Abstract
Gout/hyperuricemia is a common multifactorial disease having typical environmental risks. Recently, common dysfunctional variants of ABCG2, a urate exporter gene also known as BCRP, are revealed to be a major cause of gout/hyperuricemia. Here, we compared the influence of ABCG2 dysfunction on serum uric acid (SUA) levels with other typical risk factors in a cohort of 5,005 Japanese participants. ABCG2 dysfunction was observed in 53.3% of the population investigated, and its population-attributable risk percent (PAR%) for hyperuricemia was 29.2%, much higher than those of the other typical environmental risks, i.e. overweight/obesity (BMI ≥ 25.0; PAR% = 18.7%), heavy drinking (>196 g/week (male) or >98 g/week (female) of pure alcohol; PAR% = 15.4%), and aging (≥60 years old; PAR% = 5.74%). SUA significantly increased as the ABCG2 function decreased (P = 5.99 × 10−19). A regression analysis revealed that ABCG2 dysfunction had a stronger effect than other factors; a 25% decrease in ABCG2 function was equivalent to “an increase of BMI by 1.97-point” or “552.1 g/week alcohol intake as pure ethanol” in terms of ability to increase SUA. Therefore, ABCG2 dysfunction originating from common genetic variants has a much stronger impact on the progression of hyperuricemia than other familiar risks. Our study provides a better understanding of common genetic factors for common diseases.
Gout, which is characterized by acute arthritis, is a common disease as a consequence of hyperuricemia1. In addition to sex, several environmental factors are well-known typical risks of hyperuricemia and gout2 such as obesity, alcohol consumption, and aging; all of which were first reported by Hippocrates 2,500 years ago3 and confirmed by modern public health studies4,5,6. Recently, common dysfunctional variants in ABCG2 gene (also known as BCRP gene) that encodes a high-capacity urate exporter were reported to be a major genetic cause of gout in both Caucasian7 and Japanese8,9 populations, and their pathophysiological involvement was also reported10,11. However, the influence of such genetic traits on serum uric acid (SUA) levels, especially as compared with other typical environmental risk factors, remains to be clarified. In the present study, we show that the dysfunctional ABCG2 variants, the major genetic factors of SUA, have stronger effects on the risk of hyperuricemia progression than other typical environmental factors.
Results
Genetic risk factor of hyperuricemia in the population
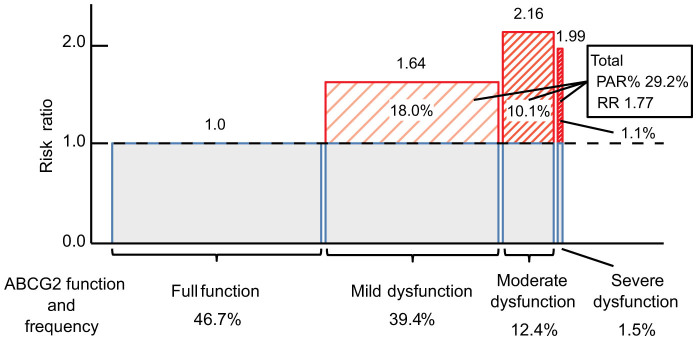
