Abstract
Insulin-like growth factors control numerous processes, namely somatic growth, metabolism and stress resistance, connecting this pathway to aging and age-related diseases. Insulin-like growth factor signaling also impacts on neurogenesis, neuronal survival and structural plasticity. Recent reports demonstrated that diminished insulin-like growth factor signaling confers increased stress resistance in brain and other tissues. To better understand the role of neuronal insulin-like growth factor signaling in neuroprotection, we inactivated insulin-like growth factor type-1-receptor in forebrain neurons using conditional Cre-LoxP-mediated gene targeting. We found that brain structure and function, including memory performance, were preserved in insulin-like growth factor receptor mutants, and that certain characteristics improved, notably synaptic transmission in hippocampal neurons. To reveal stress-related roles of insulin-like growth factor signaling, we challenged the brain using a stroke-like insult. Importantly, when charged with hypoxia-ischemia, mutant brains were broadly protected from cell damage, neuroinflammation and cerebral edema. We also found that in mice with insulin-like growth factor receptor knockout specifically in forebrain neurons, a substantial systemic upregulation of growth hormone and insulin-like growth factor-I occurred, which was associated with significant somatic overgrowth. Collectively, we found strong evidence that blocking neuronal insulin-like growth factor signaling increases peripheral somatotropic tone and simultaneously protects the brain against hypoxic–ischemic injury, findings that may contribute to developing new therapeutic concepts preventing the disabling consequences of stroke.
Keywords: Cerebral edema, hypoxia-ischemia, insulin-like growth factor, neuroinflammation, neuroprotection
Introduction
Neurons in the mammalian brain remain functional over very long time periods and many are maintained intact throughout life. To ensure long-term survival and integrity of neurons and to prevent accidental loss under challenging conditions, neurotrophic factors and hormones protect the brain. Insulin-like growth factors (IGFs) play key roles in these processes.1,2 They form part of evolutionary conserved endocrine pathways and comprise the structurally and functionally related peptides IGF-I and IGF-II. IGFs activate their cognate tyrosine kinase receptor IGF-1R and initiate signal transduction through phosphatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) molecular cascades. These ubiquitous pathways in turn regulate cell homeostasis, stress resistance and rescue from apoptosis.1 During development, IGF-I and -II coordinate growth in all tissues through the control of cell cycle and proliferation.3 While IGF-II plays a predominantly embryonic and early postnatal role, IGF-I is also abundantly expressed in many adult tissues, exerting perennial functions in energy metabolism and in maintenance of cell structure and function.4,5 In adult neuroendocrine physiology, IGF-I serves as a key effector of the somatotropic hormone axis and is expressed by several tissues, notably liver, under narrow control of pituitary growth hormone (GH). IGF-I reaches target cells through endocrine routes (mainly as liver cell-derived IGF-I) and through paracrine secretion. In the brain, IGF-I is locally produced and to some extent imported across the blood–brain barrier.6–8 More specifically, IGF-I modulates important neurophysiological aspects in the central nervous system (CNS), including neurogenesis, synaptic plasticity and complex cognitive functions.8–10 During acute brain injury, IGF-I is locally produced by neurons and glial cells, and exerts significant neuroprotection.11,12 Consistently, central IGF-I administration after ischemic brain injury leads to reduction in cortical infarction.13–15 These and related findings have generated considerable interest in IGFs as potential CNS therapeutic agents,16,17 and raised expectations that treatment of stroke and neurodegenerative diseases may benefit from systemic or local IGF supplementation.16,18–20 However, molecular mechanism and cellular targets of IGF-dependent neuroprotective pathways are still not well identified. Meanwhile, several studies suggest that in stark contrast to previous views, diminishing or even completely ablating IGF-I signaling may also confer protection from several types of brain injury. Indeed, chronic systemic delivery of IGF-I increases the size of brain lesions following middle cerebral artery occlusion (MCAO).21 Moreover, in dwarf rodent models with constitutively decreased IGF-I activity, MCAO produced smaller brain lesion, reduced brain edema and less astrocytic infiltration.21,22 In addition to that, several models of constitutive and conditional IGF-1R knockout confer protection against oxidant-induced lung injury,23 hepatocellular damage during experimental cholestasis,24 or proteotoxicity, including harmful aggregating β-amyloid peptides.19,25 Mechanistically, protection could be linked to the observation that reducing GH and IGF signaling in vitro renders postmitotic cells resistant to oxidative damage or DNA mutation.26,27 Nevertheless, little is known about the consequences of definitively suppressing IGF signaling selectively from adult neurons of the CNS. Therefore, and to specifically address the multiple roles of neuronal IGF signaling in the adult mammalian brain, we generated a new mouse model with conditional genetic inactivation of neuronal IGF-1R. We used a Cre recombinase transgene driven by the CaMKIIα promoter to efficiently target differentiated forebrain neurons, namely in cerebral cortex, hippocampus and striatum (caudate putamen). Subsequent comprehensive functional phenotyping and in particular submitting the mutant to a murine stroke model based on combined application of ischemia and hypoxia revealed that blocking IGF signaling specifically in neurons of the forebrain confers significant neuronal protection from experimentally induced ischemic–hypoxic insult. Present findings also revealed that IGF-1R in forebrain neurons efficiently controls neuroendocrine regulation of the GH/IGF-I hormone axis. Collectively, we show that neuronal IGF-1R knockout protects the brain from experimental stroke, underscoring that long-term suppression of IGF signaling in adult neurons can be protective under stressful conditions.
Materials and methods
Animals
Igf1rflox/flox mutants4 and CamkCre4+/0 transgenics28 were backcrossed >20 generations to C57BL/6J prior to experiments. By mating Igf1rflox/flox females with CamkCre4+/0 males, we generated CamkCre4+/0; Igf1rflox/WT males that were again crossed with Igf1rflox/flox females, producing CamkCre4+/0; Igf1rflox/flox founder males. They were bred with Igf1rflox/flox females to produce male and female cohorts of CamkCre4+/0; Igf1rflox/flox mutants (named nIGF1RKO) and Igf1rflox/flox littermate controls. CamkCre4+/0 transgene was transmitted in Mendelian proportion. Mice lived under specific pathogen-free (SPF) conditions in individually ventilated cages at 23℃, 14/10-h light/dark cycle and free access to water and rodent pellet chow (A04; UAR, Villemoisson, France), containing 49% carbohydrates, 24% proteins, 5% lipids, 12% moisture, 10% minerals and fiber. Mice were separated from mothers on day 30 and housed six males or six females per cage, both control and mutant genotypes in each cage. All cages were equipped with a plastic refuge and hiding box (brand name “mouse house”) from Tecniplast (Milan, Italy), designed to enhance social and prevent aggressive behavior in mice.
All experiments were conducted according to the EU Council Directive 2010/63/EU for the care and use of animals for experimental procedures and complied with the regulations of the Comité d'Ethique pour l'Expérimentation Animale ‘Charles Darwin’, registered at the Comité National de Réflexion Ethique sur l'Expérimentation Animale (IdF, Paris, N°5); Agreement 75-444 to MH, approved by the Direction des Services Vétérinaires, Paris, France) and ARRIVE guidelines. All efforts were made to minimize animal suffering.
Genotyping
Mice were genotyped by multiplex PCR from skin biopsies. Primers 5′-CCATGGGTGTTAAATGTAATGGC-3′, 5′-ATGAATGCTGGTGAGGGTTGTCTT-3′ and 5′-ATCTTGGAGTGGTGGGTCTGTTTC-3′ were used to simultaneously amplify DNA from floxed (312 bp) and Cre-lox recombined (204 bp) Igf1r alleles. CamkCre4 transgene was detected using primers for Cre (5′-CCTGGAAAATGCTTCTGTCCG-3′, 5′-CAGGGTGTTATAAGCAATCCC-3′, 392 bp amplicon) and positive control Gabra1 (5′-AACACACACTGGCAGGACTGGCTAGG-3′, 5′-CAATGGTAGGCTCACTCTGGGAGATGATA-3′, 292 bp).
Monitoring genomic Cre-lox recombination
We performed multiplex PCR on DNA from brain and peripheral tissues (DNeasy, Qiagen, Courtaboeuf, France). Products were size-separated and recombination efficiency established comparing Igf1r− with Igf1rflox bands. We performed LacZ staining on 16 µm coronal cryosections from mutants harboring CamkCre4+/0 and Rosa26R+/0 Cre-lox reporter transgenes.29 Rosa26Ractivated/0 mutants served as positive controls. Tissue sections were fixed in 4% paraformaldehyde (PAF) and stained with X-Gal and eosin.
