Abstract
Sexually dimorphic phenotypes arise largely from sex-specific gene expression, which has mainly been characterized in sexually naïve adults. However, we expect sexual dimorphism in transcription to be dynamic and dependent on factors such as reproductive status. Mating induces many behavioral and physiological changes distinct to each sex and is therefore expected to activate regulatory changes in many sex-biased genes. Here, we first characterized sexual dimorphism in gene expression in Callosobruchus maculatus seed beetles. We then examined how females and males respond to mating and how it affects sex-biased expression, both in sex-limited (abdomen) and sex-shared (head and thorax) tissues. Mating responses were largely sex-specific and, as expected, females showed more genes responding compared with males (∼2,000 vs. ∼300 genes in the abdomen, ∼500 vs. ∼400 in the head and thorax, respectively). Of the sex-biased genes present in virgins, 16% (1,041 genes) in the abdomen and 17% (243 genes) in the head and thorax altered their relative expression between the sexes as a result of mating. Sex-bias status changed in 2% of the genes in the abdomen and 4% in the head and thorax following mating. Mating responses involved de-feminization of females and, to a lesser extent, de-masculinization of males relative to their virgin state: mating decreased rather than increased dimorphic expression of sex-biased genes. The fact that regulatory changes of both types of sex-biased genes occurred in both sexes suggests that male- and female-specific selection is not restricted to male- and female-biased genes, respectively, as is sometimes assumed.
Keywords: RNA-Seq, transcriptome, sex-biased expression, sex-specific selection, alternative splicing
Introduction
Differences in morphology, physiology and behavior between the sexes are nearly ubiquitous in sexually reproducing organisms and offer some of the most striking examples of intraspecific variation in the natural world. Evolution of sexual dimorphism requires both distinct selection in the sexes and a sex-specific genetic architecture. Because the sexes largely share the genome, the majority of sexually dimorphic phenotypic variation arises and persists via differential regulation of gene expression in females and males (Ellegren and Parsch 2007; Williams and Carroll 2009; Parsch and Ellegren 2013). This has now been demonstrated in several taxa (Arbeitman et al. 2002; Hahn and Lanzaro 2005; Yang et al. 2006a; Lebo et al. 2009; Prince et al. 2010; Naurin et al. 2011). Sex determination hierarchy (Williams and Carroll 2009), sex-linkage (Meisel et al. 2012) and miRNA’s (Fagegaltier et al. 2014) participate in the generation and maintenance of sex-biased expression. Genes expressed at a higher level in males (i.e., male-biased genes) are thought to evolve primarily under male-specific selection and those with higher expression in females (i.e., female-biased genes) primarily under female-specific selection (Ellegren and Parsch 2007). In line with this, both comparative genomic (Harrison et al. 2015) and experimental evolution (Hollis et al. 2014; Immonen et al. 2014) studies have shown that sex-biased genes evolve regulatory differences in response to variation in the mating system. Studies across taxa have also demonstrated that male-biased genes generally tend to evolve faster at both regulatory and sequence levels than female- and un-biased genes (Zhang et al. 2004, 2007; Grath et al. 2009; Harrison et al. 2015), presumably due to stronger sexual selection in males.
In addition to the sex of the organism, changes to the internal (De Gregorio et al. 2001; Zhou et al. 2014) and external abiotic environments (Smith et al. 2013; Hoang et al. 2015) as well as social conditions (Carney 2007; Cummings et al. 2008; Immonen and Ritchie 2012) are major causes of variation in the gene expression patterns. A static genome can track environmental changes by altering the regulation of gene expression, and understanding the transcriptomic basis of phenotypic plasticity can thus provide important insights into how a single genotype can generate different phenotypes (Zhou et al. 2012; Kijimoto et al. 2014; Perry and Mank 2014). Thus far, studies have explored to what extent sexual dimorphism changes across ontogeny (Arbeitman et al. 2002; Perry et al. 2014) and under different developmental conditions (Wyman et al. 2010; Ledon-Rettig and Moczek 2016). The likely possibility that the pattern of sex-biased expression changes during adult life, due to changes in reproductive state, has to our knowledge not been previously investigated.
Here, we test for plasticity in gene expression between the sexes in response to mating in Callosobruchus maculatus seed beetles, a model species in sexual selection and conflict studies (Zuk et al. 2014). Mating triggers major physiological and behavioral changes that differ between the sexes, and is therefore likely to affect the expression of sex-biased genes. Molecular social interactions that occur during and after mating are predicted to evolve under sex-specific selection due to sperm competition, cryptic female choice and interlocus sexual conflict (Rice and Gavrilets 2014). For example, males transfer a cocktail of seminal fluid proteins and peptides (Sfps) to females, many of which exercise physiological effects in females (Wolfner 2009). In C. maculatus, 98 Sfps have thus far been identified (Bayram et al. 2016). Many of these proteins differ in their abundance across populations and are associated with variation in male sperm competitiveness and ability to influence female fecundity and lifespan (Goenaga et al. 2015). Studies in D. melanogaster have shown that females undergo dramatic changes not only in egg production following mating (Chapman et al. 2001), but also in sleeping and locomotion (Isaac et al. 2010), eating (Carvalho et al. 2006), re-mating (LaFlamme et al. 2012), immunity (Peng et al. 2005; Innocenti and Morrow 2009) and ageing (Chapman et al. 1995). Whilst such a comprehensive analysis of female mating responses in seed beetles is yet to be undertaken, SFPs injected into females alter egg laying rate, female remating rate and male competitive fertilization success (Yamane et al. 2015). These phenotypes likely result from postmating changes in expression of hundreds to thousands of genes in females in both reproductive and somatic tissues (Lawniczak and Begun 2004; McGraw et al. 2004, 2008; Mack et al. 2006; Innocenti and Morrow 2009; Dalton et al. 2010; Gioti et al. 2012).
Compared with females, male postmating physiological and molecular changes are poorly understood. In male C. maculatus, high mating frequency results in a rapid decline in the ejaculate volume (Rönn et al. 2008), which should require a subsequent renewal of the ejaculate components (i.e., lipids, proteins and polysaccharides). In line with this, males show a postmating increase in metabolic rate (Immonen et al. 2016b). A handful of genes are known to change expression following mating in male D. melanogaster (Ellis and Carney 2010) and Ceratitis capitata (Gomulski et al. 2012). Studying the transcriptomic basis of mating responses in each sex allows identifying candidate genes and genetic pathways underlying reproductive phenotypes. Thus far, however, our knowledge of such mating responses is largely limited to females of D. melanogaster (McGraw et al. 2004, 2008; Mack et al. 2006; Innocenti and Morrow 2009; Dalton et al. 2010; Zhou et al. 2014), Anopheles mosquitoes (Kocher et al. 2008; Rogers et al. 2008; Alfonso-Parra et al. 2016) and Ceratitis capitata fruit flies (Gomulski et al. 2012).
In the present study, we tested how gene expression dimorphism changes as a result of mating, using RNA-seq. First, we characterized sexual dimorphism in transcript abundance in virgin beetles, comparing both reproductive and somatic tissues. We also explored whether any genes show sex differences in isoform-specific expression due to alternative splicing. This is the first study to characterize sex-biased expression in C. maculatus. Second, we investigated how mating changes gene expression, specifically testing how each sex may alter the patterns of sexually dimorphic expression. Under the premise that female reproduction involves primarily female-biased genes and male reproduction male-biased genes, we predicted that females more commonly upregulate female- and males upregulate male-biased genes in response to mating. This would subsequently result in an increase in sexual dimorphism relative to the virgin state.
Materials and Methods
Sample Preparation
Callosobruchus maculatus is a facultatively aphagous pest of legume plants, occurring in the subtropical and tropical regions throughout the world. It has a polygynandrous mating system. Females lay eggs on seeds and the larval development and pupation are completed within the seeds. Adults are ready to mate as soon as they emerge from the seed. A near isogenic line (from “South-India” stock population, generated by 5 generations of inbreeding) was used in this experiment. The beetles were reared on mung beans (Vigna radiata) in laboratory climate cabinets at 29°C, 60% RH and a 12-L:12-D light cycle. The parental generation laid eggs over a 24-h period in noncrowded conditions with a surplus of host beans. Beans were isolated prior to adult hatching and virgin males and females were subsequently collected and stored in isolation. In the mating treatment group, 1-day-old males and females were briefly paired and were separated after mating was terminated. Same-aged mated and virgin beetles were kept individually for 24 h with beans, after which they were snap-frozen with liquid nitrogen and stored in −80°C. This time point was chosen as it corresponds to the time when females lay most of their eggs (Fox 1993; Immonen et al. 2016a) and males still undergo increased postmating metabolism, presumably due to ejaculate renewal (Immonen et al. 2016b). We separated the head and thorax (the somatic tissues) from the abdomen (the reproductive tissues) under ice, pooled tissues from six individuals per sample and extracted total RNA using RNeasy Mini Kit (Qiagen), following the manufacturer’s protocol. DNase digestion was applied using DNase I (RNase-Free DNase set by Qiagen). The RNA quality and quantity was assessed and affirmed using NanoDrop, Qubit and Bioanalyzer. Three replicate samples were prepared per sex, treatment and tissue type, resulting in a total of 24 samples.
cDNA Library Generation and Illumina Sequencing
The RNA-seq libraries were prepared using the Illumina TruSeq stranded mRNA sample preparation kit according to the manufacturer’s guidelines (Illumina 2013). Poly-A containing RNA was purified from total RNA using poly-T oligo attached magnetic beads, after which mRNA was fragmented and reverse transcribed to first strand cDNA using random primers. The cDNA fragments were ligated to adapters and purified cDNA libraries enriched with PCR. All sequencing was performed using Illumina HiSeq 2500 sequencing technology producing 100-bp length paired-end reads. All libraries were sequenced in two lanes. The two technical replicates of each sample were subsequently pooled in the assembly to maximize the coverage. The library generation and sequencing were performed by SNP&SEQ Technology Platform at Uppsala University.
Transcriptome Assembly and Annotation
Due to the lack of closely related reference genome, the transcriptome was de novo assembled, using all libraries as well as additional samples from different developmental stages. All samples were deeply sequenced to cover high and low expressed genes. In total, more than 492 million read pairs were sequenced and used to generate a reference transcriptome with Trinity assembler (Grabherr et al. 2011; Haas et al. 2013). The de novo assembly has been described in detail in Sayadi et al. (2016). We separately mapped back the reads from each sample to the assembled transcriptome in order to obtain the read counts as the measure of expression using RSEM method. Trinity transcript (gene isoform) clusters with the longest isoform were selected as representatives of genes in the subsequent gene-level analysis (hereafter referred to as genes for convenience). The assembled transcriptome generated 218,192 transcripts which correspond to 145,883 putative genes. De novo transcriptome assemblies generate far more transcripts than what is a biologically realistic number of genes for several reasons (Sayadi et al. 2016). We thus considered only the predicted genes that contained an ORF (a total of 29,812 genes). Further, we required a gene to be expressed by at least 2 counts per million reads (cpm) in at least three libraries. This reduced the dataset into 12,412 expressed genes in the abdomen libraries and 11,143 in the head and thorax libraries for the gene-level analysis for sex-biased expression. The female libraries used for the mating response analyses contained 12,333 expressed genes and the male libraries 12,855 expressed genes.
Statistical Analyses
The analyses were performed using edgeR v.3.10.5 (Robinson et al. 2010) and limma v.3.24.15 (Smyth 2005) packages within Bioconductor (Gentleman et al. 2004), using R v.3.2.2 (RDevelopmentCoreTeam 2011). We followed the pipeline offered in edgeR (Robinson et al. 2010): after normalization (by computing scaling factors using the trimmed mean of M values, or TMM, and adjusting the library sizes accordingly to account for putative differences in the RNA composition between libraries) a generalized linear model with likelihood ratio tests was used, where a negative binomial model is fitted with a Cox-Reid profile-adjusted likelihood ratio method to estimate tag-wise dispersion. Putative differential exon usage between the sexes (i.e., alternative splicing) was tested by fitting a negative binomial generalized log-linear model at the exon level and subsequently comparing the log fold change of each exon to that of the entire gene, as implemented in edgeR. In all analyses, we used a statistical significance threshold of 5% False Discovery Rate (FDR) unless stated otherwise (Benjamini and Hochberg 1995). Overrepresentation of Gene Ontology terms (Biological Process and Molecular Function) was tested with the GOstats package v.2.34.0, using a conditional hypergeometric test with a P value cutoff < 0.05 (Falcon and Gentleman 2007). The gene universe was defined as all the expressed genes in a given condition.
