Abstract
This study aimed to detect the association between the MLX interacting protein-like (MLXIPL), BUD13 homolog (BUD13) and zinc finger protein 259 (ZNF259) single nucleotide polymorphisms (SNPs) and serum lipid levels in the Chinese Mulao and Han populations. Genotyping of 9 SNPs was performed in 825 Mulao and 781 Han participants. The genotype and allele frequencies of ZNF259 rs2075290 and rs964184, and BUD13 rs10790162 SNPs were different between the Mulao and Han populations (P < 0.001). The SNPs of ZNF259 rs2075290 and BUD13 rs10790162 were associated with serum total cholesterol levels; ZNF259 rs2075290 and rs964184, BUD13 rs10790162, and MLXIPL rs3812316 and rs13235543 were associated with triglyceride (TG); and MLXIPL rs35332062 was associated with apolipoprotein (Apo) A1 in the Mulaos (P < 0.006–0.001). However, in the Hans, the SNPs of ZNF259 rs2075290 and BUD13 rs10790162 were associated with serum TG levels; ZNF259 rs2075290 was associated with low-density lipoprotein cholesterol and the ApoA1/ApoB ratio (P < 0.006–0.001). Significant linkage disequilibria were noted among ZNF259 rs2075290 and rs964184 and BUD13 rs10790162, and between MLXIPL rs3812316 and rs13235543 (r2 > 0.05, P < 0.001). The haplotypes of A-C-G-A-C (rs2075290A-rs964184C-rs10790162G-rs17119975A-rs11556024C) and C-C-C-C (rs799161C-rs35332062C-rs3812316C-rs13235543C) accounted for over half of the % haplotype of each ethnic group.
Atherosclerotic cardiovascular disease (CVD) is a major disease burden worldwide1,2 and lipid modification plays an important role in the reduction of CVD risk2. Although lipid modification was mainly focused on reducing the low-density lipoprotein cholesterol (LDL-C) level in the past, lowering both triglyceride (TG) and LDL-C levels was found to be more beneficial than lowering LDL-C alone in recent years3. Consequently, several research efforts have been made to control serum TG levels. Serum TG concentration is a complex polygenic trait that is determined by environmental and genetic factors including common and rare variants in multiple genes4,5,6,7. Therefore, the understanding of the variants modulating the serum TG level has become crucial in the development of novel markers for risk prediction, diagnosis, and prognosis of CVD.
Recent genome-wide association studies (GWASs) have identified a great number of TG-related loci8,9,10,11. The MLX interacting protein-like (MLXIPL; Gene ID: 51085; OMIM: 605678) gene, formerly known as carbohydrate response element binding protein (ChREBP), is located on chromosome 7q11.23 and encodes a basic helix-loop-helix leucine zipper transcription factor of the Myc/Max/Mad superfamily. ChREBP regulates the expression of pyruvate kinase, which channels glycolytic pyruvate into lipogenesis through the conversion of dietary carbohydrate to storage fat in the liver12. Suppression of ChREBP could diminish aerobic glycolysis and de novo lipogenesis by switching aerobic glycolysis to oxidative phosphorylation13. The BUD13 homolog (BUD13; Gene ID: 84811; HGNC: 28199) and zinc finger protein 259 (ZNF259; Gene ID: 8882; OMIM: 603901) genes are located on 11q23.3 and encode for BUD13 homolog protein and zinc finger protein (ZPR1), respectively. BUD13 is one of the subunits of the RES complex, which was previously identified in yeast as a splicing factor that affects nuclear pre-mRNA retention14. ZPR1 is an essential protein required for normal nucleolar function in proliferating cells15.
Single nucleotide polymorphisms (SNPs) in the BUD13 and ZNF259 have been associated with serum lipid levels, especially with TG in western populations8,9,16,17; likewise, the MLXIPL SNPs were also associated with TG level in European and Indian Asian populations18. However, little is known about the association of these SNPs and serum lipid levels in Southern Chinese populations. Therefore, this study was undertaken to determine the association of the MLXIPL (rs35332062 A358V, rs3812316 Q241H, rs13235543 P342P and rs799161 g.11092833T>C), BUD13 (rs10790162 +1741T>C, rs17119975 -575A>G and rs11556024 *147C>T) and ZNF259 (rs2075290 -336G>A and rs964184 +359C>G) SNPs with serum lipid levels in the Mulao and Han populations.
Results
General and biochemical characteristics of the subjects
As shown in Table 1, the value of BMI was lower and the levels of apolipoprotein (Apo) B and the percentage of subjects who consumed alcohol were higher in Mulao than in Han (P < 0.01–0.001). There were no significant differences in the levels of total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), LDL-C and ApoA1 levels and the ratio of ApoA1 to ApoB between the two ethnic groups (P > 0.05 for all).
Table 1. Comparison of demographic, lifestyle characteristics and serum lipid levels between the Mulao and Han populations.
| Characteristics | Mulao | Han | t (χ2) | P-value |
|---|---|---|---|---|
| Number | 825 | 781 | ||
| Male/female | 354/471 | 307/474 | −1.466 | 0.143 |
| Age (years) | 49.18 ± 16.13 | 49.25 ± 16.21 | −0.082 | 0.934 |
| Height (cm) | 155.38 ± 8.05 | 154.6 ± 8.04 | 1.948 | 0.052 |
| Weight (kg) | 52.61 ± 9.41 | 53.43 ± 8.82 | −1.808 | 0.071 |
| Body mass index (kg/m2) | 21.72 ± 3.07 | 22.36 ± 3.48 | −3.894 | 1 × 10−4 |
| Waist circumference (cm) | 74.73 ± 8.59 | 75.03 ± 7.85 | −0.720 | 0.472 |
| Systolic blood pressure (mmHg) | 127.81 ± 21.12 | 128.03 ± 18.91 | −0.219 | 0.827 |
| Diastolic blood pressure (mmHg) | 80.35 ± 11.43 | 81.41 ± 11.19 | −1.871 | 0.061 |
| Pulse pressure (mmHg) | 47.46 ± 15.77 | 46.62 ± 13.95 | 1.130 | 0.259 |
| Cigarette smoking [n (%)] | ||||
| Nonsmoker | 649 (78.7) | 622 (79.7) | ||
| ≤20 Cigarette smoking/day | 145 (17.6) | 140 (17.9) | 0.877 | 0.381 |
| >20 Cigarette smoking/day | 31 (3.7) | 19 (2.4) | ||
| Alcohol consumption [n (%)] | ||||
| Nondrinker | 642 (77.8) | 623(79.8) | ||
| ≤25 g/day | 68(8.2) | 79(10.1) | 2.877 | 0.004 |
| >25 g/day | 115(13.9) | 79(10.1) | ||
| Blood glucose level (mmol/L) | 5.91 ± 1.56 | 5.92 ± 1.48 | −0.183 | 0.854 |
| Total cholesterol (mmol/L) | 4.94 ± 1.14 | 4.90 ± 1.00 | 0.702 | 0.483 |
| Triglyceride (mmol/L) | 1.04 (0.75) | 1.03 (0.85) | −0.432 | 0.666 |
| High-density lipoprotein cholesterol (mmol/L) | 1.74 ± 0.47 | 1.73 ± 0.52 | 0.404 | 0.687 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.91 ± 0.86 | 2.84 ± 0.83 | 1.614 | 0.107 |
| Apolipoprotein (Apo) A1 (g/L) | 1.30 ± 0.40 | 1.33 ± 0.26 | −1.631 | 0.103 |
| ApoB (g/L) | 0.97 ± 0.58 | 0.84 ± 0.20 | 6.246 | <1 × 10−7 |
| ApoA1/ApoB | 1.62 ± 0.99 | 1.67 ± 0.50 | −1.296 | 0.195 |
The continuous variables were presented as the mean ± standard deviation and their differences between the two ethnic groups were tested by t-test. The categorical variables were presented as the frequencies or percentages and their differences between the groups were tested by Chi square tests. The values of triglyceride were presented as the median (interquartile range) and their differences between the ethnic groups were determined by the Wilcoxon-Mann-Whitney test.
Results of electrophoresis
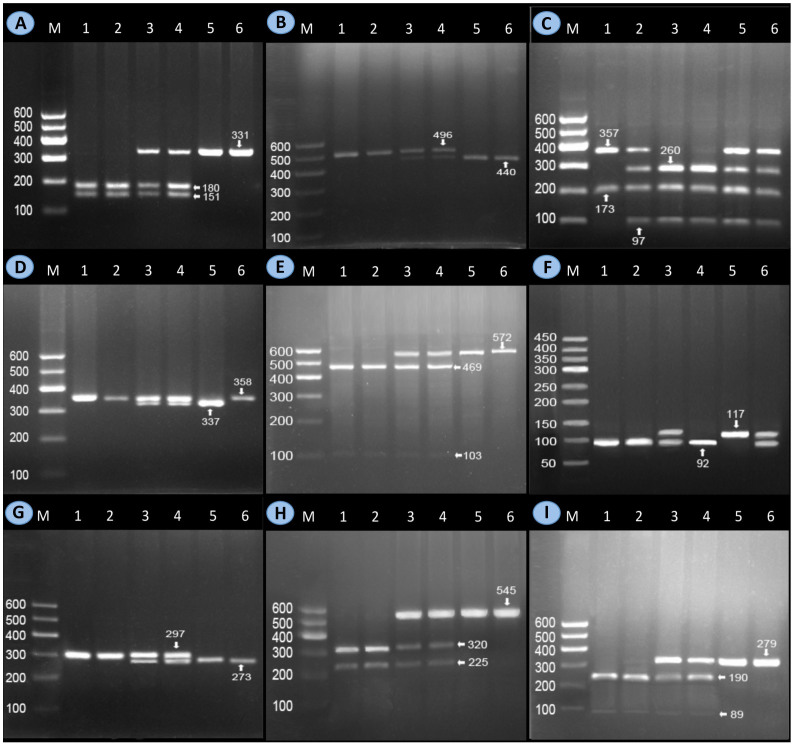
The polymerase chain reaction (PCR) products of rs2075290, rs964184, rs17119975, rs11556024, rs10790162, rs35332062, rs3812316, rs13235543 and rs799161 SNPs were 331-, 496-, 530-, 358-, 572-, 117-, 297-, 545- and 279-bp nucleotide sequences; respectively. After the restriction fragment length polymorphism (RFLP) reaction combined with electrophoresis, the genotypes were identified according to the number and length of the enzyme digestion fragments (Figure 1).
Figure 1. Genotyping of the MLXIPL, BUD13 and ZNF259 SNPs.
