Abstract
The delivery of most therapeutic agents is rendered ineffective for the treatment of brain diseases due to the presence of the blood–brain barrier (BBB). The goal of this study was to investigate the effect of pre-infusion focused ultrasound (FUS) and microbubbles on the distribution of direct brain infusion in vivo. A single-element FUS transducer was used in all sonications, which were carried out immediately prior to direct infusion procedures. Mice received direct infusion of either Gadolinium-labeled albumin (Gd-albumin, 74 kDa) or adeno-associated virus (AAV, ∼4 MDa). The volumes of Gd-albumin at 30 min were deemed comparable (P = 0.334) between the direct infusion (DI)-only group and the FUS + DI group. At 120 min, the FUS + DI group showed significantly higher contrast-enhanced volume (9.76 ± 0.74 mm3) than the DI-only group (7.14 ± 0.34 mm3). For mice infused with AAV, the total volume of transduction was estimated as GFP-positive regions and FUS + DI group demonstrated significantly higher (P = 0.017) transduction efficiency in vivo. In conclusion, enhanced bio-distribution of directly infused agents was observed when the targeted region was pre-conditioned with FUS and microbubbles. Focused ultrasound has the potential, as an adjuvant technique, to significantly enhance direct brain infusion and achieve the desired therapeutic outcomes.
Keywords: Ultrasound, gene therapy, microscopy, MRI, neurosurgery
Introduction
The delivery of therapeutic agents for the treatment of Central Nervous System (CNS) diseases is greatly limited due to the existence of the blood-brain barrier (BBB). The BBB is primarily composed of tightly connected endothelial cells, surrounding astrocytes and pericytes.1 While preventing toxins from entering the brain parenchyma, the BBB precludes the delivery of most therapeutic agents of 400 Da or larger.2 To circumvent this limitation, direct infusion can be applied, where agents are administered to the targeted brain region through an inserted cannula at a constant rate. However, the relatively high intracranial pressure often leads to inefficient infusion as well as significant back flow.3 One technique that has been widely implemented to augment the delivery efficiency of macromolecules is convection-enhanced delivery (CED), where a pressure gradient is maintained during the infusion procedure by adjusting the infusion rate.4 Nevertheless, this increased pressure leads to tissue deformation, which in turn causes back flow around the inserted cannula.5
Albeit these limitations, CED is a useful approach for the treatment of CNS diseases (such as glioblastoma) and implementing CNS gene therapeutic strategies. In the case of glioblastoma, traditional chemotherapy is hindered by the presence of the Blood–Tumor Barrier (BTB). Convection-enhanced delivery of chemotherapeutic agents can be applied following tumor resection to achieve more effective therapeutic outcomes.6 In CNS gene therapy, viral vectors carrying therapeutic genes are infused to the desired brain targets for long-term expression. For instance, adeno-associated virus (AAV) carrying human aromatic L-amino acid decarboxylase (hAADC) was used in a phase I clinical trial for the treatment of Parkinson’s disease (PD).7 Lentivirus encoding hAADC has also been studied in an open-label, phase I/II trial for its long-term safety and tolerability.8 More recently, direct infusion of AAV2-neurturin was carried out in a double-blinded, randomized clinical trial for the treatment of PD.9 Similar to glioblastoma treatment, the failure of these trials may be attributed to insufficient distribution of the infused viral vectors. Therefore, techniques that can further enhance macromolecule delivery efficiency to the brain are highly desirable.
