Abstract
We sought to determine whether patients with posterior cortical atrophy (PCA) demonstrate a pattern of binding to translocator protein 18 kDa (TSPO), a marker of microglial activation, that is distinct from that in patients with amnestic presentation of Alzheimer’s disease (AD). Eleven PCA patients, 11 amnestic AD patients, and 15 age-matched controls underwent positron emission tomography with 11C-PBR28 to measure TSPO. PCA patients showed greater 11C-PBR28 binding than controls in occipital, posterior parietal, and temporal regions. In contrast, amnestic AD patients showed greater 11C-PBR28 binding in inferior and medial temporal cortex. Increased 11C-PBR28 binding overlapped with reduced cortical volume for both PCA and amnestic AD patients, and with areas of reduced glucose metabolism in PCA patients. While both patient groups showed diffuse amyloid binding, PCA patients showed greater binding than amnestic AD patients in bilateral occipital cortex. These results suggest that microglial activation is closely associated with neurodegeneration across different subtypes of AD.
Keywords: Alzheimer’s disease, neuroinflammation, PET imaging
1. INTRODUCTION
Posterior cortical atrophy (PCA) is an atypical variant of Alzheimer’s disease (AD) and most reported cases have evidence of Alzheimer’s pathology on positron emission tomography (PET) imaging (Ossenkoppele et al., 2016; Rosenbloom et al., 2011), cerebrospinal fluid analysis (Baumann et al., 2010), or autopsy (Tang-Wai et al., 2004). Most PET studies have shown no difference in either the regional distribution or amount of amyloid binding between PCA and amnestic AD (Ossenkoppele et al., 2016; Rosenbloom et al., 2011). In contrast, the glucose hypometabolism and tau deposition seen in PCA are regionally distinct from that seen in amnestic AD (Ossenkoppele et al., 2016; Tang-Wai et al., 2004), suggesting that these clinical subtypes have divergent pathways of neurodegeneration not driven by amyloid burden.
Neuroimmune responses, i.e., activation of microglia and astrocytes, are a proposed contributor to AD pathology (McGeer and McGeer, 2013). Activated microglia over-express the 18 kDa translocator protein (TSPO) (Cosenza-Nashat et al., 2009), which can be measured in vivo using PET imaging and 11C-PBR28 (Fujita et al., 2008; Kreisl et al., 2013a). In earlier studies using 11C-PBR28, we demonstrated that TSPO correlates with AD severity (Kreisl et al., 2013b; Lyoo et al., 2015) and increases with progression of disease (Kreisl et al., 2016). However, TSPO imaging has never been compared between different clinical subtypes of AD.
We sought to determine whether patients with PCA have a different pattern of neuroimmune activation—defined by increased TSPO and measured with 11C-PBR28 PET—than patients with typical amnestic presentation of AD.
2. MATERIALS AND METHODS
2.1. Subject selection
Subjects were recruited by the Molecular Imaging Branch of the National Institute of Mental Health (NIMH) Intramural Research Program. All participants or their surrogate gave informed consent. Patients without capacity to consent gave written assent to participate and designated the surrogate in accordance with the NIMH Institutional Review Board.
The Supplementary Materials provides evaluation procedures. Briefly, PCA patients met proposed clinical and radiographic criteria (Tang-Wai et al., 2004), and ophthalmological causes of visual impairment were ruled out by history and examination. Amnestic patients met criteria for either probable Alzheimer’s disease dementia with evidence of pathophysiological processes (McKhann et al., 2011) or mild cognitive impairment due to Alzheimer’s disease of high or intermediate likelihood (Albert, et al., 2011). All amnestic AD patients had memory complaint as the initial clinical presentation of their disease and none met criteria for logopenic progressive aphasia (Gorno-Tempini et al., 2011).
All subjects had brain MRI and PET imaging with 11C-Pittsburgh Compound B (PIB). Only “PIB-positive” patients and “PIB-negative” controls underwent 11C-PBR28 imaging. 18F-fluorodeoxyglucose (FDG) PET was performed in all PCA patients and in two amnestic AD patients. FDG images from 15 age-matched cognitively normal subjects (seven female, age 64 ±5 years, MMSE score 29.5 ± 0.6) were selected from a historical cohort to compare to the PCA patients. MR data from FDG controls were compared to those from other subject groups to confirm comparability (see Supplementary Material). MRIs were performed within 70 days of initial evaluation. Subjects were re-evaluated if over 1 year passed since initial visit. All scans were thus completed within 6 months of evaluation by study neurologist or neuropsychologist. MRI was repeated if necessary to ensure PET scans were done within 1 year of MRI.
2.2. Imaging procedures
MRI, PIB PET, and 11C-PBR28 PET procedures and pre-preprocessing steps were performed as described previously (Kreisl et al., 2013b; Kreisl et al., 2016; Lyoo et al., 2015). See Supplementary Materials for detailed description. Two PCA patients and one amnestic AD patient had 11C-PBR28 imaging performed without arterial sampling. For FDG scans, mean activity of 296 MBq were injected; subjects then rested in a dark room with their eyes closed. A single static 10-minute FDG image was acquired approximately 45 minutes post-injection.
2.3. Image analysis
2.3.1. 11C-PBR28 Region of Interest (ROI)-based analysis
For the ROI-based analysis, the realigned 11C-PBR28 PET images were coregistered to the MR image. Using binary mask images for all regions, we corrected partial volume effects of each PET image time frame with a region-based voxel-wise correction technique programmed in MATLAB (Thomas et al., 2011). A 3D Gaussian kernel with 7 mm full-width at half-maximum (FWHM) was used as a point-spread function correcting the spill-in and spill-over. Time-activity curves were then extracted from partial volume-corrected images.
We first performed the “gold standard” method of 11C-PBR28 analysis using kinetic modeling (Fujita et al., 2008). Metabolite-corrected plasma and whole blood input functions were fitted to a tri-exponential function. Time delay between the radial artery and the brain was calculated from the volume-weighted average time-activity curve of all grey matter regions. With parent input functions and time activity curves, total distribution volume (VT) of the two tissue-compartmental model was calculated for each brain region and corrected for free fraction of radioligand in plasma (fP).
