Abstract
Dragonflies of the genus Orthetrum are members of the suborder Anisoptera, family Libellulidae. There are species pairs whose members are not easily separated from each other by morphological characters. In the present study, the DNA nucleotide sequences of mitochondrial and nuclear genes were employed to elucidate the phylogeny and systematics of Orthetrum dragonflies. Phylogenetic analyses could not resolve the various subfamilies of the family Libellulidae unequivocally. The nuclear 28S rRNA gene is highly conserved and could not resolve congeneric species of Orthetrum. Individual mitochondrial genes (COI, COII, and 16S rRNA) and combination of these genes as well as the nuclear ITS1&2 genes clearly differentiate morphologically similar species, such as the reddish species pairs O. chrysis and O. testaceum, and the bluish-coloured species O. glaucum and O. luzonicum. This study also reveals distinct genetic lineages between O. pruinosum schneideri (occurring in Malaysia) and O. pruinosum neglectum (occurring north of Peninsular Malaysia from India to Japan), indicating these taxa are cryptic species.
Dragonflies of the genus Orthetrum Newman, 1833 are members of the suborder Anisoptera, family Libellulidae. The genus contains some 61 species spread across the Old World1. Among these Orthetrum dragonflies, there are species pairs whose members are not easily separated from each other by morphological characters, e.g. the reddish-coloured species O. chrysis and O. testaceum, and the bluish-coloured species O. glaucum and O. luzonicum.
The Crimson-tailed Marsh Hawk Orthetrum pruinosum (Burmeister, 1839) is a widespread species occurring from west India to Japan and south to Malaysia and the Sunda Islands. The subspecies in Malaysia is O. p. schneideri Förster, 1903 and that north of Peninsular Malaysia (India to Japan) is O. p. neglectum (Rambur, 1842).
The DNA nucleotide sequences of mitochondrial and nuclear genes have been employed to elucidate the phylogeny and systematics of Orthetrum dragonflies2,3. To-date the most comprehensive phylogenetic study of Orthetrum dragonflies involves all the nine Japanese species2. In the present study, the DNA nucleotide sequences of mitochondrial and nuclear genes were employed to elucidate the phylogeny and systematics of Orthetrum dragonflies. This study, covering a more extensive taxon sampling, provides a new insight to the evolutionary relationships of Orthetrum dragonflies. The molecular phylogeny based on ITS1&2, COI, COII and 16S nucleotide sequences, reveals the occurrence of cryptic species in O. pruinosum.
Results
Aligned sequences and genetic divergence
The total length for each aligned sequences for various molecular markers and their parsimony information are sumarised in Supplementary Table 1. The uncorrected ‘p'-distance between Orthetrum species based on 16S rDNA, COI, combined COI + 16S rDNA, combined COI + COII + 16S rDNA, ITS1&2, and combined COI + COII + 16S rDNA + 28S rDNA + ITS1&2 nucleotide sequences are summarized in supplementary Tables 2–6 respectively. The interspecific ‘p' distance was many folds larger than intraspecific ‘p' distance. For COI, the intraspecific p-distance ranged from 0.00–3.99% (highest in O. melania), while interspecific p-distance ranged from 3.33% (O. melania and O. triangulare) to 17.29% (O. chrysis and O. sabina) (Supplementary Table 2). For 16S rDNA, the intraspecific p-distance ranged from 0.00–2.10% (highest in O. glaucum); the interspecific p-distance ranged from 0.60% (O. melania and O. triangulare) to 9.92% (O. abbotti and O. poecilops) (Supplementary Table 2).
The intraspecific p-distance for ITS1&2 sequences ranged from 0.00–5.05% (highest in O. luzonicum); the interspecific p-distance ranged from 1.14% (O. pruinosum neglectum and O. testaceum) to 21.12% (O. sabina and O. chrysostigma) (Supplementary Table 3).
The intraspecific p-distance for the combined COI + 16S rDNA sequences ranged from 0.00–1.78% (highest in O. sabina); the interspecific p-distance ranged from 1.15% (O. pruinosum neglectum and O. testaceum) to 12.23% (O. chrysis and O. Sabina; O. japonicum and O. Sabina) (Supplementary Table 4). For the combined mitochondrial markers (COI + COII + 16S rDNA) the intraspecific p-distance ranged from 0.00–1.94% (highest in O. pruinosum schneideri); the interspecific p-distance ranged from 7.32% (O. chrysis and O. pruinosum schneideri) to 12.58% (O. chrysis and O. sabina) (Supplementary Table 5).