Our inferential tests include: (1) a test of differential expression between virgin males and females within each tissue type, to identify sex-biased genes. To call sex-bias, we required at least a 2-fold difference in expression between the sexes (i.e., log2FC ≥ 1 or −1) (Montgomery and Mank 2016), in addition to the statistical significance threshold. Considering only genes with large expression difference between the sexes limits the cases where sex-bias in expression may result from differences in the composition of the shared tissues rather than from regulatory differences per se (Montgomery and Mank 2016). Expression differences due to different reproductive organs naturally remain. We present the data on expression differences between females and males with different fold-change cut-offs in the supplementary figure S1, Supplementary Material online. (2) We examined patterns of sex-biased expression in genes with a single versus multiple expressed transcripts to test whether any genes show sex differences in isoform-specific expression in virgins, due to alternative splicing, separately for the head and thorax and abdomen. (3) To achieve our main goal of identifying mating induced changes in sexually dimorphic expression, we fitted a two-way ANOVA-type model (in edgeR) with a specific contrast to test for the interaction effect between sex and mating status, separately for each tissue class. Here, interactions signify effects of mating status on sexual dimorphism. We then compared gene expression changes in response to mating within each sex (for each tissue class). These results were combined to assess how each sex contributes to changes in sex-bias (i.e., a switch from male- to female-bias, or vice versa, or to un-biased expression). For identifying genes that change sexually dimorphic expression due to mating, we required that they show a significant mating-by-sex interaction and respond significantly to mating in at least one of the sexes. The additional criterion of the main effect of mating allows investigating which sex is causing the putative sex-bias plasticity with high statistical confidence. Moreover, for classifying those plastic genes that switch to opposite sex-bias due to mating we also required at least a 2-fold expression difference (with 5% FDR) between the sexes in both virgin and mated states, for the reasons stated earlier. Proportion tests were used for assessing whether sex-biased genes were overrepresented among the mating responsive genes in either sex, as well as to test for deviations in the up/downregulation patterns of sex-biased mating responsive genes from the expected (the expected patterns were calculated as the proportion of all up/downregulated genes).
We refer to a pattern of expression where mated females change to more resemble males as de-feminization (i.e., when female-biased genes decrease and/or male-biased genes increase expression relative to virgin females). We refer to de-masculinization of males as a pattern where mated males increase the expression of female-biased and/or decrease male-biased genes relative to their virgin state.
Results
Sexual Dimorphism of the C. maculatus Virgin Transcriptome
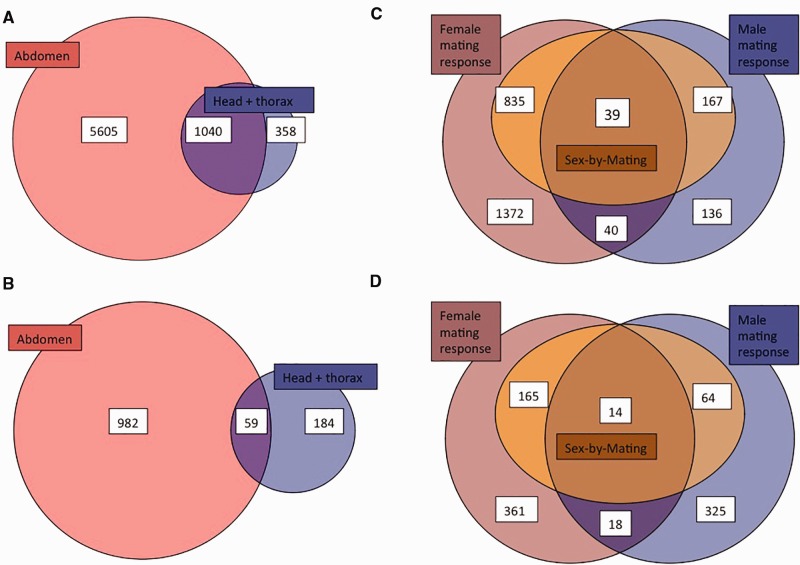
First, we analysed male and female transcriptomes separately in the abdomen and head/thorax in virgin beetles, in order to characterize how many and what types of genes differ in expression between the sexes in somatic and reproductive tissues of C. maculatus. We identified a total of 6,645 differentially expressed genes in the abdomen (54% of the 12,412 genes expressed in the abdomen, supplementary fig. S1a, Supplementary Material online) and 1,398 genes in the head and thorax (13% of the 11,143 expressed genes, supplementary fig. S1b, Supplementary Material online), with at least 2-fold expression difference between virgin females and males (FDR 5%). Even when using a highly stringent statistical criteria of 0.1% FDR (i.e., Padj≥ 0.001), 51% (6,343 genes) and 9% (1,018 genes) of the transcriptome was sex-biased in the abdomen and head/thorax tissues, respectively. In the abdomen, there were more male- than female-biased genes (≥ 2-fold change: 54% vs. 46% of the sex-biased genes, respectively). This was due to a higher number of highly (≥ 10-fold change) male-biased genes (10%) in the transcriptome compared with female-biased (4%) (see supplementary fig. S1a, Supplementary Material online). The opposite was true in the head and thorax, where 65% of the sex-biased genes showed higher expression in the females, and this was the case even for the highly sex-biased genes (≥10-fold change: 2% female- vs. 0.5% male-biased, supplementary fig. S1b, Supplementary Material online). There was a large overlap of sex-biased genes between the reproductive and somatic tissues (1,040 genes, fig. 1a). This pattern was, however, greater for the female-biased genes, of which 23% were shared between the tissues in contrast to 7% of the male-biased genes (supplementary fig. S2, Supplementary Material online). The overlap of both types of sex-biased genes between the tissues was mainly due to the head and thorax: 74% of the sex-biased genes expressed in the head and thorax were also sex-biased in the abdomen, while only 16% of the abdomen sex-biased genes were shared between the tissue classes (fig. 1a). The 50 most sex-biased genes of both tissue classes are presented in tables 1–4.
Fig. 1.—
(A) A Venn diagram of the numbers of sex-biased genes in virgins (i.e., Padj <0.05 and at least 2-fold sex-bias) in the abdomen and the head + thorax. (B) Numbers of genes showing plasticity in sex-biased expression due to mating (i.e., sex-by-mating interaction and mating response at least in one sex with Padj < 0.05) in each tissue class. Overlap of the genes that respond to mating in each sex and show sex-bias plasticity in (C) the abdomen and (D) the head and thorax tissues.
Table 1.
The 50 Most Sex-Biased Genes with Annotations. Female-Biased (log2FC >1) Genes in the Abdomen
| Gene ID | logFC | FDR | GO | Type | Term | BLAST hit |
|---|---|---|---|---|---|---|
| TR72008|c0_g2 | 13.1 | 1E-117 | ||||
| TR70149|c0_g1 | 12.5 | 2E-243 | ||||
| TR59852|c0_g2 | 12.1 | 0E-00 | GO:0005634 | CC | Nucleus | Uncharacterized protein LOC663065 isoform X2, Tribolium castaneum |
| GO:0016567 | BP | Protein ubiquitination | ||||
| GO:0004842 | MF | Ubiquitin-protein transferase activity | ||||
| GO:0016874 | MF | Ligase activity | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| GO:0008270 | MF | Zinc ion binding | ||||
| GO:0006511 | BP | Ubiquitin-dependent protein catabolic process | ||||
| GO:0007275 | BP | Multicellular organismal development | ||||
| TR19738|c0_g2 | 11.7 | 0E-00 | ||||
| TR18182|c9_g3 | 11.7 | 9E-207 | ||||
| TR11356|c0_g2 | 11.6 | 7E-138 | ||||
| TR74056|c0_g1 | 11.5 | 2E-171 | ||||
| TR64757|c0_g1 | 11.4 | 1E-42 | ||||
| TR29702|c2_g6 | 11.3 | 0E-00 | ||||
| TR44608|c4_g2 | 11.2 | 5E-258 | ||||
| TR25775|c0_g2 | 11.2 | 2E-36 | ||||
| TR38999|c0_g1 | 11.1 | 0E-00 | GO:0006468 | BP | Protein phosphorylation | Hypothetical protein YQE_12596, partial, Dendroctonus ponderosae |
| GO:0004672 | MF | Protein kinase activity | ||||
| GO:0005524 | MF | ATP binding | ||||
| TR41216|c5_g4 | 11.0 | 9E-128 | ||||
| TR44677|c1_g2 | 11.0 | 6E-49 | ||||
| TR55432|c1_g2 | 11.0 | 0E-00 | ||||
| TR32008|c0_g1 | 11.0 | 0E-00 | GO:0006685 | BP | Sphingomyelin catabolic process | Hypothetical protein D910_08519, Dendroctonus ponderosae |
| GO:0016798 | MF | Hydrolase activity, acting on glycosyl bonds | ||||
| GO:0008152 | BP | Metabolic process | ||||
| GO:0016787 | MF | Hydrolase activity | ||||
| GO:0004767 | MF | Sphingomyelin phosphodiesterase activity | ||||
| TR64521|c0_g2 | 10.9 | 0E-00 | ||||
| TR66638|c0_g1 | 10.9 | 0E-00 | GO:0003723 | MF | RNA binding | Protein bicaudal C, Tribolium castaneum |
| GO:0003676 | MF | Nucleic acid binding | ||||
| TR66019|c0_g2 | 10.8 | 9E-09 | ||||
| TR64213|c0_g1 | 10.7 | 2E-240 | ||||
| TR23777|c0_g3 | 10.7 | 1E-110 | ||||
| TR25403|c5_g2 | 10.6 | 3E-84 | ||||
| TR61942|c0_g1 | 10.5 | 2E-289 | ||||
| TR54080|c0_g1 | 10.4 | 0E-00 | GO:0016787 | MF | Hydrolase activity | Esterase, partial, Leptinotarsa decemlineata |
| TR54080|c0_g1 | 10.5 | 2E-14 | GO:0008152 | BP | Metabolic process | |
| TR73901|c6_g2 | 10.5 | 2E-279 | ||||
| TR34425|c0_g3 | 10.6 | 5E-42 | ||||
| TR33970|c0_g1 | 10.4 | 0E-00 | GO:0008233 | MF | Peptidase activity | Cathepsin B-like cysteine protease, Callosobruchus maculates |
| GO:0006508 | BP | Proteolysis | ||||
| GO:0050790 | BP | Regulation of catalytic activity | ||||
| GO:0004197 | MF | Cysteine-type endopeptidase activity | ||||
| GO:0008234 | MF | Cysteine-type peptidase activity | ||||
| TR10926|c0_g1 | 10.3 | 0E-00 | ||||
| TR57758|c0_g1 | 10.5 | 6E-57 | ||||
| TR10388|c4_g1 | 10.3 | 0E-00 | GO:0016787 | MF | Hydrolase activity | Trypsin-1 isoform X1, Bactrocera cucurbitae |
| GO:0008233 | MF | Peptidase activity | ||||
| GO:0008236 | MF | Serine-type peptidase activity | ||||
| GO:0004252 | MF | Serine-type endopeptidase activity | ||||
| GO:0006508 | BP | Proteolysis | ||||
| TR36924|c3_g1 | 10.4 | 2E-43 | ||||
| TR27866|c0_g3 | 9.9 | 0E-00 | ||||
| TR52542|c5_g1 | 9.9 | 0E-00 | ||||
| TR3585|c2_g1 | 9.9 | 2E-02 | ||||
| TR7023|c1_g2 | 10.0 | 7E-55 | ||||
| TR18182|c4_g1 | 9.8 | 5E-160 | ||||
| TR28153|c0_g1 | 9.8 | 0E-00 | ||||
| TR62432|c0_g1 | 9.8 | 4E-195 | ||||
| TR9715|c0_g2 | 9.8 | 3E-04 | ||||
| TR68457|c0_g1 | 9.4 | 3E-33 | GO:0046983 | MF | Protein dimerization activity | Hairy, Leptinotarsa decemlineata |
| GO:0006355 | BP | Regulation of transcription, DNA-templated | ||||
| GO:0003677 | MF | DNA binding | ||||
| TR29717|c0_g3 | 9.7 | 0E-00 | ||||
| TR12240|c0_g1 | 9.6 | 9E-71 | ||||
| TR68765|c0_g2 | 9.5 | 0E-00 | GO:0055114 | BP | Oxidation–reduction process | Salicyl alcohol oxidase-like protein, Phaedon cochleariae |
| GO:0050660 | MF | Flavin adenine dinucleotide binding | ||||
| TR10416|c0_g2 | 9.4 | 5E-26 | ||||
| TR58271|c0_g2 | 9.5 | 3E-158 | ||||
| TR36192|c2_g2 | 9.5 | 3E-269 | GO:0006508 | BP | Proteolysis | Cathepsin B-like proteinase, Triatoma vitticeps |
| GO:0004197 | MF | Cysteine-type endopeptidase activity | ||||
| GO:0016787 | MF | Hydrolase activity | ||||
| GO:0008233 | MF | Peptidase activity | ||||
| GO:0050790 | BP | Regulation of catalytic activity | ||||
| GO:0008234 | MF | Cysteine-type peptidase activity | ||||
| TR16870|c0_g2 | 9.5 | 4E-21 | ||||
| TR45052|c0_g1 | 9.5 | 4E-38 | ||||
| TR24355|c0_g1 | 9.4 | 5E-244 |
CC, cellular component; MF, molecular function; BP, biological process.
