Abstract
Intracranial hypertension is a common final pathway in many acute neurological conditions. However, the cerebral haemodynamic response to acute intracranial hypertension is poorly understood. We assessed cerebral haemodynamics (arterial blood pressure, intracranial pressure, laser Doppler flowmetry, basilar artery Doppler flow velocity, and vascular wall tension) in 27 basilar artery-dependent rabbits during experimental (artificial CSF infusion) intracranial hypertension. From baseline (∼9 mmHg; SE 1.5) to moderate intracranial pressure (∼41 mmHg; SE 2.2), mean flow velocity remained unchanged (47 to 45 cm/s; p = 0.38), arterial blood pressure increased (88.8 to 94.2 mmHg; p < 0.01), whereas laser Doppler flowmetry and wall tension decreased (laser Doppler flowmetry 100 to 39.1% p < 0.001; wall tension 19.3 to 9.8 mmHg, p < 0.001). From moderate to high intracranial pressure (∼75 mmHg; SE 3.7), both mean flow velocity and laser Doppler flowmetry decreased (45 to 31.3 cm/s p < 0.001, laser Doppler flowmetry 39.1 to 13.3%, p < 0.001), arterial blood pressure increased still further (94.2 to 114.5 mmHg; p < 0.001), while wall tension was unchanged (9.7 to 9.6 mmHg; p = 0.35).This animal model of acute intracranial hypertension demonstrated two intracranial pressure-dependent cerebroprotective mechanisms: with moderate increases in intracranial pressure, wall tension decreased, and arterial blood pressure increased, while with severe increases in intracranial pressure, an arterial blood pressure increase predominated. Clinical monitoring of such phenomena could help individualise the management of neurocritical patients.
Keywords: Intracranial hypertension, cerebral haemodynamics, pressure reactivity, vascular function, cerebral blood flow, intracranial pressure
Introduction
Acutely raised intracranial pressure (ICP) or intracranial hypertension is a final pathophysiologic feature common to many neurological conditions including intracranial haemorrhage, traumatic brain injury (TBI) or acute shunt blockage in hydrocephalus.1 Such an intracranial hypertension is universally detrimental; it impairs cerebral blood flow (CBF),2 electrical activity3 and metabolism.4,5 Given these pathophysiological sequelae of intracranial hypertension, it is not surprising that active control of ICP plays a pivotal role in the management of neurological disorders including TBI.6
The haemodynamic response to raised ICP is, however, incompletely described. Sudden increases in ICP decrease cerebral perfusion pressure (CPP; calculated as ABP – ICP) and thus can limit perfusion to the brain.7 However, depending on the nature of the pathology, this decrease in CPP can be somewhat compensated for by an active cerebral vasodilation such that CBF can be maintained despite decreases in CPP – a process known as cerebral autoregulation.8–11 Recent studies indicate that the CBF response to a sustained decrease in CPP caused by a decrease in arterial blood pressure (ABP) or an increase in ICP has key physiological differences.12–14 For example, Bragin et al.12,13 measured the cerebral haemodynamic response to sustained decrements of CPP (30 minutes at each level of ICP) and found when the decrease in CPP was brought about by an increased ICP, the lower limit of autoregulation was around 30 mm Hg, whereas with decreases in CPP brought about by a decreased ABP, the lower limit of autoregulation was around 50 mm Hg.
In addition, the haemodynamic response to increased ICP is complicated by the presence of the ‘Cushing response’ – a triad of arterial hypertension, changes in heart rate (HR), and breathing abnormalities.15 This particular combination of physiologic responses suggests involvement of vital centres within the brainstem and indeed rendering certain areas of the brainstem hypoxic (the nucleus tractus solitarii, for example) results in significant increases in ABP and changes in HR.16 The physiologic relevance of the Cushing response to cerebral haemodynamics, however, remains unclear.
In this study, we revisited the question of haemodynamic responses to graded and extreme elevations in ICP. Utilizing recent advances in mathematical modelling of cerebral haemodynamics, combined with complementary global and local assessments of cerebral perfusion, we aimed to describe cerebral haemodynamics in a rabbit model of intracranial hypertension. Specifically, we hypothesised that with moderate increases in ICP, cerebral vascular wall tension (WT) would decrease in attempt to maintain brain perfusion and that only with severe increases in ICP, would ABP increase.
Methods
Animals and ethics
These experiments were carried out in 1995 and 1996, in accordance with the standards provided by the UK Animals Scientific Procedures act of 1986 under a UK home office license and with permission from the institutional animal care and use committee at Cambridge University.
Physiological recordings from lumbar cerebrospinal fluid (CSF) infusions in 28 NZ white rabbits (7 female, 21 male; weight 2.7–3.7 kg) were retrospectively analysed.17 General anaesthesia was induced using intravenous alphaxalone/alphadalone (Saffan, Pitman-Moore, Uxbridge, UK, 0.2 mL/kg) and maintained using 1–3% halothane in 3:1 nitrous oxide/oxygen. Rabbits had their common carotid artery ligated so that blood flow to the brain was basilar artery dependent. This made a Doppler assessment of global blood flow possible through the insonation of only the basilar artery.
Two weeks after common carotid artery ligation, anaesthesia was repeated as above. The jugular vein was cannulated and a tracheostomy was performed. ABP was measured through in the dorsal aorta after catheter insertion in the femoral artery (GaelTec, Dunvegan, UK). Cerebral blood velocity was measured using an 8 MHz Doppler ultrasound probe (PCDop 842, SciMed, Bristol, UK) positioned over the basilar artery (accessed through a 7 mm burr-hole at the bregma as described in Nelson et al).18 ICP was monitored using an intraparenchymal microsensor (Codman and Shurtleff, Raynham, MA, USA) inserted through a right frontal burr-hole, and a laser Doppler flowmetry (LDF) probe was placed epidurally through a further right frontal burr-hole (Moor Instruments, Axbridge, Devon, UK).