Participants of this study were 5,005 Japanese individuals (Supplementary Table S1) including 831 hyperuricemia patients. Based on the previous studies8,11, all of the participants were divided into four groups by the combination of common dysfunctional variants of ABCG2, non-functional Q126X (rs72552713) and half-functional Q141K (rs2231142), as follows: full function (normal function), 3/4 function (mild dysfunction), 1/2 function (moderate dysfunction) and ≤1/4 function (severe dysfunction) (see Supplementary Figure S1 and Table S2). ABCG2 dysfunction (≤3/4 function) was observed in 53.3% of the total population investigated without obvious difference between sexes (Table 1). We then calculated the population-attributable risk percent (PAR%) of ABCG2 dysfunction for hyperuricemia, which indicates the percentage of hyperuricemic patients originated from ABCG2 dysfunction in the population. Also, PAR% of other typical risks including sex (male), aging (≥60 years old), overweight/obesity (BMI ≥ 25.0), and heavy drinking (more than 196 g/week of pure alcohol for male, and more than 98 g/week of pure alcohol for female)12,13 was calculated. PAR% of ABCG2 dysfunction for hyperuricemia was 29.2% (95% CI, 22.7–35.5) with a risk ratio (RR) of 1.77 (95% CI, 1.55–2.03; P = 6.83 × 10−18) (Fig. 1), which was much higher than those of other environmental factors, i.e. overweight/obesity (PAR% = 18.7% [95% CI, 14.9–22.6]; RR = 2.01 [95% CI, 1.78–2.28; P = 1.52 × 10−27]), heavy drinking (PAR% = 15.4% [95% CI, 11.5–19.2]; RR = 1.79 [95% CI, 1.57–2.04; P = 4.03 × 10−18]), and aging (PAR% = 5.74% [95% CI, 2.27–9.29]; RR = 1.28 [95% CI, 1.11–1.47; P = 5.81 × 10−4]), although sex difference has the strongest effect (PAR% = 91.7% [95% CI, 88.3–94.9]; RR = 17.3 [95% CI, 11.40–26.38; P = 5.22 × 10−88]). Each dysfunctional group of ABCG2 has PAR% with significant RRs as shown in Fig. 1 (PAR% = 18.0% [95% CI, 12.8–23.2]; RR = 1.64 [95% CI, 1.42–1.90; P = 5.61 × 10−12] for mild dysfunctional group; PAR% = 10.1% [95% CI, 7.36–13.0]; RR = 2.16 [95% CI, 1.81–2.57; P = 1.61 × 10−17] for moderate dysfunctional group; and PAR% = 1.1% [95% CI, 0.194–2.05]; RR = 1.99 [95% CI, 1.31–3.02; P = 2.13 × 10−3] for severe dysfunctional group).
Table 1. ABCG2 functions of participants.
| Total | Male | Female | ||||
|---|---|---|---|---|---|---|
| Function of ABCG2 | N | % | N | % | N | % |
| Full function | 2,338 | 46.7 | 1,592 | 46.8 | 746 | 46.5 |
| 3/4 function (mild dysfunction) | 1,971 | 39.4 | 1,332 | 39.2 | 639 | 39.8 |
| 1/2 function (moderate dysfunction) | 619 | 12.4 | 424 | 12.5 | 195 | 12.2 |
| ≤1/4 function (severe dysfunction) | 77 | 1.5 | 53 | 1.6 | 24 | 1.5 |
| Total | 5,005 | 100.0 | 3,401 | 100.0 | 1,604 | 100.0 |
Figure 1. Population-attributable risk percent (PAR%) of ABCG2 dysfunction for hyperuricemia in 5,005 participants.
For the boxes, the red shaded area means PAR% of ABCG2 dysfunction; the width represents the frequency of ABCG2 dysfunction in the population, and the height shows the risk ratio.