Morris water maze test
Spatial and cued learning were tested as described.30 Briefly, during training phase of the standard place-learning version of the maze, mice learned the fixed position of a hidden platform (6 cm diameter) using extra-maze cues arranged in the room around the pool. Animals received one habituation trial on day 1 and then two trials per day for seven days. Mice were left undisturbed in their home cage for the 90-min interval between trials. On day 9, mice performed one last trial and were then submitted to the 60-s probe test with the platform removed. Distance travelled in each quadrant and number of times the animal crossed each of the four possible platform sites was calculated based on video tracking. During the cued version of the maze, mice were trained to find a 9-cm platform tagged by a dark 45-mm diameter ball fixed 11 cm above the platform. In this version, animals were trained for two days, and platform and animal starting positions pseudo-randomized for each trial.
Electrophysiology
Mice were decapitated under halothane anesthesia and hippocampus quickly removed. Slices of 400 µm were incubated at 31℃ in artificial cerebrospinal fluid (aCSF: 124 mM NaCl, 3.5 mM KCl, 1.5 mM MgSO4, 2.3 mM CaCl2, 26.2 mM NaHCO3, 1.2 mM NaH2PO4, 11 mM glucose; 95% O2, 5% CO2 at pH 7.44). After 1 h, single slices were transferred on a grid in the submerged slice chamber and perfused with 2 mL/min aCSF. Extracellular recordings were obtained at 25–28℃ from the apical dendritic layer of area CA1. Field excitatory postsynaptic potentials (fEPSP) were evoked every 10 s by electrical stimulation of afferent fibers (Schaffer collaterals and commissural fibers). Stimuli (20 or 100 µs) were applied using a bipolar electrode inserted into the tissue and in the aCSF just above the slice.
Synaptic transmission
Input/output (I/O) curves were established to assess responsiveness of AMPA/kainate subtype of glutamate receptors to electrical stimulation. The slope of three averaged presynaptic fiber volleys (PFVs) and fEPSPs was plotted against intensity of stimulation. I/O curves of NMDAR-mediated synaptic responses were also constructed. Slices were perfused with 0.1 mM Mg2+ aCSF supplemented with 10 µM AMPA/kainate receptor antagonist NBQX and 10 µM picrotoxin, to block inhibitory neurotransmission. A knife-cut separated CA3 and CA1 to prevent epileptiform discharges.
Synaptic plasticity
To investigate long-term potentiation (LTP) of synaptic transmission, test stimuli were applied every 15 s and adjusted to obtain a baseline fEPSP of 0.1 mV/s. The initial slope of fEPSP was measured for 15 min prior to delivery of a theta-burst stimulation (TBS; 5 trains of 4 pulses at 100 Hz separated by 200 ms at test intensity). This sequence was repeated three times with an interburst interval of 10 s. After conditioning stimulation, testing with single pulse was resumed for 60 min to determine stable LTP. Long-term depression (LTD) was induced by low-frequency stimulation (LFS; pulses delivered at 2 Hz for 10 min and at test intensity). This conditioning stimulation was induced after 10 min of baseline recording with test fEPSP adjusted to 0.2 mV/s. Testing with single pulse was resumed for 40 min after LFS. NBQX and 2-APV (Tocris, Bristol, UK) were dissolved in water, and picrotoxin (Sigma-Aldrich, Saint-Quentin-Fallavier, France) was dissolved in dimethyl sulfoxide (DMSO).
Vascular surgery
Ten-month-old mice were challenged by combining hypoxia with ischemia (H/I) following the procedure originally described by Levine31 and Vannucci et al.,32 adapted to adult animals. Mice were anaesthetized (4% isoflurane for induction, 2% for maintenance) and body core temperature kept constant using a heated pad. The right common carotid artery was exposed, double-ligated proximal and distal, and sectioned, to interrupt blood flow. Isoflurane anesthesia was disconnected immediately at the end of surgery. After 3-h recovery, operated mice were exposed to hypoxia by placing them individually into a chamber ventilated with humidified 8% O2 and 92% N2. We achieved comparable brain damage and similar 72-h post-operative mortality in both sexes by exposing females for 22 min and males for 17 min to hypoxia. Different duration for males and females is explained in Vannucci et al.32 Body core temperature during hypoxia was continuously monitored and kept at 37.3 ± 0.5℃ by heating the walls of the chamber. Animals were allowed to recover in room air. Of note, permanent ligation of right (or left) common carotid artery is by itself insufficient at creating substantial cerebral ischemia, mainly because in the mouse, blood flow compensates efficiently through the anastomotic Willis circle. Neither does 8% O2 hypoxia alone produce brain injury.
Histology
Seventy-two hours after H/I, mice were anaesthetized with pentobarbital i.p., and blood collected by cardiac puncture for hormones and biochemistry. The brain was quickly removed, frozen in −50℃ isopentane and stored at −80℃. Coronal 16 µm cryosections between Bregma +2.46 and −3.10 were collected in multiple series to represent each brain by 15 sections per series (distance between sections 368 µm). After H/I, infarct and edema were determined histomorphometrically from H/E-staining. Micrographs were taken using Leica (Wetzlar, Germany) MZ7.5 stereomicroscope with Canon EOS 400-D camera and MP-E65 lens and analyzed using ImageJ. Ipsilateral cortex, hippocampus and striatum (caudate putamen) were graded for infarct histology: 1, no damage (no obvious cell death); 2, limited damage (some pyknotic cells); 3, substantial damage (non-confluent multiple areas of pan-necrosis, numerous pyknotic cells) or 4, severe damage (large zone of pan-necrosis with mostly pyknotic cells). Surface area of damaged tissue in the ipsilateral hemisphere was computed across sections between Bregma +2.46 and −3.10, and brain edema determined as % volumetric increase of ipsi- vs. contralateral hemisphere.33
Immunohistochemistry and TUNEL staining
Sections were fixed for 10 min at 4℃ in 4% PAF for neuron- and microglia-specific IHC or for 5 min at room temperature with 95% ethanol for astrocyte and apoptosis detection. Sections were permeabilized and blocked for 30 min in phosphate-buffered saline (PBS) with 0.25% Triton X-100 (TX) and 5% BSA (bovine serum albumin; Fraction V, Sigma-Aldrich, Saint-Quentin-Fallavier, France). For simultaneous labeling of neurons and microglia, sections were incubated overnight at 4℃ with anti-NeuN (Millipore, Molsheim, France, 1:100) and anti-CD11b (Serotec-BioRad, Colmar, France, 1:100) primary antibodies in PBS-BSA-TX. They were incubated 1 h at room temperature with secondary antibodies (Invitrogen, Carlsbad CA, USA), respectively, conjugated to Alexa 594 (1:900) and Alexa 488 (1:800) in PBS-BSA. For simultaneous labeling of astrocytes and apoptotic cells, sections were incubated overnight at room temperature with Cy-3-conjugated glial fibrillary acidic protein (GFAP) antibody (Sigma-Aldrich, 1:500). To detect DNA fragmentation, we used TUNEL (Roche, Basel, Switzerland) following instructions of the provider. We counterstained with Hoechst 33258 (Sigma-Aldrich) at 5 µg/mL in PBS and mounted with DAKO medium. Immunofluorescence was observed using Leica DM5000b microscope, and micrographs were taken with Leica DFC 300FX CCD.
To estimate prevalence of astrocytes, we measured anti-GFAP-immunofluorescence per area of interest, which was more accurate than identifying and counting astrocytes individually. In operated animals, astrocytic reactivity was evaluated measuring density of GFAP immunostaining in the cortical penumbra, area bordering the zone of intense TUNEL staining. To control for interindividual variation in GFAP immunoreactivity, GFAP density was also measured in the contralateral cortex, and results normalized by expressing them as ratio of ipsi- to contralateral density. This was necessary as we observed substantial interindividual differences in basal astrogliosis and in response to hypoxia that were efficiently reduced by normalizing GFAP signal. In non-operated animals, astrocytic populations were evaluated in hippocampus by measuring density of GFAP immunostaining. Cell death was evaluated at basal condition and in operated animals by TUNEL staining. For microglial density, we counted CD11b-positive cells per area of interest in the right cortex of operated and non-operated animals. We used three micrographs per animal taken from cerebral cortex sections at Bregma −0.08, −0.45 and −0.82. Mean density of microglia per genotype was established from average individual densities. For neuronal density, we used nine micrographs of right cortical sections at Bregma −0.08, −0.45 and −0.82 (three micrographs per Bregma level) and counted neurons manually per area of interest. The same procedure was applied to the contralateral side, and ipsi- to contralateral ratio calculated. Average ratio at each Bregma was used to establish mean ratio per animal. Using CD31-immunoreaction, average microvessel density and surface area in cortex were measured from sections at Bregma −1.56, −1.93, and −2.30. For each section, micrographs were taken from three representative cortical levels.
