Abstract
Phase contrast magnetic resonance imaging (PC-MRI) and color-coded duplex ultrasonography (CDUS) are commonly used for measuring cerebral blood flow in the internal carotid (ICA) and vertebral arteries. However, agreement between the two methods has been controversial. Recent development of high spatial and temporal resolution blood vessel wall edge-detection and wall-tracking methods with CDUS increased the accuracy and reliability of blood vessel diameter, hence cerebral blood flow measurement. The aim of this study was to compare the improved CDUS method with 3 T PC-MRI for cerebral blood flow measurements. We found that cerebral blood flow velocity measured in the ICA was lower using PC-MRI than CDUS (left ICA: PC-MRI, 18.0 ± 4.2 vs. CDUS, 25.6 ± 8.6 cm/s; right ICA: PC-MRI, 18.5 ± 4.8 vs. CDUS, 26.6 ± 6.7 cm/s, both p < 0.01). However, ICA diameters measured using PC-MRI were larger (left ICA: PC-MRI, 4.7 ± 0.50 vs. CDUS, 4.1 ± 0.46 mm; right ICA: PC-MRI, 4.5 ± 0.49 vs. CDUS, 4.0 ± 0.45 mm, both p < 0.01). Cerebral blood flow velocity measured in the left vertebral artery with PC-MRI was also lower than CDUS, but no differences in vertebral artery diameter were observed between the methods. Dynamic changes and/or intrinsic physiological fluctuations may have caused these differences in vessel diameter and velocity measurements between the methods. However, estimation of volumetric cerebral blood flow was similar and correlated between the methods despite the presence of large individual differences. These findings support the use of CDUS for cerebral blood flow measurements in the ICA and vertebral artery.
Keywords: Cerebral blood flow, phase contrast magnetic resonance imaging, ultrasonography, echo-tracking, internal carotid artery, vertebral artery
Introduction
Measurement of cerebral blood flow (CBF) is important for assessment of cerebrovascular function.1 CBF can be measured directly from the major cerebral feeding arteries at the neck including the internal carotid (ICA) and vertebral (VA) arteries with either phase contrast magnetic resonance imaging (PC-MRI) or color-coded duplex ultrasonography (CDUS).2,3
Both PC-MRI and CDUS are used commonly in clinic and research studies.4,5 In addition, PC-MRI can be gated with electrocardiogram (ECG) to measure pulsatile CBF, referred to as gated PC-MRI.6 CBF also can be measured with non-gated PC-MRI to obtain mean CBF averaging over multiple time points of a cardiac cycle.6,7 Previous studies have shown that for measurement of mean CBF, non-gated PC-MRI is as accurate as the gated PC-MRI, but has advantages of requiring a much shorter scan time, which is important for clinical application.8 Compared with PC-MRI, Doppler ultrasonography has a high temporal resolution (∼10 ms) for measuring continuous blood flow and is bedside available,9 thus can be used under conditions which may not be feasible for MRI.2,3
Understanding the agreement between PC-MRI and Doppler ultrasonography to measure CBF is essential for comparisons of studies using different methods. In this aspect, previous studies have reported that the correlation between PC-MRI and Doppler ultrasonography measurements of peak blood flow velocity in the aorta and pulmonary artery was relatively low (r = 0.69), even though a high correlation (r > 0.99) was observed in vitro using a blood-mimicking flow phantom.10 In vivo and in vitro studies also reported that measurements of CBF were overestimated with Doppler ultrasonography when compared with PC-MRI,11,12 despite inconsistent findings from others.13,14
The discrepancies between PC-MRI and Doppler ultrasonography measurements of CBF are likely caused by the inherent methodological limitations for measuring complex blood flow patterns in the large arteries.15–17 In addition, pulsatile changes of blood vessel diameters may influence volumetric CBF measurements.2 Finally, the presence of intrinsic CBF fluctuations may lead to significant differences in CBF measurement even using the same method in the same subjects.18
With the advent of high spatial and temporal resolution of blood vessel wall edge-detection and wall echo-tracking methods using CDUS, pulsatile movements of arterial wall can be measured precisely on a beat-to-beat basis to improve the accuracy and reliability of CBF measurement.2 Furthermore, PC-MRI with a high magnetic field strength such as 3 T can improve CBF measurement when compared with that using 1 or 1.5 T in previous studies.12,15,17,19
Therefore, the purpose of this study is to compare measurements of CBF in the ICA and VA using the improved CDUS methods with arterial wall edge-detection and echo-tracking with 3 T PC-MRI. We hypothesized that using these improved methods, measurements of CBF with CDUS are comparable with those using PC-MRI.