A lumbar laminectomy was performed to allow the positioning of a permanent catheter (sealed with cyanoacrylate after introduction) into the lumbar subarachnoid space. This facilitated the controlled infusion of artificial cerebrospinal fluid during the experimental protocol. Rectal temperature was monitored and the animals were placed on a padded warming blanket. The rabbits were given an intravenous infusion of pancuronium (pavulon, 0.5 mg/kg/h) and ventilation was controlled according to arterial PCO2 via periodic arterial blood gas analyses. The animals were supported in the Sphinx position using a purpose-built head frame with three-point skull fixation. All experiments were performed in an animal laboratory at the same time of day. Experiments are reported in compliance with the ARRIVE guidelines for the reporting of animal experiments.19
Protocol
Following 20 min of rest, a 5 min of baseline data were recorded. Thereafter, ICP was artificially increased by infusion of Hartmanns solution into the lumbar CSF space. Infusion rates were initially 0.1 mL/min. ICP increased to reach a plateau of around 40 mm Hg after approximately 10 min, thereafter the infusion rate was increased to rates between 0.2 and 2 ml/min to produce severe intracranial hypertension. ICP was increased until the point where diastolic flow velocity approached zero, which corresponded to an ICP of between 60 and 100 mm Hg (mean 75 mm Hg) at the termination of the experiment. Rabbits were euthanised with thiopental at the conclusion of the test.
Data acquisition and analysis
ABP, ICP, basilar artery flow velocity (Fv) and expired CO2 signals were recorded digitally at a sampling frequency of 50 Hz. Data were subsequently analysed off-line using custom-built, commercially available data analysis software (ICM+®; http://www.neurosurg.cam.ac.uk/icmplus). HR was calculated by estimating the fundamental frequency of the ABP spectra in a moving 10 s window. Diastolic and systolic values of blood pressure and flow velocity were calculated by estimating the minimum and maximum values over a 0.5 s window, shifted each 0.25 s. Mean values were then calculated over 10-s period. LDF was expressed as a.u. minus the ‘biological zero’ as measured in the asystolic rabbit at the conclusion of the experiment. Mean values of ABP, ICP, LDF, and Fv were calculated every 10 s. Fundamental amplitudes of ICP, ABP, and Fv were calculated using spectral analysis within the range of the rabbits HR (100–400 bpm). End-tidal CO2 values were corrected using the arterial PCO2 concentration from the blood gas analysis to account for any end-tidal to arterial PCO2 gradients.
Cerebral vascular resistance (CVR) was calculated as the CPP divided by the basilar artery flow velocity. Cerebral arterial compliance (Ca) was calculated as the derived cerebral arterial volume pulse amplitude divided by the ABP pulse amplitude as described previously.20
Critical closing pressure (CrCP) was calculated using both measured (HR, ABP, HR, CPP) and modelled variables (Ca – cerebral arterial compliance and CVRi – cerebrovascular resistance) as described in Varsos et al.21 and shown below
where CrCP – critical closing pressure, ABP – arterial blood pressure, CPP – cerebral perfusion pressure, CVRi – cerebrovascular resistance (invasive, i.e. calculated as CPP divided by mean blood flow velocity), Ca – cerebral arterial compliance, HR – heart rate. Arterial WT was calculated as CrCP – ICP.
Although data across the whole recording period are presented, three points of interest were identified to allow statistical comparison of intracranial haemodynamics: baseline before any infusion (‘baseline ICP’, during a plateau ICP immediately prior to the final steep rise in ICP (‘moderate ICP’), and in the period after the final rise in ICP (‘high ICP’).
The relationships between the changes in CPP and cerebral perfusion were analysed by first referencing each rabbits CPP to their CPP at baseline. To compare cerebral perfusion as measured by LDF and Doppler ultrasound, both LDF and Fv were expressed as a percentage of their baseline value. For each rabbit, the average LDF (% of baseline) and Fv (% of baseline) was calculated in 5 mm Hg wide intervals of decreasing CPP or increasing ICP using Microsoft excel (Microsoft Corp., Redmond, USA) and ICM + tools (Monash University, Melbourne, Australia).
Statistical analyses
Data are presented as mean and associated standard error of the mean. Pairwise comparisons of haemodynamic parameters between the three different ICP conditions (‘low’, ‘moderate’, and ‘high’) were performed using Student's T-Test. The alpha value was set at 0.05 and no corrections were made for multiple comparisons. Of the available 28 experiments, one was excluded from analysis due to an inability to increase ICP above 30 mm Hg with CSF infusion, leaving 27 rabbits for the final analysis. As the nature of this study was retrospective and the analytical techniques applied were not available at the time the experiments were designed, no formal sample size calculations were carried out, rather, all available valid data were used. Statistical analysis was performed using IBM SPSS version 21.0 software (IBM Corp., Armonk, USA).
Results
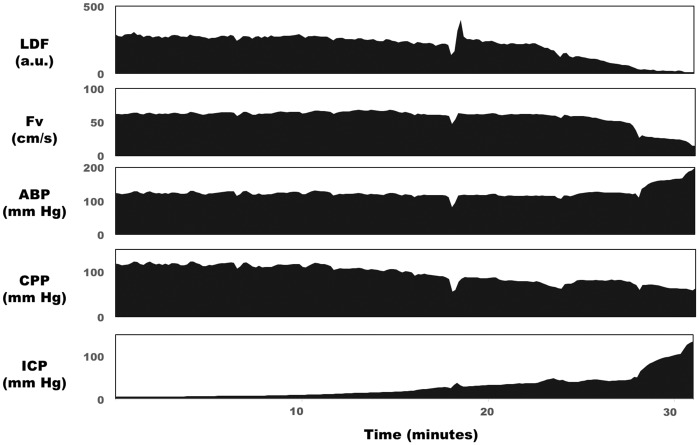
No adverse effects of the common carotid artery ligation were apparent. The mean length of the protocol was 28.2 min (SD 8.4). Monitored variables during a typical CSF infusion experiment are depicted in Figure 1. ICP increased due to incremental increases in the rate of CSF infusion. In this example, cortical perfusion, as indicated by LDF, showed a monotonic decrease with increasing ICP, whereas global perfusion, as assessed by basilar artery flow velocity, was well maintained until ICP reached values greater than 40 mm Hg. Mean ABP in this case remained stable until ICP increased above 30 mm Hg, at which point it increased and eventually reached values of 200 mm Hg.
