BMKNE) inhibited the survival of gastric cancer cells through both the blockade of the TRPM7 channel's activity and MAPK signaling.83 Therefore, we suggested that these extracts regulate the survival of gastric cancer cells through TRPM7 channels. However, the roles of TRPM7 in migration, invasion, and metastasis in gastric cancer have yet to be addressed (Fig. 1).
Fig. 1.
Summary of the roles of TRPM7 channel during the proliferation, migration, invasion, and metastasis of various cancers.
Question mark (?) means little researched until now.
TRPM7, transient receptor potential melastatin 7.
4. Conclusion
Gastric cancer remains a major health problem because of its incidence, poor prognosis, and limited treatment options. Although the age-adjusted mortality of gastric cancer has decreased over the past few decades, more studies are required to understand the mechanisms underlying known antigastric cancer effects. Ion channels play important roles in a variety of biological processes and diseases, and of these, a number of Ca2+ channels have been linked with cancer cell behaviors and with the pathophysiological hallmarks of cancer. Changes in activation and/or in the expressions of Ca2+-transporting proteins not only change global Ca2+ homeostasis, but also may cause modifications in subcellular Ca2+ microdomains and localized Ca2+signals, which can affect Ca2+-dependent signaling processes relevant to tumorigenesis. Most of these Ca2+ channels are not specific to cancer cells and are expressed in numerous normal tissues, but some are more active or are overexpressed in cancer cells. Furthermore, the expressions of a variety of TRP channels are different in the same cancer types. Based on available reports, it is likely that several types of channels can contribute to and cooperate during single tumorigenic processes, or can be involved at different stages of sequences of events associated with tumorigenesis. However, as these reports do not support the idea that single channels are related to specific cancer types, it remains to be determined whether any such specific associations exist.5 In particular, although it has been established that activation of TRPM7 channel plays important roles in the growth and survival of gastric cancer cells, little is known of its molecular role during the initiation and/or progression of gastric cancer or of the involvement of calcium signaling through TRPM7 channels on the regulation of proliferation and apoptosis. In addition, there are no experiments on human gastric cancer patients or specimens. Research has only provided basic evidence for the involvement of TRPM7 channels in gastric cancer. In the future, widespread pharmacological interest on the link between TRPM7 and gastric cancer will create opportunities for researchers to test these mechanisms clinically in gastric patients over the next few years. The specific antagonists for TRPM7 channels have also been described in the basic and preclinical area, and are progressing into clinical evaluation. With these specific antagonists for TRPM7 channels, TRPM7 channels will provide the therapeutic mechanisms for the treatment of gastric cancer patients. In our opinion, studies on the anticancer effects of ion channels are likely to result in the development of novel anticancer therapy protocols with improved outcomes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Basic Science Research Program through the Korean National Research Foundation (NRF) funded by the Ministry of Education (grant no. NRF-2014R1A1A2054469).
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Monteith G.R., McAndrew D., Faddy H.M., Roberts-Thomson S.J. Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer. 2007;7:519–530. doi: 10.1038/nrc2171. [DOI] [PubMed] [Google Scholar]
- 4.Roderick H.L., Cook S.J. Ca2+ signalling check points in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 5.Déliot N., Constantin B. Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848:2512–2522. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Pardo L.A., Contreras-Jurado C., Zientkowska M., Alves F., Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205:115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- 7.Bodding M. TRP proteins and cancer. Cell Signal. 2007;19:617–624. doi: 10.1016/j.cellsig.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 9.Smani T., Shapovalov G., Skryma R., Prevarskaya N., Rosado J.A. Functional and physiopathological implications of TRP channels. Biochim Biophys Acta. 2015;1853:1772–1782. doi: 10.1016/j.bbamcr.