For the combined five markers (COI + COII + 16S rDNA + 28S rDNA + ITS1&2) the intraspecific p-distance ranged from 0.00–1.55% (highest in O. pruinosum schneideri); the interspecific p-distance ranged from 4.20% (O. chrysis and O. sabina) to 9.51% (O. chrysis and O. sabina) (Supplementary Table 6).
Phylogenetic relationships based on 28S rDNA nucleotide sequences
There were no distinct nucleotide sequence divergence among the congeners of Orthetrum (supplementary Fig. 1). The various subfamilies of the family Libellulidae were not resolved unequivocally.
Phylogenetic relationships based on 16S rDNA nucleotide sequences
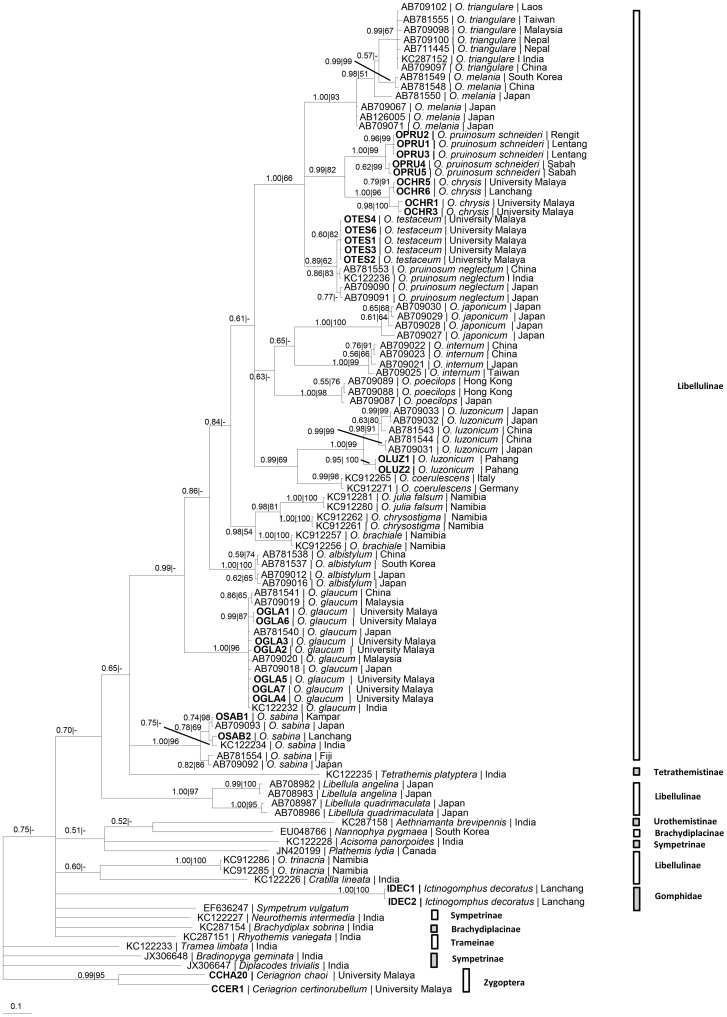
Orthetrum pruinosum schneideri clustered with O. chrysis and both were distinctly separated from O. testaceum and O. pruinosum neglectum (Fig. 1). O. sabina from Peninsular Malaysia was not grouped together with O. sabina of India, Japan and Fiji. Additionally, O. luzonicum from Peninsular Malaysia was distinct from O. luzonicum of China and Japan.
Figure 1. BI tree based on 16S rDNA nucleotide sequences.
Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap.
