Abstract
[11C]Preladenant was developed as a novel adenosine A2A receptor positron emission tomography radioligand. The present study aims to evaluate the suitability of [11C]preladenant positron emission tomography for the quantification of striatal A2A receptor density and the assessment of striatal A2A receptor occupancy by KW-6002. Sixty- or ninety-minute dynamic positron emission tomography imaging was performed on rats. Tracer kinetics was quantified by the two-tissue compartment model, Logan graphical analysis and several reference tissue-based models. Test–retest reproducibility was assessed by repeated imaging on two consecutive days. Two-tissue compartment model and Logan plot estimated comparable distribution volume (VT) values of ∼10 in the A2A receptor-rich striatum and substantially lower values in all extra-striatal regions (∼1.5–2.5). The simplified reference tissue model with midbrain or occipital cortex as the reference region proved to be the best non-invasive model for quantification of A2A receptor, showing a striatal binding potential (BPND) value of ∼5.5, and a test–retest variability of ∼5.5%. The brain metabolite analysis showed that at 60-min post injection, 17% of the radioactivity in the brain was due to radioactive metabolites. The ED50 of KW-6002 in rat striatum for i.p. injection was 0.044–0.062 mg/kg. The study demonstrates that [11C]preladenant is a suitable tracer to quantify striatal A2A receptor density and assess A2A receptor occupancy by A2A receptor-targeting molecules.
Keywords: Adenosine A2A receptors, receptor occupancy, [11C]preladenant, small-animal positron emission tomography, pharmacokinetic modeling
Introduction
Adenosine is a signaling molecule that functions via activation of four subtypes of adenosine receptors, referred to as A1, A2A, A2B, and A3. The adenosine A2A receptor (A2AR) subtype is expressed predominantly in the basal ganglia of the central nervous system (CNS).1 The A2AR plays an important role in modulating dopamine and glutamate neurotransmission, and regulating neuroinflammation.2–6 Therefore, A2AR is generally associated with neurological and psychiatric disorders related to neuroinflammation and/or disturbed dopamine/glutamate signaling pathways, such as Huntington's disease (HD), Alzheimer's disease (AD), depression, schizophrenia, and Parkinson's disease (PD).7,8
Although little is known mechanistically about the role of A2AR in brain disorders so far, it is clear that the receptor is important in CNS functioning via its effects on neurons, glial cells, and vasculature.9 This makes A2AR a potential therapeutic target, for example, in AD, HD, schizophrenia, and PD.10–13 In addition, A2AR may act as a diagnostic biomarker in, for instance, HD and PD,14–17 which could allow monitoring of disease progression.
Positron emission tomography (PET) with a suitable A2AR radioligand can be used to exploit the potential of A2AR as a biomarker by measuring its distribution and density. Furthermore, PET may also be a suitable technique to monitor changes in A2AR expression in the brain during the course of the disease, or to assess A2AR occupancy after administration of an (investigational) drug. The latter could be important for drug development, e.g. for establishing the optimal dosing regimen. [18F]MNI-444, [11C]TMSX, [11C]KW-6002, and [11C]SCH442416 are A2AR PET ligands that have been studied in human subjects.16–22 [18F]MNI-444 displayed best properties among these tracers, with binding potential (BPND) values ranging from 2.6 to 4.9 in A2AR-rich regions, and an average test–retest variability (TRV) of less than 10%.18 Other tracers are hardly useful for A2AR quantification, because of the disadvantages such as low BPND in striatum, high extra-striatal binding and low target-to-non-target ratios.16,17,19–22
We have recently developed [11C]preladenant, the C-11 labeled analog of the drug preladenant. The in vivo assessment of this tracer in rats showed a better contrast in the PET images (i.e. larger striatum-to-cerebellum ratio) than published for other A2AR radioligands in rat studies.23–27 The results suggest a great potential of [11C]preladenant to image A2AR in the brain. Another advantage of [11C]preladenant is that the toxicological profile of preladenant in humans is already known from clinical phase I/II/III studies, in which preladenant was investigated as a drug.28 Therefore, expensive toxicity studies are not required anymore and the costs and timeline of tracer development can be reduced.
To determine the suitability of [11C]preladenant PET for A2AR quantification, we further evaluated the tracer by assessing pharmacokinetic modeling, test–retest reproducibility, and the feasibility of measuring A2AR occupancy in the rat brain.
Materials and methods
(E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dhydro-1H-purine-2,6-dione (KW-6002) was purchased from Axon Medchem BV (Groningen, The Netherlands).
Radiosynthesis
[11C]Preladenant was prepared according to the method described by Zhou et al.27 The specific activity of [11C]preladenant was 81 ± 33 GBq/µmol (n = 26), and the radiochemical purity was always greater than 98%. The tracer was formulated in ∼15% ethanol in phosphate buffered saline as final product.
Animals
Adult male Wistar rats (n = 37, Hsd/Cpb:WU, 9–11 weeks age, 300–400 g, Harlan, The Netherlands) were housed in groups at a 12 h light/12 h dark circle and were fed with standard laboratory chow (RMH-B, The Netherlands) and water ad libitum. After arrival from the supplier, rats were acclimatized for at least 7 days. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Groningen (DEC 6689B and DEC 6689G) and conducted in accordance with the Law on Animal Experiments of The Netherlands. All the animal study data were reported according to ARRIVE guidelines (Animal Research: Reporting In Vivo Experiment).
Brain metabolite analysis
The animals were sacrificed by extirpation of the heart at 60-min post injection. The brain was extracted and half of the brain (sagittal section) was homogenized with 1 mL of acetonitrile and then centrifuged at 3000 g for 3 min. The supernatant was filtered through a 0.45 µm Durapore (PVDF) filter (Millipore, Billerica, MA, USA). Samples (100 µL) were subsequently injected to a ultra-high performance liquid chromatography (UPLC) using an Acquity UPLC HSS T3 UHPLC column (1.8 µm, 3.0 × 50 mm2) at a column temperature of 40℃ and a gradient containing water (pH = 2 with HClO4) and acetonitrile as mobile phase with flow rate of 1.1 mL/ min. The eluted fractions were collected every 30 s and measured with an automated well-counter (Compugamma 1282 CS, LKB-Wallac, Turku, Finland). The percentage of radioactive metabolites in the brain tissue was calculated as 100% − (total activity of intact tracer − activity of intact tracer in blood at 60-min post injection × 5%)/(total activity − total activity in blood at 60-min post injection × 5%) × 100%.