Focused ultrasound (FUS) is an emerging technology, where acoustic waves converge at a focal spot by applying appropriate delays.10 As the acoustic wave propagates through biological tissues, it interacts with the medium via various mechanisms. If the acoustic energy is applied in the form of short pulses, radiation forces could displace tissue from its original location.11 When xenograft tumors were exposed with pulsed FUS, enhanced delivery of monoclonal antibodies was reported in rodents.12 In brain applications, however, the presence of the skull has to be taken into consideration. Different from soft tissues, the impedance mismatch between bone and soft tissue limits the propagation of acoustic energy. Ultrasound contrast agents (microbubbles) have been used to intensify the mechanical effects of FUS in the brain. Driven at the frequency of the ultrasound pulses, microbubbles oscillate inside the brain capillaries resulting in BBB opening via either stable or inertial cavitation.13,14 Numerous studies have demonstrated the BBB can be transiently opened using FUS in the presence of microbubbles.15,16 Moreover, this technique has been proposed to facilitate the delivery of systemically administered therapeutic agents for the treatment of brain tumors.17,18
The intrinsic non-invasive property, relatively deep penetration, and portability have made FUS an ideal candidate to condition biological tissues. Lewis et al. developed an ultrasound cannula assembly, where direct infusion and ultrasound exposure can simultaneously be carried out.19 Their results indicated that the application of ultrasound during infusion improved volumetric distribution of Evans blue in the rat brain by a 2.24–3.25 fold. Olbricht et al. applied time-reversal acoustics to achieve effective ultrasound focusing in rat brains while simultaneously infusing tracer molecules.20 They concluded that exposing the rat brain to FUS significantly increased the penetration of infused tracers, while mixing tracer molecules with microbubbles further enhanced the effect of ultrasound.
In this study, FUS in the presence of systemically administered microbubbles (via the tail vein) was applied through the intact skull prior to direct infusion. Our hypothesis is that the permeabilized brain (resulted from FUS and microbubble conditioning) would lead to greater molecular diffusion from the subsequent direct infusion. The proposed paradigm here is different from previously reported intravenous (IV) administration of microbubbles and molecules of interest (as in the case for FUS mediated BBB opening) or direct infusion of a mixture of microbubbles and molecules of interest. By carrying out the FUS and microbubble treatment first, we ensure that the shielding effects from inadvertently introduced air (such as that from cannula insertion) were eliminated. In addition, infusion to the FUS-conditioned brain tissue directly minimizes the systemic toxicity or the potential limitation of circulation half-life of the infused molecules. Specifically in this study, two macromolecules were used: gadolinium-labeled albumin (74 kDa) and AAV vectors (∼4 MDa). The former was used so that the progression of its distribution can be monitored with magnetic resonance imaging (MRI), while AAV was selected due to its clinical relevance. Our hypothesis is that pre-infusion FUS and microbubble treatment could facilitate the distribution of the subsequently infused macromolecules.
Materials and methods
Macromolecules
Two types of macromolecules were used to study the infusion efficiency. To monitor the progression of molecular diffusion, Gadolinium-labeled albumin (Gd-albumin) (BioPAL, Inc., Worcester, MA, USA) was used (total n = 10). Gd-albumin is a MR contrast agent, which has a molecular weight of 74 kDa. For a different group of mice (total n = 6), adeno-associated virus serotype 1 (AAV1) carrying green fluorescent protein gene (AAV1-CAG-GFP, Vector Biolabs, Philadelphia, PA, USA) was infused instead, which has a molecular weight of approximately 4 MDa. The titer of the AAV vectors was 5.3 × 1013 GC/ml.
Animal preparation
All animal experimental procedures were approved by the Columbia University Institutional Animal Care and Use Committee, Columbia University's Research and Compliance Administration System and performed in accordance with the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines. All efforts were made to minimize animal suffering and to reduce the number of subjects used. Wild-type male mice of 15 weeks old (Strain: C57BL/6, Harlan, Indianapolis, IN, USA) were used in this study. Each mouse was anesthetized with a mixture of oxygen and 1–3% isoflurane (SurgiVet, Smiths Medical PM, Inc., WI, USA) and placed prone with its head immobilized by a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The hair on the mouse head was removed using an electrical trimmer and depilatory cream to minimize impedance mismatch for ultrasound exposures. For each type of macromolecule under investigation, mice were randomly divided into two groups: direct infusion (DI) (n = 3–5) only and FUS and microbubble pre-treatment followed by direct infusion (FUS + DI) (n = 3–5). Mice that did not receive FUS and microbubble treatment were also shaved and left under anesthesia for 15 min (the approximate time needed for the sonication) before the beginning of the direct infusion procedures.