Western blot
Immunoprecipitation and Western blotting were performed as described.34 Antibodies: anti-IGF-1Rβ-subunit (C20, Santa Cruz, Dallas, TX, USA), anti-phospho-tyrosine (PY20, Transduction Laboratories, BD Biosciences, Franklin Lakes NJ, USA), anti-mouse P-Jak2 Tyr1007/1008 (Cell Signaling, Saint Quentin Yvelines, France) and anti-IR (Transduction Laboratories, BD Biosciences). Anti-vinculin (Sigma-Aldrich) was used to confirm equal loading and efficient transfer. Antibodies were revealed using peroxidase-conjugated secondary antibodies and electrochemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway NJ, USA). Signals were quantified using Scion Image 4.0.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from hypothalamus with phenol–chloroform (RNAble, Eurobio, Montpellier, France) and 1-µg aliquots reverse transcribed (Transcriptor, Roche, Basel, Switzerland) using random hexamers (Promega, Madison WI, USA) and RNAsine (Promega). Duplicates of 10 ng cDNA were used for RT-PCR (Life Technologies, Carlsbad CA, USA; PCR 7300; GHRH, Mm00439100_m1; SRIH, Mm00436671_m1; β-actin, 4352933E; 18S rRNA, 4333760F). We quantified GHRH and SRIH mRNA using the standard curve method and normalized using β-actin mRNA and 18S rRNA.
Other methods
Behavioral tests, biochemistry, laser Doppler flowmetry, vGLUT analysis and other phenotyping protocols are described in supplementary information (SI), section 2.
Statistical analysis
We used SPSS 11.5 (IBM, Bois-Colombes, France) and StatView (SAS, Brie Comte Robert, France) software. Normally distributed data (Kolmogorov-Smirnov test) were analyzed using two-tailed Student’s t-test. For electrophysiology data, we also used RM-ANOVA and Tukey's post hoc test. Mortality data were analyzed using Fisher’s exact test. Non-normally distributed data were analyzed using Mann-Whitney U-test. Levels of significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, not significant (P ≥ 0.05).
Results
Intact brain despite loss of neuronal IGF-1R
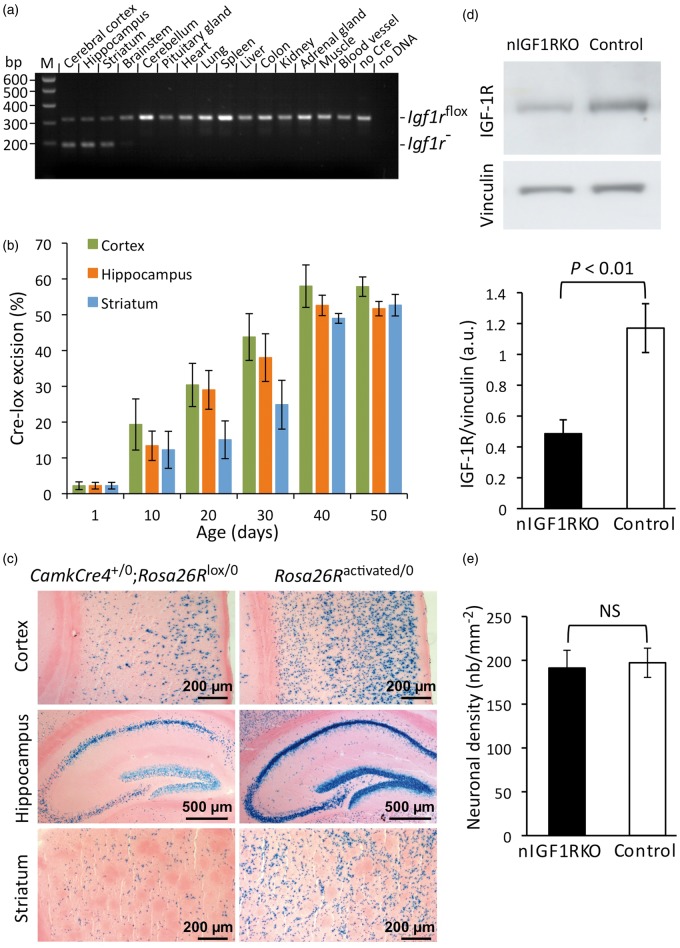
To assess the long-term role of IGF-1R signaling in adult neurons of the brain, we generated neuron-specific IGF-1R knockout mice (CamkCre4+/0; Igf1rflox/flox) that express transgenic Cre recombinase from the calmodulin-dependent protein kinase (Camk) IIα promoter in homozygous Igf1rflox/flox background. In these nIGF1RKO mice, efficient IGF-1R knockout occurred in neurons of cerebral cortex, hippocampus and striatum, while recombination was minor or undetectable in other brain regions, and absent from peripheral tissues (Figure 1(a)). In neurons of postnatal cortex, hippocampus and striatum, Igf1r inactivation took place progressively due to selective expression of CamkCre4 by postmitotic, differentiated forebrain neurons (Figure 1(b)). Seven weeks after birth, IGF-1R knockout was complete in nIGF1RKO forebrain neurons. Using a Rosa26R reporter transgene in CamkCre4 mice, we confirmed widespread Cre-lox recombination among neurons in adult cortex, hippocampus and striatum (Figure 1(c)). IGF-1R protein abundance was reduced by 58% in nIGF1RKO cortex compared with controls (Figure 1(d)), the remaining IGF-1 receptors being expressed by glial and endothelial cells.
Figure 1.
Forebrain-specific neuronal inactivation of Igf1r gene (nIGF1RKO). (a) PCR of CamkCre4+/0; Igf1rflox/flox tissues showing efficient Cre-lox excision of floxed Igf1r alleles in forebrain but not in other tissues. M: marker; no Cre, DNA from control (Igf1rflox/flox) forebrain. (b) Postnatal CamkCre4-induced excision of floxed Igf1r alleles in cortex, hippocampus and striatum (caudate putamen); n = 3 for each time point. (c) X-Gal staining of Cre-lox recombination in coronal sections from 90-day-old CamkCre4+/0; Rosa26R+/0 mice (left panels). Constitutively activated Rosa26R reporter mouse served as positive control (right panels). (d) IGF-1R levels were decreased in nIGF1RKO cortex. Western blot; n = 4 per group; a.u., arbitrary units. (e) NeuN immunolabel in adult cortex revealed equal neuronal density in nIGF1RKO and control; n = 6 per group; Mann-Whitney U-test; Error bars, SEM.
Brains from adult nIGF1RKO mice had the same weight as brains from control (Igf1rflox/flox) littermates (control 386 ± 9 mg vs. nIGF1RKO 370 ± 8 mg, at six months of age, n = 20 per group, NS). nIGF1RKO brains had no structural abnormalities, except for slightly diminished cross-sectional surface of the brain and reduced width of hippocampus (online supplementary Figure 1, supplementary Table 1). Absence of IGF-1R from adult forebrain neurons did not significantly change neuronal density (Figure 1(e)), and using TUNEL staining, we found no evidence for increased neuronal death. Similarly, astrocyte (online supplementary Figure 2(a) and (b)) and microglial density (online supplementary Figure 2(c)) did not differ significantly between nIGF1RKO mice and controls. Together, this indicates that deletion of IGF-1R from forebrain neurons during postnatal life does not induce major changes in CNS histoanatomy.