PET imaging
Prior to PET imaging, animals were anesthetized with isoflurane in medical air (5% isoflurane for induction, 1.0–2.5% isoflurane for maintenance) and kept on electronic heating pads during the study to avoid hypothermia. Cannulas were placed in a femoral vein for tracer injection and in a femoral artery for blood sampling. Six rats were i.p. injected with vehicle (50% dimethylacetamide (DMA) in saline) and six rats were i.p. injected with KW-6002 (1 mg/kg) in a 1 mg/mL solution of 50% DMA in saline 7–10 min prior to tracer injection. PET images were acquired using a Focus220 MicroPET scanner (Preclinical solutions, Siemens Healthcare Molecular Imaging, USA Inc.). Two rats were scanned simultaneously. The brains of both rats were positioned close to the center of the field of view. A transmission scan with a 57Co point source was made for attenuation correction. Rats were i.v. injected with 66 ± 23 MBq (2.1 ± 1.7 nmol) [11C]preladenant at a speed of 1 mL/min with an infusion pump for 1 min, and a 60-min dynamic PET scan was started. The injected mass was estimated to occupy 1.6 ± 1.2% A2ARs in rat striatum at the maximum uptake, being a standardized uptake value (SUV) of 2.2, and an A2AR density of 953 fmol/mg protein.29 Blood samples (each sample of 0.10–0.13 mL, 1.5–1.8 mL in total) were drawn from the femoral artery at 10, 20, 30, 40, 50, 60, 90 s and 2, 3, 5, 7.5, 10, 15, 30, 60 min after tracer administration. In all, 0.10–0.13 mL saline with 1% heparin was infused into the artery after each sampling to compensate for the blood loss. Radioactivity in 25 µL whole blood and 25 µL plasma (acquired by centrifugation of blood samples for 5 min at 1000 g) were measured with an automated well-counter and used as an arterial input function (with metabolite correction). List mode acquisition data was divided in 21 frames (6 × 10, 4 × 30, 2 × 60, 1 × 120, 1 × 180, 4 × 300, and 3 × 600 s). The data were reconstructed per time frame using an attenuation-weighted 2-dimensional ordered-subset expectation maximization algorithm (AW-OSEM2D). The 95 sagittal slices with a slice thickness of 0.8 mm were separated into one image for both rats with a 128 × 128 matrix and a pixel size of 0.47 × 0.47 mm2. Datasets were fully corrected for random coincidences, decay, scatter, and attenuation.
PET data analysis
Summed PET images were manually aligned to a T2 magnetic resonance imaging (MRI) template of a rat brain with predefined volumes of interest (VOIs) for whole brain, total cortex, frontal cortex, occipital cortex, parietal cortex, striatum, midbrain, thalamus, hippocampus, and cerebellum. Time-activity curves (TACs, expressed as Bq/cc) for different VOIs were generated using Inveon Research Workplace software (Siemens Medical Solutions, Knoxville, TN), and were normalized to body weight (g) and injected dose (Bq) to obtain dynamic SUVs.
Tracer kinetic modeling
TAC data were analyzed using PMOD software (version 3.5, PMOD Technologies, Zürich, Switzerland). A mono-exponential function was fitted to a population-based intact tracer fraction obtained from our previous study.27 The blood volume in the brain was fixed to 5%, as the volume did not significantly affect distribution volume (VT) estimation, being ∼1% difference between 5% (ref. 30,31) and 3.6% (ref. 32) fits, and ∼4% difference between 5% and 0% fits, whereas 5% blood volume fit gave smaller Akaike Information Criterion (AIC) values compared with 3.6% or 0% blood volume fits. A standard two-parameter (K1, k2) one-tissue compartment model (1TCM) and a four-parameter (K1, K1/k2, k3, k4) two-tissue compartment model (2TCM), both with a metabolite corrected plasma input function, were used to fit the TACs. The best fitting model was selected based on AIC values. The VT was obtained by modeling with the 2TCM and the Logan graphic analysis with t* set to 10 min. Several reference tissue-based models, including simplified reference tissue model (SRTM), Ichise's multilinear reference tissue model (MRTM) with t* set to 1 min, Ichise's multilinear reference tissue model 2 (MRTM2) with t* set to 1 min, and the reference tissue Logan plot (RLogan) with t* set to 5 min were used to estimate the BPND in striatum. The t* was selected based on the goodness of fit, resulting VT or BPND value (larger is better), and coefficient of variation (COV) of VT or BPND (smaller is better). Cerebellum, midbrain, hippocampus, and occipital cortex were tested as reference regions. BPND obtained from the reference tissue-based models were compared with BPND obtained from k3/k4 (direct method) and calculated from the VT determined with the 2TCM and the Logan plot using the formula (ref. 33). The best reference regions were selected based upon the test–retest reliability, the BPND value in striatum, and between-subject variability of BPND.
Test–retest
To estimate test–retest reliability, five rats underwent two PET scans on consecutive days. The PET scans were performed as described above but without cannulation, blood sampling and KW-6002 or vehicle pretreatment. BPND in striatum was determined using SRTM, RLogan, MRTM, MRTM2, and SUV ratio (SUVr) – 1, using different reference regions. The SUV was the average SUV value from 25 till 60-min post injection. TRV was defined as
The test–retest reliability was quantified using intra-class correlation coefficient (ICC) with a one-way random effects model ICC (1, 1) (ref. 34):
where BMS is the mean sum of squares between subjects, WMS is the mean sum of squares within subjects, and k is the number of measurements, being 2 for test–retest. ICC was measured on a scale ranging from −1 to 1. One represents perfect reliability, whereas −1 indicates no reliability. An ICC ≥ 0.75 is considered as a good reliability.35 The ICC was computed using R (http://www.r-project.org/).
A2AR occupancy
Eight baseline PET scans and 12 scans after administration of the selective A2AR antagonist KW-6002 were performed as described above but now without cannulation and blood sampling. The emission scan was extended to 90 min. The dose of KW-6002 (0.01, 0.03 (in duplicate), 0.04, 0.05, 0.06, 0.1 (in duplicate), 0.15, 0.3, 0.5, or 1 mg/kg) in a solution of 50% DMA in saline was i.p. administered 15–25 min before tracer injection. List mode acquisition data was divided in 23 frames (6 × 10, 4 × 30, 2 × 60, 1 × 120, 1 × 180, 4 × 300, 3 × 600, and 2 × 900 s). Receptor occupancy was calculated by the following equation:
where BPND was derived from SRTM, RLogan, SUVr – 1, and modified SUV ratio (mSUVr) – 1, using midbrain, cerebellum, or occipital cortex as the reference region. The modification factors for mSUVr – 1 were determined by Deming linear regression between SUVr – 1 and SRTM or RLogan derived BPND. The occupancy (%) was plotted against the drug dose and the dose-occupancy curve was fitted in GraphPad Prism (version 5.01, GraphPad Software, Inc.) with a one site-specific binding model using the following formula:
where Occmax is the maximum occupancy, ED50 is the drug dose which corresponds to 50% occupancy, and D is the drug dose. The striatal BPND and ED50 were estimated from the 90-min acquisition as well as from the first 60 min of this acquisition. The values from both estimates were compared.
