Abstract
Aims
The aim of this study was to determine the diagnostic performance of single-photon emission computed tomography (SPECT), stress echocardiography (SE), invasive coronary angiography (ICA), coronary computed tomography angiography (CCTA), fractional flow reserve (FFR) derived from CCTA (FFRCT), and cardiac magnetic resonance (MRI) imaging when directly compared with an FFR reference standard.
Method and results
PubMed and Web of Knowledge were searched for investigations published between 1 January 2002 and 28 February 2015. Studies performing FFR in at least 75% of coronary vessels for the diagnosis of ischaemic coronary artery disease (CAD) were included. Twenty-three articles reporting on 3788 patients and 5323 vessels were identified. Meta-analysis was performed for pooled sensitivity, specificity, likelihood ratios (LR), diagnostic odds ratio, and summary receiver operating characteristic curves. In contrast to ICA, CCTA, and FFRCT reports, studies evaluating SPECT, SE, and MRI were largely retrospective, single-centre and with generally smaller study samples. On a per-patient basis, the sensitivity of CCTA (90%, 95% CI: 86–93), FFRCT (90%, 95% CI: 85–93), and MRI (90%, 95% CI: 75–97) were higher than for SPECT (70%, 95% CI: 59–80), SE (77%, 95% CI: 61–88), and ICA (69%, 95% CI: 65–75). The highest and lowest per-patient specificity was observed for MRI (94%, 95% CI: 79–99) and for CCTA (39%, 95% CI: 34–44), respectively. Similar specificities were noted for SPECT (78%, 95% CI: 68–87), SE (75%, 95% CI: 63–85), FFRCT (71%, 95% CI: 65–75%), and ICA (67%, 95% CI: 63–71). On a per-vessel basis, the highest sensitivity was for CCTA (pooled sensitivity, 91%: 88–93), MRI (91%: 84–95), and FFRCT (83%, 78–87), with lower sensitivities for ICA (71%, 69–74), and SPECT (57%: 49–64). Per-vessel specificity was highest for MRI (85%, 79–89), FFRCT (78%: 78–81), and SPECT (75%: 69–80), whereas ICA (66%: 64–68) and CCTA (58%: 55–61) yielded a lower specificity.
Conclusions
In this meta-analysis comparing cardiac imaging methods directly to FFR, MRI had the highest performance for diagnosis of ischaemia-causing CAD, with lower performance for SPECT and SE. Anatomic methods of CCTA and ICA yielded lower specificity, with functional assessment of coronary atherosclerosis by SE, SPECT, and FFRCT improving accuracy.
Keywords: Meta-analysis, Diagnostic accuracy, Cardiac imaging, Fractional flow reserve
Introduction
Fractional flow reserve (FFR), a method to determine hyperemic pressure differences across coronary artery stenosis, is considered the ‘gold standard’ for diagnosis of ischaemia-causing coronary artery disease (CAD).1–3 The use of FFR to guide coronary revascularization, when compared with a coronary stenosis-guided strategy, improves event-free survival.4–6
Numerous cardiac imaging methods exist to diagnosis ischaemia-causing CAD, including single-photon emission computed tomography (SPECT), stress echocardiography (SE), cardiac magnetic resonance imaging (MRI), coronary CT angiography (CCTA), fractional flow reserve derived from CCTA (FFRCT), and invasive coronary angiography (ICA). To date, non-invasive cardiac imaging methods have been assessed for their diagnostic performance largely against an ICA reference standard. Numerous reports7–10 individually examining the performance of cardiac imaging methods against FFR have been hampered by incomplete performance of FFR and mixed FFR-ICA reference standards.11 There is mounting evidence that the assessment of CAD severity by ICA is flawed, because the angiographic severity of a given epicardial stenosis does not necessarily commensurate with its functional significance.12,13 Therefore, assumptions regarding the hemodynamic relevance of stenosis based on their mere angiographic appearance results in erroneous interpretations with important clinical importance. The tailoring of revascularizations according to their angiographic severity of epicardial disease conveys no symptomatic or prognostic benefit to patients and is even detrimental.4–6
We thus performed a meta-analysis comparing cardiac imaging methods for diagnosis haemodynamically significant CAD using FFR as a reference standard.
Methods
PubMed and the ISI Web of Knowledge were systematically searched for published investigations between January 2002 to February 2015 for articles in English using pre-defined search criteria (Table 1). A manual reference check of included articles was performed to identify potential studies missed by our search strategy. Reports that employed duplicative cohorts or overlapping data were excluded (I.D. and C.K.Z.), and the study with the largest population was included. Final screening of reports for inclusion in the meta-analysis was performed by three independent reviewers (I.D., B.L.N., and C.K.Z.).
Table 1.