Subjects and methods
Participants
Thirty-eight healthy subjects were recruited. Subjects were screened to exclude clinical history of stroke, major medical and psychiatric disorders, unstable heart disease, uncontrolled hypertension, and diabetes mellitus. Individuals with carotid and vertebral artery deformation and stenosis (>50%) were excluded after screening with ultrasonography. All subjects signed informed consent and the study protocols were approved by the Institutional Review Boards of the UT Southwestern Medical Center and Texas Health Presbyterian Hospital of Dallas.
Study design
PC-MRI and CDUS were performed during two visits separated by ∼30 days. Subjects rested in the supine position for at least 10 min to allow stabilization of blood pressure and heart rate before acquiring images and hemodynamic data in both settings. Subjects were asked to refrain from high intensity exercise, alcohol, or caffeinated beverage at least 12 h before the tests.
MRI data collection and analysis
MRI data were collected on Achieva 3.0 T (Philips Medical Systems, Best, The Netherlands) using an eight-channel sensitivity encoding (SENSE) head coil. First, a three-dimensional (3-D) time of flight (TOF) MR angiographic image of the extracranial vessels (ICA and VA) was acquired with the following parameters: TR = 23 ms, TE = 3.5 ms, resolution = 0.3 × 0.3 ×1.5 mm3, FOV = 160 × 160 mm2, 47 slices, FA = 18°. A venous saturation slab was placed above the imaging slab, scan duration = 83.6 s. Second, for PC-MRI, a transverse imaging plane was placed perpendicular to the ICA and VA above the carotid bifurcation and below vertebral artery bend using both coronal and sagittal TOF MR images to guide the plan selection process (this location corresponds to a level between C2 and C4 of the vertebrae similar to that used for CDUS measurement of ICA blood flow) (Figure 1(a)). Non-gated PC-MRI was collected using the following parameters: TR = 20 ms, TE = 6.9 ms, in-plan resolution = 0.45 × 0.45 mm2, slice thickness = 5 mm, FOV = 230 × 230 × 5 mm, FA = 15°, maximum velocity encoding, VENC = 80 cm/s in the through-plane direction (i.e., foot-to-head direction) for both the ICA and VA, and NEX (signal average) = 4. Total scan time for non-gated PC-MRI was ∼30 s. The VENC value was selected based on a balance between maximizing measurement precisions for those of low blood flow velocity values near the edge of the vessel wall and minimizing potential phase wrapping in the voxels with high velocity values such as those of peak velocity at the center of arteries; velocity aliasing correction was performed if identified.17
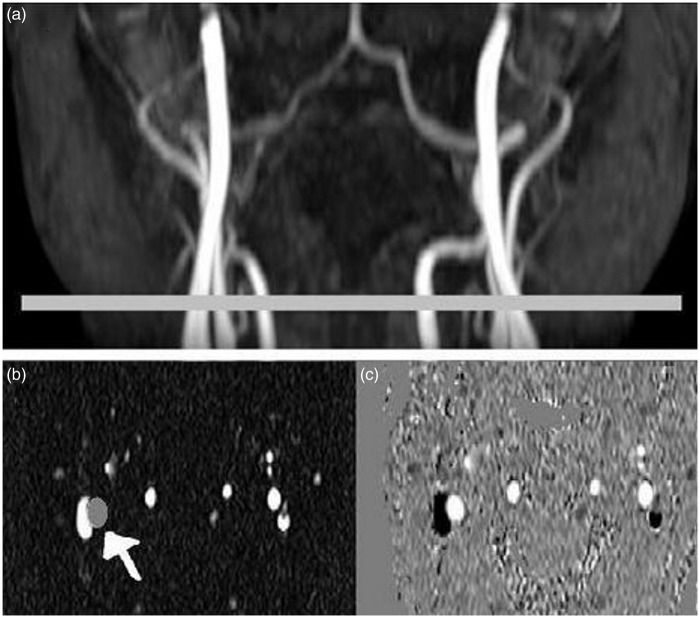
Figure 1.
