Abstract
Positron emission tomography (PET) can, when used with appropriate radioligands, non-invasively generate temporal and spatial information about acute changes in brain neurotransmitter systems. We for the first time evaluate the novel 5-HT2A receptor agonist PET radioligand, [11C]Cimbi-36, for its sensitivity to detect changes in endogenous cerebral 5-HT levels, as induced by different pharmacological challenges. To enable a direct translation of PET imaging data to changes in brain 5-HT levels, we calibrated the [11C]Cimbi-36 PET signal in the pig brain by simultaneous measurements of extracellular 5-HT levels with microdialysis and [11C]Cimbi-36 PET after various acute interventions (saline, citalopram, citalopram + pindolol, fenfluramine). In a subset of pigs, para-chlorophenylalanine pretreatment was given to deplete cerebral 5-HT. The interventions increased the cerebral extracellular 5-HT levels to 2–11 times baseline, with fenfluramine being the most potent pharmacological enhancer of 5-HT release, and induced a varying degree of decline in [11C]Cimbi-36 binding in the brain, consistent with the occupancy competition model. The observed correlation between changes in the extracellular 5-HT level in the pig brain and the 5-HT2A receptor occupancy indicates that [11C]Cimbi-36 binding is sensitive to changes in endogenous 5-HT levels, although only detectable with PET when the 5-HT release is sufficiently high.
Keywords: Positron emission tomography, 5-HT, brain imaging, kinetic modelling, neurosurgery
Introduction
Positron emission tomography (PET), when used with appropriate radioligands can non-invasively generate temporal and spatial information about acute changes in neurotransmitter systems in the brain; in particular, imaging of changes in the cerebral dopamine levels. For example, the PET radioligands [11C]-raclopride and [123I]IBZM have expanded the knowledge of the dopaminergic mechanisms involved in disorders such as schizophrenia, Parkinson’s, and stimulant abuse.1 Only to a lesser extent has this approach been successfully applied to imaging of other neurotransmitter systems, such as the opioid,2 serotonin,3 and other neurotransmitter systems.4
Neuropsychiatric disorders such as depression and migraine are ameliorated by pharmacological modulation of the serotonin (5-HT) system and this constitutes an established treatment for these disorders.5 PET imaging of the cerebral dynamic changes of the 5-HT level would make an important contribution to the understanding of the serotonergic mechanisms involved in these disorders, the underlying mechanisms behind the effect of, for example, pharmacological treatment or other interventions and could potentially lead to new treatments. Several studies have investigated PET radioligands for their ability to be displaced by endogenously released 5-HT. Five studies with the 5-HT2A antagonist radiotracer [18F]altanserin and [18F]setoperone were performed in humans, with various pharmacological challenges. A single dose of paroxetine,6 intravenous ketamine7 and pindolol plus citalopram8 did not induce any significant changes in [18F]altanserin binding or [18F]setoperone binding. Clomipramine9 and dexfenfluramine10 (40 or 60 mg single oral dose) dose-dependently decreased [18F]altanserin binding in several brain regions. In addition, during sleep in sleep-deprived individuals, [18F]altanserin binding increases, consistent with lower 5-HT levels, known to occur during sleep.11
According to the competition model, there are three factors, which determine the ability of a tracer to detect changes in synaptic neurotransmission: affinity of the neurotransmitter for its receptor, KNT, the basal neurotransmitter concentration in the interstitial fluid, FNT, and the challenge-induced change in neurotransmitter concentration, ΔFNT. The relationship between the resulting receptor occupancy and these variables is described by the equation3
| (1) |
That is, if the challenge-induced change in 5-HT concentration is much larger than KNT + FNT, then changes in radioligand binding with PET may be much more readily detected. Accordingly, we speculate that the different outcomes of the studies mentioned above7–10 may be due to the extent to which the different challenges affect synaptic 5-HT levels.
We recently developed and assessed the first 5-HT2A receptor agonist PET radioligand [11C]Cimbi36 in humans.12 Agonists have generally been suggested to be more sensitive to neurotransmitter release as compared to antagonist radioligands13,14 and preliminary data in non-human primates suggest that [11C]Cimbi36 binding is reduced following intravenous fenfluramine (5 mg/kg).15 For clinical studies, however, fenfluramine cannot be given at such high doses, and instead the 5-HT transporter selective inhibitor (es)citalopram has often been used as a tool to induce 5-HT increases in the human brain. Two recent PET-studies in humans question, however, if intravenous citalopram is capable of acutely increasing 5-HT levels and they both suggest that due to the 5-HT autoreceptors, an acute SSRI intervention results in a decrease in brain interstitial fluid 5-HT levels.16,17 In the absence of a direct measure of brain interstitial 5-HT concentration, such as done by state-of-the-art in vivo cerebral microdialysis,18 the matter is, however, difficult to settle.
The aim of the present study was to measure the effect of different pharmacological interventions on 5-HT by cerebral microdialysis and to correlate this outcome to the occupancy of the 5-HT2A receptor agonist radioligand [11C]Cimbi36. We measured pharmacologically induced changes in 5-HT levels in the medial prefrontal cortex (mPFC) in vivo in pigs and assessed simultaneously changes in [11C]Cimbi36 binding. We hypothesized that the different challenges would induce varying increases in the regional 5-HT brain level as measured with microdialysis, and a corresponding decline in [11C]Cimbi36 receptor binding in the brain.
