Abstract
The enslavement of an alpha-proteobacterial endosymbiont by the last common eukaryotic ancestor resulted in large-scale gene transfer of endosymbiont genes to the host nucleus as the endosymbiont transitioned into the mitochondrion. Mitochondrial genomes have experienced widespread gene loss and genome reduction within eukaryotes and DNA sequencing has revealed that most of these gene losses occurred early in eukaryotic lineage diversification. On a broad scale, more recent modifications to organelle genomes appear to be conserved and phylogenetically informative. The first red algal mitochondrial genome was sequenced more than 20 years ago, and an additional 29 Florideophyceae mitochondria have been added over the past decade. A total of 32 genes have been described to have been missing or considered non-functional pseudogenes from these Florideophyceae mitochondria. These losses have been attributed to endosymbiotic gene transfer or the evolution of a parasitic life strategy. Here we sequenced the mitochondrial genomes from the red algal parasite Choreocolax polysiphoniae and its host Vertebrata lanosa and found them to be complete and conserved in structure with other Florideophyceae mitochondria. This result led us to resequence the previously published parasite Gracilariophila oryzoides and its host Gracilariopsis andersonii, as well as reevaluate reported gene losses from published Florideophyceae mitochondria. Multiple independent losses of rpl20 and a single loss of rps11 can be verified. However by reannotating published data and resequencing specimens when possible, we were able to identify the majority of genes that have been reported as lost or pseudogenes from Florideophyceae mitochondria.
Keywords: Rhodophyta, mitochondria, gene loss, parasite, atp8, rpl20
Introduction
Endosymbiotic events have had a profound impact on eukaryotic evolution (Lane and Archibald 2008; Keeling 2010; Koonin 2010; Zimorski et al. 2014; Martin et al. 2015). All eukaryotes [with one recent exception (Karnkowska et al. 2016)] possess a mitochondrion or mitochondrion-related organelle (MRO) that was initially acquired from an alpha-proteobacteria endosymbiont (Gray et al. 1999; Lang et al. 1999; Koonin 2010; Gray 2012). Additionally, photosynthetic lineages maintain a plastid that originated as a cyanobacterial endosymbiont in the shared ancestor of Glaucophytes, Rhodophytes, and Viridiplantae (Chlorophytes and Streptophytes), and was subsequently spread through the eukaryotic tree of life via secondary and tertiary endosymbiotic events (Bhattacharya et al. 2004; Keeling 2004; Stiller 2007; Gould et al. 2008; Lane and Archibald 2008; Keeling 2010; Stiller et al. 2014). There is evidence of massive gene transfer from the endosymbiont to the host nucleus upon the initial acquisition of these organelles, resulting in host control and regulation of the organelle’s function (Martin et al. 1998; Timmis et al. 2004; Qiu et al. 2013; Ku, Nelson-Sathi, Roettger, Garg, et al. 2015; Ku, Nelson-Sathi, Roettger, Sousa, et al. 2015). Further organellar genome modifications appear to be mostly lineage specific, with gene losses and transfers being restricted within lineages (Tucker 2013; Janouškovec et al. 2013; Ku, Nelson-Sathi, Roettger, Garg, et al. 2015; Ku, Nelson-Sathi, Roettger, Sousa, et al. 2015; Qiu et al. 2015; Tanifuji et al. 2016). The conservation among organellar genomes, in addition to their being inherited predominately uniparentally, has made organelles prime targets for understanding evolutionary relationships across and within the eukaryotic tree of life.
Red algae (phylum Rhodophyta) diversified from their last common ancestor, shared with green algae, more than 1 billion years ago (Yoon et al. 2004). There are ∼7,100 currently described species of rhodophytes that are divided into seven classes; Bangiophyceae, Compsopogonophyceae, Cyanidiophyceae, Florideophyceae, Porpyridiophyceae, Rhodellophyceae, and Stylonematophyceae (Guiry MD and Guiry GM 2016). The Florideophyceae exhibit a wide range of morphological complexity and are by far the most species rich class, containing ∼6,750 species spread across 30 orders (Guiry MD and Guiry GM 2016). Understanding the evolutionary relationships within the Florideophyceae has traditionally been complicated by phenotypic plasticity (Cianciola et al. 2010). More recently, molecular data have been analyzed and great progress has been made in describing new genera and species (Cianciola et al. 2010; Saunders and McDonald 2010; Le Gall and Saunders 2010). However, teasing apart the evolutionary histories of red algal orders has proven quite difficult even with the abundance of sequence data currently available (Verbruggen et al. 2010; Lam et al. 2016). Resolving the evolutionary relationships among florideophytes will provide a robust framework for asking a wide range of evolutionary questions including, but not limited to, transitions from marine to freshwater habitats, the evolution of the complex triphasic life-cycle found in many Florideophyceae orders, and the evolution of parasitism, a life strategy that has arisen many times among the Florideophyceae (Blouin and Lane 2012; Salomaki and Lane 2014; Blouin and Lane 2015; Lam et al. 2016). The use of the maternally inherited mitochondrial genome to resolve evolutionary relationships among the Florideophyceae shows promise (Yang et al. 2015).
The number of sequenced red algal organellar genomes has been increasing exponentially over the past decade. In part, this is a result decreasing sequencing costs allow for increasing use of next-generation sequencing technologies. Currently there are 30 published Florideophyceae mitochondrial genomes species available on GenBank (table 1). However, only 16 of the 30 florideophycean orders are represented in these data, and 10 of those orders are represented by a single mitochondrion genome sequence.
Table 1.
Table of all currently available Florideophyceae mitochondrion genomes that were examined in this study with GenBank Accession, genome length, and A/T%
| Species | GenBank Accession | Length | AT Content (%) | Reported Missing Genes | Notes |
|---|---|---|---|---|---|
| Ah. plicata | KF649303 | 32,878 | 66.6 | atp8, rpl20 |
|
| A. taxiformis | KJ398158 | 26,097 | 73.3 | sdhD |
|
| Ce. japonicum | KJ398159 | 26,200 | 71.5 | atp4 (as ymf39), sdhC, sdhD, TatC (as secY), rpl20 |
|
| Ce. sungminbooi | KU145004 and KU145005 | 24,508 | 71.2 | noted as partial in article (Hughey and Boo 2016) |
|
| 24,494 | 71.2 | ||||
| Ch. crispus | NC_001677 | 25,836 | 72.1 | — |
|
| C. polysiphoniae | KX687877 | 25,357 | 79.4 | rpl20 |
|
| Co. compressa | KU053956 | 25,391 | 74.3 | — |
|
| Corallina officinalis | KU641510 | 26,504 | 69.9 |
|
|
| D. binghamiae | KX247283 | 26,052 | 77.4 |
|
|
| G. elegans | KF290995 | 24,922 | 70.5 | rpl20 |
|
| G. vagum | KC875854 | 24,901 | 69.5 | rpl20 |
|
| Gracilaria chilensis | KP728466 | 26,898 | 72.4 | — |
|
| Gracilaria salicornia | KF852534 | 25,272 | 71.6 | — |
|
| G. vermiculophylla | KJ526627 | 25,973 | 71.9 | — |
|
| Gr. oryzoides | NC_014771 and KX687879 | 25,161 | 71.9 | atp8, sdhC |
|
| G. andersonii | NC_014772 and KX687878 | 27,036 | 72 | atp4 (as ymf39) |
|
| G. chorda | NC_023251 | 26,534 | 72.4 | atp4 (as ymf39) |
|
| Gracilariopsis lemaneiformis | JQ071938 | 25,883 | 72.5 | — |
|
| Gra. angusta | NC_023094 | 27,943 | 69.8 | — |
|
| Grateloupia taiwanensis | KM999231 | 28,906 | 68.6 | — |
|
| H. rubra | KF649304 | 33,066 | 67.8 | atp4, atp8, rpl20 |
|
| K. striatus | KF833365 | 25,242 | 69.9 | atp4 (as ymf39) |
|
| Mastocarpus papillatus | KX525587 | 25,906 | 65.0 |
|
|
| P. palmata | KF649305 | 29,735 | 67.8 | — |
|
| P. pulvinata | HQ586061 | 25,894 | 76.1 | atp8, nad4L |
|
| Pl. cartilagineum | KJ398160 | 26,431 | 76.4 | atp8, nad4L |
|
| R. pseudopalmata | KC875852 | 26,351 | 70.5 | rpl20 |
|
| Riquetophycus sp. | KJ398161 | 26,351 | 74.3 | — |
|
| S. schousboei | KJ398162 | 25,906 | 73.3 | rpl20 |
|
| Sc. dubyi | KJ398163 | 26,438 | 74.1 | atp4 (as ymf39), rpl20 |
|
| S. flabellata | KJ398164 | 26,767 | 71.5 | atp4 (as ymf39), sdhC |
|
| Sp. durum | KF186230 | 26,202 | 71.6 | rps11, rpl20 |
|
| V. lanosa | KX687880 | 25,119 | 71.7 | rpl20 |
|
Note.—Genes previously reported as missing are listed along with notes regarding their status as a result of this study.
