Is there still a need to reduce myocardial infarct size in patients with ST-segment elevation myocardial infarction?
Ischaemic heart disease (IHD) remains the leading cause of death and disability in Europe and worldwide. A major cause of morbidity and mortality in IHD patients is an acute ST-segment elevation myocardial infarction (STEMI), which despite prompt reperfusion by primary percutaneous coronary intervention (PPCI) has significant mortality (7% death at 1 year) and morbidity (22% prolonged or new hospitalization for heart failure at 1 year) in patients with large infarcts.1 When high-risk STEMI patients presenting with cardiogenic shock are not excluded, mortality at 1 year is even higher, at 12% after 1 year.2 As such, there remains an urgent need to discover novel therapies which can be given prior to or at the time of PPCI to reduce myocardial infarct (MI) size in order to preserve left ventricular (LV) systolic function, prevent the onset of heart failure, and improve survival in reperfused STEMI patients. In patients presenting with STEMI, rapid access to the emergency medical services and timely reperfusion by PPCI minimize the total ischaemic time, a major determinant of MI size.
Although myocardial reperfusion is essential to salvage myocardium following a STEMI, the process of restoring coronary blood flow to the ischaemic tissue can, in itself, induce myocardial injury and cardiomyocyte death, a phenomenon which is known as ‘myocardial reperfusion injury’.3,4 Crucially, there is currently no effective therapy for reducing myocardial reperfusion injury in STEMI patients, and therefore, it remains a valid target for cardioprotection. However, the search for an effective therapy capable of targeting myocardial reperfusion injury and reducing MI size has been quite challenging, with a large number of failures to translate novel cardioprotective therapies into the clinical setting.5,6 In this consensus article, we highlight the importance of myocardial reperfusion injury as a viable target for cardioprotection and discuss the potential reasons underlying the neutral results of recent clinical cardioprotection trials and explore the future possibilities for reducing MI size and improving clinical outcomes in patients with IHD.
Why has it been so difficult to prevent myocardial reperfusion injury in patients with ST-segment elevation myocardial infarction?
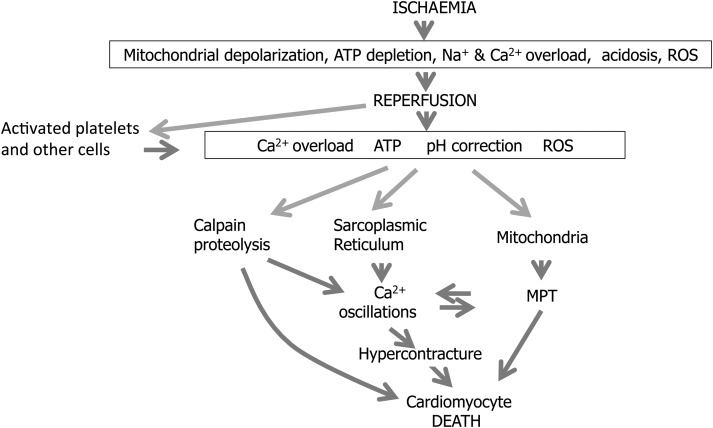
One major factor is an incomplete understanding of the mechanisms underlying myocardial reperfusion injury, with variable reperfusion times, multiple pathophysiological factors (calcium overload, oxidative stress, inflammation, and mitochondrial dysfunction), and multiple players (cardiomyocytes, microvasculature, inflammatory cells, and platelets), making it a complex phenomenon to target effectively.4,7,8 There is general agreement that a large part of the cell death caused by myocardial reperfusion injury occurs during the first few minutes of reperfusion, and that early treatment is required to prevent it.4,7 The most important aspect of reperfusion injury is cardiomyocyte cell death, which depends mainly on phenomena occurring within cardiomyocytes themselves, as it is possible to recapitulate reperfusion injury and demonstrate cardioprotection in isolated cardiomyocytes9 (Figure 1). However, other cells can also contribute to cardiomyocyte cell death during reperfusion injury. This is particularly clear in the case of platelets, the activation and adhesion of which increase cell death independently of aggregation and of any effects on myocardial flow.10 Activated resident cardiac fibroblasts may also exacerbate the local inflammatory reaction and aggravate reperfusion damage to cardiomyocytes.11,12 Microvascular injury and microvascular obstruction may prevent the restoration of myocardial blood flow despite restoration of coronary artery patency in patients with STEMI, and its extent is associated with larger MI size, adverse LV remodelling,13 and worse prognosis,14,15 but up to what a point it is a cause or consequence of the existence of large infarcts needs to be clarified—and it may depend on the circumstances. Furthermore, increased endothelial permeability and subsequent recruitment of inflammatory cells into the site of infarction may also contribute to acute ischaemia/reperfusion injury—a number of clinical studies that have investigated anti-inflammatory therapies administered at the time of reperfusion to reduce MI size have had neutral results.16,17
Figure 1.