Figure 1.
Measured haemodynamic response to lumbar CSF infusion in the rabbit. ICP was increased over the period of 30 min from baseline levels (10 mm Hg) to high levels (>100 mm Hg). Cortical LDF (top panel) decreased with this increasing ICP, whereas Fv in the basilar artery remained stable until ICP was above 50 mm Hg. Mean arterial blood pressure increased when ICP increased above 30 mm Hg as part of the Cushing reflex.
LDF: laser Doppler flowmetry; Fv: flow velocity in the basilar artery; ABP: arterial blood pressure; CPP: cerebral perfusion pressure; ICP: intracranial pressure.
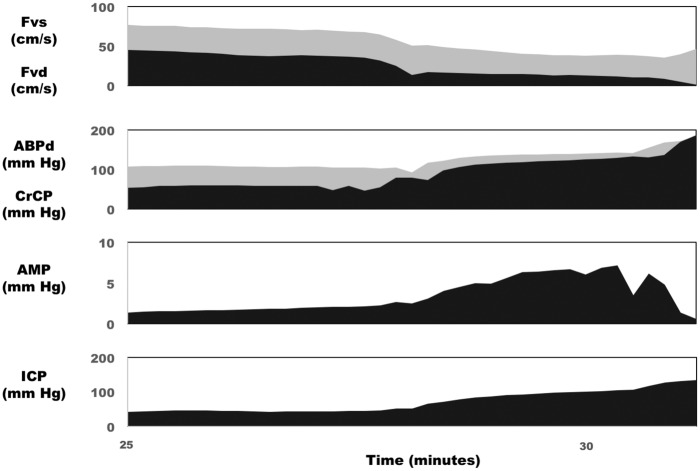
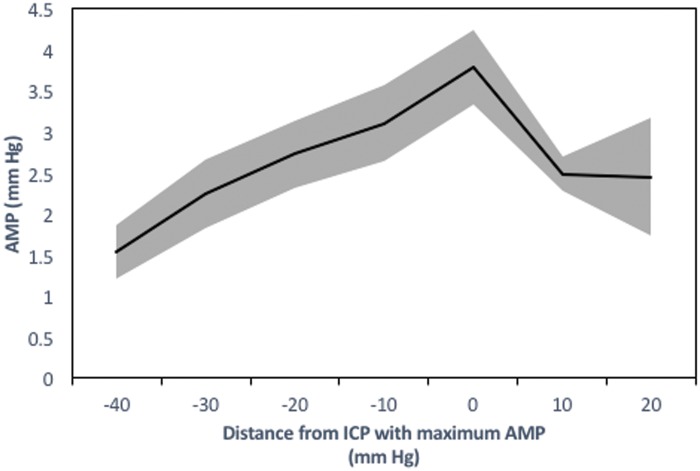
Figure 2 shows selected derived haemodynamic parameters in the same rabbit during the final segment of the experiment (ICP increasing above 40 mm Hg). As a result of this raised ICP, calculated CrCP increased and eventually reached values equivalent to the diastolic ABP. At this point, the diastolic closing margin (diastolic ABP minus CrCP) is equal to zero or negative indicating that the forces acting to close the microvasculature are greater than the forces keeping the vessels open. This time point coincided with a cessation of diastolic flow velocity in this case. The pulse amplitude of ICP (AMP) increased with increasing mean ICP with the exception of at very high levels of mean ICP at which point AMP appeared to decrease with increasing ICP in this rabbit. This phenomenon was observed in 11 of the 27 rabbits. The relationship between AMP and mean ICP in these 11 rabbits is presented in Figure 3, which depicts an ‘upper breakpoint’ at a mean ICP=78 mm Hg (range 55 to 118 mm Hg equivalent to CPP=39 mm Hg; range 28 to 63 mm Hg). The diastolic closing margin at this ‘upper break point’ was close to zero (9.5 mm Hg) and diastolic flow velocity was low (12.6 cm/s or 30.5% of baseline).
Figure 2.
Derived haemodynamic parameters in response to lumbar CSF infusion in the rabbit. In the same rabbit as Figure 1, progressive increases in ICP caused an increase in CrCP, and ABP diastolic, as well as a corresponding decrease in the difference between them – the diastolic closing margin (second panel). At very high ICP, CrCP approached ABPd, which was associated with a drop of diastolic flow velocity to zero (top panel). AMP of ICP increased along with mean ICP until very high ICP, at which point AMP began to decrease (third panel).
CrCP: critical closing pressure; ABPd: diastolic arterial blood pressure; Fvs: systolic flow velocity in the basilar artery; Fvd: diastolic flow velocity in the basilar artery; AMP: pulse amplitude of ICP; ICP: intracranial pressure.
Figure 3.
Upper breakpoint of intracranial mean pressure–amplitude relationship (n=27; mean ± SE).
In 11 rabbits, an upper breakpoint of the intracranial mean pressure–amplitude relationship was observed whereby further increases in mean ICP resulted in decreases in the pulse amplitude of ICP. This upper breakpoint was associated with low diastolic closing margins (9.5 mm Hg) and diastolic flow velocities (12.6 cm/s – 30.5% of baseline).
ICP: intracranial pressure; AMP: pulse amplitude of intracranial pressure.
Table 1 outlines all recorded and derived parameters at baseline, moderate, and high ICP. The Cushing response is reflected by the marked increase in ABP, and decrease in HR between moderate and high ICP. However, increases in ABP were also observed between baseline and moderate increases in ICP. The Cushing vasopressor response occurred in 25 out of 27 experiments and was initiated at an average ICP of 26.2 mm Hg (range 10 to 45 mm Hg). As a result of the Cushing vasopressor response, despite an increase in ICP by 35 mm Hg between the ‘moderate’ and ‘high’ ICP conditions, CPP only decreased by 14 mm Hg (Table 1).
Table 1.