Analyses of mitochondrion and MRO genomes across the tree of life have shown they are highly variable in gene content, arrangement, and structure (Smith and Keeling 2015). More recently, the oxymonad Monocercomonoides was shown to have entirely lost its MRO and all genes of mitochondrial origin that had been transferred to the nucleus (Karnkowska et al. 2016). Wide variability of mitochondrial genome content and structure has been implicated in the Florideophyceae as well. A study investigating the impact of adopting a parasitic life strategy on mitochondrial genomes of red algae described the atp8 and sdhC genes of red algal parasite Gracilariophila oryzoides as pseudogenes, and that the atp8 gene in the parasite Plocamiocolax pulvinata has been lost entirely (Hancock et al. 2010). The authors concluded that the products of these genes may be provided either from the parasite nucleus as a result of endosymbiotic gene transfer (EGT), or perhaps the proteins are being obtained from their hosts. More recently, Yang et al. (2015) sequenced 11 Florideophyceae mitochondrial genomes. Analysis of their data, in combination with all previously sequenced red algal mitochondria led them to describe multiple independent losses of atp8, nad4L, rpl20, rps11, sdhC, sdhD, secY, and ymf39 across the Florideophyceae (Yang et al. 2015).
Prior to this study, 30 Florideophyceae mitochondrial genomes have been sequenced. Of those, 19 are reported to be missing a functional copy of at least one gene. A total of eight different genes have been reported as pseudogenes or missing entirely from a Florideophyceae mitochondrial genome. Previous speculation on what is driving gene loss from Florideophyceae mitochondria include EGT from the mitochondrion to the nucleus (Hancock et al. 2010; Yang et al. 2015), and decreasing selective pressures in parasite mitochondria as a parasite may be obtaining products of those genes from the host (Hancock et al. 2010). Both explanations seem plausible, with the later hypothesis being directly responsible for the sequencing of the mitochondrial genome from the parasitic red alga, Choreocolax polysiphoniae and its host Vertebrata lanosa (this study).
The mitochondrial genomes of the parasitic red alga, C. polysiphoniae and its host V. lanosa represent the first mitochondrial genomes available from the family Rhodomelaceae, which comprises ∼1/7th of species diversity within the phylum Rhodophyta (Guiry MD and Guiry GM 2016). In 2010, our lab reported that two mitochondrial respiratory protein-coding genes were degraded in the red algal parasites, Gr. oryzoides and P. pulvinata (Hancock et al. 2010). Unexpectedly, the C. polysiphoniae mitochondrion has no degradation of respiratory mitochondrial genes. To reconcile these datasets we resequenced the mitochondrial genomes of the parasite Gr. oryzoides and its host G. andersonii. Furthermore, we systematically reevaluate the described gene losses from the other 30 previously published Florideophyceae mitochondrial genomes, revealing that more than two-thirds of the described losses are the result of errors in sequencing or downstream analyses. We find Florideophyceae mitochondrial genomes to be highly conserved and that gene losses are rare and predominately, if not entirely, observed in genes encoding ribosomal proteins.
Materials and Methods
Mitochondrial Genome Sequencing
Specimens of V. lanosa and C. polysiphoniae were collected from Beavertail State Park, Jamestown, Rhode Island, USA (voucher RI 0423). Gracilariopsis andersonii and Gr. oryzoides were collected at Pigeon Point, Pescadero, California, USA (voucher CL031613). Representatives of these parasite and host pair populations are retained as vouchers in the Lane Lab herbarium at the University of Rhode Island. Vegetative tissue from V. lanosa and G. andersonii was inspected for parasites and epiphytes under a dissecting microscope and clean tissue was ground under liquid nitrogen. Erumpent pustules of C. polysiphoniae (n = 50) and Gr. oryzoides (n = 10) were excised from the thallus of their hosts V. lanosa, and G. andersonii, respectively, and collected in a 1.5 ml microcentrifuge tube. The parasite tissue was hand-ground using a Corning Axygen PES-15-B-SI disposable tissue grinder pestle in a 1.5 ml microcentrifuge tube while submerged in 100 µl of DNA extraction buffer (Saunders 1993). DNA was extracted from all specimens using a standard phenol/chloroform extraction (Saunders 1993).
All DNA libraries were prepared for Illumina sequencing on the Apollo 324 robot using the PrepX ILM DNA Library Kit (Wafergen Biosystems, Freemont, California). The G. andersonii library was sequenced on a full-cell of an Illumina MiSeq paired-end 250 × 250 basepair (bp) run yielding 30,330,114 sequences in pairs. The Gr. oryzoides and C. polysiphoniae libraries were each sequenced on full-cells of an Illumina MiSeq paired-end 300 × 300 bp run yielding 26,097,992 and 29,355,470 sequences in pairs, respectively. The V. lanosa library was sequenced on a partial-cell of an Illumina MiSeq paired-end 300 × 300 bp run yielding 12,888,082 sequences in pairs. For all datasets, sequences with PHRED scores <30 were removed and the remaining reads were trimmed of adapter sequences. Additionally, fifteen 5′ and five 3′ nucleotides were trimmed from the remaining reads and all reads under 100 nucleotides were removed from the dataset. All trimming was completed using CLC Genomics Workbench v. 8.5.1 (CLC Bio-Qiagen, Aarhus, Denmark) and the remaining reads were assembled using default parameters in CLC Genomics Workbench v. 8.5.1. Trimmed sequencing reads for the G. andersonii and Gr. oryzoides mitochondria were mapped back to the previously published mitochondrion to compare the two assemblies and confirm support for differences.
Open reading frame (ORF) prediction on the V. lanosa and C. polysiphoniae mitochondrion sequences was done using translation table 4 (Protazoa Mitochondrion) using ATG as a start-codon in Geneious Pro v9.1 (Kearse et al. 2012). The resulting ORFs were manually annotated using blastN against GenBank. If blastN was insufficient for annotating an ORF, the ORF was translated to an amino acid sequence and then searched against the non-redundant protein sequence database (nr) in GenBank using blastP and the Pfam database (Finn et al. 2010, 2015). If no conserved domain or sequence similarity could be found after searches using blastP or Pfam, the ORF remained without further annotation. Mitochondrion genome sequences were submitted to the tRNAscan-SE online server v1.21 for identification of tRNA sequences (Schattner et al. 2005). Ribosomal RNA predictions were based on annotations produced by MFannot (http://megasun.bch.umontreal.ca/cgi-bin/mfannot/mfannotInterface.pl).
Red Algal Mitochondrial Genome Conservation
All 30 currently available Florideophyceae mitochondrial genomes (table 1) were downloaded from GenBank and imported into GeneiousPro v9.1. These mitochondrial genomes were combined with those from V. lanosa and C. polysiphoniae to create a database of Florideophyceae mitochondrial genomes. Sequences that have previously been found to have missing genes or pseudogenes were reanalyzed for ORFs in GeneiousPro v9.1. In cases where an ORF was found in a conserved location that had not previously been annotated as a gene, the ORF was translated and searched against the non-redundant protein sequence database (nr) in GenBank using blastP and Pfam. If this was insufficient to annotate an ORF in a conserved location, representatives of the missing genes were mapped back to the genome of interest for further evaluation and the region was manually curated. Translations of ORFs from locations of missing genes were aligned with annotated copies of those genes to manually assess annotation. When an apparent premature stop codon was found in a conserved location for a missing gene or pseudogene, the region was resequenced using PCR amplification for confirmation when material or DNA from that species could be obtained.