Material and methods
Animals
We used 13 female 9- to 10-week-old Danish Landrace pigs weighing 24 ± 1.5 kg (mean ± SD) in this study. All animal experiments were performed in accordance with the European Communities Council Resolves of 22 September 2010 (2010/63/EU) and approved by the Danish Veterinary and Food Administration’s Council for Animal Experimentation (Journal No. 2012-15-2934-00156), and is in compliance with the ARRIVE guidelines (www.nc3rs.org.uk/arrive-guidelines). We housed the pigs and performed tranquilization, anesthesia, intubation, installation of arterial and venous intravenous lines in the morning, monitoring and sacrifice of animals late afternoon as previously described.19 On the day of investigation, we implanted microdialysis guide cannulas bilaterally into the medial prefrontal cortex (mPFC). After midline sagittal incision and exposure of the skull, we placed two burr holes 25 mm anterior and 8 mm lateral to each side of bregma, incised dura and secured hemostasis. Then we placed an anchor screw anterolateral to the right burr hole and decorticated the skull to allow fixation of the 10 mm CMA 12 guide cannulas (CMA, Kista, Sweden), which we introduced through each burr hole in a direction perpendicular to the skull and fixed with Dentalon Plus Cement (Heraeus Kulzer GmbH, Hanau, Germany). After surgery, we inserted a CMA 12 metal-free microdialysis probe (CMA Microdialysis) with a membrane length of 4 mm and a molecular weight cut-off value of 20 kDa through each of the cannulas. The coordinates allowed full embedment of the membrane in grey matter of mPFC. A postoperative MRI from a 3.0 T Siemens VERIO MR scanner, which we analyzed with the Brainlab Stereotactic Planning software and manual co-registration to a histology slice, served to confirm the correct position of the probe in the grey matter of mPFC of the pig brain.
Study design
All baseline and challenge PET scans were conducted on the same day, in fixed order. Microdialysis probes were implanted bilaterally in the mPFC and we confirmed the correct position with a postoperative brain MRI, as described above. A 2-h wash-out period was allowed for the microdialysis to reach a stable baseline level. The two PET scans, each lasting 90 min, were conducted with at least 30 min apart. After completion of the first PET scan, we administered the pharmacological interventions intravenously, with the aim to acutely increase the extracellular 5-HT levels.
After the last PET scan, we excised tissue from the mPFC where the microdialysis probe had been implanted. The tissue was quickly frozen on dry ice and stored in a −80℃ freezer until further analyzed for either content of 5-HT or sliced in a microtome and stained to obtain a histology slice that could be compared to the MRI, for establishment of probe placement in the grey matter of mPFC.
Pharmacological interventions
To confirm that a change in radioligand binding was not caused by direct interaction between fenfluramine and the 5-HT2A receptor20,21 we gave two pigs that later received fenfluramine intramuscular para-chlorophenylalanine (pPCA)22 prior to the day of the PET-scan. pCPA is an irreversible inhibitor of the enzyme tryptophan hydroxylase, which catalyzes the rate-limiting step in 5-HT biosynthesis, and we have previously shown that pCPA substantially reduces 5-HT level in the pig brain without altering 5-HT2A receptor density.23 Thus, nine of the 13 pigs received a four-day pretreatment of intramuscular saline 10 mL (N = 7) or pCPA 100 mg/kg (N = 2) in a solution of 2 ml/kg. The intramuscular injections required sedation of the pigs with a solution of dexmedetomidin 50 µg/kg, butorphanol 0.2 mg/kg and midazolam 0.15 mg/kg on the first day. On the following days, propofol 4 mg/kg and midazolam 0.1 mg/kg were given intravenously in a catheter placed in the ear. After the procedures, the pigs were given atipamezole 200 µg/kg for reversal of anesthesia.
In pilot experiments prior to the ones described here, we tested the effect of three different doses of fenfluramine; 0.05 mg/kg, 0.5 mg/kg and 2.0 mg/kg, but when 2 mg/kg was given, the pig started to shiver and showed a large increase in vital signs, so the injection was interrupted after ¾ had been given. Since the dose of 0.05 mg/kg did not induce any significant alterations in microdialysate 5-HT levels, we chose to give a final dose of 0.5 mg/kg.
Interventions between the two PET-scans were given 15 min prior to the second scan and consisted of either
Saline (Controls, N = 2)
Citalopram 2 mg/kg (Selective 5-HT reuptake inhibitor (SSRI), N = 2)
Citalopram 2 mg/kg, preceded by pindolol 1 mg/kg, given 30 min prior to the second scan (5-HT1A autoreceptor antagonist, N = 3)
Fenfluramine 0.5 mg/kg (5-HT releaser, N = 4)
Fenfluramine 0.5 mg/kg with pCPA pre-treatment (5-HT depletion, N = 2)
Unexpectedly, two pigs assigned for fenfluramine 0.5 mg/kg intervention, did not respond to the challenge as evaluated by both microdialysate 5-HT levels and PET measures. These studies were conducted five months after the initial experiments, which raised the concern that in between the experiments, the fenfluramine had either decomposed or the content had been replaced by some unknown 5-HT inactive material. We acquired a new batch of fenfluramine and compared it to the remaining powder in the vial. Visual inspection, HPLC analysis and new trials in mice and pigs supported that the vial content had been replaced. Accordingly, we excluded these two pigs from the group analyses of pharmacological interventions with fenfluramine, but we did not exclude them from the correlation analysis of microdialysis and PET measures.
Microdialysis and 5-HT measurements
We placed the pig in the PET scanner and allowed a 2-h washout period to obtain steady baseline microdialysis level. The probes were perfused with a standard Ringer solution (147 mM NaCl, 4 mM KCl and 2.3 mM CaCl2, adjusted to pH 6.5) at a flow rate of 1.0 µL/min. From both probes, we collected 15 min time-series of samples of extracellular fluid to be analyzed off-line for monoamines by HPLC. After a 2-h washout period, we collected 16 samples with 15 min interval. The microdialysate samples were quickly frozen on dry ice and stored in a −80℃ freezer until further analyzed.