We next performed a simplified ratio method of analysis, using cerebellum as the pseudo-reference region, according to recently validated methods (Lyoo et al., 2015). For each of the nine non-cerebellum ROIs, concentration of radioactivity from 60–90 minutes post-injection was divided by that from the cerebellum ROI to create standardized uptake value ratios (SUVRs).
2.3.2. 11C-PBR28 statistical parametric mapping (SPM) analysis
For the SPM analysis, we first created parametric SUVR images by averaging the 60–90 minute time frames and then dividing each voxel by the average concentration of radioactivity in cerebellar gray matter over that time period. SUVR images, uncorrected for partial volume effects, were then normalized and smoothed (with an 8-mm FWHM Gaussian filter) before voxel-wise analysis using the full factorial model in SPM8. Diagnosis and TSPO genotype were included as fixed factors with age and education as covariates. SPM analysis was performed at voxel-level with P<0.005 as threshold. Cluster-wise correction was then applied such that only clusters that survive correction for multiple comparisons with threshold P<0.05 were reported.
2.3.3. PIB analysis
PIB PET data were analyzed in three ways. First, a 40–60-minute PIB SUVR image was obtained using cerebellar gray matter as the reference region (Jack et al., 2008). Binding in a composite target region was used to determine amyloid-positivity using an SUVR threshold of 1.5 (Jack et al., 2008). This conservative threshold was used to exclude non-Alzheimer’s pathophysiology as the underlying cause of impairment in PCA and amnestic patients. Positive and negative PIB scans were additionally confirmed using a visual binary read of averaged images using 40–60 minute scan data. Second, PIB DVR images were created using the Logan reference model in PMOD version 3.1 (PMOD Technologies Ltd.) (Price et al., 2005). Image data 35–60 minutes post-injection were used, with cerebellum as reference and k2′=0.149 min−1 (Lopresti et al., 2005). DVR images, uncorrected for partial volume effects, were then normalized and smoothed. Voxel-wise comparison was performed using the full factorial model in SPM8 with age and education as covariates. SPM analysis was performed at voxel-level with P<0.005 as threshold. Cluster-wise correction was then applied such that only clusters that survive correction for multiple comparisons with threshold P<0.05 were reported. Third, partial volume correction was applied to the DVR images to create regional DVR values for correlative analysis with regional 11C-PBR28 SUVR values (see Section 2.5.).
2.3.4. FDG analysis
FDG images were spatially normalized using the same procedures as PIB and 11C-PBR28. Activity in each voxel was then corrected for concentration of radioactivity in pons using SPM8. The resulting images were then corrected for partial volume effects using the same region-based voxel-wise method and smoothed. The full factorial model was applied to both the partial volume corrected and uncorrected SUVR images with age as covariate. Education information was not available for the historical controls that had FDG PET. T-statistic maps were created by using the family-wise error P<0.05 to correct for multiple comparisons.
2.3.5. Cortical thickness analysis
Cortical thickness was measured as the distance between each vertex for the cortical gray matter surface and its corresponding vertex of white matter surface, then mapped on the white matter surface. Cortical surface maps were spatially normalized to the averaged template surface and smoothed by the Gaussian kernel with 8 mm FWHM. A general linear model implemented to FreeSurfer with age and education as covariates was used to compare the groups. Multiple comparisons were corrected by running the Monte-Carlo simulation of 5,000 times of permutation; only clusters with cluster-level P<0.05 were considered significant.
2.4. TSPO affinity determination
11C-PBR28 shows differential binding to TSPO caused by the rs6971 polymorphism on the TSPO gene (Owen et al., 2012). Co-dominant expression results in three binding patterns: high affinity binding (HAB) in those without the polymorphism, low affinity binding (LAB) in those homozygous for the polymorphism, and mixed affinity binding (MAB) in heterozygotes. In vitro binding to TSPO on peripheral leukocytes was used to determine TSPO affinity status (Kreisl et al., 2013a) for all subjects, except in one PCA patient who did not have blood available for testing. For 21 subjects (five PCA, four amnestic AD, 12 controls), the genotype of the rs6971 polymorphism within the TSPO gene was additionally confirmed (Owen et al., 2012). We previously demonstrated 100% concordance between in vitro binding and TSPO genotype results (Kreisl et al., 2013a). Low affinity binders were excluded from the study.
2.5. Statistical analysis
Data were analyzed using IBM SPSS Statistics 23. Demographic variables were compared using one-way ANOVA with Tukey’s post-hoc test for multiple group comparisons. For ROI-based 11C-PBR28 analysis, we used univariate ANOVA with diagnosis and TSPO genotype as fixed factors, and age and education as covariates; Bonferroni correction was used for multiple group comparisons. To compare the amount of asymmetry among groups, we calculated an asymmetry index (asymmetry index (AI%)=200 × (R−L)/(R+L) for 11C-PBR28 and PIB images, and AI%=−200 × (R−L)/(R+L) for FDG (Ossenkoppele et al., 2016). Therefore, a positive AI% represents a right-sided abnormality (greater 11C-PBR28 or PIB binding or lower FDG uptake in right than left) and a negative AI% represents a left-sided abnormality. For AI%, PET image data were extracted from partial volume-uncorrected 11C-PBR28 (SUVR) and PIB (DVR) images, and from partial volume corrected FDG (SUVR) images. Univariate ANOVA with diagnosis as a fixed factor and age (and education for 11C-PBR28 and PIB) as covariate were used for AI% comparison, with Bonferroni correction for multiple group comparisons. To determine the relationship between TSPO and volume loss and amyloid load, we performed linear correlation analysis between 11C-PBR28 SUVR values, voxel count (i.e., number of mm3 voxels within each ROI), and PIB DVR values. We also performed correlation analysis between 11C-PBR28 binding and available scores on Block Design and Delayed Recall from the Hopkins Verbal Learning Test – Revised to look for regional associations between TSPO binding and impaired performance on visuospatial and memory tests. For three PCA patients who underwent testing but were too impaired to perform the Block Design test, we imputed zero as the raw score to derive the age-based t score. Correlations were first performed on ROI image data on PCA and amnestic patients combined to increase sample size. To correct for TSPO genotype, age, and education, we performed partial correlations between the individual outcome measures, with age, education, and TSPO genotype as covariates, to calculate Pearson R correlation coefficients. For correlations between 11C-PBR28 and PIB binding, we performed partial correlations additionally controlling for voxel count and duration of illness.