Three-dimensional TOF angiographic image of the ICA and VA (a). Horizontal bar shows the PC-MRI image position perpendicular to the ICA and VA. PC-MRI images: magnitude (b) and phase velocity map (c). Gray circle indicated by the white arrow shows the ROI drawn on the left ICA, which was masked on the corresponding phase images to measure blood flow velocity and vessel area. Same imaging processing procedures were repeated for each ICA and VA.
TOF: time of flight; ICA: internal carotid artery; VA: vertebral artery; PC-MRI: phase contrast magnetic resonance imaging.
PC-MRI data were analyzed using a region-of-interest (ROI) method.8 Briefly, ROIs were drawn manually and ICA and VA cross-sectional areas were obtained using magnitude images. Vessel diameters were calculated using A = π(d/2)2 with A represents the vessel cross-sectional area; d, vessel diameter (Figure 1(b)). Vessel masks generated using the ROIs from the magnitude images (Figure 1(b)) were applied to phase images to obtain mean blood flow velocity within each of the ROIs (Figure 1(c)). Blood flow was calculated as the product of mean blood flow velocity and vessel cross-sectional area.
CDUS data collection and analysis
Blood flow velocity and diameters of the ICA and VA were measured using a 3–12 MHz linear array transducer (CX-50, Phillips Healthcare). The ICA was imaged at least 1 cm above the carotid bifurcation at a level between C2 and C4 of the vertebrae (Figure 2(a)), whereas VA was imaged at a level between C4 and C6 intertransverse segments (Figure 2(b)). These locations were selected based on the considerations of imaging accessibility and quality, vessel morphometry as well as the proximity close to the brain.2 To ensure a laminar flow pattern and enhance the uniformity of Doppler signal, a straight segment of at least 0.5 cm of ICA or VA with a viewable parallel vessel wall and the largest longitudinal sectional area, perpendicular to the vessel lumen (Figure 2(a) and (b)) was selected to estimate the vessel diameter.2 To measure the angle-corrected and spatially averaged blood flow velocity, the Doppler sample volume was positioned across the entire vessel lumen. Mean blood flow velocity was determined from both spatially and time averaged velocity over a minimum of five consecutive cardiac cycles (Figure 2(e) and (f)).2,20 Longitudinal vessel wall images were recorded continuously for 5 cardiac cycles using B-mode video with a 21 Hz frame rate. To quantify pulsatile changes of vessel diameter, the distance between the parallel internal layers of the blood vessel, perpendicular to the lumen, was measured using a wall edge-detection and wall-tracking software (Brachial Analyzer, Medical Imaging Applications). Time-averaged vessel diameter was calculated from 3∼5 consecutive cardiac cycles (Figure 2(c) and (d)). Both blood flow velocity and vessel diameter calculations were averaged over three separate measurements over a time period of 10 to 15 min to obtain mean values for statistical analysis. This procedure was taken to minimize potential influences of spontaneous fluctuations associated with respiratory and other low frequency oscillations on CBF measurement.18 Blood flow was calculated for each of the vessels as the product of mean blood flow velocity and mean cross-sectional area. Total CBF was calculated as a sum of the measurements from bilateral ICA and VA.