Search syntax
| Source | Search terms | Filters |
|---|---|---|
| PubMed | (noninvasive fractional flow reserve OR noninvasive FFR OR coronary CT angiography OR coronary computed tomography angiography OR coronary angiography OR nuclear myocardial perfusion OR magnetic resonance perfusion OR myocardial perfusion scintigraphy OR SPECT OR stress echocardiography OR stress perfusion OR stress myocardial perfusion OR dobutamine stress) AND (fractional flow reserve OR FFR) | Humans and clinical trial |
| Web of Science | (‘noninvasive fractional flow reserve’ OR ‘noninvasive FFR’ OR ‘coronary CT angiography’ OR ‘coronary computed tomography angiography’ OR ‘coronary angiography’ OR ‘nuclear myocardial perfusion’ OR ‘magnetic resonance perfusion’ OR ‘myocardial perfusion scintigraphy’ OR ‘SPECT’ OR ‘stress echocardiography’ OR ‘stress perfusion’ OR ‘stress myocardial perfusion’ OR ‘dobutamine stress’) AND (‘fractional flow reserve’ OR ‘FFR’) | Cardiovascular systems and ‘article’ |
FFR, fractional flow reserve; SPECT, single-photon emission computed tomography.
Study eligibility
The inclusion criteria for studies in the analysis were as follows: (i) FFR served as the reference test and was measured in a minimum of 75% of patients, arteries and/or coronary segments included in the analyses of the respective studies. The threshold of 75% was chosen to provide for maximum inclusion of studies in which FFR was directly measured in the patients, vessels, or segments considered in the primary endpoint analysis. This same level has been previously used by Norgaard et al.11
Data collection
Data extraction was initially performed by one reviewer (C.K.Z.) and subsequently verified by the two reviewers (I.D. and B.L.N.). For each eligible study, the following data were collected: year of publication; patient demographics; type of cardiac imaging evaluated; criteria for an abnormal scan defining ischaemia; FFR threshold used to describe ischaemia; and number of patients, vessels and/or segments compared with FFR. For the meta-analysis, absolute numbers of true and false positive, and true and false negative results were extracted from the articles or otherwise derived from the data provided in the articles. The findings were summarized in a 2 × 2 table. Subsequently, studies were grouped according to the cardiac imaging method, which included SPECT, SE, CCTA, FFRCT, MRI, and ICA. If a study compared more than one modality to FFR, each test was evaluated separately. The quality of the included studies was evaluated by two independent reviewers (I.D. and C.Z.) and conformed to the revised version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS).14 Discrepancies in quality assessment were resolved by consensus discussion.
Statistical analysis
Intra-observer agreement between reviewers with regard to the quality assessment of the studies was assessed by the Cohen kappa test. On the basis of the results from the 2 × 2 tables, pooled measures for sensitivity, specificity, diagnostic odds ratio (DOR), and area under the curves (AUC) along with their 95% confidence intervals (CIs) were calculated using DerSimonian Lair methodology.15 Based on the pooled DOR of each index, test summary receiver-operator curves (sROC) were reconstructed using Moses–Shapiro–Littenberg methodology.16 The DOR reflects the ability of a test to distinguish, in this case, haemodynamic and non-heamodynamic significant CAD. A DOR of 1 indicates that the test has no discriminative power. The higher the DOR, the better the diagnostic ability of the imaging modality. To evaluate heterogeneity between studies, a Cochran Q statistic and the I2 index was used. A substantial I2 index indicates heterogeneity beyond sampling variation. A meta-regression analysis was performed to identify pre-defined sources of heterogeneity (age, gender, prevalence of diabetes, prevalence of hypertension, prior myocardial infarctions, prior revascularizations, and multivessel disease). Analyses were performed using Meta-DiSc 1.4.17
Results
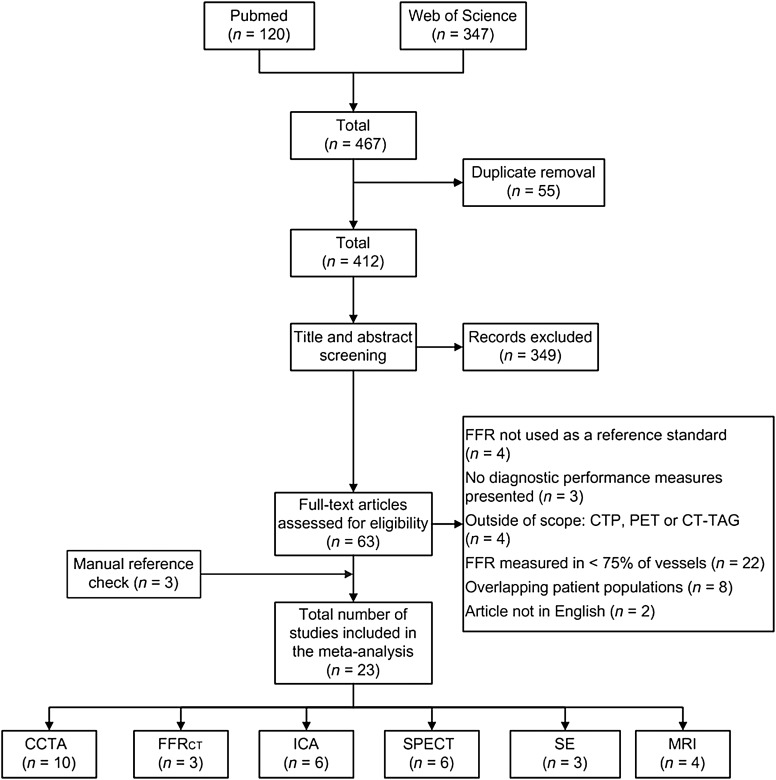
Systemic search resulted in 467 potentially relevant articles. After removal of duplicates and screening by title and abstract, 63 full articles were retrieved and were read full-text. The flowchart of the article search and selection process is demonstrated in Figure 1. A total of 23 eligible studies met the study criteria and were included: 10 CCTA (n = 1167 patients), 3 SE (n = 141 patients), 3 FFRCT (n = 609 patients), 6 ICA (n = 2610 patients), 4 MRI (n = 132 patients), and 6 SPECT studies (n = 282 patients). Inclusion of cardiac imaging methods in each study, along with the demographics of the study populations, is listed in Supplementary material online, Table S1. Finally, 1696 individuals were analysed, with 4740 vessels included for the per-vessel analysis. Haemodynamic significant CAD defined by FFR was identified in 720 (42%) patients and 1613 (34%) arteries. In 18 (78%) studies, FFR values were obtained in all coronary vessels that were included for analysis, with 5 studies obtaining FFR vessels in >75% of coronary vessels (see Supplementary material online, Table S2).