Phylogenetic relationships based on COI nucleotide sequences
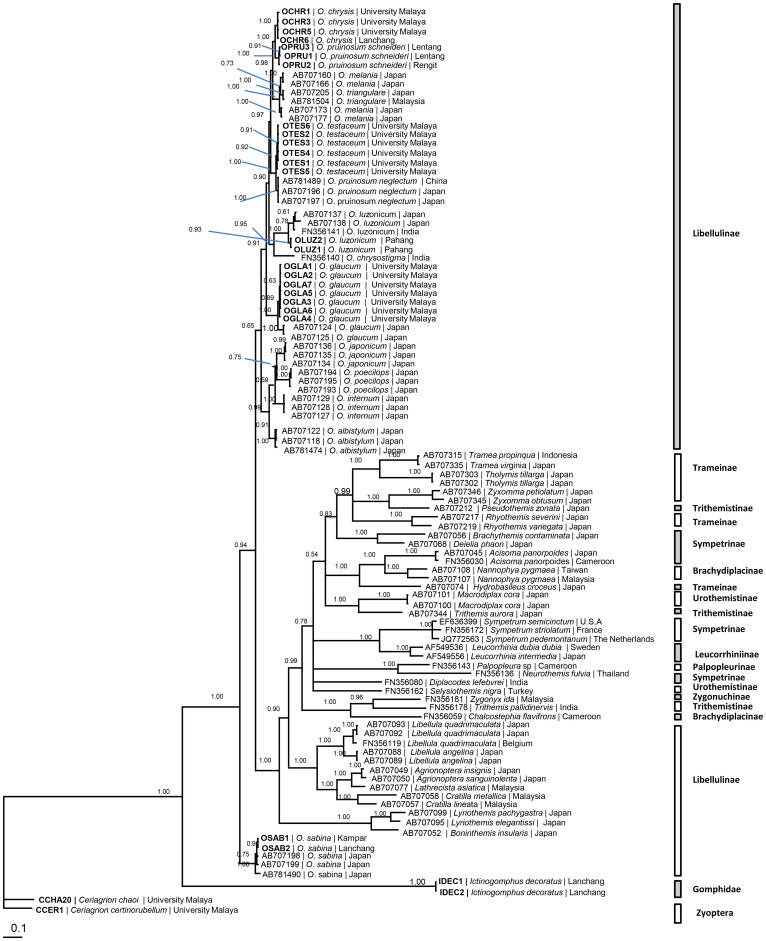
Orthetrum pruinosum schneideri clustered with O. chrysis and both were distinctly separated from O. testaceum and O. pruinosum neglectum (Fig. 2). The peninsular Malaysian taxon of O. luzonicum clustered with those of China and Japan. Likewise, O. sabina from Peninsular Malaysia clustered with O. sabina of India, Japan and Fiji.
Figure 2. BI tree based on COI nucleotide sequences.
Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap.
Phylogenetic relationships based on COII nucleotide sequences
There were two major clusters of Orthetrum species (supplementary Fig. 2): (I) [O. pruinosum schneideri, O. chrysis], O. testaceum, O. melania, O. luzonicum, O. glaucum, O. albistylum with weak support posterior probability (PP = 0.51) values and no support from maximum likelihood (ML); and (II) O. sabina.
Phylogenetic relationships based on ITS1 and ITS2 nucleotide sequences
The ITS nuDNA nucleotide sequences clearly separated O. pruinosum schneideri and O. pruinosum neglectum (Fig. 3) indicating distinct genetic lineages. O. pruinosum schneideri nested with O. chrysis while O. pruinosum neglectum nested with O. testaceum. The component taxa of Orthetrum were grouped in two distinct clades separated by a clade of other Libellulid genera. O. sabina was not nested with other Orthetrum taxa. The genus Orthetrum and the Libellulid subfamilies were not monophyletic.
Figure 3. BI tree based on ITS1&2 nucleotide sequences.
Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap.
Phylogenetic relationships based on combined nucleotide sequences
The combined COI and COII sequences yielded three major clusters (Fig. 4): (I) [O. pruinosum schneideri, O. chrysis], O. testaceum, O. triangulare, O. luzonicum with PP supoprt of 0.92 and no support from ML; (II) O. glaucum; and (III) O. sabina. Similar topology resulted from the combined COI + COII + 16S rDNA nucleotide sequences (supplementary Fig. 3). The combined 5 markers (supplementary Fig. 4) showed three clades: (I) O. chrysis, O. pruinosum schneideri, O. testaceum; (II) O. glaucum, O. sabina; and (III) O. luzonicum.
Figure 4. BI tree based on COI + COII nucleotide sequences.
Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap.
The combined COI + 16S rDNA sequences of Orthetrum taxa formed five major clusters (Fig 5): (I) [O. pruinosum schneideri, O. chrysis], O. testaceum, O. pruinosum neglectum, O. melania; (II) [O. internum, O. japonicum], O. poecilops, O. albistylum; (III) O. luzonicum; (IV) O. glaucum; and (V) O. sabina. The first four clusters (I–IV) had full PP and high ML support except cluster V with moderate support of PP = 0.79 and ML = 79%.
Figure 5. BI tree based on COI + 16S rDNA nucleotide sequences.
Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap.
Discussion
The phylogeny of the dragonflies (suborder Anisoptera) has been extensively studied4,5,6,7,8,9,10. Nine genera of Libellulidae have been reported to be monophyletic11. In the present study with more extensive taxon sampling, the various subfamilies of the family Libellulidae as well as the component taxa of the genus Orthetrum were not resolved unequivocally as monophyletic by the 28S rDNA (supplementary Fig. 1), 16S rDNA (Fig. 1), COI (Fig. 2), and ITS1&2 (Fig. 3) nucleotide sequences.
Species complexes in the genus Orthetrum have been uncovered by DNA sequence analyses. Based on molecular phylogeny and morphological characteristics, Orthetrum internum McLachlan, 1894 (previously regarded as O. japonicum internum McLachlan, 1894) is resolved as a genuine/distinct species from O. japonicum japonicum (Uhler, 1858)2,12. Likewise, O. triangulare and the allied taxon O. melania are well separated by the nuclear (ITS1 and ITS2) and mitochondrial (COI and 16S rRNA) genes3. Additionally, O. melania is separated into four subgroups: O. m. melania (mainland Japan), O. m. continentale (China, Korea and Taiwan), O. m. yaeyamense (Yaeyama Island, Japan), and O. m. ryukyuense (Amami, Kerama, Okinawa and Tokara, Japan).
In the present study, the nuclear 28S rDNA nucleotide sequences were highly conserved and could not resolve congeneric species of Orthetrum (supplementary Fig. 1). The 28S rRNA gene has been found to be better for resolving deep branching in the Odonata13. However, the mitochondrial genes (COI, COII and 16S) and the nuclear ITS1&2 genes unequivocally separated morphologically similar species, such as the reddish-coloured O. chrysis and O. testaceum and the bluish-coloued species O. glaucum and O. luzonicum (Figs. 1,2,3,4, Supplementary Fig. 2). Additionally, the 16S rDNA sequences revealed distinct genetic lineages of (1) O. luzonicum from Peninsular Malaysia and China-Japan, and (2) O. sabina of Peninsular Malaysia and India-Japan-Fiji (Fig. 1).
In the phylogeny based on nine Japanese Orthetrum species, O. pruinosum neglectum clusters with O. melania2. The present study based on the ITS1&2 (Fig. 3), COI (Fig. 2), 16S rDNA (Fig. 1) and combined COI + 16S rDNA (Fig. 5) nucleotide sequences and with more extensive taxon sampling indicates that O. pruinosum neglectum clusters nearer to O. testaceum than O. melania. The allied/sibling taxon O. pruinosum schneideri is grouped with O. chrysis (Figs. 1,2,3,4,5, Supplementary Figs. 2–4). It is distinctly separated from O. pruinosum neglectum. The two taxa are, without reasonable doubt, cryptic species of a species complex. In the African dragonfly genus Trithemis, COI and ND1 genes reveal three distinct genetic clusters of T. stricta but these taxa could not be identified by using classical taxonomic characters14.
In summary, phylogenetic analyses of a more extensive taxon sampling based on nucleotide sequences of mitochondrial and nuclear genes indicate that the various subfamilies of the family Libellulidae and the genus Orthetrum are not resolved unequivocally as monophyletic. The nuclear 28S rRNA gene is highly conserved and could not resolve congeneric species of Orthetrum. Individual mitochondrial genes (COI, COII, and 16S rRNA) and combination of these genes as well as the nuclear ITS1&2 genes clearly differentiate morphologically similar species, such as the reddish species pairs O. chrysis and O. testaceum, and the bluish-coloured species O. glaucum and O. luzonicum. This study also reveals distinct genetic lineages between O. pruinosum schneideri (occurring in Malaysia) and O. pruinosum neglectum (occurring north of Peninsular Malaysia from India to Japan), indicating these taxa are cryptic species. The finding of O. pruinosum occurring as a species complex paves the way for an in-depth phylogeographical study to determine the systematic status of the component taxa. Likewise, phylogeographical studies are needed for O. luzonicum and O. sabina.
Methods
Ethics statement
No specific permits were required for the described field studies. The dragonflies were collected in disturbed habitats such as open ditches and ponds. No specific permissions were required and the dragonflies are not endangered or protected species.