To determine the AT content (%) and non-synonymous to synonymous substitution ratio (dN/dS ratio) (table 2), all protein-coding genes widely shared throughout the 32 Florideophyceae mitochondrial genomes were aligned using GeneiousPro v9.1. The average AT content (%) was calculated for each gene across the Florideophyceae in GeneiousPro v9.1. The CODEML program in PAML v. 4.8 (Yang 2007) was utilized to estimate the pairwise dN/dS ratio across all published Florideophyceae mitochondrion genes. For each gene analyzed, a nucleotide alignment was created using the translation align function in GeneiousPro v9.1 utilizing the Blosum62 cost matrix. Additionally, a Neighbor-Joining tree was constructed from the alignment in GeneiousPro v9.1 using the Tamura-Nei substitution model and the gene from Hildenbrandia rubra was used to root the tree. For the rpl20 gene, which has been lost in H. rubra, Palmaria palmata was used to root the tree. The alignment and Neighbor-Joining tree were used as input files and the following parameters were specified in CODEML: runmode = 0; seqtype = 1; codonfreq = 0; model = 0; icode = 4; and omega (measures dN/dS ratio) and kappa (measures transitions/transverstions) were estimated.
Table 2.
A/T%, non-synonymous to synonymous mutation (dN/dS) ratio and individual dN and dS values for genes encoded on the Florideophyceae mitochondrion genomes
| Gene | AT Content (%) | dN/dS ratio | dN | dS | Species with pseudogene only |
|---|---|---|---|---|---|
| atp4 | 79.8 | 0.51645 | 022882 | 0.44307 | |
| atp6 | 72.5 | 0.12864 | 0.07486 | 0.58195 | |
| atp8 | 78.8 | 0.51248 | 0.20110 | 0.39241 | |
| atp9 | 66.1 | 0.01550 | 0.00212 | 0.13673 | |
| cob | 70.3 | 0.11691 | 0.05443 | 0.46555 | |
| cox1a | 67.2 | 0.06936 | 0.01711 | 0.24675 | |
| cox2 | 69.5 | 0.14959 | 0.04549 | 0.30414 | |
| cox3 | 68.3 | 0.12819 | 0.07186 | 0.56058 | |
| nad1 | 69.0 | 0.09454 | 0.06667 | 0.70520 | |
| nad2 | 74.6 | 0.31481 | 0.15877 | 0.50434 | |
| nad3b | 74.5 | 0.15718 | 0.07060 | 0.44919 | |
| nad4 | 72.7 | 0.18548 | 0.07790 | 0.41999 | |
| nad4L | 75.3 | 0.14619 | 0.05654 | 0.38677 | |
| nad5 | 71.9 | 0.21716 | 0.08547 | 0.39356 | |
| nad6 | 75.1 | 0.26439 | 0.17651 | 0.66762 | |
| rpl16 | 75.9 | 0.36193 | 0.13664 | 0.37752 | |
| rpl20 | 79.1 | 0.62160 | 0.46012 | 0.74023 | Ce. japonicum |
| Ce. sungminbooi | |||||
| C. polysiphoniae | |||||
| Co. compressa | |||||
| D. binghamiaed | |||||
| G. elegans | |||||
| G. vagum | |||||
| H. rubra | |||||
| V. lanosa | |||||
| rps3c | 76.9 | 0.51571 | 0.28532 | 0.55326 | |
| rps11 | 77.3 | 0.45177 | 0.19375 | 0.42886 | Sp. durum |
| rps12 | 67.5 | 0.20794 | 0.11986 | 0.57642 | |
| sdhB | 71.7 | 0.20603 | 0.10707 | 0.51969 | |
| sdhC | 78.6 | 0.47084 | 0.17487 | 0.37140 | |
| sdhD | 78.2 | 0.46556 | 0.26263 | 0.56412 | |
| TatC | 80.0 | 0.50046 | 0.34550 | 0.69037 | Ce. japonicum |
Note.—Species with a pseudogene, rather than a functional copy of the gene, are listed in the far right column and were left out of calculations of A/T% and dN/dS ratio.
aIntrons removed and CDSs only were used for A/T% and dN/dS analysis.
bCe. japonicum nad3 left out of dN/dS analysis.
cD. binghamiae rps3 left out of dN/dS analysis.
dNo evidence for remnant pseudogene, appears to be a complete loss.
Results and Discussion
Description of a Red Algal Alloparasite and Host Mitochondrion
The mitochondrial genomes of the red algal alloparasite, C. polysiphoniae (KX687877) and its host, V. lanosa (KX687880) represent the first mitochondrial genomes sequenced from the Rhodomelaceae (Florideophyceae, Rhodophyta), further expanding the diversity of available red algal mitochondrial genomes. The C. polysiphoniae mitochondrial genome is a 25,357 bp long circular molecule with an AT content of 79.4%. The mitochondrial genome of C. polysiphoniae encodes 23 protein-coding genes, 2 rRNAs and 20 tRNAs and contains only 9.9% intergenic, non-coding DNA. The V. lanosa mitochondrial genome is also a circular molecule that is 25,337 bp long, has an AT content of 76.4%, and encodes 23 protein-coding genes, 2 rRNAs and 19 tRNAs with 10.4% of the mitochondrial DNA being non-coding. Both the C. polysiphoniae and V. lanosa mitochondrial genomes maintain a similar genome architecture to other published red algal mitochondria.
Parasitic Red Algal Mitochondrial Genomes Are Conserved
The atp8 gene has previously been reported missing from five Florideophyceae mitochondria (Hancock et al. 2010; Yang et al. 2015) including the parasites Gr. oryzoides and P. pulvinata. A re-annotation of the mitochondrion of P. pulvinata, identified the atp8 as an ORF that was annotated only as a hypothetical protein coding sequence (CDS) in the sequence downloaded from GenBank (tables 1 and 3). Subsequently, resequencing the Gr. oryzoides mitochondrial genome (KX687879) revealed a complete copy of the atp8 gene, rather than the pseudogene that was previously reported using both Illumina and Sanger sequencing (Hancock et al. 2010). The sdhC gene was also previously reported to be a pseudogene in the adelphoparasite Gr. oryzoides (Hancock et al. 2010). As with atp8, resequencing of the Gr. oryzoides mitochondrion (KX687879) demonstrated that there was no frameshift mutation, as originally published, and that sdhC remains complete in red algal parasite mitochondria. These findings indicate that red algal parasites have not found alternative mechanisms for acquiring mitochondrion proteins and rely on their own mitochondrion for generating cellular energy as was previously hypothesized (Hancock et al. 2010).
Table 3.
Current status of Florideophyceae mitochondrial genes previously reported as missing in Hancock et al. (2010) and Yang et al. (2015) or otherwise unannotated
| Gene | Current Status |
||
|---|---|---|---|
| Gene Present | Location in Published Sequence or GenBank Accession Number | Pseudogene | |
| atp8 | Ah. plicata | KX687876 | |
| Gr. oryzoides | 20,431 > 20,024d | ||
| H. rubra | 24,587 > 24,213 | ||
| P. pulvinata | 20,389 > 19,985 | ||
| Pl. cartilagineum | 20,528 > 20,127 | ||
| nad4L | P. pulvinata | 25,592 > 25,894 | |
| Pl. cartilagineum | 26,129 > 26,474 (43) | ||
| rpl20 | Ah. plicataa | 30,851 > 31,093 | Ce. japonicum |
| R. pseudopalmataa | 24,127 > 24,351 | Ce. sungminbooi | |
| S. schousboei | 23.912 > 24,148 | C. polysiphoniae | |
| Sc. dubyi | 24,440 > 24,709 | Co. compressa | |
| Sp. duruma | 24,248 > 24,487 | D. binghamiaef | |
| G. elegans | |||
| G. vagum | |||
| H. rubra | |||
| V. lanosa | |||
| rps11 | Sp. durum | ||
| sdhC | Ce. japonicum | 12,331 > 12,044 | |
| Ce. sungminbooi | 10,977 > 10,678 | ||
| D. binghamiae | 21,403 > 21,789 | ||
| Gr. oryzoides | 11,593 > 11,234d | ||
| S. flabellataa | 11,358 > 10,966 | ||
| sdhD | A. taxiformis | 15,759 > 15,514 | |
| Ce. japonicum | 16,636 > 16,403 | ||
| Ce. sungminbooi | 15,284 > 15,036 | ||
| TatC | Ce. sungminbooi | 15,277 > 15,029 | Ce. japonicumg |
| atp4 | Ce. japonicumb | 6,935 > 7,489 | |
| G. andersonii | 7,560 > 8,102e | ||
| G. chordac | 7,238 > 7,780 | ||
| H. rubra | 13,225 > 13,785 | ||
| Sc. dubyib | 7,261 > 7,803 | ||
| S. flabellatab | 7,234 > 7,776 | ||
aIndicates presence of functional gene is dependent on non-ATG start codon, alternatively these could be pseudogenes. RNA sequence data would be required to confirm gene function.