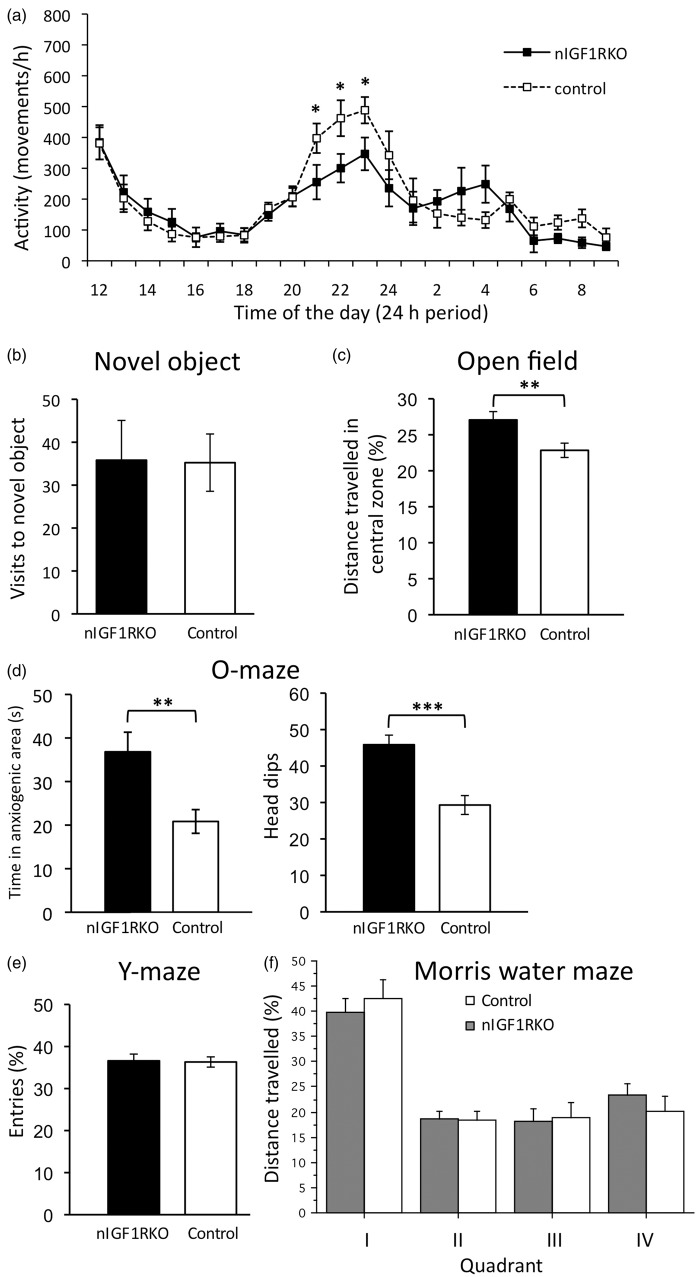
We next thought that despite absence of overt histoanatomical changes, investigating animal behavior and cognition might reveal functional deficits due to experimentally induced IGF resistance and submitted animals to a battery of behavioral tests. We found that nIGF1RKO mutants had normal locomotor activity, except for a decreased nocturnal peak activity, performed normally in novel object test (Figure 2(a) and (b)), and showed less anxiety in open-field test and O-maze (Figure 2(c) and (d)). Importantly, short-term memory was intact as revealed by unchanged performance in Y-maze test (Figure 2(e) and online supplementary information, section 1.1.). Moreover, spatial memory performance in Morris water maze was also preserved in nIGF1RKO mice (Figure 2(f)).
Figure 2.
Behavioral analysis. (a) Circadian locomotor activity in nIGF1RKO and control mice. (b) Novel object test to assess spontaneous interest. (c) Open-field: nIGF1RKO mice were more attracted to the center than controls. (d) O-maze: nIGF1RKO spent more time in unprotected sectors (left panel) and performed more explorative head-dips (right panel), indicative of reduced anxiety. (e) nIGF1RKO and controls performed normally in Y-maze test. (a to e) n = 15 nIGF1RKO vs. 25 controls; *P < 0.05; **P < 0.01; ***P < 0.001; Student’s t-test. (f) Analysis using Morris water maze revealed preserved spatial memory in nIGF1RKO mice, as demonstrated by similar performance in the final quadrant test; n = 17 nIGF1RKO vs. 18 controls; NS; ANOVA; error bars, SEM.
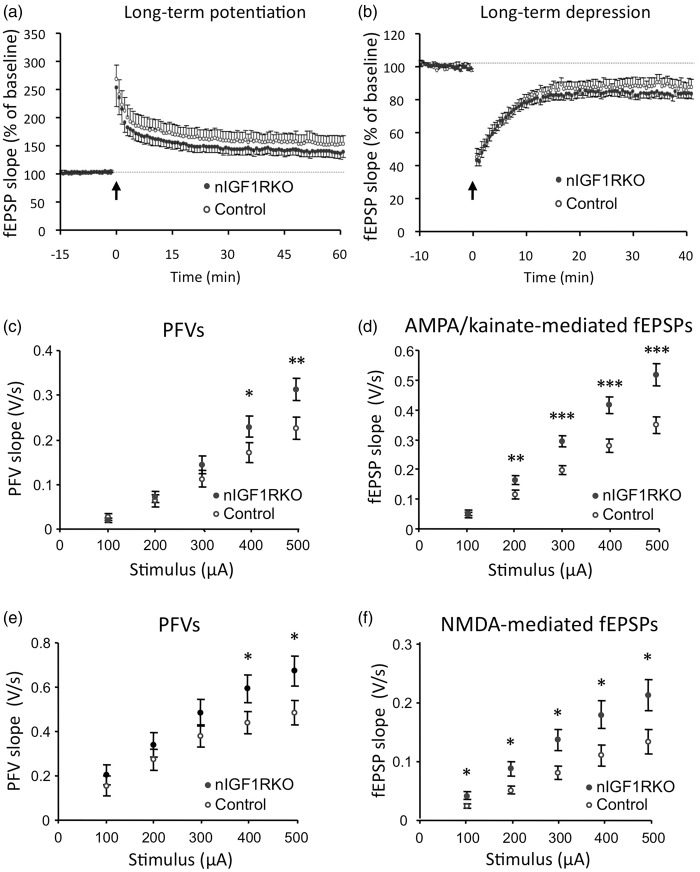
Enhanced synaptic transmission and preserved plasticity in nIGF1RKO mutants
Even in the absence of alterations in behavior or memory performance, electrophysiological changes could still exist, and we therefore applied several established tests. Checking for possible cell autonomous effects of IGF-1R knockout in adult neurons, we measured LTP of synaptic transmission in hippocampal slices in response to TBS of glutamatergic afferents. In nIGF1RKO and controls, TBS induced a strong potentiation of fEPSP slope that progressively recovered (Figure 3(a)). No differences were found between groups in magnitude and time course of LTP. Figure 3(b) shows the effect of LFS on evoked fEPSP recorded in CA1. LFS induced a strong depression of fEPSP that slightly increased during the first minutes and progressively recovered, but remained significantly depressed 40 min after LFS, in nIGF1RKO and control mice. Hence, magnitude and time course of LTD were not significantly altered in nIGF1RKO mice, and we concluded that synaptic plasticity remained essentially unchanged in nIGF1RKO mice.
Figure 3.
Electrophysiology in nIGF1RKO and control mice. (a) Time-course of long-term potentiation (LTP) induced by high frequency stimulation of glutamatergic afferents (arrow). (b) Comparison of long-term depression (LTD) induced by low-frequency stimulation of glutamatergic afferents (arrow). RM-ANOVA used for data in panels A and B, followed by Tukey's post hoc test applied to the last 15 min of recording. (c) Mean presynaptic fiber volley (PFV) as a function of current stimulus intensity in control and nIGF1RKO mice. (d) Mean AMPAR-mediated synaptic responses as a function of increased stimulus intensity. (e) Mean PFV as a function of current stimulus intensity. Tissue slices were supplemented with NBQX and picrotoxin. (f) Mean isolated NMDAR-mediated synaptic responses as a function of increased stimulus intensity. *P < 0.05; **P < 0.01; ***P < 0.001; n = 6 nIGF1RKO vs. 9 controls; panels c-f: Student’s t-test; Error bars, SEM. fEPSP: field excitatory postsynaptic potentials.
We then performed experiments to evaluate synaptic transmission in hippocampal slices from controls and nIGF1RKO mutants. In standard artificial cerebrospinal fluid, stimulation of the stratum radiatum induced a PFV followed by an AMPA/kainate receptor-mediated field excitatory postsynaptic potential (fEPSP) that was completely suppressed by adding 10 µM NBQX at the end of the recording. I/O curves for PFVs and fEPSPs showed significant interaction between genotype and stimulus for PFV, indicating that electrical stimulation was more efficient in recruiting afferent fibers in mutant mice (Figure 3(c)). Importantly, postsynaptic AMPA/kainate receptor-mediated responses were significantly higher in nIGF1RKO mice (Figure 3(d)). Next, we constructed I/O curves of isolated N-methyl-D-aspartate (NMDA)-receptor-mediated fEPSPs from slices submerged with a low Mg2+ medium supplemented with NBQX and picrotoxin, to block AMPA/kainate receptor-mediated and inhibitory synaptic responses, respectively. Under these conditions, electrical stimulation of Schaffer collaterals-induced synaptic responses that were abolished by 30 µM of NMDAR antagonist 2-APV, added at the end of the recording. Comparison in control and nIGF1RKO mice revealed significant interaction between genotype and stimulus for PFV (Figure 3(e)) as reported above for AMPA/kainate receptor-mediated synaptic responses. This result confirmed the higher efficacy of stimulation in activating presynaptic afferent fibers. Regarding postsynaptic responses, NMDAR-mediated synaptic potentials were significantly higher in nIGF1RKO mice (Figure 3(f)), and we concluded that synaptic transmission was significantly altered in nIGF1RKO mutants.