We determined the relative changes in 5-HT concentrations in the dialysates and the absolute 5-HT concentration in brain tissue samples by HPLC, with electrochemical detection. The column was a Prodigy 3 µ ODS (3) C18 (DA 2 mm × 100 mm, particle size 3 µm, Phenomenex, Torrance, CA, USA). The mobile phase consisted of 55 mM sodium acetate, 1 mM octanesulfonic acid, 0.1 mM Na2EDTA and 8% acetonitrile, adjusted to pH 3.2 with 0.1 M acetic acid, and was degassed with an on-line degasser. Ten microliters of the dialysate samples was injected, and the flow rate was 0.15 mL/min. The electrochemical detection was done with an amperometric detector (Antec Decade, Antec, Leiden, the Netherlands) with a glassy carbon electrode set at 0.8 V, with an Ag/AgCl reference electrode.
The excised pig brain blocks were homogenised in perchloric acid 0.1 N. After centrifugation at 14,000 r/min for 30 min, 200 µL of the supernatant was filtered through a glass 0.22 µm filter Avantec 13CP020AS. The 5-HT tissue concentrations in the pig brain homogenate was calculated as 5-HT ug/g brain tissue and represent as mean ± SEM for the saline and the pCPA-treated group.
The output of the HPLC was recorded by the program “LC solution” (Simadzu, Columbia, MD, USA), which also was used to calculate the peak areas. We determined the baseline level on the basis of the mean peak area obtained by the HPLC from the three samples preceding the pharmacological intervention. We used the baseline level to calculate the relative change in the 5-HT levels of the following samples. In 10 of the 13 pigs, stable baseline 5-HT levels were achieved on both sides; in three pigs, only one probe returned stable baseline levels.
PET scanning protocol
PET scans were obtained in list mode with a high-resolution research tomography (HRRT) scanner, with the pig in the prone position. [11C]Cimbi36 was given as an intravenous bolus injection and data acquisition began at the time of injection. The synthesis and radiochemical labeling of [11C]Cimbi36 has previously been described.12
The mean injected [11C]Cimbi36 was 471 MBq (range, 197–510 MBq; N = 26), and the average injected mass was 1.20 µg (range 0.10–3.55 µg; N = 26). During the first 20 min of the PET scan, whole blood radioactivity was continuously measured using an ABSS autosampler (Allogg Technology, Mariefred, Sweden) counting coincidences in a lead-shielded detector. In addition, blood samples were manually drawn at 2.5, 5, 10, 20, 30, 50, 70 and 90 min and the radioactivity in whole blood and plasma was measured in a well counter (Cobra 5003, Packard Instruments, PerkinElmer, Skovlunde, Denmark) that was cross-calibrated to the HRRT scanner and autosampler. Radiolabeled parent compound and metabolites were measured in plasma using HPLC with online radioactivity detection as previously described.24 An average parent compound fraction-time curve was generated, based on all baseline scans in this study and the data were fitted to a bi-exponential function. This function was used with the individual plasma radioactivity concentration curves from the combined baseline and intervention scans to generate the individual plasma parent compound input functions.
Quantification of PET data
The [11C]Cimbi36 HRRT PET data were reconstructed into 38 frames of increasing length (6 × 10, 6 × 20, 4 × 30, 9 × 60, 2 × 180, 8 × 300, 3 × 600 s). Images consisted of 207 planes of 256 × 256 voxels of 1.22 × 1.22 × 1.22 mm. Summed images of all counts in the 90 min scan were reconstructed for each pig and used for co-registration to a standardized MRI-based atlas of the Danish Landrace pig brain, similar to that previously published for the Göttingen minipig,25 using the software Register, as previously described.26
Each coregistration was verified by visual inspection before extraction of time-radioactivity curves (TACs) from the volumes of interest (VOIs), and adjusted if needed. The TACs were determined for the following VOIs: Neocortex and cortical white matter, cerebellum, hippocampus, striatum (caudate and putamen) and thalamus (medial and lateral thalamus).
We quantified the 5-HT2A receptor binding with [11C]Cimbi36 both with the 2TC and Logan invasive modeling; the latter model generated a higher number of converging regional fitting and was thus chosen for further analysis. We calculated the volumes of distribution (VT) for the VOIs with PMOD software (version 3.0; PMOD Technologies, Zürich, Switzerland). The VT of the baseline and intervention scan was used to determine occupancy by use of the occupancy plot.27,28 The standard coefficient of variance (COV) was below 10% for all regional VTs. Data that did not fulfill this criterion were not included in the analysis.
Statistical analysis
We correlated the occupancy as measured by PET with the highest peak increase (of the left or right microdialysis probe) in extracellular 5-HT levels. Unless performed with very time-consuming methods29 incompatible with the use of short-lived radioisotypes and the need to conduct two PET studies, microdialysis experiments do not generate absolute 5-HT concentrations. Instead, we assumed a fairly stable baseline concentration of the pig cerebral interstitial fluid 5-HT of about 1.7 nM, equal to what has been found in mice,30–34 and ΔFNT was then computed as 1.7 nM times the relative peak increase in 5-HT level minus 1.7 nM. The data were fitted to the model given in equation (1) with a non-linear regression analysis. The groups of pre-treatment with saline or pCPA were compared with unpaired two-tailed t-test.