Lane M, 100 bp marker ladder; (A) ZNF259 rs2075290: lanes 1 and 2, AA genotype (180- and 151-bp) lanes 3 and 4, GA genotype (331-, 180- and 151-bp); and lanes 5 and 6, GG genotype (331 bp). (B) ZNF259 rs964184: lanes 1 and 2, CC genotype (496 bp); lanes 3 and 4, CG genotype (496-, 440- and 56-bp); and lanes 5 and 6, GG genotype (440- and 56-bp). (C) BUD13 rs10790162: lane 1, AA genotype (357- and 173-bp); lanes 2, 5 and 6, AG genotype (357-, 260-, 173- and 97-bp); and lanes 3 and 4, GG genotype (260-, 173- and 97-bp). (D) BUD13 rs17119975 SNP: lanes 1, 2 and 6, AA genotype (358 bp); lanes 3 and 4, AG genotype (358-, 337- and 21-bp); and lane 5, GG genotype (337- and 21-bp). (E) BUD13 rs11556024: lanes 1 and 2, CC genotype (469- and 103-bp); lanes 3 and 4, CT genotype (572-, 469- and 103-bp); and lanes 5 and 6, TT genotype (572 bp). (F) MLXIPL rs799161: lanes 1, 2 and 4, CC genotype (92- and 25-bp); lanes 3 and 6, CT genotype (117-, 92- and 25-bp); and lane 5, TT genotype (117 bp). (G) MLXIPL rs35332062: lanes 1 and 2, CC genotype (297 bp); lanes 3 and 4, CT genotype (297-, 273- and 24-bp); and lanes 5 and 6, TT genotype (273- and 24-bp). (H) MLXIPL rs3812316: lanes 1 and 2, GG genotype (320- and 225-bp); lanes 3 and 4, CG genotype (545-, 320- and 225-bp); and lanes 5 and 6, CC genotype (545 bp). (I) MLXIPL rs13235543: lanes 1 and 2, CC genotype (190- and 89-bp); lanes 3 and 4, CT genotype (279-, 190- and 89-bp); and lanes 5 and 6, TT genotype (279 bp). The bands less than 90-bp fragments were not visible in the gel owing to their fast migration speed.
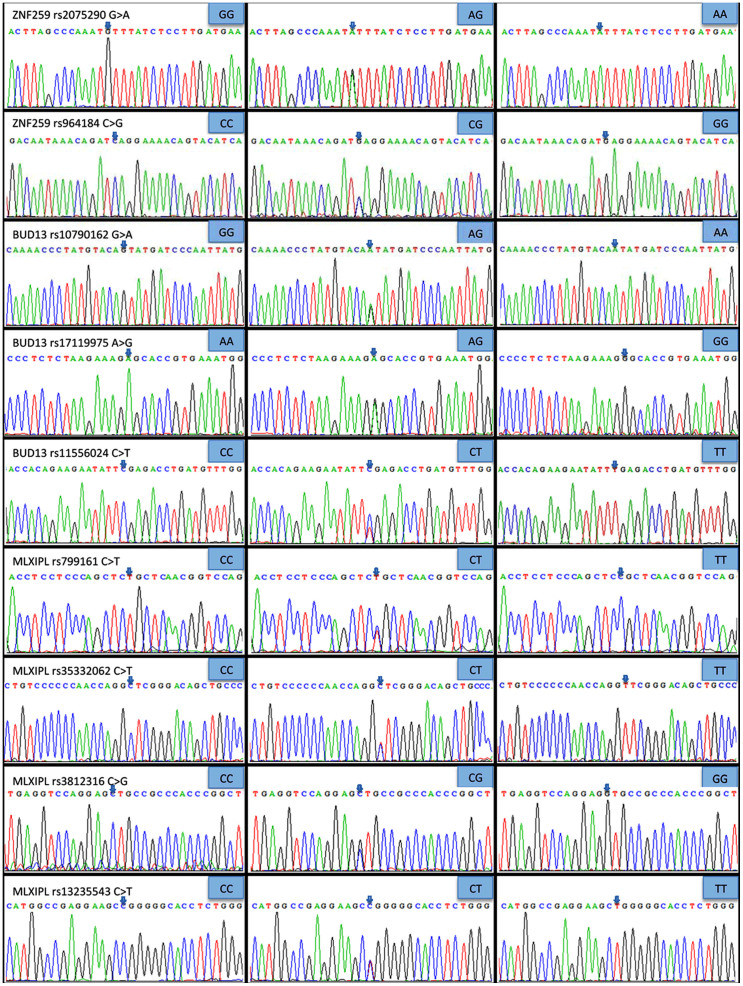
DNA sequencing
The genotypes detected by PCR-RFLP were also confirmed by direct sequencing (Figure 2). The sequencing results were directly submitted to GenBank's Gene Expression Omnibus (GEO) database. The GenBank accession numbers for the DNA sequences of the ZNF259 rs2075290 AA/AG/GG genotypes were KF306313-306315, the ZNF259 rs964184 CC/CG/GG genotypes were KF306310-306312, the BUD13 rs10790162 GG/AG/AA genotypes were KF306302-306304, the BUD13 rs17119975 AA/AG/GG genotypes were KF306316-306318, the BUD13 rs11556024 CC/CT/TT genotypes were KF306305-306307, the MLXIPL rs799161 TT/CT/CC genotypes were KF306319-306321, the MLXIPL rs35332062 CC/CT/TT genotypes were KF306325-306327, the MLXIPL rs3812316 CC/CG/GG genotypes were KC853060-853062, and the MLXIPL rs13235543 CC/CT/TT genotypes were KF306322-306324, respectively.
Figure 2. A part of the nucleotide sequences of the MLXIPL, BUD13 and ZNF259 SNPs by direct sequencing.
MLXIPL: MLX interacting protein-like, BUD13: BUD13 homolog and ZNF259: zinc finger protein 259.
Genotypic and allelic frequencies
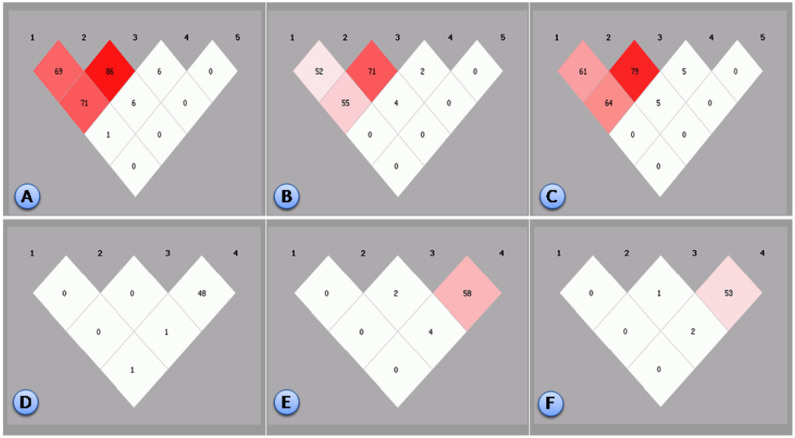
As shown in Table 2, of the 9 SNPs, the genotype and allele frequencies of the ZNF259 rs2075290 and rs964184, and BUD13 rs10790162 SNPs were different between the Mulao and Han populations (P < 0.001 for each). The genotype frequencies but not the allele frequencies of BUD13 rs17119975 SNP were different between the Mulao and Han populations (P < 0.05). All SNPs (except MLXIPL rs799161) were in the Hardy-Weinberg equilibrium (P > 0.05). Significant linkage disequilibria (LD) were found between ZNF259 rs2075290 and rs964184 (r2 = 0.699 in Mulao, r2 = 0.526 in Han, P < 0.001); ZNF259 rs2075290 and rs10790162 (r2 = 0.715 in Mulao, r2 = 0.558 in Han, P < 0.001); ZNF259 rs964184 and BUD13 rs10790162 (r2 = 0.866 in Mulao, r2 = 0.718 in Han, P < 0.001); and MLXIPL rs3812316 and rs13235543 (r2 = 0.482 in Mulao, r2 = 0.588 in Han, P < 0.001; Figure 3).
Table 2. Comparison of genotype and allele frequencies between the Mulao and Han populations [n (%)].
| Mulao | Han | ||||
|---|---|---|---|---|---|
| SNP | Genotype/Allele | (n = 825) | (n = 781) | χ2 | P-value |
| ZNF259 rs2075290 | AA/GA/GG | 413(50.1)/348(42.2)/64(7.7) | 460(58.9)/279(35.7)/42(5.4) | 13.494 | 0.001 |
| A/G | 1174(71.2)/476(28.8) | 1199(76.8)/363(23.2) | 13.082 | 3 × 10−4 | |
| ZNF259 rs964184 | CC/CG/GG | 467(56.6)/306(37.1)/52(6.3) | 515(65.9)/234(30.0)/32(4.1) | 15.514 | 4 × 10−4 |
| C/G | 1240(75.2)/410(24.8) | 1264(80.9)/298(19.1) | 15.548 | 8 × 10−5 | |
| BUD13 rs10790162 | GG/GA/AA | 472(57.2)/295(35.8)/58(7.0) | 519(66.5)/230(29.4)/32(4.1) | 16.595 | 2 × 10−4 |
| G/A | 1239 (75.1)/411 (24.9) | 1268 (81.2)/294 (18.8) | 17.355 | 3 × 10−5 | |
| BUD13 rs17119975 | AA/AG/GG | 537(65.1)/254(30.8)/34(4.1) | 472(60.4)/284(36.4)/25(3.2) | 6.032 | 0.049 |
| A/G | 1328 (80.5)/322 (19.5) | 1228 (78.6)/334 (21.4) | 1.722 | 0.189 | |
| BUD13 rs11556024 | CC/CT/TT | 700(84.9)/120(14.5)/5(0.6) | 671(85.9)/103(13.2)/7(0.9) | 1.038 | 0.595 |
| C/T | 1520 (92.1)/130 (7.9) | 1445 (92.5)/117 (7.5) | 0.171 | 0.680 | |
| MLXIPL rs799161 | CC/CT/TT | 361(43.8)/390(47.2)/74(9.0) | 345(44.2)/378(48.4)/58(7.4) | 1.285 | 0.526 |
| C/T | 1112 (67.4)/538 (32.6) | 1068 (68.4)/494 (31.6) | 0.353 | 0.552 | |
| MLXIPL rs35332062 | CC/CT/TT | 717(86.9)/98(11.9)/10(1.2) | 692(88.6)/83(10.6)/6(0.8) | 1.482 | 0.477 |
| C/T | 1532 (92.8)/118 (7.2) | 1467 (93.9)/95 (6.1) | 1.483 | 0.223 | |
| MLXIPL rs3812316 | CC/CG/GG | 751(91.0)/67(8.1)/7(0.9) | 703(90.0)/76(9.7)/2(0.3) | 3.726 | 0.155 |
| C/G | 1569 (95.1)/81 (4.9) | 1482 (94.9)/80 (5.1) | 0.076 | 0.783 | |
| MLXIPL rs13235543 | CC/CT/TT | 704(85.3)/114(13.8)/7(0.9) | 682(87.3)/94(12.0)/5(0.7) | 1.401 | 0.496 |
| C/T | 1522 (92.2)/128 (7.8) | 1458 (93.3)/104 (6.7) | 1.447 | 0.229 |
ZNF259, zinc finger protein 259; BUD13, BUD13 homolog; MLXIPL, MLX interacting protein-like.