Table 2.
The 50 Most Sex-Biased Genes with Annotations. Female-Biased (log2FC >1) Genes in the Head and Thorax
| Gene | logFC | FDR | GO | Type | Term | BLASTHit |
|---|---|---|---|---|---|---|
| TR45052|c0_g1 | 9.8 | 7E-27 | ||||
| TR7171|c0_g2 | 9.7 | 1.E 96 | GO:0016491 | MF | Oxidoreductase activity | Cytochrome P450 412a2, Leptinotarsa decemlineata |
| GO:0005506 | MF | Iron ion binding | ||||
| GO:0004497 | MF | Monooxyge se activity | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| GO:0020037 | MF | Heme binding | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| TR37635|c0_g1 | 9.5 | 2E-21 | ||||
| TR23129|c0_g2 | 9.4 | 4E-27 | ||||
| TR38999|c0_g1 | 9.4 | 1E-67 | GO:0006468 | BP | Protein phosphorylation | Hypothetical protein YQE_12596, Dendroctonus ponderosae |
| GO:0005524 | MF | ATP binding | ||||
| GO:0004672 | MF | Protein kinase activity | ||||
| TR74056|c0_g1 | 9.1 | 7E-20 | ||||
| TR20045|c4_g3 | 8.9 | 9E-173 | ||||
| TR72359|c0_g1 | 8.9 | 4E-05 | ||||
| TR33787|c0_g4 | 8.9 | 2E-21 | GO:0016874 | MF | Ligase activity | Hypothetical protein TcasGA2_TC010120, Tribolium castaneum |
| GO:0008152 | BP | Metabolic process | ||||
| TR25835|c0_g2 | 8.8 | 1E-14 | ||||
| TR68289|c3_g2 | 8.7 | 1E-109 | ||||
| TR28694|c0_g1 | 8.7 | 7E-20 | ||||
| TR54960|c0_g3 | 8.7 | 4E-14 | GO:0003676 | MF | nucleic acid binding | Hypothetical protein TcasGA2_TC012080, T. castaneum |
| GO:0003723 | MF | RNA binding | ||||
| GO:0000166 | MF | Nucleotide binding | ||||
| TR58271|c0_g2 | 8.5 | 5E-11 | ||||
| TR35084|c0_g1 | 8.5 | 5E-18 | ||||
| TR29702|c2_g6 | 8.4 | 1E-61 | ||||
| TR18180|c0_g1 | 8.4 | 6E-56 | ||||
| TR44554|c0_g1 | 8.3 | 2E-76 | ||||
| TR18182|c4_g1 | 8.3 | 5E-54 | ||||
| TR12240|c0_g1 | 8.3 | 1E-53 | ||||
| TR18182|c9_g4 | 8.2 | 7E-49 | ||||
| TR44608|c4_g2 | 8.2 | 0.0003 | ||||
| TR28684|c0_g4 | 8.2 | 1E-12 | GO:0004861 | MF | Cyclin-dependent protein serine/threonine kinase inhibition | Protein HEXIM, T. castaneum |
| GO:0000122 | BP | Negative regulation of transcription from RNA polymerase II promoter | ||||
| GO:0017069 | MF | snRNA binding | ||||
| GO:0071901 | BP | Negative regulation of protein serine/threonine kinase | ||||
| GO:0005634 | CC | Nucleus | ||||
| GO:0005737 | CC | Cytoplasm | ||||
| TR57758|c0_g1 | 8.1 | 9E-14 | ||||
| TR4301|c0_g2 | 7.9 | 5E-12 | ||||
| TR66638|c0_g1 | 7.9 | 1E-48 | GO:0003676 | MF | Nucleic acid binding | Protein bicaudal C, T. castaneum |
| GO:0003723 | MF | RNA binding | ||||
| TR61957|c3_g2 | 7.8 | 0E+00 | ||||
| TR75409|c0_g1 | 7.8 | 2E-37 | GO:0046872 | MF | Metal ion binding | Uncharacterized protein LOC103314013, T. castaneum |
| TR62432|c0_g1 | 7.8 | 7E-22 | ||||
| TR69060|c0_g3 | 7.8 | 3E-14 | ||||
| TR59852|c0_g2 | 7.8 | 6E-61 | GO:0016567 | BP | Protein ubiquitination | Uncharacterized protein LOC663065 isoform X2, T. castaneum |
| GO:0005634 | CC | Nucleus | ||||
| GO:0016874 | MF | Ligase activity | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| GO:0008270 | MF | Zinc ion binding | ||||
| GO:0006511 | BP | Ubiquitin-dependent protein catabolic process | ||||
| GO:0007275 | BP | Multicellular organismal development | ||||
| GO:0004842 | MF | Ubiquitin-protein transferase activity | ||||
| TR44673|c0_g9 | 7.7 | 1E-16 | ||||
| TR44247|c0_g1 | 7.7 | 4E-50 | ||||
| TR55946|c0_g2 | 7.6 | 7E-75 | ||||
| TR45396|c0_g1 | 7.5 | 9E-24 | ||||
| TR1169|c3_g9 | 7.5 | 8E-23 | ||||
| TR54908|c0_g1 | 7.5 | 8E-107 | ||||
| TR49895|c0_g1 | 7.4 | 1E-11 | GO:0003743 | MF | Translation initiation factor activity | Eukaryotic translation initiation factor 2 subunit 1, T. castaneum |
| GO:0003723 | MF | RNA binding | ||||
| GO:0006413 | BP | Translation l initiation | ||||
| GO:0003676 | MF | Nucleic acid binding | ||||
| GO:0005850 | CC | Eukaryotic translation initiation factor 2 complex | ||||
| TR57251|c0_g1 | 7.4 | 7E-55 | ||||
| TR18182|c9_g3 | 7.4 | 9E-23 | ||||
| TR71884|c0_g1 | 7.2 | 5E-39 | GO:0005634 | CC | Nucleus | G2/mitotic-specific cyclin-A, T. castaneum |
| TR44662|c0_g1 | 7.1 | 4E-36 | GO:0020037 | MF | Heme binding | Hypothetical protein TcasGA2_TC000751, T. castaneum |
| GO:0004601 | MF | Peroxidase activity | ||||
| GO:0006979 | BP | Response to oxidative stress | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| TR36341|c0_g2 | 7.0 | 1E-16 | ||||
| TR63881|c0_g1 | 7.0 | 3E-20 | ||||
| TR29995|c3_g9 | 6.9 | 4E-16 | ||||
| TR64757|c0_g1 | 6.9 | 3E-05 | ||||
| TR8233|c0_g2 | 6.8 | 3E-33 | ||||
| TR1205|c0_g3 | 6.7 | 8E-16 | GO:0016021 | CC | Integral component of membrane | Hypothetical protein YQE_10313, D. ponderosae |
| GO:0022857 | MF | Transmembrane transporter activity | ||||
| GO:0006810 | BP | Transport | ||||
| GO:0055085 | BP | Transmembrane transport | ||||
| GO:0005215 | MF | Transporter activity | ||||
| GO:0022891 | MF | Substrate-specific transmembrane transporter activity | ||||
| GO:0016020 | CC | Membrane | ||||
| TR61942|c0_g1 | 6.7 | 0.0001 | ||||
| TR68765|c0_g1 | 6.6 | 4.E-160 | GO:0050660 | MF | Flavin adenine dinucleotide binding | Hypothetical protein TcasGA2_TC015713, T. castaneum |
| GO:0055114 | BP | Oxidation–reduction process |
CC, cellular component; MF, molecular function; BP, biological process.
Table 3.