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Venkatachalam K., Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteilh-Zoller M.K., Hermosura M.C., Nadler M.J., Scharenberg A.M., Penner R., Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paravicini T.M., Chubanov V., Gudermann T. TRPM7: a unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol. 2012;44:1381–1384. doi: 10.1016/j.biocel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Schlingmann K.P., Weber S., Peters M., Niemann Nejsum L., Vitzthum H., Klingel K. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz C., Dorovkov M.V., Zhao X., Davenport B.J., Ryazanov A.G., Perraud A.L. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 15.Li M., Jiang J., Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakriya M., Lewis R.S. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parnas M., Peters M., Dadon D., Lev S., Vertkin I., Slutsky I. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009;45:300–309. doi: 10.1016/j.ceca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H.C., Xie J., Zhang Z., Su L.T., Yue L., Runnels L.W. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010;5:e11161. doi: 10.1371/journal.pone.0011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Numata T., Li M., Mori Y., Orser B.A., Jackson M.F. The modulation of TRPM7 currents by nafamostat mesilate depends directly upon extracellular concentrations of divalent cations. Mol Brain. 2010;3:38. doi: 10.1186/1756-6606-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chubanov V., Mederos y Schnitzler M., Meißner M., Schäfer S., Abstiens K., Hofmann T. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol. 2012;166:1357–1376. doi: 10.1111/j.1476-5381.2012.01855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zierler S., Yao G., Zhang Z., Kuo W.C., Pörzgen P., Penner R. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem. 2011;286:39328–39335. doi: 10.1074/jbc.M111.264341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin X., Yue Z., Sun B., Yang W., Xie J., Ni E. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol. 2013;168:1294–1312. doi: 10.1111/bph.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Du J., Jiang J., Ratzan W., Su L.T., Runnels L.W. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007;282:25817–25830. doi: 10.1074/jbc.M608972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang J., Li M., Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005;126:137–150. doi: 10.1085/jgp.200409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang J., Li M.H., Inoue K., Chu X.P., Seeds J., Xiong Z.G. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Numata T., Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008;283:15097–15103. doi: 10.1074/jbc.M709261200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae C.Y., Sun H.S. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin. 2011;32:725–733. doi: 10.1038/aps.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H.S., Hong C., Kim B.J., So I. The pathophysiologic roles of TRPM7 channel. Korean J Physiol Pharmacol. 2014;18:15–23. doi: 10.4196/kjpp.2014.18.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fonfria E., Murdock P.R., Cusdin F.S., Benham C.D., Kelsell R.E., McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006;26:159–178. doi: 10.1080/10799890600637506. [DOI] [PubMed] [Google Scholar]
- 30.Runnels L.W., Yue L., Clapham D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 31.Nadler M.J., Hermosura M.C., Inabe K., Perraud A.L., Zhu Q., Stokes A.J. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 32.He Y., Yao G., Savoia C., Touyz R.M. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 33.Hanano T., Hara Y., Shi J., Morita H., Umebayashi C., Mori E. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- 34.Elizondo M.R., Arduini B.L., Paulsen J., MacDonald E.L., Sabel J.L., Henion P.D. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Su L.T., Agapito M.A., Li M., Simonson W.T., Huttenlocher A., Habas R. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A.G., Figdor C.G. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006;25:290–301. doi: 10.1038/sj.emboj.7600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krapivinsky G., Mochida S., Krapivinsky L., Cibulsky S.M., Clapham D.E. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 38.Sah R., Mesirca P., Mason X., Gibson W., Bates-Withers C., Van den Boogert M. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation. 