bThe atp4 gene was annotated as hypothetical protein CDS in GenBank but considered as atp4 in Yang et al. (2015), figure 2.
cThe atp4 gene was annotated as hypothetical protein CDS in GenBank but considered as ymf39 in Yang et al. (2015), figure 2.
dLocation in newly sequenced Gr. oryzoides mitochondrion (GenBank KX687879).
eLocation in newly sequenced G. andersonii mitochondrion (GenBank KX687878).
fNo evidence for remnant pseudogene.
gThe Ce. japonicum TatC gene seems likely to be the result of homopolymer sequence error, though possibility of pseudogene remains. Due to high levels of variation in length and sequence of Florideophyceae TatC genes, we continue to recognize the Ce. japonicum TatC gene as a pseudogene until firm evidence contradicts this.
Gene Loss in Other Red Algal Mitochondria
As a result of identifying conserved copies of genes originally reported to have been lost, we reevaluated all reported gene losses from red algal mitochondria. Investigation of the other reported atp8 losses revealed that the gene was an ORF annotated as a hypothetical protein CDS in Plocamium cartilagineum, and that an ORF corresponding to atp8 could be found in the published H. rubra sequence data that were not previously annotated (tables 1 and 3). The Ahnfeltia plicata mitochondrion had a premature stop codon resulting in a pseudogene where atp8 is normally found, however targeted PCR and sequencing showed the gene (KX687876) is complete. Analysis of the ratio of non-synonymous to synonymous substitutions (dN/dS ratio) in Florideophyceae copies of the atp8 gene show a higher rate on non-synonymous mutations in atp8 than atp6 and atp9, which combine with atp8 to make up the F0 domain of the F1F0-ATP synthase complex involved in ATP synthesis (table 2). However the dN/dS ratio of all three proteins remains <1 indicating that purifying selection is acting on the mitochondrial F1F0-ATP synthase complex in red algae, as is expected from genes essential for mitochondrion function.
Table 4.
The use of alternative start codons by gene based on published literature and the proximity to the closest in-frame ATG initiation codon. Alternative initiation codons that are supported by the lack of a nearby ATG initiation codon and conserved gene start location based on alignment are indicated in bold
| Gene | Species | Location with Alternative Initiation Codon | Location with ATG Initiation Codon | Difference in Gene Length in nucleotides (Amino Acids) |
|---|---|---|---|---|
| atp4 | A. taxiformis | 7,298 (ATT) | 7,304 | 6 (2) |
| atp6 | Gra. angusta | 23,475 (ATT) | 23,466 | 9 (3) |
| Sp. durum | 21,462 (ATT) | 21,465 | 3 (1) | |
| atp8 | — | — | — | — |
| atp9 | — | — | — | — |
| cob | S. flabellataa | 8,070 (ATT) | 8,196 | 126 (42) |
| cox1 | Sc. dubyi | 3,889 (ATT) | 3,904 | 15 (5) |
| cox2 | Gra. angusta | 7,845 (TTG) | 7,818 | 27 (9) |
| P. palmata | 6,556 (ATT) | 6,535 | 21 (7) | |
| cox3 | — | — | — | — |
| nad1 | — | — | — | — |
| nad2 | — | — | — | — |
| nad3 | — | — | — | — |
| nad4 | H. rubra | 22,165 | 22,150 | 15 (5) |
| nad4L | — | — | — | — |
| nad5 | — | — | — | — |
| nad6 | — | — | — | — |
| rpl16 | Ah. plicataa | 5,899 (TTA) | 6,169 | 270 (90) |
| Gra. angustaa | 3,324 (TTA) | 3,585 | 261 (87) | |
| rpl20 | Ah. plicataa,c | 30,851 (ATA) | 30,947 | 96 (32) |
| R. pseudopalmataa | 24,127 (ATT) | 24,235 | 108 (36) | |
| Sp. duruma | 24,248 (ATT) | — | — | |
| rps3 | Pl. cartilagineum | 2,693 (ATC) | 2,732 | 39 (13) |
| Sp. durum | 2,574 (ATA) | — | — | |
| rps11 | Ah. plicataa | 17,436 (ATA) | 17,277 | 159 (53) |
| G. chilensis | 10,426 (ATT) | 10,423 | 3 (1) | |
| Gra. angusta | 14,946 (ATT) | 14,938 | 9 (3) | |
| P. palmataa | 14,083 (TTA) | — | — | |
| rps12 | — | — | — | — |
| sdhB | Gra. angustaa | 13,459 (TTA) | 13,312 | 147 (49) |
| sdhC | Gra. angusta | 13,852 (TTG) | 13,849 | 3 (1) |
| H. rubraa,b | 17,061 (ATT) | 17,022 | 39 (13) | |
| Sc. dubyi | 11,319 (CTT) | 11,310 | 9 (3) | |
| sdhD | Ah. plicata | 20,718 (TTA) | 20,733 | 15 (5) |
| H. rubraa | 20,662 (ATA) | — | — | |
| TatC | Ah. plicata | 29,469 (ATT) | 29,487 | 18 (6) |
| Ch. crispusa,d | 348 (GTT) | 681 | 333 (111) | |
| Gra. angustaa | 24,671 (ATT) | 25,031 | 360 (120) | |
| H. rubraa | 29,085 (ATA) | — | — | |
| K. striatusa | 24,437 (TTG) | 24,176 | 261 (87) | |
| P. palmataa | 24,152 (ATC) | 23,687 | 465 (155) | |
| R. pseudopalmataa | 22,834 (TTA) | 23,350 | 516 (172) | |
| Schimmelmania schousboeia | 22,636 (ATT) | 23,188 | 552 (184) | |
| S. flabellataa | 23,492 (TTA) | 23,546 | 54 (18) | |
| Sp. durum | 22,915 (ATC) | 22,942 | 27 (9) |
aIndicates examples where other non-ATG initiation codons from translation table 4 (Protozoa Mitochondrion) are also possible locations for the gene to start although no ATG codon is found within 30 nucleotides (10 amino acid residues) upstream or downstream from the start of the currently annotated gene.
bThe H. rubra sdhC gene annotation is longer than other copies of sdhC and the beginning of the gene overlaps with a tRNA. Starting annotation at ATG makes the gene much more similar in length to other Florideophyceae copies of sdhC.
cGene not previously annotated in GenBank.
dThe Ch. crispus TatC (ymf16) gene is currently annotated with a GTT initiation codon, which is not found for any other Florideophyceae mitochondrion gene nor is it a start codon in translation table 4 (Protozoa Mitochondrion). Four other ORFs in the same reading frame that use either ATA or TTA as a start codon for TatC gene are found from 12 to 39 nucleotides downstream of the GTT codon.
Although the biological implications of losing the nad4L gene was not discussed in previous literature, the gene was noted as being absent in the mitochondrial genomes of both P. pulvinata and Pl. cartilagineum (Hancock et al. 2010; Yang et al. 2015). In P. pulvinata an ORF was identified in the same location as other red algal copies of nad4L, between the 16S ribosomal RNA and the 26S ribosomal RNA, and both Pfam sequence search and blastP search of the translation strongly supports it coding for a functional nad4L. The published sequence for the mitochondrial genome of Pl. cartilagineum splits an ORF here identified as the nad4L gene in two pieces, with the 5′ portion of the sequence found from bases 26,172–26,431 and the 3′ portion of the sequence is located from bases 1 to 43. With a dN/dS ratio of 0.14619, the nad4L gene remains under strong purifying selection in red algal mitochondria. Therefore, the loss of nad4L in any red algal mitochondria would represent a strong departure from this heavy selective pressure.