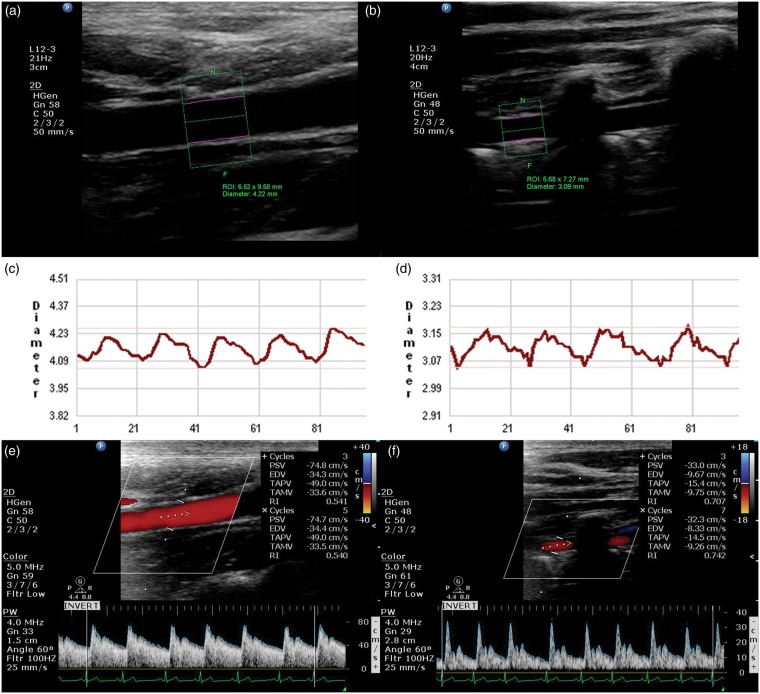
Figure 2.
Measurement of blood flow in the ICA and VA with color-coded duplex ultrasonography. High-resolution B-mode imaging of the ICA (a) and VA (b). ROIs, represented by the green rectangles, are manually selected to cover a segment of ICA or VA, the double pink lines are the detected vessel inner walls used to track the wall movements and to measure beat-by-beat pulsatile changes of vessel diameter for the ICA (c) and VA (d). Beat-by-beat recordings of blood flow velocity at the sites of diameter measurement are presented for the ICA (e) and VA (f).
ICA: internal carotid artery; VA: vertebral artery; ROIs: regions of interest.
Statistical analysis
Comparisons between PC-MRI and CDUS measurements in the ICA and VA, and between the left and right-side measurements from the same vessels using the same methods were performed using paired t-tests. The relationships between the measurements using PC-MRI and CDUS were examined with the Pearson product-moment correlation coefficient analysis. Bland–Altman plots were used to examine the agreement between the two methods. Data were expressed as mean ± standard deviation (SD). Statistical significance level was accepted at p < 0.05.
Results
For technical reasons, high quality image data could not be obtained in five subjects with PC-MRI and two subjects with CDUS. Thus, data from 31 subjects are reported. Demographic characteristics of study participants are presented in Table 1.
Table 1.
Demographic characteristics of study participants.
| Age (years) | 48 ± 15 |
| Education (years) | 16 ± 2 |
| Gender (M/F) | 15/16 |
| Body mass index (kg/m2) | 26 ± 4 |
| Systolic blood pressure (mmHg) | 112 ± 10 |
| Diastolic blood pressure (mmHg) | 71 ± 9 |
| Heart rate (beats/min) | 62 ± 7 |
Values are mean ± SD. N = 31.
Blood flow velocity
Blood flow velocities in both ICAs and left VA measured with PC-MRI were significantly lower by ∼30% when compared with those with CDUS (Table 2). Measurements of blood flow velocity between the methods showed significant correlations in the VA (left VA, R2 = 0.25, p < 0.01; right VA, R2 = 0.50, p < 0.01), but not in ICA (left ICA, R2 = 0.003, p = 0.80; right ICA, R2 = 0.08, p = 0.08). No differences in blood flow velocity between the left and right ICA were observed using the same method. However, blood flow velocity in the left VA was lower by ∼12% than that of the right VA with PC-MRI (Table 2).
Table 2.