Haemodynamic parameters during experimental intracranial hypertension in rabbits (n=27).
| Baseline |
Moderate ICP |
High ICP |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Significance compared to baseline | Mean | SE | Significance compared to baseline | Significance compared to moderate ICP | |
| ABP mean (mm Hg) | 88.84 | 3.29 | 94.15 | 3.21 | * | 114.54 | 3.67 | ** | ** |
| ABP systolic (mm Hg) | 116.07 | 3.90 | 121.81 | 3.77 | 143.78 | 4.32 | ** | ** | |
| ABP diastolic (mm Hg) | 72.77 | 3.25 | 77.84 | 3.04 | * | 96.27 | 3.36 | ** | ** |
| HR (BPM) | 273.27 | 6.96 | 262.73 | 7.64 | * | 227.21 | 11.13 | ** | ** |
| ICP mean (mm Hg) | 9.00 | 1.51 | 40.75 | 2.17 | ** | 75.05 | 3.65 | ** | ** |
| AMP (mm Hg) | 0.40 | 0.10 | 1.81 | 0.17 | ** | 3.59 | 0.33 | ** | ** |
| CPP (mm Hg) | 79.71 | 3.46 | 53.08 | 2.77 | ** | 39.64 | 2.16 | ** | ** |
| PET CO2 (mm Hg) | 32.29 | 0.56 | 31.74 | 0.69 | 31.49 | 0.77 | |||
| Fv mean (cm/s) | 47.04 | 2.99 | 44.99 | 2.82 | 31.33 | 2.45 | ** | ** | |
| Fv diastolic (cm/s) | 34.17 | 2.76 | 30.49 | 2.58 | * | 15.59 | 1.67 | ** | ** |
| Fv systolic (cms/s) | 66.93 | 3.64 | 65.51 | 3.33 | 50.97 | 2.66 | ** | ** | |
| Ca (%) | 100.00 | 0.00 | 130.81 | 14.87 | * | 166.90 | 20.46 | * | * |
| CVR (%) | 100.00 | 0.00 | 72.70 | 3.05 | ** | 89.41 | 8.67 | * | |
| WT (mm Hg) | 19.27 | 1.84 | 9.75 | 1.27 | ** | 9.58 | 1.36 | ** | |
| CrCP (mm Hg) | 28.42 | 2.05 | 50.61 | 2.12 | ** | 85.09 | 3.99 | ** | ** |
| LDF (%) | 100.00 | 0.00 | 39.10 | 29.82 | ** | 13.27 | 5.19 | ** | ** |
| Cortical CVR (%) | 100.00 | 0.00 | 157.10 | 85.01 | * | 231.35 | 37.56 | * | * |
*p = <0.05, **p < 0.001.
ABP: arterial blood pressure; HR: heart rate; ICP: intracranial pressure; AMP: pulse amplitude of ICP; CPP: cerebral perfusion pressure; Fv: flow velocity in the basilar artery; Ca: cerebral arterial compliance; CVR: cerebral vascular resistance; WT: wall tension; CrCP: critical closing pressure; LDF: laser Doppler flowmetry; PET CO2: pressure of end-tidal CO2
Mean flow velocity of the basilar artery was not significantly changed from baseline to moderate ICP, whereas cortical LDF and diastolic flow velocity decreased. Cerebral vascular resistance and WT decreased from baseline to moderate ICP, and arterial compliance increased from low, to moderate to high ICP conditions. Consistent with the increased ICP, CrCP increased throughout the infusion. End-tidal CO2 did not change significantly during the experiment.
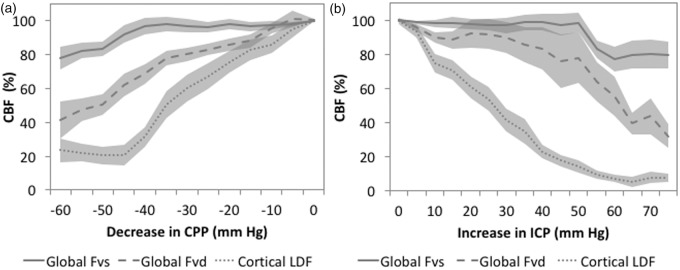
The comparison of cortical LDF and basilar artery Fv in response to raised ICP is shown in Figure 4(a). This demonstrates that cortical LDF was most sensitive to increases in ICP followed by diastolic velocity and then systolic velocity. When expressed as a function of CPP, a similar response was observed; LDF was most sensitive, systolic velocity least sensitive, and diastolic velocity moderately sensitive to decreases in CPP (Figure 4(b)).
Figure 4.
Cerebral blood flow during lumbar artificial CSF infusion in rabbits (n=27 mean ± SE). (a) Global (as assessed by basilar artery Fv) and cortical (as assessed by LDF) CBF are normalised to baseline CBF and then expressed relative to changes in ICP produced by the lumbar CSF infusion. LDF appears most sensitive to increases in ICP, followed by diastolic flow velocity and then systolic flow velocity. When CBF is plotted against changes in CPP. (b) Again, cortical LDF appears most sensitive to decreases in CPP, followed by diastolic flow velocity and then systolic flow velocity.
CBF: cerebral blood flow; Fv: flow velocity; Fvs: systolic flow velocity in the basilar artery; Fvd: diastolic flow velocity in basilar artery; LDF: laser Doppler flowmetry
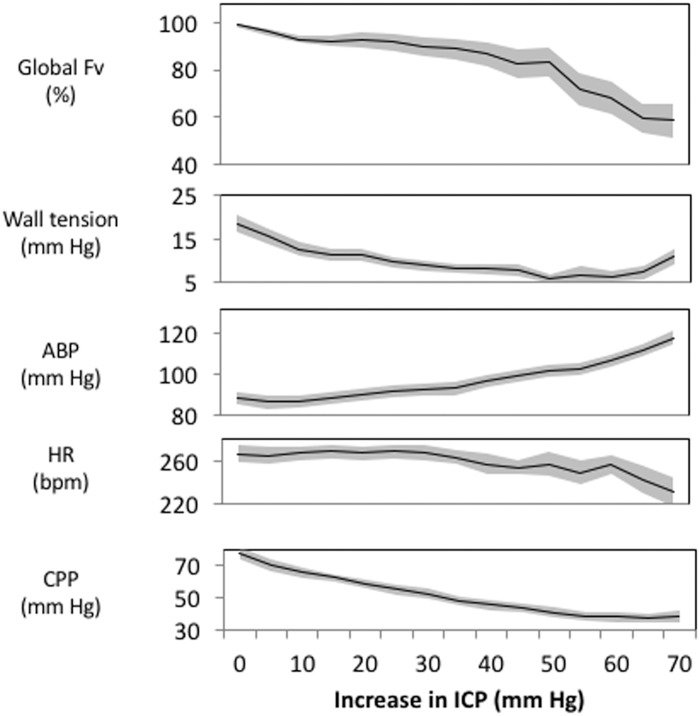
The inter-relationships between changes in ICP, CBF, vascular WT, ABP, CPP, and HR in all 27 rabbits are shown in Figure 5. CBF appears well maintained with up to 60 mm Hg increases in ICP. This maintenance of CBF was contributed by both decreases in WT and increases in ABP. WT progressively decreased with increasing ICP particularly during early increases in ICP. At the highest levels of ICP, WT started to increase.