Specimens
Specimens of the Orthetrum dragonflies for the present study were collected using sweep net or plastic bag. They were identified with established literature15,16. In addition, Ictinogomphus decoratus (Anisoptera, Gomphidae) was included for comparison. Two species of Ceriagrion (Zygoptera, Coenagrionidae) were used as outgroup. Details of the species studied are listed in Table 1.
Table 1. Nucleotide sequences of COI, COII, 16S rRNA, 28S rRNA, ITS1 and/or ITS2 sequences for the taxa of Orthetrum of the family Libellulidae used in the present study. Ictinogomphus decoratus (family Gomphidae), Ceriagrion chaoi and C. cerinorubellum (suborder Zygoptera) were used as outgroups. NA, not available.
| No. | Sample Name | Sampling Location | Collection Code | GenBank/DDBJ Accession Number | |||||
|---|---|---|---|---|---|---|---|---|---|
| COI | COII | 16S | 28S | ITS1 | ITS2 | ||||
| Samples derived from this study | |||||||||
| Odonata | |||||||||
| Libellulidae | |||||||||
| 1 | Orthetrum chrysis | University Malaya | OCHR1 | AB860015 | AB860042 | AB860069 | AB860097 | KJ802958 | KJ802986 |
| 2 | Orthetrum chrysis | University Malaya | OCHR3 | AB860016 | AB860043 | AB860070 | AB860098 | KJ802959 | KJ802987 |
| 3 | Orthetrum chrysis | University Malaya | OCHR5 | AB860017 | AB860044 | AB860071 | AB860099 | KJ802960 | KJ802988 |
| 4 | Orthetrum chrysis | Lanchang, Pahang | OCHR6 | AB860018 | AB860045 | AB860072 | AB860100 | KJ802961 | KJ802989 |
| 5 | Orthetrum glaucum | University Malaya | OGLA1 | AB860019 | AB860046 | AB860073 | AB860101 | KJ802962 | KJ802990 |
| 6 | Orthetrum glaucum | University Malaya | OGLA2 | AB860020 | AB860047 | AB860074 | AB860102 | KJ802963 | KJ802991 |
| 7 | Orthetrum glaucum | University Malaya | OGLA3 | AB860021 | AB860048 | AB860075 | AB860103 | KJ802964 | KJ802992 |
| 8 | Orthetrum glaucum | University Malaya | OGLA4 | AB860022 | AB860049 | AB860076 | AB860104 | KJ802965 | KJ802993 |
| 9 | Orthetrum glaucum | University Malaya | OGLA5 | AB860308 | KF248113 | KF248140 | KF581186 | KJ802966 | KJ802994 |
| 10 | Orthetrum glaucum | University Malaya | OGLA6 | AB860023 | AB860050 | AB860077 | AB860106 | KJ802967 | KJ802995 |
| 11 | Orthetrum glaucum | Lentang, Pahang | OLGA7 | AB860024 | AB860051 | AB860078 | AB860107 | KJ802968 | KJ802996 |
| 12 | Orthetrum testaceum | University Malaya | OTES1 | AB860025 | AB860052 | AB860079 | AB860108 | KJ802969 | KJ802997 |
| 13 | Orthetrum testaceum | University Malaya | OTES2 | AB860026 | AB860053 | AB860080 | AB860109 | KJ802970 | KJ802998 |
| 14 | Orthetrum testaceum | University Malaya | OTES3 | AB860027 | AB860054 | AB860081 | AB860110 | KJ802971 | KJ802999 |
| 15 | Orthetrum testaceum | University Malaya | OTES4 | AB860028 | KF248112 | KF248139 | KF581185 | KJ802972 | KJ803000 |
| 16 | Orthetrum testaceum | University Malaya | OTES5 | - | - | - | - | KJ802973 | KJ803001 |
| 17 | Orthetrum testaceum | University Malaya | OTES6 | AB860029 | AB860056 | AB860083 | AB860112 | KJ802974 | KJ803002 |
| 18 | Orthetrum luzonicum | Pahang | OLUZ1 | AB860037 | AB860064 | AB860091 | AB860118 | KJ802980 | KJ803008 |
| 19 | Orthetrum luzonicum | Pahang | OLUZ2 | AB860038 | AB860065 | AB860092 | AB860119 | KJ802981 | KJ803009 |
