Abstract
The changes in the availability of striatal dopamine transporter and dopamine D2 receptor after mild focal ischemia in rats were measured using a small animal positron emission tomography system. Mild focal ischemia was induced by 20-minute middle cerebral artery occlusion. [11C]PE2I binding to dopamine transporter was transiently increased on the ipsilateral side of the striatum at 2 days after middle cerebral artery occlusion. On day 7 and 14 after middle cerebral artery occlusion, [11C]PE2I binding levels were decreased. In contrast, [11C]raclopride binding to dopamine D2 receptor in the ipsilateral striatum had not changed at 2 days after middle cerebral artery occlusion. [11C]Raclopride binding was significantly decreased on the ischemic side of the striatum at 7 and 14 days after middle cerebral artery occlusion. Moreover, on day 1 and 2 after middle cerebral artery occlusion, significant circling behavior to the contralateral direction was induced by amphetamine challenge. This behavior disappeared at 7 days after middle cerebral artery occlusion. At 14 days, circling behavior to the ipsilateral direction (middle cerebral artery occlusion side) was significantly increased, and that to the contralateral direction also appeared again. The present study suggested that amphetamine-induced circling behavior indicated striatal dopaminergic alterations and that dopamine transporter and dopamine D2 receptor binding could be key markers for predicting motor dysfunction after mild focal ischemia.
Keywords: Middle cerebral artery occlusion, dopamine transporter, dopamine D2 receptor, positron emission tomography, circling behavior
Introduction
Cerebral ischemia is known to produce brain damage and related behavioral deficits such as motor dysfunction and memory deficits. Neuronal damage after brief episodes of cerebral ischemia has remained difficult to evaluate using clinical and preclinical imaging techniques such as magnetic resonance imaging (MRI), SPECT, and positron emission tomography (PET). However, it might be possible to predict changes of the dopaminergic system, because available PET probes for dopamine transporter (DAT) and dopamine (DA) receptors have been documented in previous reports. What is needed is an imaging method that can detect predictive markers of neuronal dysfunction after ischemia.
Much work has been done on evaluating the changes in neurological function after ischemia. Recent studies have demonstrated that free radical and chemical mediators, such as cytokine and endothelin, are important chemicals in the mechanism of ischemic brain injury.1–3 Other studies have shown the increase of neurotransmitters, such as DA and glutamate, in ischemic pathological conditions.4,5 In the DA nervous system, ischemia-induced cell damage enhances the DA level.6 In addition, the levels of extracellular DA and serotonin are strongly involved in the severity of the ischemia insult.7 Thus, dopaminergic neural transmission plays an important role in mediating ischemic brain damage. On the other hand, it has been reported that DA D2 receptor agonists, such as pergolide, bromocriptine, and lisuride, can offer neuro-protection against ischemic brain injury.8 This report also noted the importance of dopaminergic neural transmission in brain injury induced by ischemia.
MRI studies have suggested delayed neurodegeneration after mild ischemia.9–11 Although mild ischemia has not precisely been defined in rat, middle cerebral artery occlusion (MCAO) was performed for 15 min in these studies, which focused on the striatal hyper intensity of the T1-weighted image, which appeared from 7 days after ischemia/reperfusion. Interestingly, progressive increase in circling behavior induced by apomorphine (non-selective DA agonist) was also observed at this time point (8 and 16 days after ischemia/reperfusion). In contrast, no significant finding was obtained for both the T1-weighted image and circling behavior until 4 days. In 6-OHDA-induced DA lesioned rat, d-amphetamine-induced ipsilateral turnings and apomorphine-induced contralateral turnings are well documented.12–15 Thus, these results indicated that dopaminergic imbalance between the damaged and the non-damaged hemispheres induced circling behavior following systemic administration of DA enhancer. In fact, clinical study has shown the relation between abnormal behavior and striatal dysfunction of the dopaminergic nervous system in neurodegenerative disease such as Parkinson’s disease and Huntington’s disease. To apply the results of animal experiments to clinical studies, evaluating both behavior and striatal dysfunction is also important to understand the details of the pathological conditions of focal ischemia.
A previous article reported the time course of progression of DA D2 receptor binding after ischemia measured by in vivo [11C]raclopride PET.16 This study revealed that the ipsilateral- to contralateral-[11C]raclopride binding ratio was significantly decreased from 7 days after MCAO. Rats were occluded for 2 h in this study. Other reports demonstrated a decrease of DA D1 receptor binding measured using [3H]SCH23390 after ischemia/reperfusion.17,18 The MCAO period in these studies was 3 or 24 h. These studies measuring DA D2 and D1 receptor binding clearly showed that the availability of the DA D2 and D1 receptor had changed after ischemia/reperfusion. However, to our knowledge, little has been reported on the longitudinal changes in the availability of the DA D2 receptor and DAT after mild focal ischemia, that does not induce cerebral infarction.
