Abstract
DMPU/HF (HF content 65 wt %/wt) is an ideal nucleophilic fluorination reagent for the diastereoselective synthesis of substituted 4-fluorotetrahydropyrans and 4-fluoropiperidines via a fluoro-Prins reaction. When compared to classical nucleophilic fluorination reagents like pyridine/HF, DMPU/HF gives both higher yields and better diastereoselectivity.
Graphical Abstract

Incorporation of fluorine into organic compounds is known to impart useful and important properties to these compounds.1,1e Hydrogen fluoride is regarded as one of the most atom-economical nucleophilic fluorination reagents, but its gaseous state at ambient conditions and toxicity hinder its wider use.2 In order to ease its manipulation, hydrogen fluoride gas is mixed with amine bases to form complexes such as Olah’s reagent (pyridine·9HF) and triethylamine/HF (Et3N·3HF).3 However, these amine bases reduce the acidity of the system and may decrease reactivity in reactions that need high acidity. We have recently reported that HF could form acidic stable complexes with potential hydrogen-bond acceptors.4 Indeed, 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone (DMPU) can form a stable complex with up to 11 equiv of HF.5 This acidic complex has been demonstrated to be an optimal fluorination reagent in the gold-catalyzed mono- and difluorination of alkynes.5 DMPU/HF can be prepared in 65% yield (wt/wt HF content, mole ratio of DMPU/HF = 1:11.9) or at lower concentrations (e.g., 34%, mole ratio of DMPU/HF = 1:3.3).
Since the DMPU/HF reagent is more acidic than Olah’s reagent (pyridine·9HF) or triethylamine HF (Et3N·3HF), we proposed that its use could be advantageous in fluorination reactions that require a highly acidic medium. Herein, we report an improved diastereoselective synthesis of fluorinated tetrahydropyrans and piperidines using DMPU/HF.
The Prins reaction6 of a homoallylic alcohol and an aldehyde in the presence of an acid is a well-established synthetic methodology for the preparation of tetrahydropyrans.7 However, there are only a few reports on the Prins reaction for the synthesis of fluorinated tetrahydropyrans.8 Most of the reported syntheses of fluorinated tetrahydropyrans utilize a strong Lewis acid, BF3· OEt2, as the fluorine source. Hence, they suffer from low yields and especially low diastereoselectivity.8b Fuchigami and coworkers reported the synthesis of 4-fluorotetrahydropyrans with HF salts in liquid form, but a large excess of HF was needed (HF as solvent).8a Because the Prins reaction requires an acidic medium, the more acidic HF/DMPU system should improve the efficiency of Prins cyclization.
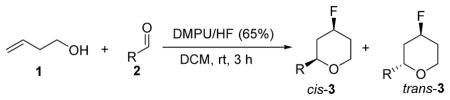
We were pleased to find that the reaction of homoallylic alcohol 1 and benzaldehyde 2a in the presence of DMPU/HF produced the expected 4-fluorotetrahydropyran 3a (Table 1). Reactions in a number of nonpolar solvents (hexane, toluene, and DCM) provided high efficiency and excellent diastereoselectivity (Table 1, entries 1–3). A lower concentration of HF in the reaction medium (34% HF/DMPU wt/wt, DMPU/HF = 1:3.3) slowed the reaction, but the diastereoselectivity was maintained (Table 1, entry 4). A complete replacement of solvent by DMPU resulted in a much slower conversion and eroded the diastereomeric ratio (Table 1, entry 5). Reactions were completely shut down in Lewis basic solvents, including THF and DMF (Table 1, entries 6 and 7).
Table 1.
Optimization of the Fluoro-Prins Reactiona

| ||||
|---|---|---|---|---|
|
| ||||
| entry | solvent | time (h) | conversion (%) | cis/transb |
| 1 | hexane | 3 | 100 | 17:1 |
| 2 | toluene | 3 | 100 | 17:1 |
| 3 | DCM | 3 | 96 | 17:1 |
| 4c | DCM | 9 | 90 | 17:1 |
| 5 | DMPU | 3 | 42 | 10:1 |
| 6 | THF | 9 | 0 | |
| 7 | DMF | 9 | 0 | |
1 (0.2 mmol), 2 (0.2 mmol), DMPU/HF (2.1 mmol of HF), and solvent (0.5 mL) were mixed in a polyethylene vial and then stirred for 3–9 h at rt.
Determined by 19F NMR.
34% DMPU–HF.
We also compared the reactivity and selectivity of Olah’s reagent and HF/DMPU in the Prins reaction of 2-naphthaldehyde eq 1. The more acidic DMPU/HF reagent enabled a faster conversion and much better diastereoselectivity than Olah’s reagent.
 |
(eq 1) |
To explore the general applicability of our methodology, several aldehydes were subjected to our optimized reaction conditions (Table 2). Aromatic and aliphatic aldehydes gave the corresponding fluorinated tetrahydropyrans in good yields and good diastereoselectivity. A more electron-rich aldehyde, such as 4-hydroxy-3,5-dimethoxybenzaldehyde, did not react under these conditions. The same phenomenon was also observed in the BF3·OEt2-mediated Prins cyclization8b (Table 2, entry 10).