| 20 | Orthetrum pruinosum schneideri | Lentang, Pahang | OPRU1 | AB860032 | AB860059 | AB860086 | AB860115 | KJ802977 | KJ803005 |
| 21 | Orthetrum pruinosum schneideri | Rengit, Pahang | OPRU2 | AB860033 | AB860060 | AB860087 | AB860116 | KJ802978 | KJ803006 |
| 22 | Orthetrum pruinosum schneideri | Lentang, Pahang | OPRU3 | AB860034 | AB860061 | AB860088 | AB860117 | KJ802979 | KJ803007 |
| 23 | Orthetrum pruinosum schneideri | Maliau, Sabah | OPRU4 | AB860035 | AB860062 | AB860089 | - | - | - |
| 24 | Orthetrum pruinosum schneideri | Maliau, Sabah | OPRU5 | AB860036 | AB860063 | AB860090 | - | - | - |
| 25 | Orthetrum sabina | Kampar, Perak | OSAB1 | AB860030 | AB860057 | AB860084 | AB860113 | KJ802975 | KJ803003 |
| 26 | Orthetrum sabina | Lanchang Pahang | OSAB2 | AB860031 | AB860058 | AB860085 | AB860114 | KJ802976 | KJ803004 |
| Odonata | |||||||||
| Gomphidae | |||||||||
| 27 | Ictinogomphus decoratus | Lanchang, Pahang | IDEC1 | AB860039 | AB860066 | AB860093 | AB860120 | KJ802982 | KJ803010 |
| 28 | Ictinogomphus decoratus | Lanchang, Pahang | IDEC2 | AB860040 | AB860067 | AB860094 | AB860121 | KJ802983 | KJ803011 |
| Odonata | |||||||||
| Coenagrionidae | |||||||||
| 29 | Ceriagrion chaoi | University Malaya | CCHA20 | AB860041 | AB860068 | AB860095 | AB860122 | KJ802984 | KJ803012 |
| 30 | Ceriagrion cerinorubellum | University Malaya | CCER1 | AB860310 | AB860307 | AB860096 | AB860123 | KJ802985 | KJ803013 |
DNA extraction, PCR amplifications and DNA sequencing
The genomic DNA was extracted and PCR amplification was performed as described in Lim et al.17 except with variations in annealing temperature for different primers. The primers and annealing temperature for PCR were: COI –F: 5′- ATAATTGGRGGRTTYGGRAAY TG-3′ and R: 5′- CCAAARAATCAAAATAARTGT TG-3′18, at 50°C; COII: C2-J-3102: 5′-AAATGGCAACATGAGCACAAYT-3′ and TK-N-3773: 5′-GAGACCAGTACTTGCTTTCAGTCATC-3′19 at 50°C; 16S rDNA: 5′-TTGACTGTACAAAGGTAGC-3′ and 5′-GATATTACGCTGTTATCCC-3′20 at 50°C; 28S rDNA: 28sf, 5′-AAGGTAGCCAAATGCCTCATC-3′ and 28sr, 5′-AGTAGGGTAAAACTAACCT-3′ at 52°C13; ITS1: CAS18sF,5′- TACACACCGCCCGTCGCTACTA-3′ and CAS5p8sB1d, 5′- ATGTGCGTTCRAAATGTCGATGTTCA-3′21 at 67°C; and ITS2: CAS5p8sFc, 5′-TGAACATCGACATTTYGAACGCACAT-3′ and CAS28sB1d, 5′-TTCTTTTCCTCCSCTTAYTRATATGCTTAA-3′21 at 55°C.
The PCR products were assayed by electrophoresis on 1.0% agarose mini gels stained with SYBR® Safe DNA gel stain (Invitrogen, USA) and visualised under UV light. The amplicons were isolated and purified using the LaboPassTM PCR purification kit (Cosmo Genetech, South Korea). The purified PCR products were sent to a commercial company for sequencing. The same set of PCR primers were used for DNA sequencing. Samples were sequenced using BigDyeH Terminator v3.1 Sequencing Kit and analysed on an ABI PRISMH 377 Genetic Analyser.
Genetic divergence
To assess the parsimony information of the sequences of the data sets and species level variation of Orthetrum species, selected specimens were used to measure the uncorrected (p) pairwise genetic distances using PAUP* 4.0b10 software22. All individual markers and combined mitochondrial markers (COI + 16S rDNA; COI + COII + 16S rDNA; and COI + COII + 16S rDNA + 28S rDNA) were used to estimate uncorrected (p) pairwise genetic distances.