Figure 1.
Flow chart showing the process of literature search and selection algorithm. A total of 23 studies were selected. Of note, there are studies that investigated multiple imaging modalities. FFR, fractional flow reserve; CTP, computed tomography perfusion; PET, positron emission tomography; CT-TAG, CT-derived transluminal attenuation gradient; CCTA, coronary computed tomography angiography; FFRCT, computed fractional flow reserve derived from CCTA; ICA, invasive coronary angiography; SPECT, single-photon emission computed tomography; SE, stress echocardiography; MRI, magnetic resonance imaging.
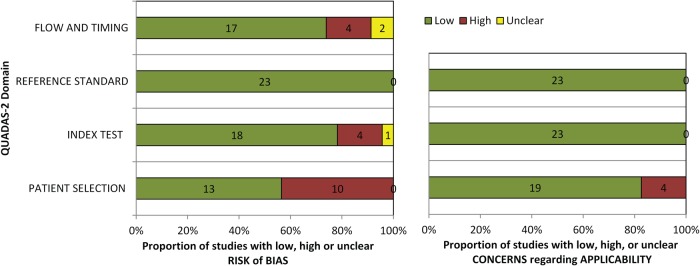
The methodological quality of the included studies was assessed by two independent reviewers using the QUADAS-2 score with a good inter-rater reliability (κ = 0.86). Supplementary material online, Table S3 summarizes the QUADAS-2 quality score for each included study. Supplementary material online, Figure S1 displays the specific quality study items evaluated by the QUADAS-2 tool. The included studies rated generally poor for patient selection, suggesting a high risk of bias and concerns of applicability (Figure 2 and Supplementary material online, Table S3).
Figure 2.
Assessment of methodological quality of included studies using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) revised criteria. Stacked bars represent the number of studies with a low risk of bias (green), unclear risk of bias (yellow), or high risk of bias (red) with regard to patient selection, utilized reference standard, and imaging modality (index test).
Per-patient diagnostic performance of cardiac imaging methods compared with FFR
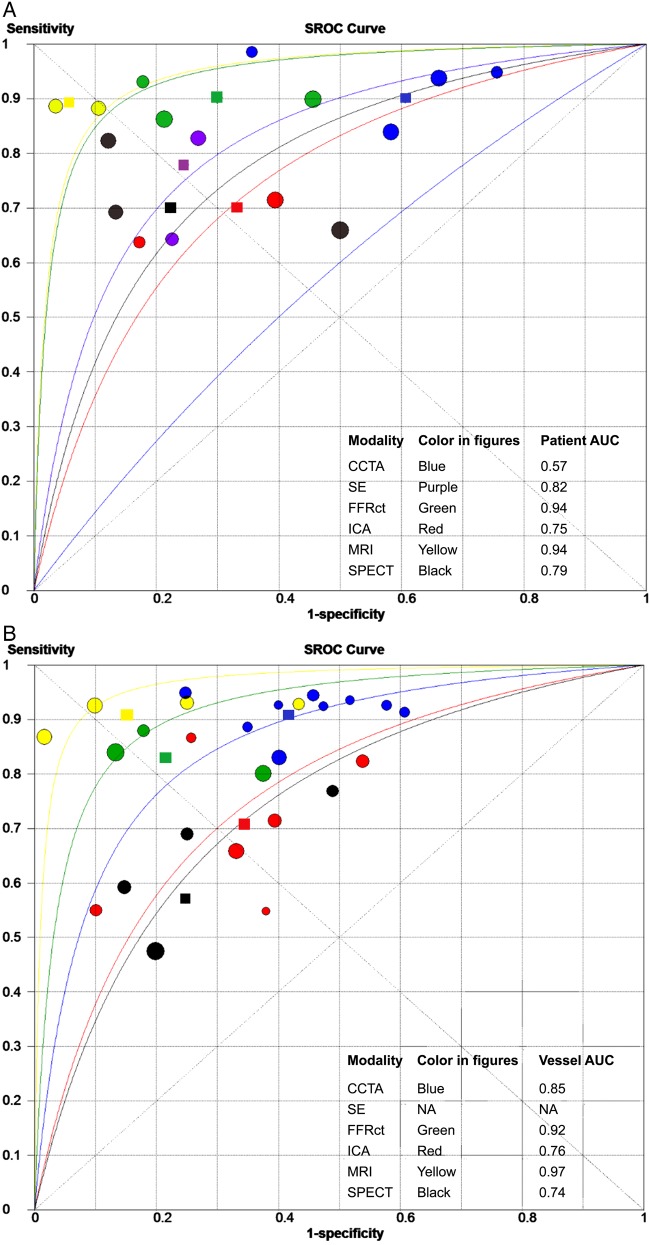
Pooled estimates of sensitivity, specificity, on both a per-patient and per-vessel level are summarized in Table 2. Forest plots for sensitivity and specificity are shown in Supplementary material online, Figure S2. At the patient-level CCTA (90%, 95% CI: 86–93), FFRCT (90%, 95% CI: 85–93), and MRI (90%, 95% CI: 75–97) had the highest sensitivity, with lower sensitivity for SPECT (70%, 95% CI: 59–80), SE (77%, 95% CI: 61–88), and ICA (69%, 95% CI: 65–75). The highest and lowest specificity was seen for MRI (94%, 95% CI: 79–99) and CCTA (39%, 95% CI: 34–44), respectively. Intermediate specificity was observed for SPECT, SE, FFRCT, and ICA (Table 2 and Supplementary material online, Figure S2). The NLR of MRI (12, 95% CI: 5–30), FFRCT (16, 95% CI: 