Table 2.
Scope of the Fluoro-Prins Reactiona
| ||||
|---|---|---|---|---|
|
| ||||
| entry | R | 3 | yield (%) | cis-3/trans-3b |
| 1 | C6H5– | 3a | 75 | 17:1 |
| 2 | 2-naphtyl | 3b | 74 | >20:1 |
| 3 | 4-ClC6H4– | 3c | 87 | >20:1 |
| 4 | 4-BrC6H4– | 3d | 91 | >20:1 |
| 5 | 4-NO2C6H4– | 3e | 81 | >20:1 |
| 6 | 4-CF3C6H4– | 3f | 92 | >20:1 |
| 7 | 4-i-PrC6H4– | 3g | 78 | >20:1 |
| 8 | 4-MeC6H4– | 3h | 72 | >20:1 |
| 9 | 2-NO2C6H4– | 3i | 76 | >20:1 |
| 10 | 4-OH-3,5-dimethoxy-C6H2 | 3j | ro rxn | |
| 11 | 6-Br-2-OH-3-MeO-C6H2 | 3k | 56 | > 20:1 |
| 12 | cyclohexyl– | 3l | 88 | 20:1 |
1 (0.2 mmol), 2 (0.2 mmol), and DMPU/HF (2.1 mmol HF) in DCM (0.5 mL) was mixed in a plastic vial and then stirred for 3 h at rt.
Determined by 19F NMR.
We also investigated the aza-Prins cyclization of aldehyde and N-tosyl homoallyl amine in the presence of our DMPU/HF reagent. As shown in Table 3, the reaction of N-tosyl homoallyl amine 4 with aliphatic aldehydes furnished the corresponding fluoropiperidines 5 in excellent yields and good diastereoselectivity after a few hours. Similar to previous literature reports,9 this reaction did not proceed well with aryl aldehydes (Table 3, entries 3–5), and longer reaction times were needed in order to achieve a full conversion. The reaction became very sluggish with an electron-rich aromatic aldehyde (e.g., anisaldehyde), and only a trace amount of product was obtained even after an extended reaction time (Table 3, entry 6).
Table 3.
Scope of the Aza-Prins Fluorocyclizationa

| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | R | time (h) | 5 | yield (%) | cis-5/trans-5b |
| 1 | cyclohexyl– | 4 | 5a | 100 | 10:1 |
| 2 | n-C5H11– | 4 | 5b | 100 | 8.5:1 |
| 3 | Ph– | 24 | 5c | 96 | 2:1 |
| 4 | 4-Br-C6H4– | 24 | 5d | 90 | 2.5:1 |
| 5 | 4-NO2C6H4– | 24 | 5e | 42 | 2:1 |
| 6 | 4-MeOC6H4– | 48 | 5f | 0 | |
4 (0.2 mmol), 2 (0.2 mmol), and DMPU/HF (2.1 mmol of HF) in DCE (0.5 mL) was mixed in a polyethylene vial and then stirred at 55 °C.
Determined by 19F NMR.
Room temperature.
Determined by 19F NMR using PhCF3 as internal standard.
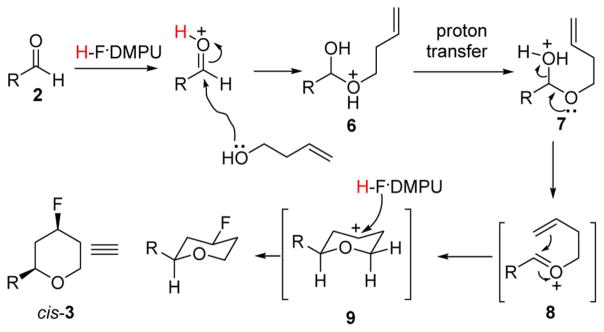
The proposed mechanism of the fluoro-Prins cyclization reaction is shown in Scheme 1. First, HF/DMPU activates the aldehyde 2, which then reacts with the homoallylic alcohol. Subsequent elimination of water results in the formation of the intermediate oxonium ion 8 that then cyclizes into carbocation 9. The nucleophilic fluorine in HF/DMPU quenches intermediate 9 to give the fluorinated product 3.10
Scheme 1.
Proposed Mechanism for the Fluoro-Prins Cyclization
In summary, DMPU/HF is a suitable nucleophilic fluorination reagent for the diastereoselective synthesis of substituted 4-fluorotetrahydropyrans and 4-fluoropiperidines via the Prins reaction. When compared to other commonly used nucleophilic fluorination reagents like pyridine/HF, DMPU/HF gives both higher yields and better cis/trans selectivity. The experimental procedure is simple and is amenable to scale-up.
Supplementary Material
Acknowledgments
We are grateful to the National Institutes of Health for financial support (R15 GM101604-01). B.X. is grateful to the National Science Foundation of China for financial support (NSFC-21472018). O.E.O. is grateful to the University of Louisville for a McSweeney Diversity Endowed Fellowship and to Dr. Zhou Li (University of Louisville) for his support in the preparation of this manuscript.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.5b01919.