Effect size of ABCG2 dysfunction on SUA
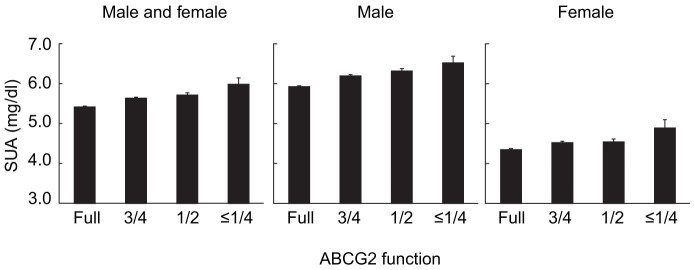
To evaluate the effect size on SUA by each factor, 4,857 individuals, who received no treatment for gout and/or hyperuricemia, were selected from 5,005 participants, and further regression analysis was performed. As shown in Fig. 2 and Supplementary Table S2, SUA was trending upward both in males and females as ABCG2 function decreased. A regression analysis was performed to examine the significance of the effect size of ABCG2 dysfunction as well as other typical factors, which revealed that SUA was significantly affected by both ABCG2 dysfunction and typical risk factors (Table 2). The effect size on SUA, i.e. regression coefficient (β) by a 25% decrease in ABCG2 dysfunction was a gain of 0.193 mg/dl, whereas the effect of other environmental factors were as follows: 1.46 mg/dl between sexes, 4.0 × 10−3 mg/dl by a year-old in age, 0.098 mg/dl by a point of BMI, and 3.5 × 10−4 mg/dl by a gram per week of pure alcohol in alcohol consumption. The ratio of regression coefficients (βABCG2/β: effect size on SUA by a 25% decrease in ABCG2 dysfunction vs. by each risk factor) showed that ABCG2 dysfunction had a stronger effect than other environmental factors; a 25% decrease in ABCG2 function showed an effect equivalent to “an increase of BMI by 1.97-point,” “552.1 g/week alcohol intake as pure ethanol,” or “47.6 years aging” in terms of ability to increase SUA levels.
Figure 2. Serum uric acid (SUA) levels according to each ABCG2 function.
All bars show mean ± s.e.m.
Table 2. Effect of ABCG2 dysfunction and other risk factors on SUA levels in 4,857 individuals.
| Risk factors | β‡ (regression coefficient) | 95% CI | P value | βABCG2/β (ratio of regression coefficients) |
|---|---|---|---|---|
| ABCG2 function* | 0.193 | 0.150–0.235 | 5.99 × 10−19 | 1.00 |
| Sex† | 1.46 | 1.38–1.53 | 2.34 × 10−296 | 0.13 |
| Age, years | 4.0 × 10−3 | 4.5 × 10−4–7.6 × 10−3 | 0.028 | 47.6 |
| BMI, kg/m2 | 0.098 | 0.087–0.108 | 1.29 × 10−68 | 1.97 |
| Alcohol consumption, g/week of pure alcohol | 3.5 × 10−4 | 1.7 × 10−4–5.3 × 10−4 | 1.77 ×10−4 | 552.1 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
*Calculation for ABCG2 function was conducted for full function as 1, 3/4 function (mild dysfunction) as 2, 1/2 function (moderate dysfunction) as 3, and ≤1/4 function (severe dysfunction) as 4.
†Calculation for sex was conducted for female as 1 and male as 2.
‡“β” indicates the increase of SUA (mg/dl) per unit of each risk factor. The ratio of regression coefficients (βABCG2/β) was calculated from the β of ABCG2 function divided by that of each risk factor, showing an effect equivalent to a 25% decrease in ABCG2 function in terms of ability to increase SUA levels.
Discussion
Our study revealed that ABCG2 dysfunction originating from common genetic variants has a much stronger impact on the progression of hyperuricemia than other familiar risk factors except sex. To our knowledge, this is the first study to report that common genetic variants of a common disease showed a stronger effect than typical environmental factors.
ABCG2, also known as a drug exporter BCRP, is expressed on the epithelial cells of small intestine14 and renal tubules15. We have previously shown that ABCG2 is a high-capacity urate transporter which physiologically excretes urate for the regulation of SUA8,10. We also found that ABCG2 has two common dysfunctional variants: a nonsense variant Q126X and a missense variant Q141K8. Functional analyses revealed that Q126X is a nonfunctional variant and Q141K is a half-functional variant due to the halved ABCG2 expression on the membrane8. Since haplotype frequency analyses demonstrated no simultaneous presence of the minor alleles of Q126X and Q141K in one haplotype, the combination of nonfunctional variant Q126X and half-functional variant Q141K makes it possible to estimate dysfunctional levels of ABCG28,10 (Supplementary Figure S1 and Table S2). In the present study, any ABCG2 dysfunction was proved to be commonly observed in the Japanese population (53.3%). Such a high-frequency dysfunction of ABCG2 implies the importance of ABCG2 as a risk factor for these common diseases, hyperuricemia and gout, and therefore indicates the usefulness of screening high-risk individuals.