Figure 3. Linkage disequilibrium statuses of the MLXIPL, BUD13 and ZNF259 SNPs.
Linkage disequilibrium among the (1) ZNF259 rs2075290, (2) ZNF259 rs964184 and (3) BUD13 rs10790162, (4) BUD13 rs17119975 and (5) BUD13 rs11556024 SNPs in the Mulao (A), Han (B) and combined Mulao and Han populations (C). Linkage disequilibrium among the (1) MLXIPL rs799161, (2) MLXIPL rs35332062, (3) MLXIPL rs3812316 and (4) MLXIPL rs13235543 SNPs in the Mulao (D), Han (E) and combined Mulao and Han populations (F). The linkage disequilibrium status is illustrated by the magnitude of the r2 value.
The frequencies of haplotypes are listed in Table 3. Six haplotypes (among 5 SNPs of BUD13/ZNF259) and 7 haplotypes (among 4 SNPs of MLXIPL) with a frequency >1% were identified in the Mulao and Han populations respectively. We combined 17 haplotypes (among 5 SNPs of BUD13/ZNF259) and 13 haplotypes (among 4 SNPs of MLXIPL) with frequencies less than 3% into one group, called “rare_hap”. The haplotypes of A-C-G-A-C (among the ZNF259 rs2075290 and rs964184, and BUD13 rs10790162, rs17119975 and rs11556024 SNPs) and C-C-C-C (among the MLXIPL rs799161, rs35332062, rs3812316 and rs13235543 SNPs) accounted for over half of the % haplotype of each ethnic group. The frequencies of the A-C-G-A-C and G-G-A-A-C haplotypes were significantly different between the two ethnic groups (P < 0.01 for each).
Table 3. Haplotype frequencies among 5 SNPs of the BUD13/ZNF259 genes and 4 SNPs of the MLXIPL gene between the Mulao and Han populations [n(%)].
| Haplotype | Mulao | Han | χ2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| ZNF259 rs2075290 | ZNF259 rs964184 | BUD13 rs10790162 | BUD13 rs17119975 | BUD13 rs11556024 | ||||
| A | C | G | A | C | 761 (54.0) | 761 (54.7) | 7.276 | 0.007 |
| G | G | A | A | C | 323 (22.9) | 216 (15.5) | 24.739 | 0.000 |
| A | C | G | G | C | 213 (15.1) | 243 (17.5) | 3.078 | 0.079 |
| G | C | G | G | C | 51 (3.6) | 51 (3.7) | 0.002 | 0.963 |
| Rare Hap (<3%) | 62 (4.4) | 119 (8.6) | 6.129 | 0.013 | ||||
ZNF259, zinc finger protein 259; BUD13, BUD13 homolog; MLXIPL, MLX interacting protein-like.
ZNF259, zinc finger protein 259; BUD13, BUD13 homolog; MLXIPL, MLX interacting protein-like.
Genotypes and serum lipid levels
As shown in Table 4, the levels of TG (ZNF259 rs2075290 and rs964184, BUD13 rs10790162, and MLXIPL rs13235543), ApoA1 (MLXIPL rs35332062), ApoB (MLXIPL rs13235543) in the Mulao population were significantly different among the three genotypes (P < 0.006–0.001), whereas the levels of TG (BUD13 rs10790162) and ApoA1 (MLXIPL rs11556024) in the Han population were different among the genotypes (P < 0.006–0.001). When the minor homozygous genotype was combined with the heterozygous genotype to enhance power, the levels of TC (ZNF259 rs2075290 and BUD13 rs10790162), TG (ZNF259 rs2075290 and rs964184, BUD13 rs10790162, and MLXIPL rs3812316 and rs13235543) and ApoA1 (MLXIPL rs35332062) in the Mulao population were found to be significantly different between the two genotypes (P < 0.006–0.001); whereas, the levels of TG (ZNF259 rs2075290 and BUD13 rs10790162), LDL-C and the ratio of ApoA1/ApoB (ZNF259 rs2075290) in the Han population were different between the genotypes (P < 0.006–0.001).
Table 4. Comparison of serum lipid levels among the genotypes in the Mulao and Han populations.
| Genotype | n | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ApoA1 (g/L) | ApoB (g/L) | ApoA1/ApoB |
|---|---|---|---|---|---|---|---|---|
| ZNF259 rs2075290 G>A | ||||||||
| Mulao | ||||||||
| AA | 413 | 4.83 ± 1.15 | 0.97(0.65) | 1.72 ± 0.43 | 2.86 ± 0.84 | 1.32 ± 0.38 | 0.96 ± 0.56 | 1.70 ± 1.22 |
| GA | 348 | 4.96 ± 1.10 | 1.09(0.83) | 1.76 ± 0.53 | 2.93 ± 0.88 | 1.28 ± 0.41 | 0.98 ± 0.60 | 1.54 ± 0.69 |
| GG | 64 | 5.26 ± 1.14 | 1.33(1.46) | 1.69 ± 0.39 | 3.07 ± 0.85 | 1.31 ± 0.39 | 0.95 ± 0.47 | 1.62 ± 0.77 |
| F | 4.126 | 10.208 | 0.971 | 2.591 | 0.641 | 0.257 | 2.247 | |
| P | 0.017 | 0.001 | 0.379 | 0.076 | 0.527 | 0.774 | 0.106 | |
| AA | 413 | 4.83 ± 1.15 | 0.97(0.65) | 1.72 ± 0.43 | 2.86 ± 0.84 | 1.32 ± 0.38 | 0.96 ± 0.56 | 1.70 ± 1.22 |
| GA/GG | 412 | 5.11 ± 1.11 | 1.12(0.83) | 1.73 ± 0.51 | 3.00 ± 0.87 | 1.30 ± 0.41 | 0.97 ± 0.20 | 1.58 ± 0.71 |
| F | 8.127 | −4.157 | 0.030 | 3.764 | 0.244 | 0.043 | 1.895 | |
| P | 0.004 | 2 × 10−5 | 0.862 | 0.053 | 0.622 | 0.836 | 0.169 | |
| Han | ||||||||
| AA | 460 | 4.90 ± 0.98 | 0.970.82) | 1.76 ± 0.59 | 2.87 ± 0.82 | 1.34 ± 0.26 | 0.84 ± 0.20 | 1.70 ± 0.52 |
| GA | 279 | 4.86 ± 1.09 | 1.15(0.85) | 1.67 ± 0.39 | 2.77 ± 0.89 | 1.31 ± 0.26 | 0.83 ± 0.19 | 1.65 ± 0.45 |
| GG | 42 | 5.16 ± 0.73 | 1.10(1.51) | 1.63 ± 0.43 | 3.05 ± 0.61 | 1.30 ± 0.21 | 0.89 ± 0.18 | 1.49 ± 0.41 |
| F | 1.899 | 6.073 | 3.310 | 3.569 | 1.174 | 2.844 | 4.233 | |
| P | 0.150 | 0.014 | 0.037 | 0.029 | 0.310 | 0.059 | 0.015 | |
| AA | 460 | 4.90 ± 0.98 | 0.97(0.82) | 1.76 ± 0.59 | 2.87 ± 0.82 | 1.34 ± 0.26 | 0.84 ± 0.20 | 1.70 ± 0.52 |
| GA/GG | 321 | 5.01 ± 1.05 | 1.15(0.87) | 1.65 ± 0.39 | 2.91 ± 0.86 | 1.30 ± 0.25 | 0.86 ± 0.19 | 1.57 ± 0.46 |
| F | 1.491 | −3.017 | 5.101 | 7.266 | 1.993 | 2.273 | 8.307 | |
| P | 0.222 | 0.003 | 0.024 | 0.007 | 0.158 | 0.132 | 0.004 | |
| ZNF259 rs964184 C>G | ||||||||
| Mulao | ||||||||
| CC | 467 | 4.86 ± 1.14 | 0.97(0.66) | 1.72 ± 0.41 | 2.89 ± 0.84 | 1.29 ± 0.39 | 0.95 ± 0.55 | 1.62 ± 0.83 |
| CG | 306 | 5.03 ± 1.10 | 1.00(0.83) | 1.78 ± 0.55 | 2.97 ± 0.88 | 1.31 ± 0.41 | 0.99 ± 0.59 | 1.58 ± 0.73 |
| GG | 52 | 5.14 ± 1.20 | 1.11(1.29) | 1.65 ± 0.41 | 3.01 ± 0.88 | 1.31 ± 0.36 | 0.94 ± 0.38 | 1.53 ± 0.61 |
| F | 2.724 | 10.903 | 2.376 | 1.164 | 0.146 | 0.501 | 0.372 | |
| P | 0.066 | 0.001 | 0.094 | 0.313 | 0.864 | 0.606 | 0.689 | |
| CC | 467 | 4.86 ± 1.14 | 0.97(0.66) | 1.72 ± 0.41 | 2.89 ± 0.84 | 1.29 ± 0.39 | 0.95 ± 0.55 | 1.62 ± 0.83 |
| CG/GG | 358 | 5.08 ± 1.11 | 1.14(0.89) | 1.71 ± 0.54 | 2.99 ± 0.88 | 1.31 ± 0.41 | 0.96 ± 0.57 | 1.56 ± 0.71 |
| F | 4.690 | −4.025 | 0.002 | 1.910 | 0.180 | 0.111 | 0.681 | |