Statistics
All results are expressed as mean ± standard deviation (SD). Unpaired two-tailed t-test with Bonferroni correction for multiple comparisons was used to assess the difference in plasma activity between vehicle and KW-6002 pretreatment groups. Paired two-tailed t-test with Bonferroni correction for multiple comparisons was used to assess the difference between BPND and (m)SUVr – 1, the difference in BPND between direct method and indirect methods, and the difference in COV between 60- and 90-min acquisitions. Two-way ANOVA with Bonferroni post-tests was used to evaluate the effects of KW-6002 pretreatment and VOIs on VT and BPND. Repeated measures two-way ANOVA with Bonferroni post-tests was used to assess the difference in AIC, VT, and BPND between models and VOIs, the difference in BPND between test–retest and VOIs/models, and the difference in BPND between acquisition times and VOIs. Bland–Altman plot (difference (Δ) vs. mean) and one sample t-test were used to judge the bias (Δ) in BPND obtained from Logan plot and reference tissue-based modeling methods as compared with BPND calculated from 2TCM. Δ (%) was computed as
where BPND,1 was derived from 2TCM, Logan plot, SRTM, MRTM, and RLogan, and BPND,2 was always calculated from 2TCM. Deming linear regression was used to compare acquisition durations, and SUVr – 1 and BPND. A probability value (p) < 0.05 was considered statistically significant.
Results
Plasma clearance and brain metabolism of [11C]preladenant
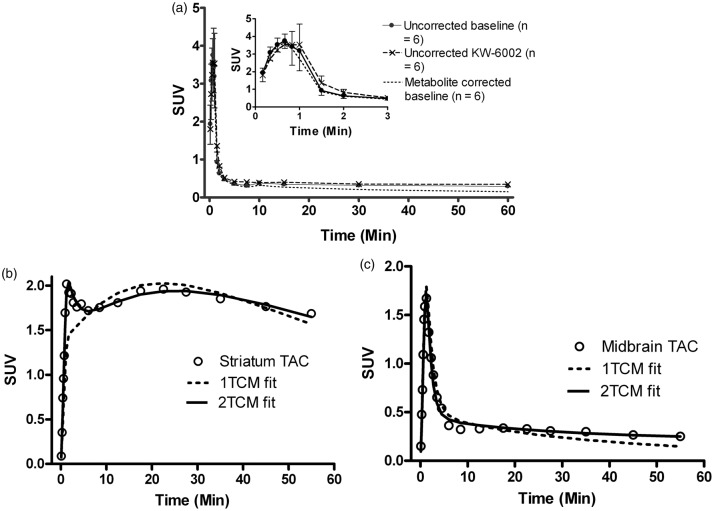
Figure 1(a) shows the plasma clearance of the tracer during the 60-min scan. KW-6002 pretreatment did not significantly affect the tracer kinetics in plasma. The plasma curves were corrected with a mono-exponential fitted population-based intact-tracer function. The metabolite corrected plasma curve at baseline was well described with a two-phase exponential function, with a t1/2α of 0.59 ± 0.37 min, and t1/2β of 22.24 ± 4.68 min (n = 6). At 60-min post tracer injection, 17 ± 5% (n = 3) of the total activity in the brain tissue was due to the radioactive metabolites.
Figure 1.
(a) Kinetics of [11C]preladenant in rat plasma. The insert shows the first 3 min of the plasma kinetics. Error bars indicate standard deviation. (b, c) Representative TACs and modeling fits of individual brain regions at baseline. (b) TAC of striatum with 1TCM and 2TCM fits with a fixed blood volume of 5%. (c) TAC of midbrain with 1TCM and 2TCM fits with a fixed blood volume of 5%.
TACs: time-activity curves; 1TCM: one-tissue compartment model; 2TCM: two-tissue compartment model.
Tracer kinetic modeling
The tracer kinetics in rat brain is better described with a 2TCM with a fixed blood volume of 5% than a 1TCM with a fixed blood volume of 5% (Figure 1(b) and (c)). The 2TCM fits showed significantly (p < 0.001) lower AIC values compared with 1TCM fits (Bonferroni post-tests), whereas no difference in average AIC values was observed between brain regions, and there was no interaction between model and VOI (repeated measures two-way ANOVA). For the striatal TAC (Figure 1(b)), the 2TCM fitted the data much better than the 1TCM, particularly for the first 10 min. In the extra-striatal regions, 1TCM failed to describe the later time points of TACs (Figure 1(c)).
VT was estimated by 2TCM and Logan graphic analysis (Table 1). The effects of model and brain region on VT at baseline were analyzed. There was a strong interaction between model and brain region (p < 0.001), and both model and VOI significantly (p < 0.001) affected average VT (repeated measures two-way ANOVA). Striatal VT determined by the 2TCM was 10.5 ± 1.9, which was slightly (5.8%) but significantly (p < 0.001) higher compared with VT estimation by Logan plot (9.9 ± 1.7). Both models provided comparable VT values of ∼1.5 in extra-striatal regions (Bonferroni post-tests). KW-6002 pretreatment significantly (p < 0.001) reduced the VT in striatum to ∼2.0, whereas no significant difference was found between vehicle and KW-6002 pretreatment in other brain regions (two-way ANOVA with Bonferroni post-tests). The VT data of parietal cortex, frontal cortex, thalamus, cortex total, and whole brain are listed in Supplementary Table 1 and are not discussed here, because these brain regions are affected by spill-over from striatum and harderian glands with high tracer uptake. Several reference regions, including midbrain, hippocampus, cerebellum, and occipital cortex were selected and tested in reference region models to predict BPND. These selected regions are relatively large brain structures lacking A2AR specific binding sites.36 Furthermore, they are away from striatum and harderian glands, so that the spill-over effect is avoided. Therefore, the VT values obtained from these regions were smaller and more stable than the values of other extra-striatal regions (VT = ∼1.5 vs. VT = ∼1.5–2.5, COV = 6–14% vs. COV = 8–20%) and were not affected by the KW-6002 pretreatment (Table 1 and Supplementary Table 1). Such properties make them the suitable candidates as reference regions to calculate striatal BPND.
Table 1.
VT (mean ± SD) obtained from 2TCM and Logan plot (n = 6).
| Brain region | 2TCM-baseline | 2TCM-blocking | Logan-baseline | Logan-blocking |
|---|---|---|---|---|
| Striatum | 10.50 ± 1.91 | 2.04 ± 0.38*** | 9.88 ± 1.74 | 2.00 ± 0.36*** |
| Midbrain | 1.47 ± 0.17 | 1.51 ± 0.18 | 1.47 ± 0.13 | 1.38 ± 0.15 |
| Hippocampus | 1.58 ± 0.22 | 1.52 ± 0.30 | 1.48 ± 0.17 | 1.35 ± 0.10 |
| Cerebellum | 1.58 ± 0.14 | 1.41 ± 0.27 | 1.57 ± 0.10 | 1.40 ± 0.23 |
| Occipital cortex | 1.55 ± 0.15 | 1.46 ± 0.16 | 1.50 ± 0.10 | 1.45 ± 0.14 |
Significant differences against baseline are indicated by ***p < 0.001.