PAR% of ABCG2 dysfunction was 29.2% and much higher than those of other typical risk factors, including overweight/obesity, heavy drinking, and aging. This result indicates that about 30% of hyperuricemia patients in the Japanese population originate from ABCG2 dysfunction, and other environmental factors did not show such impact. As shown in Fig. 1, RRs of hyperuricemia increased as the ABCG2 function decreased. Lower RR in severe ABCG2 dysfunction as compared with that in moderate dysfunction may be ascribed to the relatively few hyperuricemia patients with severe dysfunction (n = 18). As for the relationship between SNPs and the risk of hyperuricemia, Woodward et al. so far reported that the PAR% of Q141K for gout was 10% in Caucasians7, and Yamagishi et al. indicated that PAR% for gout and/or hyperuricemia was 19% in Japanese16. Ours is the first report to show the PAR% of ABCG2 dysfunction using the combination of Q126X and Q141K for functional evaluation. It is reasonable that the PAR% of Q141K in Caucasians would be lower than that in Japanese, because the minor allele frequency in Caucasians (0.11 according to Woodward et al.7) is lower than those of Japanese (0.31 by Yamagishi et al.16 and 0.29 in the present study). When we re-calculated PAR% according to the definition of hyperuricemia in Yamagishi et al.16 (SUA ≥ 7.0 mg/dl), the resulting PAR% of Q141K was 22.2%, which is comparable to that in Yamagishi et al.16. In the present study, we defined hyperuricemia as SUA >7.0 mg/dl17 and obtained the PAR% of Q141K and Q126X as 23.5% and 2.6%, respectively. Moreover, the results imply that our approach using the combination of the two variants (PAR% = 29.2%) is more effective and useful than using each variant.
Accordingly, common dysfunctional ABCG2 in the population and high PAR%, imply the importance of ABCG2 genotyping for the screening of high-risk individuals for hyperuricemia/gout.
Subsequent regression analysis revealed that ABCG2 dysfunction defined by the combination of Q126X and Q141K significantly increased SUA, while previous studies showed the association of SUA and only Q141K7,8. The effect size, or regression coefficient (β), of a 25% decrease in ABCG2 function for SUA was 0.193 mg/dl, and mean SUA of participants who have severe dysfunction (≤1/4 function) and full function are 5.98 mg/dl and 5.41 mg/dl, respectively, a difference of 0.57 mg/dl (Supplementary Table S2). Hyperuricemia (SUA > 7.0 mg/dl) is more common in males due to the SUA-lowering effect by female hormone. Considering that the difference of SUA between sexes is approximately 1.5 mg/dl, the effect of ABCG2 function is strong enough as a factor in SUA increase. The effect of age (4.0 × 10−3 mg/dl), BMI (0.098 mg/dl), or alcohol consumption (3.5 × 10−4 mg/dl) was a significant factor in the increase of SUA, but we found that the effect of ABCG2 dysfunction was stronger than those of the typical environmental factors.
The ratios of regression coefficient (βABCG2/β) for BMI and alcohol consumption were 1.97 and 552.1, respectively. This indicates that a decrease of 25% in ABCG2 function had the power to raise SUA levels comparable to “gaining a body weight of 5.7 kg for a 170 cm-tall man,” or “drinking 1.7 L of whiskey every week.” Although both obesity/overweight and drinking alcohol are especially targeted as the first step for assessment of gout/hyperuricemia in guidelines17,18,19, genotyping of ABCG2 is revealed to be essential for the risk estimation of gout/hyperuricemia.