2013;128:101–114. doi: 10.1161/CIRCULATIONAHA.112.000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sah R., Mesirca P., Van den Boogert M., Rosen J., Mably J., Mangoni M.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A. 2013;110:E3037–E3046. doi: 10.1073/pnas.1311865110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Dou Y., Li Y., Chen J., Wu S., Xiao X., Xie S. Inhibition of cancer cell proliferation by midazolam by targeting transient receptor potential melastatin 7. Oncol Lett. 2013;5:1010–1016. doi: 10.3892/ol.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhennin-Duthille I., Gautier M., Faouzi M., Guilbert A., Brevet M., Vaudry D. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011;28:813–822. doi: 10.1159/000335795. [DOI] [PubMed] [Google Scholar]
- 43.Kim B.J., Park E.J., Lee J.H., Jeon J.H., Kim S.J., So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99:2502–2509. doi: 10.1111/j.1349-7006.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauren P. The two histological main types of gastric carcinoma: diffuse and so called intestinal-type carcinoma. An Attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 45.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19:S37–S43. [PubMed] [Google Scholar]
- 46.McLean M.H., El-Omar E.M. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014;11:664–674. doi: 10.1038/nrgastro.2014.143. [DOI] [PubMed] [Google Scholar]
- 47.Rybarczyk P., Gautier M., Hague F., Dhennin-Duthille I., Chatelain D., Kerr-Conte J. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int J Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 48.Yee N.S., Zhou W., Lee M., Yee R.K. Targeted silencing of TRPM7 ion channel induces replicative senescence and produces enhanced cytotoxicity with gemcitabine in pancreatic adenocarcinoma. Cancer Lett. 2012;318:99–105. doi: 10.1016/j.canlet.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilbert A., Gautier M., Dhennin-Duthille I., Haren N., Sevestre H., Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297:C493–C502. doi: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 50.Meng X., Cai C., Wu J., Cai S., Ye C., Chen H. TRPM7 mediates breast cancer cell migration and invasion through the MAPK pathway. Cancer Lett. 2013;333:96–102. doi: 10.1016/j.canlet.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Guilbert A., Gautier M., Dhennin-Duthille I., Rybarczyk P., Sahni J., Sevestre H. Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatin breast cancer cells migration through its kinase domain. Eur J Cancer. 2013;49:3694–3707. doi: 10.1016/j.ejca.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Chen J.P., Luan Y., You C.X., Chen X.H., Luo R.C., Li R. TRPM7 regulates the migration of human nasopharyngeal carcinoma cell by mediating Ca(2+) influx. Cell Calcium. 2010;47:425–432. doi: 10.1016/j.ceca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Gao H., Chen X., Du X., Guan B., Liu Y., Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium. 2011;50:559–568. doi: 10.1016/j.ceca.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y., Sukumaran P., Varma A., Derry S., Sahmoun A.E., Singh B.B. Cholesterol-induced activation of TRPM7 regulates cell proliferation, migration, and viability of human prostate cells. Biochim Biophys Acta. 2014;1843:1839–1850. doi: 10.1016/j.bbamcr.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelton K., Freeman M.R., Solomon K.R. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012;12:751–759. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno H., Suzuki Y., Watanabe M., Sokabe T., Yamamoto T., Hattori R. Potential role of transient receptor potential (TRP) channels in bladder cancer cells. J Physiol Sci. 2014;64:305–314. doi: 10.1007/s12576-014-0319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Liao Q.J., Zhang Y., Zhou H., Luo C.H., Tang J. TRPM7 is required for ovarian cancer cell growth, migration and invasion. Biochem Biophys Res Commun. 2014;454:547–553. doi: 10.1016/j.bbrc.2014.10.118. [DOI] [PubMed] [Google Scholar]
- 58.Middelbeek J., Visser D., Henneman L., Kamermans A., Kuipers A.J., Hoogerbrugge P.M. TRPM7 maintains progenitor-like features of neuroblastoma cells: implications for metastasis formation. Oncotarget. 2015;6:8760–8776. doi: 10.18632/oncotarget.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitz C., Perraud A.L., Johnson C.O., Inabe K., Smith M.K., Penner R. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 60.Voets T., Nilius B., Hoefs S., van der Kemp A.W., Droogmans G., Bindels R.J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 61.Kim B.J. The role of ginseng total saponin in transient receptor potential melastatin type 7 channels. Anim Cells Syst. 2012;16:376–384. [Google Scholar]