Results
Probe placement
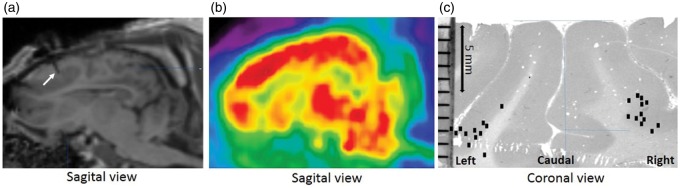
Figure 1 illustrates the placement of the tip of the probe, from which the membrane extends 4 mm upwards in the grey matter. Except for one pig with one misplaced probe, both probes were correctly positioned in all pigs.
Figure 1.
(a) MRI of the pig brain showing the implanted microdialysis probe (white arrow) and (b) [11C]Cimbi36 PET image of the pig brain. The placement of the tip of the microdialysis probes in the mPFC, as identified from the MRI scan, is indicated on a histology slice (c) and marked with a black dot. The microdialysis probe extends from the tip (black dot) and 4 mm cortically, meaning that the relevant part of the probe is embedded within the grey matter of the mPFC.
Tissue concentrations of 5-HT
The mean tissue concentrations of 5-HT in pigs pre-treated with saline was 0.25 ± 0.05 µg/g tissue (mean ± SD, N = 7) cortical tissue. Pre-treatment with pCPA resulted in lower 5-HT tissue concentrations, 0.05 µg/g tissue (N = 2), in line with previous observations.23
In vivo microdialysis
Figure 2 illustrates time-dependent effects of the interventions on changes in cerebral 5-HT as measured by microdialysis in the mPFC. Fenfluramine produced an immediate and powerful increase in extracellular 5-HT level to a peak 1123 ± 144% (N = 2) relative to baseline level (100%). The 5-HT releasing effect of fenfluramine was blunted by pCPA pre-treatment, with a peak 5-HT increase to 516 ± 159% (N = 2). Citalopram produced an immediate increase in 5-HT, to 217% (N = 2), that was normalized or perhaps even reversed after 30 min. The combination of pindolol and citalopram intervention prolonged and enhanced the serotonergic response as compared to citalopram alone, leading to a sustained mean increase of up to 441 ± 78% (N = 3) of 5-HT baseline level. Saline intervention did not change the 5-HT level in the pig brain relative to baseline level.
Figure 2.
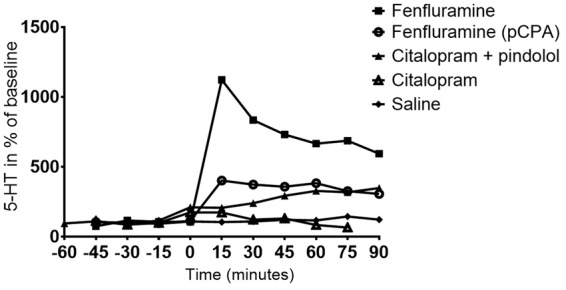
Time-dependent intervention effects on the changes in the relative cerebral extracellular 5-HT levels as measured by microdialysis in the mPFC. Values are given as means of 2–3 measurements. All VT values are listed in the Supplementary Material, Table 1.
In vivo PET imaging
The VT in the baseline scans was 7.3 ± 1.7 mL/cm3 (N = 13) in the neocortex grey matter and 3.6 ± 1.0 mL/cm3 (N = 13) in the cerebellum. The 5-HT occupancy was quantified using the Lassen plot in each individual pig. The 5-HT2A receptor occupancy was 17 ± 5% (N = 2) with saline, whereas the occupancy after pharmacological intervention with citalopram was 19 ± 2% (N = 2), with combined citalopram and pindolol 28 ± 4% (N = 3), fenfluramine with pCPA pre-treatment 38 ± 3% (N = 2) and fenfluramine with no pre-treatment 44 ± 3% (N = 2). Based on the baseline and the intervention scans, the non-displaceable distribution volume (VND) of [11C]Cimbi36 was determined to 2.7 ± 0.5 mL/cm3 (N = 9).
Correlation of microdialysis and PET measures
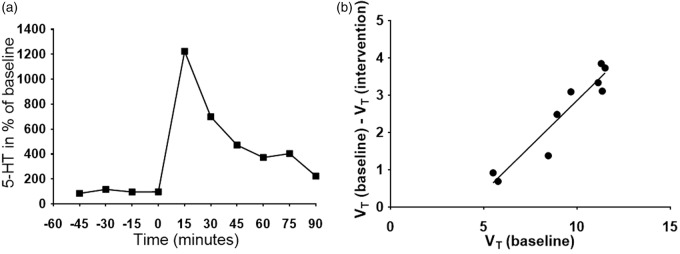
Figure 3 shows an example of microdialysis data and an occupancy plot obtained in one pig. The corresponding measures of the PET occupancy and the ΔFNT were fitted to equation (1) (Figure 4). KNT can be calculated from equation (1) and was determined to be 14.3 nM, i.e. about eight times higher than the baseline brain interstitial 5-HT concentration. The observed correlation measures complied with the competition model (equation (1)).
Figure 3.
Example of microdialysis time course and an occupancy plot in a single pig. Panel A shows the relative change in 5-HT (given in % of baseline levels) over time, after fenfluramine 0.5 mg/kg i.v. was given at time=0 min. Panel B shows the corresponding occupancy plot based on volumes of distribution (VT) in the left and right VOIs (mL/cm3).
Figure 4.