The 50 Most Sex-Biased Genes with Annotations. Male-Biased (log2FC < −1) Genes in the Abdomen
| Gene ID | logFC | FDR | GO | Type | Term | BLAST hit |
|---|---|---|---|---|---|---|
| TR6663|c0_g1 | −16.5 | 0E+00 | ||||
| TR27296|c0_g1 | −14.8 | 0E+00 | ||||
| TR2855|c0_g1 | −14.4 | 0E+00 | ||||
| TR19004|c0_g5 | −14.4 | 0E+00 | ||||
| TR64284|c2_g6 | −14.3 | 0E+00 | GO:0016491 | MF | Oxidoreductase activity | Unknown, Dendroctonus ponderosae |
| GO:0055114 | BP | Oxidation–reduction process | ||||
| TR6663|c0_g5 | −14.3 | 0E+00 | ||||
| TR71857|c0_g2 | −13.9 | 0E+00 | GO:0005737 | CC | Cytoplasm | Unknown, Dendroctonus ponderosae |
| GO:0004177 | MF | Aminopeptidase activity | ||||
| GO:0030145 | MF | Manganese ion binding | ||||
| GO:0008235 | MF | Metalloexopeptidase activity | ||||
| GO:0019538 | BP | Protein metabolic process | ||||
| GO:0006508 | BP | Proteolysis | ||||
| TR27305|c0_g1 | −13.8 | 0E+00 | ||||
| TR47060|c0_g1 | −13.6 | 0E+00 | ||||
| TR34460|c0_g3 | −13.6 | 0E+00 | ||||
| TR64463|c0_g1 | −13.4 | 0E+00 | ||||
| TR51551|c0_g1 | −13.4 | 0E+00 | ||||
| TR17032|c0_g1 | −13.4 | 0E+00 | GO:0005509 | MF | Calcium ion binding | Unknown, Dendroctonus ponderosae |
| TR43223|c0_g1 | −13.3 | 0E+00 | ||||
| TR49381|c0_g1 | −13.3 | 1E-276 | ||||
| TR29424|c0_g1 | −13.2 | 7E-270 | GO:0005737 | CC | Cytoplasm | Unknown, Dendroctonus ponderosae |
| GO:0005622 | CC | Intracellular | ||||
| GO:0008235 | MF | Metalloexopeptidase activity | ||||
| GO:0004177 | MF | Aminopeptidase activity | ||||
| GO:0030145 | MF | Manganese ion binding | ||||
| GO:0019538 | BP | Protein metabolic process | ||||
| GO:0006508 | BP | Proteolysis | ||||
| TR7979|c0_g2 | −13.2 | 7E-251 | ||||
| TR55513|c0_g1 | −13.1 | 0E+00 | ||||
| TR29301|c0_g2 | −13.0 | 0E+00 | ||||
| TR73858|c0_g1 | −13.0 | 0E+00 | ||||
| TR44273|c0_g1 | −13.1 | 3E-150 | GO:0005524 | MF | ATP binding | Heat shock cognate 71 kDa protein-like isoform X1, Tribolium castaneum |
| GO:0000166 | MF | Nucleotide binding | ||||
| TR66585|c0_g1 | −13.0 | 4E-268 | GO:0005634 | CC | Nucleus | Hypothetical protein YQE_11918, partial, Dendroctonus ponderosae |
| GO:0006334 | BP | Nucleosome assembly | ||||
| TR17086|c0_g1 | −13.0 | 0E+00 | ||||
| TR70731|c0_g1 | −13.0 | 0E+00 | ||||
| TR16878|c0_g1 | −12.9 | 0E+00 | ||||
| TR27262|c0_g1 | −12.9 | 0E+00 | ||||
| TR22315|c0_g1 | −12.7 | 0E+00 | ||||
| TR28238|c1_g4 | −12.8 | 2E-282 | ||||
| TR30376|c0_g1 | −12.7 | 0E+00 | ||||
| TR38674|c0_g1 | −12.9 | 6E-234 | GO:0005506 | MF | Iron ion binding | Unknown, Aedes aegypti |
| GO:0016491 | MF | Oxidoreductase activity | ||||
| GO:0020037 | MF | Heme binding | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| GO:0016705 | MF | Oxidoreductase activity | ||||
| GO:0004497 | MF | Monooxygenase activity | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| TR12307|c0_g1 | −12.9 | 1E-282 | ||||
| TR41652|c0_g1 | −12.7 | 0E+00 | ||||
| TR18203|c0_g1 | −12.6 | 0E+00 | ||||
| TR39204|c1_g1 | −12.7 | 0E+00 | ||||
| TR74166|c0_g2 | −12.6 | 0E+00 | GO:0005737 | CC | Cytoplasm | Unknown, Dendroctonus ponderosae |
| GO:0016301 | MF | Kinase activity | ||||
| GO:0016740 | MF | Transferase activity | ||||
| GO:0016773 | MF | Phosphotransferase activity | ||||
| GO:0004335 | MF | Galactokinase activity | ||||
| GO:0005524 | MF | ATP binding | ||||
| GO:0000166 | MF | Nucleotide binding | ||||
| GO:0008152 | BP | Metabolic process | ||||
| GO:0016310 | BP | Phosphorylation | ||||
| GO:0006012 | BP | Galactose metabolic process | ||||
| GO:0046835 | BP | |||||
| TR49828|c0_g2 | −12.6 | 0E+00 | GO:0016491 | MF | Oxidoreductase activity | Hypothetical protein TcasGA2_TC010329, Tribolium castaneum] |
| GO:0008152 | BP | Metabolic process | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| TR59474|c0_g1 | −12.7 | 6E-259 | ||||
| TR3816|c0_g1 | −12.7 | 6E-308 | GO:0005874 | CC | Microtubule | Hypothetical protein D910_07655, Dendroctonus ponderosae |
| GO:0005871 | CC | Kinesin complex | ||||
| GO:0000166 | MF | Nucleotide binding | ||||
| GO:0005524 | MF | ATP binding | ||||
| GO:0008017 | MF | Microtubule binding | ||||
| GO:0003777 | MF | Microtubule motor activity | ||||
| GO:0008152 | BP | Metabolic process | ||||
| GO:0007018 | BP | |||||
| TR14537|c0_g1 | −12.5 | 0E+00 | ||||
| TR69544|c0_g1 | −12.6 | 6E-261 | GO:0008270 | MF | Zinc ion binding | Hypothetical protein YQE_01746, partial, Dendroctonus ponderosae |
| GO:0004181 | MF | |||||
| GO:0006508 | BP | Proteolysis | ||||
| TR22773|c0_g1 | −12.6 | 2E-239 | ||||
| TR36788|c0_g2 | −12.6 | 0E+00 | ||||
| TR810|c0_g2 | −12.6 | 4E-207 | ||||
| TR57874|c0_g3 | −12.5 | 0E+00 | GO:0004252 | MF | Serine-type endopeptidase activity | Hypothetical protein D910_08079, Dendroctonus ponderosae |
| GO:0006508 | BP | Proteolysis | ||||
| TR47457|c0_g1 | −12.5 | 7E-264 | ||||
| TR15902|c0_g1 | −12.4 | 0E+00 | ||||
| TR15343|c0_g2 | −12.5 | 3E-312 | ||||
| TR31224|c1_g1 | −12.4 | 0.0E+00 | GO:0004252 | MF | Serine-type endopeptidase activity | Hypothetical protein D910_03668, Dendroctonus ponderosae |
| GO:0006508 | BP | Proteolysis | ||||
| TR24113|c2_g5 | −12.5 | 0E+00 | ||||
| TR38284|c0_g1 | −12.4 | 2E-228 | GO:0008177 | MF | Succinate dehydrogenase activity | Succinate dehydrogenase flavoprotein subunit, mitochondrial like, Tribolium castaneum |
| GO:0006099 | BP | Tricarboxylica acid cycle | ||||
| GO:0016020 | CC | Membrane | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| GO:0016491 | MF | Oxidoreductase activity | ||||
| GO:0022900 | BP | Electron transport chain | ||||
| GO:0050660 | MF | Flavin adenine dinucleotide binding | ||||
| GO:0005743 | CC | Mitochondrial inner membrane |
CC, cellular component; MF, molecular function; BP, biological process.
Table 4.
The 50 Most Sex-Biased Genes with Annotations. Male-Biased (log2FC < −1) Genes in the Head and Thorax
| Gene ID | logFC | FDR | GO | Type | Term | BLAST hit |
|---|---|---|---|---|---|---|
| TR45430|c1_g5 | −9.7 | 3E-08 | ||||
| TR43196|c0_g1 | −8.6 | 3E-23 | GO:0055085 | BP | Transmembrane transport | Putative inorganic phosphate cotransporter, Tribolium castaneum |
| GO:0016021 | CC | Integral component of membrane | ||||
| TR45058|c0_g1 | −8.4 | 3E-09 | ||||
| TR60993|c0_g2 | −8.2 | 5E-12 | ||||
| TR12288|c0_g1 | −8.1 | 3E-14 | ||||
| TR45430|c1_g4 | −7.7 | 4E-11 | ||||
| TR68274|c1_g3 | −6.0 | 1E-44 | ||||
| TR7204|c0_g1 | −6.0 | 2E-10 | ||||
| TR7035|c2_g4 | −5.7 | 0.0003 | GO:0005576 | CC | Extracellular region | Attacin-like immune protein, Diabrotica virgifera virgifera |
| TR56173|c0_g1 | −5.5 | 1E-11 | ||||
| TR48097|c0_g1 | −5.5 | 0.0005 | ||||
| TR14545|c0_g1 | −5.3 | 3E-23 | GO:0008234 | MF | Cysteine-type peptidase activity | Putative gut cathepsin L-like cysteine protease, Callosobruchus maculates |
| GO:0016787 | MF | Hydrolase activity | ||||
| GO:0008233 | MF | Peptidase activity | ||||
| GO:0006508 | BP | Proteolysis | ||||
| TR57561|c0_g2 | −5.1 | 1E-19 | GO:0008152 | BP | Metabolic process | Esterase, Leptinotarsa decemlineata |
| GO:0016787 | MF | Hydrolase activity | ||||
| TR34187|c0_g1 | −4.9 | 1E-19 | GO:0006030 | BP | Chitin metabolic process | Peritrophic matrix protein 2-C precursor, T. castaneum |
| GO:0008061 | MF | Chitin binding | ||||
| GO:0005576 | CC | Extracellular region | ||||
| TR52512|c0_g4 | −4.9 | 2E-07 | ||||
| TR68287|c1_g1 | −4.8 | 4E-21 | ||||
| TR33586|c1_g1 | −4.8 | 6E-10 | ||||
| TR29249|c0_g1 | −4.8 | 8E-51 | ||||
| TR7634|c0_g1 | −4.7 | 5E-23 | GO:0016787 | MF | Hydrolase activity | Esterase, L. decemlineata |
| GO:0008152 | BP | Metabolic process | ||||
| TR50077|c0_g2 | −4.6 | 3E-17 | GO:0005549 | MF | Odorant binding | Odorant receptor 124, partial, T. castaneum |
| GO:0016020 | CC | Membrane | ||||
| GO:0007608 | BP | Sensory perception of smell | ||||
| GO:0004984 | MF | Olfactory receptor activity | ||||
| GO:0050911 | BP | Detection of chemical stimulus involved in sensory perception of smell | ||||
| TR48099|c0_g1 | −4.5 | 3E-10 | GO:0005975 | BP | Carbohydrate metabolic process | Hypothetical protein YQE_09695, partial, D. ponderosae |
| GO:0016020 | CC | Membrane | ||||
| GO:0016798 | MF | Hydrolase activity, acting on glycosyl bonds | ||||
| TR28685|c0_g2 | −4.4 | 0.0003 | GO:0016740 | MF | Transferase activity | Glutathione S-transferase D2, T. castaneum |
| GO:0008152 | BP | Metabolic process | ||||
| GO:0004364 | MF | Glutathione transferase activity | ||||
| TR56870|c0_g5 | −4.4 | 0.0197 | ||||
| TR53421|c0_g1 | −4.2 | 9E-22 | GO:0003993 | MF | Acid phosphatase activity | Venom acid phosphatase Acph-1-like, T. castaneum |
| GO:0006470 | BP | Protein dephosphorylation | ||||
| TR32496|c0_g1 | −4.2 | 0.0032 | ||||
| TR10366|c0_g1 | −4.2 | 1E-24 | ||||
| TR7204|c0_g2 | −4.2 | 1E-09 | ||||
| TR72160|c0_g1 | −4.1 | 7E-25 | GO:0004497 | MF | Monooxyge se activity | Cytochrome P450 412a2, L. decemlineata |
| GO:0005506 | MF | Iron ion binding | ||||
| GO:0055114 | BP | Oxidation–reduction process | ||||
| GO:0020037 | MF | Heme binding | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| GO:0016491 | MF | Oxidoreductase activity | ||||
| GO:0016705 | MF | Oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen | ||||
| TR33543|c0_g2 | −4.0 | 0.00043 | GO:0004222 | MF | Metalloendopeptidase activity | Zinc metalloproteinase s-13, T. castaneum |
| GO:0008233 | MF | Peptidase activity | ||||
| GO:0008237 | MF | Metallopeptidase activity | ||||
| GO:0016787 | MF | Hydrolase activity | ||||
| GO:0008270 | MF | Zinc ion binding | ||||
| GO:0006508 | BP | Proteolysis | ||||
| GO:0046872 | MF | Metal ion binding | ||||
| TR29759|c1_g1 | −4.0 | 2E-14 | ||||
| TR17569|c0_g1 | −4.0 | 1E-09 | ||||
| TR64329|c5_g1 | −4.0 | 3E-30 | ||||
| TR57575|c0_g1 | −4.0 | 0.0120 | GO:0005856 | CC | Cytoskeleton | Hpothetical protein YQE_08828, partial, D. ponderosae |
| GO:0005525 | MF | GTP binding | ||||
| GO:0051258 | BP | Protein polymerization | ||||
| GO:0000166 | MF | Nucleotide binding | ||||
| GO:0043234 | CC | Protein complex | ||||
| GO:0005737 | CC | Cytoplasm | ||||
| GO:0008152 | BP | Metabolic process | ||||
| GO:0003924 | MF | GTPase activity | ||||
| GO:0005200 | MF | Structural constituent of cytoskeleton | ||||
| GO:0007017 | BP | Microtubule-based process | ||||
| GO:0005874 | CC | Microtubule | ||||
| TR37369|c3_g7 | −3.9 | 1E-49 | GO:0055114 | BP | Oxidation–reduction process | Acyl-CoA Delta(11) desaturase-like, T. castaneum |
| GO:0006629 | BP | Lipid metabolic process | ||||
| GO:0006633 | BP | Fatty acid biosynthetic process | ||||
| GO:0016491 | MF | Oxidoreductase activity | ||||
| GO:0016021 | CC | Integral component of membrane | ||||
| TR7035|c2_g2 | −3.9 | 8E-05 | GO:0005576 | CC | Extracellular region | Attacin-like immune protein, D. virgifera virgifera |
| TR1055|c0_g1 | −3.9 | 0.00906 | GO:0030145 | MF | Manganese ion binding | Hypothetical protein D910_02561, D. ponderosae |
| GO:0005737 | CC | Cytoplasm | ||||
| GO:0008235 | MF | Metalloexopeptidase activity | ||||
| GO:0004177 | MF | Aminopeptidase activity | ||||
| GO:0004672 | MF | Protein kinase activity | ||||
| GO:0006508 | BP | Proteolysis | ||||
| GO:0019538 | BP | Protein metabolic process | ||||
| GO:0005622 | CC | Intracellular | ||||
| GO:0006468 | BP | Protein phosphorylation | ||||
| TR56982|c4_g2 | −3.8 | 2E-20 | GO:0016787 | MF | Hydrolase activity | Putative glycosyl hydrolase, Chrysomela lapponica |
| GO:0008152 | BP | Metabolic process | ||||
| GO:0005975 | BP | Carbohydrate metabolic process | ||||
| TR34693|c1_g1 | −3.8 | 8E-19 | GO:0016787 | MF | Hydrolase activity | Glycoside hydrolase family protein 28, C. maculatus |
| GO:0008152 | BP | Metabolic process | ||||
| GO:0071555 | BP | Cell wall organization | ||||
| GO:0005975 | BP | Carbohydrate metabolic process | ||||
| GO:0004650 | MF | Polygalacturonase activity | ||||
| GO:0005576 | CC | Extracellular region | ||||
| TR52603|c0_g2 | –3.7 | 0.02513 | ||||
| TR33725|c0_g1 | −3.7 | 3E-11 | ||||
| TR28685|c0_g1 | −3.7 | 2E-50 | GO:0004364 | MF | Glutathione transferase activity | Glutathione S-transferase D2, T. castaneum |
| GO:0016740 | MF | Transferase activity | ||||
| GO:0008152 | BP | Metabolic process | ||||
| TR37895|c0_g1 | −3.7 | 8E-19 | ||||
| TR57761|c0_g1 | −3.6 | 3E-23 | ||||
| TR10418|c0_g2 | −3.5 | 3E-43 | ||||
| TR19023|c0_g2 | −3.4 | 1E-27 | GO:0003993 | MF | Acid phosphatase activity | Hypothetical protein YQE_11758, partial, D. ponderosae |
| TR19023|c0_g2 | −3.4 | 1E-27 | GO:0006470 | BP | Protein dephosphorylation | |
| TR12918|c3_g8 | −3.4 | 4E-07 | ||||
| TR10887|c0_g1 | −3.4 | 7E-25 | ||||
| TR15912|c4_g11 | −3.4 | 7E-06 | ||||
| TR38223|c0_g1 | −3.3 | 2E-11 | GO:0005549 | MF | Odorant binding | Pheromone-binding protein-related protein 3, Zootermopsis nevadensis |
CC, cellular component; MF, molecular function; BP, biological process.