Positron emission tomography (PET) has been widely used to assess the availability of DA receptors and transporter in human studies as well as in rodent experiments. PET makes possible non-invasive in vivo measurement. [11C]PE2I and [11C]raclopride have been used as selective PET probes to evaluate the availability of DAT and DA D2 receptor, respectively. In addition, to date, the method of kinetic analysis of these probes has been established for both human and non-human brain. Since some studies demonstrated that a simplified reference tissue model (SRTM) could be applied, BPND values have been useful parameters for assessing the neuronal functions.
The aim of this study was to assess the relation between amphetamine-induced behavior and striatal DAT and DA D2 receptor binding after mild focal ischemia using PET.
Materials and methods
Animals
Male Wistar rats (8–10 weeks old) were purchased from Japan Clea (Tokyo, Japan). Rats were housed under a 12-h dark–light cycle and had free access to food and water. All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Shionogi Research Laboratories (Osaka, Japan) and Osaka University Graduate School of Medicine (Osaka, Japan), and were consistent with the internal guidelines for animal experiments and in adherence to the ethics policies. Experiments were reported according to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Cerebral ischemic model
MCAO model rats were used in this study. Adult male Wistar rats weighing about 300 g were initially anaesthetized with 5% isoflurane and maintained under anesthesia with 2.0% isoflurane. Rats were placed on a heat pad system (38℃) during the operation to cause MCAO, which was done using almost the same method as in our previous studies.17,19 In brief, a 4-0 monofilament nylon suture thread (Natsume Seisakusho, Tokyo, Japan) coated with silicon (Heraseus, Kulzer, Germany) was inserted via the common carotid artery into the internal carotid artery and then into the circle of Willis. The suture thread was inserted 20 to 22 mm from the bifurcation of the common carotid artery. After the intraluminal suture thread had been positioned, the neck incision was closed with a silk suture. The duration of the occlusion period was 15 min, and reperfusion after various periods was carried out by removing the intraluminal suture thread. All operations were done under 2% isoflurane anesthesia.
Preparation of [11C]PE2I
[11C]PE2I was produced by the same method as described previously with minor modifications.20 In brief, precursor (PE2I acid; 0.5 mg) was added to 10% TBAOH of methanol solution (5 µL) in anhydrous DMF (0.4 mL). After trapping of [11C]CH3I, the reaction mixture was heated for 3 min at 80℃. Purification was performed by HPLC. The purified fraction was evaporated to dryness, and the residue was dissolved in saline containing 7% EtOH, 1% polysorbate 80, and sodium ascorbate (20 mg). Specific activity ranging from 20 to 60 GBq/µmol at the end of synthesis was used in the animal experiments. Radiochemical purity was higher than 99%.
Preparation of [11C]raclopride
[11C]Raclopride was produced by the same method as described previously with minor modifications.21 In brief, precursor (desmethyl raclopride; 0.5 mg) was added to 2 M NaOH (10 µL) in anhydrous acetone (0.4 mL). After trapping of [11C]CH3OTf, the reaction mixture was heated for 3 min at 40℃. Purification was performed by HPLC. The purified fraction was evaporated to dryness, and the residue was dissolved in saline containing sodium ascorbate (20 mg). Specific activity ranging from 20 to 80 GBq/µmol at the end of synthesis was used in the animal experiments. Radiochemical purity was higher than 99%.
PET/computed tomography (CT) measurements
PET scans of MCAO rats were performed under 2% isoflurane anesthesia using Pre-Clinical Imaging System Triumph LabPET12/CT (TriFoil Imaging Inc., Chatsworth, USA). Approximately 20–40 MBq of [11C]PE2I or [11C]raclopride (0.3–0.5 mL) was injected into the tail vein through the cannula, and emission data were collected at the same time as the start of the tracer injection. PET data of both ligands were dynamically acquired for 60 min. After the PET scans, CT scans were done to acquire anatomical information and to obtain data for attenuation correction of the PET images. Next, CT images were reconstructed by the filtered back-projection method (512 slices), and PET images were reconstructed by the 3D-MLEM method with CT-based attenuation correction. CT and PET images were automatically fused by AMIDE software, which is a free tool for viewing and analyzing medical imaging. Using this software, striatal regions of interest (ROI; 2.5 mm × 2.5 mm × 0.5 mm) and the cerebellum (ROI: 6.0 mm × 2.5 mm × 0.5 mm) were manually placed on the PET images, and the radioactivity concentrations in each region were determined. Ischemic rats at each time point (at 2, 7, and 14 days after ischemia/reperfusion) were separately assigned, and rats were immediately sacrificed after the PET scan.