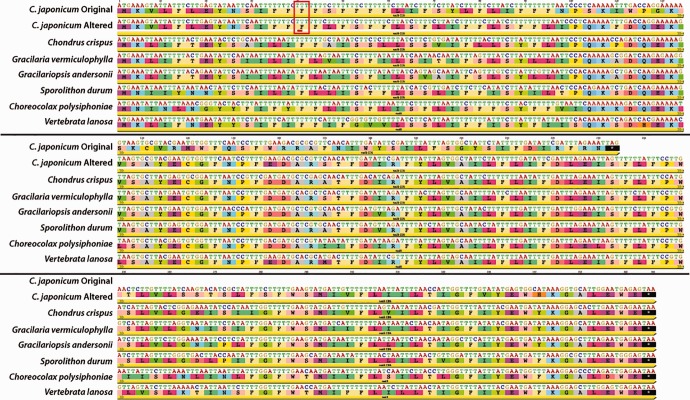
The sdhD gene encodes an essential protein that serves to anchor the succinate dehydrogenase complex II to the inner-membrane of the mitochondrion (Elorza et al. 2004; Bayley et al. 2005, 2006). The sdhD gene was reported missing from the mitochondria of Ceramium japonicum and Asparagopsis taxiformis (Yang et al. 2015) and the gene is also not annotated in the more recently published mitochondrial genome of Ceramium sungminbooi (Hughey and Boo 2016). Upon reanalysis, an ORF was identified between nad4 and nad2, in the conserved Florideophyceae location of sdhD in the published mitochondrial genomes for all three species (see tables 1 and 3). Furthermore, a translated alignment of these ORFs with other Florideophyceae copies of sdhD show they are conserved in frame, retaining several critical conserved residues (fig. 1), and therefore should be annotated as sdhD.
Fig. 1.—
Translated alignment of sdhD genes from florideophycean mitochondria showing A. taxiformis, Ce. japonicum, and Ce. sungminbooi (top three sequences) share critical conserved residues with all other Florideophyceae sdhD genes.
Four mitochondria are reportedly missing copies of sdhC. Similarly to our findings with the sdhD genes, unannotated ORFs that are conserved with other Florideophyceae sdhC genes were identified from the mitochondrial genomes of Ce. sungminbooi and Dasya bingamiae (table 3). Based on the published Sebdenia flabellata mitochondrial genome, using an ATG as the only start-codon, there is no ORF that can be attributed to sdhC. However, using all start-codons in translation table 4 (Protozoa Mitochondrion) an ORF that is highly conserved in comparison with other Florideophyceae copies of sdhC is found with a TTA start-codon (tables 1 and 3). Alternative start codons have previously been invoked for annotating red algal mitochondrion genes with variable support, which is discussed in more detail below (and see table 4). The S. flabellata sdhC appears to be a well-justified case for using an alternative initiation codon.
The Ce. japonicum mitochondrion is the other reported case of an sdhC gene loss (Yang et al. 2015). Although it appears to be highly conserved throughout the 5′ region in comparison to other species, the Ce. japonicum sdhC is truncated by ∼81 nucleotides (27 amino acids) at the 3′ end when aligned with copies of the sdhC gene from other Florideophyceae. The Coeloseira compressa sdhC is similarly conserved at the 5′ region and truncated at the 3′ end. A Pfam search of the Ce. japonicum and Co. compressa sequences, translated to amino acids, confirms their identity as Succinate dehydrogenase/Fumarate reductase transmembrane subunit proteins though suggests they may be truncated as well. Although material was not available for experimental validation, we speculate that this observed truncation has little effect on the functionality of sdhC as an anchor protein in succinate dehydrogenase complex II. The length of Florideophyceae sdhC genes (excluding Ce. japonicum and Co. compressa) is quite variable, ranging from 339 bp in P. pulvinata to 411 bp in A. taxiformis. Furthermore, the dN/dS ratio remains at 0.47084 indicating that purifying selection is acting fairly strongly on deleterious mutations in sdhC. The alternative would seem that the sdhC gene in Ce. japonicum and Co. compressa is losing its functional capacity, which would hinder the ability of these free-living species to generate cellular energy.
Although not reported as a loss, the published G. andersonii rps11 gene is inverted in comparison with all other Florideophyceae copies of the gene (Hancock et al. 2010; Yang et al. 2015). Resequencing this genome revealed an ORF in the conserved location between nad3 and atp9 that was not inverted and maintained strong homology with red algal rps11 genes. Analysis of this ORF in comparison to the previously published G. andersonii mitochondrion identified a string of seven “A”s stretching from bases 9,158 to 9,164 correspond to only six “A”s in the newly sequenced mitochondrion. This apparent frameshift mutation resulted in a premature stop codon in the conserved direction that led to identifying an ORF in the same location but inverted as rps11 in the earlier publication. The rps11 gene in the resequenced G. andersonii mitochondrion, extending from bases 14,568 to 14,209, maintains strong homology with, and is encoded in the same direction as other Florideophyceae copies of rps11.
Although no genes were explicitly described as being lost in the recently sequenced D. binghamiae mitochondrial genome (Tamayo and Hughey 2016), annotations for rpl20 and sdhC are absent from the published sequence. Additionally, alignments demonstrate that the cox3, rps3 and TatC genes are truncated in comparison with other Florideophyceae. Perhaps even more interesting is the report of two inverted multi-gene rearrangements that are unprecedented in light of the highly conserved synteny in florideophycean mitochondria. Unfortunately a thorough evaluation of the losses, truncations and rearrangements in this mitochondrial genome is difficult as the publication is extremely brief (<500 words) and lacks essential details such as the sequencing platform from the materials and methods.
Frameshift Mutations Are Overstated
In addition to the annotation of an inverted rps11 in G. andersonii, frameshift mutations have been described as the cause for genes being lost or becoming pseudogenes in Florideophyceae mitochondria including atp8 in Ah. plicata and Gr. oryzoides and sdhC in Gr. oryzoides. The Gracilaropsis andersonii rps12 gene is another case of an apparent frameshift mutation causing a gene to be truncated. In G. andersonii, the rps12 gene is annotated at 240 nucleotides in length while other red algal copies of the gene range from 366 to 390 bp long. As a part of this study we resequenced the G. andersonii mitochondrion (KX687878) and identified that the “CT” found at bases 25,864–25,865 in the previously published G. andersonii mitochondrion appears to be the result of sequencing or assembly error. Without these additional bases, the rps12 gene remains conserved and is 369 bp long.
At first glance, the Ce. japonicum nad3 gene appears to be an instance of a frameshift mutation causing a gene to be truncated. Although the Ce. japonicum nad3 gene is annotated in its mitochondrial genome, it is only 234 bp long, whereas all other Florideophyceae nad3 genes are either 363 or 366 bp long. An alignment of other Florideophyceae nad3 genes to the Ce. japonicum nad3 region indicates that this truncation is the result of a frameshift mutation in a string of 26 “T” and 3 “C” between 32 and 60 bp from the start codon. A translated alignment of the annotated Ce. japonicum nad3 with all other Florideophyceae nad3 genes shows little conservation in the annotated Ce. japonicum nad3. Additionally, a blastP search of the NCBI nr database, and a Pfam sequence search of the translated original annotation shows the region is not homologous with any gene sequenced to date. However, manually deleting a “T” from the previously mentioned string yields a 366 bp nad3 gene that is highly conserved with copies of the nad3 gene sequenced from other Ceramiales mitochondria (fig. 2) and is homologous with nad3 genes in the NCBI nr database and Pfam database. Long homopolymer runs are notoriously challenging for both sequencing and assembly (Kieleczawa 2006; Gilles et al. 2011; Loman et al. 2012; Laehnemann et al. 2016) but is a more likely explanation than a frameshift resulting in two conserved sections of the gene.
Fig. 2.—
Alignment of the original Ce. japonicum nad3 gene with the modified Ce. japonicum nad3 (“T” deleted from base 36; red box) and copies of the nad3 gene from Ch. crispus, Gracilaria vermiculophylla, G. andersonii, Sp. durum, C. polysiphoniae, and V. lanosa. Manual deletion of one ‘T’ from the string of 26 “T”s and 3 “C”s between 32 and 60 bp from the start codon restores conservation of the length and sequence of the Ce. japonicum nad3 gene. Genes are shown with the amino acid translation below.