Microbubbles and FUS sonications
Each mouse undergoing pre-infusion FUS, a bolus (approximately 25 µl) of lipid-shelled polydisperse microbubbles was injected via the tail vein immediately prior to sonication. The microbubbles were produced in-house, following a previously published protocol.21 Briefly, 1, 2-distearoyl-sn-glycero-3-phosphocholine and polyethylene glycol 2000 were mixed at a 9:1 ratio. Ten milligrams of the mixture was dissolved in a 10 ml solution consisting of filtered phosphate-buffered saline/glycerol (10% volume)/propyleneglycol(10% volume) using a sonicator (Model 1510, Branson Ultrasonics, Danbury, CT, USA). Two milliliters of the solution were transferred to a 10 ml vial, which was filled with decafluorobutane (C4F10) gas. Each vial was activated using a mechanical shaker (VialMix®, N. Billerica, MA) for 45 s at room temperature. The diameter of the activated microbubbles ranged from 0.6 to 18 µm (mean diameter 1.048 µm) as measured by a Multisizer (Beckman Coulter, Fullerton, CA, USA).
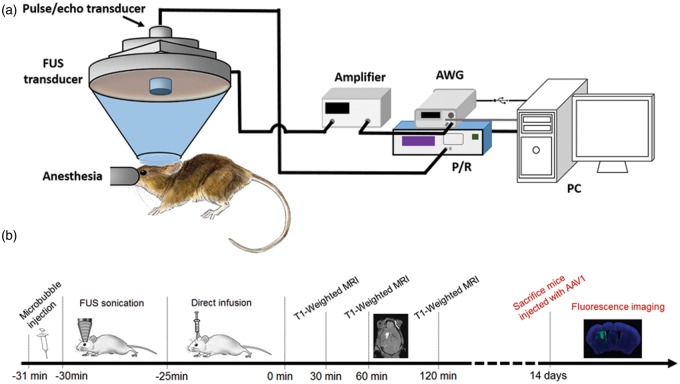
The FUS system was composed of a single element focused ultrasound transducer (Imasonic, Voray-sur-l’Ognon, France) and a pulse-echo transducer (Olympus NDT, Waltham, MA, USA). The FUS transducer, which operates at a center frequency of 1.5 MHz, has a focal length of 60 mm and aperture of 60 mm. The −6 dB focus of the FUS transducer was measured with a hydrophone (Onda, Sunnyvale, CA, USA) in degassed water to be 7.5 × 1 × 1 mm3. The pulse-echo transducer (radius = 11.2 mm, focal length = 60 mm, center frequency = 10 MHz) was confocally mounted through the center opening of the FUS transducer as shown in Figure 1(a). The FUS transducer was driven by a computer-controlled function generator (Agilent, PaloAlto, CA, USA) and a 50-dB power amplifier (ENI, Rochester, NY, USA). A 3-D positioner (Velmex, Inc., Bloomfield, NY, USA) was used to manipulate the transducer system in all experiments.
Figure 1.
Experimental setup/timeline. (a) Experimental setup for the FUS transducer and controlling system. (b) Experimental timeline for two different macromolecule delivery: mice infused with Gd-albumin underwent MRI scans at different time points (black), while mice infused with AAV were survived for 14 days (red).
The left caudate-putamen (CPu) was the selected target in this study, which was achieved via a metallic grid scanning method described in greater detail elsewhere.22 Briefly, a metallic grid was placed on top of the lambda suture and ultrasound C-scans were performed to locate the center of the grid. The coordinates used for CPu targeting were anterior–posterior (AP) −6 mm, medial-lateral (ML) −2.2 mm, while the FUS focus was placed 3 mm below the skull. A single bolus of (approximately 8 × 108) microbubbles was injected via the tail vein immediately prior to the sonication. The acoustic parameters used in this study include: the free field (i.e. in water) peak-rarefactional pressure (PRP) of 0.72 MPa; pulse repetition frequency of 5 Hz; pulse length of 10 ms; and a total duration of 300 s.
Direct infusion
Direct infusion was performed using a stereotaxic frame (David KOPF Instruments, Tujunga, CA, USA). After exposing the skull, the CPu was located using the following coordinates centered at the bregma: AP +0.5 mm, ML −2.2 mm and dorsal-ventral (DV) +3.0 mm. A 34 G needle (Hamilton, Reno, NV, USA) was used to infuse either of the two macromolecules. The total volume infused was 0.8 µl and the infusion rate was maintained at approximately 0.003 µl/s. Upon completion, the needle was left in place for an additional 5 min. Animals (four in total) with mistargeted infusion or noticeable back flow were excluded from the study.