Mean blood flow velocity, vessel diameter, and volumetric blood flow measurements using CDUS and PC-MRI.
| Velocity (cm/s) |
Diameter (mm) |
Flow (ml/min) |
||||
|---|---|---|---|---|---|---|
| PC-MRI | CDUS | PC-MRI | CDUS | PC-MRI | CDUS | |
| Left-ICA | 18.0 ± 4.2 | 25.6 ± 8.6* | 4.7 ± 0.50 | 4.1 ± 0.46* | 222 ± 56 | 202 ± 67 |
| Right-ICA | 18.5 ± 4.8 | 26.6 ± 6.7 * | 4.5 ± 0.49 | 4.0 ± 0.45* | 213 ± 63 | 197 ± 59 |
| Left-VA | 12.3 ± 3.1 | 16.7 ± 4.2 * | 3.3 ± 0.61 | 3.2 ± 0.57 | 84 ± 38 | 87 ± 39 |
| Right-VA | 14.0 ± 3.3† | 15.0 ± 3.7 | 2.8 ± 0.68† | 2.9 ± 0.56† | 59 ± 34† | 63 ± 31† |
| Total | 599 ± 133 | 557 ± 130 | ||||
Values are the mean ± SD, N = 31.
p < 0.05, comparisons between the methods of the same vessel.
p < 0.05, comparisons between the left and right side of the vessel using the same method.
CDUS: color-coded duplex ultrasonography; PC MRI: phase contrast magnetic resonance imaging; ICA: internal carotid artery; VA: vertebral artery.
Blood vessel diameter
Measurements of ICA diameter with PC-MRI were significantly larger by ∼12% when compared with those using CDUS (p < 0.01), whereas no differences were observed in the VA (Table 2). Measurements of vessel diameters between the methods showed significant correlations in the VA (left VA, R2 = 0.46, p < 0.01; right VA, R2 = 0.36, p < 0.01), but not in ICA (left ICA, R2 = 0.001, p = 0.90; right ICA, R2 = 0.04, p = 0.27). No differences in the vessel diameters between the left and right ICA were observed using the same method. However, the right VA diameter was lower by ∼9% than that of the left VA with CDUS, and lower by ∼15% with PC-MRI (Table 2).
Measurement of volumetric blood flow
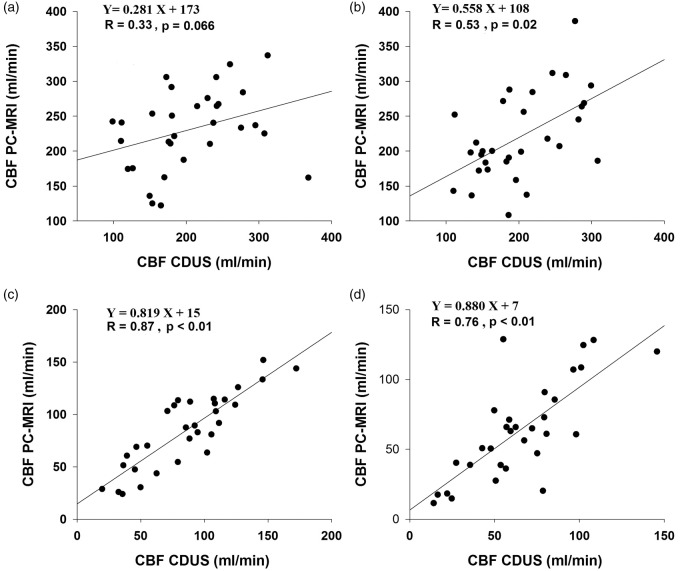
No differences in volumetric blood flow were observed either in the ICA or VA between the methods (Table 2). In addition, blood flow in all four arteries were correlated between the methods, while the observed correlations appeared to be stronger for the VA than ICA (Figure 3). Total CBF measured with CDUS appeared to be lower by ∼7% when compared with PC-MRI although this difference was not statistically significant. In addition, blood flow was lower by ∼29% in the right VA than that in the left VA regardless of the method used (Table 2).
Figure 3.
Relationships between CBF measurements using CDUS and PC-MRI. Left: internal carotid artery (a); right: internal carotid artery (b); left: vertebral artery (c); right: vertebral artery (d).
CBF: cerebral blood flow; CDUS: color-coded duplex ultrasonography; PC-MRI: phase contrast magnetic resonance imaging.