- 62.Kim B.J. Transient receptor potential melastatin type 7 channels are involved in zinc-induced apoptosis in gastric cancer. Anim Cells Syst. 2011;15:123–130. [Google Scholar]
- 63.Kim B.J. Involvement of melastatin type transient receptor potential 7 channels in ginsenoside Rd-induced apoptosis in gastric and breast cancer cells. J Ginseng Res. 2013;37:201–209. doi: 10.5142/jgr.2013.37.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calderone A., Jover T., Mashiko T., Noh K.M., Tanaka H., Bennett M.V. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koh J.Y., Suh S.W., Gwag B.J., He Y.Y., Hsu C.Y., Choi D.W. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 66.Beyersmann D., Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K., Branigan D., Xiong Z.G. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;5:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim B.J., Kim S.Y., Lee S., Jeon J.H., Matsui H., Kwon Y.K. The role of transient receptor potential channel blockers in human gastric cancer cell viability. Can J Physiol Pharmacol. 2012;90:175–186. doi: 10.1139/y11-114. [DOI] [PubMed] [Google Scholar]
- 69.Kim B.J., Nam J.H., Kwon Y.K., So I., Kim S.J. The role of waixenicin A as transient receptor potential melastatin 7 blocker. Basic Clin Pharmacol Toxicol. 2013;112:83–89. doi: 10.1111/j.1742-7843.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 70.Kiyonaka S., Kato K., Nishida M., Mio K., Numaga T., Sawaguchi Y. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzales A.L., Garcia Z.I., Amberg G.C., Earley S. Pharmacological inhibition of TRPM4 hyperpolarizes vascular smooth muscle. Am J Physiol Cell Physiol. 2010;299:C1195–C1202. doi: 10.1152/ajpcell.00269.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishizawa K., Yoshizumi M., Kawai Y., Terao J., Kihira Y., Ikeda Y. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. J Pharmacol Sci. 2011;115:466–470. doi: 10.1254/jphs.10r38fm. [DOI] [PubMed] [Google Scholar]
- 73.Kim M.C., Lee H.J., Lim B., Ha K.T., Kim S.Y., So I. Quercetin induces apoptosis by inhibiting MAPKs and TRPM7 channels in AGS cells. Int J Mol Med. 2014;33:1657–1663. doi: 10.3892/ijmm.2014.1704. [DOI] [PubMed] [Google Scholar]
- 74.Safarzadeh E., Sandoghchian Shotorbani S., Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4:421–427. doi: 10.5681/apb.2014.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Feng Y., Wang N., Cheung F., Tan H.Y., Zhong S. Chinese medicines induce cell death. The molecular and cellular mechanisms for cancer therapy. Biomed Res Int. 2014;2014:530342. doi: 10.1155/2014/530342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qiu J. Back to the future for Chinese herbal medicines. Nat Rev Drug Discov. 2007;6:506–507. doi: 10.1038/nrd2350. [DOI] [PubMed] [Google Scholar]
- 77.Wang L., Zhou G.B., Liu P., Song J.H., Liang Y., Yan X.J. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H.S., Ryu D.S., Lee G.S., Lee D.S. Anti-inflammatory effects of dichloromethane fraction from Orostachys japonicus in RAW 264.7 cells: suppression of NF-κB activation and MAPK signaling. J Ethnopharmacol. 2012;140:271–276. doi: 10.1016/j.jep.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Ryu D.S., Lee H.S., Lee G.S., Lee D.S. Effects of the ethylacetate extract of Orostachys japonicus on induction of apoptosis through the p53-mediated signaling pathway in human gastric cancer cells. Biol Pharm Bull. 2012;35:660–665. doi: 10.1248/bpb.35.660. [DOI] [PubMed] [Google Scholar]
- 80.Hwang M.W., Kim H.W., Kim B.J. Involvement of transient receptor potential melastatin 7 channels in Orostachys japonicus-induced apoptosis in cancer cells. Int J Pharmacol. 2012;8:638–646. [Google Scholar]
- 81.Kim B.J. Involvement of transient receptor potential melastatin 7 channels in Sophorae Radix-induced apoptosis in cancer cells. J Pharmacopuncture. 2012;15:031–38. doi: 10.3831/KPI.2012.15.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lim B., Lee H.J., Kim M.C., Kim B.J. Effects of ulmi pumilae cortex on AGS gastric cancer cells. J Pharmacopuncture. 2013;16:056–63. doi: 10.3831/KPI.2013.16.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee H.J., Kim M.C., Lim B., Kim B.J. Buxus microphylla var. Koreana Nakai extract for the treatment of gastric cancer. J Pharmacopuncture. 2013;16:039–45. doi: 10.3831/KPI.2013.16.016. [DOI] [PMC free article] [PubMed] [Google Scholar]