Our results in the present study show that genetic factor ABCG2 should be considered to be one of the common risks of hyperuricemia/gout, which is stronger than other typical environmental risks. Since ABCG2 dysfunction can be estimated easily by genotyping only two variants8,9,10,11, our findings will help to recognize a trait of hyperuricemia at a very early stage and to assist prevention and treatment for hyperuricemia and ultimately for gout.
Methods
Study participants
All procedures involved in this study were approved by the institutional ethical committees (National Defense Medical College and Nagoya University), and were performed in accordance with the Declaration of Helsinki. All of the 5,005 Japanese individuals in this study were recruited from Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study)20. Written informed consent was obtained from all subjects. Hyperuricemia was defined as the SUA level that exceeds 7.0 mg/dl ( = 416.36 μmol/l). Alcohol consumption was calculated from the participants' written questionnaires as shown in Supplementary Table S3. Among the 5,005 participants, those who were under treatment for or had past histories of gout/hyperuricemia were excluded; then multiple regression analysis was performed for 4,857 individuals to evaluate the relationship among SUA levels, ABCG2 dysfunction, and other risk factors.
Genetic analysis
Genomic DNA was extracted from whole peripheral blood cells21. Genotyping of the two variants in ABCG2 gene, Q126X and Q141K, was performed with a LightCycler 480 (Roche Diagnostics) by high resolution melting (HRM) analysis22. To confirm the genotypes, more than one hundred samples were subjected to direct sequencing. DNA sequencing analysis was performed with a 3130xl Genetic Analyzer (Applied Biosystems)23. The MAFs of Q126X and Q141K were 0.025 and 0.294, respectively, and both variants were in Hardy-Weinberg equilibrium (P > 0.05). ABCG2 function was estimated from the genotype combination as shown in Supplementary Figure S1 and Table S2.
Statistical analysis
For all calculations in the statistical analysis, a software R (version 3.0.2) (http://www.r-project.org/) and a software SPSS v.17.0J (IBM Japan Inc., Tokyo, Japan) were used. The PAR% of ABCG2 dysfunction and other typical risk factors for hyperuricemia was calculated from the following equation:
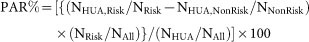 |
(“NHUA,Risk” and “NHUA,NonRisk” indicate the numbers of hyperuricemia patients in the risk group and non-risk group, respectively. “NRisk” and “NNonRisk” represent the numbers of individuals in the risk group and non-risk group, respectively. “NHUA” and “NAll” mean the number of all hyperuricemia patients and all participants, respectively.)
For the robustness of statistics, random resampling methods with computer simulation are often applied24,25. In this study, to evaluate the 95% CI of PAR%, the bootstrap method25 was used for random resampling of all participants' data set with replacement for 10,000 times.
The linear regression analysis was conducted with the model in which ABCG2 dysfunction, sex, age, BMI, and alcohol consumption were included.
Supplementary Material
SUPPLEMENTARY INFORMATION
Acknowledgments
We would like to thank all the participants involved in this study. We also thank K. Gotanda, H. Amaki, Y. Morimoto, N. Katsuta, C. Okada, H. Inoue, H. Ogata, S. Tatsukawa, and Y. Tanahashi (National Defense Medical College, Tokorozawa, Japan), M. Naito and N. Hamajima (Nagoya University, Nagoya, Japan) for collecting samples and genetic analysis. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (including the Grant-in-Aid for Scientific Research on Innovative Areas “Genome Science”), the Ministry of Health, Labour and Welfare of Japan, the Ministry of Defense of Japan, the Japan Society for the Promotion of Science, the Takeda Science Foundation, the AstraZeneca VRI Research Grant, the Kawano Masanori Memorial Foundation for Promotion of Pediatrics, and the Gout Research Foundation of Japan.
Footnotes
There is potential competing interest: H.M., T.N., T. Takada, K.I. and N.S. have a patent pending based on the work reported in this paper. The other authors declare that they have no conflict of interest.