SD: standard deviation; 2TCM: two-tissue compartment model.
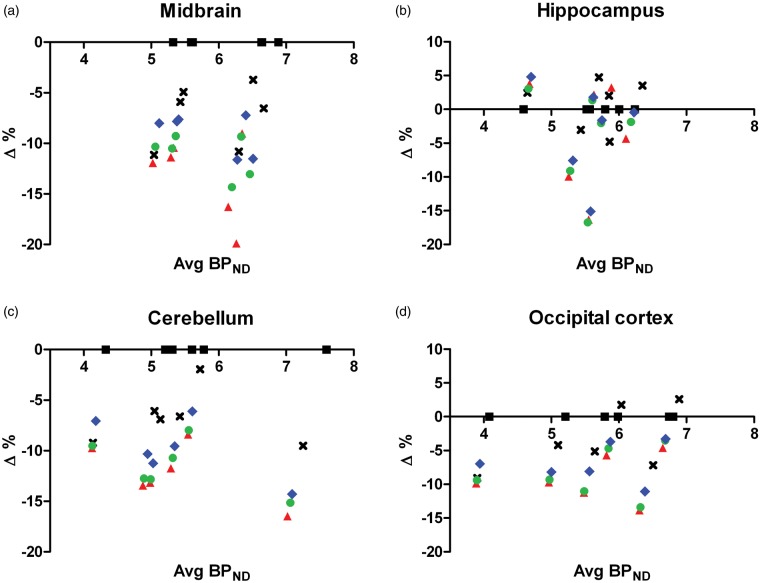
BPND values estimated by k3/k4 (direct method) are shown in Supplementary Table 2. The BPND values were significantly (p < 0.001) different among brain regions for vehicle/KW-6002 treatment. Both treatment and VOI strongly (p < 0.001) affected average BPND (two-way ANOVA). Striatal BPND in vehicle treated animals was 9.17 ± 1.90, the value was significantly (p < 0.001) reduced to ∼1.38 ± 0.63 with KW-6002 pretreatment, whereas the pretreatment did not alter the BPND in reference regions (Bonferroni post-tests). Striatal BPND at baseline was also obtained by indirect methods using a reference region (Supplementary Table 3). Indirect methods estimated similar BPND values of 5.0–6.1, which were ∼40% smaller than BPND calculate from the direct method. However, the between-subject variability with indirect methods (8–19% COV) was smaller than the variability of the direct method (20% COV). Indirect methods with midbrain or hippocampus as the reference tissue seemed to produce less variable results (∼10% COV), compared with other reference regions (∼17% COV) (Supplementary Table 3 and Figure 2). Midbrain as the reference region estimated significantly (p < 0.05) higher BPND values in striatum, regardless of the model used (repeated measures two-way ANOVA with Bonferroni post-tests). We compared various reference regions for the striatal BPND values at baseline obtained from Logan plot and reference tissue-based modeling methods with the BPND calculated from 2TCM as the gold standard. Relative to the 2TCM, a significantly (p < 0.05) negative bias (Δ) (up to 13.2%) was found with Logan plot and reference tissue-based methods for all reference regions in most cases (one-sample t-test) (Figure 2 and Supplementary Table 4). Such bias was also demonstrated by the linear regression of the striatal BPND values obtained from SRTM and RLogan against BPND values calculated from 2TCM, showing slopes of 0.91 for RLogan and 0.87 for SRTM when the midbrain was used as the reference region (Supplementary Figure 1).
Figure 2.
Bland–Altman plot for midbrain (a), hippocampus (b), cerebellum (c), and occipital cortex (d) with different modeling methods to obtain striatal BPND. , , where BPND,1 was derived from 2TCM (black square), Logan plot (black cross), SRTM (red triangle), MRTM (green circle), and RLogan (blue diamond), and BPND,2 was always calculated from 2TCM. Each point represents the data of an individual animal (n = 6). BPND obtained from 2TCM and Logan plot was calculated from the VT using the formula .
2TCM: two-tissue compartment model; SRTM: simplified reference tissue model; MRTM: multilinear reference tissue model; RLogan: reference tissue Logan plot.
SRTM and MRTM estimated comparable k2' values (Supplementary Table 5). The variability was slightly smaller with SRTM than MRTM. Therefore, k2' values determined by SRTM (0.39 ± 0.06 − 0.45 ± 0.02 min−1) were used in RLogan and MRTM2.
Test–retest
Test and retest BPND values for SRTM, RLogan, and SUVr – 1 are presented in Table 2. TRV and ICC for all methods are listed in Supplementary Table 6. The average retest BPND values were significantly (p < 0.01) smaller than the test results, and such difference was comparable between VOIs or models, whereas differences in VOI or model did not significantly affect average BPND (repeated measures two-way ANOVA), nor the test and retest BPND values (Bonferroni post-tests). The average TRV was less than 10%, which was similar across VOIs and models. The ICC values were fairly homogenous for all VOIs and models, ranging from 0.83 to 0.94, except for models with hippocampus as the reference region, showing an ICC of 0.38–0.43. Because of lacking of test–retest reliability, hippocampus was no longer assessed in the occupancy study. Occipital cortex displayed the lowest within-subject variability (TRV = 4.6–6.4%) and highest test–retest reliability (ICC = 0.91–0.94) compared with other reference regions. Since MRTM and MRTM2 did not show better results compared with SRTM and RLogan in terms of resulting BPND value and the inter-/intra-subject variability of BPND, these bi-linear regression methods were not assessed in the occupancy study.
Table 2.
Test and retest striatal BPND (mean ± SD) values obtained from different models and reference regions (n = 5).
| Reference region | SRTM |
RLogan |
aSUVr −1 |
|||
|---|---|---|---|---|---|---|
| Test | Retest | Test | Retest | Test | Retest | |
| Midbrain | 5.81 ± 0.85 | 5.49 ± 0.94 | 5.96 ± 0.90 | 5.50 ± 1.04 | 6.37 ± 0.93 | 5.79 ± 1.10 |
| Hippocampus | 5.38 ± 0.38 | 5.02 ± 0.67 | 5.46 ± 0.39 | 5.10 ± 0.68 | 5.64 ± 0.42 | 5.34 ± 0.67 |
| Cerebellum | 5.72 ± 1.23 | 5.38 ± 1.15 | 5.87 ± 1.30 | 5.55 ± 1.16 | 6.37 ± 0.93 | 5.94 ± 1.40 |
| Occipital cortex | 5.69 ± 1.01 | 5.39 ± 0.89 | 5.79 ± 1.09 | 5.44 ± 0.94 | 6.05 ± 1.22 | 5.68 ± 1.24 |
.