Kinetic analysis of [11C]raclopride and [11C]PE2I
Time activity curves in the contralateral and ipsilateral striatum, which were determined by AMIDE software, were analyzed by a SRTM using the radioactivity concentration in the cerebellum as the reference tissue. This method is a two-tissue reversible compartment model for a target tissue, which is the striatum in this study. SRTM analysis was done using PMOD software (PMOD Technologies Ltd, Zurich, Switzerland), and then the binding potential (BPND) in the ipsilateral and the contralateral striatum were calculated and compared.
Amphetamine-induced circling behavior test
The amphetamine-induced circling test was carried out at 1, 2, 7, and 14 days after reperfusion. Rats were intraperitoneally injected with 1 mg/kg amphetamine (Sigma, St. Louis, MO) dissolved in water. The rats were then immediately placed in bowls, and their spontaneous circling activity was measured for 30 min. Circling behavior events to the ipsilateral direction and the contralateral direction were separately counted, and the average count was summarized.
Hematoxylin–eosin (HE) staining
The rats were sacrificed by decapitation at 2, 7, and 14 days after ischemia/reperfusion. The brains were quickly removed and frozen, and stored at −80℃ until use. Brain slices (20 µm) contained in the striatal regions were prepared using a cryostat, and sections were stained with HE. HE staining images in the striatum were obtained using a microscope (BZ-9000, Keyence Japan).
Statistical analysis
Statistical analysis was performed by the unpaired t-test. P < 0.01 was considered as statistically significant in this study.
Results
[11C]PE2I binding in MCAO rats
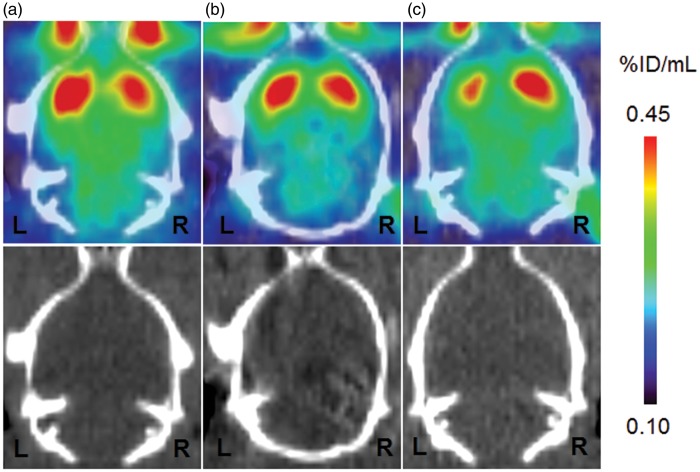
[11C]PE2I binding to DAT in the striatum is summarized in Figure 1. PET images at 2, 7, and 14 days after ischemia/reperfusion are shown in Figure 1(A) to (C). Quantitative analysis of [11C]PE2I binding was performed using BPND values calculated by the SRTM method. As summarized in Figure 2, [11C]PE2I binding transiently increased by 45% in the ipsilateral striatum (% of the contralateral striatum) at 2 days after ischemia/reperfusion. After 7 and 14 days, [11C]PE2I binding was reduced to 96% and 78%, respectively.
Figure 1.
Typical PET images of [11C]PE2I uptake in ischemia/reperfusion rat brain. The figure shows PET images (0- to 60-min summation images) and the corresponding X-CT images. Scale bar represents % injected dose/mL (% ID/mL). PET and CT images are for 2 days (A), 7 days (B), and 14 days (C) after ischemia/reperfusion. The left side on the images is the ipsilateral side of the striatum.
PET: positron emission tomography; CT: computed tomography.
Figure 2.