Female-biased genes of the abdomen were enriched with biological processes related to metabolism, cellular response to stress, response to hormones and regulation of transcription, for example, supplementary table S1a, Supplementary Material online. Similar terms were also enriched among the female-biased genes of the head and thorax (supplementary table S1c, Supplementary Material online). Male-biased genes of the abdomen showed overrepresentation of biological processes related to oxidation–reduction, carbohydrate metabolism and G-protein coupled receptor signaling pathway, for example, supplementary table S2a, Supplementary Material online. Male-biased genes in the head and thorax were enriched, for example, with genes involved in visual perception, detection of chemical stimulus and neurotransmitter transport (supplementary table S2c, Supplementary Material online). We note that the GO analyses are a useful exploratory tool, but should be interpreted as guidance rather than as conclusive evidence of functional differences (Pavlidis et al. 2012). The lack of closely related and well-annotated genomes can mean that some of the potentially most interesting genes under sex-specific selection may lack annotation due to faster divergence. In addition to the Gene Ontology annotations, we also explored whether the recently identified Sfp encoding genes of C. maculatus (Bayram et al. 2016) were overrepresented among the male-biased genes of the abdomen. These 98 genes were more common than expected among the male-biased genes when considering all the genes with the Padj < 0.05 (1.0% vs. 0.78%; X2 = 111, P = 0.0078, supplementary fig. S3, Supplementary Material online), but they were not significantly overrepresented among those with >2-fold sex difference. Although 15 of these 98 genes were indeed strongly male-biased, most were only moderately male-biased in expression and some even showed female-bias (supplementary fig. S3, Supplementary Material online).
In addition to sex differences in the amount of expression, males and females can also differ in the patterns of gene splicing. In order to test for such putative differences in alternative splicing, we examined changes in the isoform-specific expression differences between males and females. We considered transcripts that show at least a 2-fold expression difference as sex-biased, with 5% FDR. In the abdomen, 20,683 transcripts were retained in the analysis after pre-filtering (transcripts with > 2 cpm in at least three libraries were retained), corresponding to 11,839 genes. The majority of the genes had only a single expressed transcript in our dataset (7,258 genes) and 54% of these were sex-biased (3,903 genes). Among the genes with multiple expressed transcripts (4,581), the average number of isoforms per gene was 2 with a maximum of 17. 68% of these genes (3,128) had at least one sex-biased isoform. This is a significantly greater proportion than for the single-transcript genes (X2 = 245.05, df = 1, P value < 2.2e-16). We also compared the numbers of sex-biased transcripts from multi- versus single-transcript genes: 61% (6,080) of the sex-biased transcripts were from the former, while 3,903 transcripts (39%) were from the single-transcript genes (X2 = 949.48, df = 1, P value < 2.2e-16). These patterns are consistent with the notion that the evolution of sex-biased expression is less constrained in multi-exon compared with single-exon genes. This interesting observation should, however, be verified with a well-annotated genome. We found that 28% of all the multi-transcript genes (1,371, 12% of the whole transcriptome) showed significant sex differences in the isoform-specific expression in the abdomen, in line with alternative splicing. However, the proportion of these genes was lower compared with the multi-transcript genes that showed a uniform pattern of sex-bias across the isoforms (1,837 genes, 40%; X2 = 144, df = 1, P value < 2.2e-16). The putative alternatively spliced genes of the abdomen were enriched with biological processes related to metabolic processes and oxidation–reduction (supplementary table S3a, Supplementary Material online). Among the Sfp genes of C. maculatus, 27 out of 98 showed signs of alternative splicing. For 14 genes, the transcripts showed a mixture of male-biased and un-biased expression (supplementary table S4a, Supplementary Material online), two showed different degrees of male-bias (supplementary table S4a, Supplementary Material online), two a mixture of male-, female- and un-biased expression (supplementary table S4b, Supplementary Material online), two a mixture of male- and female-bias (supplementary table S4b, Supplementary Material online) while the remaining 8 showed a mixture of female- and un-biased expression (supplementary table S4c, Supplementary Material online).
In the head and thorax, 18,278 expressed transcripts (from 10,551 genes) were retained after filtering (transcripts with > 2 cpm in at least three libraries were retained), of which the majority had only one expressed transcript per gene (6,586). Overall, 3,965 genes had more than one expressed transcript (on an average there were 2 expressed transcripts per gene, with a maximum of 16). Similar to the abdomen, multi-transcript genes of the head and thorax showed sex-biased expression (in at least one of the transcripts) more often than the single-transcript genes (16% vs. 11%, respectively: X2= 61.914, df = 1, P value = 3.588e-15). A total of 316 (6.6% of the multi-transcript genes and 3% of the whole transcriptome) genes showed sex differences in isoform-specific expression in the head and thorax. Similar to the abdomen, this is a lower proportion than of those multi-transcript genes that showed a uniform sex-bias across the isoforms (390 genes, 10%: X2= 27.8, df = 1, P value = 1.312e-07). The alternatively spliced genes in the head and thorax were enriched with biological processes related to carbohydrate metabolism (supplementary table S3c, Supplementary Material online). The alternatively spliced genes were largely distinct in each tissue class (supplementary fig. S4, Supplementary Material online).
Plasticity in Gene Expression Due to Mating
Our main goal was to study whether and how the sex-biased genes identified in virgins respond to mating in each sex. We did this by first testing for significant two-way interactions between mating treatment and sex: 1,305 genes showed a significant interaction in the abdomen and 309 in the head and thorax. Of these, 1,041 genes (15.7% of the virgin sex-biased genes) in the abdomen and 243 (17.4%) in the head and thorax also showed a significant main effect of mating in at least one of the sexes and were thus considered further as genes showing plasticity in the degree of sexual dimorphism due to mating. The abdomen and the head and thorax showed largely different responses to mating: only 59 genes showed a similar interaction effect in both tissue groups (fig. 1b), despite the large shared proportion of the sex-biased genes between them (fig. 1a). Females and males both contributed to the sex-bias plasticity but largely of different genes (fig. 1c and d).
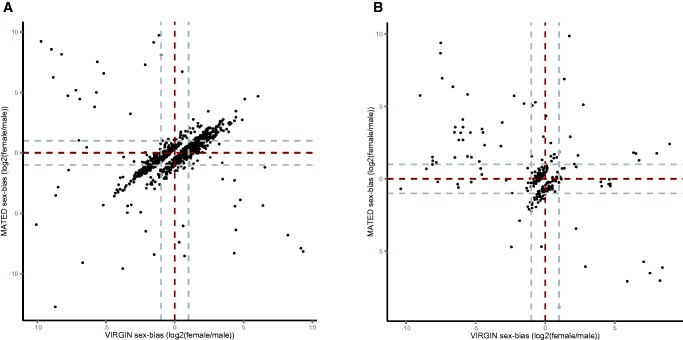
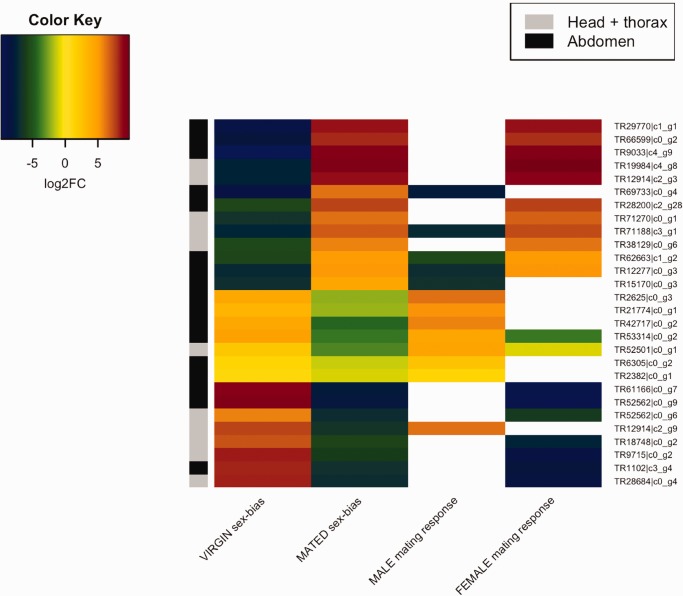
Next, we estimated how many of the identified “plastic” sex-biased genes changed status from the virgin state. The majority of the genes showed subtle changes in the degree of sex-bias, but in 2% of the genes in the abdomen and 4% in the head and thorax the sex-bias status were altered (fig. 2a and b;table 5). Most of such cases changed from showing sex-biased expression to showing no bias (79 genes in the abdomen and 48 genes in the head and thorax, table 5). However we also identified 9 and 6 genes that changed from female- to male-biased expression, while 8 and 5 genes changed from male- to female-biased expression in the abdomen and head + thorax, respectively (i.e., showed significant sex-bias by 2-fold as both virgin and mated; table 5 and fig. 3). Only a minority of these genes (5 out of 28) switched sex-bias due a simultaneous but opposite expression change in both sexes. In 6 genes, the switch to male-bias occurred due to upregulation by mated males whereas in 7 genes there was a strong downregulation in the mated females without any change in the male expression. Switches to female-bias occurred mainly due to increased expression only in females (6 genes) but 2 genes switched to female-bias by strong downregulation only in males (fig. 3). See supplementary table S5, Supplementary Material online, for the data and annotations for these genes that switch sex-bias.