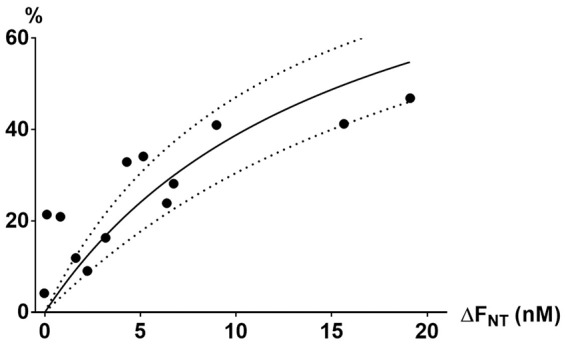
The ΔFNT (nM) and the corresponding 5-HT2A receptor occupancy (%) with 95% confidence interval of all 13 pigs were fitted with nonlinear regression analysis according to the occupation model (equation (1)). The points did not deviate significantly from the model.
Discussion
We here show, first, how different pharmacological interventions directly change the cerebral interstitial fluid concentration of 5-HT in the pig brain. Second, we demonstrate how the cerebral binding of 5-HT2A receptor agonist radioligand [11C]Cimbi36 changes in response to these interventions.
The pharmacological interventions induced a dose-dependent increase in cerebral 5-HT release of 2–11-fold of baseline level in the mPFC in the pig brain. This is in line with microdialysis studies in rats and non-human primates where the increase in the cerebral 5-HT level was 4–35 fold with fenfluramine (1–10 mg/kg)35–39 and we observed an 11-fold increase with fenfluramine (0.5 mg/kg). Further, in rats citalopram (5–10 mg/kg)40 induced a 2–4-fold increase in 5-HT level compared to the 2-fold increase with citalopram (2 mg/kg) in our study. A lower dose of citalopram (1 mg/kg) did not change the extracellular 5-HT level in the rat, but when the 5-HT1/2 receptor blocker methiothepin was added, 5-HT levels increased four times as compared to the baseline level.41 This increase was equivalent to the four-fold increase with citalopram (2 mg/kg) plus pindolol (1 mg/kg) used in our study. Although we used smaller doses of all the pharmacological interventions in the pig as compared to the studies in the rat, we observed an increase in extracellular 5-HT level comparable to those in the rats. This efficacy of lower doses could be due to species difference, or could be ascribed to the absence of anaesthesia in some of the rodent studies or differences in the administration route of the drugs.35–37,39 In any instance, we found that a dose of 1.5 mg/kg was the maximal dose of fenfluramine that the pigs tolerated.
pCPA-pretreatment effectively reduced the brain 5-HT levels to ∼20%, which is in line with Ettrup et al.23 Although a higher percentage of depletion (5–10%) was achieved in rats with pCPA-pretreatment of 200–400 mg/kg,42 Ettrup et al. did not in pigs find an added effect of increasing pCPA dose from 50 to 100 mg/kg.23 This lack of a clear dose–response relationship has previously been ascribed to a threshold effect or to a limited bioavailability from intramuscular administration in pigs compared to intraperitoneal administration in rats. Somewhat unexpectedly, this vast reduction in total 5-HT level only reduced the fenfluramine induced 5-HT release to about half as much as compared to saline-pretreated pigs. This could be caused by the serotonergic neurons more efficiently storing and releasing 5-HT in the vesicles of the synaptic terminal. Alternatively, given that our microdialysis measurements only generate relative increases in interstitial fluid 5-HT, if pCPA-treated pigs had reduced interstitial fluid baseline 5-HT levels to 20% of the saline-pretreated pigs, then the absolute amounts of released 5-HT could have been substantially smaller. Based on the occupancy plot (Figure 4), the latter did, however, not seem to be the case.
Depending on the pharmacological intervention, the 5-HT2A receptor occupancy as measured by the PET radioligand [11C]Cimbi36 ranged between 19 and 44%. The paired data on 5-HT microdialysis and [11C]Cimbi36 occupancy measures comply with the competition model (equation (1)) and indicate that [11C]Cimbi36 was sensitive to changes in endogenous 5-HT levels, but that was only detectable with PET when the 5-HT release was sufficiently high. As anticipated, we observed a higher occupancy with more powerful serotonergic challenges and also a higher occupancy when citalopram was combined with pindolol.
Based on the data shown in Figure 4, we estimated KNT (the affinity of 5-HT to the 5-HT2A receptor) to be about 14.3 nM. For comparison, the concentration of 3H-5-HT that labels 50% of cloned human 5-HT2A receptors is 21 nM (PDSP Ki database).
To put our findings in perspective, one can compare the sensitivity of [11C]-Cimbi36 to 5-HT to that of [11C]-raclopride to dopamine, a much used approach to detect in vivo change in cerebral dopamine. From microdialysis studies in non-human primates, it is known that when amphetamine 0.3 mg/kg is given intravenously, the interstitial dopamine concentration increases approximately 8-fold.43–45 In non-human primates, an 8-fold increase in dopamine results in a decrease in [11C]-raclopride BPnd of 13%.46 This is consistent with findings in humans, where intravenous amphetamine 0.2–0.3 mg/kg results in a decrease in striatal BPnd of 15.5%46 or 13%.47–49 From Figure 4 it can be seen that when interstitial 5-HT is pharmacologically increased by 8-fold, and [11C]-Cimbi36 BPnd decreases by 46% (CI 38-55%), i.e. [11C]-Cimbi36 is over three times more sensitive to changes in 5-HT than [11C]-raclopride is to dopamine. Moreover, while [11C]-raclopride can only be used to reliably assess dopamine changes in striatum, [11C]-Cimbi36 can – due to the widespread and relatively uniform distribution of the 5-HT2AR – theoretically be used to determine regional 5-HT changes in the brain.