Interestingly, abundance of vesicular glutamate transporters vGLUT1 and 2 was within the normal range in mutants and controls (online supplementary Figure 3 and supplementary information, section 1.2.), excluding that increased synaptic transmission was due to changes in vGLUT. These data demonstrate that differentiated neurons tolerate the complete loss of IGF-1R signaling and that neuronal function and behavioral performance are clearly maintained or even enhanced in mutant mice.
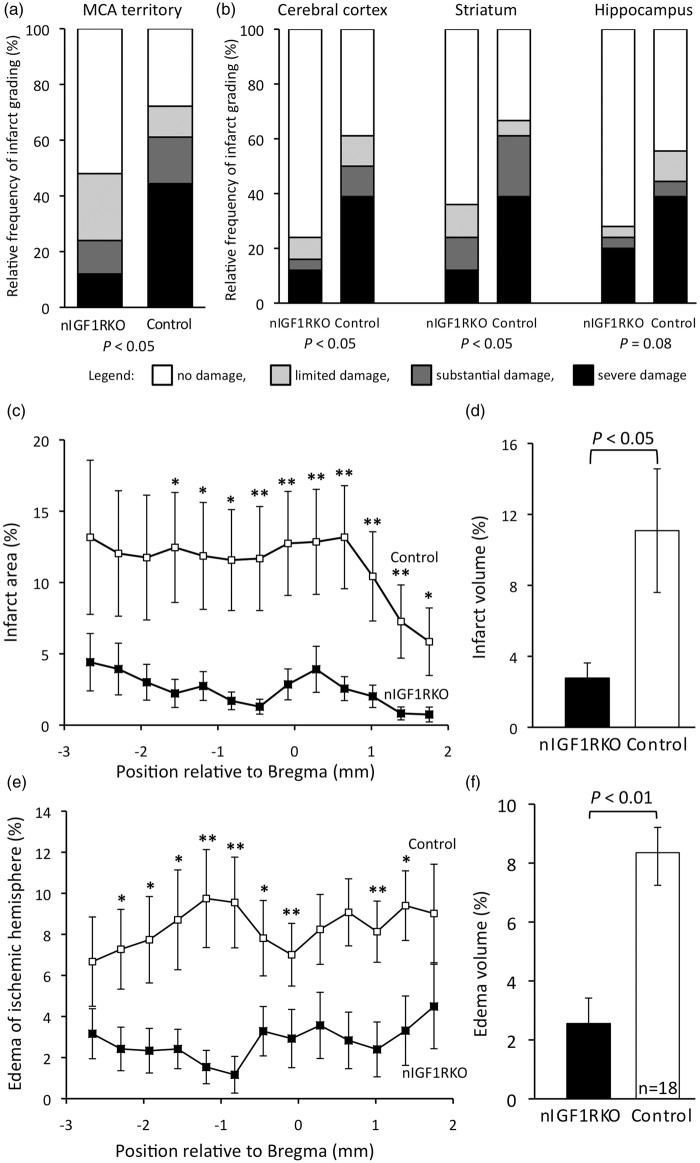
nIGF1RKO mice are protected from stroke
Recent reports showed that in various tissues, cells develop increased stress resistance when IGF signaling is diminished or suppressed.19,23–26,35–37 To test whether this was also true for acute hypoxia–ischemia challenge in brain tissue, we submitted nIGF1RKO mutants to a stroke-modeling protocol based on vascular surgery-induced cerebral H/I. We ligated the right common carotid artery of 10-month-old nIGF1RKO mice and exposed males for 17 min and females for 22 min to a breathing air reduced to 8% oxygen.32 Postoperative mortality did not differ between genotypes (Table 1), and weight loss was also the same in both groups (control −6.9% ± 1.9, n = 10; nIGF1RKO −8.1% ± 1.2, n = 13; NS). Importantly, when brain damage within the territory of the right middle cerebral artery was evaluated three days after H/I insult, the proportion of animals that had developed severe infarct was three times lower in nIGF1RKO mice as compared with controls (Figure 4(a)). This was also evident when damage was evaluated separately for cortex, striatum and hippocampus (Figure 4(b)). When males and females were evaluated separately, there was significantly less infarct damage in nIGF1RKO males (severe and substantial damage combined: control 57% vs. nIGF1RKO 10%, P = 0.05), and we observed a conspicuous tendency to lower grade injury in nIGF1RKO females (control 64% vs. nIGF1RKO 33%, P = 0.20). Measuring the damaged area in all sections throughout the anterior–posterior axis revealed that nIGF1RKO mice had dramatically smaller infarcts than controls (P < 0.05; Figure 4(c)). Accordingly, the reconstructed infarct volume was markedly reduced in nIGF1RKO mice compared with controls (−75%, P < 0.05; Figure 4(d)). Taken together, these analyses indicated that nIGF1RKO mouse brain is well protected from infarction in this experimental model of stroke.
Table 1.
Mortality within 72 h after hypoxia-ischemia (H/I).
| Genotype | Mice submitted to H/Ia | Mice alive after 72 h | Surviving fractionb |
|---|---|---|---|
| nIGF1RKO | 34 | 26 | 0.76 |
| Control | 24 | 18 | 0.75 |
Age 10 months at time of operation.
Difference between genotypes not significant; Fisher’s exact test.
Figure 4.
H/I-induced forebrain damage in 10-month-old nIGF1RKO mice. Damage was evaluated from brain histology three days after H/I challenge. (a) Histological damage grading was coded: no detectable damage (white), limited (grey), substantial (dark grey), or severe (black). When the entire territory of the right middle cerebral artery (MCA) was evaluated, three-times less nIGF1RKO mice developed severe damage and twice as many nIGF1RKO mice showed no damage compared with controls. (b) When anatomical regions were evaluated separately, significantly fewer nIGF1RKO mice showed severe damage in ipsilateral cortex and striatum. (c) Infarct size was significantly smaller in nIGF1RKO mice in 10 out of 13 anterior–posterior brain areas (position of coronal sections relative to Bregma). (d) In nIGF1RKO mutants, infarct volume reconstituted from serial sections was 75% reduced compared with controls. (e) nIGF1RKO brains showed less edema (ipsi- vs. contralateral hemisphere size) in 9 out of 13 anterior–posterior areas, and (f) a strong reduction in edema volume. *P < 0.05; **P < 0.01; Mann-Whitney U-test; n = 25 nIGF1RKO and 18 controls; Error bars, SEM.
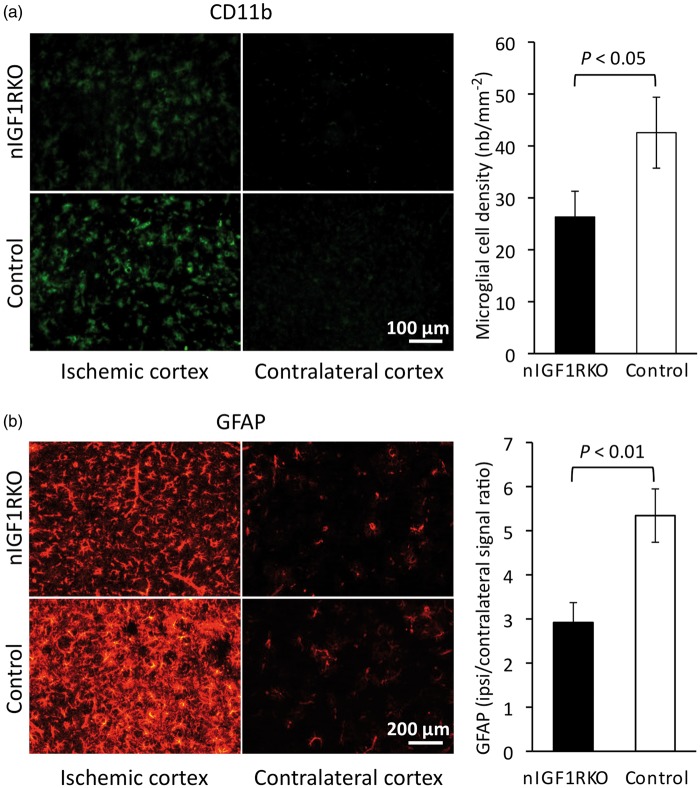
Cell edema develops in ischemic areas as an early consequence of infarct. Using histomorphometry, we showed that three days after H/I, edema was significantly smaller in nIGF1RKO mice as compared with controls (Figure 4(e) and (f)), indicative of protection from severe consequences of H/I. Meanwhile, we found no increase in preoperative cerebral vascularization nor increased blood flow before or after ischemia that could have explained the observed neuroprotection (online supplementary information, section 1.3. and online supplementary Figure 4). We next characterized the consequences of H/I at the cellular level. As expected, in sham-operated animals, no cell death was observed by TUNEL in both nIGF1RKO and control groups. However, three days after H/I, the ipsi- to contralateral ratio of NeuN immunoreactive cells revealed a 27% loss of neurons in control cortex (n = 18), while significantly less (8.8%) neurons were lost in nIGF1RKO cortex (n = 25; P < 0.05; Mann-Whitney U-test). We then asked whether enhanced neuroprotection and reduced edema in nIGF1RKO mice were associated with decreased inflammation, and tested microglial activation by CD11b-immunostaining. While sham-operated animals from both genotypes did not exhibit microglial activation, operated control mice showed strong reactivity (Figure 5(a)). Compared with controls, operated nIGF1RKO mice displayed 38% less microglial activation (P < 0.05). Importantly, we found markedly fewer GFAP-positive astrocytes in nIGF1RKO cortex (−45%, P < 0.01, Figure 5(b)). Thus, glial markers were not different between genotypes before stroke, but nIGF1RKO mice showed less inflammation afterwards confirming the previously observed neuroprotective and anti-inflammatory effect in mutants.