The Ce. japonicum TatC (secY) initially appears to be another case of a Florideophyceae mitochondrion gene losing function and becoming a pseudogene due to a frameshift mutation, and again, pinpointing the exact location of the mutation is difficult. By manually manipulating the sequence and deleting a nucleotide from a sting of 43 T’s and 7 C’s between 23,501 and 23,550 bp into the published sequence, an ORF that is highly conserved with other Florideophyceae TatC genes containing an ATT initiation codon is observed. Due to high levels of variation in length and sequence of Florideophyceae TatC genes, we continue to recognize the Ce. japonicum TatC gene as a pseudogene until firm evidence contradicts this. However, based on our findings that all frameshift mutations previously discussed in this manuscript were the result of sequencing error or downstream analysis, it seems likely that is again the case here. Resequencing of this region is essential before considering TatC (SecY) as a true loss in Ce. japonicum and the addition of RNA sequence data would help to confirm or reject this hypothesis.
Some Genes Have Degraded into Pseudogenes
Even though secondary analysis of published sequences combined with subsequent PCR and resequencing efforts have found many of the genes that have been reported missing, this is not the case for all losses. The rpl20 gene seems to blur the lines of deciphering when a gene is lost, and it appears to be the least conserved gene in Florideophyceae mitochondria. Interestingly, aside from its presence in red algal mitochondria, the only other lineage of eukaryotes reported to maintain rpl20 are the jakobids (Burger and Nedelcu 2012). Retaining up to 67 genes, the most of any known mitochondria, Jakobid mitochondria are considered to most closely resemble the alpha-proteobacteria endosymbiont that became the contemporary mitochondrion (Gray et al. 2004; Burger and Nedelcu 2012; Burger et al. 2013). In the Florideophyceae, rpl20 has been reported missing or a pseudogene in 11 species including the two new additions from this study.
Annotation of rpl20 has been complicated because, in addition to ATG, which is the most commonly used initiation-codon for Florideophyceae mitochondrion genes, it appears that ATA may serve as an initiation-codon for rpl20 in Ah. plicata, and ATT in Rhodymenia pseudopalmata and Sporolithon durum (table 4). Without these alternative initiation-codons, rpl20 is likely a pseudogene in Ah. plicata, R. pseudopalmata, and Sp. durum. In addition to the aforementioned species, a conserved copy of rpl20 using the ATG start codon was located in Schimmelmannia schousboei (previously not annotated) and Schizymenia dubyi (previously annotated as a hypothetical protein CDS).
In Ce. japonicum, Ce. sungminbooi, C. polysiphoniae, Gelidium elegans, Gelidium vagum, H. rubra, and V. lanosa, the 3′ region of rpl20 gene remains somewhat conserved, however the 5′ end of the sequence is laden with stop codons, or appears to be missing entirely. Therefore rpl20 is considered a pseudogene in these species. No substantial region in the D. binghamiae mitochondrial genome appears to be homologous to the rpl20 gene. Furthermore, rpl20 is annotated as a gene/CDS in the Co. compressa mitochondrial genome, however the 3′ region is slightly truncated and not highly conserved with other rpl20 copies, suggesting that perhaps this also is a pseudogene. This wide variability in rpl20 initiation codons and conservation cause annotation to be extremely difficult. Confirming the presence or absence of a functional rpl20 localized in the mitochondrion is difficult and will likely require RNA sequencing and nuclear genome sequencing to identify possible cases of EGT.
The only unique Florideophyceae mitochondrion gene loss that appears to stand up to further scrutiny also encodes a ribosomal protein. Based on the published sequence of the Sp. durum mitochondrion, rps11 has degraded to a pseudogene. In all other Florideophyceae, rps11 is found adjacent to the 3′ end of nad3; however in Sp. durum, this region contains no ORFs that can be attributed to a full-length copy of rps11. As in other genes, a frameshift mutation appears to be initially responsible for rps11 becoming a pseudogene. However, in all previously discussed frameshift derived pseudogenes, it was apparent that the insertion or deletion of a nucleotide or two would ‘repair’ the gene and result in a conserved copy that could then subsequently be confirmed by PCR. In the case of the Sp. durum rps11, artificially “fixing” the gene could restore a conserved 3′ end of the gene; however, a six residue gap upstream of this “fix” remained in translated alignments adding further support that rps11 is no longer functional in Sp. durum. RNA and nuclear genome sequencing work remains necessary to identify whether this is a complete loss or a case of EGT from mitochondrion to the nucleus.
The Importance of Nomenclature
Identifying gene losses in Florideophyceae mitochondrial genomes has been further complicated by the use of two different names for a homologous gene. In Yang et al. (2015), the ymf39 gene was reported as the most widely lost gene in Florideophyceae mitochondria, and was noted as being absent in six species: Ce. japonicum, G. andersonii, H. rubra, Kappaphycus striatus, Sc. dubyi, and S. flabellata. Furthermore, this gene is annotated only as a hypothetical protein CDS in Gracilariopsis chorda. Resequencing of the G. andersonii (KX687878) and reanalysis of the published H. rubra data reveals that the ymf39 gene is present in both mitochondria. Interestingly, the other four species lacking ymf39 are also the only Florideophyceae mitochondria with an annotated atp4 gene, which is found between the cox3 and cob genes, the same location as ymf39 in other Florideophyceae mitochondria (Yang et al. 2015). According to Burger et al. (2003), ymf39 encodes subunit b of mitochondrial F0F1-ATP synthase and should formally be designated as atp4.
Although it has not led to reports of gene loss, it is of note that three names have been applied to the TatC gene in Florideophyceae mitochondria. In Chondrus crispus the gene currently annotated as ymf16 was initially described as a gene of unknown function called ORF 262 (Leblanc et al. 1995). In the publication of the Porphyra purpurea mitochondrial genome it was noted that ymf16 is recognized as a homolog of E. coli TatC encoding a protein in the Sec-independent protein translocation pathway (Burger et al. 1999). Coeloseira compressa, D. binghamiae, and K. striatus use the name TatC, which is a sec-independent protein translocase protein. In all other published Florideophyceae mitochondria this gene is called SecY, a sec dependent protein translocase protein. When these sequences are searched against the Pfam database, all similarity hits match sec-independent protein translocase protein (TatC). This gene was initially incorrectly annotated as SecY with the publication of the second, third, and fourth florideophycean mitochondrion genomes (Hancock et al. 2010) with subsequent sequencing efforts transferring that nomenclature throughout the Florideophyceae. Furthermore, in their review of algal mitochondrial genomes, Burger and Nedelcu (2012) note that SecY is not found in the mitochondrial DNA of algae.
It seems reasonable for annotation efforts to rely largely upon previous publications as a reference, however in the case of atp4, our understanding of gene function surpassed the nomenclatural usage. The annotation of the TatC gene as SecY may have been the result of available comparative data or knowledge of mitochondrial translocase proteins at the time of the initial publication. In this effort to correct the course of mitochondrial genome annotations we support following the recommendations of Burger et al. (2003) that all ymf39 annotations in Florideophyceae mitochondria be updated to atp4 to reduce further confusion. Additionally, it is recommended that SecY and ymf16 annotations be changed to TatC.
The Use of Alternative Initiation-Codons
The most widespread initiation-codon in Florideophyceae mitochondrion genes is ATG, though some exceptions have been previously proposed (table 4). For example, in the Grateloupia angusta mitochondria the use of ATT, TTA, or TTG as initiation-codons was reported for nine genes (Kim et al. 2014). Further examination of the published Gra. angusta mitochondrial genome revealed that seven of the genes reported with an alternative initiation-codon (atp4 (as ymf39), atp6, cox2, orf-Gang5, rps11, sdhB, and sdhC), an ORF starting with ATG could be found within a few basepairs of the previously annotated gene, and the current Gra. angusta atp4 (as ymf39) gene annotation on GenBank does use an ATG start-codon. The reasoning behind the decision to opt for an alternative codon rather than ATG at the beginning of the gene was not described in the genome announcement.
Alternative start-codons have been suggested in a few other Florideophyceae mitochondrion genes besides Gra. angusta. For example, in A. taxiformis the atp4 gene is annotated with the initiation-codon ATT, yet 6 bp (two amino acid residues) away in the same reading frame is an ATG, which could also serve as the initiation-codon (table 4). The Gra. angusta and Sp. durum copies of atp6 are both annotated to start with ATT codons that are 9 and 3 bp (3 and 1 amino acid residues), respectively, upstream of an ATG (table 4). A complete assessment of Florideophyceae mitochondrion genes that have been annotated using non-ATG protist mitochondrion initiation-codons and their proximity to a potential ATG start-codon is shown in table 4.