SD: standard deviation; SRTM: simplified reference tissue model; RLogan: reference tissue Logan plot.
A2AR occupancy
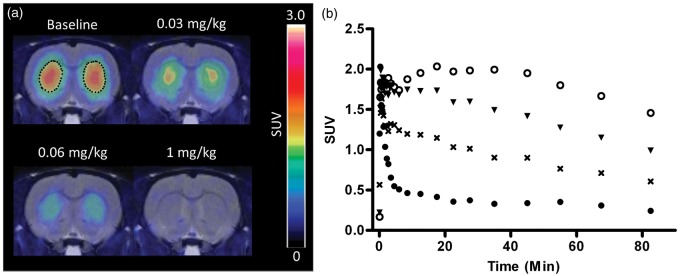
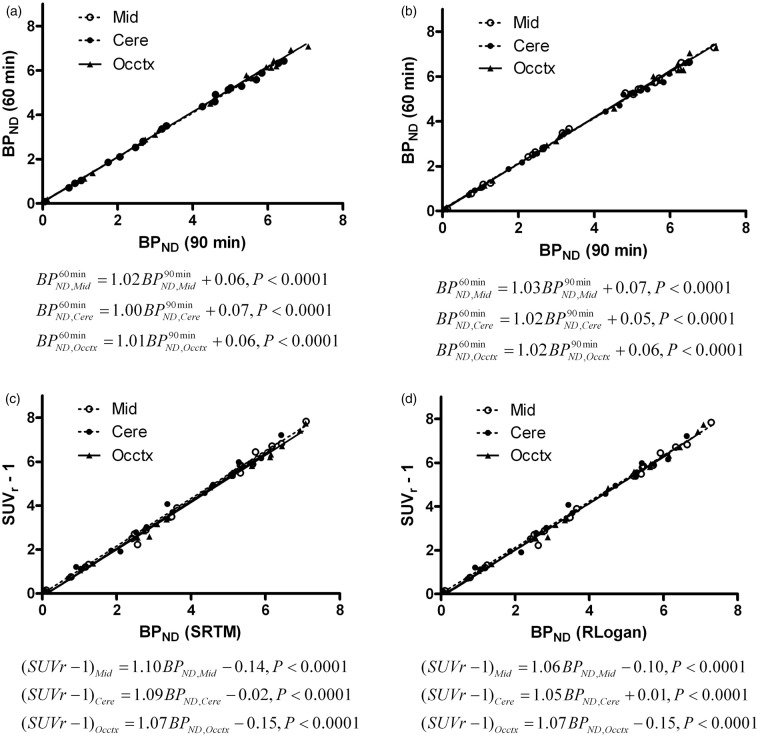
Pretreatment of KW-6002 at 15–25 min before administration of [11C]preladenant decreased the tracer uptake in striatum in a dose-dependent manner (Figure 3). BPND in striatum was determined by SRTM, RLogan, and (m)SUVr − 1 with a static scan duration of 35 min, starting at 25-min post injection, using midbrain, cerebellum, or occipital cortex as the reference region. The correlation between striatal BPND obtained from 90-min acquisition and from 60-min acquisition was studied using Deming linear regression. The BPND values at 60 min were plotted against BPND values at 90 min (Figure 4(a) and (b)). Both SRTM and RLogan showed a strong correlation between acquisition times, with slopes close to 1 (1.00–1.03) and a very small y-intercept of around 0.06. In comparison with the 60-min acquisition, the 90-min acquisition estimated marginally smaller (1.8–3.6%) BPND values at baseline. Deming linear regression was also used to assess the correlation between SUVr – 1 and BPND derived from pharmacokinetic modeling for 60-min acquisition (Figure 4(c) and (d)). A high agreement was found between these methods, indicating that SUVr – 1 is also a robust parameter to predict BPND. The striatal BPND can be calculated from SUVr – 1 corrected by the following equation:
where the y-intercept and slope were obtained from Deming linear regression (Figure 4(c) and (d)).
Figure 3.
Representative PET images of the coronal view of the rat brain (summed from 30 to 90 min) (a) and corresponding TACs in striatum (b) at baseline (open circle) and after i.p. injection of KW-6002 at 0.03 (triangle), 0.06 (cross), and 1 (closed circle) mg/kg (occupancy of 37%, 67%, and 98%, respectively). The PET images were merged onto the MRI template. Striatum at baseline were outlined by dots.
PET: positron emission tomography; TACs: time-activity curves; MRI: magnetic resonance imaging.
Figure 4.
Correlation of striatal BPND obtained from 60-min acquisition with BPND obtained from 90-min acquisition (a, b), and correlation of SUVr – 1 with BPND obtained from 60-min acquisition (c, d). (a, c) BPND was predicted by SRTM; (b, d) BPND was predicted by RLogan. .
Mid: midbrain, Cere: cerebellum, Occtx: occipital cortex; SRTM: simplified reference tissue model; RLogan: reference tissue Logan plot.
To estimate the ED50 of KW-6002, the dose-occupancy curve was fitted with the equation:
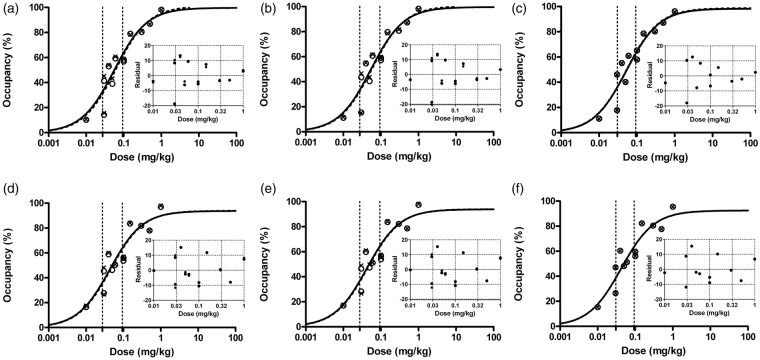
The hillslope was calculated to be approximately 1, indicating the binding pattern of monomer one-site binding. Then the hillslope value was fixed to 1 for all calculations. Occmax (maximum occupancy) was estimated to be ∼87–103% with a standard error of ∼4–6%, and a 95% confidence interval of 73–123% for the tested reference regions and models. A2AR occupancy as well as ED50 estimates were similar between models and scan durations with the same reference regions (<10% difference between models and <5% difference between acquisition times) (Figure 5). However, larger differences in ED50 estimation were observed between reference regions: KW-6002 ED50 estimates for i.p. injection were 0.056 ± 0.003, 0.062 ± 0.002, and 0.044 ± 0.002 mg/kg for midbrain, cerebellum, and occipital cortex as the reference region, respectively.
Figure 5.