Figure 5.
Cerebral haemodynamic response to increasing ICP (n=27 mean ± SE). In this study, group averaged global Fv was well maintained until an ICP 55 mm Hg above baseline (top panel). This maintained Fv was achieved by both a decrease in wall tension and an increase in ABP (panels 2 and 3). HR did not show major changes until relatively large increases in ICP (60–75 mm Hg above baseline; panel 4). As a consequence of the vasopressor response, CPP was relatively buffered especially during relatively large increases in ICP. From baseline ICP to ICP increases of 15–20 mm Hg, CPP decreased from a mean of 78 to 58 mm Hg, whereas from ICP increases of 25 to 30 mm Hg to ICP increases of 35–40 mm Hg CPP only decreased from 54 to 45 mm Hg.
Discussion
We detail three novel insights into the cerebrovascular response to raised ICP. First, two intrinsic and mechanistically distinct compensatory adaptations to raised ICP were observed: (1) with moderate increases in ICP, CBF is maintained by decreases in vascular WT; and (2) at higher levels of ICP, cerebral perfusion is protected by the Cushing vasopressor response that maintains CPP and keeps cerebral vessels open. Second, regional differences in the control of cerebral perfusion seem to exist with cortical blood flow being more sensitive to an increase in ICP than global blood flow. Third, at dramatically high ICP (and low CPP) decreases in the amplitude of ICP pulsations can be observed. Herein, we discuss the possible physiologic underpinnings and clinical implications of these observed phenomena.
ICP-dependent cerebroprotection: Vascular WT and the Cushing response
During increases in ICP, global CBF was maintained until ICP had increased by 55 mm Hg above baseline ICP (Figures 4 and 5). CBF was maintained by two mechanisms: a decrease in vascular WT (at more moderate levels of ICP) and increases in ABP. During moderate increases in ICP, estimated WT progressively decreased (Figure 5, Table 1). This decrease in WT represents a decrease in cerebral vasomotor tone and thus an autoregulatory response that acts to maintain CBF in the face of a decreased CPP. Similar decreases in calculated WT have also been described in response to hypercapnia, arterial hypotension, and plateau waves of ICP.21,22 During the increase in ICP, estimated WT decreased and reached a plateau during ICP increases between 30 and 60 mm Hg. (Figure 5, Table 1). This plateau of vascular WT (at around 6–8 mm Hg) may represent a condition of maximum vasodilation biologically fixed by the rigid collagen fibres in the tunica adventitia.23
Further increases in ICP caused a vigorous Cushing response as reflected by reductions in HR and increases in ABP (mean ABP increasing by > 30 mm Hg in 13 out of 27 rabbits). However, in contrast to our hypothesis, this hypertensive response was also present with moderate ICP increases (in 12 out of 27 individuals the hypertensive response was observed with increases of ICP of less than 25 mm Hg). This relatively ‘early’ increase in ICP may indicate that the ICP-induced increases in ABP play a protective role in maintaining perfusion rather than merely signifying irreversible neurologic damage. In support of this finding, a similar increase ABP in humans has been observed at even moderate ICP during lumbar CSF infusion studies.24,25
Although it has been over 100 years since Harvey Cushing described this response,15 whether it is beneficial or detrimental is still debated.26 The current data indicate two possible mechanisms by which the vasopressor response may be beneficial. First by increasing the ABP, the driving pressure for CBF – the CPP – is maintained. Second, by increasing the intravascular pressure, the vessels are more resistant to the collapsing force of ICP (i.e. the difference between the ABP and the CrCP is maintained).
The Cushing vasopressor response keeps vessels open by maintaining ABP above the CrCP. CrCP is the ABP at which cerebral blood flow approaches zero27 and reflects closing forces acting on the vessel, namely ICP and WT.23 Therefore, CrCP is an important parameter during any conditions where WT or ICP may change (such as syncope,28 the Valsalva maneuver,29 or plateau waves of ICP22). The difference between the ‘opening’ force ABP and ‘closing’ force of CrCP is the force that keeps vessels open and has been denoted the ‘closing margin’.30 If this closing margin is reduced to zero, vessels will collapse resulting in cessation of flow. By increasing ABP, the Cushing vasopressor response acts to maintain this closing margin.
In cases where, despite the Cushing response, closing margin during diastole is equal to zero, we observed a complete cessation of cerebral diastolic flow (Figure 2). The abolition of the diastolic closing margin seems to have two consequences observable with haemodynamic monitoring; vessels collapse during diastole giving zero flow velocity and the amplitude of the ICP pressure pulse (itself determined by the pulsatile addition of blood volume) decreases (Figure 2).
Thus, it seems that the brain has two intrinsic mechanisms to protect itself from hypoperfusion during intracranial hypertension: decreases of cerebral arterial WT at low to moderate ICP and a Cushing vasopressor response that predominates at high ICP. A practical application of these findings is that these intrinsic mechanisms are the main therapeutic measures that can be used to adjust CBF: decreasing vascular WT (using pharmacological or ventilator intervention) or increasing ABP (using vasopressors). However, inspection of Figure 5 indicates that the efficacy of each intervention may depend on the prevailing cerebrovascular factors that are only appreciated through multimodality monitoring; if WT is already close to zero, further attempts at vasodilation are unlikely to improve perfusion as the vessels may already be maximally dilated. In these situations, increasing ABP may be the only viable way to increase CBF.