| P | 0.031 | 6 × 10−5 | 0.968 | 0.167 | 0.671 | 0.739 | 0.410 | |
| Han | ||||||||
| CC | 515 | 4.85 ± 0.96 | 0.99(0.86) | 1.75 ± 0.58 | 2.80 ± 0.82 | 1.33 ± 0.26 | 0.82 ± 0.19 | 1.71 ± 0.51 |
| CG | 234 | 4.96 ± 1.15 | 1.11(0.86) | 1.66 ± 0.38 | 2.89 ± 0.92 | 1.31 ± 0.24 | 0.86 ± 0.20 | 1.59 ± 0.42 |
| GG | 32 | 5.20 ± 0.71 | 1.02(1.14) | 1.74 ± 0.37 | 3.08 ± 0.65 | 1.35 ± 0.19 | 0.87 ± 0.19 | 1.67 ± 0.67 |
| F | 2.739 | 6.087 | 2.119 | 2.450 | 1.153 | 3.677 | 4.912 | |
| P | 0.065 | 0.014 | 0.121 | 0.087 | 0.316 | 0.026 | 0.008 | |
| CC | 515 | 4.85 ± 0.96 | 0.99(0.86) | 1.75 ± 0.58 | 2.80 ± 0.82 | 1.33 ± 0.26 | 0.82 ± 0.19 | 1.71 ± 0.51 |
| CG/GG | 266 | 5.07 ± 1.11 | 1.07(0.86) | 1.70 ± 0.38 | 2.99 ± 0.89 | 1.33 ± 0.23 | 0.86 ± 0.20 | 1.63 ± 0.46 |
| F | 5.416 | −2.522 | 0.687 | 4.782 | 0.064 | 4.698 | 2.411 | |
| P | 0.020 | 0.012 | 0.407 | 0.029 | 0.801 | 0.031 | 0.121 | |
| BUD13 rs10790162 G>A | ||||||||
| Mulao | ||||||||
| GG | 472 | 4.83 ± 1.15 | 0.96(0.66) | 1.73 ± 0.42 | 2.86 ± 0.82 | 1.30 ± 0.39 | 0.95 ± 0.57 | 1.64 ± 0.84 |
| AG | 295 | 4.98 ± 1.13 | 1.12(0.79) | 1.77 ± 0.55 | 2.92 ± 0.90 | 1.29 ± 0.41 | 0.97 ± 0.57 | 1.58 ± 0.72 |
| AA | 58 | 5.25 ± 1.20 | 1.41(1.39) | 1.63 ± 0.40 | 3.11 ± 0.91 | 1.31 ± 0.35 | 1.00 ± 0.48 | 1.50 ± 0.63 |
| F | 3.783 | 15.444 | 2.079 | 2.196 | 0.036 | 0.314 | 1.021 | |
| P | 0.023 | 9 × 10−5 | 0.126 | 0.112 | 0.964 | 0.730 | 0.361 | |
| GG | 472 | 4.83 ± 1.15 | 0.96(0.66) | 1.73 ± 0.42 | 2.86 ± 0.82 | 1.30 ± 0.39 | 0.95 ± 0.57 | 1.64 ± 0.84 |
| AG/AA | 353 | 5.11 ± 1.14 | 1.15(0.90) | 1.70 ± 0.53 | 3.02 ± 0.90 | 1.30 ± 0.40 | 0.97 ± 0.56 | 1.54 ± 0.71 |
| F | 7.562 | −5.000 | 0.361 | 4.320 | 0.013 | 0.608 | 2.008 | |
| P | 0.006 | 1 × 10−6 | 0.548 | 0.038 | 0.911 | 0.436 | 0.157 | |
| Han | ||||||||
| GG | 519 | 4.86 ± 0.96 | 0.97(0.80) | 1.77 ± 0.59 | 2.81 ± 0.82 | 1.34 ± 0.26 | 0.82 ± 0.19 | 1.71 ± 0.51 |
| AG | 230 | 4.96 ± 1.14 | 1.17(0.70) | 1.66 ± 0.37 | 2.89 ± 0.92 | 1.31 ± 0.24 | 0.86 ± 0.20 | 1.60 ± 0.42 |
| AA | 32 | 5.07 ± 0.65 | 1.16(0.67) | 1.67 ± 0.40 | 2.96 ± 0.62 | 1.29 ± 0.21 | 0.87 ± 0.20 | 1.62 ± 0.73 |
| F | 1.415 | 13.752 | 3.480 | 1.050 | 1.541 | 3.141 | 4.634 | |
| P | 0.244 | 2 × 10−4 | 0.031 | 0.350 | 0.215 | 0.044 | 0.010 | |
| GG | 519 | 4.86 ± 0.96 | 0.97(0.80) | 1.77 ± 0.59 | 2.81 ± 0.82 | 1.34 ± 0.26 | 0.82 ± 0.19 | 1.71 ± 0.51 |
| AG/AA | 262 | 5.02 ± 1.09 | 1.17(1.02) | 1.66 ± 0.38 | 2.93 ± 0.89 | 1.30 ± 0.24 | 0.86 ± 0.20 | 1.61 ± 0.47 |
| F | 2.363 | −3.989 | 3.367 | 1.675 | 2.354 | 4.250 | 4.176 | |
| P | 0.125 | 7 × 10−5 | 0.067 | 0.196 | 0.125 | 0.040 | 0.041 | |
| BUD13 rs17119975 A>G | ||||||||
| Mulao | ||||||||
| AA | 537 | 4.96 ± 1.17 | 1.07(0.79) | 1.74 ± 0.50 | 2.93 ± 0.88 | 1.32 ± 0.39 | 0.95 ± 0.53 | 1.62 ± 0.77 |
| AG | 254 | 4.90 ± 1.06 | 1.01(0.62) | 1.73 ± 0.42 | 2.90 ± 0.83 | 1.25 ± 0.42 | 1.00 ± 0.64 | 1.62 ± 1.42 |
| GG | 36 | 4.57 ± 1.08 | 0.88(0.79) | 1.68 ± 0.49 | 2.62 ± 0.66 | 1.37 ± 0.31 | 1.06 ± 0.81 | 1.65 ± 0.52 |
| F | 1.700 | 4.592 | 0.284 | 1.765 | 2.547 | 1.151 | 0.012 | |
| P | 0.183 | 0.032 | 0.753 | 0.172 | 0.079 | 0.317 | 0.988 | |
| AA | 537 | 4.96 ± 1.17 | 1.07(0.79) | 1.74 ± 0.50 | 2.93 ± 0.88 | 1.32 ± 0.39 | 0.95 ± 0.53 | 1.62 ± 0.77 |
| AG/GG | 290 | 4.74 ± 1.07 | 1.00(0.63) | 1.74 ± 0.43 | 2.76 ± 0.81 | 1.31 ± 0.41 | 1.03 ± 0.66 | 1.64 ± 1.36 |
| F | 3.345 | −2.480 | 0.551 | 3.312 | 0.020 | 1.840 | 0.023 | |
| P | 0.068 | 0.013 | 0.458 | 0.069 | 0.886 | 0.175 | 0.880 | |
| Han | ||||||||
| AA | 472 | 4.95 ± 0.99 | 1.01(0.73) | 1.74 ± 0.58 | 2.90 ± 0.82 | 1.33 ± 0.23 | 0.84 ± 0.20 | 1.66 ± 0.46 |
| AG | 284 | 4.85 ± 1.06 | 1.14(1.06) | 1.69 ± 0.44 | 2.78 ± 0.89 | 1.33 ± 0.29 | 0.83 ± 0.18 | 1.66 ± 0.54 |
| GG | 25 | 4.99 ± 0.98 | 0.84(0.47) | 1.77 ± 0.28 | 2.94 ± 0.97 | 1.31 ± 0.14 | 0.84 ± 0.23 | 1.71 ± 0.59 |
| F | 0.988 | 5.879 | 0.938 | 2.024 | 0.034 | 0.110 | 0.085 | |
| P | 0.373 | 0.015 | 0.392 | 0.133 | 0.967 | 0.896 | 0.919 | |
| AA | 472 | 4.95 ± 0.99 | 1.01(0.73) | 1.74 ± 0.58 | 2.90 ± 0.82 | 1.33 ± 0.23 | 0.84 ± 0.20 | 1.66 ± 0.46 |
| AG/GG | 309 | 4.91 ± 1.06 | 1.10(1.04) | 1.73 ± 0.43 | 2.86 ± 0.89 | 1.32 ± 0.29 | 0.84 ± 0.18 | 1.68 ± 0.55 |
| F | 0.074 | −1.860 | 0.036 | 0.168 | 0.053 | 0.005 | 0.130 | |
| P | 0.786 | 0.063 | 0.851 | 0.682 | 0.819 | 0.944 | 0.719 | |
| BUD13 rs11556024 C>T | ||||||||
| Mulao | ||||||||
| CC | 700 | 4.92 ± 1.14 | 1.04(0.77) | 1.72 ± 0.48 | 2.90 ± 0.86 | 1.29 ± 0.41 | 0.96 ± 0.57 | 1.61 ± 0.99 |
| CT | 120 | 4.91 ± 1.20 | 1.05(0.66) | 1.78 ± 0.45 | 2.91 ± 0.95 | 1.34 ± 0.34 | 0.97 ± 0.58 | 1.74 ± 1.15 |
| TT | 5 | 5.64 ± 0.44 | 0.88(0.36) | 1.83 ± 0.34 | 3.58 ± 0.48 | 1.58 ± 0.16 | 1.37 ± 0.74 | 1.39 ± 0.66 |
| F | 0.854 | 0.434 | 0.662 | 1.280 | 1.978 | 1.062 | 0.805 | |
| P | 0.426 | 0.510 | 0.516 | 0.279 | 0.139 | 0.346 | 0.447 | |
| CC | 700 | 4.92 ± 1.14 | 1.04(0.77) | 1.72 ± 0.48 | 2.90 ± 0.86 | 1.29 ± 0.41 | 0.96 ± 0.57 | 1.61 ± 0.99 |
| CT/TT | 125 | 5.27 ± 1.19 | 1.04(0.64) | 1.80 ± 0.44 | 3.24 ± 0.94 | 1.46 ± 0.34 | 1.17 ± 0.58 | 1.56 ± 1.14 |
| F | 1.534 | −0.779 | 0.454 | 2.522 | 2.993 | 2.097 | 0.034 | |
| P | 0.216 | 0.436 | 0.501 | 0.113 | 0.084 | 0.148 | 0.853 | |
| Han | ||||||||
| CC | 671 | 4.87 ± 1.01 | 1.05(0.84) | 1.70 ± 0.54 | 2.84 ± 0.86 | 1.31 ± 0.25 | 0.84 ± 0.19 | 1.65 ± 0.51 |
| CT | 103 | 4.98 ± 1.03 | 0.90(0.75) | 1.86 ± 0.44 | 2.84 ± 0.74 | 1.42 ± 0.27a | 0.81 ± 0.19 | 1.80 ± 0.39 |
| TT | 7 | 4.44 ± 0.46 | 0.83(0.88) | 1.57 ± 0.18 | 2.55 ± 0.56 | 1.27 ± 0.05 | 0.82 ± 0.15 | 1.61 ± 0.34 |
| F | 1.218 | 1.573 | 3.855 | 0.373 | 7.668 | 0.877 | 4.450 | |
| P | 0.297 | 0.210 | 0.022 | 0.689 | 0.001 | 0.416 | 0.012 | |
| CC | 671 | 4.87 ± 1.01 | 1.05(0.84) | 1.70 ± 0.54 | 2.84 ± 0.86 | 1.31 ± 0.25 | 0.84 ± 0.19 | 1.65 ± 0.51 |
| CT/TT | 110 | 4.71 ± 1.03 | 0.90(0.75) | 1.71 ± 0.43 | 2.69 ± 0.74 | 1.34 ± 0.27 | 0.81 ± 0.19 | 1.71 ± 0.39 |
| F | 0.626 | −1.342 | 0.004 | 0.693 | 0.338 | 0.351 | 0.314 | |