MRI and analysis
For mice infused with Gd-albumin, MRI scans were performed during the same session at 30 min, 60 min, and 120 min post injection with a 9.4 T MRI system (Bruker Medical, Boston, MA, USA). A representative experimental timeline is illustrated in Figure 1(b). All acquisitions were carried out with a T1-weighted 2D FLASH sequence (TR/TE 230/3.3 ms, flip angle 70°, 20 slices, 10 averages, bandwidth 100 kHz, matrix size 256 × 256, resolution 100 µm × 100 µm × 400 µm). The distribution of Gd-albumin was quantified as the total contrast-enhanced volume using a custom-written program (MATLAB R2013b, The MathWorks, Natick, MA, USA).23 For each MR image, the background (brain regions outside CPu) intensity was first calculated. Both sides of CPu regions were manually outlined and contrast-enhanced pixels were segmented if the intensity of a pixel is 2.5 standard deviation (SD) above the background intensity. The segmented pixels from the contralateral side were subtracted from the direct infusion side to exclude the effect of blood vessels and ventricles.
Histology
Mice receiving Gd-albumin infusion were sacrificed and transcardially perfused with 30 ml phosphate-buffered saline and 60 ml 4% paraformaldehyde 3 h post-surgery. Hematoxylin and Eosin (H&E) staining was performed on paraffin-embedded brain sections with a slice thickness of 6 microm. Stained sections were examined using a bright field microscope for potential tissue damage (Olympus, Shinjuku, Tokyo).
Fluorescence imaging and quantification
In the case of mice infused with AAV1-CAG-GFP vectors, no MRI scan was performed. Instead, mice were allowed to survive for two weeks followed by transcardial perfusion. The brains were fixed in 4% paraformaldehyde for 24 h and then cryo-protected in 30% sucrose solution for three days. Frozen brain sections were collected at 30 µm in thickness with an inter-sectional distance of 210 µm. The sections were mounted with ProLong Gold with DAPI (Life Technologies, Grand Island, NY, USA), which labels cell nuclei. Successful AAV transduction was signified by GFP expression, which was imaged using a confocal microscope (Nikon Instruments Inc., Melville, NY, USA). Whole brain fluorescence images were obtained by stitching tile images taken with a 10× objective lens. The volume of transduction was quantified using a custom-written program (MATLAB R2013b, The MathWorks, Natick, MA, USA). The CPu region was manually outlined based on the DAPI channel and pixels above the threshold within the CPu region were counted. The inter-sectional volumes were obtained by linear extrapolation and the final volume was calculated by summing the pixels over a range of 10 sections (2.4 mm in thickness). All quantitative analyses were performed by an independent expert, who was blinded to the group assignment of the mice.
Statistics
All statistical analyses were performed using GraphPad software (GraphPad Software, Inc., La Jolla, CA, USA). Unpaired two-tailed Student’s t-test was used to compare the volume of macromolecule delivery, and P < 0.05 was considered statistically significant.
Results
Gd-albumin infusion
The distribution of infused Gd-albumin was revealed as contrast enhancement in the T1-weighted MRI scans, as shown in Figure 2. For the DI-only mice, the enhancement was relatively confined where the greater intensity signifies higher Gd-albumin concentration. In contrast, the whole CPu region was highlighted in the FUS + DI treated brain, where the contrast agent seemed to diffuse to and accumulate along the corpus callosum. Given the same volume of infused Gd-albumin, lower intensity and larger volume of enhancement indicated a more profound diffusion.
Figure 2.
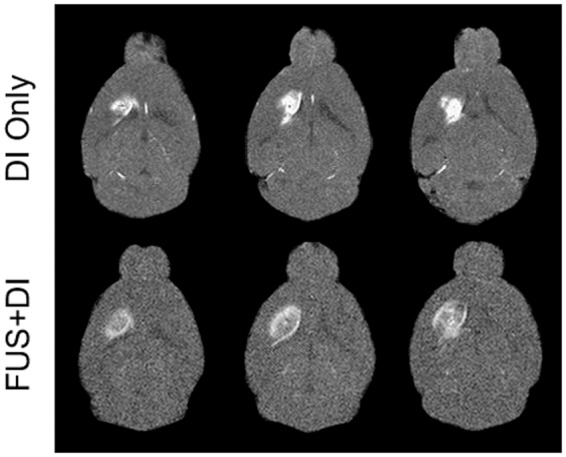
Series of T1-weighted MRI scans revealed the diffusion of infused Gd-albumin in the CPu regions (bright pixels) at 120 min.