Fig. 2.—
Sex-biased expression in virgin and mated beetles for the plastic genes in (A) the abdomen (1,041 genes) and (B) the head and thorax (243 genes). Grey dashed lines indicate a 2-fold difference in expression between the sexes (i.e., logFC = 1 or − 1. Positive values indicate female- and negative male-biased expression). Off diagonal segments show the genes that have changed sex-bias status due to mating (upper left segment: from male- to female-bias, lower right segment: from female- to male-bias).
Table 5.
Numbers of Genes Showing a Significant Switch in Sex-Bias Status Due To Mating
| Tissue | F-b to M-b | F-b to Nb | Nb to F-b | M-b to F-b | M-b to Nb | Nb to M-b |
|---|---|---|---|---|---|---|
| Abdomen | 9 | 49 | 10 | 8 | 30 | 19 |
| H+T | 6 | 13 | 11 | 5 | 35 | 10 |
Note.—The columns denote changes in sex-bias from virgin to mated state.
F-b, female-bias; M-b, male-bias; Nb, no sex-bias.
Fig. 3.—
Expression response to mating in males and females for the genes that switch sex-biased expression in the two tissue groups (i.e., sex-biased ≥ 2-fold in both virgin and mated beetles, with FDR 5%). Two left-most columns show the degree of sex-bias in the virgin and mated beetles (positive log2FC indicates female-biased expression) and the two right-most columns the degree of mating response in males and females for the same genes (positive log2FC indicates higher expression in the mated beetles). Absence of a record (white) indicates no significant difference between mated and virgin individuals.
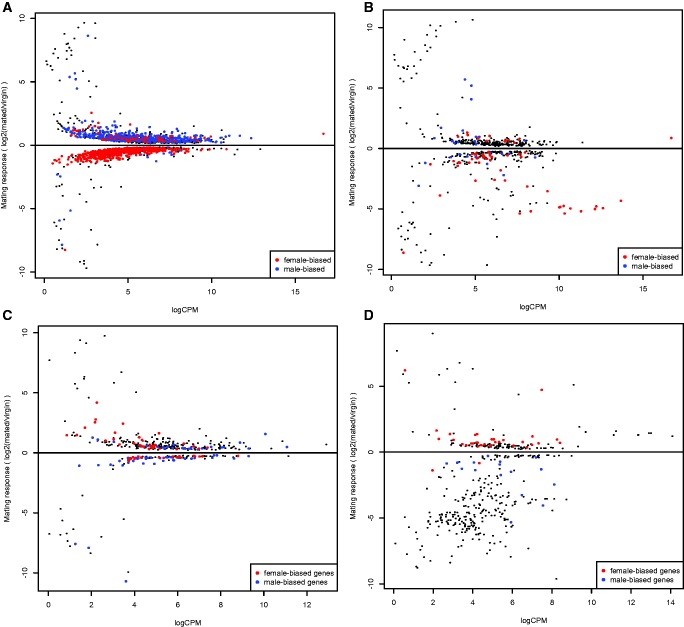
To further test how each sex responds to mating and how this might alter sexually dimorphic expression, we focused on examining the transcriptomic changes of sex-biased genes (as identified in virgins) separately within each sex in each tissue class. Nearly 6 times more genes responded to mating in the female abdomen compared with males: 2,286 genes significantly changed expression in the females and 382 genes in the males (Padj < 0.05). Female mating response was enriched with virgin sex-biased genes: 59% (1,351) were sex-biased, a significantly greater proportion than in the whole abdomen transcriptome (X2df= 24.11, P < 0.0001). Female-biased genes were more common than expected relative to their frequency among the virgin sex-biased genes (771 genes, 57% of the sex-biased mating responsive genes: X2df= 61.41, P < 0.0001). However, opposite to our prediction, a great majority of these female-biased genes were downregulated in mated relative to virgin females (707 genes, 92%: X2df= 452.21, P < 0.0001), whereas male-biased genes responded mainly by upregulation (541 genes, 93%: X2df= 509.11, P < 0.0001) (fig. 4a), resulting in de-feminization of the female abdomen relative to the virgin state. The genes that increased expression due to mating were enriched for example with biological processes involved in oxidation–reduction, carbohydrate metabolism, lipid metabolism and proteolysis (supplementary table S6a, Supplementary Material online), while the downregulated genes showed for example overrepresentation of protein modification process, regulation of signal transduction and DNA repair (supplementary table S6b, Supplementary Material online).
Fig. 4.—
Mating response in the female (A and B) and male (C and D) abdomen and the head + thorax, respectively, as a function of average expression (logCPM). Sex-biased genes in virgins (Padj < 0.05 and at least 2-fold sex-bias) are highlighted. Positive values indicate a higher expression in the mated state relative to virgin.
In contrast to female abdomen, in the male abdomen the virgin sex-biased genes were significantly underrepresented among the mating responsive genes (118 genes, 31%: X2df = 76.3, P < 0.0001; fig. 4b). Male-biased genes were also not more common than expected (68 genes, 57%: X2df = 0.785, P = 0.3756). However, those male-biased genes that did respond were more often downregulated than expected (41 genes, 60%: X2df= 25.71, P < 0.0001), which is again opposite to our prediction. Female-biased genes were, however, not more commonly upregulated than expected in mated males’ abdomen (31 genes, 62%: X2df= 0.921, P = 0.33). Functional enrichment analysis showed that males also increased expression of oxidation–reduction genes in the abdomen (supplementary table S7a, Supplementary Material online), for example. We note that these were different redox genes than those that responded in females (results not shown).
In the head and thorax, 558 genes responded to mating in females and 421 in males (Padj < 0.05). Sex-biased genes were more common than expected among the female mating responsive genes (87 genes, 16%: X2df = 4.41, P = 0.0350). Although female-biased genes were not overrepresented relative to their expected proportion (61 genes, 70%: X2df = 0.99 1, P = 0.3192), they were much more commonly downregulated due to mating (48 genes, 79%: X2df= 25.0 1, P < 0.0001). This is a similar pattern to the abdomen female-biased genes. Male-biased genes did not show a deviant pattern from expected (14 genes, 54%: X2df= 0.0041, P = 0.9495) (fig. 4c). Females’ upregulated, for example, signal transduction genes and, in contrast to the abdomen, downregulated metabolic and oxidation–reduction genes in the head and thorax (supplementary table S7c and d, Supplementary Material online).
Similar to the male abdomen, sex-biased genes were not particularly common among the mating responsive genes in the male head and thorax (50 genes, 12%: X2df= 0.17 1, P = 0.6837), and male-biased genes were also not overrepresented (16 genes, 32%: X2df = 0.089 1, P = 0.7653). However, the male-biased genes that did change expression responded exclusively by downregulation (16 genes, 100% X2df= 8.5 1, P = 0.00356) and a great majority of female-biased genes by upregulation (32 genes, 94%: X2df= 53.0 1, P < 0.0001) (fig. 4d), suggesting a de-masculinization of the male somatic tissues due to mating. See supplementary table S7b and c, Supplementary Material online, for the enriched Gene Ontology terms.
The full result outputs for the sex-biased genes of virgins, genes that show plasticity in sex-bias, as well as the mating responsive genes of females and males are presented in supplementary table S8, Supplementary Material online.
Discussion
Characterizing sex-specific patterns of plasticity in adult gene expression represents a gap in our understanding the functional operation and evolutionary dynamics of sex-biased genes. Here, we addressed this by investigating how stable sex-bias is across different reproductive states and how sex-biased genes are regulated within each sex. We examined sex-biased expression in reproductive and somatic tissues of C. maculatus in order to investigate how genes with sex-biased expression respond to changes in reproductive status. Mating clearly induces major sex-specific behavioral and physiological changes. We predicted that females would primarily increase the expression of female- and males of male-biased genes, under the premise that the degree of relative expression between the sexes reflects the functional importance of the focal gene product for that sex. This is generally assumed in molecular evolutionary studies which predict that male-biased genes evolve under male-specific and female-biased genes under female-specific selection (Ellegren and Parsch 2007). Our findings yield a number of novel insights. We show that mating affects the expression of hundreds of genes in a largely sex-specific manner (fig. 1c and d). This results in a change in the magnitude of sexually dimorphic expression in both reproductive and somatic tissues (fig. 2). However, the status of sex-bias does not change due to mating for the majority of the virgin sex-biased genes. Both sexes respond to mating by changing the expression of both male- and female-biased genes but do so in largely opposite directions (fig. 4). Contrary to our prediction, mating decreases dimorphic expression of sex-biased genes rather than increases it. Below, we will present the characteristics of sexually dimorphic gene expression in C. maculatus and discuss in detail how each sex responds mating.
Sexually Dimorphic Transcriptome of C. maculatus
Advances in high transcriptome profiling have afforded new insight into the widespread occurrence on sex-biased gene expression in diverse species including brown alga (Martins et al. 2013), nematodes (Albritton et al. 2014), Drosophila (Parisi et al. 2004; Zhang et al. 2004, 2007; McIntyre et al. 2006; Baker et al. 2007; Assis et al. 2012; Allen et al. 2013), mosquitoes (Hahn and Lanzaro 2005; Baker et al. 2011), birds (Mank et al. 2007), zebrafish (Small et al. 2009), and mice (Yang et al. 2006a). However, despite the fact that beetles are uniquely diverse, and include many pest species, genomic tools in this large insect order are still poor. Characterization of sexual dimorphism in the transcriptome has thus far only been conducted in the red flour beetle Tribolium castaneoum (Prince et al. 2010) and the horned beetle Onthophagus taurus (Kijimoto et al. 2014). In line with previous studies, we found extensive sex differences in gene expression: in the abdomen, 54% of the expressed genes were sex-biased and in the head and thorax 13% showed sex-biased expression (supplementary fig. S1, Supplementary Material online). Extremely male-biased genes (by at least 10-fold) were more common in the abdomen than extremely female-biased genes. This is in accordance with many other studies that find more genes with stronger male-biased expression, particularly in the reproductive tissues (reviewed in Ellegren and Parsch 2007; Zhang et al. 2007; Small et al. 2009; Martins et al. 2013). In contrast, we find that in the somatic tissues of C. maculatus female-biased genes were nearly twice as numerous as male-biased genes (supplementary figs. S1b and S2b, Supplementary Material online). This could reflect the generally more pleiotropic nature of female-biased genes, which in Drosophila show a wider expression breadth than male-biased genes (Assis et al. 2012; Meisel et al. 2012). In accordance, we found that the overlap between the female-biased genes of the abdomen and the head and thorax was far greater than for the male-biased genes (supplementary fig. S2, Supplementary Material online), suggesting that female-biased genes are less specific to sex-limited tissues also in C. maculatus.
Functional analysis revealed sex-specific variation in metabolism of carbohydrates and nitrogen compounds, and in oxidation–reduction processes. One of the most female-biased genes include the gene Cathepsin B-like cysteine protease (CmCatB) of C. maculatus (logFC = 10.4) (table 1). This gene is expressed in the larval gut where it is involved in the hydrolysis of proteins and counter-defence of plant dietary toxins (Koo et al. 2008). It is nearly 2,000 times higher expression in the abdomen of both virgin and mated females suggests that females have specific dietary requirements compared with males. We studied the transcriptome of C. maculatus under aphagous conditions, and therefore CmCatB is not involved in the digestion of food but rather in the decomposition of stored macromolecules, perhaps in the fat body where it is expressed in the moth Helicoverpa armigera (Yang et al. 2006b). Given the importance of diet and metabolism in sex-specific life-history evolution, the metabolic genes with dimorphic expression make interesting, yet under-appreciated, candidates under sex-specific selection for future studies.