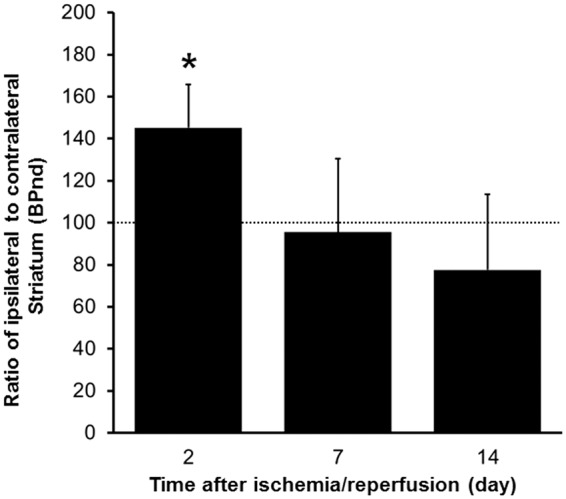
Results of quantitative analysis of [11C]PE2I uptake in ischemia/reperfusion rat brain. Values represent the BPND and are expressed as means ± SD of 4 (2 days) or 6 (7 and 14 days) animals.
SD: standard deviation.
[11C]Raclopride binding in MCAO rats
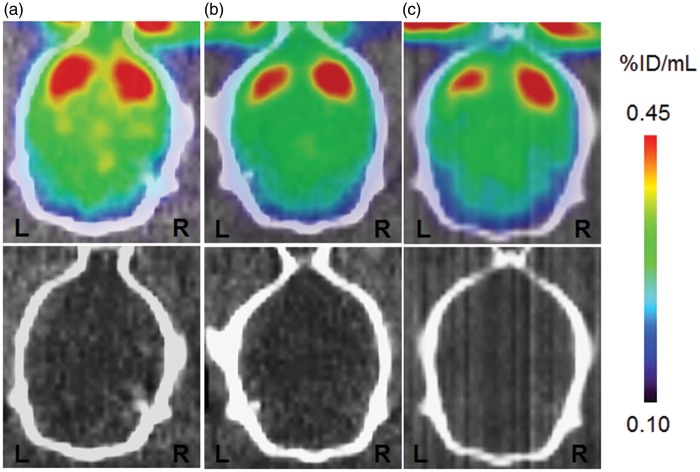
[11C]Raclopride binding to DA D2 receptor in the striatum is summarized in Figure 3, and PET images are shown in Figure 3(A) to (C). To quantitatively compare the [11C]raclopride binding in the ipsilateral and the contralateral striatum, the same kinetic analysis method used for [11C]PE2I was applied. The results of the quantitative analysis are shown in Figure 4. After 2, 7, and 14 days, [11C]raclopride binding levels in the ipsilateral striatum were reduced to 93%, 62%, and 49% of that in the contralateral striatum, respectively.
Figure 3.
Typical PET images of [11C]raclopride uptake in ischemia/reperfusion rat brain. The figure shows PET images (0- to 60-min summation images) and the corresponding X-CT images. Scale bar represents % injected dose/mL (% ID/mL). PET and CT images are for 2 days (A), 7 days (B), and 14 days (C) after ischemia/reperfusion. Images on the left side are for the ipsilateral side of the striatum.
PET: positron emission tomography; CT: computed tomography.
Figure 4.
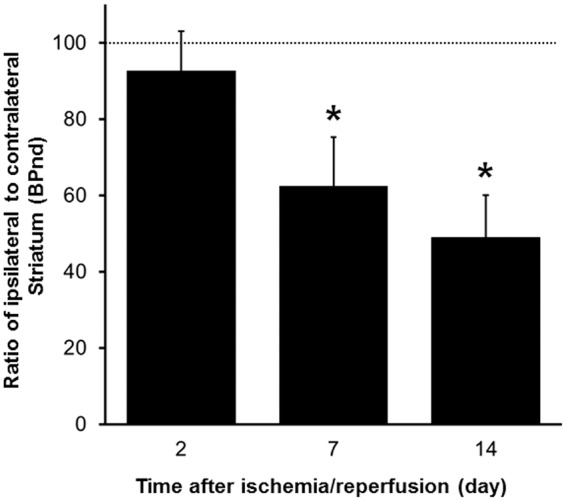
Results of quantitative analysis of [11C]raclopride uptake in ischemia/reperfusion rat brain. Values represent the BPND, expressed as means ± SD of 6 (2days) or 7 (7 and 14 days) animals.
SD: standard deviation.
Circling behavior
The results of amphetamine-induced circling behavior in MCAO rats are shown in Figure 5. At 1 and 2 days after ischemia/reperfusion, the number of circling behavior events to the contralateral direction was observed (27.6 ± 12.4 at 1 day, and 19.1 ± 14.3 at 2 days). This behavior had almost disappeared at 7 days (3.6 ± 1.7). After 14 days, circling behavior to the contralateral direction was observed again (9.0 ± 5.5). The number of circling behavior events to the ipsilateral direction progressively increased over time (0.6 ± 0.8 at 1 day, 1.6 ± 0.8 at 2 days, 3.6 ± 1.5 at 7 days, and 20.4 ± 11.4 at 14 days).