As expected, saline intervention did not change microdialysis 5-HT brain levels, and still we somewhat puzzlingly observed 5-HT2AR occupancy in the two saline-treated animals, 12 and 21% (average 17%). In two animals that received inactive fenfluramine, however, the 5-HT2AR occupancy was only 4% and 9%. We have no explanation for this variability in occupancy, but speculate that anesthesia or a better peripheral pain alleviation instituted after the initial scans could play a role.50
In a previous PET study in humans using the 5-HT2A receptor antagonist [18F]altanserin, intervention with citalopram + pindolol did not change the occupancy.8 This difference could, however, also be ascribed to a higher sensitivity to 5-HT competition with the 5-HT2A receptor agonist [11C]Cimbi36 compared to the antagonist [18F]altanserin, which binds to both low and high affinity receptors. Our data suggest that the reason why a reduction in [18F]altanserin PET was seen after dexfenfluramine10,15 but not after citalopram + pindolol8 is because dexfenfluramine is a much stronger elicitor of 5-HT.
Three studies in humans tested the sensitivity of the 5-HT1A receptor radioligand [11C]CUMI-10116,51 and the partial 5-HT1B agonist radioligand [11C]AZ1041936917 to pharmacological changes with escitalopram or citalopram. While Pinborg et al.51 did not observe any significant change in [11C]CUMI-101 binding following an intravenous infusion of citalopram (∼0.15 mg/kg), Selvaraj et al.,16 using a similar dose of citalopram (∼0.15 mg/kg) found a mean increase of 7% in BPND in several cortical regions, but no change in the dorsal raphe nucleus (DRN). Likewise, in humans, Nord et al.17 reported increased binding of [11C]AZ10419369 in serotonergic projection areas following a clinically relevant peroral dose of 20 mg escitalopram (∼0.28 mg/kg), whereas there was a statistical trend for a decreased raphe nuclei binding. Thus, two studies in humans showed an increase in [11C]CUMI-10116 and [11C] AZ1041936917 binding, rather than the expected decrease. This was in both cases interpreted as a consequence of the pharmacological challenge inducing a temporary decrease in 5-HT, due to 5-HT1A autoreceptor regulation. Although in a different species, our data do not lend support for an acute decrease in cerebral interstitial 5-HT levels, at least not when giving what corresponds to a 3–4 times higher dose of citalopram. Currently, no studies have investigated the effect of lower doses of citalopram on brain interstitial 5-HT levels.
A limitation of this study is that microdialysis allows only for measurements in a few selected brain regions, while PET, on the other hand, can generate information about changes in the 5-HT of the entire brain. Other microdialysis studies41,52–55 show that the SSRI-induced elevation in the extracellular 5-HT level is higher in the DRN compared to striatal and cortical projection areas, where the 5-HT increase is lower or even absent. This region-dependent pattern may be caused by a higher density of the 5-HT transporter and 5-HT1A receptors in the DRN56,57 and inhibition of DRN firing rate to projection areas.55,58,59 Therefore, the Δ[5-HT], as measured by microdialysis in the mPFC, may not necessarily reflect the different regional changes in the interstitial 5-HT level. By using the occupancy plot, we implicitly assume a uniform release of 5-HT throughout the brain. While this assumption may not be justified, we nevertheless found that the occupancy plots generally had statistically significant correlations with good r-square values. The low number of pigs (2–3) in each group of the serotonergic challenges did not allow for a statistical comparison of the different pharmacological intervention effects, but that was not the main goal of the study. Rather, we wanted to asses the sensitivity of [11C]Cimbi36 to detect changes in 5-HT brain levels.
In conclusion, we demonstrate that the change in the [11C]Cimbi36 PET signal correlates to pharmacologically induced changes in interstitial 5-HT brain level. The observed correlation between changes in the extracellular 5-HT level in the pig brain and the 5-HT2A receptor occupancy indicates that [11C]Cimbi36 is sensitive to changes in endogenous 5-HT levels, but that is only detectable with PET when the 5-HT release is sufficiently high. Differences in earlier studies may thus be ascribed to the efficacy of the pharmacological interventions to change interstitial brain 5-HT levels. Verifying the direct correlation between pharmacologically induced changes in 5-HT and [11C]Cimbi36 PET occupancy is an important step prior to conduction of clinical trials and the calibration allows for estimating the regional relative change in interstitial 5-HT in patients in future studies.