Striatal A2AR occupancy against KW-6002 doses. (a–c) Occupancy was determined by SRTM (a), RLogan (b), and mSUVr – 1 (c), using midbrain as the reference region. (d–f) Occupancy was determined by SRTM (d), RLogan (e), and mSUVr – 1 (f), using occipital cortex as the reference region. (a, b, d, e) Circle: occupancy for 60-min acquisition; Cross: occupancy for 90-min acquisition; Curved dotted line: 60-min acquisition fit; Solid line: 90-min acquisition fit. (c, f) Circle: occupancy for mSUVr – 1, based on RLogan correction; Cross: occupancy for mSUVr – 1, based on SRTM correction; Curved dotted line: RLogan corrected mSUVr – 1 fit, Solid line: SRTM corrected mSUVr – 1 fit. The areas between vertical dotted lines represent 95% confidence interval of ED50. The inserts indicate residuals of regression. Correction factors for mSUVr – 1 were determined by Deming linear regression between SUVr – 1 and SRTM or RLogan derived BPND.
A2AR: A2A receptor; SRTM: simplified reference tissue model; RLogan: reference tissue Logan plot.
Discussion
This study demonstrates that [11C]preladenant is a suitable PET tracer for the quantification of available A2AR binding sites in the rat brain. The tracer displayed high uptake in striatum and low and homogenous uptake in all extra-striatal regions. The regional distribution of [11C]preladenant is in agreement with the known A2AR expression in the rat brain,36 as the receptors are predominantly expressed in striatum only. An A2AR subtype selective antagonist, KW-6002 (Ki value: A2AR = 2.2 nM, A1R = 150 nM (ref. 37)) significantly reduced the tracer uptake in striatum by 80%. However, the VT in striatum was till 40% higher than the values in the reference brain regions (Table 1). A complete blockade could be achieved with higher doses of KW-6002. However, 1 mg/kg was the highest dose without adverse effects. Test–retest results indicate that [11C]preladenant PET is highly reproducible for the imaging of A2ARs. The A2AR occupancy study reveals that the tracer uptake is sensitive to the changes in available A2ARs. The sufficiently large dynamic range (BPND ≈ 5.5 at baseline and < 0.5 with complete blockade, Supplementary Table 3) allows [11C]preladenant to estimate A2AR occupancy in striatum by KW-6002 as well as other A2AR-targeting molecules with high accuracy. Thus, [11C]preladenant is a good tool to aid the development of A2AR drugs if confirmed in humans.
Several plasma input-dependent (2TCM and Logan plot) and reference tissue-based (SRTM, MRTM, MRTM2, and RLogan) modeling methods as well as (m)SUVr – 1 were applied to calculate VT and/or BPND. Regarding the BPND estimation, four reference regions have been tested with indirect models. There are two purposes to test different reference regions: (a) to find proper reference regions with negligible receptor expression, which are able to estimate target BPND with high value, low standard error, and a high test–retest reproducibility; and (b) to have suitable alternative reference regions. Because A2ARs are also expressed in glial cells that are involved in regulating neuroinflammation, A2AR tracers could also be used to study A2AR changes in animal models with neuroinflammation, for example, the herpes simplex virus infected rat model. In this case, midbrain and cerebellum are not proper reference regions anymore, because these regions are affected by the virus and associated with an increased level of A2AR. Then, we may choose a less infected region (occipital cortex) as the reference region instead.
VT and BPND predictions with all models and reference regions were in agreement with each other, except for the striatal BPND estimation with the direct method, which was about 60% larger than the BPND estimated by indirect methods. Indirect methods might have underestimated BPND because of (a) the presence of radioactive metabolites in the brain, which contributed ∼17% of the total activity in the brain at 60-min post injection. The brain metabolites reduced the target-to-background ratio, leading to an underestimation in BPND when the indirect methods were applied. When the VT values were corrected by subtracting the VT contribution by 17% radioactive metabolites, the BPND calculated from became ∼8.9, which was comparable with k3/k4 of 9.2. Also because of the radioactive metabolites in the brain, when the 2TCM was used to fit tracer TACs, a specific distribution volume of 0.6–1.1 was found in reference regions which was not affected by KW-6002 blockade. The presence of radioactive metabolites could also explain the difference between the direct method and indirect methods derived BPND in striatum with KW-6002 pretreatment (Supplementary Tables 2 and 3). The brain metabolite could theoretically be a problem with this tracer. However, it didn't show much effect on the BPND stability, as only ∼3% difference was observed in BPND between 90-min and 60-min acquisition data; (b) noise-induced negative bias with graphical analysis.38 For Ichise's multilinear regression, it was shown that a 5% average noise in the TAC can result in a 75% underestimation of BPND at a true BPND of 3 (ref. 39). Such underestimation is more pronounced at high noise level and high actual VT and BPND; (c) Violations of assumptions with SRTM fit.40 For example, the tracer kinetics could be fitted well with the 2TCM, but not with the 1TCM. Such violation resulted in an underestimation of BPND. On the other hand, the BPND calculated from the direct method might be biased due to the polar radioactive metabolites penetrating the blood-brain-barrier. Reference tissue-based BPND values displayed much lower variability (∼10% COV) compared with the variability of k3/k4 and VT (∼18–20% COV) in striatum. This is a well-known aspect of reference tissue models and is related to the number of fit parameters. VT seems to be more variable than BPND, probably due to the variability in the plasma sample measurements. Therefore, we consider that BPND predicted from indirect methods is more robust than k3/k4 and VT.
In addition, we compared the striatal BPND values predicted from different reference tissue models, using 2TCM as the gold standard. BPND estimated from reference tissue-based models correlated well (R2 > 0.99) with 2TCM estimation (Supplementary Figure 1). However, a small (up to 13.2%) negative bias (Supplementary Table 4) was observed with reference tissue-based methods. Here, we consider that the accuracy in BPND prediction is less important than other properties like the robustness of model-parameter values and the model complexity. Therefore, BPND derived from reference tissue-based modeling methods was used to study the test–retest reproducibility of [11C]preladenant PET and to characterize A2AR occupancy.
The test–retest experiment showed that the striatal BPND values for the retest were slightly lower (<10%) with ∼10% larger between-subject variability compared with test values. The receptor occupancy by the PET tracer during the test scan was estimated to be ∼2–7%, provided an A2AR density of 300–953 fmol/mg protein in striatum.1,29,41 We assume that the occupancy decreased at least 60% after 24 h (ref. 42), then ∼1–3% of the receptors were occupied by the cold tracer. However, this number cannot completely explain the underestimation of 4–9% with retest. Therefore, we consider that other factors such as anesthesia during the test scan may lead to the underestimation of BPND with retest.