11–23), and CCTA (22, 95% CI: 10–50) were better than SPECT (40, 95% CI: 19–83) and SE (34, 95% CI: 17–66), as described in Table 2. The diagnostic odds ratio (DOR) on a per-patient basis was the highest for MRI and FFRCT (Table 2 and Supplementary material online, Figure S3). Figure 3A depicts the sensitivity and specificity of the individual studies in an ROC space and the summary ROC curves for CCTA, SE, FFRCT, ICA, MRI, and SPECT imaging.
Table 2.
Diagnostic performance of CCTA, SE, FFRCT, ICA, MRI, and SPECT for the detection of haemodynamic significant coronary artery disease
| Index test | Na | Sensitivity | Specificity | PLR | NLR | DOR |
|---|---|---|---|---|---|---|
| Patient-based analysis | ||||||
| CCTA | 694 | 0.90 (0.86–0.93) | 0.39 (0.34–0.44) | 1.54 (1.25–1.90) | 0.22 (0.10–0.50) | 6.91 (2.80–17.03) |
| SE | 115 | 0.77 (0.61–0.88) | 0.75 (0.63–0.85) | 3.00 (1.94–4.65) | 0.34 (0.17–0.66) | 9.51 (3.87–23.38) |
| FFRCT | 609 | 0.90 (0.85–0.93) | 0.71 (0.65–0.75) | 3.34 (1.78–6.25) | 0.16 (0.11–0.23) | 21.94 (9.07–53.07) |
| ICA | 954 | 0.69 (0.65–0.75) | 0.67 (0.63–0.71) | 2.54 (1.25–5.13) | 0.46 (0.39–0.55) | 5.46 (2.54–11.76) |
| MRI | 70 | 0.90 (0.75–0.97) | 0.94 (0.79–0.99) | 10.31 (3.14–33.88) | 0.12 (0.05–0.30) | 92.15 (16.35–519.42) |
| SPECT | 110 | 0.70 (0.59–0.80) | 0.78 (0.68–0.87) | 3.40 (1.04–11.08) | 0.40 (0.19–0.83) | 9.06 (1.48–55.54) |
| Vessel-based analysis | ||||||
| CCTA | 2085 | 0.91 (0.88–0.93) | 0.58 (0.55–0.61) | 2.09 (1.74–2.49) | 0.17 (0.12–0.24) | 13.15 (8.47–20.41) |
| SE | NA | – | – | – | – | – |
| FFRCT | 1050 | 0.83 (0.78–0.87) | 0.78 (0.78–0.81) | 4.02 (1.84–8.80) | 0.22 (0.13–0.35) | 19.15 (5.73–63.95) |
| ICA | 3196 | 0.71 (0.69–0.74) | 0.66 (0.64–0.68) | 2,26 (1.71–2.99) | 0.45 (0.36–0.56) | 5.34 (3.38–8.45) |
| MRI | 371 | 0.91 (0.84–0.95) | 0.85 (0.79–0.89) | 6.16 (2.10–18.02) | 0.11 (0.06–0.20) | 73.53 (22.17–243.82) |
| SPECT | 470 | 0.57 (0.49–0.64) | 0.75 (0.69–0.80) | 2.34 (1.61–3.42) | 0.55 (0.44–0.69) | 4.72 (2.99–7.45) |
PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; NA, not available. Other abbreviations are as in Figure 1.
aNumber of patients might differ from the total patients included in this meta-analysis, due to the difference in studies included in either the patient or vessel-based analysis (Supplementary material online, Tables S4 and S5 provide detailed information on the studies included in the patient- and vessel-based analysis).
Figure 3.
SROC curves of the diagnostic accuracy of cardiac imaging compared with fractional flow reserve. Summary receiver operating characteristic (SROC) curve of the diagnostic accuracy of (A) per-patient and (B) per-vessel data of studies comparing CCTA, FFRCT, ICA, MRI, and SPECT to fractional flow reserve. Each study shows sensitivity and specificity of the different imaging modalities. Area under curve (AUC). The circles show the performance of the separate studies, while the diamond shapes reflect the pooled diagnostic performance of each imaging modality. Abbreviations as in Figure 1.
On a per-patient level, significant heterogeneity for sensitivity was observed for CCTA (I2 = 80%, P < 0.01). Significant heterogeneity for specificity was found for CCTA, FFRCT, ICA, and SPECT. With regard to specificity, SE showed no heterogeneity (I2 = 0%; P = 0.68), whereas slight heterogeneity was seen for MRI (I2 = 54%, P = 0.14) (see Supplementary material online, Figure S2).
Per-vessel diagnostic performance of cardiac imaging methods compared with FFR
At the vessel level, CCTA (91%, 95% CI: 88–93) and MRI (91%, 95% CI: 84–95) exhibited the highest sensitivity, with the lowest sensitivity observed for SPECT (57%, 95% CI: 49–64) (Table 2 and Supplementary material online, Figure S4). The NLR for MRI (11, 95% CI: 6–20), CCTA (17, 95% CI: 12–24), and FFRCT (0.22, 95% CI: 13–35) was better than for SPECT (55, 95% CI: 44–69) and ICA (45, 95% CI: 36–56) (see Supplementary material online, Table S5). With regard to specificity, functional techniques were superior to anatomic methods for diagnosis of ischaemia: MRI (85%, 95% CI: 79–89), FFRCT (78%, 95% CI: 78–81), and SPECT (75%, 95% CI: 69–80) vs. CCTA (58%, 95% CI: 55–61) and ICA (66%, 95% CI: 64–68) (see Supplementary material online, Figure S4). The highest PLR was observed for MRI (6.16, 95% CI: 2.10–18.02) and FFRCT (4.02, 95% CI: 1.84–8.80), with lower values for SPECT (2.34, 95% CI: 1.61–3.42), CCTA (2.09, 95% CI: 1.74–2.49), and ICA (2.26, 95% CI: 1.71–2.99). The per-vessel DOR is shown in Supplementary material online, Figure S5, whereas the summarized ROC curves on a per-vessel basis are shown in Figure 3B.
At the artery level, significant heterogeneity for sensitivity was seen only for ICA studies (I2 = 90%, P < 0.001), whereas the heterogeneity was significant for specificity for all imaging modalities (see Supplementary material online, Figure S4).
Predictors of study heterogeneity
Meta-regression analysis to identify factors impacting heterogeneity was only performed for CCTA, ICA, MRI, and SPECT studies on a per-vessel level, since these were the only imaging modalities that had included more than three studies. Age (P = 0.01) and prevalence of diabetes (P = 0.02) were identified as predictors of heterogeneity for CCTA studies. For the ICA studies, meta-regression analysis revealed that the year of publication (P < 0.01), age (P < 0.01), percentage males (P < 0.01), prevalence of diabetes (P < 0.01), and hypertension (P < 0.01) were independent predictors of heterogeneity. Only prevalence of hypertension was a significant predictor (P = 0.02) of heterogeneity in SPECT.
Discussion
The results of this present meta-analysis show a high performance for MRI for the diagnosis of hemodynamically significant CAD on both a per-patient as well as per-vessel basis, when compared directly with an FFR reference standard. Both CCTA and FFRCT yielded high diagnosis sensitivity, with low specificity for CCTA. Diagnostic performance for SPECT, SE, and ICA was generally poorer.
Our study findings are novel, and directly additive to prior reports.7–10 Specifically, our study differs from prior published investigations in that we constrained our analyses solely to studies that evaluated cardiac imaging methods to an invasive FFR standard. Prior reports—including meta-analyses—have included admixtures of study designs that included individuals undergoing imaging methods that were compared with ICA or to convenience samples that comprised FFR and, when absent, ICA standards.7–10 Indeed, in a recently published meta-analysis by Takx et al., 19 out of the 37 (51%) included studies had measured FFR in <75% of coronary vessels, while the routine interrogation of all arteries by FFR was only performed in 16 (43%) studies.10 As such, the routine assessment of FFR has not been fully exploited yet, while studies have unveiled an important discordance between the angiographic severity of CAD and its haemodynamic significance. Data from the landmark FAME trial taught us that 20% of stenosis in the range of 70–90% were not severe enough to impede coronary flow.12 Similarly, the recently published FAMOUS-NSTEMI trail that has been conducted among 350 patients with non-ST-elevation myocardial infarctions showed discordance between angiography and FFR in 32% of cases.18 Notably, they also found that the 70% threshold by ICA failed to delineate the haemodynamic significance in 47% of epicardial lesions.18 Interestingly, even at both ends of the angiographic spectrum, namely the ‘low’ (<30%) and ‘high-grade’ (>90%) stenosis, assumptions on the functional relevance based on their mere angiographic appearance may be misleading. In fact, abnormal FFR values in the absence of focal epicardial disease are not uncommon and were observed in 18% of coronary arteries by De Bruyne et al.13 On the other hand, 19% of high-grade lesions, which are generally considered flow-limiting, were shown to underestimate the pathophysiologic consequences as indicated by FFR.19 Therefore, the angiographic appearance of coronary atherosclerosis does not always commensurate with its functional significance and may lead to erroneous interpretations with important clinical implications. Indeed, tailoring of revascularizations according to their functional relevance, as indicated by FFR, rather than on their mere angiographic appearance improves event-free survival,4–6 whereas angiography-guided revascularizations convey neither symptomatic nor prognostic benefit to patients and may be even detrimental.4 As such, we restricted our analyses to studies that performed invasive FFR in at least 75% of study subjects to avoid confounding related to a mixed anatomic/physiologic endpoint.
The study results are of considerable importance, given the availability of multiple cardiac imaging methods to diagnosis haemodynamically significant CAD, which serve as guides to consider coronary revascularization. Importantly, our present study findings considerably add to the extant literature, which have largely compared individual modalities with FFR without adequate consideration of the totality of available cardiac imaging methods. These data may inform the use of testing, particularly in the non-invasive setting, to identify individuals who may be appropriately referred for further invasive testing. Traditionally, non-invasive stress tests evaluating myocardial ischaemia have served as the mainstay of diagnosis of CAD, and are a surrogate marker of coronary stenosis that cause ischaemia. More than 10 million stress tests are performed in the USA annually, with SPECT most commonly performed and comprising 90% of stress tests.20,21 Interestingly, both SE (DOR 9.51) and SPECT (DOR 9.06) appeared to be more accurate than ICA (DOR 5.46) for the depiction of lesion-specific ischaemia as reflected by a higher DOR, questioning the validity of prior studies that refereed SE and SPECT against ICA. Surprisingly, ICA exhibits both a low sensitivity (69%) and specificity (67%). This finding emphasizes the role of non-invasive imaging to guide clinical decision-making and questions the role of ICA for the initial diagnostic work-up of patients suspected of CAD. Consequently, the reliance on anatomical measures, even when supplied by ICA, is unreliable (see below), and hence referral to the ‘cath lab’ should be ischaemia-driven.
The present meta-analysis shows that the accuracy of SPECT myocardial perfusion imaging to detect haemodynamic significant CAD as indicated by FFR is moderate. Interestingly, SPECT is performing poorly on a per-vessel level (sensitivity 57 vs. 70% on a per-patient basis), which is arguably attributable to the lack of anatomical information. Indeed, Schindler et al.1 showed a mismatch between SPECT defined myocardial territories and real coronary anatomy in more than half of the cases. Furthermore, in the presence of multivessel CAD, SPECT either misses ischaemia due to the presence of balanced ischaemia or recognizes only the most severe region. As such, in the latter scenario, the other vessel regions are neglected, which may have resulted in the lower sensitivity at the per-vessel region. Notably, the performance of SE in this study was comparable with SPECT, despite the operator-dependent bias of SE. In light of its high diagnostic accuracy, cardiac MRI might become a potential alternative for SPECT and SE. The reasons for MRI superiority are unknown, but may be related to its high spatial resolution, which may encourage the identification of subendocardial ischaemia that may be missed by SPECT. When considering SE, MRI may be superior owing to its diagnostic capabilities at an earlier point than may be present to induce stress-induced regional wall motion abnormalities, which occur conceptually at a later point in the ischaemic cascade.22 Yet, it is important to note that the number of studies directly comparing to FFR were the lowest for MRI, a finding that may underscore the limited availability of MRI to select and skilled centres. Paradoxically, a lower degree of accuracy is expected when cardiac MRI becomes more clinically available. Compared with study protocols, in routine clinical practice inclusion criteria are usually broad, which comes at a cost of lower accuracy. Therefore, large prospective multicentre studies are required to further elucidate the diagnostic value of MRI for the detection of myocardial ischaemia.
Importantly, we observed a high diagnostic sensitivity by CCTA, which is associated with a high NLR, rendering it an excellent technique for the exclusion of hemodynamically significant CAD. However, the specificity of CCTA as well as ICA is low, and emphasizes the discordance between stenosis severity and ischaemia-causing coronary artery lesions. If identification of haemodynamically compromising CAD is the objective, the reliance upon visualized coronary luminal compromise thus appears to be inappropriate. Interestingly, FFRCT emerged as a new tool for the non-invasive diagnosis of ischaemia-causing CAD by applying computational fluid dynamics on conventional CCTA images. In this study, we observed a high sensitivity of FFRCT with moderate specificity, when compared with an invasive FFR reference standard. It is worthy to note that, in the included studies, FFRCT was evaluated in isolation and did not assess the combination of FFRCT to CCTA. This combination would be expected to significantly improve the diagnostic specificity of CCTA, given the coupling of anatomic and functional measures. In this regard, the recently published PLATFORM (Prospective LongitudinAl Trial of FFRct: Outcome and Resource IMpacts) trial demonstrated that the addition of FFRCT to CCTA increased the diagnostic certainty as reflected by a cancellation of 61% of ICAs in patients who were initially planned for an invasive procedure.23 Notably, the incorporation of FFRCT in the diagnostic strategy resulted in a substantially lower ‘cath’ normalcy rate in patients referred for ICA.23 It should be noted, however, that in patients planned for non-invasive testing FFRCT did not result in lower normalcy rates, while associated with higher radiation exposure compared with stress testing or conventional CCTA.2 Therefore, whether FFRCT-guided revascularization improves outcome analogue to invasive FFR remains to be elucidated in future studies.