3. RESULTS
3.1. Subject characteristics
Demographic information for subjects who completed study procedures are listed in Table 1. One PCA patient was determined to be low affinity binder at screen and did not undergo 11C-PBR28 PET. Eleven PCA patients, 11 amnestic AD patients, and 15 controls underwent 11C-PBR28 PET imaging. Two PCA patients and one amnestic AD patients met criteria for mild cognitive impairment while the rest had more severe functional impairment and met criteria for dementia. For both patient groups, scores on delayed recall for Hopkins Verbal Learning Test – Revised (HVMT-R) and Brief Visuospatial Memory Test – Revised (BVMT-R) were greater than two SDs below age- and education-adjusted norms. Mini Mental State Exam, HVLT-R, BVMT,-R and verbal fluency scores did not differ between PCA and amnestic AD patients. However, PCA patients performed worse on Block Design, a measure of visuospatial function. In fact, four PCA patients were so visually impaired they could not perform the Block Design task at all, while all 11 amnestic AD patients were able to complete it.
Table 1.
Subject characteristics.
| PCA | AD | HC | |
|---|---|---|---|
|
|
|||
| N | 11 | 11 | 15 |
| Age (years) | 64.3 ± 7.0 | 65.6 ± 7.3 | 63.7 ± 4.7 |
| Sex | 6F, 5M | 5F, 6M | 3F, 12M |
| Education (years) | 15.5 ± 2.7 | 16.5 ± 2.1 | 16.1 ± 2.4 |
| Duration of symptoms (years) | 5.5 ± 3.1 | 3.4 ± 1.4 | N/A |
| TSPO genotype (HAB:MAB)a | 7:4 | 3:8 | 4:11 |
| Non-steroidal anti-inflammatory drug use | 27.3% | 27.3% | 40.0% |
| Cholinesterase inhibitor use | 54.5% | 81.8% | 0% |
| Mini Mental State Exam score | 18.4 ± 6.2 | 21.5 ± 4.7 | 29.9 ± 0.4 b |
| WAIS-III Block Designc | 29.8 ± 5.9d | 43.8 ± 9.5 | |
| % unable to perform Block Design | 36% | 0% | |
| HVLT-R Delayed Recallc | 24.1 ± 12.1 | 22 ± 6.0 | |
| BVMT-R Delayed Recallc | 21.4 ± 6.4 | 20 ± 3.3 | |
| Phonemic (FAS) Fluencyc | 34.1 ± 14.6 | 46.2 ± 17.1 | |
| Category Fluencyc | 22.3 ± 10.2 | 28.6 ± 14.5 | |
One PCA patient did not have TSPO binding status determined and was assumed to be HAB based on cerebellar binding compared to MABs.
P < 0.0001 vs. PCA and AD (Oneway ANOVA with Tukey post-hoc test)
Test not performed or unable to be completed in some PCA patients
P = 0.009 vs. AD (two-tailed t test)
PCA = Posterior cortical atrophy, AD = amnestic Alzheimer’s disease, HC = healthy controls, HAB = High affinity binder, MAB = mixed affinity binder, MMSE – Mini Mental State Exam, HVLT-R – Hopkins Verbal Learning Test - Revised, BVMT-R – Brief Visuospatial Memory Test - Revised. WAIS-III – Wechsler Adult Intelligence Scale, Third Edition. Data given as mean ± SD.
For the 21 subjects who had TSPO genotype determined, there was 100% concordance between genotype and binding assay results. One PCA patient did not have blood available for TSPO affinity determination. This subject was assumed to be an HAB because his cerebellar VT/fP was 2% greater than the mean for HAB patients (combined PCA and amnestic AD) and greater than two SDs above the mean for MAB patients (Supplementary Fig 2).
3.2. Patterns of TSPO binding
3.2.1. ROI-based analysis
Using kinetic modeling with the metabolite-corrected arterial input function, PCA patients had greater 11C-PBR28 binding than controls in parietal and occipital regions, after correcting for age, education, and TSPO genotype (Table 2). In contrast, amnestic AD patients had greater binding than controls in middle and inferior temporal cortex and entorhinal cortex. 11C-PBR28 binding was the same in cerebellum among PCA patients, amnestic AD patients, and controls (P=1.00, univariate ANOVA). These differences remained after correcting for sex.
Table 2.
Regional 11C-PBR28 binding in patients with posterior cortical atrophy, amnestic Alzheimer’s disease, and in healthy controls.