Figure 5.
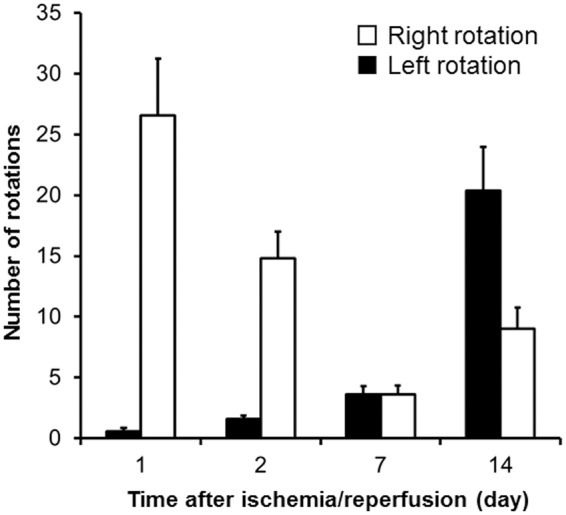
Results of circling behavior test in ischemia/reperfusion rat. Values are expressed as means ± SE of 7 (1 day), 5 (7 days), or 10 (2 and 14 days) animals.
SE: standard error.
HE staining
Typical images of HE staining are shown in Figure 6. At 2 days after reperfusion, significant cell loss in the ipsilateral striatum could not be detected compared with that in the contralateral striatum. On the other hand, at 7 and 14 days, the number of living neural cells progressively decreased over time.
Figure 6.
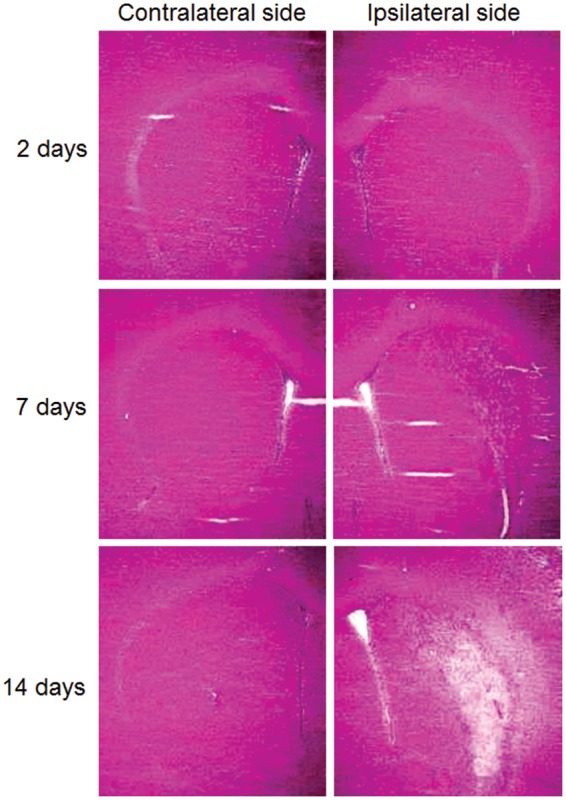
Typical images of HE staining in rat striatum. Images are for 2 days, 7 days, and 14 days after ischemia/reperfusion.
HE: hematoxylin–eosin.
Discussion
A previous study showed that brief focal ischemia induces delayed ischemic hyper intensity on T1-weighted MRI in the striatum of patients.11 These patients showed delayed cognitive impairments. Striatal T1-weighted hyper intensity was also observed in mild focal ischemia rat, but the mechanism remains unclear. One possibility raised recently is that striatal neurodegeneration is related to late cognitive impairment.22 Brief MCAO induced cell-type specific injury in the striatum and the selective loss of medium-sized spinous projection neurons in the striatum have been reported.23 Another report using MCAO model rat noted that neural degeneration induced by ischemia was observed in the ischemic core (striatum) at early time points after injury.24 To clarify the progressive neurodegeneration mechanism after mild ischemia, we focused on the striatal dopaminergic system following mild MCAO, because striatal dopaminergic neuro-transmission and behavioral changes are closely related in ischemic dysfunction. Thus, in the present study, the availabilities of DAT and DA D2 receptor were measured using PET, and the amphetamine-induced circling behavior was also observed in the mild ischemia rat.