Experimental procedures and analytical data for all new compounds (PDF)
The authors declare no competing financial interest.
References
- 1.(a) O’Hagan D. Chem Soc Rev. 2008;37:308–319. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]; (b) Chambers RD. Fluorine in Organic Chemistry. Blackwell Publishing Ltd/CRC Press; Boca Raton, FL: 2004. [Google Scholar]; (c) Kirsch P. Modern Fluoroorganic Chemistry. Wiley-VCH; Weinheim: 2004. [Google Scholar]; (d) Uneyama K. Organofluorine Chemistry. Blackwell Publishing; Oxford: 2006. [Google Scholar]; (e) Müller K, Faeh C, Diederich F. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.(a) Wade TN. J Org Chem. 1980;45:5328–5333. [Google Scholar]; (b) Alvernhe GM, Ennakoua CM, Lacombe SM, Laurent AJ. J Org Chem. 1981;46:4938–4948. [Google Scholar]
- 3.(a) Olah GA, Welch JT, Vankar YD, Nojima M, Kerekes I, Olah JA. J Org Chem. 1979;44:3872–3881. [Google Scholar]; (b) Olah GA, Li X-Y, Wang Q, Surya Prakash GK. Synthesis. 1993;1993:693–699. [Google Scholar]; (c) Haufe G. J Prakt Chem/Chem-Ztg. 1996;338:99–113. [Google Scholar]
- 4.Laurence C, Brameld KA, Graton J, Le Questel JY, Renault E. J Med Chem. 2009;52:4073–4086. doi: 10.1021/jm801331y. [DOI] [PubMed] [Google Scholar]
- 5.Okoromoba OE, Han J, Hammond GB, Xu B. J Am Chem Soc. 2014;136:14381–14384. doi: 10.1021/ja508369z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Yadav JS, Reddy BVS, Reddy MS, Niranjan N, Prasad AR. Eur J Org Chem. 2003;2003:1779–1783. [Google Scholar]; (b) Yadav JS, Reddy BVS, Reddy MS, Niranjan N. J Mol Catal A: Chem. 2004;210:99–103. [Google Scholar]; (c) Zhao XL, Liu L, Chen YJ, Wang D. Tetrahedron. 2006;62:7113–7120. [Google Scholar]; (d) White JD, Blakemore PR, Browder CC, Hong J, Lincoln CM, Nagornyy PA, Robarge LA, Wardrop DJ. J Am Chem Soc. 2001;123:8593–8595. doi: 10.1021/ja011256n. [DOI] [PubMed] [Google Scholar]; (e) Tian X, Jaber JJ, Rychnovsky SD. J Org Chem. 2006;71:3176–3183. doi: 10.1021/jo060094g. [DOI] [PubMed] [Google Scholar]; (f) Nannei R, Dallavalle S, Merlini L, Bava A, Nasini G. J Org Chem. 2006;71:6277–6280. doi: 10.1021/jo060839i. [DOI] [PubMed] [Google Scholar]
- 7.(a) Alder RW, Harvey JN, Oakley MT. J Am Chem Soc. 2002;124:4960–4961. doi: 10.1021/ja025902+. [DOI] [PubMed] [Google Scholar]; (b) Arundale E, Mikeska LA. Chem Rev. 1952;51:505–555. [Google Scholar]
- 8.(a) Kishi Y, Inagi S, Fuchigami T. Eur J Org Chem. 2009;2009:103–109. [Google Scholar]; (b) Launay GG, Slawin AMZ, O’Hagan D. Beilstein J Org Chem. 2010 doi: 10.3762/bjoc.6.41. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bondalapati S, Reddy UC, Kundu DS, Saikia AK. J Fluorine Chem. 2010;131:320–324. [Google Scholar]; (d) Yadav JS, Subba Reddy BV, Anusha B, Subba Reddy UV, Bhadra Reddy VV. Tetrahedron Lett. 2010;51:2872–2874. [Google Scholar]
- 9.(a) Yoshida J-i, Sugawara M, Tatsumi M, Kise N. J Org Chem. 1998;63:5950–5961. doi: 10.1021/jo980601x. [DOI] [PubMed] [Google Scholar]; (b) Yoshida J, Ishichi Y, Isoe S. J Am Chem Soc. 1992;114:7594–7595. [Google Scholar]
- 10.(a) Al-Mutairi EH, Crosby SR, Darzi J, Harding JR, Hughes RA, King CD, Simpson TJ, Smith RW, Willis CL. Chem Commun. 2001:835–836. [Google Scholar]; (b) Jaber JJ, Mitsui K, Rychnovsky SD. J Org Chem. 2001;66:4679–4686. doi: 10.1021/jo010232w. [DOI] [PubMed] [Google Scholar]; (c) Damera K, Yu B, Wang B. J Org Chem. 2015;80:5457–5463. doi: 10.1021/acs.joc.5b00249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.