|
VT/fP (mL · cm−3)
|
SUVR
|
|||||
|---|---|---|---|---|---|---|
| PCA (n = 11) | AD (n = 11) | HC (n = 15) | PCA (n = 11) | AD (n = 11) | HC (n = 15) | |
|
| ||||||
| Prefrontal | 181.5 ± 56.5 | 192.8 ± 52.6 | 158.0 ± 52.0 | 1.57 ± 0.17 | 1.61 ± 0.18g | 1.40 ± 0.18 |
| Superior parietal | 263.3 ± 86.1d | 185.5 ± 80.6 | 159.0 ± 79.7 | 2.09 ± 0.24c,f | 1.58 ± 0.26 | 1.42 ± 0.26 |
| Inferior parietal | 234.7 ± 61.3b | 194.2 ± 57.3 | 147.0 ± 56.6 | 1.89 ± 0.20c,e | 1.60 ± 0.22h | 1.31 ± 0.22 |
| Precuneus | 214.3 ± 65.9a | 185.9 ± 61.4 | 139.6 ± 60.7 | 1.77 ± 0.22c | 1.57 ± 0.24h | 1.26 ± 0.24 |
| Occipital | 239.6 ± 66.9a | 180.1 ± 61.7 | 166.3 ± 61.0 | 2.12 ± 0.20c,f | 1.55 ± 0.22 | 1.48 ± 0.22 |
| Superior temporal | 181.5 ± 57.1 | 184.3 ± 53.3 | 150.4 ± 52.7 | 1.55 ± 0.14b | 1.56 ± 0.15h | 1.34 ± 0.15 |
| Mid & inf temporal | 188.1 ± 58.3 | 204.8 ± 54.4g | 146.0 ± 53.8 | 1.60 ± 0.15c | 1.63 ± 0.16i | 1.29 ± 0.16 |
| Hippocampus | 109.5 ± 44.1 | 130.5 ± 41.2 | 89.1 ± 40.8 | 0.94 ± 0.12a | 1.07 ± 0.12i | 0.81 ± 0.12 |
| Entorhinal | 196.9 ± 87.3 | 256.1 ± 77.6h | 149.3 ± 80.5 | 1.41 ± 0.19 | 1.69 ± 0.21i,j | 1.22 ± 0.21 |
| Cerebellum | 111.7 ± 37.2 | 113.5 ± 34.8 | 111.2 ± 34.4 | N/A | N/A | N/A |
Group differences assessed using used univariate ANOVA with diagnosis and TSPO genotype as fixed factors, and age and education as covariates.
PCA > HC, P < 0.05;
PCA > HC, P < 0.01;
PCA > HC, P < 0.001
PCA > AD, P < 0.05;
PCA > AD, P < 0.01;
PCA > AD, P < 0.001
AD > HC, P < 0.05;
AD > HC, P < 0.01;
AD > HC, P < 0.001
AD > PCA, P < 0.01
PCA = posterior cortical atrophy, AD = amnestic Alzheimer’s disease, HC = healthy controls, VT/fP = total distribution volume/free fraction of radioligand, SUVR = standardized uptake value ratio. 11C-PBR28 binding values corrected for age, education, and TSPO genotype. Data are presented as mean ± SD.
Using the simplified ratio (SUVR) method, PCA patients had greater 11C-PBR28 binding than controls in a similar pattern to that seen with kinetic modeling, with significant increases additionally found in lateral temporal cortex and hippocampus (Table 2). Further, PCA patients had greater binding than amnestic AD patients in parietal and occipital regions. Amnestic AD patients had greater 11C-PBR28 binding than controls in several regions, including prefrontal, parietal, lateral, and medial temporal regions. Finally, amnestic AD patients had greater binding than PCA patients in entorhinal cortex (P=0.007, univariate ANOVA). These distinct patterns of binding could be appreciated on a single-subject basis (Fig 1).
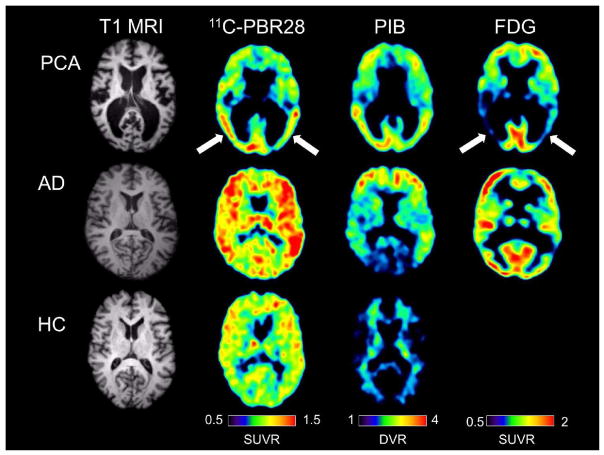
Fig 1.
Single subject images from a patient with posterior cortical atrophy (top row), a patient with amnestic Alzheimer’s disease (center row), and a healthy control subject (bottom row). All three subjects were mixed affinity binders for TSPO. The posterior cortical atrophy subject showed focal occipito-temporal 11C-PBR28 binding, with FDG hypometabolism in the same region (arrows). While the posterior cortical atrophy subjects showed occipito-temporal PIB binding, PIB binding was also found in frontal cortex. The subject with amnestic Alzheimer’s disease showed more diffuse 11C-PBR28 binding, with occipital sparing on PIB and classic bilateral temporo-parietal hypometabolism on FDG imaging. The control subject showed low amounts of diffuse 11C-PBR28 binding and absence of cortical PIB binding.
3.2.2. Voxel-wise analysis
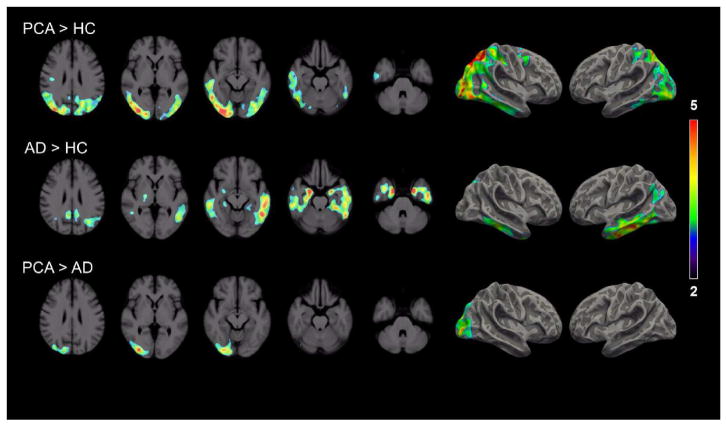
Voxel-wise comparison of 11C-PBR28 was performed using SPM, using cluster-wise correction for multiple comparisons. PCA patients showed significant clusters of greater 11C-PBR28 binding than controls in posterior parietal, posterior temporal, and occipital regions, with differences greater on the right than left (Fig 2). PCA patients showed greater binding than amnestic AD patients in right occipito-parietal cortex. Amnestic AD patients, on the other hand, showed greater 11C-PBR28 binding than controls in lateral and medial temporal cortex, with differences greater on the left than the right.