Supplementary Material
Acknowledgements
The authors gratefully thank Jytte Rasmussen, Bente Dall, Szabolcs Lehel, Gerda Thomsen, Svitlana Olsen, and Agnete Dyssegaard and for their excellent technical assistance. Ling Feng is thanked for his assistance with the graphical representation of Figure 4.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study received grant funding from the Lundbeck Foundation (R170-2014-994) for running costs and for PhD salary (LMJ) and from the Aase and Ejnar Danielsens Fund (10-001296).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
All authors revised and gave final approval of the manuscript. LMJ, PW, AE and GMK contributed to the conception of the article, and LMJ, PW, HDH and GMK contributed to the drafting of the article). All authors contributed to the design (with exception of HDH and JV), acquisition of data (with exception of HDH and GMK) and analysis and interpretation of data (with the exception of JV, AOB and FLA)
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2000; 20: 423–451. [DOI] [PubMed] [Google Scholar]
- 2.Sprenger T, Berthele A, Platzer S, et al. What to learn from in vivo opioidergic brain imaging? Eur J Pain 2005; 9: 117–121. [DOI] [PubMed] [Google Scholar]
- 3.Paterson LM, Tyacke RJ, Nutt DJ, et al. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab 2010; 30: 1682–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnema SJ, Scheinin M, Shahid M, et al. Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology (Berl) 2015; 232(21–22): 4129–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller CP, Jacobs BL. Handbook of the behavioral neurobiology of serotonin, 1st ed London: Elsevier BV, 2010. [Google Scholar]
- 6.Meyer JH, Cho R, Kennedy S, et al. The effects of single dose nefazodone and paroxetine upon 5-HT[sub 2A] binding potential in humans using [[sup 18] F]-setoperone PET. Psychopharmacology (Berl) 1999; 144: 279. [DOI] [PubMed] [Google Scholar]
- 7.Matusch A, Hurlemann R, Rota Kops E, et al. Acute S-ketamine application does not alter cerebral [18F]altanserin binding: a pilot PET study in humans. J Neural Transm Vienna Austria 2007; 114: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 8.Pinborg LH, Adams KH, Yndgaard S, et al. [18F]altanserin binding to human 5HT2A receptors is unaltered after citalopram and pindolol challenge. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2004; 24: 1037–1045. [DOI] [PubMed] [Google Scholar]
- 9.Larisch R, Klimke A, Hamacher K, et al. Influence of synaptic serotonin level on [18F]altanserin binding to 5HT2 receptors in man. Behav Brain Res 2003; 139: 21–29. [DOI] [PubMed] [Google Scholar]
- 10.Quednow BB, Treyer V, Hasler F, et al. Assessment of serotonin release capacity in the human brain using dexfenfluramine challenge and [18F]altanserin positron emission tomography. NeuroImage 2012; 59: 3922–3932. [DOI] [PubMed] [Google Scholar]
- 11.Elmenhorst D, Kroll T, Matusch A, et al. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep 2012; 35: 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettrup A, da Cunha-Bang S, McMahon B, et al. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36. J Cereb Blood Flow Metab 2014; 34: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narendran R, Hwang D-R, Slifstein M, et al. In vivo vulnerability to competition by endogenous dopamine: Comparison of the D2 receptor agonist radiotracer (–)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 2004; 52: 188–208. [DOI] [PubMed] [Google Scholar]
- 14.Willeit M, Ginovart N, Graff A, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 2007; 33: 279–289. [DOI] [PubMed] [Google Scholar]
- 15.Finnema S, Ettrup A, Stepanov V, et al. Pilot study on receptor binding and serotonin sensitivity of [11C]CIMBI-36 in monkey brain. Soc Nucl Med Annu Meet Abstr 2011; 52: 495. [Google Scholar]
- 16.Selvaraj S, Turkheimer F, Rosso L, et al. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry 2012; 17: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 17.Nord M, Finnema SJ, Halldin C, et al. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol 2013; 16: 1577–1586. [DOI] [PubMed] [Google Scholar]
- 18.Cosford RJ. Quantitative microdialysis of serotonin and norepinephrine: pharmacological influences on in vivo extraction fraction. J Neurosci Meth 1996; 68: 39–47. [DOI] [PubMed] [Google Scholar]
- 19.Andersen VL, Hansen HD, Herth MM, et al. 11C-labeling and preliminary evaluation of pimavanserin as a 5-HT2A receptor PET-radioligand. Bioorg Med Chem Lett 2015; 25: 1053–1056. [DOI] [PubMed] [Google Scholar]
- 20.Mennini T, Fracasso C, Cagnotto A, et al. In vitro and in vivo effects of the anorectic agent dexfenfluramine on the central serotoninergic neuronal systems of non-human primates. A comparison with the rat. Naunyn Schmiedebergs Arch Pharmacol 1996; 353: 641–647. [DOI] [PubMed] [Google Scholar]
- 21.Finnema SJ, Varrone A, Hwang T-J, et al. Confirmation of fenfluramine effect on 5-HT1B receptor binding of [11C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab 2012; 32: 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javed A, Van De Kar LD, Gray TS. p-Chlorophenylalanine and fluoxetine inhibit d-fenfluramine-induced Fos expression in the paraventricular nucleus, cingulate cortex and frontal cortex but not in other forebrain and brainstem regions. Brain Res 1997; 774: 94–105. [DOI] [PubMed] [Google Scholar]
- 23.Ettrup A, Kornum BR, Weikop P, et al. An approach for serotonin depletion in pigs: Effects on serotonin receptor binding. Synapse 2011; 65: 136–145. [DOI] [PubMed] [Google Scholar]
- 24.Gillings N. A restricted access material for rapid analysis of [11C]-labeled radiopharmaceuticals and their metabolites in plasma. Nucl Med Biol 2009; 36: 961–965. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe H, Andersen F, Simonsen CZ, et al. MR-based statistical atlas of the Göttingen minipig brain. NeuroImage 2001; 14: 1089–1096. [DOI] [PubMed] [Google Scholar]