Even though some of the alternative start-codon usage is questionable, though not necessarily incorrect, there appear to be several Florideophyceae mitochondrion genes that likely are using alternative start-codons (table 4). In several of these cases the alternative hypothesis is that the genes are severely truncated and have been rendered non-functional. For example, it seems much more reasonable to believe that S. flabellata utilizes ATT as a start-codon for cob as opposed to losing the need to transcribe the first 42 amino acids of the protein. A similar situation occurs with the sdhB gene in Gra. angusta, where reliance on an ATG initiation-codon would result in the first 49 amino acids not being transcribed. Furthermore, maintaining a functional TatC (annotated as SecY) and rpl16 in the Gra. angusta mitochondrion is dependent on the use of alternative initiation-codons (ATT and TTA, respectively) (table 4).
The Ch. crispus TatC (annotated as ymf16) gene is also likely reliant on an alternative start codon. Currently the Ch. crispus TatC gene is annotated with a GTT initiation codon. However, GTT has not been used as an initiation codon in any other Florideophyceae mitochondrion gene, nor is it one of the start codon options in translation table 4 (Protozoa Mitochondrion). Four other ORFs in the same reading frame that use either ATA or TTA as a start codon for TatC gene are found from 12 to 39 nucleotides downstream of the GTT codon. All Ch. crispus ORFs that can be reasonably attributed to TatC use a start-codon other than ATG, suggesting that this is another reliable instance for invoking an alternative, however the use of GTT is questionable.
Why Were Genes Reported Missing?
There are several technical and biological reasons that could explain the previous results of missing genes in Florideophyceae mitochondrial genomes. Each cell maintains numerous mitochondria and some of these may in fact maintain the frameshift mutations that have led to gene losses being reported in published literature (Hancock et al. 2010; Yang et al. 2015). Preferential amplification of these mitochondrial genomes, or segments of the genome when using targeted PCR, would lead to the aforementioned findings even in cases where other mitochondria in the cell remain fully functional. The first four Florideophyceae mitochondrial genomes to be sequenced were completed primarily using nuclease digestions or PCR amplification and Sanger sequencing methods to assemble the genome at ∼2× depth (Leblanc et al. 1995; Hancock et al. 2010). The advances in sequencing technologies and reduction in costs since the Ch. crispus mitochondrion was first sequenced over 20 years ago have enabled much greater sequencing depths. For example, the V. lanosa mitochondrial genome published here has average read coverage of 391×. This increased depth allows for the correction of “errors” either in the biology or technical aspects of sequencing by utilizing the deeper coverage of sequencing reads when forming a consensus sequence. It is noteworthy that all frameshift mutations that have been reported and led to missing genes, pseudogenes or the inversion of the G. andersonii rps11 were found in long homopolymer regions. Assembling sequences containing long regions of low-complexity, often dominated by a single nucleotide, has been recognized as a major complication for sequencing (Kieleczawa 2006; Laehnemann et al. 2016) and was especially difficult for the 454 FLX technology used in Hancock et al. (2010) (Gilles et al. 2011; Loman et al. 2012).
RNA data can sometimes help identify problematic annotation or assembly. The apparent atp8 and sdhC pseudogenes observed in their Gr. oryzoides DNA data were confusing to the authors as they noted that both genes were still being transcribed based on RNA sequencing efforts (Hancock et al. 2010). However, transfer to the nucleus was invoked at the time as a possible explanation. In retrospect, the RNA data was a strong indication to reexamine the data assembly. The sequencing errors reported in the first few published red algal mitochondrial genomes formed the foundation that was used as a reference for the annotation of subsequently sequenced Florideophyceae mitochondrial genomes. The apparent flexibility of mitochondrial genomes based on early sequencing efforts set a precedent for gene loss in Florideophyceae mitochondria. Building on a flawed foundation has allowed for the gene loss to be overstated without a deeper reanalysis of results. This is in no way meant as a criticism of the researchers themselves and it is plausible that results shown in data being published with current technology will be revised with future advances.
Conclusions
A detailed investigation of previously reported gene losses in Florideophyceae mitochondria reveals that losses are much less common and widespread than the published literature indicates. Prior to this study there genes had been either described as lost, or annotations were overlooked from 18 of the 30 published mitochondrial genomes (tables 1 and 3). Thoroughly examining each loss using the available published sequence data, in combination with resequencing those specimens that we could obtain material from, has positively identified 23 of the “missing” genes. Overwhelmingly, the “missing” genes or pseudogenes were the result of overlooked ORFs in the available sequence data and artificial frameshift mutations that resulted from sequencing and/or downstream assembly and analysis. In light of these findings, it is essential to thoroughly investigate results that indicate genes are degrading into pseudogenes or being lost entirely.
The Ce. japonicum mitochondrion was described as having lost five genes (atp4, rpl20, sdhC, sdhD, and TatC) of which three were identified here. Additionally, the translation of the existing annotation of the nad3 gene shares no homology with any other gene, though that homology is restored through the manual deletion of a “T” in a low complexity region of that gene. Furthermore a gap in the sequence is annotated between cob and nad6. Resequencing this mitochondrion to confirm the presence or absence of the rpl20 and TatC, and close the gap is essential prior to inferring biological relevance resulting from these losses. However it does seem likely that at least rpl20 remains absent from the Ce. japonicum mitochondrion considering it has also been truncated in all other Ceramiales mitochondrial genomes.
It is logical that gene losses would be rare in red algal mitochondria since the core genes encoded are essential for cellular respiration and oxidative phosphorylation. The loss of genes involved in these processes would interfere with the organisms’ ability to produce cellular energy and would likely be a lethal mutation. The ribosomal proteins rps11 and rpl20 have been lost in the mitochondria of other lineages (Burger and Nedelcu 2012) and may be examples of gene transfer from the mitochondrion to the nucleus. Additional red algal genome data will allow for the identification of nuclear-encoded, mitochondrial target proteins.
Acknowledgments
Funding for this work was provided to CL under grant no. 1257472 from the National Science Foundation. This research is based in part upon work conducted using the Rhode Island Genomics and Sequencing Center which is supported in part by the National Science Foundation (MRI grant no. DBI-0215393 and EPSCoR Grant Nos. 0554548 and EPS-1004057), the US Department of Agriculture (Grant Nos. 2002-34438-12688 and 2003-34438-13111), and the University of Rhode Island.
Literature Cited
- Bayley J-P, Devilee P, Taschner PEM. 2005. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med Genet. 6:39.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley J-P, et al. 2006. Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet. 7:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Yoon HS, Hackett JD. 2004. Photosynthetic eukaryotes unite: endosymbiosis connects the dots. Bioessays. 26:50–60. [DOI] [PubMed] [Google Scholar]
- Blouin NA, Lane CE. 2012. Red algal parasites: models for a life history evolution that leaves photosynthesis behind again and again. Bioessays 34:226–235. [DOI] [PubMed] [Google Scholar]
- Blouin NA, Lane CE. 2015. Red algae provide fertile ground for exploring parasite evolution. Perspect Phycol. 3:11–19. [Google Scholar]
- Burger G, Gray MW, Forget L, Lang BF. 2013. Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jakobid protists. Genome Biol Evol. 5:418–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G, Lang BF, Braun HP, Marx S. 2003. The enigmatic mitochondrial ORF ymf39 codes for ATP synthase chain b. Nucleic Acids Res. 31:2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger G, Nedelcu AM. 2012. Mitochondrial Genomes of Algae In: Bock R, Knoop V, editors. Genomics of Chloroplasts and Mitochondria (Advances in photosynthesis and respiration). Dordrecht (The Netherlands): Springer, p. 127–157. [Google Scholar]
- Burger G, Saint-Louis D, Gray MW, Lang BF. 1999. Complete sequence of the mitochondrial DNA of the red alga Porphyra purpurea. Cyanobacterial introns and shared ancestry of red and green algae. Plant Cell 11:1675–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciola EN, Popolizio TR, Schneider CW, Lane CE. 2010. Using molecular-assisted alpha taxonomy to better understand red algal biodiversity in Bermuda. Diversity 2:946–958. [Google Scholar]
- Elorza A, et al. 2004. Nuclear SDH2-1 and SDH2-2 genes, encoding the iron-sulfur subunit of mitochondrial complex II in Arabidopsis, have distinct cell-specific expression patterns and promoter activities. Plant Physiol. 136:4072–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, et al. 2010. The Pfam protein families database. Nucleic Acids Res. 38:D211–D222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, et al. 2015. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44:D279–D285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles A, et al. 2011. Accuracy and quality assessment of 454 GS-FLX Titanium pyrosequencing. BMC Genomics 12:245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould SB, Waller RR, McFadden GI. 2008. Plastid evolution. Annu Rev Plant Biol. 59:491–517. [DOI] [PubMed] [Google Scholar]
- Gray MW. 2012. Mitochondrial Evolution. Cold Spring Harb Perspect Biol. 4:a011403.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MW, Burger G, Lang BF. 1999. Mitochondrial evolution. Science 283:1476–1482. [DOI] [PubMed] [Google Scholar]
- Gray MW, Lang BF, Burger G. 2004. Mitochondria of protists. Annu Rev Genet. 38:477–524. [DOI] [PubMed] [Google Scholar]
- Guiry MD, Guiry GM. 2016. Algaebase. Ireland, Galway: World-wide Electron. Publ. Natl. Univ; Avialable from: http//www.algaebase.org. searched on 21 July 2016. [Google Scholar]
- Hancock L, Goff LJ, Lane CE. 2010. Red algae lose key mitochondrial genes in response to becoming parasitic. Genome Biol Evol. 2:897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughey JR, Boo GH. 2016. Genomic and phylogenetic analysis of Ceramium cimbricum (Ceramiales, Rhodophyta) from the Atlantic and Pacific Oceans supports the naming of a new invasive Pacific entity Ceramium sungminbooi sp. nov. Bot Mar. 59:211–222. [Google Scholar]
- Janouškovec J, et al. 2013. Evolution of red algal plastid genomes: ancient architectures, introns, horizontal gene transfer, and taxonomic utility of plastid markers. PLoS One 8:e59001.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnkowska A, et al. 2016. A eukaryote without a mitochondrial organelle. Curr Biol. 26:1274–1284. [DOI] [PubMed] [Google Scholar]
- Kearse M, et al. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling PJ. 2004. Diversity and evolutionary history of plastids and their hosts. Am J Bot. 91:1481–1493. [DOI] [PubMed] [Google Scholar]
- Keeling PJ. 2010. The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond B Biol Sci. 365:729–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieleczawa J. 2006. Fundamentals of sequencing of difficult templates-An overview. J Biomol Tech. 17:207–217. [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Yang EC, Boo SM, Yoon HS. 2014. Complete mitochondrial genome of the marine red alga Grateloupia angusta (Halymeniales). Mitochondrial DNA. 25:269–270. [DOI] [PubMed] [Google Scholar]
- Koonin EV. 2010. The origin and early evolution of eukaryotes in the light of phylogenomics. Genome Biol. 11:209.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C, Nelson-Sathi S, Roettger M, Garg S, et al. 2015. Endosymbiotic gene transfer from prokaryotic pangenomes: Inherited chimerism in eukaryotes. Proc Natl Acad Sci. USA 112:10139–10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C, Nelson-Sathi S, Roettger M, Sousa FL, et al. 2015. Endosymbiotic origin and differential loss of eukaryotic genes. Nature 524:427–432. [DOI] [PubMed] [Google Scholar]
- Laehnemann D, Borkhardt A, McHardy AC. 2016. Denoising DNA deep sequencing data-high-throughput sequencing errors and their correction. Brief Bioinform. 17:154–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DW, Verbruggen H, Saunders GW, Vis ML. 2016. Multigene phylogeny of the red algal subclass Nemaliophycidae. Mol Phylogenet Evol. 94:730–736. [DOI] [PubMed] [Google Scholar]
- Lane CE, Archibald JM. 2008. The eukaryotic tree of life: endosymbiosis takes its TOL. Trends Ecol Evol. 23:268–275. [DOI] [PubMed] [Google Scholar]
- Lang BF, Gray MW, Burger G. 1999. Mitochondrial genome evolution and the origin of Eukaryotes. Annu Rev Genet. 33:351–397. [DOI] [PubMed] [Google Scholar]
- Leblanc C, Kloareg B, Loiseaux-de Goër S, Boyen C. 1995. DNA sequence, structure, and phylogenetic relationship of the mitochondrial small-subunit rRNA from the red alga Chondrus crispus (Gigartinales, Rhodophytes). J Mol Evol. 41:196–202. [DOI] [PubMed] [Google Scholar]
- Le Gall L, Saunders GW. 2010. DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. J Phycol. 46:374–389. [Google Scholar]
- Loman NJ, et al. 2012. Performance comparison of benchtop high-throughput sequencing platforms. Nat Biotechnol. 30:434–439. [DOI] [PubMed] [Google Scholar]
- Martin W, et al. 1998. Gene transfer to the nucleus and the evolution of chloroplasts. Nature 393:162–165. [DOI] [PubMed] [Google Scholar]
- Martin WF, Garg S, Zimorski V. 2015. Endosymbiotic theories for eukaryote origin. Philos Trans R Soc B. 370:20140330.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, et al. 2013. Assessing the bacterial contribution to the plastid proteome. Trends Plant Sci. 18:680–687. [DOI] [PubMed] [Google Scholar]
- Qiu H, Price DC, Yang EC, Yoon HS, Bhattacharya D. 2015. Evidence of ancient genome reduction in red algae (Rhodophyta). J Phycol. 51:624–636. [DOI] [PubMed] [Google Scholar]
- Salomaki ED, Lane CE. 2014. Are all red algal parasites cut from the same cloth?. Acta Soc Bot Pol. 83:369–375. [Google Scholar]
- Saunders GW. 1993. Gel purification of red algal genomic DNA: an inexpensive and rapid method for the isolation of polymerase chain reaction-friendly DNA. J Phycol. 29:251–254. [Google Scholar]
- Saunders GW, McDonald B. 2010. DNA barcoding reveals multiple overlooked Australian species of the red algal order Rhodymeniales (Florideophyceae), with resurrection of Halopeltis J. Agardh and description of Pseudohalopeltis gen. nov. Bot Botanique. 88:639–667. [Google Scholar]
- Schattner P, Brooks AN, Lowe TM. 2005. The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res. 33:686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Keeling PJ. 2015. Mitochondrial and plastid genome architecture: reoccurring themes, but significant differences at the extremes. Proc Natl Acad Sci. USA 112:10177–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller JW. 2007. Plastid endosymbiosis, genome evolution and the origin of green plants. Trends Plant Sci. 12:391–396. [DOI] [PubMed] [Google Scholar]
- Stiller JW, et al. 2014. The evolution of photosynthesis in chromist algae through serial endosymbioses. Nat Commun. 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo DA, Hughey JR. 2016. Organellar genome analysis of the marine red alga Dasya binghamiae (Dasyaceae, Rhodophyta) reveals an uncharacteristic florideophyte mitogenome structure. Mitochondrial DNA Part B. 1:510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanifuji G, Archibald JM, Hashimoto T. 2016. Comparative genomics of mitochondria in chlorarachniophyte algae: endosymbiotic gene transfer and organellar genome dynamics. Sci Rep. 6:21016.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis JN, Ayliffe MA, Huang CY, Martin W. 2004. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 5:123–135. [DOI] [PubMed] [Google Scholar]
- Tucker RP. 2013. Horizontal gene transfer in choanoflagellates. J Exp Zool B Mol Dev Evol. 320:1–9. [DOI] [PubMed] [Google Scholar]
- Verbruggen H, et al. 2010. Data mining approach identifies research priorities and data requirements for resolving the red algal tree of life. BMC Evol Biol. 10:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, et al. 2015. Highly conserved mitochondrial genomes among multicellular red algae of the Florideophyceae. Genome. Biol Evol. 7:2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: Phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24:1586–1591. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Hackett JD, Ciniglia C, Pinto G, Bhattacharya D. 2004. A molecular timeline for the origin of photosynthetic eukaryotes. Mol Biol Evol. 21:809–818. [DOI] [PubMed] [Google Scholar]
- Zimorski V, Ku C, Martin WF, Gould SB. 2014. Endosymbiotic theory for organelle origins. Curr Opin Microbiol. 22:38–48. [DOI] [PubMed] [Google Scholar]