Fig 2.
Axial (left) and surface-based (right) projection maps showing differences in 11C-PBR28 binding (SUVR) among patients with posterior cortical atrophy and amnestic Alzheimer’s disease and in healthy controls. Only the clusters with cluster-level threshold P < 0.05 for multiple comparisons are displayed. Contrast threshold P < 0.05 after family-wise correction for multiple comparisons and TSPO genotype, age, and education as covariates. Color bars denote T-values.
3.3. Patterns of neurodegeneration
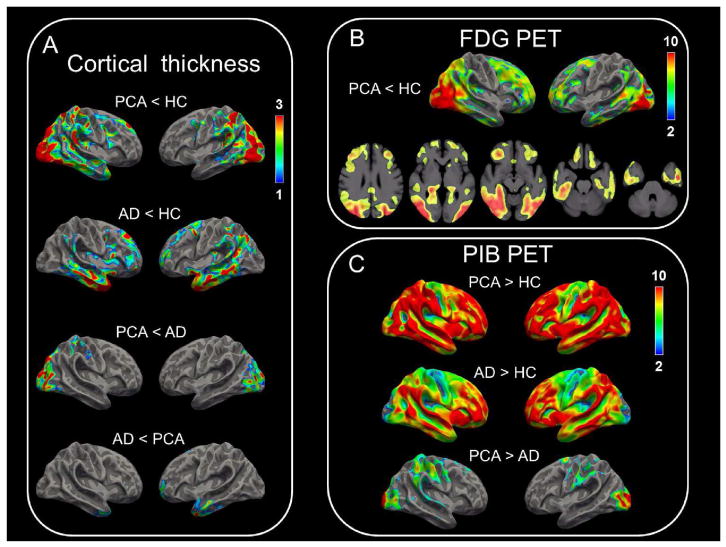
PCA patients showed reduced cortical thickness on MRI compared to controls in posterior brain regions (Fig 3A). Voxel-wise comparison of FDG, using family-wise error correction for multiple comparisons, showed that PCA patients had lower FDG metabolism than controls in posterior brain regions and, to a lesser extent, in bilateral prefrontal cortex (Fig 3B). The greatest overlap between cortical volume loss and FDG hypometabolism was in occipital cortex. PCA patients showed reduced cortical thickness than amnestic AD patients in occipital cortex. Amnestic AD patients showed reduced cortical thickness than controls in peri-Sylvian regions bilaterally, and reduced cortical thickness than PCA patients in inferior temporal cortex (Fig 3A).
Fig 3.
Surface-based projection maps show differences in cortical thickness (A), FDG hypometabolism (B), and PIB binding (C). For (A), clusters with threshold P < 0.05 for multiple comparisons are displayed and color bars denote P-values in logarithmic scale (− log10P). For (B), FDG PET images from 15 age-matched historical controls were compared to those from the posterior cortical atrophy patients; PET images were corrected for partial volume effects. Voxels with threshold P < 0.05 correcting for family-wise error are displayed and color bars denote T-values. For (C), PIB PET images were not corrected for partial volume effects. Clusters with threshold P < 0.05 for multiple comparisons are displayed, and color bars denote T-values.
3.4. Patterns of amyloid retention
On an individual basis, all PCA and amnestic AD patients were “amyloid positive” based on global SUVR>1.5, while all controls were “amyloid negative.” Voxel-wise comparison of PIB binding was performed using cluster-wise correction for multiple comparisons. On a group basis, both PCA and amnestic AD patients showed greater PIB retention than controls in a diffuse pattern, with greatest PIB binding seen in prefrontal, temporal, and parietal cortices (Fig 3C). PCA patients showed greater PIB binding than amnestic AD patients in bilateral occipital cortex. Amnestic AD patients did not have greater PIB binding than PCA patients in any brain region. To confirm the results of SPM analysis, we compared PIB binding in PCA and amnestic AD patients with a ROI-analysis. The ROI-analysis also showed greater PIB binding in PCA patients than amnestic AD patients in occipital cortex only. This difference remained significant after correcting for duration of illness (P < 0.0001, univariate ANOVA).
3.5. Patterns of asymmetry
Because results of voxel-wise analysis showed greater increases in 11C-PBR28 binding on the right side of the brain in PCA patients and on the left side of the brain in amnestic AD patients, we calculated AI% for 11C-PBR28, PIB, and FDG PET images and for voxel count from MR images. PCA patients showed right > left 11C-PBR28 binding, right > left FDG hypometabolism, and right > left atrophy on MRI (Table 3). No subject group showed asymmetry in PIB binding.
Table 3.
Regional asymmetry indices (AI) for 11C-PBR28 binding, voxel count, FDG, and PIB.
| AI (%)
|
|||
|---|---|---|---|
| PCA | AD | HC | |
|
| |||
| 11C-PBR28 (SUVR) | |||
| Superior parietal | 3.5 ± 4.2 | −0.4 ± 4.7 | 0.1 ± 3.2 |
| Inferior parietal | 3.8 ± 4.2a | −0.4 ± 4.6 | 0.7 ± 2.6 |
| Occipital | 3.7 ± 3.7b,c | −1.3 ± 3.0 | 0.2 ± 2.0 |
| Superior temporal | 1.0 ± 2.2 | −0.5 ± 4.4 | 0.6 ± 2.6 |
| Mid & inf temporal | 2.7 ± 2.1b | −1.7 ± 4.2 | 0.0 ± 2.9 |
| FDG (SUVR) | |||
| Superior parietal | 7.6 ± 9.5d | N/A | −1.1 ± 1.6 |
| Inferior parietal | 7.0 ± 9.5d | N/A | −0.4 ± 2.5 |
| Occipital | 5.3 ± 8.5d | N/A | −1.3 ± 2.8 |
| Superior temporal | 1.4 ± 2.8 | N/A | −0.1 ± 1.8 |
| Mid & inf temporal | 2.2 ± 5.9 | N/A | −0.6 ± 2.1 |
| PIB (DVR) | |||
| Superior parietal | −0.2 ± 2.9 | −1.7 ± 5.4 | 1.7 ± 4.2 |
| Inferior parietal | −0.4 ± 4.3 | −1.2 ± 4.6 | 2.4 ± 4.9 |
| Occipital | 0.9 ± 6.1 | −1.8 ± 6.6 | 0.0 ± 3.9 |
| Superior temporal | 0.3 ± 5.2 | −2.1 ± 7.1 | 0.4 ± 5.5 |
| Mid & inf temporal | −2.4 ± 4.1 | −4.2 ± 3.7 | 0.6 ± 4.8 |
Group differences tested with univariate ANOVA with diagnosis as fixed factor and age and education as covariates. AI (%) = 200 × (R − L)/(R + L) for 11C-PBR28 and PIB; AI (%) = −200 × (R − L)/(R + L) for voxel count and FDG. Therefore, a positive AI% represents a right-sided abnormality (greater 11C-PBR28 or PIB binding or lower FDG uptake in right than left) and a negative AI% represents a left-sided abnormality.
P < 0.05 vs. AD,
P < 0.01 vs. AD,
P < 0.05 vs. HC,
P < 0.01 vs. HC
PCA = posterior cortical atrophy, AD = amnestic Alzheimer’s disease, HC = healthy controls, SUVR = standardized uptake value ratio, DVR = distribution volume ratio. N/A = FDG data not available for AD patients. Data are presented as mean ± SD.
3.6. Correlation analyses
When PCA and AD patients were combined (n = 19), we found significant negative correlations between 11C-PBR28 binding and performance on Block Design in superior parietal lobule (R = −0.653, P = 0.002), inferior parietal lobule (R = −0.631, P = 0.004), precuneus (R = −0.472, P = 0.041), and occipital cortex (R = −0.742, P = 0.0003). We also found a positive correlation such that greater 11C-PBR28 binding in entorhinal cortex was associated with better performance on Block Design (R = 0.547, P = 0.015). For superior parietal lobule and occipital cortex, correlations were primarily driven by PCA patients (n = 8), whereas for inferior parietal lobule, the correlation was driven by AD patients (n = 11, Supplementary Fig 3). No correlation was seen between 11C-PBR28 binding and performance on the Delayed Recall of the Hopkins Verbal Learning Test.
We performed correlative analysis between 11C-PBR28 binding and voxel count using ROI data. We found that greater 11C-PBR28 binding correlated with lower voxel count in superior parietal lobule (R = −0.691, P = 0.0004), precuneus (R = −0.481, P = 0.023), occipital cortex (R = −0.707, P = 0.0002), and entorhinal cortex (R = −0.423, P = 0.0498). For superior parietal lobule, precuneus, and occipital cortex, correlations were primarily driven by PCA patients, whereas for entorhinal cortex the correlation was driven by AD patients (Supplementary Fig 4). These correlations remained significant after correcting voxel count for total gray matter.
We performed correlative analysis between 11C-PBR28 and PIB and found correlation in precuneus (R = 0.531, P = 0.028) when all patients were combined.
4. DISCUSSION
To our knowledge, this is the first PET study to compare TSPO binding in different clinical subtypes of AD. The distribution of increased 11C-PBR28 binding corresponded to clinical phenotype, with greatest change in visual association cortices in PCA patients and in limbic regions in amnestic AD patients. Increased 11C-PBR28 binding also mirrored reduced cortical thickness and FDG metabolism. In contrast, PIB binding was greater in both PCA and amnestic AD patients than in controls throughout neocortex. Taken together, these results suggest that TSPO overexpression is more closely related to neurodegeneration and clinical manifestation in AD than is amyloid burden.
11C-PBR28 binding correlated with reduced performance on Block Design in occipital and parietal brain regions. These results suggest that increased 11C-PBR28 binding is associated with worse visuospatial cognitive performance. We also found that higher binding in entorhinal cortex was associated with better performance on Block Design. This correlation was likely driven by the amnestic AD patients, who as a group had greater entorhinal binding and better Block Design performance than PCA patients. 11C-PBR28 binding correlated with small gray matter volume (voxel count) in parietal, occipital, entorhinal cortices. Interestingly, the relationship between 11C-PBR28 binding and reduced voxel count was stronger in PCA patients in parietal and occipital cortices and stronger in amnestic AD patients in entorhinal cortex. We additionally found that 11C-PBR28 binding correlated with PIB binding only in precuneus. This brain region accumulates PIB early in AD (Mintun et al., 2006) and showed increased 11C-PBR28 and PIB binding in both PCA and amnestic AD patients. No correlations with PIB were found in regions with phenotype-specific increase in 11C-PBR28 (e.g., occipital cortex for PCA, entorhinal cortex in amnestic AD). Therefore, phenotype-specific increase in regional TPSO appears to be more closely related to neurodegeneration than regional amyloid load.
Results from several studies have established a link between neuroimmune activation and Alzheimer’s pathology. Autopsy studies have demonstrated the presence of TSPO-expressing microglia proximal to neuritic plaques in AD brain (Cosenza-Nashat et al., 2009). In addition, in vitro studies have shown that paired helical filamental tau can activate microglia (Ghosh, et al., 2013; Morales et al., 2013). Amyloid and tau may be additive such that microglial activation is greatest where both proteinopathies are present. This may explain why 11C-PBR28 binding is more regionally specific than PIB. Some studies have shown more widespread increase in TSPO in AD (Edison et al., 2008; Fan et al., 2015; Kreisl et al., 2013). In our present study, we selected amnestic AD patients who were relatively intact in non-memory domains to best distinguish from PCA patients. Studies that found more diffuse increase in TSPO may have included AD patients with more generalized impairment and more widespread tau burden.