| P | 0.429 | 0.179 | 0.951 | 0.405 | 0.561 | 0.554 | 0.575 | |
| MLXIPL rs799161 C>T | ||||||||
| Mulao | ||||||||
| CC | 361 | 4.96 ± 1.11 | 1.07(0.77) | 1.74 ± 0.51 | 2.94 ± 0.84 | 1.30 ± 0.40 | 0.93 ± 0.50 | 1.61 ± 0.82 |
| CT | 390 | 4.90 ± 1.17 | 1.03(0.75) | 1.72 ± 0.44 | 2.88 ± 0.89 | 1.30 ± 0.39 | 1.00 ± 0.63 | 1.64 ± 1.19 |
| TT | 74 | 4.89 ± 1.20 | 0.97(0.71) | 1.72 ± 0.51 | 2.92 ± 0.85 | 1.30 ± 0.39 | 0.94 ± 0.50 | 1.62 ± 0.72 |
| F | 0.270 | 0.760 | 0.196 | 0.493 | 0.009 | 1.229 | 0.067 | |
| P | 0.763 | 0.383 | 0.822 | 0.611 | 0.991 | 0.293 | 0.935 | |
| CC | 361 | 4.96 ± 1.11 | 1.07(0.77) | 1.74 ± 0.51 | 2.94 ± 0.84 | 1.30 ± 0.40 | 0.93 ± 0.50 | 1.61 ± 0.82 |
| CT/TT | 464 | 4.89 ± 1.18 | 1.03(0.71) | 1.72 ± 0.44 | 2.90 ± 0.88 | 1.30 ± 0.39 | 0.97 ± 0.62 | 1.63 ± 1.13 |
| F | 0.448 | −1.251 | 0.287 | 0.342 | 0.010 | 0.585 | 0.052 | |
| P | 0.503 | 0.211 | 0.592 | 0.559 | 0.920 | 0.444 | 0.820 | |
| Han | ||||||||
| CC | 345 | 4.84 ± 1.08 | 1.02(0.83) | 1.75 ± 0.65 | 2.78 ± 0.89 | 1.34 ± 0.29 | 0.82 ± 0.21 | 1.72 ± 0.54 |
| CT | 378 | 4.93 ± 0.94 | 1.05(0.90) | 1.71 ± 0.38 | 2.88 ± 0.77 | 1.32 ± 0.22 | 0.84 ± 0.18 | 1.64 ± 0.46a |
| TT | 58 | 4.97 ± 1.12 | 1.00(0.71) | 1.65 ± 0.46 | 2.99 ± 1.01 | 1.27 ± 0.17 | 0.84 ± 0.20 | 1.60 ± 0.40 |
| F | 0.868 | 0.150 | 1.043 | 2.480 | 2.060 | 0.839 | 3.380 | |
| P | 0.420 | 0.698 | 0.353 | 0.084 | 0.128 | 0.433 | 0.035 | |
| CC | 345 | 4.84 ± 1.08 | 1.02(0.83) | 1.75 ± 0.65 | 2.78 ± 0.89 | 1.34 ± 0.29 | 0.82 ± 0.21 | 1.72 ± 0.54 |
| CT/TT | 436 | 4.95 ± 0.96 | 1.04(0.87) | 1.68 ± 0.39 | 2.94 ± 0.80 | 1.29 ± 0.21 | 0.84 ± 0.18 | 1.62 ± 0.45 |
| F | 1.479 | −0.181 | 2.082 | 4.771 | 3.882 | 0.881 | 5.762 | |
| P | 0.224 | 0.856 | 0.149 | 0.029 | 0.049 | 0.348 | 0.017 | |
| MLXIPL rs35332062 C>T | ||||||||
| Mulao | ||||||||
| CC | 717 | 4.91 ± 1.15 | 1.03(0.76) | 1.73 ± 0.48 | 2.89 ± 0.88 | 1.29 ± 0.41 | 0.95 ± 0.54 | 1.62 ± 1.04 |
| CT | 98 | 4.91 ± 0.98 | 1.19(0.70) | 1.68 ± 0.39 | 2.91 ± 0.73 | 1.34 ± 0.30 | 1.05 ± 0.67 | 1.54 ± 0.59 |
| TT | 10 | 5.75 ± 0.58 | 1.53(0.54) | 1.96 ± 0.51 | 3.48 ± 0.52 | 1.74 ± 0.51ab | 1.37 ± 1.23 | 1.75 ± 0.88 |
| F | 2.333 | 4.991 | 1.549 | 1.970 | 5.780 | 3.393 | 0.393 | |
| P | 0.098 | 0.025 | 0.213 | 0.140 | 0.003 | 0.034 | 0.675 | |
| CC | 717 | 4.91 ± 1.15 | 1.03(0.76) | 1.73 ± 0.48 | 2.89 ± 0.88 | 1.29 ± 0.41 | 0.95 ± 0.54 | 1.62 ± 1.04 |
| CT/TT | 108 | 5.33 ± 0.98 | 1.22(0.80) | 1.82 ± 0.40 | 3.19 ± 0.73 | 1.54 ± 0.33 | 1.21 ± 0.73 | 1.64 ± 0.62 |
| F | 4.206 | −2.681 | 1.012 | 3.664 | 11.554 | 6.049 | 0.009 | |
| P | 0.041 | 0.007 | 0.315 | 0.056 | 0.001 | 6.049 | 0.923 | |
| Han | ||||||||
| CC | 692 | 4.89 ± 1.04 | 1.04(0.84) | 1.71 ± 0.42 | 2.83 ± 0.86 | 1.33 ± 0.26 | 0.84 ± 0.20 | 1.67 ± 0.51 |
| CT | 83 | 4.99 ± 0.84 | 0.98(0.85) | 1.88 ± 1.03 | 3.02 ± 0.73 | 1.32 ± 0.17 | 0.84 ± 0.15 | 1.64 ± 0.38 |
| TT | 6 | 4.29 ± 0.15 | 2.28(1.52) | 1.41 ± 0.26 | 2.18 ± 0.14 | 1.20 ± 0.09 | 0.74 ± 0.05 | 1.66 ± 0.27 |
| F | 1.461 | 0.196 | 4.883 | 3.824 | 0.718 | 0.738 | 0.218 | |
| P | 0.233 | 0.658 | 0.008 | 0.022 | 0.488 | 0.478 | 0.804 | |
| CC | 692 | 4.89 ± 1.04 | 1.04(0.84) | 1.71 ± 0.42 | 2.83 ± 0.86 | 1.33 ± 0.26 | 0.84 ± 0.20 | 1.67 ± 0.51 |
| CT/TT | 89 | 4.64 ± 0.85 | 0.98(0.95) | 1.64 ± 1.01 | 2.60 ± 0.75 | 1.26 ± 0.17 | 0.79 ± 0.15 | 1.65 ± 0.50 |
| F | 1.258 | −0.207 | 0.284 | 1.563 | 1.411 | 1.271 | 0.061 | |
| P | 0.262 | 0.836 | 0.594 | 0.212 | 0.235 | 0.260 | 0.806 | |
| MLXIPL rs3812316 C>G | ||||||||
| Mulao | ||||||||
| CC | 751 | 4.93 ± 1.16 | 1.03(0.74) | 1.74 ± 0.48 | 2.91 ± 0.88 | 1.30 ± 0.42 | 0.95 ± 0.54 | 1.61 ± 0.80 |
| CG | 67 | 4.78 ± 1.04 | 1.24(0.78) | 1.66 ± 0.40 | 2.80 ± 0.75 | 1.32 ± 0.31 | 1.06 ± 0.75 | 1.54 ± 0.66 |
| GG | 7 | 4.85 ± 1.16 | 1.19(0.84) | 2.44 ± 0.42 | 2.86 ± 0.72 | 1.33 ± 0.32 | 1.09 ± 0.74 | 1.55 ± 0.65 |
| F | 2.733 | 7.293 | 2.029 | 2.054 | 2.756 | 1.168 | 0.128 | |
| P | 0.066 | 0.007 | 0.132 | 0.129 | 0.064 | 0.312 | 0.880 | |
| CC | 751 | 4.93 ± 1.16 | 1.03(0.74) | 1.74 ± 0.48 | 2.91 ± 0.88 | 1.30 ± 0.42 | 0.95 ± 0.54 | 1.61 ± 0.80 |
| CG/GG | 74 | 6.02 ± 1.07 | 1.25(0.77) | 2.05 ± 0.42 | 3.60 ± 0.72 | 1.77 ± 0.32 | 1.13 ± 0.74 | 1.75 ± 0.65 |
| F | 3.694 | −2.751 | 1.708 | 2.593 | 5.486 | 0.403 | 0.120 | |
| P | 0.055 | 0.006 | 0.192 | 0.108 | 0.019 | 0.526 | 0.730 | |
| Han | ||||||||
| CC | 703 | 4.90 ± 1.03 | 1.03(0.81) | 1.73 ± 0.54 | 2.83 ± 0.85 | 1.33 ± 0.25 | 0.84 ± 0.20 | 1.67 ± 0.51 |
| CG | 76 | 4.97 ± 0.91 | 1.16(0.89) | 1.71 ± 0.38 | 2.95 ± 0.78 | 1.30 ± 0.22 | 0.83 ± 0.15 | 1.62 ± 0.39 |
| GG | 2 | 4.92 ± 1.02 | 1.10(0.68) | 1.71 ± 0.38 | 2.90 ± 0.78 | 1.31 ± 0.22 | 0.82 ± 0.15 | 1.64 ± 0.39 |
| F | 0.448 | 0.008 | 0.145 | 1.301 | 0.722 | 0.042 | 1.054 | |
| P | 0.503 | 0.929 | 0.704 | 0.254 | 0.396 | 0.838 | 0.305 | |
| CC | 703 | 4.90 ± 1.03 | 1.03(0.81) | 1.73 ± 0.54 | 2.83 ± 0.85 | 1.33 ± 0.25 | 0.84 ± 0.20 | 1.67 ± 0.51 |
| CG/GG | 78 | 4.97 ± 0.91 | 1.16(0.89) | 1.70 ± 0.38 | 2.95 ± 0.78 | 1.30 ± 0.22 | 0.83 ± 0.82 | 1.62 ± 0.39 |
| F | 0.448 | −0.207 | 0.145 | 1.301 | 0.722 | 0.042 | 1.054 | |
| P | 0.503 | 0.836 | 0.704 | 0.254 | 0.396 | 0.838 | 0.305 | |
| MLXIPL rs13235543 C>T | ||||||||
| Mulao | ||||||||
| CC | 704 | 4.90 ± 1.16 | 1.01(0.71) | 1.74 ± 0.49 | 2.89 ± 0.88 | 1.29 ± 0.41 | 0.94 ± 0.53 | 1.65 ± 1.05 |
| CT | 114 | 5.02 ± 1.00 | 1.22(0.73) | 1.70 ± 0.37 | 2.96 ± 0.79 | 1.32 ± 0.33 | 1.10 ± 0.70a | 1.48 ± 0.60 |
| TT | 7 | 5.41 ± 1.05 | 1.42(2.20) | 1.76 ± 0.41 | 3.11 ± 0.77 | 1.53 ± 0.35 | 1.32 ± 0.85 | 1.46 ± 0.57 |
| F | 1.093 | 13.227 | 0.488 | 0.511 | 1.299 | 5.094 | 1.498 | |
| P | 0.336 | 3 × 10−4 | 0.614 | 0.600 | 0.274 | 0.006 | 0.224 | |
| CC | 704 | 4.90 ± 1.16 | 1.01(0.71) | 1.74 ± 0.49 | 2.89 ± 0.88 | 1.29 ± 0.41 | 0.94 ± 0.53 | 1.65 ± 1.05 |
| CT/TT | 121 | 5.22 ± 1.00 | 1.23(0.73) | 1.73 ± 0.37 | 3.04 ± 0.78 | 1.43 ± 0.33 | 1.21 ± 0.71 | 1.47 ± 0.60 |
| F | 1.741 | −3.862 | 0.017 | 0.628 | 2.538 | 5.190 | 0.750 | |
| P | 0.187 | 1 × 10−4 | 0.897 | 0.429 | 0.112 | 0.023 | 0.387 | |