Phylogenetic analysis
To elucidate the phylogenetic relationship among the different species of Orthetrum species, sequences generated from this study were combined with GenBank sequences (Table 1 and Supplementary Table 7) to construct phylogenetic trees. The generated forward and reverse sequences were manually edited and assembled using ChromasPro v1.5 (Technelysium Pty Ltd., Australia) software. The datasets for all genetic markers were aligned using ClustalX23. In the preliminary alignment for ITS1 and ITS2, the flanking sequences of 18S rDNA and 5.8S rDNA were included as the guide and were only being trimmed off after final alignment before subjected for phylogenetic analysis. For 28S and 16S, the sequences were aligned using MAFFT 624, with Q-INS-i strategy in order to take into account the secondary structure of the RNA. The generated aligned sequences were subjected for the search of the best model to be used for maximum likelihood (ML) and Bayesian Inference (BI) analyses using Kakusan v. 325. Best fit models were evaluated using the corrected Akaike Information Criterion for ML and the Bayesian Information Criterion (BIC) for BI with nonpartitioned on the whole sequence. The selected models for ML and BI of each data set are summarised in Supplementary Table 1. ML analysis was performed via Treefinder version October26 and BI analysis was performed using MrBayes 3.1.227. Bayesian analyses were initiated with a random starting tree and two parallel runs, each of which consisted of running four chains of Markov chain Monte Carlo (MCMC) iterations for 6x106 generations. The trees in each chain were sampled every 200th generation. Likelihood values for all post-analysis trees and parameters were evaluated for convergence and burn-in using the “sump” command in MrBayes and the computer program Tracer ver. 1.5 (http://tree.bio.ed.ac.uk/software/tracer/). The first 30,000 trees were discarded as burn-in (where the likelihood values were stabilized prior before the burn in), and the remaining trees after burn-in were used to calculate posterior probabilities using the “sumt” command.
Supplementary Material
Supplementary Tables and Figures
Acknowledgments
This study was funded in part by MoHE-HIR Grant (H-50001-00-A000025) and the University of Malaya (H-5620009). We thank our institutions for providing various research facilities and other support.
Footnotes
The authors declare no competing financial interests.
Author Contributions H.S.Y. and P.E.L. conceived the research in collaboration with J.T., Y.F.N., P.E. and I.W.S. H.S.Y., Y.F.N. and I.W.S. collected the specimens. H.S.Y. identified the specimens. J.T. conducted the PCR and P.E.L., J.T. and P.E. performed the phylogenetic analyses. H.S.Y. and P.E.L. wrote the paper in collaboration with the co-authors. H.S.Y. and P.E.L. were responsible for the final manuscript version.
References
- Silsby J. Dragonflies of the world (CSIRO Publishing, Collingwood, 2001). [Google Scholar]
- Karube H., Futahashi R., Sasamoto A. & Kawashima I. Taxonomic revision of Japanese odonate species, based on nuclear and mitochondrial gene genealogies and morphological comparison with allied species. Part I. Tombo, Fukui 54, 75–106 (2012). [Google Scholar]
- Sasamoto A. & Futahashi R. Taxonomic revision of the status of Orthetrum triangulare and melania group (Anisoptera: Libellulidae) based on molecular phylogenetic analyses and morphological comparisons, with a description of three new subspecies of melania. Tombo, Fukui 55, 57–82 (2013). [Google Scholar]
- Artiss T., Schultz T. R., Polhemus D. A. & Simon C. Molecular phylogenetic analysis of the dragonfly genera Libellula, Ladona, and Plathemis (Odonata: Libellulidae) based on mitochondrial cytochrome oxidase I and 16S rRNA sequence data. Mol. Phylogenet. Evol. 18, 348–361 (2001). [DOI] [PubMed] [Google Scholar]
- Ware J., May M. & Kjer K. Phylogeny of the higher Libelluloidea (Anisoptera: Odonata): an exploration of the most speciose superfamily of dragonflies. Mol. Phylogenet. Evol. 45, 289–310 (2007). [DOI] [PubMed] [Google Scholar]
- Fleck G., Brenk M. & Misof B. Larval and molecular characters help to solve phylogenetic puzzles in the highly diverse dragonfly family Libellulidae (Insecta: Odonata: Anisoptera): The Tetrathemistinae are a polyphyletic group. Org. Divers. Evol. 8, 1–16 (2008). [Google Scholar]