Author Contributions A.N., H.M. and N.S. conceived and designed this study. H. Nakaoka, T.N., H. Nakashima, Y. Sakurai and K.I. assisted with research design. K.W., S.K., R.O. and T. Tamura collected samples and analyzed clinical data. H.M., Y.T., Y.O., M.S., S.S., Y.K., T.C., J.A., Y. Shichijo and A.A. performed genetic analysis. A.N., H. Nakaoka, T.N. and H. Nakashima performed statistical analysis. A.N., H.M., H. Nakaoka, T.N., H. Nakashima analyzed data. T. Takada, H.S., T.H., Y. Sakurai and K.I. provided intellectual input and assisted with the preparation of the manuscript. A.N., H.M. and N.S. wrote the manuscript. A.N. and H.M. contributed equally to this work.
References
- Rott K. T. & Agudelo C. A. Gout. JAMA 289, 2857–2860 (2003). [DOI] [PubMed] [Google Scholar]
- Wortmann R. L. in Harrison's Principles of Internal Medicine (eds Anthony, S. et al.) 2444–2449 (McGraw-Hill, 2008). [Google Scholar]
- Adams F. & Society S. The Genuine Works of Hippocrates. (Sydenham Society, 1849). [Google Scholar]
- Williams P. T. Effects of diet, physical activity and performance, and body weight on incident gout in ostensibly healthy, vigorously active men. Am. J. Clin. Nutr. 87, 1480–1487 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K., Atkinson K., Karlson E. W., Willett W. & Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet 363, 1277–1281 (2004). [DOI] [PubMed] [Google Scholar]
- Mikuls T. R. et al. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann. Rheum. Dis. 64, 267–272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward O. M. et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. U. S. A. 106, 10338–10342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H. et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci. Transl. Med. 1, 5ra11 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuo H. et al. Common dysfunctional variants in ABCG2 are a major cause of early-onset gout. Sci. Rep. 3, 2014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida K. et al. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 3, 764 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H. et al. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 4, 3755 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (US). Helping patients who drink too much: a clinician's guide: updated 2005 edition. [Rev. Jan. 2007] edn, (U.S. Dept. of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, 2007). [Google Scholar]
- Dawson D. A., Grant B. F. & Li T.-K. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol. Clin. Exp. Res. 29, 902–908 (2005). [DOI] [PubMed] [Google Scholar]
- Maliepaard M. et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 61, 3458–3464 (2001). [PubMed] [Google Scholar]
- Huls M. et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 73, 220–225 (2008). [DOI] [PubMed] [Google Scholar]
- Yamagishi K. et al. The rs2231142 variant of the ABCG2 gene is associated with uric acid levels and gout among Japanese people. Rheumatology (Oxford). 49, 1461–1465 (2010). [DOI] [PubMed] [Google Scholar]
- The guideline revising committee of the Japanese Society of Gout and Nucleic Acid Metabolism. in Guideline for the Management of Hyperuricemia and Gout (ed The guideline revising committee of the Japanese Society of Gout and Nucleic Acid Metabolism.) (Medical Review, 2010). [Google Scholar]
- Khanna D. et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 64, 1431–1446 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. et al. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 65, 1312–1324 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima N. & J-MICC Study Group. The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac. J. Cancer Prev. 8, 317–323 (2007). [PubMed] [Google Scholar]
- Matsuo H. et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am. J. Hum. Genet. 83, 744–751 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margraf R. L., Mao R. & Wittwer C. T. Rapid diagnosis of MEN2B using unlabeled probe melting analysis and the LightCycler 480 instrument. J. Mol. Diagn. 10, 123–128 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama A. et al. A common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum. Cell 26, 133–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Identification of high-quality cancer prognostic markers and metastasis network modules. Nat. Commun. 1, 34 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 7, 1–26 (1979). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFORMATION