This editorial refers to ‘Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors’†, by X.S. Li et al., on page 814.
The gut microbiota, comprising the trillions of bugs inhabiting the gastrointestinal tract, may be considered a complex bioreactor with several metabolic and immunological effects that extend beyond the gut itself, and, in the last decade, an increasing amount of evidence has linked functions and alterations of the gut microbiota to cardiometabolic diseases.1
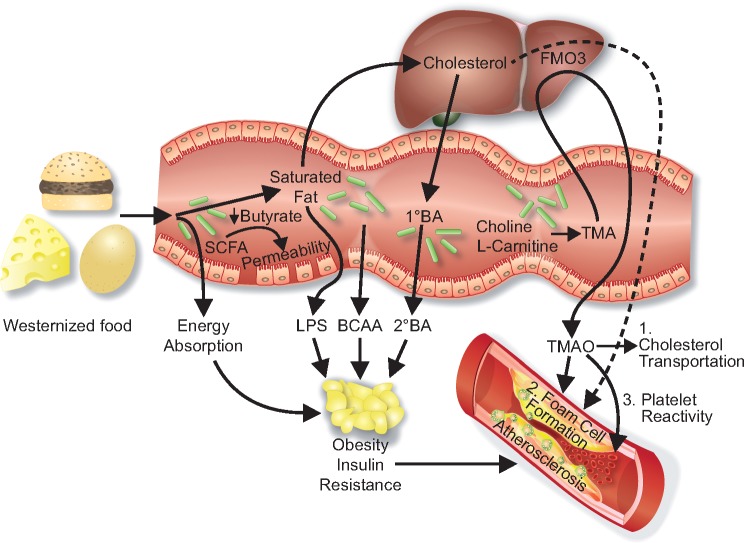
Several mechanisms and interactions between dietary factors, gut microbes, and host metabolism are involved (Figure 1). First, increased absorption of energy from the gut content may contribute to obesity, a trait that is transmissible in animal models, suggesting a vital role for the gut microbiome.3 This effect is partly mediated by short chain fatty acids (SCFAs), which are end-products of microbial fermentation of dietary fibres, and involved in energy harvest from the gut as well as maintaining the integrity of the gut mucosa.1 Hence, reduction of certain SCFAs, in particular butyrate, may result in a dysfunctional gut mucosal barrier, facilitating passive leakage of microbial toxins such as lipopolysaccharides (LPSs) from the gut to surrounding adipose tissue and circulation, triggering inflammation and insulin resistance.1,4 Of note, LPSs may also be actively co-transported across the gut membrane together with triglyceride-rich particles such as chylomicrons.1
Figure 1.
Diet-gut interactions and cardiovascular risk. Bacterial production of short chain fatty acids (SCFAs) may interfere with energy absorption promoting obesity, and gut barrier function promoting leakage of lipopolysaccharides (LPS) to visceral adipose tissue. Microbial metabolites such as branched chain amino acids (BCAAs) and secondary bile acids (BAs) may interfere with several metabolic pathways, contributing to obesity and insulin resistance. Trimethylamine-N-oxide (TMAO) may directly enhance atherosclerosis by interfering with cholesterol transportation and foam cell formation, as well as inducing platelet reactivity, promoting thrombosis and acute coronary events.
In addition, the gut microbes produce a large range of metabolites which act not only in the gut, but also systemically, and this large pool of known and unknown metabolites is not fully explored. As an example, a link between gut microbiota biosynthesis of branched chain amino acids (BCAAs), serum levels of BCAAs, and insulin resistance was recently reported from a large study of serum metabolomics and gut microbiome analyses.5 Furthermore, primary bile acids (BAs) are produced from cholesterol in the liver and transformed in the gut to secondary BAs which regulate numerous metabolic pathways via receptor signalling and by modulating the gut microbiota composition.1
Whereas the above-mentioned mechanisms are mainly linked to obesity and metabolic disturbances, which in turn may contribute to cardiovascular risk, the metabolite trimethylamine N-oxide (TMAO) is the first potentially direct link between the gut microbiota and atherosclerotic heart disease. Trimethylamine (TMA) is produced by the gut microbiota from nutrients containing l-carnitine, choline, and phosphatidylcholine, and is subsequently oxidized by hepatic flavin-containing monooxygenases to TMAO.5 TMAO has been proposed to interfere with cholesterol transportation, and TMAO precursors promote foam cell formation and atherosclerosis in animal models, but not when adding antibiotics to the drinking water, suggesting a microbiota-dependent mechanism.5 Of note, elevated plasma levels of TMAO have, in several independent cohorts from the USA and Europe, been shown to predict incident myocardial infarction, stroke, and all-cause mortality, as well as poor clinical outcome in heart failure patients.5–8
In this issue of the journal, Li and colleagues extend the field further, by investigating TMAO as a potential risk stratification marker in the setting of acute coronary syndromes (ACS).9 In two independent cohorts of patients presenting with ACS in the USA and Switzerland, the upper quartile of TMAO levels were found independently to predict major coronary events (MACE) at different time points. Interestingly, in the US cohort, TMAO predicted incident MACE at 30 days and 6 months beyond traditional risk factors and electrocardiographic data, even in subjects who were initially negative for troponin T. The authors conclude that TMAO measurement may be of clinical utility in risk stratification among subjects presenting with suspected ACS.
So, is it time to start measuring TMAO in the emergency department? One argument favouring this view is the stability of TMAO measurement applying high-performance liquid chromatography, which could give rapid and reproducible TMAO measurements.9 This could in theory be useful for risk stratification, in particular in patients with negative troponin T levels. Of note, such stratification could be mechanistically and clinically relevant in light of recent studies revealing interaction between TMAO and calcium signalling in platelets, which could enhance thrombosis potential in vivo.10
On the other hand, as pointed out by the authors, TMAO has not been predictive of cardiovascular events in all published studies, and, as a biomarker, TMAO is not without its paradoxes. First, whereas TMAO has been proposed as a potentially microbiota-dependent link between Westernized diet including red meat and cardiovascular disease,6 TMAO levels are also elevated in several types of fish and sea food,7 which are considered to be cardioprotective. Interestingly, the US cohort had somewhat higher overall levels of TMAO in the study by Li et al., possibly reflecting dietary differences between the two study populations, opening up the possibility that the prognostic power of TMAO may vary from population to population, depending on background dietary habits.
Secondly, two independent cohorts have reported that TMAO levels increased markedly after bariatric surgery, an intervention known to reduce cardiovascular risk, possibly due to major shifts in microbiota composition resulting in a more aerobic gut environment after surgery.11 Thirdly, TMAO may be confounded by renal and liver function, the first being linked to elimination of TMAO by the kidneys, and the latter being linked to hepatic oxidation of TMA. Hence, other metabolites in the TMAO pathway, such as γ-butyrobetaine which is not dependent on hepatic oxidation and perhaps more directly related to intake of red meat as it is a metabolite of l-carnitine, could be of relevance.12
Although TMAO may have its limitations as a biomarker in certain populations, this should not restrict mechanistically plausible studies with the potential for revealing novel therapeutic principles. Thus, the clinical relevance of TMAO may be strengthened in light of recently developed TMA inhibitors, which in animal models inhibit microbial production of TMA. This ‘drug the bug’ strategy reduces TMAO levels and reverses atherosclerosis in mice, apparently without killing the microbes.13 It remains to be demonstrated in humans that such a strategy is safe and of clinical benefit. In addition to TMA inhibitors, dietary interventions might be highly relevant, as diet shapes the gut microbiota and affects cardiovascular risk. Interestingly, high adherence to a Mediterranean diet was associated with lower urinary TMAO levels, irrespective of background diet (vegan, vegetarian, or omnivorous).14
In this respect it should be noted that the composition of gut microbiota is highly variable from person to person, and one strategy that works in one person will not necessarily work in another. This was elegantly demonstrated in a recent work targeting post-prandial glycaemic responses. It is noteworthy that the glycaemic response to standardized food items was highly variable from individual to individual, but, when stratifying for gut microbiota composition in a computerized meal prediction model, the prediction of individual glycaemic responses was substantially improved.15
Could this variation in the gut microbiota composition be used to stratify treatment in a clinical setting, such as ACS? The next step should be adequately powered clinical trials targeting the gut microbiota and related metabolites, to establish a causal and clinically relevant relationship between the gut microbiome and atherosclerosis. Stratification by TMAO levels to select high-risk candidates for rapid coronary angiography and/or double platelet inhibition could be one approach. TMAO could also be used as a biomarker in clinical trials of TMA inhibitors, dietary interventions, or other strategies for targeting the gut microbiome in order to reduce clinical events. Hence, the work by Li etal. is an important contribution to bringing clinical microbiota medicine closer to the emergency room, so far as a rationale for properly designed clinical trials.
Conflicts of interest: none declared.
References
- 1. Sonnenburg JL, Backhed F.. Diet–microbiota interactions as moderators of human metabolism. Nature 2016;535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI.. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- 3. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R.. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772. [DOI] [PubMed] [Google Scholar]
- 4. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K MetaHIT Consortium Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O.. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016;535:376–381. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL.. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL.. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL.. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal BHalvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A.. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015;277:717–26. [DOI] [PubMed] [Google Scholar]
- 9. Li XS, Obeid S,, Klingenberg R,, Gencer B,, Mach F,, Räber L,, Windecker S,, Rodondi N,, Nanchen D,, Muller O, Melroy X,, Mirand MX,, Matter CM,, Wu Y,, Li L,, Wang Z,, Alamri HS,, Gogonea V,, Chung YM,, Tang WH,, Hazen SL,, Lüscher TM.. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL.. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F.. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab 2015;22: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skagen K, Troseid M, Ueland T, Holm S, Abbas A, Gregersen I, Kummen M, Bjerkeli V, Reier-Nilsen F, Russell D, Svardal A, Karlsen TH, Aukrust P, Berge RK, Hov JE, Halvorsen B, Skjelland M.. The carnitine–butyrobetaine–trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016;247:64–69. [DOI] [PubMed] [Google Scholar]
- 13. Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL.. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 2015;163:1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, Serrazanetti DI, Di Cagno R, Ferrocino I, Lazzi C, Turroni S, Cocolin L, Brigidi P, Neviani E, Gobbetti M, O’Toole PW, Ercolini D.. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 15. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E.. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–1094. [DOI] [PubMed] [Google Scholar]