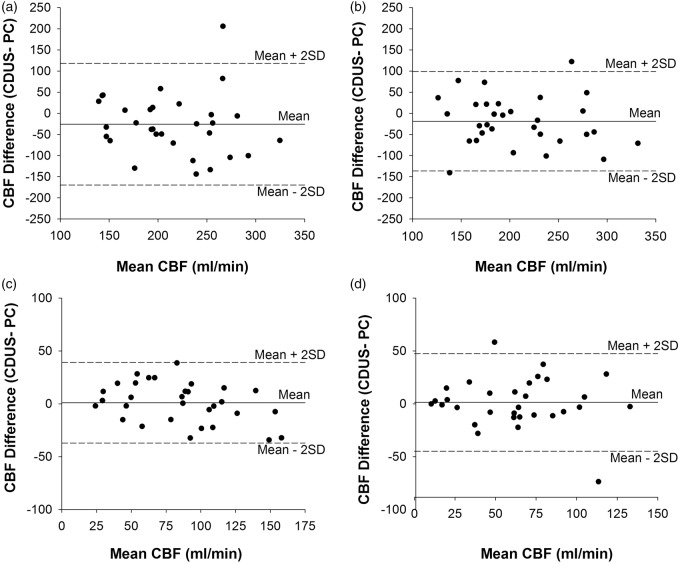
Finally, Bland–Altman plots showed that differences in blood flow measurements between PC-MRI and CDUS were not systemically biased (Figure 4). However, the wide range of confidence interval for the mean differences (±2 standard deviation) showed large individual variability of CBF measurements between the methods.
Figure 4.
Bland–Altman plots of differences in CBF measurements between CDUS and PC-MRI. Left: internal carotid artery (a); right: internal carotid artery (b); left: vertebral artery (c); and right: vertebral artery (d).
CBF: cerebral blood flow; CDUS: color-coded duplex ultrasonography; PC-MRI: phase contrast magnetic resonance imaging.
Discussion
The primary finding of this study is that measurements of CBF in the ICA and VA with CDUS arterial wall edge-detection and wall-tracking method are comparable to and correlated with PC-MRI. However, measurements of vessel diameters and blood flow velocity, particularly in the ICA, showed significant individual differences between the methods. The presence of these differences likely reflects methodological limitations for measuring CBF as well as influences of physiological fluctuations on CBF measurement, which are discussed in the following.
Previous studies compared gated and non-gated PC-MRI with Doppler ultrasonography for CBF measurement.11,12 These studies, consistent with the findings of the present study, showed that overall there was a correlation between the CBF measurements using these two methods. However, in contrast to the present study, Doppler ultrasonography measurement of CBF appeared to be overestimated when compared with PC-MRI.11,12 Of note, pulsatile changes in blood vessel diameters were not measured in these studies. Instead, the color-coded Doppler imaging was used to measure artery diameters, which had a poor spatial resolution and the spread of the color-overlay during vessel lumen imaging may lead to a significant bias in the measurement of vessel diameter and thus an overestimated CBF.11,12
Using high spatial (<0.01 mm) and temporal resolutions (<0.05 s) along with arterial wall edge-detection and wall-tracking technology, we acquired the longitudinal views of both ICA and VA and measured beat-to-beat pulsatile changes of vessel diameters to calculate volumetric CBF. This procedure dramatically reduced intra-observer variability in measuring blood vessel diameters in the peripheral circulation.20 Recently, we have used this method to measure CBF in the ICA and VA and observed a coefficient of variation of ∼4.7% between the repeated measurements.2 Findings of the present study suggest that measurement of CBF using CDUS method is comparable with and correlated with PC-MRI, although measurement of total CBF appeared to be slightly lower than PC-MRI by ∼7%. Pulsatile changes of ICA and VA diameters cannot be measured with PC-MRI used in the present study, which may contribute to the observed differences between the methods.
The importance of accurate and reliable measurement of CBF velocity also should be highlighted. For CDUS, mean CBF velocity in the ICA and VA was obtained using both spatial and temporal averaging methods.2 Specifically, spatial average of blood flow velocity was performed across the whole blood vessel lumen to account for the velocity profile of laminar flow in the ICA and VA, and temporal average was performed over at least five complete cardiac cycles obtained from three separate measurements over a time period of 10 to 15 min to reduce the impact of intrinsic spontaneous fluctuations on measuring mean CBF.18 Of note, this procedure of temporal average is likely to be important, since the magnitude of physiologic fluctuations of blood flow velocity in the conduit cerebral arteries can be as large as 20 to 50% of its mean values over a time scale of several minutes.18,20 Mean blood flow velocity with non-gated PC-MRI was obtained from averaging of four imaging scans during an acquisition period of ∼30 s and spatial average was performed within the ROI. These procedures, in principle, are similar to those used with CDUS. However, differences in the time frames used for signal acquisition and averaging, spatial and temporal resolutions used for measuring temporal and spatial velocity profiles in the blood vessels may lead to the observed differences between the methods.