In addition, we compared BPND and ED50 values obtained from the 90-min acquisition with the values obtained from the 60-min acquisition in the occupancy study. The BPND as well as ED50 predictions were very similar between models and acquisition times (Figures 4 and 5). The slightly (<3%) higher BPND values at baseline obtained from the 60-min acquisition could be explained by the slow accumulation of radioactive metabolites in the brain, leading to a reduced BPND value for 90-min acquisition. Furthermore, 60-min acquisition showed improved precision in BPND prediction, with significantly (p < 0.05) lower COV compared with COV of 90-min acquisition. Therefore, 60-min acquisition provides better estimation of BPND in rat brain, compared with 90-min acquisition.
The kinetic modeling and test–retest experiments showed that all reference tissue-based models, including SRTM, MRTM, MRTM2, and RLogan with different reference brain regions, including midbrain, cerebellum, and occipital cortex provided comparable results, with a high BPND value in striatum with small between-subject variability and high test–retest reliability. However, in comparison with midbrain and occipital cortex, cerebellum seems to be less favorable, with a slightly higher between-subject and TRV than the other two reference regions.
In the A2AR occupancy study, ∼30% difference was found in ED50 prediction between modeling methods using occipital cortex as the reference region and models using midbrain or cerebellum as the reference region. It is difficult to determine which value is more reliable and which reference region is better than the rest in the A2AR occupancy study, since the 95% confidence interval for ED50 is quite wide for all methods, being around 0.02–0.09 mg/kg (Figure 5). Taking into account factors like the need of blood sampling, complexity of the model, intra- and inter-individual variability, and the value of BPND in striatum, we consider that SRTM with midbrain or occipital cortex as the reference tissue to be the preferable model for striatal A2AR quantification, as SRTM is simpler than RLogan and MRTM2, which require a priori k2' estimation, and SRTM is slightly more robust than MRTM (Supplementary Table 6).
An interesting finding in this study is the suitability of SUVr to predict striatal BPND, in terms of comparable COV and test–retest reliability between SUVr – 1 and BPND (Table 2 and Supplementary Table 6), a strong correlation between the two parameters (Figure 4(c) and (d)), and the feasibility of (m)SUVr – 1 to study A2AR occupancy (Figure 5(c) and (f)). In the occupancy study, both mSUVr – 1 and SUVr – 1 predicted comparable ED50 of KW-6002 with the value obtained from BPND with pharmacokinetic modeling, indicating that mSUVr − 1 and SUVr – 1 are robust parameters to predict BPND and study A2AR occupancy in striatum. Therefore, the PET acquisition procedure can be further reduced to a 35-min static scan, starting at 25-min post tracer injection. However, we should use (m)SUVr – 1 with caution especially in pathological conditions. Since the regional perfusion might have changed under such conditions and thus might have different effects on SUVr and BPND. The usefulness of (m)SUVr – 1 for striatal A2AR quantification in other experimental setups needs to be further explored.
During the last two decades, many PET tracers have been developed for the imaging of A2ARs in the brain. The most promising [18F]MNI-444 was recently tested in the rhesus monkeys42 and human subjects.18 The tracer showed good brain penetration, high BPND values in A2AR-rich regions and high test–retest reproducibility. However, slow kinetics might be the problem with this tracer. The tracer uptake peaked in A2AR-rich regions at around 40- to 60-min post injection in the human and monkey brain. For the monkey study, a 120-min acquisition is required to quantify the tracer kinetics, with a 6–9% overestimation of striatal BPND with 120-min acquisition compared with 180-min acquisition. For the human study, the 90-min acquisition overestimated striatal BPND by ∼9% as compared with 210-min acquisition. In our rat study, [11C]preladenant showed faster kinetics, with the highest uptake in striatum at 22.5-min post injection. A 60-min acquisition is sufficient to estimate BPND, with marginally overestimation of BPND of <3% on average as compared with BPND values measured with 90-min acquisition. However, the direct comparisons between studies are difficult because of the different species and experimental setups in the studies.
Conclusions
This study shows that [11C]preladenant selectively binds to A2ARs in the brain. The tracer has a favorable pharmacokinetic profile. The tracer displayed high BPND in striatum with excellent test–retest reproducibility and ability to assess the changes of available A2ARs in the brain. A 60-min acquisition protocol using the SRTM with midbrain or occipital cortex as the reference region for kinetic modeling is the preferred method to quantify A2ARs in the brain. The acquisition protocol can be further reduced to a 35-min static scan to estimate BPND with high accuracy since BPND and SUVr are closely correlated. The results indicate that [11C]preladenant is a very promising A2AR tracer that warrants further validation in non-human primates and human subjects.
Supplementary Material
Acknowledgments
We thank Andrea Parente, Marianne Schepers, Anja P Huizing, Rolf Zijlma, and Jurgen Sijbesma for technical assistance; Michel Koole and Cindy Casteels for suggestions on kinetic modeling.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Xiaoyun Zhou, Rudi AJO Dierckx, Philip H Elsinga, and Erik FJ de Vries conceived and designed the experiment and were involved in interpretation of the data. Xiaoyun Zhou and Shivashankar Khanapur carried out the experiments. Xiaoyun Zhou, Johan R de Jong, and Antoon TM Willemsen analyzed and interpreted the experimental data. Xiaoyun Zhou drafted the manuscript. All authors corrected and improved the contents of the manuscript. All authors agreed to publish the manuscript in the Journal of Cerebral Blood Flow & Metabolism.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Jarvis MF, Schulz R, Hutchison AJ, et al. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther 1989; 251: 888–893. [PubMed] [Google Scholar]
- 2.Ferré S, Quiroz C, Orru M, et al. Adenosine A(2A) receptors and A(2A) receptor heteromers as key players in striatal function. Front Neuroanat 2011; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishi A, Liu F, Matsuyama S, et al. Metabotropic mGlu5 receptors regulate adenosine A2A receptor signaling. Proc Natl Acad Sci USA 2003; 100: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffmann SN, Fisone G, Moresco R, et al. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 2007; 83: 277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haskó G, Pacher P, Vizi ES, et al. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci 2005; 26: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebola N, Simões AP, Canas PM, et al. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 2011; 117: 100–111. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro JA, Sebastião AM, de Mendonça A. Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 2002; 68: 377–392. [DOI] [PubMed] [Google Scholar]