Figure 5.
Fewer inflammatory cells in nIGF1RKO mice 3 days after H/I. (a) In the cortical infarct, microglial CD11b-immunoreactivity was significantly lower in nIGF1RKO mice. (b) Astrocyte activation, assessed by GFAP immunolabel, was significantly reduced in ipsilateral cortex of nIGF1RKO mice. Representative micrographs for nIGF1RKO and controls are shown in left panels. Mann-Whitney U-test; n = 25 nIGF1RKO and 18 controls; Error bars, SEM.
Loss of neuronal IGF-1R stimulates neuroendocrine production of somatotropin
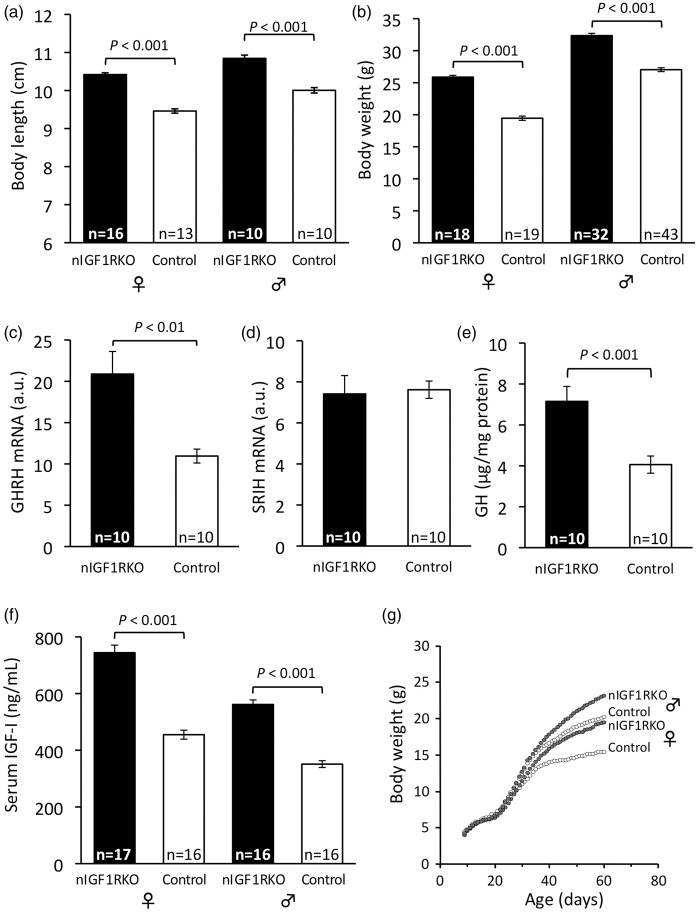
Adult nIGF1RKO were healthy, fertile and had normal food intake and energy metabolism (online supplementary Table 2). However, they showed faster growth and markedly increased adult body size (Figure 6(a) and (b)). Overgrowth was ubiquitous, all major peripheral organs being larger in nIGF1RKO (online supplementary Table 3). This pointed to endocrine changes in nIGF1RKO mouse growth control, and we thus checked somatotropic hormones. Expression of GH releasing hormone (GHRH, the principal determinant of GH secretion) was 1.8-fold increased in hypothalamus at three months, whereas expression of somatostatin (SRIH; somatotropin release-inhibiting hormone) was unchanged (Figure 6(c) and (d)). nIGF1RKO pituitaries were enlarged (control 1.88 ± 0.04 mg vs. nIGF1RKO 2.79 ± 0.04 mg, n = 10 per group, +49%; P < 0.001) and pituitary GH contents 76% increased (control 4.06 ± 0.42 µg GH per mg protein vs. nIGF1RKO 7.15 ± 0.73 µg/mg, P < 0.001; Figure 6(e)). Consistent with high GHRH and GH, circulating IGF-I was strongly increased in nIGF1RKO mice (males: +60%, females: +64%, both P < 0.001; Figure 6(f)), explaining enhanced growth (Figure 6(g)). Blood biochemistry of nIGF1RKO was normal except for a few changes attributable to GH and IGF-I actions (online supplementary Table 4). Collectively, these data demonstrate that suppression of IGF-1R in CamkIIα-expressing neurons leads to increased GH and IGF-I and significant peripheral overgrowth.
Figure 6.
Body size and somatotropic hormones in nIGF1RKO mice. (a) Body length and (b) weight were increased in adult nIGF1RKO mutants. (c) Hypothalamic GHRH was highly expressed in mutants as compared with controls, whereas (d) SRIH was similar; a.u., arbitrary units. (e) Pituitary GH and (f) plasma IGF-I were strongly increased in adult nIGF1RKO mice. (g) Postnatal growth trajectories of nIGF1RKO and control groups diverge after 40 days of age; Number of individuals is indicated in each panel (n in panel g identical to panel b); Student’s t-test; Error bars, SEM.
Blood-borne IGF-I might contribute to non-neuronal IGF-1R signaling in the brain,7,10 yet there is unfortunately no convincing way to demonstrate this directly in nIGF1RKO, where a large proportion of forebrain IGF-1R is knocked out. However, we were able to show that Janus kinase 2 (Jak 2), downstream of GH receptor was hyperphosphorylated in nIGF1RKO cortex (control 1.13 ± 0.08 vs. nIGF1RKO 1.42 ± 0.08 a.u., n = 4 per group, P < 0.05; Mann-Whitney U-test), indicative of increased GH action in nIGF1RKO CNS, possibly at glial cell level.
Discussion
Here, we found that suppression of IGF-1R from differentiated forebrain neurons did not provoke gross neurobiological impairment nor did it otherwise negatively affect the CNS of adult mice. Instead, we observed that neuronal function was preserved in nIGF1RKO mutants and some traits even improved. Moreover, we demonstrated that nIGF1RKO brain was markedly less susceptible to H/I damage in a mouse model of stroke. Absence of spontaneous defects may surprise, but it should be reminded that this IGF-1R conditional mutant was carefully designed to circumvent developmental defects. CamkCre4 is expressed only by mature forebrain neurons, and not while neurons are born, migrate or establish synaptic contacts; only as they become functional, neurons progressively lost IGF-1R, avoiding developmental imperfections. With respect to effects of IGFs on neuronal homeostasis, we would like to emphasize that paradigms are changing, and at least concerning the long-term consequences of IGF signaling on adult tissues, it seems now that less activation is protective for cells under stressful conditions.