Large individual differences were observed not only in the ICA blood flow velocity but also ICA diameters between the CDUS and PC-MRI methods. Interestingly, significant correlations of blood flow velocity and vessel diameters between the methods were observed in the VA, but not ICA, and CBF variability between the methods appeared to be larger in the ICA than VA (Figures 3 and 4). These observations collectively suggest that differences in the vessel anatomy between the ICA and VA may have significant impact on CBF measurements. Specifically, ICA is likely to be more compliant to the transient changes in transmural pressure and less restricted by the surrounding tissue and bone structures than the VA, thus both the ICA diameter and blood flow velocity measurements are likely to be more prone to the pulsatile and low frequency changes in arterial pressure.2
The observed differences in blood flow velocity and vessel diameter measurements between the methods also may be related to the differences in the imaging planes used for PC-MRI and Doppler ultrasonography. Indeed, opposite differences between blood flow velocity and vessel diameter measurements were observed in the ICA, but not in the VA using the two methods suggesting that VA morphology is likely to be more uniform along the cervical spine whereas ICA is not. Notably, bilateral blood flow measured in the ICA using the same method was similar, and the observed lower blood flow in the right VA regardless of the method used is consistent with previous findings (Table 2).21
The major limitation of this study is our inclusion of only healthy participants. Thus, translation of these findings into patients with carotid stenosis or other cerebrovascular disease should be made with caution. In addition, we used a fast (<2 min) and non-gated PC-MRI to measure mean CBF because its potential advantages for clinical use.17 However, this method cannot reveal pulsatile changes in blood flow velocity as well as spatial velocity distribution. In this aspect, the time-resolved PC-MRI with 3-D velocity encoding may provide further information for understanding the differences in CBF measurement between the methods observed in the present study.15,22
In conclusion, using advanced duplex ultrasonography with accurate and reliable vessel diameter quantification, we have shown that blood flow measurements in the ICA and VA are in agreement with those measured with the PC-MRI. Since duplex ultrasonography can be used under less demanding clinical conditions than MRI and other imaging modalities, findings of the present study support its use for measuring CBF in large cerebral arteries. Furthermore, findings of this study may facilitate data interpretation and comparison of CBF measurements using duplex ultrasonography and PC-MRI. Finally, the observed differences in the vessel diameters, blood flow velocity, and CBF between the methods highlight the potential impact of dynamic changes in blood vessel diameters as well as intrinsic physiological fluctuations on CBF measurements.
Acknowledgements
We thank our study participants for their willingness, time, and effort devoted to this study and Mr. Kyle Armstrong, Ms. Rosemary Parker, Ms. Rani Varghese, Mr. Salvador Pena, and all other members of our team for their excellent technical support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by the NIH grant R01HL102457, R01AG033106, and AHA grant 12POST8910003.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Muhammad Ayaz Khan performed experiments, conducted data analysis, and wrote the manuscript. Jie Liu and Takashi Tarumi performed experiments, conducted data analysis and manuscript revision. Justin Stevan Lawley and Peiying Liu discussed results and commented on the data acquisition, analysis, and interpretation. David C. Zhu, Hanzhang Lu, and Rong Zhang involved in the study design, discussed results, and manuscript revision. All authors edited and revised the manuscript and approved final submission.