| Han | ||||||||
| CC | 682 | 4.89 ± 1.03 | 1.05(0.88) | 1.72 ± 0.54 | 2.83 ± 0.86 | 1.33 ± 0.26 | 0.83 ± 0.20 | 1.67 ± 0.51 |
| CT | 94 | 4.99 ± 0.85 | 0.98(0.75) | 1.74 ± 0.36 | 2.97 ± 0.72 | 1.32 ± 0.21 | 0.83 ± 0.14 | 1.63 ± 0.37 |
| TT | 5 | 4.29 ± 0.15 | 2.28(1.52) | 1.41 ± 0.26 | 2.17 ± 0.14 | 1.20 ± 0.09 | 0.74 ± 0.05 | 1.65 ± 0.27 |
| F | 1.589 | 0.305 | 0.994 | 3.101 | 0.773 | 0.733 | 0.385 | |
| P | 0.205 | 0.581 | 0.370 | 0.046 | 0.462 | 0.481 | 0.680 | |
| CC | 682 | 4.89 ± 1.03 | 1.05(0.88) | 1.72 ± 0.54 | 2.83 ± 0.86 | 1.33 ± 0.26 | 0.83 ± 0.20 | 1.67 ± 0.51 |
| CT/TT | 99 | 4.64 ± 0.85 | 0.98(0.73) | 1.57 ± 0.37 | 2.57 ± 0.73 | 1.26 ± 0.25 | 0.79 ± 0.14 | 1.64 ± 0.37 |
| F | 1.223 | −0.331 | 1.540 | 1.944 | 1.542 | 1.389 | 0.091 | |
| P | 0.269 | 0.741 | 0.215 | 0.164 | 0.215 | 0.239 | 0.763 | |
TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoA1/ApoB, the ratio of ApoA1 to ApoB. The association of genotypes and serum lipid parameters (TC, HDL-C, LDL-C, ApoA1, ApoB and ApoA1/ApoB) was tested by analysis of covariance (ANCOVA). Age, sex, body mass index (BMI), smoking and alcohol consumption were adjusted for the statistical analysis. The values of triglyceride were presented as the median (interquartile range), and the difference among the genotypes was determined by the Kruskal-Wallis test or the Wilcoxon-Mann-Whitney test.
F: F value determined by analysis of covariance (ANCOVA) or U value determined by the Kruskal-Wallis test or the Wilcoxon-Mann-Whitney test.
A P-value of less than 0.006, adjusted by Bonferroni correction, was considered statistically significant.
aP < 0.006 in comparison with the major homozygous genotype in the same ethnic group, analyzed by post-hoc test.
bP < 0.006 in comparison with the heterozygous genotype in the same ethnic group, analyzed by post-hoc test.
Table 5 shows the magnitude and direction of correlation between serum lipid levels and genotypes in the two populations. Many of the examining SNPs showed significant correlation with serum lipid levels in multiple linear regression analysis; although, these SNPs did not show significant association with serum lipid levels in the analysis of covariance (ANCOVA).
Table 5. Correlation between the genotypes of the MLXIPL, BUD13 and ZNF259 SNPs and serum lipid levels in the Mulao and Han populations.
| Lipid | SNP | Affected allele/Other allele | Affected genotype/Other genotype | Beta | Std. error | t | P-value |
|---|---|---|---|---|---|---|---|
| Mulao plus Han | |||||||
| TC | BUD13 rs10790162 | AA, GA/GG | 0.167 | 0.049 | 3.407 | 0.001 | |
| TG | BUD13 rs10790162 | A/G | 0.248 | 0.048 | 5.163 | <1 × 10−7 | |
| LDL-C | BUD13 rs17119975 | G/A | 0.513 | 0.183 | 2.800 | 0.005 | |
| BUD13 rs10790162 | A/G | 0.099 | 0.039 | 2.546 | 0.011 | ||
| BUD13 rs13235543 | T/C | 0.149 | 0.069 | 2.164 | 0.031 | ||
| ApoA1 | BUD13 rs13235543 | TT, CT/CC | 0.097 | 0.031 | 3.093 | 0.002 | |
| BUD13 rs11556024 | T/C | 0.060 | 0.027 | 2.234 | 0.025 | ||
| ApoB | BUD13 rs13235543 | TT, CT/CC | 0.097 | 0.031 | 3.093 | 0.002 | |
| BUD13 rs10790162 | A/G | 0.058 | 0.024 | 2.405 | 0.016 | ||
| ApoA1/ApoB | BUD13 rs10790162 | AA, GA/GG | −0.083 | 0.030 | −2.754 | 0.006 | |
| BUD13 rs11556024 | T/C | 0.133 | 0.051 | 2.611 | 0.009 | ||
| BUD13 rs13235543 | T/C | −0.113 | 0.053 | −2.138 | 0.033 | ||
| Mulao | |||||||
| TC | BUD13 rs10790162 | AA, GA/GG | 0.200 | 0.071 | 2.836 | 0.005 | |
| TG | BUD13 rs10790162 | AA, AG/GG | 0.292 | 0.062 | 4.744 | 2 × 10−5 | |
| BUD13 rs13235543 | TT, CT/CC | 0.248 | 0.101 | 2.450 | 0.015 | ||
| ZNF259 rs964184 | GG, CG/CC | −0.401 | 0.192 | −2.083 | 0.037 | ||
| LDL-C | BUD13 rs10790162 | A/G | 0.167 | 0.067 | 2.485 | 0.013 | |
| ApoA1 | MLXIPL rs35332062 | TT, CT/CC | 0.510 | 0.155 | 3.284 | 0.001 | |
| MLXIPL rs35332062 | T/C | −0.427 | 0.179 | −2.392 | 0.017 | ||
| ApoB | BUD13 rs13235543 | TT, CT/CC | 0.214 | 0.059 | 3.608 | 4 × 10−4 | |
| ApoA1/ApoB | BUD13 rs13235543 | T/C | −0.180 | 0.090 | −1.999 | 0.046 | |
| BUD13 rs10790162 | A/G | −0.124 | 0.063 | −1.978 | 0.048 | ||
| Han | |||||||
| TC | MLXIPL rs799161 | T/C | 0.127 | 0.062 | 2.042 | 0.042 | |
| TG | BUD13 rs17119975 | G/A | 0.308 | 0.081 | 3.826 | 1 × 10−4 | |
| BUD13 rs10790162 | A/G | 0.268 | 0.083 | 3.211 | 0.001 | ||
| BUD13 rs17119975 | GG, AG/AA | −0.561 | 0.240 | −2.340 | 0.020 | ||
| HDL-C | MLXIPL rs35332062 | T/C | 0.788 | 0.127 | 6.218 | <1 × 10−7 | |
| BUD13 rs13235543 | T/C | −0.407 | 0.116 | −3.501 | 5 × 10−4 | ||
| BUD13 rs11556024 | TT, CT/CC | 0.175 | 0.061 | 2.852 | 0.004 | ||
| MLXIPL rs3812316 | GG, CG/CC | −0.329 | 0.126 | −2.614 | 0.009 | ||
| LDL-C | MLXIPL rs799161 | T/C | 0.196 | 0.066 | 2.990 | 0.003 | |
| ApoA1 | BUD13 rs11556024 | T/C | 0.095 | 0.028 | 3.465 | 0.001 | |
| ApoB | BUD13 rs10790162 | A/G | 0.055 | 0.017 | 3.198 | 0.001 | |
| ZNF259 rs2075290 | GG, AG/AA | −0.048 | 0.020 | −2.396 | 0.017 | ||
| BUD13 rs11556024 | TT, CT/CC | −0.039 | 0.019 | −2.018 | 0.044 | ||
| ApoA1/ApoB | BUD13 rs11556024 | TT, CT/CC | 0.182 | 0.050 | 3.642 | 2 × 10−4 | |
| BUD13 rs10790162 | AA, GA/GG | −0.091 | 0.031 | −2.974 | 0.003 | ||
| MLXIPL rs799161 | T/C | −0.107 | 0.036 | −2.999 | 0.003 |
Multivariable linear regression analyses with stepwise modeling were performed to assess the correlation between serum lipid levels and genotypes in Mulao, Han, and combined the Mulao and Han populations.
Discussion
The main findings of this study are as follows: we successfully replicated the association of MLXIPL rs3812316, ZNF259 rs2075290 and rs964184 SNPs with serum TG in the Mulao population and of ZNF259 rs2075290 and BUD13 rs10790162 with serum TG in the Han population; and we explored a previously unreported association of BUD13 rs11556024, and MLXIPL rs35332062 and rs13235543 SNPs with serum lipid levels. In addition, we reported the linkage disequilibrium status and the possible haplotype frequencies of these SNPs.