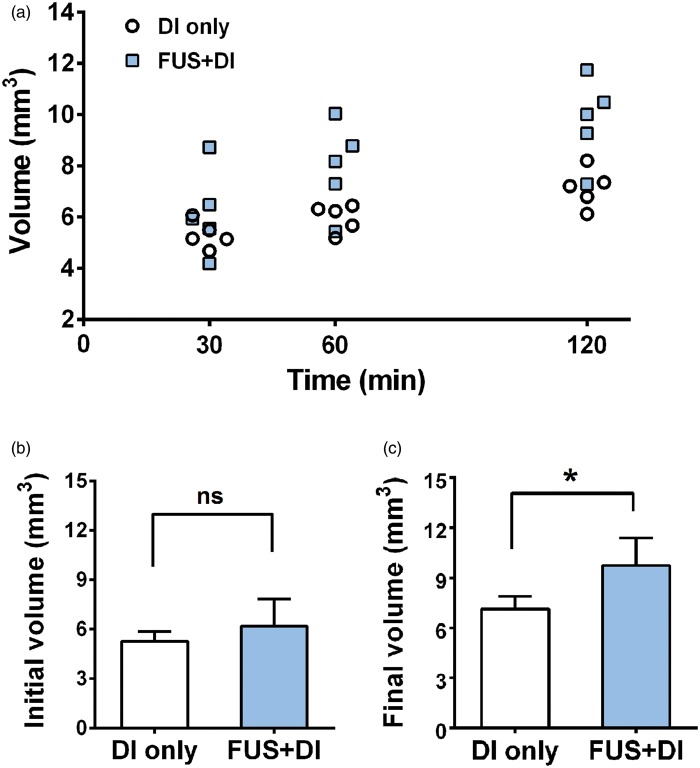
Figure 3 shows the volume of Gd-albumin enhancement at three distinct time points. In both groups, the total volume increased from 30 min to 120 min. At 30 min, no significant difference was observed between the DI-only group and FUS + DI group. Nonetheless, the volumes of enhancement became significantly different between the two groups as time progressed, specifically at 60 min (P = 0.025) and 120 min (P = 0.012). Quantitatively, the volume at 120 min of the FUS + DI group was 9.76 ± 0.74 mm3 compared to 7.14 ± 0.34 mm3 of the DI-only group. The volume increase from 30 min to 120 min was significantly higher (P = 0.008) for the FUS + DI group (3.58 ± 0.40 mm3) than the DI-only group (1.82 ± 0.16 mm3). The mean percent volume increased for the DI-only and FUS + DI groups were 34.42% and 61.75%, respectively.
Figure 3.
Gd-albumin diffusion monitoring up to 120 min. (a) The volumes of Gd-albumin diffusion at 30 min, 60 min, and 120 min. (b) The volumes of Gd-albumin at 30 min showed no significant difference between the DI-only and FUS + DI group (P = 0.334). (c) The volume at 120 min of the FUS + DI group was significant higher than the DI-only group (P = 0.012).