Changes in the availability of DAT after mild ischemia
One of the major findings in the present study is the prominent increase of DAT binding with [11C]PE2I observed at 2 days after the ischemia/reperfusion. This increase of [11C]PE2I binding to DAT might be due to a compensatory mechanism against enhanced DA release after ischemia/reperfusion. A previous study demonstrated that the extracellular DA level was increased in the striatum of cerebral ischemic rat brain.25 This previous report supports our finding of a transient increase of [11C]PE2I binding to DAT at 2 days after the ischemia/reperfusion. A change of DAT binding was also found in gerbil brain that had been exposed to 10-min ischemia.26 In that report, DAT binding measured by an in vitro autoradiographic method using [125I]β-CIT was significantly increased in the striatum at 1 and 4 days after ischemia. This finding suggests that DAT had increased in the ischemic gerbil brain, as the in vitro binding assay using radioactive ligand mainly reflected the number of target binding sites (Bmax value). Thus, an increase of in vivo [11C]PE2I binding in the present study might be mainly due to a change of DAT Bmax, although further in vitro saturation experiments will be needed to confirm this.
Some studies have reported that the extracellular DA level dynamically increased during ischemia and that DA release directly contributed to cell death in the ischemic brain.7,27 Another human study found that the pathology caused by an anterior cerebral infarct is closely linked to a change in the DA system in the subcortical area.28 Consideration of these findings suggests the possibility that DAT in the subcortical area works in a compensatory manner to prevent cell damage at 2 days after the ischemia/reperfusion. Thus, we consider that successful detection of an increase in the availability of DAT under in vivo condition is important for detecting dopaminergic dysfunction in the early stage of ischemia/reperfusion injury.
Changes in the availability of DA D2 receptor after mild ischemia
A recent study showed the time course of progression of DA D2 receptor binding after cerebral ischemia.16 In this report, ischemia produced by 2 h of MCAO in rat, and [11C]raclopride binding was measured at 1, 3, 7, 14, 21, and 28 days after the reperfusion. The BPND ratio of the ipsilateral to contralateral striatum did not change until 3 days after the reperfusion. Our data showed no significant change of [11C]raclopride binding in the ipsilateral striatum at 2 days after ischemia/reperfusion. It is well known that [11C]raclopride binding reflects the change of DA concentration in the brain.29 Our data indicated that the level of endogenous DA did not differ between the contralateral and ipsilateral striatum at 2 days after ischemia/reperfusion. It might be considered that the DA level temporarily increased in an early phase after the ischemia/reperfusion, and then rapidly returned to a normal level at 2 days after ischemia/reperfusion. On the other hand, [11C]raclopride binding to DA D2 receptor was decreased at the late phase after ischemia/reperfusion (from 7 days) in the present study. Martin et al.16 reported a significant decrease of the BPND ratio at 7 days after MCAO (2-h occlusion). Although the occlusion period in our study was much shorter than theirs, reduction of [11C]raclopride binding started at the same time point after the reperfusion. Moreover, the BPND ratio of [11C]raclopride decreased by 51% in our study and by approximately 80% theirs at 14 days after ischemia/reperfusion. These findings could be explained by the difference of the MCAO periods. Thus, changes in the availability of DA D2 receptor induced by ischemia might depend on the occlusion time or extent of neuron damage in the late phase after ischemia/reperfusion.
Fujioka et al.11 reported delayed neurodegeneration after mild ischemia. In their study, the middle cerebral artery (MCA) of the rat was occluded for 15 min, and HE staining to detect neurons was carried out from 4 h to 16 weeks. The number of neurons in MCA occluded rat did not change compared with those in the sham-treated rat until 3 days. In contrast, neurologic deficit was observed from 7 days after MCAO. We obtained similar results in the present study as shown in Figure 6. Therefore, we considered that the decrease of in vivo DA D2 receptor binding at 7 and 14 days after ischemia/reperfusion was due to neuronal cell loss in the striatum. This is an important observation because delayed dopaminergic dysfunction including DA neurologic deficit induced by mild ischemic injury could be dynamically visualized by [11C]raclopride PET in the in vivo condition.
Circling behavior induced by amphetamine
The administration of amphetamine has been known to induce DA release in native striatum. The spontaneous circling behavior induced by amphetamine challenge is often used to evaluate dopaminergic abnormalities. Using this method, Parkinson’s disease model rats infused with 6-OHDA, which produces severe loss of presynaptic DA neurons, have been examined to evaluate dopaminergic abnormalities in many previous studies.