- 8.Gomes CV, Kaster MP, Tomé AR, et al. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 2011; 1808: 1380–1399. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm BB, Chen JF, Cunha RA, et al. Adenosine and brain function. Int Rev Neurobiol 2005; 63: 191–270. [DOI] [PubMed] [Google Scholar]
- 10.Dall'Igna OP, Porciúncula LO, Souza DO, et al. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol 2003; 138: 1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum D, Hourez R, Galas MC, et al. Adenosine receptors and Huntington's disease: implications for pathogenesis and therapeutics. Lancet Neurol 2003; 2: 366–374. [DOI] [PubMed] [Google Scholar]
- 12.Cunha RA, Ferré S, Vaugeoi JM, et al. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des 2008; 14: 1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzschild MA, Agnati L, Fuxe K, et al. Targeting adenosine A2A receptors in Parkinson's disease. Trends Neurosci 2006; 29: 647–654. [DOI] [PubMed] [Google Scholar]
- 14.Varani K, Abbracchio MP, Cannella M, et al. Aberrant A2A receptor function in peripheral blood cells in Huntington's disease. FASEB J 2003; 17: 2148–2150. [DOI] [PubMed] [Google Scholar]
- 15.Ishiwata K, Ogi N, Hayakawa N, et al. Adenosine A2A receptor imaging with [11C]KF18446 PET in the rat brain after quinolinic acid lesion: comparison with the dopamine receptor imaging. Ann Nucl Med 2002; 16: 467–475. [DOI] [PubMed] [Google Scholar]
- 16.Mishina M, Ishiwata K, Naganawa M, et al. Adenosine A(2A) receptors measured with [11C]TMSX PET in the striata of Parkinson's disease patients. PLoS One 2011; 6: e17338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramlackhansingh AF, Bose SK, Ahmed I, et al. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 2011; 76: 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barret O, Hannestad J, Vala C, et al. Characterization in humans of 18F-MNI-444, a PET radiotracer for brain adenosine 2A receptors. J Nucl Med 2015; 56: 586–591. [DOI] [PubMed] [Google Scholar]
- 19.Naganawa M, Kimura Y, Mishina M, et al. Quantification of adenosine A2A receptors in the human brain using [11C]TMSX and positron emission tomography. Eur J Nucl Med Mol Imaging 2007; 34: 679–687. [DOI] [PubMed] [Google Scholar]
- 20.Brooks DJ, Doder M, Osman S, et al. Positron emission tomography analysis of [11C]KW-6002 binding to human and rat adenosine A2A receptors in the brain. Synapse 2008; 62: 671–681. [DOI] [PubMed] [Google Scholar]
- 21.Mishina M, Ishiwata K, Kimura Y, et al. Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse 2007; 61: 778–784. [DOI] [PubMed] [Google Scholar]
- 22.Naganawa M, Mishina M, Sakata M, et al. Test-retest variability of adenosine A2A binding in the human brain with 11C-TMSX and PET. EJNMMI Res 2014; 4: 76 [aq]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moresco RM, Todde S, Belloli S, et al. In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416. Eur J Nucl Med Mol Imaging 2005; 32: 405–413. [DOI] [PubMed] [Google Scholar]
- 24.Hirani E, Gillies J, Karasawa A, et al. Evaluation of [4-O-methyl-(11)C]KW-6002 as a potential PET ligand for mapping central adenosine A(2A) receptors in rats. Synapse 2001; 42: 164–176. [DOI] [PubMed] [Google Scholar]
- 25.Ishiwata K, Noguchi J, Wakabayashi S, et al. 11C-labeled KF18446: a potential central nervous system adenosine A2a receptor ligand. J Nucl Med 2000; 41: 345–354. [PubMed] [Google Scholar]
- 26.Khanapur S, Paul S, Shah A, et al. Development of [18F]-labeled Pyrazolo [4, 3-e]-1, 2, 4-triazolo [1, 5-c] pyrimidine (SCH442416) analogs for the imaging of Cerebral Adenosine A2A receptors with Positron Emission Tomography. J Med Chem 2014; 57: 6765–6780. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Khanapur S, Huizing AP, et al. Synthesis and preclinical evaluation of 2-(2-furanyl)-7-[2-[4-[4-(2-[11C]methoxyethoxy)phenyl]-1-piperazinyl]ethyl]7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine-5-amine ([11C]Preladenant) as a PET tracer for the imaging of cerebral adenosine A2A receptors. J Med Chem 2014; 57: 9204–9210. [DOI] [PubMed] [Google Scholar]
- 28.Cutler DL, Tendolkar A, Grachev ID. Safety, tolerability and pharmacokinetics after single and multiple doses of preladenant (SCH420814) administered in healthy subjects. J Clin Pharm Ther 2012; 37: 578–587. [DOI] [PubMed] [Google Scholar]
- 29.Alfaro TM, Vigia E, Oliveira CR, et al. Effect of free radicals on adenosine A(2A) and dopamine D2 receptors in the striatum of young adult and aged rats. Neurochem Int 2004; 45: 733–738. [DOI] [PubMed] [Google Scholar]
- 30.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990; 113: 27–47. [DOI] [PubMed] [Google Scholar]
- 31.Bazan NG, Braquet P, Ginsberg MD. Neurochemical correlates of cerebral ischemia. In: Ginsberg MD, Braquet P, Bazan (eds). Introduction: Current biochemical and molecular approaches to the study of cerebral ischemia, New York, NY: Plenum Press, 1992, pp. 1–8. . [Google Scholar]
- 32.Julien-Dolbec C, Tropres I, Montigon O, et al. Regional response of cerebral blood volume to graded hypoxic hypoxia in rat brain. Br J Anaesth 2002; 89: 287–293. [DOI] [PubMed] [Google Scholar]
- 33.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 34. The design and analysis of clinical experiments. In: Fleiss J. (ed). In: Reliability of measurement, Danvers, MA: John Wiley & Sons, Inc, 1986, pp. 1–32. [Google Scholar]
- 35.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979; 86: 420–428. [DOI] [PubMed] [Google Scholar]
- 36.Parkinson FE, Fredholm BB. Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn Schmiedebergs Arch Pharmacol 1990; 342: 85–89. [DOI] [PubMed] [Google Scholar]
- 37.Shimada J, Koike N, Nonaka H, et al. Adenosine A2A antagonists with potent anti-cataleptic activity. Bioorg Med Chem Lett 1997; 7: 2349–2352. [Google Scholar]
- 38.Slifstein M, Laruelle M. Effects of statistical noise on graphic analysis of PET neuroreceptor studies. J Nucl Med 2000; 41: 2083–2088. [PubMed] [Google Scholar]
- 39.Buchert R, Varga J, Mester J. Limitations of bi-linear regression analysis for the determination of receptor occupancy with positron emission tomography. Nucl Med Commun 2004; 25: 451–459. [DOI] [PubMed] [Google Scholar]
- 40.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4(3 Pt 1): 153–158. [DOI] [PubMed] [Google Scholar]
- 41.Parsons B, Togasaki DM, Kassir S, et al. Neuroleptics up-regulate adenosine A2a receptors in rat striatum: implications for the mechanism and the treatment of tardive dyskinesia. J Neurochem 1995; 65: 2057–2064. [DOI] [PubMed] [Google Scholar]
- 42.Barret O, Hannestad J, Alagille D, et al. Adenosine 2A receptor occupancy by tozadenant and preladenant in Rhesus monkeys. J Nucl Med 2014; 55: 1712–1718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.