IGF-I signaling is initiated by autophosphorylation of the IGF-1R β subunit, which activates downstream effectors PI3K/Akt/mTOR and MAPK/ERK. The PI3K/Akt pathway is pivotal for IGF signal transduction and controls protein homeostasis by facilitating translation and inhibiting autophagy. The PI3K/Akt cascade also activates the NF-κB pathway that inhibits apoptosis and triggers inflammation. In agreement with our findings, in experimental stroke, reducing mTOR signaling using rapamycin improved motor deficits and reduced the volume of brain lesions,38 while ERK activation mediates neuronal damage.39 Thus, efficient and durable suppression of IGF signaling in postnatal neurons likely supports neuroprotective mechanisms. Interestingly, excitotoxic metabolites produced during ischemic injury seem to specifically target the IGF pathway at the cellular level. Indeed, reactive oxygen species such as superoxide anions, or genetic inactivation of antioxidant enzyme SOD2, repress IGF signaling in dermal fibroblasts.40 Similarly, glutamate excitotoxicity attenuates IGF signaling in cultured neurons41 and in vivo in intact mouse brain.14 Since nIGF1RKO mice also display increased AMPA and NMDA-dependent excitability of hippocampal neurons, our findings suggest a neuronal cross-talk between IGF and glutamate signaling that could play a role in neuroprotective effects alleviating the consequences of ischemic insults. With respect to effects of IGF-1R knockout on electrophysiology, we found enhanced synaptic transmission but unchanged synaptic plasticity, which may be considered unusual. However, several other mouse mutants display similar combination and the inverse situation also exists.42,43 Importantly, we measured synaptic transmission separately for AMPAR and NMDAR, and we studied basal synaptic transmission using low frequency stimulation involving AMPA subtype of glutamate receptors, with Mg blocking NMDAR. In that setup, increased transmission may reflect recruitment of presynaptic fibers, facilitated glutamate release or changes in postsynaptic AMPAR. In synaptic plasticity paradigms, synaptic transmission is leveled in control and transgenic animals before stimulating at high frequency. Thus, changes in LTP or LTD between groups could reflect altered NMDAR activation or expression. In our model, increased NMDAR activation did not lead to increased synaptic plasticity, suggesting pathways involved in LTP and LTD expression are inhibited and counteract NMDAR activation. We searched for neuromorphological changes that could explain electrophysiological findings, but could not substantiate any obvious alterations. Interestingly, in two related models of conditional IGF-1R inactivation in neurons, we did find morphological changes, namely reduced cell size and short primary dendrite that may cause a gain-of-function neuronal phenotype.25,44
In the model of stroke that we used to challenge nIGF1RKO brains, neurons are directly injured by H/I insult, whereas secondary damage is caused by the accompanying neuroinflammatory response.45 As glial cells are not targeted by the Igf1r gene deletion in nIGF1RKO mice, they preserve IGF-1R expression and may benefit postoperatively from increased circulating IGF-I. Increased permeability of the blood–brain barrier during the first hours after H/I insult allows circulating IGF-I to enter the brain parenchyma, where astrocytes and microglial cells are potential protagonists of IGF-I-mediated neuroprotection.46,47 It is known that IGF-I regulates glutamate uptake by astrocytes, thereby reducing postinfarct excitotoxicity in the infarct penumbra.48 Conversely, during sustained inflammation, astrocytes and microglia can exert deleterious effects on surviving neurons by releasing pro-inflammatory and cytotoxic cytokines.45,49,50 Here, we report that nIGF1RKO mice show fewer microglial cells in the infarct and less astrocytic activation in the penumbra, traits that are clearly associated with neuroprotection. This suggests that high plasma IGF-I in nIGF1RKO mice limited inflammation and potentiated neuroprotection possibly via astrocyte and microglia regulation. However, neuroinflammation being secondary to neuronal insults, we cannot rule out that reduced gliosis could be the direct consequence of reduced ischemic insult and limited neuronal damage. Moreover, increased plasma GH eventually reaching the brain parenchyma in nIGF1RKO mice may be neuroprotective by triggering additional local IGF-I production.51 Together, our data support the idea that in the context of IGF-I resistance of central neurons, increased levels of IGF-I in the brain may contribute to neuroprotection through glial cell response.
Infarct-induced cell death releases cytotoxic solutes that also act on vascular components. This local cytotoxicity compromises integrity of the blood–brain barrier, leading to vasogenic edema. Importantly, IGF-I stimulates angiogenesis and vascular endothelial growth factor production by endothelial cells, accelerating blood vessel remodeling after brain injury.52 This hormonal action could be one of the mechanisms explaining how increased endocrine IGF-I contributed to reducing cerebral edema in nIGF1RKO mice. Still, it is not clear how this could occur in the absence of changes in blood vessel density in nIGF1RKO mice. Because edema can provoke acute secondary ischemia,53 stabilizing vascular endothelia is by itself neuroprotective and likely to contribute to the observed infarct prevention in nIGF1RKO mice. Interestingly, in mice with partial IGF-1R inactivation in glial cells and in neurons, H/I during prenatal brain development exacerbated neuronal apoptosis and cerebral edema.12 In that model, IGF-1R knockout occurred early during embryonic development, thus mice exhibited persistent brain growth retardation, which could in turn explain the vulnerability to H/I. These results also shed light on the role of IGF signaling in glial cells and vascular epithelia. Complementary to the results presented here, gene inactivation specifically in glial cells or in vascular cells of the brain will help to further dissect the phenotypic contribution of different CNS cell compartments.
We found that experimentally induced neuronal IGF resistance entails systemic upregulation of somatotropins. This suggests that IGF-sensing neurons in cortex, striatum or hippocampus participate in neuroendocrine control of GH and IGF-I, and efficiently regulate GHRH-producing networks in the arcuate nucleus (ARC).54 Neuronal projections from cortex to hypothalamus, relayed through thalamic or anterior hypothalamic nuclei to ARC may be involved in these processes.55 Direct implication of ARC neurons could be an alternative explanation, given that CamkCre4-driven Cre-lox recombination also occurred in a small number of ARC neurons. Results obtained using tamoxifen-inducible neuron-specific IGF-1R knockout25 suggest that increased somatotropic tone after neuronal IGF-1R inactivation is due to altered neuroendocrine feedback in the adult brain, and does not represent a defect in late postnatal development. Interestingly, IGF-1R knockout targeted to adipocytes using AP2-Cre; Igf1rflox/flox mutant mice56 results in similarly high somatotropic tone and peripheral overgrowth. These AP2-Cre; Igf1rflox/flox mice present with ectopic IGF-1R knockout in the brain resulting in 40% loss of IGF-1R protein in the CNS. This diminished abundance of brain IGF-1R most likely caused increased IGF and overgrowth through a similar mechanism as we observed in nIGF1RKO mice. To further elucidate neuroendocrine action regulated by IGF signaling in the brain, designing other cell type-specific IGF-1R knockout in the CNS will be necessary, targeting, e.g. specific nuclei or particular classes of forebrain neurons. Similar strategies will help elucidating the specific roles of glial and vascular cells in neuronal resistance to H/I insult.
Neuron-specific IGF-1R knockout is a powerful means to study brain IGF signaling in a physiological context, but the relevance of this experimental IGF resistance targeted to the CNS would increase if in addition it mimicked naturally occurring biological circumstances, including disease phenotypes. Interestingly, insulin resistance specifically in the brain has been proposed in connection with pathogenesis of Alzheimer disease (AD), and it was recently demonstrated that IGF resistance is a feature of the AD brain as well.57–59 Talbot et al.59 showed that hippocampus from non-diabetic AD patients exhibited greatly reduced responses to insulin and IGF-I. In this work, brain insulin resistance accompanied by IGF-I resistance could be interpreted as a consequence of AD, but also as an active neuronal defense mechanism against proteotoxicity. The latter has recently been shown directly using inducible neuron-specific IGF-1R knockout.25 Authors showed that efficient ablation of neuronal IGF-1R during adulthood alleviated amyloid pathology and cognitive deficits in AD mice. Implication of this pathway in AD-related proteotoxicity and neurodegeneration has been confirmed also at the level of downstream S6K1 recently.60 Together with the findings presented here, this strongly suggests that molecular mechanisms induced by neuronal IGF-I resistance during brain aging confers neuroprotection against several types of insults, from Aβ proteotoxicity to ischemic damage.