Differential control of cortical compared to global blood flow
Using in-vivo global and cortical CBF measurement during dynamic changes in ICP, we demonstrated that cortical blood flow decreased almost linearly with increasing ICP, whereas global flow (basilar artery flow velocity) was well maintained until a very high ICP (∼60 mm Hg). A similar phenomenon is observed when CBF was expressed as a function of CPP. In addition, global CVRi decreased with increasing ICP, while cortical vascular resistance increased consistent with differential autoregulatory control of cortical and global blood flow (Table 1).
A potential explanation for the vulnerability of cortical compared to global CBF could lie in a differential vascular reactivity of cortical compared with non-cortical brain. Such a discrepancy could theoretically result in a ‘vascular steal’ phenomena whereby, during periods of reduced CPP, vessels within actively autoregulating subcortical areas dilate, while those in the cortex do not change calibre. This topographic difference in resistance would result in CBF being diverted away from the cortex. Such a vascular steal phenomena has been previously described in the context of cerebrovascular CO2 reactivity.31 In support of a topographic differences in autoregulatory capacity, Horsfield et al.32 demonstrated an intrinsic difference in the autoregulatory efficiency of the grey matter compared to white matter of healthy humans. They demonstrated that in response to a transient decrease in CPP induced by leg cuff release, CBF recovered more quickly in the white matter compared to the grey matter.
Further support for a differential autoregulatory capacity of the cortical compared to non-cortical areas comes from clinical monitoring studies. In a group of severe TBI patients with monitored ABP, ICP, LDF, and middle cerebral artery Fv, Zweifel et al.33 assessed the correlation between slow changes in CPP and slow changes in CBF. In this situation, there was a higher correlation between CPP and LDF than between CPP and middle cerebral artery Fv. Moreover, investigations combining near infrared spectroscopy derived cortical oxygenation and transcranial Doppler derived middle cerebral artery Fv also indicate a cortical vulnerability. Budohoski et al.34 found that the correlation between ABP and Fv seems to be lower than the correlation between ABP and NIRS oxygenation, while Kuriyama35 demonstrated that prior to the onset of syncope, cortical oxygenation decreases, while middle cerebral artery Fv remains constant. From a teleological point of view, such cortical sensitivity to high ICP could be a mechanism for diverting blood flow to areas of the brain most crucial for survival; the brainstem cardiorespiratory nuclei.
The mechanisms underlying this differential response of cortical and global perfusion to increased ICP are unclear. It could reflect intrinsic differences in vascular biology such as increased vascularity in the white matter compared with the grey matter or reflect regional differences in the hydrodynamic response to CSF infusion. For example, an increase in ICP via a CSF infusion could cause a greater increase in ICP in regions closer to the CSF space, including the cortex. Regional impairment of CBF has been demonstrated in the periventricular spaces during CSF infusion in normal pressure hydrocephalus patients.36 In this latter study, infusion of artificial CSF into the subarachnoid space resulted in a decrease in CBF that was most prominent in the watershed areas close to the ventricles. Whether such autoregulatory gradients to CSF infusion also exist in relation to distance from the subarachnoid spaces is unknown.
Relationship between ICP pulsatility and mean ICP
The linear relationship between pulse amplitude and mean ICP has been described many years ago37 and is one of most specific landmarks of ICP signal. It has been classically interpreted by loss of cerebrospinal compensatory reserve with increasing mean ICP, elegantly interpreted by the exponential pressure–volume relationship. Avezaat et al.38 observed a right side deflection point of the volume–pressure response at very high levels of ICP, experimentally studied with an inflated epidural balloon. The same phenomenon can be observed as the upper breakpoint of the amplitude – mean ICP relationship and has been observed clinically in rare cases of patients after TBI who died of refractory intracranial hypertension39 or in the paediatric neurosurgical population.40 It is an uncommon appearance, counterbalanced by the Cushing increase of ABP, and its aetiology is unclear. It could be related to terminal closing of the cerebral arterial bed when the CrCP approaches ABP. In support of this, the observed upper breakpoint was associated with a low diastolic closing margin and diastolic flow velocity.
Perspectives
ICP monitoring has become a standard of care after severe traumatic brain injury; however, we do not yet have any class 1 evidence that ICP or more extensive haemodynamic monitoring improves patient outcome.41 Typically, fixed thresholds of ICP or CPP are used as a guide for therapy,42 despite it being unlikely that a single threshold of either ICP or CPP is optimal for every patient. Unfortunately, highly individual haemodynamic and metabolic physiology hamper the use of ‘one size fits all’ ICP or CPP thresholds. The current study highlights that modest (±10 mm Hg) alterations in ICP decrease cortical perfusion and result in decreases in vascular WT. Thus, even in the controlled experimental condition, just knowing the ICP or CPP is unlikely to indicate if the CBF is adequate. Probably only by integrating global (ICP, CPP, pressure reactivity, Doppler derived WT, CrCP and Fv) and local (microdialysis, brain oxygen pressure) physiologic markers will we be able to clearly understand the state of the patients cerebral circulation and how it may be optimised.
Limitations
The current results reflect the cerebral haemodynamic response to raising ICP through the addition of CSF volume. Although a practical model for interrogating the cerebral haemodynamic response to raised ICP, the observed haemodynamic response may differ somewhat to those observed clinically in the injured, oedematous brain where changes in volume of the intracranial blood, CSF, or parenchymal compartments are all common. Clinical observational studies in TBI patients may help to confirm the current results. Application of cortical LDF during an infusion of fluid into the subarachnoid space raises the possibility of LDF probe displacement by the infusion. Although gross displacement of the LDF probe could easily be ruled out visually, subtle changes in orientation of the LDF probe would be more difficult to detect and could result in decreases in flux.