- 26.Kornum BR, Lind NM, Gillings N, et al. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Göttingen minipig. J Cereb Blood Flow Metab 2008; 29: 186–196. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham VJ, Rabiner EA, Slifstein M, et al. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2010; 30: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen HD, Herth MM, Ettrup A, et al. Radiosynthesis and in vivo evaluation of novel radioligands for PET imaging of cerebral 5-HT7 receptors. J Nucl Med 2014; 55: 640–646. [DOI] [PubMed] [Google Scholar]
- 29.Olson RJ, Justice JB. Quantitative microdialysis under transient conditions. Anal Chem 1993; 65: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 30.Gardier AM, David DJ, Jego G, et al. Effects of chronic paroxetine treatment on dialysate serotonin in 5-HT1B receptor knockout mice. J Neurochem 2003; 86: 13–24. [DOI] [PubMed] [Google Scholar]
- 31.Tao R, Ma Z, Auerbach SB. Differential effect of local infusion of serotonin reuptake inhibitors in the Raphe versus Forebrain and the role of depolarization-induced release in increased extracellular serotonin. J Pharmacol Exp Ther 2000; 294: 571–579. [PubMed] [Google Scholar]
- 32.Guiard BP, David DJP, Deltheil T, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol 2008; 11: 79–92. [DOI] [PubMed] [Google Scholar]
- 33.Calcagno E, Canetta A, Guzzetti S, et al. Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J Neurochem 2007; 103: 1111–1120. [DOI] [PubMed] [Google Scholar]
- 34.Deltheil T, Guiard BP, Cerdan J, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology 2008; 55: 1006–1014. [DOI] [PubMed] [Google Scholar]
- 35.Tao R, Fray A, Aspley S, et al. Effects on serotonin in rat hypothalamus of d-fenfluramine, aminorex, phentermine and fluoxetine. Eur J Pharmacol 2002; 445: 69–81. [DOI] [PubMed] [Google Scholar]
- 36.Udo de Haes JI, Harada N, Elsinga PH, et al. Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse 2006; 59: 18–26. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz D, Hernandez L, Hoebel BG. Fenfluramine administered systemically or locally increases extracellular serotonin in the lateral hypothalamus as measured by microdialysis. Brain Res 1989; 482: 261–270. [DOI] [PubMed] [Google Scholar]
- 38.Laferrere B, Wurtman RJ. Effect of d-fenfluramine on serotonin release in brains of anaesthetized rats. Brain Res 1989; 504: 258–263. [DOI] [PubMed] [Google Scholar]
- 39.Udo de Haes JI, Cremers TIFH, Bosker F-J, et al. Effect of increased serotonin levels on [18F]MPPF binding in rat brain: fenfluramine vs the combination of citalopram and ketanserin. Neuropsychopharmacology 2005; 30: 1624–1631. [DOI] [PubMed] [Google Scholar]
- 40.Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci 1994; 55: 163–167. [DOI] [PubMed] [Google Scholar]
- 41.Invernizzi R, Belli S, Samanin R. Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res 1992; 584: 322–324. [DOI] [PubMed] [Google Scholar]
- 42.Kornum BR, Licht CL, Weikop P, et al. Central serotonin depletion affects rat brain areas differently: a qualitative and quantitative comparison between different treatment schemes. Neurosci Lett 2006; 392: 129–134. [DOI] [PubMed] [Google Scholar]
- 43.Laruelle M, Iyer RN, Al-Tikriti MS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997; 25: 1–14. [DOI] [PubMed] [Google Scholar]
- 44.Tsukada H, Nishiyama S, Kakiuchi T, et al. Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C]raclopride?: PET studies combined with microdialysis in conscious monkeys. Brain Res 1999; 841: 160–169. [DOI] [PubMed] [Google Scholar]
- 45.Narendran R, Jedema HP, Lopresti BJ, et al. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry 2014; 19: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breier A, Su T-P, Saunders R, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 1997; 94: 2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez D, Gil R, Slifstein M, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 2005; 58: 779–786. [DOI] [PubMed] [Google Scholar]
- 48.Martinez D, Narendran R, Foltin RW, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 2007; 164: 622–629. [DOI] [PubMed] [Google Scholar]
- 49.Schneier FR, Abi-Dargham A, Martinez D, et al. Dopamine transporters, D2 receptors, and dopamine release in generalized social anxiety disorder. Depress Anxiety 2009; 26: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kupers R, Frokjaer VG, Naert A, et al. A PET [18F]altanserin study of 5-HT2A receptor binding in the human brain and responses to painful heat stimulation. NeuroImage 2009; 44: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 51.Pinborg LH, Feng L, Haahr ME, et al. No change in [11C]CUMI-101 binding to 5-HT1A receptors after intravenous citalopram in human. Synapse 2012; 66: 880–884. [DOI] [PubMed] [Google Scholar]
- 52.Adell A, Artigas F. Differential effects of clomipramine given locally or systemically on extracellular 5-hydroxytryptamine in raphe nuclei and frontal cortex. Naunyn Schmiedebergs Arch Pharmacol 1991; 343: 237–244. [DOI] [PubMed] [Google Scholar]
- 53.Bel N, Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: An in vivo microdialysis study. Eur J Pharmacol 1992; 229: 101–103. [DOI] [PubMed] [Google Scholar]
- 54.Malagié I, Trillat A-C, Jacquot C, et al. Effects of acute fluoxetine on extracellular serotonin levels in the raphe: an in vivo microdialysis study. Eur J Pharmacol 1995; 286: 213–217. [DOI] [PubMed] [Google Scholar]
- 55.Gartside SE, Umbers V, Hajós M, et al. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br J Pharmacol 1995; 115: 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortés R, Soriano E, Pazos A, et al. Autoradiography of antidepressant binding sites in the human brain: localization using [3h]imipramine and [3h]paroxetine. Neuroscience 1988; 27: 473–496. [DOI] [PubMed] [Google Scholar]
- 57.Hrdina PD, Foy B, Hepner A, et al. Antidepressant binding sites in brain: autoradiographic comparison of [3H]paroxetine and [3H]imipramine localization and relationship to serotonin transporter. J Pharmacol Exp Ther 1990; 252: 410–418. [PubMed] [Google Scholar]
- 58.Artigas F, Romero L, de Montigny C, et al. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 1996; 19: 378–383. [DOI] [PubMed] [Google Scholar]
- 59.Romero L, Celada P, Artigas F. Reduction of in vivo striatal 5-hydroxytryptamine release by 8-OH-DPAT after inactivation of Gi/Go proteins in dorsal raphe nucleus. Eur J Pharmacol 1994; 265: 103–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.