It has been noted that the genotype and allele frequencies of several SNPs are not consistent among different populations8,9,19,20,21,22. The G allele frequency of MLXIPL rs3812316 (Q241H) SNP was 0.05 in Mexicans41, 0.10 in Europeans18 and 0.09 in Indian Asians18 and Japanese individuals23. Nakayama K, et al. found that in a worldwide survey, individuals from Africa (0.05), South Asia (0.06), East Asia (0.11) and South-East Asia (0.12) had lower frequencies of the minor G allele compared to those from Central Asian populations (0.21 to 0.26), including Mongolian, Tibetan and Uyghur16. The minor allele frequencies of our study populations (0.049 in Mulao, 0.051 in Han) were much closer to those of the African and South Asian populations. The genotype and allele frequencies of ZNF259 rs2075290 and rs964184 and BUD13 rs10790162 (P < 0.05 for each) were significantly different between Mulao and Han. The genotype frequencies but not the allele frequencies of BUD13 rs17119975 were different between the Mulao and Han populations (P > 0.05). The minor allele frequencies of the MLXIPL, BUD13 and ZNF259 SNPs of our Han population were in close proximity to those of CHB from the international haplotype map (HapMap; http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap24_B36/) data. Generally, the minor allele frequencies of the 9 observed SNPs were lower in European ancestries than in Asian ancestries8,9,24,25. These findings suggest that the genotype and allele frequencies of the MLXIPL, BUD13 and ZNF259 SNPs are different among diverse ethnic groups.
The association of variants in the MLXIPL gene and serum lipid levels among different ethnic populations is still controversial. The MLXIPL rs3812316-G allele was reported to be associated with decreased plasma TG levels in Asians16,18,23 and in combined Northern Europeans and Indian Asians26. It was also reported to be related to the risk of CAD in the Han Chinese27 and Japanese populations23. However, a notable absence of association was found between low- and high- triglyceridemia individuals in the central Europe white population28 or between type 2 diabetes and normal controls of the North India Sikh population26. In contrast to previous studies, our results showed that the minor allele of MLXIPL rs3812316 SNP was associated with higher TG levels in the Mulao but not the Han population.
Many GWASs have reported that the G allele of rs964184 at the ZNF259 region was strongly associated with increased serum TC, TG and LDL-C but was associated with decreased HDL-C in the European population8,9,16,17 and resulted in a 1.13 fold increased in the risk of CAD and metabolic syndrome28,29,30. The G allele was also associated with decreased HDL-C in a combined population of white European and Asian Indian11 and with a 1.8-times and 3.28-times increased risk of hypertriglyceridemia in Mexican41 and European populations31, respectively. Partially consistent with previous studies, we replicated the association of ZNF259 rs964184 G allele with serum TC and TG levels in Mulao (but not in Han) population, but we did not find its association with the serum HDL-C level in our study population. The STAMPEED Consortium, which included 13 independent studies of European ancestry, reported that ZNF259 rs2075290 and BUD13 rs10790162 were correlated with TG, HDL-C, waist circumference levels and metabolic syndrome32; however, the mechanism of association is not well understood. In our study, we found that the minor allele carriers of ZNF259 rs2075290 and BUD13 rs10790162 were associated with higher TG (in Mulao and Han) and TC (in Han) compared to the minor allele non-carriers, yet no association with HDL-C was noted.
The reason for the discrepancy in association of the above-mentioned SNPs with serum lipid levels among different populations is not fully understood. It could be partly due to differences in their genetic background. Compared to the Han population, the Mulao population had higher ApoB levels and apparently similar remaining serum lipid parameters. Of 56 ethnic groups in China, Han is the largest one. Mulao, on the other hand, is one of the minorities, with a population of 207,352 according to the China's fifth national census in 2000. Approximately 90% of the Mulao population dwells in the Luocheng Mulao Autonomous County, Guangxi Zhuang Autonomous Region. The Mulams are the descendants of the ancient “Baiyue tribe” in southern China. Historical data trace the history of this ethnic minority back to the Jin Dynasty (AD 265–420). Interestingly, Mulams abide by their culture of consanguineous marriage to cousins on the maternal side. Hence, the Mulao population may have same genetic background and less heterogeneity within the population. Recent molecular anthropological data showed that Mulams are genetically much closer to the other neighboring ethnic groups in Guangxi than to the Han Chinese33. Therefore, some hereditary characteristics and genotypes of lipid metabolism-related genes in this population might be somewhat different from those in Han Chinese.
Another reason could be due to the ethnic difference in their LD pattern. Kooner, et al. reported that the LD status of ZNF259 rs964184 with other SNPs were different between Europeans (high LD with 26 other SNPs) and Mexicans (not in high LD with any SNPs)41. In our study population, ZNF259 rs2075290 and BUD13 rs10790162 were in high LD with ZNF259 rs964184. Therefore, ethnic differences in the LD pattern could partially explain the discrepancy in the association of these SNPs with plasma lipids among diverse populations. The third possible reason is that several environmental factors such as diet, alcohol consumption and obesity might further modify the effect of genetic variation on serum lipid levels34,35,36,37,38,39,40. The Mulao population had a higher percentage of subjects who consumed alcohol and had a lower BMI value than the Han population (P < 0.05–0.001). Therefore, it is possible that some uncontrollable or unmeasured environmental factors might further modify the effect of genetic variation on the serum lipid levels of our study populations. In addition, this study showed the association of MLXIPL rs35332062 SNP with ApoA1, MLXIPL rs13235543 with TG and ApoB in the Mulao population, and that of MLXIPL rs11556024 with ApoA1 in the Han population. Since this study is the first attempt to detect the association of these three SNPs with serum lipid levels, we are unable to make comparison with other studies. Thus, further studies with larger sample sizes are needed for the confirmation.
This study has some limitations. The sample size was relatively low compared to many GWAS and replication studies. Hence, further studies with larger sample sizes are needed to confirm our results. Secondly, we were not able to alleviate the effect of diet and several environmental factors during the statistical analysis. Thirdly, although we have detected the effects of the MLXIPL and BUD13-ZNF259 SNPs on serum lipid levels in this study, several SNPs still remain to be studies. In addition, detecting the interactions of SNP-SNP and/or SNP-environmental is required for a clear understanding of the genetic background of plasma lipids in the Chinese population.
In summary, the SNPs of ZNF259 rs2075290 and BUD13 rs10790162 were associated with serum TC levels; ZNF259 rs2075290 and rs964184, BUD13 rs10790162, and MLXIPL rs3812316 and rs13235543 were associated with TG; and MLXIPL rs35332062 was associated with ApoA1 in the Mulao population. In Han, on the other hand, the SNPs of ZNF259 rs2075290 and BUD13 rs10790162 were associated with serum TG levels; ZNF259 rs2075290 was associated with LDL-C and the ApoA1/ApoB ratio. Several MLXIPL, BUD13 and ZNF259 SNPs were associated with different serum lipid parameters in the two ethnic groups, suggesting that the associations of these variants on serum lipid levels might have ethnic specificity.
Methods
Study populations
The current study included 825 (354 males, 42.9% and 471 females, 57.1%) unrelated subjects of Mulao nationality from Luocheng Mulao Autonomous County, Guangxi Zhuang Autonomous Region, People's Republic of China. The subjects were randomly selected from our stratified, randomized cluster samples. During the same period, 782 (307 men, 39.3% and 474 women, 60.7%) unrelated individuals of Han nationality who resided in the same villages were also randomly selected from our stratified, randomized cluster samples. All of the participants were rural agricultural workers. The ages of the subjects ranged from 15 to 80 years, with an average age of 49.18 ± 16.13 years for Mulao and 49.25 ± 16.21 years for Han. The subjects had no evidence of diseases related to kidney, thyroid, atherosclerosis, CVD and/or diabetes. None of them used lipid-lowering medication such as statins or fibrates when the blood sample was taken. All experiments were performed in accordance with relevant guidelines and regulations. Verbal informed consents and their thumbprints (to express consent) of all subjects were obtained after they received a full explanation of the study. Verbal informed consents and thumbprints were also obtained from the parents of minor participants (<18 years old) who were involved in this study. Written informed consents were not obtained because of the poor educational level of the participants. The consent procedure was also approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University. An incentive of approximately ten dollars was provided to each participant in the study19,20,21,22.
Epidemiological survey and biochemical measurements
The epidemiological survey was carried out using internationally standardized methods and following a common protocol19. Information on demographics, socioeconomic status, and lifestyle factors was collected using standardized questionnaires. The methods of measuring blood pressure, height, weight and waist circumference parameters were based on previous studies19. Fasting venous blood samples were taken and the levels of serum TC, TG, HDL-C, and LDL-C in the samples were directly determined by enzymatic methods with commercially available kits, Tcho-1, TG-LH (RANDOX Laboratories Ltd., Ardmore, Diamond Road, Crumlin Co. Antrim, United Kingdom, BT29 4QY), Cholestest N HDL, and Cholestest LDL (Daiichi Pure Chemicals Co., Ltd., Tokyo, Japan); respectively. Serum ApoA1 and ApoB levels were assessed by the immunoturbidimetric assay using a commercial kit (RANDOX Laboratories Ltd.)19,20. All determinations were performed with an autoanalyzer (Type 7170A; Hitachi Ltd., Tokyo, Japan) in the Clinical Science Experiment Center of the First Affiliated Hospital, Guangxi Medical University. The normal values of serum TC, TG, HDL-C, LDL-C, ApoA1 and ApoB levels and the ratio of ApoA1 to ApoB in our Clinical Science Experiment Center were 3.10–5.17, 0.56–1.70, 1.16–1.42, 2.70–3.10 mmol/L, 1.20–1.60, 0.80–1.05 g/L and 1.00–2.50, respectively21,22.
SNP selection
We selected SNPs in the MLXIPL, BUD13 and ZNF259 genes by three criteria: (1) Tag SNPs, which were established by Haploview (Broad Institute of MIT and Harvard, USA, version 4.2) or functional or missense SNPs (http://www.ncbi.nlm.nih.gov/SNP/snp), (2) a known minor allele frequency higher than 1% in the Human Genome Project Database, and (3) the target SNP region should be adequately replicated by PCR, and the polymorphic site should have a commercially available restriction endonuclease enzyme cleavage site to be genotyped with RFLP. The detailed procedure to establish tag SNPs is as follows. We chose the Chinese Han Bejing (CHB) population as the reference population, 11 as chromosome number and 0.8 as the r2 value in the Haploview. The software captured 122 of 122 alleles at r2 ≥ 0.8 and 100 percent of alleles with a mean r2 of 0.967 in the BUD13-ZNF259 region, using 56 Tag SNPs in 56 tests. Among the 56 tag SNPs, we finally selected those that could proxy for at least two SNPs and could be genotyped with PCR-RFLP. BUD13 rs17119975 was the proxy for BUD13 rs17119975, rs11216126 and rs11216129. BUD13 rs11556024 was the proxy for BUD13 rs11556024, rs10466588 and rs17119920. ZNF259 rs964184 was the proxy for BUD13-ZNF259 rs964184, rs180349, rs2266788, rs180326, rs6589566, rs651821 and rs3825041. ZNF259 rs2075290 and BUD13 rs10790162 were previously reported in GWASs as lipid-related loci. For the MLXIPL gene, we selected 3 missense SNPs (MLXIPL rs35332062 p.Ala358Val, rs3812316 p.Gln241His and rs13235543 p.Pro342 = ) that were located in the coding region of MLXIPL (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=51085) and one tag SNP, MLXIPL rs799161 which was the proxy for MLXIPL rs799160 and rs799161.