AAV infusion
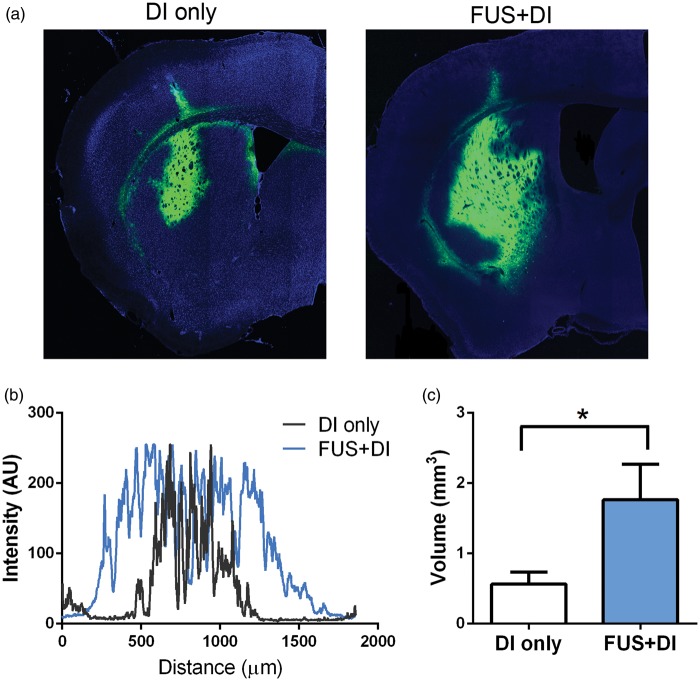
Different from Gd-albumin, AAV vectors are much larger (on the order of 4 MDa) and harder to diffuse once injected into the brain.5 Figure 4(a) illustrates the transduced brain tissue (expressing GFP) in a DI-only mouse and a FUS + DI treated mouse. From a qualitative perspective, the post-FUS infusion led to more profound AAV1 transduction in the CPu region. The maximum width of the transduction was represented in Figure 4(b). The width of the transduction from the FUS + DI mouse spanned approximately 1.5 mm, while the mouse treated with DI only had a maximum width of transduction around 0.75 mm. Quantitatively, the total transduced volume, quantified as pixels above the threshold in the green channel, was on average three times higher in the FUS + DI group (P = 0.017), shown in Figure 4(c).
Figure 4.
Distribution of directly infused AAV1-GFP vectors. (a) Fluorescence imaging revealed the distribution of AAV transduced cells. (b) The maximum width of distribution of cells expressing GFP genes. (c) The total volume of AAV transduction in the FUS + DI group was significantly higher than the DI-only group (P = 0.017).
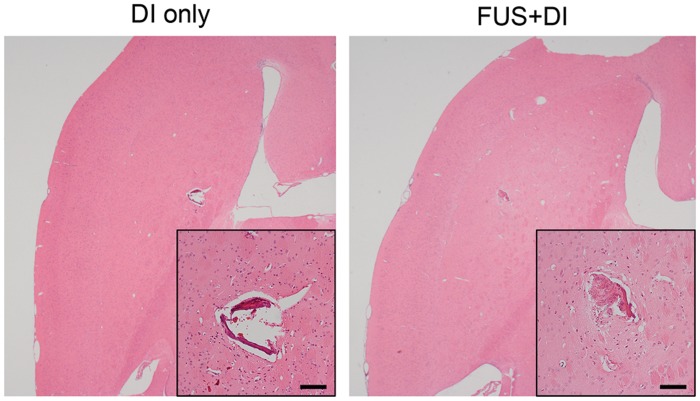
Finally, the safety of pre-infusion FUS was examined with H&E stained sections. As shown in Figure 5, the trajectory of the infusion needle is visible in the H&E images in both DI-only and FUS + DI groups. Nevertheless, no additional brain tissue damage due to the application of ultrasound was observed in all subjects studied here.
Figure 5.
H&E stained brain sections revealed no additional tissue damage was caused by the application of ultrasound compared to direct infusion only (scale bar = 100 µm).
Discussion
The existence of the BBB presents great challenges to systemic drug delivery for the treatment of CNS diseases.24 Direct brain infusion, as one promising solution, has been frequently used in pre-clinical and clinical settings for the delivery of macromolecules to the brain.4,6 Even though techniques such as CED have been proposed to enhance the delivery efficiency, inadequate diffusion remains as one of the most significant limitations. This study investigated the potential of pre-infusion FUS and microbubbles as an adjuvant therapy to facilitate direct brain infusion.
Different from previously published reports, we sought to evaluate the diffusion efficiency of macromolecules in the mouse brains pre-conditioned with FUS and microbubbles. In studies carried out by Lewis et al., ultrasound was applied simultaneously with the infusions, where the acoustic radiation force was directly applied on the dye (Evans Blue).19 These forces might have contributed to larger diffusion volume by “pushing” the molecules through the extracellular spaces. Interestingly, they did not observe enhanced Evans Blue diffusion when microbubbles were mixed with the infusate. The presence of microbubbles, in their study, might have interfered with the acoustic field by acting as ultrasound reflectors. Therefore, one of the main incentives for applying FUS before the infusion procedure was to avoid such acoustic shielding effects.