The experimental paradigm involved bilateral ligation of the common carotid artery prior to experimentation – this could affect cerebral haemodynamics per se. Indeed, recent investigations have indicated that common carotid artery ligation in NZ rabbits can lead to nascent aneurysm formation at the basilar terminus after 12 weeks43 with subtle changes in structure of the basilar terminus detectable as early as 5 days after ligation.44 While an important consideration, the current investigation analysed within individual cerebral haemodynamic changes and no neurological deficit was observed in these rabbits before testing. Moreover, rendering the rabbits basilar artery dependent ensured that flow velocity measured at the basilar artery represented a global cerebral perfusion and provided a well-controlled model to address our proposed questions.
Halothane was used for the induction and maintenance of anaesthesia, which could be relevant to the interpretation of cerebral haemodynamic data as halothane has the dose-dependent potential to cause cerebral vasodilation, intracranial hypertension, and systemic hypotension.45–47 However, after induction, the dose of anaesthesia was titrated as low as possible while maintaining adequate anaesthesia. Moreover, ICP and ABP were both monitored intensively during this study and were both normal during baseline recording (Table 1).
Finally, the current analysis is derived from the digital recordings of experiments performed (by SH, SP, HR and MC) and published in the past.17,48 The first analysis investigated the relationship between CO2 reactivity and cerebral autoregulation,17 while the second analysis compared the accuracy of three non-invasive estimates of ICP.48 Although this is potentially a limitation, it can also be argued that the multiple use of experimental material to conduct separate mathematical modelling studies is scientifically and ethically sound because it takes full advantage of recent advances in physiologic mathematical modelling while limiting the number of animals that need to be sacrificed in accordance with the 3 R principles for animal research (replacement, reduction and refinement-http://www.nc3rs.org.uk/).
Conclusion
The brain attempts to compensate for an increased ICP by both reducing cerebrovascular wall tension and increasing ABP, but the relative importance of these mechanisms depends on the prevailing ICP. In addition, cortical perfusion appears to be exquisitely sensitive to increases in ICP and this may not be detected by global measures of perfusion.
Acknowledgements
The authors would like to acknowledge Dr Hugh Richards and Dr Stefan Piechnik who contributed to data collection. JD is supported by a Woolf Fisher scholarship. GVV is supported by an A.G. Leventis Foundation Scholarship, and a Charter Studentship from St Edmund's College, Cambridge. XYL is supported by Bill Gates Scholarship, and DC is supported by a Cambridge Commonwealth, European & International Trust Scholarship (University of Cambridge). MC is supported by the National Institute of Health Research, Cambridge Biomedical Research Center (Neuroscience Theme).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ICM + software (Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus/) is a multimodal data acquisition and analysis software licensed by the University of Cambridge, Cambridge Enterprise Ltd. P.S. and M.C. have a financial interest in part of the licensing fee. The remaining authors declare no relevant competing financial interests.
Authors' contributions
JD contributed to the analysis and interpretation of data, drafting, and revising of manuscript. MC contributed to the experimental design, acquisition of data, and interpretation of data. SH contributed the experimental design, performing the experiments, and revising the manuscript. XL, GVV, CR, DC, and PNA contributed to the interpretation of the data and revision of the manuscript. PS contributed to the analysis and interpretation of data and critical revision of the manuscript. All authors approved the final manuscript before submission.
References
- 1.Bhatia A, Gupta AK. Neuromonitoring in the intensive care unit. I. Intracranial pressure and cerebral blood flow monitoring. Intensive Care Med 2007; 33: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 2.Beiner JM, Olgivy CS, DuBois AB. Cerebral blood flow changes in response to elevated intracranial pressure in rabbits and bluefish: a comparative study. Comp Biochem Physiol A Physiol 1997; 116: 245–252. [DOI] [PubMed] [Google Scholar]
- 3.Numoto M, Donaghy RM. Effects of local pressure on cortical electrical activity and cortical vessels in the dog. J Neurosurg 1970; 33: 381–387. [DOI] [PubMed] [Google Scholar]
- 4.Zwetnow NN. The influence of an increased intracranial pressure on the lactate, pyruvate, bicarbonate, phosphocreatine, ATP, ADP and AMP concentrations of the cerebral cortex of dogs. Acta Physiol Scand 1970; 79: 158–166. [DOI] [PubMed] [Google Scholar]
- 5.Timofeev I, Czosnyka M, Carpenter KLH, et al. Interaction between brain chemistry and physiology after traumatic brain injury: impact of autoregulation and microdialysis catheter location. J Neurotrauma 2011; 28: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Roux P. Physiological monitoring of the severe traumatic brain injury patient in the intensive care unit. Curr Neurol Neurosci Rep 2013; 13: 331. [DOI] [PubMed] [Google Scholar]
- 7.Miller JD, Stanek A, Langfitt TW. Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Prog Brain Res 1972; 35: 411–432. [DOI] [PubMed] [Google Scholar]
- 8.Kontos HA, Wei EP, Navari RM, et al. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol 1978; 234: H371–H383. [DOI] [PubMed] [Google Scholar]
- 9.Fog M. The relationship between the blood pressure and the tonic regulation of the pial arteries. J Neurol Psychiatry 1938; 1: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lassen N. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother 2015; 15: 169–185. [DOI] [PubMed] [Google Scholar]
- 12.Bragin DE, Statom GL, Yonas H, et al. Critical cerebral perfusion pressure at high intracranial pressure measured by induced cerebrovascular and intracranial pressure reactivity. Crit Care Med 2014; 42: 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bragin DE, Bush RC, Müller WS, et al. High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma 2011; 28: 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady KM, Lee JK, Kibler KK, et al. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg 2009; 108: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 15.Cushing H. Concerning a definite regulatory mechanism of the vaso-motor centre which controls blood pressure during cerebral compression. Johns Hopkins Hosp Bull 1901; 12: 290–292. [Google Scholar]