One particularly concerning finding of the present study is the high heterogeneity between different studies. Particularly, we identified generally biased patient selection, which by meta-analytic measures introduced a high risk of heterogeneity and concerns of applicability of our study findings. While we examined the extant literature and included the highest-quality studies available, this finding nevertheless underscores the need for high-quality, unbiased, prospective multicentre trials, which have the potential to mitigate bias related to patient referral, patient selection and centre expertise. Two such trials are ongoing. The PACIFIC (Prospective CompArison of CardIac PET/CT, SPECT/CT PerFusion imaging and CT Coronary Angiography With Invasive Coronary Angiography) trial is examining the diagnostic performance CCTA, SPECT, and PET against invasive FFR, and is employing traditional metrics such as stenosis severity and myocardial ischaemia to diagnose hemodynamically significant CAD (NCT01521468). Importantly, this study performs these methods on all patients, thus minimizing the errors in diagnostic performance related to differential testing and referral of patients to invasive FFR. The CREDENCE (Computed TomogRaphic Evaluation of Atherosclerotic DEtermiNants of Myocardial IsChEmia) trial distinctly differs from the PACIFIC trial by examining metrics beyond stenosis severity and ischaemia (NCT02173275). Anatomic measures related to atherosclerotic plaque characteristics and aggregate plaque volume are being evaluated for their incremental diagnostic efficacy,24 and the diagnostic value of FFRCT as an adjunct to CCTA is subject of future studies. Similarly, stress testing is being evaluated not only for myocardial ischaemia but will include measures of left ventricular function, stress electrocardiographic findings, and other high-risk markers (such as transient ischaemic dilatation of the left ventricle). The outcomes of these trials will be directly additive to the present study findings.
This study is not without limitations. First, while we considered many of the most commonly employed cardiac imaging tests, we did not include all of them, including positron emission tomography and transluminal attenuation gradients. This was due to the general paucity of data associated with these methods compared with those methods included in the present study. Whether these approaches are superior, or incremental, to the studies tested in this meta-analysis remains to be seen. Second, we did not include assessments by integration of anatomical and functional tests, including for a combination of SPECT, PET, or FFRCT with either CCTA or ICA. However, it is anticipated, given the high negative predictive power of CCTA and high specificity of SPECT, SE, and FFRCT, that by exploiting the synergistic capabilities of multimodality imaging, accuracy will be increased.25 Interestingly, a recently published study by Beuchel et al. reported that, among patients scheduled for elective ICA (n = 7530), the use of SPECT prior to referral to ICA improved the diagnostic yield of ICA beyond clinical risk factors and symptoms.26 Building on this, the mental integration of coronary anatomy as provided by ICA and myocardial perfusion imaging will not only improve ICA's ability to depict lesion-specific ischaemia, but will also shift its focus from a diagnostic tool to a gatekeeper for coronary revascularization. This is a particularly germane topic—particularly for FFRCT—that allows the coupling of coronary stenosis with lesion-specific ischaemia without the need for additional imaging.27 Future studies will be required to evaluate the performance of these hybrid strategies. Third, the studies included in this report were largely retrospective, single centre and small. Patients were often pre-selected for FFR measurements based on angiographic findings, which may improve sensitivity at the cost of specificity. These study characteristics may thus hamper the generalizability of their reported values. Fourth, the pooled studies in our study employed different FFR cut-off values to define ischaemia. Specifically, 8 (32%) studies used 0.75, whereas the majority used the ratio of 0.80 to define ischaemia, which is in line with current guidelines.2,3 Yet, this differential demarcation precludes definitive evaluation of functionally significant CAD in the ‘gray zone’ of FFR, namely that between 0.75 and 0.8. Finally, due to the small number of studies included for each imaging modality, we were unable to evaluate for publication bias.
Conclusion
In this comparative meta-analysis of available cardiac imaging methods directly referenced to FFR, MRI had the highest performance for diagnosis of ischaemia-causing CAD, with lower performance for SPECT and SE. Anatomic methods of CCTA and ICA yielded lower specificity, with functional assessment of coronary atherosclerosis by SE, SPECT, and FFRCT improving accuracy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors' contributions
J.S. and J.W.R.T. performed statistical analysis. I.D., C.K.Z., and B.L.N. acquired the data. J.K.M. handled funding and supervision. I.D., B.L.N, C.K.Z., P.K., and J.K.M. conceived and designed the research. I.D., J.S., B.L.N., C.Z., P.K., J.K.M. drafted the manuscript. I.D., J.S., C.K.Z., B.L.N., C.Z., P.K., and J.K.M. made critical revision of the manuscript for key intellectual content.
Funding
This manuscript was funded, in part, by grants from the National Heart Lung and Blood Institute (R01 HL111141, R01 HL115150 and >R01 HL118019), as well as from a generous gift from the Dalio Foundation. Funding to pay the Open Access publication charges for this article was provided by HeartFlow.