- Fleck G. et al. A phylogeny of anisopterous dragonflies (Insecta, Odonata) using mtRNA genes and mixed nucleotide/doublet models. J. Zool. Syst. Evol. Res. (2008) 46, 310–322 (2008). [Google Scholar]
- Dijkstra K.-D. B. & Vick G. S. Inflation by venation and the bankruptcy of traditional genera: the case of Neodythemis and Micromacromia, with keys to the continental African species and the description of two new Neodythemis species from the Albertine Rift (Odonata: Libellulidae). Int. J. Odonatol. 9, 51–70 (2006). [Google Scholar]
- Pilgrim E. M. & von Dohlen C. D. Phylogeny of the Sympetrinae (Odonata: Libellulidae): further evidence of the homoplasious nature of wing venation. Syst. Ent. 33, 159–174 (2008). [Google Scholar]
- Blanke A., Greve C., Mokso R., Beckman F. & Misof B. An updated phylogeny of Anisoptera including formal convergence analysis of morphological characters. Syst. Ent. 38, 474–490 (2013). [Google Scholar]
- Bybee S. M., Ogdenb T. H., Branhama M. A. & Whiting M. F. Molecules, morphology and fossils: a comprehensive approach to odonate phylogeny and the evolution of the odonate wing. Cladistics 24, 477–514 (2008). [DOI] [PubMed] [Google Scholar]
- Futahashi R. A revisional study of Japanese dragonflies based on DNA analyses. Tombo, Fukui 53, 67–74 (2011). (in Japanese). [Google Scholar]
- Hasegawa E. & Kasuya E. Phylogenetic analysis of the insect order Odonata using 28S and 16S rDNA sequences: a comparison between data sets with different evolutionary rates. Entomol. Sci. 9, 55–66 (2006). [Google Scholar]
- Damm S., Schierwater B. & Hadrys H. An integrative approach to species discovery in odonates: from character-based DNA barcoding to ecology. Mol. Ecol. 19, 3881–3893 (2010). [DOI] [PubMed] [Google Scholar]
- Orr A. G. Dragonflies of Peninsular Malaysia and Singapore (Natural History Publications (Borneo), Kota Kinabalu, 2005). [Google Scholar]
- Tang H. B., Wang L. K. & Hämäläinen M. A photographic guide to the dragonflies of Singapore (The Raffles Museum of Biodiversity Research, Singapore, 2010). [Google Scholar]
- Lim P. E., Tan J., Suana I. W., Eamsobhana P. & Yong H. S. Distinct genetic lineages of Bactrocera caudata (Insecta: Tephritidae) revealed by COI and 16S DNA sequences. PLoS ONE 7, e37276 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F., Dobata S. & Futahashi R. Disturbed population genetics: suspected introgressive hybridization between two Mnais damselfly species (Odonata). Zool. Sci. 22, 869–881 (2005). [DOI] [PubMed] [Google Scholar]
- Jordan S., Simon C. & Polhemus D. Molecular systematics and adaptive radiation of Hawaii's endemic damselfly genus Megalagrion (Odonata:Coenagrionidae). Syst. Biol. 52, 89–109 (2003). [DOI] [PubMed] [Google Scholar]
- Schmitz J. & Moritz R. F. A. Molecular phylogeny of Vespidae (Hymenoptera) and the evolution of sociality in wasps. Mol. Phylogenet. Evol. 9, 183–191 (1998). [DOI] [PubMed] [Google Scholar]
- Ji Y.-J., Zhang D.-X. & He L.-J. Evolutionary conservation and versatility of a new set of primers for amplifying the ribosomal internal transcribed spacer regions in insects and other invertebrates. Mol. Ecol. Notes 3, 581–585 (2003). [Google Scholar]
- Swofford D. L. PAUP: Phylogenetic analysis using parsimony (and other methods) (Sinauer Associates, Sunderland, Massachusetts, 2002). [Google Scholar]
- Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F. & Higgins D. G. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 24, 4876–4882 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Asimenos G. & Toh H. Multiple alignment of DNA sequences with MAFFT. Bioinformatics for DNA Sequence Analysis. Posada, D. (ed.) 39–54 (Humana Press, New York, 2009). [DOI] [PubMed] [Google Scholar]
- Tanabe A. S. Kakusan: a computer program to automate the selection of a nucleotide substitution model and the configuration of a mixed model on multilocus data. Mol. Ecol. Notes 7, 962–964 (2007). [Google Scholar]
- Jobb G., von Haeseler A. & Strimmer K. Treefinder: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4, 18 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Huelsenbeck J. P. & Ronquist F. MrBayes: Bayesian Inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables and Figures