References
- 1.Kirkness CJ. Cerebral blood flow monitoring in clinical practice. AACN Clin Issues 2005; 16: 476–487. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Zhu YS, Khan MA, et al. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimers Dement 2014; 10: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato K, Ogoh S, Hirasawa A, et al. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 2011; 589: 2847–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernooij MW, van der Lugt A, Ikram MA, et al. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab 2008; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 5.Scheel P, Ruge C, Petruch UR, et al. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke 2000; 31: 147–150. [DOI] [PubMed] [Google Scholar]
- 6.Enzmann D, Marks M and Relc N. Comparison of cerebral artery blood flow measuement with gated cine and ungated phase-contrast techniques. J Magn Reson Imaging 1993; 3: 705–712. [DOI] [PubMed]
- 7.Bakker CJ, Hartkamp MJ, Mali WP. Measuring blood flow by nontriggered 2D phase-contrast MR angiography. Magn Reson Imaging 1996; 14: 609–614. [DOI] [PubMed] [Google Scholar]
- 8.Xu F, Ge Y, Lu H. Noninvasive quantification of whole-brain cerebral metabolic rate of oxygen (CMRO2) by MRI. Magn Reson Med 2009; 62: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niki K, Sugawara M, Chang D, et al. A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels 2002; 17: 12–21. [DOI] [PubMed] [Google Scholar]
- 10.Lee VS, Spritzer CE, Carroll BA, et al. Flow quantification using fast cine phase-contrast MR imaging, conventional cine phase-contrast MR imaging, and Doppler sonography: In vitro and in vivo validation. Am J Roentgenol 1997; 169: 1125–1131. [DOI] [PubMed] [Google Scholar]
- 11.Ho SS, Chan YL, Yeung DK, et al. Blood flow volume quantification of cerebral ischemia: comparison of three noninvasive imaging techniques of carotid and vertebral arteries. Am J Roentgenol 2002; 178: 551–556. [DOI] [PubMed] [Google Scholar]
- 12.Oktar SO, Yucel C, Karaosmanoglu D, et al. Blood-flow volume quantification in internal carotid and vertebral arteries: comparison of 3 different ultrasound techniques with phase-contrast MR imaging. Am J Neuroradiol 2006; 27: 363–369. [PMC free article] [PubMed] [Google Scholar]
- 13.Zananiri FV, Jackson PC, Halliwell M, et al. A comparative study of velocity measurements in major blood vessels using magnetic resonance imaging and Doppler ultrasound. Br J Radiol 1993; 66: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 14.Hoppe M, Heverhagen JT, Froelich JJ, et al. Correlation of flow velocity measurements by magnetic resonance phase contrast imaging and intravascular Doppler ultrasound. Invest Radiol 1998; 33: 427–432. [DOI] [PubMed] [Google Scholar]
- 15.Bammer R, Hope TA, Aksoy M, et al. Time-resolved 3D quantitative flow MRI of the major intracranial vessels: initial experience and comparative evaluation at 1.5T and 3.0T in combination with parallel imaging. Magn Reson Med 2007; 57: 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meckel S, Leitner L, Bonati LH, et al. Intracranial artery velocity measurement using 4D PC MRI at 3 T: comparison with transcranial ultrasound techniques and 2D PC MRI. Neuroradiology 2013; 55: 389–398. [DOI] [PubMed] [Google Scholar]
- 17.Peng SL, Su P, Wang FN, et al. Optimization of phase-contrast MRI for the quantification of whole-brain cerebral blood flow. J Magn Reson Imaging 2015; 42: 1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol 2000; 278: H1848–H1855. [DOI] [PubMed] [Google Scholar]
- 19.Lotz J, Doker R, Noeske R, et al. In vitro validation of phase-contrast flow measurements at 3 T in comparison to 1.5 T: precision, accuracy, and signal-to-noise ratios. J Magn Reson Imaging 2005; 21: 604–610. [DOI] [PubMed] [Google Scholar]
- 20.Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol 1985; 11: 625–641. [DOI] [PubMed] [Google Scholar]
- 21.Schoning M, Walter J, Scheel P. Estimation of cerebral blood-flow through color duplex sonography of the carotid and vertebral arteries in healthy-adults. Stroke 1994; 25: 17–22. [DOI] [PubMed] [Google Scholar]
- 22.Harloff A, Albrecht F, Spreer J, et al. 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med 2009; 61: 65–74. [DOI] [PubMed] [Google Scholar]