Previous studies using proteomic analyses identified 98 seminal fluid proteins in C. maculatus (Goenaga et al. 2015; Bayram et al. 2016). The genes encoding these Sfps were overrepresented among the subtly male-biased genes in the abdomen. Although some of Sfp encoding genes were extremely male-biased (>10 logFC), several were also female-biased (supplementary fig. S3, Supplementary Material online). In line with our finding, many Sfp gene transcripts are expressed in the reproductive tracts of both males and females in a tiger mosquito Aedes albopictus (Boes et al. 2014). This shows that despite their important reproductive function in males, Sfp’s also play important roles in females. Indeed, it is possible that the manner by which some of these seminal fluid proteins act in mated females is by providing an extra dose of proteins that are produced endogenously within females (Arnqvist and Rowe 2005).
Male-biased genes in the head and thorax were enriched with genes involved in visual and chemical perception and neurotransmitter transport. These processes likely reflect the importance of detection of these sensory stimuli for males as part of mate searching, courtship and pre-mating competition. For example, two odorant binding genes were among the 50 most male-biased genes and showed over 25- and 10-fold higher expression in the males (odorant receptor 124, pheromone-binding protein-related protein 3, respectively, table 4). We note that the virgin males and females used in this study were naïve and held in isolation, and therefore these results stem from innate sex differences rather than direct responses to social interactions.
In addition to whole genes that are sex-biased in expression, an additional level of sexual dimorphism is found at loci that produce multiple transcripts through alternative pre-mRNA splicing or the use of alternative transcription initiation and termination sites (McIntyre et al. 2006). Sex-specific alternative splicing is a major player in sex determination including both cellular differentiation of somatic tissues (Nagoshi et al. 1988; Penalva and Sanchez 2003) and the gonads (Gan et al. 2010). In addition, pervasive sexual dimorphism in the patterns of alternative splicing in sexually mature adults is also common in Drosophila (McIntyre et al. 2006; Telonis-Scott et al. 2009), but has overall been less well explored. Here, we examined sex-biased expression of 4,581 genes with multiple expressed transcripts in the reproductive tissues. In total, 28% of these genes showed evidence for sex-specific alternative splicing, which makes up 12% of the whole expressed transcriptome. Even in the somatic tissues, 7% of the multi-transcript genes were alternatively spliced between the sexes. These results support the growing evidence of the prevalence of sex-specific alternative splicing in adults (McIntyre et al. 2006; Telonis-Scott et al. 2009; Hartmann et al. 2011). Our data also suggests that sex-biased splicing is much more common in the reproductive and sex-specific tissues of the abdomen (supplementary fig. S4, Supplementary Material online), in line with our gene-level analysis and the patterns seen in Drosophila (Telonis-Scott et al. 2009). Metabolic processes were overrepresented among the alternatively spliced genes in both tissue types.
We also specifically examined the exon-specific expression patterns among the 98 Sfp genes of C. maculatus and found that 27% showed evidence of sex-biased splicing, suggesting that alternative splicing may be a rather common way of regulating distinct phenotypes in each sex. Most of these genes exhibited a combined expression pattern of male-biased and un-biased transcripts (supplementary table S4a, Supplementary Material online). A similar mixture of male- and un-biased expression has previously been reported for a gene bmarg encoding for an arginase most likely secreted into the seminal fluid of Bombyx mori silkworm, where bmarg-r is expressed only in the male reproductive organs, while bmarg-f is expressed uniformly in the fat body and muscle of both sexes (Nagaoka et al. 2011). Alternative splicing of a gene encoding for a prostate enzyme transglutaminase 4, which is secreted into semen, has also been observed in humans (Cho et al. 2010). More detailed analyses of the candidate sex-specifically spliced Sfp genes identified here will be necessary to dissect the regulatory patterns and function of the isoforms in each sex.
Mating Induces Subtle but Numerous Changes in Sex-Biased Expression
Perhaps unsurprisingly, mating affects the expression of more genes in females than in males, and therefore the absolute contribution of females to the plasticity in virgin sex-biased expression is greater than of males (fig. 1c and d). However, this picture is more moderated when reproductive and somatic tissues are considered separately. Male mating response in the abdomen contributes proportionally more to the plasticity in virgin sex-bias than female response: 54% of the male response genes show a change in sex-bias due to mating in contrast to 38% of the female response genes (fig. 1c). The opposite is true for the head and thorax, where only 19% of the male response genes and 32% of the female response genes show plasticity in sex-bias (fig. 1d). The great majority of the sex-bias plasticity is subtle and does not change the direction of the virgin-state bias. Sex-bias is typically characterized in reproductively naïve adults for categorizing genes for molecular evolutionary analyses (Jiang and Machado 2009). This practice assumes that the sex-bias status of a gene in virgins is representative of its pattern of expression throughout the adult lifespan during which sex-specific selection may operate. A study of sex-biased expression throughout ontogeny has recently demonstrated that many genes show different evolutionary patterns depending on whether their sex-biased expression is conserved or stage-specific (Perry et al. 2014), suggesting the importance of considering the patterns of plasticity also in adults. Our results provide evidence that the sex-bias status is rather stable in the face of altered mating status and therefore offer general assurance for the way sex-bias is typically characterized in adults.
Studies of sex-biased expression typically focus on the molecular evolutionary patterns, whereas analyzing the functional roles of sex-biased genes in males and females has received less attention. Here, we attempted to bridge some of this gap by asking how sex-biased genes respond to mating in each sex. Despite the highly sex-specific nature of postmating changes in physiology and behavior, sex-biased genes were the primary targets of mating only in the female abdomen (57% of the responsive genes), although they were also more common than expected in the female head and thorax (16%). In both types of tissues, females pre-dominantly downregulated female-biased genes, and in the abdomen a great majority of the male-biased genes responded by increased expression (fig. 4a and b). This result shows that females also require the products of male-biased genes in their postmating reproductive physiology. Despite the general finding that male-biased genes are also expressed in females, they are assumed to be subject to only male-specific selection (Ellegren and Parsch 2007). Our results instead suggest that many male-biased genes may also experience female-specific selection which raises the possibility that, at least in some cases, the faster regulatory divergence often documented for male-biased genes (Ellegren and Parsch 2007) may have resulted from sex-specific selection operating in both sexes rather than in males alone. In males, the sex-biased genes responded to mating in a reversed fashion compared with females: male-biased genes were downregulated in both tissue groups, and female-biased genes more commonly upregulated than expected in the head and thorax (fig. 4c and d). Taken together, these results suggest that sex-specific phenotypes involve expression of both types of sex-biased genes in both sexes. This finding echoes a previous study on a wild turkey that shows how the degree of masculinity of males does not only depend on the expression of male- but also on the relative expression of female-biased genes (Pointer et al. 2013).
In line with several previous studies (Mack et al. 2006; Innocenti and Morrow 2009; Gomulski et al. 2012; Alfonso-Parra et al. 2016), we found that mating increases the expression of genes involved in metabolism of carbohydrates and lipids, and reduction of oxidative stress in the female abdomen (supplementary table S6a, Supplementary Material online). These are the same pathways that show higher expression in virgin males. For example, the great majority of the mating responsive redox genes in the female abdomen are male biased and show increased expression due to mating in females (supplementary fig. S5, Supplementary Material online). These patterns likely reflect a shift in metabolic requirements for males and females that depend on mating status. In C. maculatus, virgin males show a higher metabolic rate and are more active than virgin females (Berger et al. 2016), as male pre-mating activities are characterized by behaviors such as active mate searching, harassment of other males, vigorous chasing and mounting of females. In females, mating induces oogenesis and oviposition, including the search for suitable oviposition sites, no doubt increasing metabolic demands in females. Hence, the masculinization seen in females after mating is likely to large extent due to an up-regulation of the metabolic machinery in mated females. Some of the observed expression changes in metabolic and redox genes may also contribute to nourishing and protecting stored sperm from oxidative damage (Baer et al. 2009; Prokupek et al. 2009; Shaw et al. 2014). In contrast to Drosophila (McGraw et al. 2004; Innocenti and Morrow 2009), the female mating response in C. maculatus did not involve antimicrobial responses. This may reflect differences between species, but it is also possible that the timing of expression of immunity genes differs. Some immunity genes may also go undetected because of the difficulty in annotating rapidly evolving genes based on sequence similarity, such as those involved in immunity and reproduction.
Conclusions
Our results show that sexual dimorphism is a pervasive feature of the C. maculatus transcriptome that is not only limited to the reproductive tissues. Mating induces changes in the degree of sex-bias for thousands of genes, demonstrating the plastic nature of adult gene expression. Although a number of genes changed sex-bias classification, this was relatively rare (4% in head and thorax and 2% in abdomen) suggesting that characterizing sex bias from reproductively naïve individuals is generally predictive of their sex-bias status also after mating. On the other hand, our results highlight the difficulty of predicting how genes may be involved in encoding reproductive phenotypes based on their sex-bias status. The fact that regulatory changes in both types of sex-biased genes occurred in the mating response of both sexes, suggest that selection may not be limited to the sex showing overall higher expression of the focal gene. More studies should therefore aim to link sex-biased expression with reproductive phenotypes under sex-specific selection.
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank Arild Husby and three anonymous reviewers for comments on this article. Sequencing was performed by the SNP&SEQ Technology Platform of SciLifeLab at Uppsala University, Department of Medical Sciences. This work was funded by the European Research Council (AdG-294333) and the Swedish Research Council (621-2010-5266) to G.A.
Literature Cited
- Albritton SE, et al. 2014. Sex-biased gene expression and evolution of the x chromosome in nematodes. Genetics 197:865–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Parra C, et al. 2016. Mating-induced transcriptome changes in the reproductive tract of female Aedes aegypti. PLoS Negl Trop Dis. 10:e0004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SL, Bonduriansky R, Chenoweth SF. 2013. The genomic distribution of sex-biased genes in drosophila serrata: X chromosome demasculinization, feminization, and hyperexpression in both sexes. Genome Biol Evol. 5:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeitman MN, et al. 2002. Gene expression during the life cycle of Drosophila melanogaster. Science 297:2270–2275. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. 2005. Sexual conflict. New Jersey: Princeton University Press. [Google Scholar]
- Assis R, Zhou Q, Bachtrog D. 2012. Sex-biased transcriptome evolution in Drosophila. Genome Biol Evol. 4:1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Eubel H, Taylor NL, O’Toole N, Millar AH. 2009. Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera. Genome Biol. 10:r67.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Meadows LA, Wang J, Dow JA, Russell S. 2007. Variable sexually dimorphic gene expression in laboratory strains of Drosophila melanogaster. BMC Genomics 8:454.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, et al. 2011. A comprehensive gene expression atlas of sex- and tissue-specificity in the malaria vector, Anopheles gambiae. BMC Genomics 12:296. doi: 10.1186/1471-2164-12-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram H, Sayadi A, Goenaga J, Immonen E, Arnqvist G. 2016. Novel seminal fluid proteins in the seed beetle Callosobruchus maculatus identified by a proteomic and transcriptomic approach. Insect Mol Biol. 26:58–73. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 57:289–300. [Google Scholar]
- Berger D, et al. 2016. Intralocus sexual conflict and the tragedy of the commons in seed beetles. Am Nat. 188:E000. [DOI] [PubMed] [Google Scholar]
- Boes KE, et al. 2014. Identification and characterization of seminal fluid proteins in the asian tiger mosquito, Aedes albopictus. PLoS Negl Trop Dis. 8:e2946. doi: 10.1371/journal.pntd.0002946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney GE. 2007. A rapid genome-wide response to Drosophila melanogaster social interactions. BMC Genomics 8:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. 2006. Allocrine modulation of feeding behavior by the sex peptide of Drosophila. Curr Biol. 16:692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Herndon LA, Heifetz Y, Partridge L, Wolfner MF. 2001. The Acp26Aa seminal fluid protein is a modulator of early egg hatchability in Drosophila melanogaster. Proc R Soc Biol Sci. 268:1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory-gland products. Nature 373:241–244. [DOI] [PubMed] [Google Scholar]
- Cho SY, et al. 2010. Differential alternative splicing of human transglutaminase 4 in benign prostate hyperplasia and prostate cancer. Exp Mol Med. 42:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings ME, et al. 2008. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc R Soc Biol Sci. 275:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, et al. 2010. Dynamic, mating-induced gene expression changes in female head and brain tissues of Drosophila melanogaster. BMC Genomics 11:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. 2001. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. PNAS 98:12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Parsch J. 2007. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 8:689–698. [DOI] [PubMed] [Google Scholar]
- Ellis LL, Carney GE. 2010. Mating alters gene expression patterns in Drosophila melanogaster male heads. BMC Genomics 11:558.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagegaltier D, et al. 2014. A genome-wide survey of sexually dimorphic expression of Drosophila miRNAs identifies the steroid hormone-induced miRNA let-7 as a regulator of sexual identity. Genetics 198:647–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon S, Gentleman R. 2007. Using GOstats to test gene lists for GO term association. Bioinformatics 23:257–258. [DOI] [PubMed] [Google Scholar]
- Fox CW. 1993. Multiple mating, lifetime fecundity and female mortality of the Bruchid beetle, Callosobruchus maculatus (Coleoptera, Bruchidae). Funct Ecol. 7:203–208. [Google Scholar]
- Gan QA, et al. 2010. Dynamic regulation of alternative splicing and chromatin structure in Drosophila gonads revealed by RNA-seq. Cell Res. 20:763–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioti A, et al. 2012. Sex peptide of Drosophila melanogaster males is a global regulator of reproductive processes in females. Proc R Soc Biol Sci. 279:4423–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenaga J, Yamane T, Ronn J, Arnqvist G. 2015. Within-species divergence in the seminal fluid proteome and its effect on male and female reproduction in a beetle. BMC Evol Biol. 15:266. doi: 10.1186/s12862-015-0547-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomulski LM, et al. 2012. Transcriptome profiling of sexual maturation and mating in the mediterranean fruit fly, Ceratitis capitata. PLoS One 7:e30857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr MG, et al. 2011. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 29:644-U130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grath S, Baines JF, Parsch J. 2009. Molecular evolution of sex-biased genes in the Drosophila ananassae subgroup. BMC Evol Biol. 9:291.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, et al. 2013. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 8:1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Lanzaro GC. 2005. Female-biased gene expression in the malaria mosquito Anopheles gambiae. Curr Biol. 15:R192–R193. [DOI] [PubMed] [Google Scholar]
- Harrison PW, et al. 2015. Sexual selection drives evolution and rapid turnover of male gene expression. PNAS 112:4393–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B, et al. 2011. Distinct regulatory programs establish widespread sex-specific alternative splicing in Drosophila melanogaster. RNA 17:453–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang K, Matzkin LM, Bono JM. 2015. Transcriptional variation associated with cactus host plant adaptation in Drosophila mettleri populations. Mol Ecol. 24:5186–5199. [DOI] [PubMed] [Google Scholar]
- Hollis B, Houle D, Yan Z, Kawecki TJ, Keller L. 2014. Evolution under monogamy feminizes gene expression in Drosophila melanogaster. Nat Commun. 5:3482.. [DOI] [PubMed] [Google Scholar]
- Illumina. 2013. TruSeq® Stranded total RNA sample preparation guide, Illumina. Technical manual (September), 162. RS-122-9007 DOC.