- 16.Waki H, Bhuiyan MER, Gouraud SS, et al. Acute reductions in blood flow restricted to the dorsomedial medulla induce a pressor response in rats. J Hypertens 2011; 29: 1536–1545. [DOI] [PubMed] [Google Scholar]
- 17.Harland S, Richards HK, Czosnyka M, et al. Dissociation of cerebral autoregulation and CO2 reactivity following carotid occlusion in rabbits. J Cereb Blood Flow Metab 1999; 19: S636. [Google Scholar]
- 18.Nelson RJ, Perry S, Hames TK, et al. Transcranial Doppler ultrasound studies of cerebral autoregulation and subarachnoid hemorrhage in the rabbit. J Neurosurg 1990; 73: 601–610. [DOI] [PubMed] [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D-J, Kasprowicz M, Carrera E, et al. The monitoring of relative changes in compartmental compliances of brain. Physiol Meas 2009; 30: 647–659. [DOI] [PubMed] [Google Scholar]
- 21.Varsos GV, Richards H, Kasprowicz M, et al. Critical closing pressure determined with a model of cerebrovascular impedance. J Cereb Blood Flow Metab 2013; 33: 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varsos GV, De Riva N, Smielewski P, et al. Critical closing pressure during intracranial pressure plateau waves. Neurocrit Care 2013; 18: 341–348. [DOI] [PubMed] [Google Scholar]
- 23.Dewey RC, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. Vasomotor tone, critical closing pressure, and vascular bed resistance. J Neurosurg 1974; 41: 597–606. [DOI] [PubMed] [Google Scholar]
- 24.Varsos GV, Czosnyka M, Smielewski P, et al. Cerebral critical closing pressure in hydrocephalus patients undertaking infusion tests. Neurol Res 2015; 37: 674–682. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt EA, Czosnyka Z, Momjian S, et al. Intracranial baroreflex yielding an early cushing response in human. Acta Neurochir Suppl 2005; 95: 253–256. [DOI] [PubMed] [Google Scholar]
- 26.Wan WH, Ang BT, Wang E. The Cushing Response: a case for a review of its role as a physiological reflex. J Clin Neurosci 2008; 15: 223–228. [DOI] [PubMed] [Google Scholar]
- 27.Panerai R. The critical closing pressure of the cerebral circulation. Med Eng Phys 2003; 25: 621–632. [DOI] [PubMed] [Google Scholar]
- 28.Carey BJ, Manktelow BN, Panerai RB, et al. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation 2001; 104: 898–902. [DOI] [PubMed] [Google Scholar]
- 29.Dawson SL, Panerai RB, Potter JF. Critical closing pressure explains cerebral hemodynamics during the valsalva maneuver. J Appl Physiol 1999; 86: 675–680. [DOI] [PubMed] [Google Scholar]
- 30.Varsos GV, Richards HK, Kasprowicz M, et al. Cessation of diastolic cerebral blood flow velocity: the role of critical closing pressure. Neurocrit Care 2014; 20: 40–48. [DOI] [PubMed] [Google Scholar]
- 31.Sobczyk O, Battisti-Charbonney A, Fierstra J, et al. A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage 2014; 92: 56–68. [DOI] [PubMed] [Google Scholar]
- 32.Horsfield MA, Jara JL, Saeed NP, et al. Regional differences in dynamic cerebral autoregulation in the healthy brain assessed by magnetic resonance imaging. PLoS One 2013; 8: e62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zweifel C, Czosnyka M, Lavinio A, et al. A comparison study of cerebral autoregulation assessed with transcranial Doppler and cortical laser Doppler flowmetry. Neurol Res 2010; 32: 425–428. [DOI] [PubMed] [Google Scholar]
- 34.Budohoski KP, Czosnyka M, Smielewski P, et al. Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J Cereb Blood Flow Metab 2013; 33: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuriyama K. Cerebrovascular responses during lower body negative pressure-induced presyncope. Aviat Space Environ Med 2000; 71: 1033–1038. [PubMed] [Google Scholar]
- 36.Momjian S, Owler BK, Czosnyka Z, et al. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain 2004; 127: 965–72. [DOI] [PubMed] [Google Scholar]
- 37.Marmarou A. An Experimental and theoretical evaluation of the cerebrospinal system. PhD Thesis, Drexel University, USA, 1973.
- 38.Avezaat C, Van Eijndhoven J, Wyper D. Cerebrospinal fluid pulse pressure and intracranial volume-pressure relationships. J Neurol Neurosurg Psychiatry 1979; 42: 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Czosnyka M, Price DJ, Williamson M. Monitoring of cerebrospinal dynamics using continuous analysis of intracranial pressure and cerebral perfusion pressure in head injury. Acta Neurochir (Wien) 1994; 126: 113–119. [DOI] [PubMed] [Google Scholar]
- 40.Czosnyka M, Wollk-Laniewski P, Batorski L, et al. Remarks on amplitude – pressure characteristic phenomenon. Intracranial Press 1989; VII: 255–259. [Google Scholar]
- 41.Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the neurocritical care society and the European society of intensive. Intensive Care Med 2014; 40: 1189–1209. [DOI] [PubMed] [Google Scholar]
- 42.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, et al. Guidelines for the management of severe traumatic brain injury. 3rd addition. J Neurotrauma 2007; 24 Suppl 1: S37–S44. [DOI] [PubMed]
- 43.Gao L, Hoi Y, Swartz DD, et al. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 2008; 39: 2085–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng H, Metaxa E, Gao L, et al. Progressive aneurysm development following hemodynamic insult. J Neurosurg 2011; 114: 1095–1103. [DOI] [PubMed] [Google Scholar]
- 45.Todd MM, Drummond JC. A comparison of the cerebrovascular and metabolic effects of halothane and isoflurane in the cat. Anesthesiology 1984; 60: 276–282. [DOI] [PubMed] [Google Scholar]
- 46.Matta BF, Mayberg TS, Lam AM. Direct cerebrovasodilatory effects of halothane, isoflurane, and desflurane during propofol-induced isoelectric electroencephalogram in humans. Anesthesiology 1995; 83: 980–985. [DOI] [PubMed] [Google Scholar]
- 47.Fitch W, Jones JV, Graham DI, et al. Effects of hypotension induced by halothane on the cerebral circulation in baboons with experimental renovascular hypertension. Br J Anaesth 1978; 50: 119–125. [DOI] [PubMed] [Google Scholar]
- 48.Robba C, Donnelly J, Bertuetti R, et al. Doppler Non-invasive monitoring of ICP in an animal model of acute intracranial hypertension. Neurocrit Care 2015; 23: 419–426. [DOI] [PubMed] [Google Scholar]