Conflict of interest: C.K.Z. is a co-founder, Senior Vice President of Medical Affairs, and member of the Board of Directors of HeartFlow Inc.; B.L.N. has received institutional research support from Edwards Lifesciences and Siemens; J.K.M. has served on the medical advisory boards of GE Healthcare, Arineta, AstraZeneca, and Bristol-Myers Squibb; on the Speakers Bureau of GE Healthcare; received research support from GE Healthcare, Vital Images, and Phillips Healthcare; serves as a consultant to AstraZeneca, Abbott Vascular, HeartFlow, NeoGraft Technologies, MyoKardia, and CardioDx.
Supplementary Material
References
- 1. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 2. Authors/Task Force Members Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 3. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64:1929–1949. [DOI] [PubMed] [Google Scholar]
- 4. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–224. [DOI] [PubMed] [Google Scholar]
- 5. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B, Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 6. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius-Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nuesch E, Juni P, Investigators FT. Fractional flow reserve-guided PCI for stable coronary artery disease. N Engl J Med 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 7. Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, Nelemans PJ, Schalla S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol 2012;59:1719–1728. [DOI] [PubMed] [Google Scholar]
- 8. Rossi A, Papadopoulou SL, Pugliese F, Russo B, Dharampal AS, Dedic A, Kitslaar PH, Broersen A, Meijboom WB, van Geuns RJ, Wragg A, Ligthart J, Schultz C, Petersen SE, Nieman K, Krestin GP, de Feyter PJ. Quantitative computed tomographic coronary angiography: does it predict functionally significant coronary stenoses? Circ Cardiovasc Imaging 2014;7:43–51. [DOI] [PubMed] [Google Scholar]
- 9. Zhou T, Yang LF, Zhai JL, Li J, Wang QM, Zhang RJ, Wang S, Peng ZH, Li M, Sun G. SPECT myocardial perfusion versus fractional flow reserve for evaluation of functional ischemia: a meta analysis. Eur J Radiol 2014;83:951–956. [DOI] [PubMed] [Google Scholar]
- 10. Takx RA, Blomberg BA, El Aidi H, Habets J, de Jong PA, Nagel E, Hoffmann U, Leiner T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging 2015;81. [DOI] [PubMed] [Google Scholar]
- 11. Norgaard BL, Jensen JM, Leipsic J. Fractional flow reserve derived from coronary CT angiography in stable coronary disease: a new standard in non-invasive testing? Eur Radiol 2015;25:2282–2290. [DOI] [PubMed] [Google Scholar]
- 12. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–2821. [DOI] [PubMed] [Google Scholar]
- 13. De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, Gould KL, Wijns W. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but ‘Normal’ coronary angiography. Circulation 2001;104:2401–2406. [DOI] [PubMed] [Google Scholar]
- 14. Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 16. Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med 1993;12:1293–1316. [DOI] [PubMed] [Google Scholar]
- 17. Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Layland J, Oldroyd KG, Curzen N, Sood A, Balachandran K, Das R, Junejo S, Ahmed N, Lee MM, Shaukat A, O'Donnell A, Nam J, Briggs A, Henderson R, McConnachie A, Berry C; FAMOUS–NSTEMI investigators. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J 2015;36:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, Hanratty C, Banning A, Wheatcroft S, Hobson A, Chitkara K, Hildick-Smith D, McKenzie D, Calver A, Dimitrov BD, Corbett S. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain?: the RIPCORD study. Circ Cardiovasc Interv 2014;7:248–255. [DOI] [PubMed] [Google Scholar]
- 20. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berrington de Gonzalez A, Kim KP, Smith-Bindman R, McAreavey D. Myocardial perfusion scans: projected population cancer risks from current levels of use in the United States. Circulation 2010;122:2403–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leong-Poi H, Rim SJ, Le DE, Fisher NG, Wei K, Kaul S. Perfusion versus function: the ischemic cascade in demand ischemia: implications of single-vessel versus multivessel stenosis. Circulation 2002;105:987–992. [DOI] [PubMed] [Google Scholar]
- 23. Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, Curzen N, Purcell I, Gutberlet M, Rioufol G, Hink U, Schuchlenz HW, Feuchtner G, Gilard M, Andreini D, Jensen JM, Hadamitzky M, Chiswell K, Cyr D, Wilk A, Wang F, Rogers C, De Bruyne B, PLATFORM Investigators. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFRct: outcome and resource impacts study. Eur Heart J 2015;36:3359–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park HB, Heo R, o Hartaigh B, Cho I, Gransar H, Nakazato R, Leipsic J, Mancini GB, Koo BK, Otake H, Budoff MJ, Berman DS, Erglis A, Chang HJ, Min JK. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaemperli O, Bengel FM, Kaufmann PA. Cardiac hybrid imaging. Eur Heart J 2011;32:2100–2108. [DOI] [PubMed] [Google Scholar]
- 26. Buechel RR, Kaufmann BA, Tobler D, Wild D, Zellweger MJ. Non-invasive nuclear myocardial perfusion imaging improves the diagnostic yield of invasive coronary angiography. Eur Heart J Cardiovasc Imaging 2015;16:842–847. [DOI] [PubMed] [Google Scholar]
- 27. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol 2013;61:2233–2241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.