Genotyping and DNA sequencing
Genomic DNA was isolated from peripheral blood leukocytes using the phenol-chloroform method21,22. The genotyping of 9 SNPs was performed by PCR and RFLP. The characteristics of each SNP and the details of PCR-RFLP procedure including annealing temperature, length of the PCR products and corresponding restriction enzyme used for genotyping are summarized in Supplemental Tables 1 and 2, respectively. Genotypes were scored by an experienced reader who was blinded to the epidemiological data and serum lipid results. Then, for confirmation to the RFLP results, the PCR products of the 54 samples (each 2 samples of three different genotypes for 9 SNPs from the two ethnic groups) were sequenced with an ABI Prism 3100 (Applied Biosystems) at Shanghai Sangon Biological Engineering Technology & Services Co., Ltd., People's Republic of China.
Statistical analysis
Epidemiological data were recorded on a pre-designed form and managed with Excel software. The power and sample size of the study was evaluated by Quanto 1.2 software (http://biostats.usc.edu/software). This study sample size produced a power of 0.377 for recessive model and that of 0.821 for dominant model respectively. Therefore, we mainly used the results of dominant model for the discussion. The statistical analyses were performed using the statistical software package SPSS 17.0 (SPSS Inc., Chicago, Illinois). The quantitative variables were presented as the mean ± standard deviation for continuous variables (serum TG levels were presented as medians and interquartile ranges) and as frequencies or percentages for categorical variables. Chi square tests were used to compare the differences in percentages and to assess Hardy-Weinberg expectations. General characteristics between two ethnic groups were compared by Student's unpaired t-test. Pair-wise linkage disequilibria and haplotype frequencies among the SNPs were analyzed using Haploview (Broad Institute of MIT and Harvard, USA, version 4.2).
The association of genotypes and serum lipid parameters (except TG) was tested by ANCOVA and the association between subgroups was tested by a post-hoc test with the adjustment of potential confounders including sex, age, education level, physical activity, blood pressure, alcohol consumption, and cigarette smoking. As the distribution of TG levels in the general population does not follow normal distribution, non-parametric tests (Kruskal-Wallis 1 way analysis of variance ANOVA for k samples and Mann-Whitney U for 2 samples) were used to determine the association between genotypes and serum TG levels. Any variants associated with the serum lipid parameter at a value of P < 0.006 (corresponding to P < 0.05 after adjusting for nine independent tests by the Bonferroni correction) were considered statistically significant. Multivariable linear regression analyses with stepwise modeling were performed (by adjusting confounders incluidng age, gender, BMI, smoking and alcohol consumption) to assess the magnitude and direction of correlation between serum lipid levels and genotypes (common homozygote genotype = 1, heterozygote genotype = 2, rare homozygote genotype = 3) or alleles (the minor allele non-carrier = 1, the minor allele carrier = 2) in Mulao, Han and combined Mulao and Han populations.
Supplementary Material
Supplementary Dataset 1
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No: 30960130).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.H.H.A. participated in the design, carried out the epidemiological survey, collected the samples, undertook genotyping, performed statistical analyses, drafted the manuscript and edited the final manuscript. R.X.Y. conceived the study, participated in the design, carried out the epidemiological survey, collected the samples, helped to draft the manuscript and edited the final manuscript. D.F.W., J.Z.W., H.L. and W.W. carried out the epidemiological survey, and collected the samples. All authors read and approved the final manuscript.
References
- Shekelle R. B. et al. Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N. Engl. J. Med. 304, 65–70 (1981). [DOI] [PubMed] [Google Scholar]
- Wu L. L. Review of risk factors for cardiovascular diseases. Ann. Clin. Lab. Sci. 29, 127–133 (1999). [PubMed] [Google Scholar]
- Kannel W. B. & Vasan R. S. Triglycerides as vascular risk factors: new epidemiologic insights. Curr. Opin. Cardiol. 24, 345–350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched A. J. & Chan L. Nutrigenetics and nutrigenomics of atherosclerosis. Curr. Atheroscler. Rep. 15, 328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D. A., DeFaire U., Pedersen N. L., Dahlen G. & McClearn G. E. Genetic and environmental influences on serum lipid levels in twins. N. Engl. J. Med. 328, 1150–1156 (1993). [DOI] [PubMed] [Google Scholar]
- Davignon J. & Cohn J. S. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis 124 Suppl, S57–64 (1996). [DOI] [PubMed] [Google Scholar]
- Perusse L. et al. Familial resemblance of plasma lipids, lipoproteins and postheparin lipoprotein and hepatic lipases in the HERITAGE Family Study. Arterioscler. Thromb. Vasc. Biol. 17, 3263–3269 (1997). [DOI] [PubMed] [Google Scholar]
- Waterworth D. M. et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 30, 2264–2276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeemon P. et al. Implications of discoveries from genome-wide association studies in current cardiovascular practice. World J. Cardiol. 3, 230–247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T. R. et al. A replication study of GWAS-derived lipid genes in Asian Indians: the chromosomal region 11q23.3 harbors loci contributing to triglycerides. PLoS. One. 7, e37056 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa M., Ge Q., Wynn R. M., Ishii S. & Uyeda K. Coordinate regulation/localization of the carbohydrate responsive binding protein (ChREBP) by two nuclear export signal sites: discovery of a new leucine-rich nuclear export signal site. Biochem. Biophys. Res. Commun. 391, 1166–1169 (2010). [DOI] [PubMed] [Google Scholar]
- Tong X., Zhao F., Mancuso A., Gruber J. J. & Thompson C. B. The glucose-responsive transcription factor ChREBP contributes to glucose-dependent anabolic synthesis and cell proliferation. Proc. Natl. Acad. Sci. U S A. 106, 21660–21665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M. A. et al. Structure of the yeast Pml1 splicing factor and its integration into the RES complex. Nucleic. Acids. Res. 37, 129–143 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z. et al. The cytoplasmic zinc finger protein ZPR1 accumulates in the nucleolus of proliferating cells. Mol. Biol. Cell 9, 2963–2971 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. et al. High prevalence of an anti-hypertriglyceridemic variant of the MLXIPL gene in Central Asia. J. Hum. Genet. 56, 828–833 (2011). [DOI] [PubMed] [Google Scholar]
- Kathiresan S. et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41, 56–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooner J. S. et al. Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat. Genet. 40, 149–151 (2008). [DOI] [PubMed] [Google Scholar]
- Yin R. X. et al. Interactions of the apolipoprotein A5 gene polymorphisms and alcohol consumption on serum lipid levels. PLoS. One. 6, e17954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung L. H. et al. The proprotein convertase subtilisin/kexin type 9 gene E670G polymorphism and serum lipid levels in the Guangxi Bai Ku Yao and Han populations. Lipids Health Dis. 10, 5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung L. H. et al. Association of the TRIB1 tribbles homolog 1 gene rs17321515 A>G polymorphism and serum lipid levels in the Mulao and Han populations. Lipids Health Dis. 10, 230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung L. H. et al. Association of the apolipoprotein M gene polymorphisms and serum lipid levels. Mol. Biol. Rep. 40, 1843–1853 (2013). [DOI] [PubMed] [Google Scholar]
- Nakayama K. et al. Large scale replication analysis of loci associated with lipid concentrations in a Japanese population. J. Med. Genet. 46, 370–374 (2009). [DOI] [PubMed] [Google Scholar]
- Kristiansson K. et al. Genome-wide screen for metabolic syndrome susceptibility Loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ. Cardiovasc. Genet. 5, 242–249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major J. M. et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 20, 3876–3883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been L. F. et al. Replication of association between a common variant near melanocortin-4 receptor gene and obesity-related traits in Asian Sikhs. Obesity (Silver Spring) 18, 425–429 (2010). [DOI] [PubMed] [Google Scholar]
- Pan L. A. et al. G771C Polymorphism in the MLXIPL Gene Is Associated with a Risk of Coronary Artery Disease in the Chinese: A Case-Control Study. Cardiology 114, 174–178 (2009). [DOI] [PubMed] [Google Scholar]
- Vrablik M. et al. MLXIPL variant in individuals with low and high triglyceridemia in white population in Central Europe. Hum. Genet. 124, 553–555 (2008). [DOI] [PubMed] [Google Scholar]
- Schunkert H. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert H. et al. Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur. Heart J. 33, 238–251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen C. T. et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 42, 684–687 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraja A. T. et al. A bivariate genome-wide approach to metabolic syndrome: STAMPEED consortium. Diabetes 60, 1329–1339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q. et al. Genetic relationships among four minorities in Guangxi revealed by analysis of 15 STRs. J. Genet. Genomics 34, 1072–1079 (2007). [DOI] [PubMed] [Google Scholar]
- Aung L. H. et al. Proprotein convertase subtilisin/kexin type 9 gene E670G polymorphism interacts with alcohol consumption to modulate serum lipid levels. Int. J. Med. Sci. 10, 124–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Palmieri M. R. et al. Nutrient intake and serum lipids in urban and rural Puerto Rican men. Am. J. Clin. Nutr. 30, 2092–2100 (1977). [DOI] [PubMed] [Google Scholar]
- Sola R. et al. Effect of a traditional Mediterranean diet on apolipoproteins B, A-I, and their ratio: a randomized, controlled trial. Atherosclerosis 218, 174–180 (2011). [DOI] [PubMed] [Google Scholar]
- Ruixing Y. et al. Effects of demographic, dietary and other lifestyle factors on the prevalence of hyperlipidemia in Guangxi Hei Yi Zhuang and Han populations. Eur. J. Cardiovasc. Prev. Rehabil. 13, 977–984 (2006). [DOI] [PubMed] [Google Scholar]
- Ruixing Y. et al. Comparison of demography, diet, lifestyle, and serum lipid levels between the Guangxi Bai Ku Yao and Han populations. J. Lipid Res. 48, 2673–2681 (2007). [DOI] [PubMed] [Google Scholar]
- Valente E. A. et al. The effect of the addition of resistance training to a dietary education intervention on apolipoproteins and diet quality in overweight and obese older adults. Clin. Interv. Aging. 6, 235–241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield E., McPherson R. & Koski K. G. Diet and waist-to-hip ratio: important predictors of lipoprotein levels in sedentary and active young men with no evidence of cardiovascular disease. J. Am. Diet. Assoc. 99, 1373–1379 (1999). [DOI] [PubMed] [Google Scholar]
- Weissglas-Volkov D. et al. Genomic study in Mexicans identifies a new locus for triglycerides and refines European lipid loci. J. Med. Genet. 50, 289–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Dataset 1