In this study, we demonstrated the pre-infusion FUS and microbubble treatment can be used to enhance direct brain infusion of two macromolecules of clinical relevant sizes. Using Gd-albumin, a MR contrast agent and a macromolecule, the progression of the diffusion volume was monitored up to 2 h post-infusion. No significant difference in the Gd-albumin volumes was observed between the DI-only and FUS + DI groups at 30 min (Figure 3(b)). This difference became more apparent at the 60 min (P = 0.025) and 120 min (P = 0.012) time-points. Comparatively, mice that underwent infusion only exhibited an increase of 1.82 ± 0.16 mm3 during the 90-min monitoring period, which was significantly lower (P = 0.008) than the FUS + DI group. These observations are likely due to the slow diffusion rate and it is highly possible that the difference could further increase as time progresses.
Another clinically relevant molecule investigated in this study was AAV, which has been used in several clinical trials for the treatment of Parkinson’s disease.9,25,26 Viral vectors carrying different therapeutic genes were infused directly to the substantia nigra and putamen for long-term expression. It was speculated that the non-significant therapeutic outcomes might be caused in part by insufficient AAV transduction. We demonstrated here that the AAV transduction volume can be significantly enhanced (P = 0.017) with pre-infusion FUS and microbubble conditioning. As shown in Figure 4, GFP expression was far more prominent in the FUS + DI-treated mice and the special distribution of the expression was almost twice of that from the DI-only group. This result indicated that FUS and microbubble treatment might have allowed the AAV to easily navigate through the brain tissue. By analyzing H&E sections of the FUS-treated brains, it was concluded that no damage was induced by the pre-infusion FUS and microbubble treatment. Therefore, pre-conditioning brain targets with FUS in conjunction with microbubbles has the potential to serve as an adjuvant therapy to enhance the delivery efficiency of direct brain infusion or CED.
The increased macromolecule distributions reported in this study may be explained by two possible mechanisms. The intravenously infused microbubbles had an important role in transcranial ultrasound by accentuating the acoustic radiation force in the brain and serving as cavitation nuclei. Several studies investigated the effects of acoustic radiation force on the permeability of soft tissues (i.e. creating intercellular space) in the absence of microbubbles. For instance, greater monoclonal antibody penetration has been reported in xenograft tumors with pre-exposure to pulsed ultrasound;12 pulsed-FUS has also been shown to create temporary gaps between muscle fibers and increase extravasation of administered fluorophores in vivo.11 These transient, intercellular gaps created by the shear forces were shown to be reversible and can last up to 72 h post-sonication in their study. Therefore, one possible mechanism for our findings is the temporary increased intercellular permeability, which allowed greater diffusion of the infused macromolecules. Alternatively, under the parameters used in this study, the BBB in the targeted brain tissue is temporarily opened with FUS and microbubbles.27 Our group has previously showed that the vascular permeability was significantly enhanced in the brains treated with FUS and microbubbles.28 Therefore, the subsequently infused macromolecules could enter the bloodstream through the opened BBB and re-distribute with a larger diffusion area in the proximity of the infusion site.
One major limitation of this study was the size of the mouse brain, which has a total volume of approximately 0.5 cm.3,29 As a result, the total volume of macromolecules that can be infused was limited to 0.8 µl and CED was not performed. Nevertheless, it is reasonable to expect enhanced diffusion in brains pre-conditioned with FUS and microbubbles when CED-based infusion is applied. In addition, further studies will aim at elucidating the exact mechanism for this observed diffusion enhancement. Particularly, the importance of re-distribution through the opened BBB can be further examined by intravenous mannitol administration and analyzing the presence of infused molecules in the blood samples. Finally, the amount of microbubbles injected (∼25 µl) account for approximately 1% of the total blood volume in adult mice, which might lead to slight increase of blood pressure in mice that underwent FUS treatments. The increased blood pressure has the potential to facilitate the transport from the bloodstream to the brain parenchyma; however, it should have minimal effects on the diffusion of directly infused macromolecules in the extracellular space.
Conclusions
Direct brain infusion has been widely applied as the most common technique for brain drug/gene delivery. The diffusion of the infused macromolecules is often limited and the occurrence of significant backflow often leads to insufficient delivery. Focused ultrasound in combination with microbubbles was used in this study as an adjuvant therapy to enhance the diffusion efficiency of direct infusion. Our results demonstrate significant increase in Gd-albumin and AAV transduction volumes in brains pre-conditioned with FUS in conjunction with microbubbles. The findings reported herein support the application of pre-injection FUS when drug/gene delivery to large brain volumes is desired.