- Immonen E, Collet M, Goenaga J, Arnqvist G. 2016a. Direct and indirect genetic effects of sex-specific mitonuclear epistasis on reproductive ageing. Heredity 116:338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen E, Ronn J, Watson C, Berger D, Arnqvist G. 2016b. Complex mitonuclear interactions and metabolic costs of mating in male seed beetles. J Evol Biol. 29:360–370. [DOI] [PubMed] [Google Scholar]
- Immonen E, Ritchie MG. 2012. The genomic response to courtship song stimulation in female Drosophila melanogaster. Proc R Soc Biol Sci. 279:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immonen E, Snook RR, Ritchie MG. 2014. Mating system variation drives rapid evolution of the female transcriptome in Drosophila pseudoobscura. Ecol Evol. 279:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti P, Morrow EH. 2009. Immunogenic males: a genome-wide analysis of reproduction and the cost of mating in Drosophila melanogaster females. J Evol Biol. 22:964–973. [DOI] [PubMed] [Google Scholar]
- Isaac RE, Li C, Leedale AE, Shirras AD. 2010. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc R Soc Biol Sci. 277:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZF, Machado CA. 2009. Evolution of sex-dependent gene expression in three recently diverged species of Drosophila. Genetics 183:1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijimoto T, et al. 2014. The nutritionally responsive transcriptome of the polyphenic beetle Onthophagus taurus and the importance of sexual dimorphism and body region. Proc R Soc Biol Sci. 281:2014–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher SD, Richard FJ, Tarpy DR, Grozinger CM. 2008. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera). BMC Genomics 9:232.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo YD, et al. 2008. Functional expression of an insect cathepsin B-like counter-defence protein. Insect Mol Biol. 17:235–245. [DOI] [PubMed] [Google Scholar]
- LaFlamme BA, Ram KR, Wolfner MF. 2012. The Drosophila melanogaster seminal fluid protease “seminase” regulates proteolytic and post-mating reproductive processes. PLoS Genet. 8:e1002435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak MK, Begun DJ. 2004. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome 47:900–910. [DOI] [PubMed] [Google Scholar]
- Lebo MS, Sanders LE, Sun F, Arbeitman MN. 2009. Somatic, germline and sex hierarchy regulated gene expression during Drosophila metamorphosis. BMC Genomics 10:80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledon-Rettig CC, Moczek AP. 2016. The transcriptomic basis of tissue- and nutrition-dependent sexual dimorphism in the beetle. Ecol Evol. 6:1601-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, Heifetz Y, Bender M. 2006. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. PNAS 103:10358–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE, Hultin-Rosenberg L, Axelsson E, Ellegren H. 2007. Rapid evolution of female-biased, but not male-biased, genes expressed in the avian brain. Mol Biol Evol. 24:2698–2706. [DOI] [PubMed] [Google Scholar]
- Martins MJ, Mota CF, Pearson GA. 2013. Sex-biased gene expression in the brown alga Fucus vesiculosus. BMC Genomics 14:294.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Clark AG, Wolfner MF. 2008. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics 179:1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. 2004. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 14:1509–1514. [DOI] [PubMed] [Google Scholar]
- McIntyre LM, et al. 2006. Sex-specific expression of alternative transcripts in Drosophila. Genome Biol. 7:R79.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel RP, Malone JH, Clark AG. 2012. Disentangling the relationship between sex-biased gene expression and X-linkage. Genome Res. 22:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SH, Mank JE. 2016. Inferring regulatory change from gene expression: the confounding effects of tissue scaling. Mol Ecol. 25:5114–5128. [DOI] [PubMed] [Google Scholar]
- Nagaoka S, Takata Y, Kato K. 2011. Identification of two arginases generated by alternative splicing in the silkworm, Bombyx mori. Arch Insect Biochem Physiol. 76:97–113. [DOI] [PubMed] [Google Scholar]
- Nagoshi RN, Mckeown M, Burtis KC, Belote JM, Baker BS. 1988. The control of alternative splicing at genes regulating sexual-differentiation in Drosophila melanogaster. Cell 53:229–236. [DOI] [PubMed] [Google Scholar]
- Naurin S, Hansson B, Hasselquist D, Kim YH, Bensch S. 2011. The sex-biased brain: sexual dimorphism in gene expression in two species of songbirds. BMC Genomics 12:37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M, et al. 2004. A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol. 5(6):R40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsch J, Ellegren H. 2013. The evolutionary causes and consequences of sex-biased gene expression. Nat Rev Genet. 14:83–87. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Jensen JD, Stephan W, Stamatakis A. 2012. A critical assessment of storytelling: gene ontology categories and the importance of validating genomic scans. Mol Biol Evol. 29:3237–3248. [DOI] [PubMed] [Google Scholar]
- Penalva LOF, Sanchez L. 2003. RNA binding protein sex-lethal (sxl) and control of Drosophila sex determination and dosage compensation. Microbiol Mol Biol Rev. 67:343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. 2005. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 15:1690–1694. [DOI] [PubMed] [Google Scholar]
- Perry J, Mank JE. 2014. From genotype × environment to transcriptome × environment: identifying and understanding environmental Influences in the gene expression underlying sexually selected traits In: Hunt J, Hosken DJ, editors. Genotype-by-environment interactions and sexual selection. New York: Wiley-Blackwell. [Google Scholar]
- Perry JC, Harrison PW, Mank JE. 2014. The ontogeny and evolution of sex-biased gene expression in Drosophila melanogaster. Mol Biol Evol. 31:1206–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointer MA, Harrison PW, Wright AE, Mank JE. 2013. Masculinization of gene expression is associated with exaggeration of male sexual dimorphism. PLoS Genet. 9:e1003697.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince EG, Kirkland D, Demuth JP. 2010. Hyperexpression of the X chromosome in both sexes results in extensive female bias of X-linked genes in the flour beetle. Genome Biol Evol. 2:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokupek AM, Kachman SD, Ladunga I, Harshman LG. 2009. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 18:465–475. [DOI] [PubMed] [Google Scholar]
- RDevelopmentCoreTeam. 2011. R: a language and environment for statistical computing. Vienna. http://www.R-project.org/.
- Rice WR, Gavrilets S. 2014. The genetics and biology of sexual conflict. New York: Cold Spring Harbor Laboratory Press Perspectives in Biology. [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, et al. 2008. Molecular and cellular components of the mating machinery in Anopheles gambiae females. PNAS 105:19390–19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn JL, Katvala M, Arnqvist G. 2008. Interspecific variation in ejaculate allocation and associated effects on female fitness in seed beetles. J Evol Biol. 21:461–470. [DOI] [PubMed] [Google Scholar]
- Sayadi A, Immonen E, Bayram H, Arnqvist G. 2016. The de novo transcriptome and its functional annotation in the seed beetle Callosobruchus maculatus. PLoS One 11:e0158565.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WR, et al. 2014. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae. PNAS 111:5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Carney GE, Mo Q, Vannucci M, Jones AG. 2009. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: evidence for masculinization of the transcriptome. BMC Genomics 10:579.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, et al. 2013. Transcriptome-wide expression variation associated with environmental plasticity and mating success in cactophilic Drosophila mojavensis. Evolution 67:1950–1963. [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2005. Limma: linear models for microarray data In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, editors. Bioinformatics and computational biology solutions using R and bioconductor. New York: Springer; p. 397–420. [Google Scholar]
- Telonis-Scott M, Kopp A, Wayne ML, Nuzhdin SV, McIntyre LM. 2009. Sex-specific splicing in Drosophila: widespread occurrence, tissue specificity and evolutionary conservation. Genetics 181:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Carroll SB. 2009. Genetic and molecular insights into the development and evolution of sexual dimorphism. Nat Rev Genet. 10:797–804. [DOI] [PubMed] [Google Scholar]
- Wolfner MF. 2009. Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered. 100:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman MJ, Agrawal AF, Rowe L. 2010. Condition-dependence of the sexually dimorphic transcriptome in Drosophila melanogaster. Evolution 64:1836–1848. [DOI] [PubMed] [Google Scholar]
- Yamane T, Goenaga J, Ronn JL, Arnqvist G. 2015. Male seminal fluid substances affect sperm competition success and female reproductive behavior in a seed beetle. PLoS ONE 10(4):e0123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, et al. 2006a. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 16:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, et al. 2006b. Cathepsin B-like proteinase is involved in the decomposition of the adult fat body of Helicoverpa armigera. Arch Insect Biochem Phys. 62:1–10. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sturgill D, Parisi M, Kumar S, Oliver B. 2007. Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hambuch TM, Parsch J. 2004. Molecular evolution of sex-biased genes in Drosophila. Mol Biol Evol. 21:2130–2139. [DOI] [PubMed] [Google Scholar]
- Zhou S, Mackay T, Anholt RR. 2014. Transcriptional and epigenetic responses to mating and aging in Drosophila melanogaster. BMC Genomics 15:927.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SS, Campbell TG, Stone EA, Mackay TFC, Anholt RRH. 2012. Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet. 8:e1002593 doi: 10.1371/journal.pgen.1002593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk M, Garcia-Gonzalez F, Herberstein ME, Simmons LW. 2014. Model systems, taxonomic bias, and sexual selection: beyond Drosophila. Annu Rev Entomol. 59:321–338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.