The relationship between amyloid deposition and AD phenotype remains unclear. Most prior studies found no difference in topography or amount of PIB binding between PCA and amnestic AD patients (de Souza et al., 2011; Ossenkoppele et al, 2016; Rosenbloom et al., 2011; Singh et al., 2015; Wang et al., 2015). However, cases of PCA patients with focal PIB uptake in posterior brain regions have been reported (Ng et al., 2007; Kambe et al., 2010; Tenovuo et al., 2008), and in one study PCA patients had greater occipital PIB than early onset AD patients (Lehmann et al., 2013). Greater PIB binding in occipital cortex may be a marker of overall disease severity. For example, high amounts of occipital PIB uptake have been reported in the logopenic variant of AD (Whitwell et al., 2013), and one study reported that the difference in occipital PIB binding seen between PCA and amnestic AD patients could be corrected by adjusting for global PIB binding (Formaglio et al., 2011). However, we found greater occipital PIB in PCA than amnestic patients even after correcting for duration of symptoms. Inter-individual regional vulnerability to cerebral amyloid may determine the topography of tau-mediated neurodegeneration and therefore the clinical phenotype. One possibility is that regional differences in amyloid-induced neuroimmune activation promote tau aggregation. Under this hypothesis, microglial activation could influence which brain regions are preferentially affected in AD and, in turn, the resulting clinical presentation (e.g., memory vs. visuospatial impairment).
We also found modest asymmetry in patients with PCA, such that 11C-PBR28 binding was greater in right than left occipital and parietal cortices. Similar asymmetry was found for FDG hypometabolism among PCA patients. While right > left FDG hypometabolism was previously noted in PCA (Nestor et al., 2003), individual patients may demonstrate symmetric or left > right hypometabolism (Schmidtke et al., 2005). Therefore, we cannot conclude that PCA is necessarily associated with predominant right-sided neurodegeneration. However, that the asymmetry found in our study was demonstrated in 11C-PBR28 and FDG, but not in PIB imaging, further supports the argument that increased TSPO density is more strongly related to neurodegeneration than to amyloid deposition alone.
We also found greater 11C-PBR28 binding in the two AD subtypes using both kinetic modeling and a simplified ratio method. Our results therefore provide further support of the SUVR approach to 11C-PBR28 analysis, and demonstrate that this approach can be used regardless of clinical AD subtype. The SUVR method requires only 30 minutes of imaging, does not require plasma measurement, and improves power for statistical analysis over kinetic modeling (Lyoo et al., 2015).
This study has limitations, including the relatively low sample size. However, we still saw a large effect size between patients and controls using both kinetic modeling and the simplified SUVR method, suggesting we had sufficient power for this study. Another limitation is that for one patient with PCA, we were unable to determine TSPO binding status, either by in vitro binding assay or TSPO genotyping. This subject was assumed to be HAB based on absolute 11C-PBR28 binding in cerebellum (VT/fP). If this subject’s binding status was incorrectly designated, this would have increased the chance of a Type 2 error, rather than a Type 1 error, because statistical correction of the VT/fP values would have underestimated TSPO binding in this subject. Therefore, we do not believe that inclusion of this subject in the primary analyses confounded our results. Another limitation is the lack of FDG data in the amnestic AD group and non-uniform FDG imaging procedures between PCA patients and controls. Performing FDG in each amnestic patient was beyond the scope of the initial study design. Results from prior studies have shown that amnestic AD patients have bitemporal-parietal pattern of hypometabolism while PCA patients have posterior hypometabolism (Rosenbloom et al., 2011). Our use of FDG in this study therefore serves to highlight 1) that our PCA patients as a group had posterior pattern of hypometabolism, thus supporting the correct designation as PCA, and 2) that increased 11C-PBR28 binding in PCA overlaps with FDG hypometabolism, thus supporting the argument that increased TSPO is associated with neurodegeneration in the PCA subtype of AD.
We used a partial volume correction for ROI analysis because the patients, particularly those with PCA, had considerable atrophy which is expected to underestimate binding in gray matter. We previously demonstrated that the region-based voxelwise method for partial volume correction showed similar results as uncorrected data in comparing AD patients to controls with 11C-PBR28 (Kreisl et al., 2013). However, we have not previously used partial volume correction for voxelwise analysis of 11C-PBR28 images. Therefore, for the SPM analysis, we used the more conservative approach for each radioligand. Because binding is expected to be greater in patients than controls for 11C-PBR28 and PIB but lower in patients for FDG, we used partial volume-uncorrected images for voxelwise analysis of 11C-PBR28 and PIB, but partial volume-corrected images for FDG. We expect that using corrected images for PIB and PBR28 and uncorrected images for FDG would result in even larger effect sizes.
In conclusion, we found that patients with PCA and amnestic AD had distinct patterns of increased TSPO binding that mirrored neurodegeneration on MRI and FDG PET. These results suggest that neuroimmune activation is closely associated with neuronal loss across different subtypes of AD. Further studies are required to determine whether microglial response to amyloid influences the pattern of neurodegeneration and the resulting clinical expression of AD.
Supplementary Material
HIGHLIGHTS.
The first PET study to compare TSPO binding in different clinical subtypes of AD.
PCA patients had greater occipito-parieto-temporal 11C-PBR28 binding than controls
Amnestic patients showed 11C-PBR28 binding in inferior and medial temporal cortex.
Subtypes of AD have distinct patterns of TSPO binding that mirrors degeneration.
Acknowledgments
ROLE OF FUNDING SOURCE
This work was funded by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002852 and ZIAMH0022793 under clinicaltrials.gov identifiers NCT00955422 and NCT00613119). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Yi Zhang, PhD for assistance in radioligand production, Francis McMahon and Winston Corona for TSPO genotyping, Ioline Henter for editorial assistance, Angela Summers for performing neuropsychological testing, and Maria Ferraris-Araneta, Denise Rallis-Frutos, Yulin Chu, Gerald Hodges, and the NIH PET Department for assistance with PET studies.