Acknowledgement
The authors would like to thank Oluyemi Olomulade, BS, Gesthimani Samiotaki, PhD, and Li Liu, MD for their technical support and insightful discussion.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by NIH R01EB009041, NIH R01AG038961 and the Kinetics Foundation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
SW and EK designed the experiments. SW, MEK, CF, TS and CA performed experiments and collected data. SW, MEK, CF analyzed the data. SW and EK wrote the manuscript.
References
- 1.Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013; 36: 437–449. [DOI] [PubMed] [Google Scholar]
- 2.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2005; 2: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potts MB, Silvestrini MT, Lim DA. Devices for cell transplantation into the central nervous system: Design considerations and emerging technologies. Surg Neurol Int 2013; 4: S22–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 1994; 91: 2076–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen MY, Lonser RR, Morrison PF, et al. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 1999; 90: 315–320. [DOI] [PubMed] [Google Scholar]
- 6.White E, Bienemann A, Taylor H, et al. A phase I trial of carboplatin administered by convection-enhanced delivery to patients with recurrent/progressive glioblastoma multiforme. Contemp Clin Trials 2012; 33: 320–331. [DOI] [PubMed] [Google Scholar]
- 7.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008; 70: 1980–1983. [DOI] [PubMed] [Google Scholar]
- 8.Palfi S, Gurruchaga JM, Ralph GS, et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet 2014; 383: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 9.Warren Olanow C, Bartus RT, Baumann TL, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol 2015; 78: 248–257. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien WD. Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol 2007; 93: 212–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock HA, Smith LH, Cuesta J, et al. Investigations into pulsed high-intensity focused ultrasound-enhanced delivery: preliminary evidence for a novel mechanism. Ultrasound Med Biol 2009; 35: 1722–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Shin IS, Hancock H, et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J Control Release 2012; 162: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynynen K, McDannold N, Sheikov NA, et al. Local and reversible blood-brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005; 24: 12–20. [DOI] [PubMed] [Google Scholar]
- 14.Tung Y-S, Vlachos F, Feshitan JA, et al. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J Acoust Soc Am 2011; 130: 3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquet F, Teichert T, Wu S-Y, et al. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS One 2014; 9: e84310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol 2006; 51: 793–807. [DOI] [PubMed] [Google Scholar]
- 17.Couture O, Foley J, Kassell NF, et al. Review of ultrasound mediated drug delivery for cancer treatment: updates from pre-clinical studies. Transl Cancer Res 2014; 3: 494–511. [Google Scholar]
- 18.Liu H-L, Fan C-H, Ting C-Y, et al. Combining microbubbles and ultrasound for drug delivery to brain tumors: current progress and overview. Theranostics 2014; 4: 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis GK, Schulz ZR, Pannullo SC, et al. Ultrasound-assisted convection-enhanced delivery to the brain in vivo with a novel transducer cannula assembly. J Neurosurg 2012; 117: 1128–1140. [DOI] [PubMed] [Google Scholar]
- 20.Olbricht W, Sistla M, Ghandi G, et al. Time-reversal acoustics and ultrasound-assisted convection-enhanced drug delivery to the brain. J Acoust Soc Am 2013; 134: 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Samiotaki G, Olumolade O, et al. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol 2014; 40: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JJ, Wang S, Brown TR, et al. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound. Ultrason Imag 2008; 30: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samiotaki G, Vlachos F, Tung Y-S, et al. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med 2012; 67: 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pardridge W. Targeted delivery of protein and gene medicines through the blood-brain barrier. Clin Pharmacol Ther 2015; 97: 347–361. [DOI] [PubMed] [Google Scholar]
- 25.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 2011; 10: 309–319. [DOI] [PubMed] [Google Scholar]
- 26.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 2007; 369: 2097–105. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Olumolade OO, Sun T, et al. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther 2015; 22: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlachos F, Tung Y-S, Konofagou E. Permeability dependence study of the focused ultrasound-induced blood-brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magn Reson Med 2011; 66: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent TJ, Thiessen JD, Kurjewicz LM, et al. Longitudinal brain size measurements in APP/PS1 transgenic mice. Magn Reson Insight 2010; 4: 19–26. [Google Scholar]