In the present study, amphetamine challenge induced significant circling behavior to the contralateral direction at 2 days after ischemia/reperfusion, and circling behavior to the ipsilateral direction was observed at 14 days after the ischemia/reperfusion in mild MCA-ischemic rats. Circling behavior to the contralateral direction induced by amphetamine at 2 days after ischemia/reperfusion indicates dopaminergic activation in the ipsilateral striatum. At this time point, increased [11C]PE2I binding suggests hyper active presynaptic dopaminergic neural transmission in the ipsilateral striatum. At 1 day, the maximum number of circling behavior events to the contralateral direction was observed. These findings suggest that mild focal ischemia causes the dopaminergic hyper activation in the ipsilateral striatum at an early phase after the ischemia/reperfusion. It seemed that the DAT inhibiting effect of amphetamine was enhanced in the ipsilateral striatum, because increase of [11C]PE2I binding to DAT was observed.
Amphetamine-induced circling behavior to the contralateral direction almost disappeared at 7 days after ischemia/reperfusion. At 7 days, the result of [11C]PE2I binding indicated that the availability of DAT had returned to the normal level (the BPND of [11C]PE2I was almost the same value in the contralateral striatum). On the other hand, circling behavior to the ipsilateral direction was observed at 14 days after ischemia/reperfusion. At this time point, circling behavior to the ipsilateral direction induced by amphetamine administration indicates activation of dopaminergic neural transmission in the contralateral striatum. [11C]Raclopride binding was clearly decreased in the ipsilateral striatum. This reduction was due to the loss of neurons, which was indicated by HE staining as shown in Figure 6. Thus, in this case, amphetamine-induced activation of postsynaptic dopaminergic function in the contralateral striatum was higher than that in the ipsilateral striatum, resulting in rats rotating to the ipsilateral direction. The changes in DA D2 receptor binding might reflect the amount of dopaminergic neuron at the late phase after focal mild ischemia.
Conclusion
The present study showed direct changes of the availability of DAT and DA D2 receptor measured with in vivo PET after mild ischemia in rat brain. Circling behavior induced by amphetamine challenge was also measured, revealing a relation between abnormal behavior and the availability of DAT and DA D2 receptor. The data of in vivo DAT and DA D2 receptor imaging could be offer useful information for predicting dopaminergic dysfunction after mild ischemia.
Acknowledgments
The authors thank R Saika for technical support in the histological study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Substantial contributions to conception and design: SM, JH, and KA. Acquisition of data, or analysis and interpretation of data: SM, MI, NI, HY, HI, HS, and KM. Drafting the article or revising it critically for important intellectual content: SM, TW, ES, JH, and KA. Final approval of the version to be published: SM, ES, JH, and KA.
References
- 1.Sakamoto A, Ohnishi ST, Ohnishi T, et al. Relationship between free radical production and lipid peroxidation during ischemia-reperfusion injury in the rat brain. Brain Res 1991; 554: 186–192. [DOI] [PubMed] [Google Scholar]
- 2.Siesjö BK. Pathophysiology and treatment of focal cerebral ischemia. J Neurosurg 1992; 77: 169–184. [DOI] [PubMed] [Google Scholar]
- 3.Tuttolomondo A, Di Raimondo D, di Sciacca R, et al. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des 2008; 14: 3574–3589. [DOI] [PubMed] [Google Scholar]
- 4.Baker AJ, Zornow MH, Scheller MS, et al. Changes in extracellular concentrations of glutamate, aspartate, glycine, dopamine, serotonin, and dopamine metabolites after transient global ischemia in the rabbit brain. J Neurochem 1991; 57: 1370–1379. [DOI] [PubMed] [Google Scholar]
- 5.Hillered L, Hallström A, Segersvärd S, et al. Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. J Cereb Blood Flow Metab 1989; 9: 607–616. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto N, Matsumoto T, Mabe H, et al. Dopamine has inhibitory and accelerating effects on ischemia-induced neuronal cell damage in the rat striatum. Brain Res Bull 1994; 33: 281–288. [DOI] [PubMed] [Google Scholar]