Abbreviations
- AD
Alzheimer’s disease
- 11C-PBR28
[O-methyl-11C]-N-acetyl-N-(2-methoxybenzyl)-2-phenoxy-5-pyridinamine
- FDG
18F-fluorodeoxyglucose
- PCA
Posterior cortical atrophy
- PIB
11C-Pittsburgh Compound B
- TSPO
translocator protein (18 kDa)
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann TP1, Duyar H, Sollberger M, Kuhle J, Regeniter A, Gomez-Mancilla B, Schmidtke K, Monsch AU. CSF-tau and CSF-Abeta(1–42) in posterior cortical atrophy. Dement Geriatr Cogn Disord. 2010;29:530–3. doi: 10.1159/000314679. [DOI] [PubMed] [Google Scholar]
- Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, Lee SC. 2009 Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–28. doi: 10.1111/j.1365-2990.2008.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza LC, Corlier F, Habert MO, Uspenskaya O, Maroy R, Lamari F, Chupin M, Lehéricy S, Colliot O, Hahn-Barma V, Samri D, Dubois B, Bottlaender M, Sarazin M. Similar amyloid-beta burden in posterior cortical atrophy and Alzheimer’s disease. Brain. 2011;134:2036–43. doi: 10.1093/brain/awr130. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, Rossor M, Brooks DJ. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–9. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Fan Z, Okello AA, Brooks DJ, Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain. 2015;138:3685–98. doi: 10.1093/brain/awv288. [DOI] [PubMed] [Google Scholar]
- Formaglio M, Costes N, Seguin J, Tholance Y, Le Bars D, Roullet-Solignac I, Mercier B, Krolak-Salmon P, Vighetto A. In vivo demonstration of amyloid burden in posterior cortical atrophy: a case series with PET and CSF findings. J Neurol. 2011;258:1841–51. doi: 10.1007/s00415-011-6030-0. [DOI] [PubMed] [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T, Hong J, Pike VW, Innis RB. Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. NeuroImage. 2008;40:43–52. doi: 10.1016/j.neuroimage.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, LaFerla FM, Olschowka JA, O’Banion MK. Sustained interleukin-1beta overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci. 2013;33:5053–64. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambe T, Motoi Y, Ishii K, Hattori N. 2010 Posterior cortical atrophy with [11C] Pittsburgh compound B accumulation in the primary visual cortex. J Neurol. 2010;257:469–71. doi: 10.1007/s00415-009-5377-y. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB Biomarkers Consortium PET Radioligand Project Team. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013a;33:53–8. doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E, Jenko KJ, Morse CL, Zoghbi SS, Pike VW, Turner RS, Innis RB. 11C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol Aging. 2016;44:53–61. doi: 10.1016/j.neurobiolaging.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N, Corona W, Morse CL, Zoghbi SS, Pike VW, McMahon FJ, Turner RS, Innis RB Biomarkers Consortium PET Radioligand Project Team. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013b;136:2228–38. doi: 10.1093/brain/awt145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136:844–58. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Meltzer CC, Schimmel K, Tsopelas ND, DeKosky ST, Price JC. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: a comparative analysis. J Nucl Med. 2005;46:1959–72. [PubMed] [Google Scholar]
- Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW, Fujita M, Innis RB, Kreisl WC. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J Nucl Med. 2015;56:701–6. doi: 10.2967/jnumed.114.146027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol. 2013;126:479–97. doi: 10.1007/s00401-013-1177-7. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: Potential antecendant marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Morales I, Jimenez JM, Mancilla M, Maccioni RB. Tau oligomers and fibrils induce activation of microglial cells. J Alzheimers Dis. 2013;37:849–56. doi: 10.3233/JAD-131843. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer’s disease) with FDG-PET. J Neurol Neurosurg Psychiatry. 2003;74:1521–9. doi: 10.1136/jnnp.74.11.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SY, Villemagne VL, Masters CL, Rowe CC. Evaluating atypical dementia syndromes using positron emission tomography with carbon 11 labeled Pittsburgh Compound B. Arch Neurol. 2007;64:1140–4. doi: 10.1001/archneur.64.8.1140. [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M5, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–67. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011;76:1789–96. doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K, Hull M, Talazko J. Posterior cortical atrophy: variant of Alzheimer’s disease? A case series with PET findings. J Neurol. 2005;252:27–35. doi: 10.1007/s00415-005-0594-5. [DOI] [PubMed] [Google Scholar]
- Singh TD, Josephs KA, Machulda MM, Drubach DA, Apostolova LG, Lowe VJ, Whitwell JL. Clinical, FDG and amyloid PET imaging in posterior cortical atrophy. J Neurol. 2015;262:1483–92. doi: 10.1007/s00415-015-7732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, Caselli RJ, Knopman DS, Petersen RC. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Tenovuo O, Kemppainen N, Aalto S, Nagren K, Rinne JO. Posterior cortical atrophy: a rare form of dementia with in vivo evidence of amyloid-beta accumulation. J Alzheimers Dis. 2008;15:351–5. doi: 10.3233/jad-2008-15301. [DOI] [PubMed] [Google Scholar]
- Thomas BA, Erlandsson K, Modat M, Thurfjell L, Vandenberghe R, Ourselin S, Hutton BF. The importance of appropriate partial volume correction for PET quantification in Alzheimer’s disease. Eu J Nucl Med Mol Imaging. 2011;38:1104–19. doi: 10.1007/s00259-011-1745-9. [DOI] [PubMed] [Google Scholar]
- Wang XD, Lu H, Shi Z, Cai L, Liu S, Liu S, Han T, Wang Y, Zhou Y, Wang X, Gao S, Ji Y. A Pilot Study on Clinical and Neuroimaging Characteristics of Chinese Posterior Cortical Atrophy: Comparison with Typical Alzheimer’s Disease. PLoS One. 2015;10:e0134956. doi: 10.1371/journal.pone.0134956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Lowe VJ, Duffy JR, Strand EA, Machulda MM, Kantarci K, Wille SM, Senjem ML, Murphy MC, Gunter JL, Jack CR, Jr, Josephs KA. Elevated occipital beta-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. J Neurol Neurosurg Psychiatry. 2013;84:1357–64. doi: 10.1136/jnnp-2013-305628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.