Supplementary Material
Acknowledgements
The authors thank M. Clemessy, S. El Mestikawy, I. Renault and R. Taylor for their contributions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Agence Nationale pour la Recherche (NT05-3 42491), EU Network LifeSpan (036894), Groupement d’Intérêt Scientifique and Institut du Vieillissement to MH and YLB. MENRT supported CDMF. ARC and INSERM supported LK.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CDMF, LK, YLB and MH conceived and designed the study. JD conducted signaling experiments. JS performed histomorphometry. SV and CCM performed Doppler measurements. CD and MNB conducted Morris tests. JMB performed electrophysiology. LK and AB measured gene expression. CDMF carried out all other experiments. FT and BG provided resources. CDMF, LK, JD, SV, CCM, CD, MNB, JMB, SA, YLB and MH analyzed and interpreted data. CDMF, MH and SA wrote the manuscript with input from all authors.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Russo VC, Gluckman PD, Feldman EL, et al. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev 2005; 26: 916–943. [DOI] [PubMed] [Google Scholar]
- 2.Aleman A, Torres-Alemán I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol 2009; 89: 256–265. [DOI] [PubMed] [Google Scholar]
- 3.Dupont J, Holzenberger M. Biology of insulin-like growth factors in development. Birth Defects Res C 2003; 69: 257–271. [DOI] [PubMed] [Google Scholar]
- 4.Desbois-Mouthon C, Wendum D, Cadoret A, et al. Hepatocyte proliferation during liver regeneration is impaired in mice with liver-specific IGF-1R knockout. FASEB J 2006; 20: 773–775. [DOI] [PubMed] [Google Scholar]
- 5.Rowland KJ, Trivedi S, Lee D, et al. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1 receptor deletion. Gastroenterology 2011; 141: 2166–2175. [DOI] [PubMed] [Google Scholar]
- 6.Nishijima T, Piriz J, Duflot S, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 2010; 67: 834–846. [DOI] [PubMed] [Google Scholar]
- 7.Mitschelen M, Yan H, Farley JA, et al. Long-term deficiency of circulating and hippocampal insulin-like growth factor I induces depressive behavior in adult mice: a potential model of geriatric depression. Neuroscience 2011; 185: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 2012; 13: 225–239. [DOI] [PubMed] [Google Scholar]
- 9.Carro E, Trejo JL, Núñez A, et al. Brain repair and neuroprotection by serum insulin-like growth factor I. Mol Neurobiol 2003; 27: 153–162. [DOI] [PubMed] [Google Scholar]
- 10.Trejo JL, Piriz J, Llorens-Martin MV, et al. Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry 2007; 12: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell SL, Frederick TJ, Krady JK, et al. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia 2002; 39: 85–97. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, D'Ercole JA, Ye P. Blunting type 1 insulin-like growth factor receptor expression exacerbates neuronal apoptosis following hypoxic/ischemic injury. BMC Neurosci 2011; 12: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan J, Bennet L, Gluckman PD, et al. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol 2003; 70: 443–462. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, Fan Y, Hao Q, et al. Postischemic IGF-1 gene transfer promotes neurovascular regeneration after experimental stroke. J Cereb Blood Flow Metab 2009; 29: 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun C, Meng Q, Zhang L, et al. Glutamate attenuates IGF-1 receptor tyrosine phosphorylation in mouse brain: possible significance in ischemic brain damage. Neurosci Res 2012; 74: 290–297. [DOI] [PubMed] [Google Scholar]
- 16.Chen DY, Stern SA, Garcia-Osta A, et al. A critical role for IGF-II in memory consolidation and enhancement. Nature 2011; 469: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieger F, Elflein N, Saenger S, et al. Polyethylene glycol-coupled IGF1 delays motor function defects in a mouse model of spinal muscular atrophy with respiratory distress type 1. Brain 2014; 137: 1374–1393. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Aleman I. Targeting insulin-like growth factor-1 to treat Alzheimer's disease. Expert Opin Ther Targets 2007; 11: 1535–1542. [DOI] [PubMed] [Google Scholar]
- 19.Cohen E, Paulsson JF, Blinder P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice. Cell 2009; 139: 1157–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology 2012; 79: 2148–2153. [DOI] [PubMed] [Google Scholar]
- 21.Endres M, Piriz J, Gertz K, et al. Serum insulin-like growth factor I and ischemic brain injury. Brain Res 2007; 1185: 328–335. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Mitschelen M, Toth P, et al. Endothelin-1-induced focal cerebral ischemia in the growth hormone/IGF-1 deficient Lewis Dwarf rat. J Gerontol A Biol Sci Med Sci 2014; 69: 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahamed K, Epaud R, Holzenberger M, et al. Deficiency in type 1 insulin-like growth factor receptor in mice protects against oxygen-induced lung injury. Respir Res 2005; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadoret A, Rey C, Wendum D, et al. IGF-1R contributes to stress-induced hepatocellular damage in experimental cholestasis. Am J Pathol 2009; 175: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gontier G, George C, Chaker Z, et al. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through Aβ clearance. J Neurosci 2015; 35: 11500–11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003; 421: 182–187. [DOI] [PubMed] [Google Scholar]
- 27.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 2011; 3: 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantamadiotis T, Lemberger T, Bleckmann SC, et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 2002; 31: 47–54. [DOI] [PubMed] [Google Scholar]
- 29.Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A 1999; 96: 5037–5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morice E, Billard JM, Denis C, et al. Parallel loss of hippocampal LTD and cognitive flexibility in a genetic model of hyperdopaminergia. Neuropsychopharmacology 2007; 32: 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol 1960; 36: 1–17. [PMC free article] [PubMed] [Google Scholar]
- 32.Vannucci SJ, Willing LB, Goto S, et al. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab 2001; 21: 52–60. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Nair A, Krady K, et al. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest 2004; 113: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Gontier G, Chaker Z, et al. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell 2014; 13: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dikkes P, Jaffe DB, Guo WH, et al. IGF2 knockout mice are resistant to kainic acid-induced seizures and neurodegeneration. Brain Res 2012; 17: 85–95. [DOI] [PubMed] [Google Scholar]
- 36.Cohen E, Du D, Joyce D, et al. Temporal requirements of insulin/IGF-1 signaling for proteotoxicity protection. Aging Cell 2010; 9: 126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freude S, Hettich MM, Schumann C, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer’s disease. FASEB J 2009; 23: 3315–3324. [DOI] [PubMed] [Google Scholar]
- 38.Xie L, Sun F, Wang J, et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol 2014; 192: 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subramaniam S, Zirrgiebel U, von Bohlen und Halbach O, et al. ERK activation promotes neuronal degeneration predominantly through plasma membrane damage and independently of caspase-3. J Cell Biol 2004; 165: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh K, Maity P, Krug L, et al. Superoxide anion radicals induce IGF-1 resistance through concomitant activation of PTP1B and PTEN. EMBO Mol Med 2015; 7: 59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Galloway E, Arango C, Pons S, et al. Glutamate excitotoxicity attenuates insulin-like growth factor-I prosurvival signaling. Mol Cell Neurosci 2003; 24: 1027–1037. [DOI] [PubMed] [Google Scholar]
- 42.Wiera G, Szczot M, Wojtowicz T, et al. Impact of matrix metalloproteinase-9 overexpression on synaptic excitatory transmission and its plasticity in rat CA3-CA1 hippocampal pathway. J Physiol Pharmacol 2015; 66: 309–315. [PubMed] [Google Scholar]
- 43.Vnencak M, Paul MH, Hick M, et al. Deletion of the amyloid precursor-like protein 1 (APLP1) enhances excitatory synaptic transmission, reduces network inhibition but does not impair synaptic plasticity in the mouse dentate gyrus. J Comp Neurol 2015; 523: 1717–1729. [DOI] [PubMed] [Google Scholar]
- 44.Chaker Z, Aïd S, Berry H, et al. Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell 2015; 14: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci 2009; 30: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez AM, Fernandez S, Carrero P, et al. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci 2007; 27: 8745–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Genis L, Dávila D, Fernandez S, et al. Astrocytes require insulin-like growth factor I to protect neurons against oxidative injury. F1000Research 2014; 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med 2004; 4: 193–205. [DOI] [PubMed] [Google Scholar]
- 49.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007; 8: 57–69. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Z, Zhang Q, Yu Z, et al. Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia 2007; 55: 546–558. [DOI] [PubMed] [Google Scholar]
- 51.Frago LM, Paneda C, Dickson SL, et al. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology 2002; 143: 4113–4122. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A 2004; 101: 9833–9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol 2007; 83: 140–148. [DOI] [PubMed] [Google Scholar]
- 54.Balthasar N, Mery PF, Magoulas CB, et al. Growth hormone-releasing hormone (GHRH) neurons in GHRH-enhanced green fluorescent protein transgenic mice: a ventral hypothalamic network. Endocrinology 2003; 144: 2728–2740. [DOI] [PubMed] [Google Scholar]
- 55.Staiger JF, Wouterlood FG. Efferent projections from the lateral septal nucleus to the anterior hypothalamus in the rat: a study combining Phaseolus vulgaris-leucoagglutinin tracing with vasopressin immunocytochemistry. Cell Tissue Res 1990; 261: 17–23. [DOI] [PubMed] [Google Scholar]
- 56.Klöting N, Koch L, Wunderlich T, et al. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 2008; 57: 2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moloney AM, Griffin RJ, Timmons S, et al. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging 2010; 31: 224–243. [DOI] [PubMed] [Google Scholar]
- 58.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Abeta oligomers. J Clin Invest 2012; 122: 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012; 122: 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caccamo A, Branca C, Talboom JS, et al. Reducing ribosomal protein S6 kinase 1 expression improves spatial memory and synaptic plasticity in a mouse model of Alzheimer's disease. J Neurosci 2015; 35: 14042–14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.