- 7.Richards DA, Obrenovitch TP, Johonson-Mora A, et al. Effect of global ischaemia, under simulated penumbral conditions, on brain monoamine neurochemistry and subsequent neurological and histological deficits. J Neurochem 1993; 61: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill MJ, Hicks CA, Ward MA, et al. Dopamine D2 receptor agonists protect against ischaemia-induced hippocampal neurodegeneration in global cerebral ischaemia. Eur J Pharmacol 1998; 352: 37–46. [DOI] [PubMed] [Google Scholar]
- 9.Fujioka M, Taoka T, Hiramatsu KI, et al. Delayed ischemic hyperintensity on T1-weighted MRI in the caudoputamen and cerebral cortex of humans after spectacular shrinking deficit. Stroke 1999; 30: 1038–1042. [DOI] [PubMed] [Google Scholar]
- 10.Fujioka M, Taoka T, Matsuo Y, et al. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke 1999; 30: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka M, Taoka T, Matsuo Y, et al. Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol 2003; 54: 732–747. [DOI] [PubMed] [Google Scholar]
- 12.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 1970; 24: 485–493. [DOI] [PubMed] [Google Scholar]
- 13.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl 1971; 367: 95–122. [DOI] [PubMed] [Google Scholar]
- 14.Hudson JL, van Horne CG, Strömberg I, et al. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 1993; 626: 167–174. [DOI] [PubMed] [Google Scholar]
- 15.Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 1996; 50: 275–331. [DOI] [PubMed] [Google Scholar]
- 16.Martín A, Gómez-Vallejo V, San Sebastián E, et al. In vivo imaging of dopaminergic neurotransmission after transient focal ischemia in rats. J Cereb Blood Flow Metab 2013; 33: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe K, Kashiwagi Y, Tokumura M, et al. Discrepancy between cell injury and benzodiazepine receptor binding after transient middle cerebral artery occlusion in rats. Synapse 2004; 53: 234–239. [DOI] [PubMed] [Google Scholar]
- 18.Nagasawa H, Araki T, Kogure K. Alteration of dopamine D1 receptor in the strionigral system of the postischemic rat brain. Neurosci Lett 1992; 134: 271–274. [DOI] [PubMed] [Google Scholar]
- 19.Hosoi R, Kashiwagi Y, Tokumura M, et al. Sensitive reduction in 14C-acetate uptake in a short-term ischemic rat brain. J Stroke Cerebrovasc Dis 2007; 16: 77–81. [DOI] [PubMed] [Google Scholar]
- 20.Halldin C, Erixon-Lindroth N, Pauli S, et al. [11C]PE2I: a highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging 2003; 30: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 21.Langer O, Någren K, Dolle F, et al. Precursor synthesis and radiolabelling of the dopamine D2 receptor ligand [11C]raclopride from [11C]methyl triflate. J Labelled Comp Radiopharm 1999; 42: 1183–1193. [Google Scholar]
- 22.O'Callaghan C, Bertoux M, Hornberger M. Beyond and below the cortex: the contribution of striatal dysfunction to cognition and behaviour in neurodegeneration. J Neurol Neurosurg Psychiatry 2014; 85: 371–378. [DOI] [PubMed] [Google Scholar]
- 23.Nagahiro S, Uno M, Sato K, et al. Pathophysiology and treatment of cerebral ischemia. J Med Invest 1998; 45: 57–70. [PubMed] [Google Scholar]
- 24.Butler TL, Kassed CA, Sanberg PR, et al. Neurodegeneration in the rat hippocampus and striatum after middle cerebral artery occlusion. Brain Res 2002; 929: 252–260. [DOI] [PubMed] [Google Scholar]
- 25.Kondoh T, Lee SH, Low WC. Alterations in striatal dopamine release and reuptake under conditions of mild, moderate, and severe cerebral ischemia. Neurosurgery 1995; 37: 948–954. [DOI] [PubMed] [Google Scholar]
- 26.Fujita M, Shimada S, Tohyama M, et al. Visualization of ischemic insult in caudate putamen with beta-CIT. J Nucl Med 1996; 37: 1214–1218. [PubMed] [Google Scholar]
- 27.Lieb K, Andrae J, Reisert I, et al. Neurotoxicity of dopamine and protective effects of the NMDA receptor antagonist AP-5 differ between male and female dopaminergic neurons. Exp Neurol 1995; 134: 222–229. [DOI] [PubMed] [Google Scholar]
- 28.Yang CP, Huang WS, Shih HT, et al. Diminution of basal ganglia dopaminergic function may play an important role in the generation of akinetic mutism in a patient with anterior cerebral arterial infarct. Clin Neurol Neurosurg 2007; 109: 602–606. [DOI] [PubMed] [Google Scholar]
- 29.Koepp MJ, Gunn RN, Lawrence AD, et al. Evidence for striatal dopamine release during a video game. Nature 1998; 393: 266–268. [DOI] [PubMed] [Google Scholar]