Main mechanisms of cardiomyocyte cell death during myocardial reperfusion and their inter-relations.
The mitochondrial permeability transition pore (MPTP) is an important mediator of myocardial reperfusion injury,18 yet several aspects of its role remain obscure. It is not well understood how opening of the MPTP causes sarcolemmal rupture within the first few minutes of reperfusion. A potential link could be the development of hypercontracture, caused by high and oscillating Ca2+ in the presence of ATP.19 Calpain activation occurring upon normalization of intracellular pH in cells with Ca2+ has been demonstrated to contribute to cardiomyocyte death.20 Reactive oxygen species may induce MPTP opening, and interventions attenuating mitochondrial ROS production can prevent MPTP opening and reduce MI size,21 but they also have extra-mitochondrial targets, the importance of which needs to be clarified. A potentially important target of ROS is the tetrahydrobiopterin–eNOS complex, which may be dissociated by oxidation, resulting in peroxynitrite formation and reduced NO availability.22 Recent studies have proposed that RIP3-mediated programmed cell necrosis may play a role in myocardial reperfusion injury through CaMKII and the MPTP.23
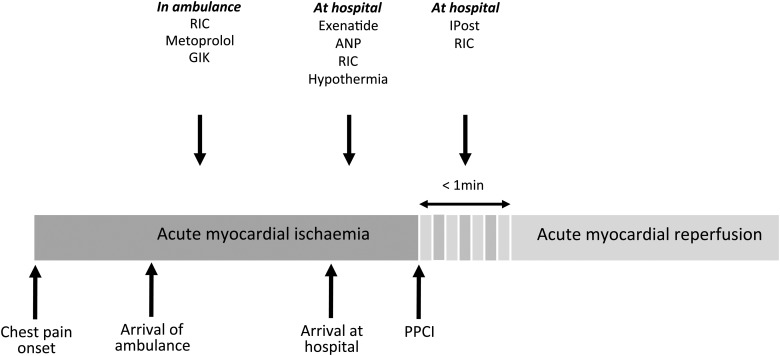
A number of mechanical and pharmacological interventions have been investigated in clinical cardioprotection studies to target myocardial reperfusion injury in reperfused STEMI patients over the last few years—these are discussed in the following sections (Table 1; Figures 1 and 2).
Table 1.
Summary of the data available for several therapeutic interventions for targeting myocardial reperfusion injury and reducing myocardial infarct size
| Ischaemic conditioning |
NO/cGMP pathway |
Mitochondria and MPTP |
Multiple targets |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPost | RIC | ANP | GIK | Exenatide | Nitric oxide and nitrite | MTP-131 | CsA | TRO40303 | PKC-δ inhibition | Hypothermia | Metoprolol | Adenosine | |
| Mechanism of cardioprotection known | + | + | + | + | + | + | + | + | +/− | +/− | + | + | + |
| Pre-clinical data shows consistent cardioprotection | + | + | + | + | + | +/− | + | +/− | +/− | +/− | + | + | + |
| Potential issues over safety | − | − | − | − | − | − | − | − | +/− | − | +/− | +/− | − |
| Clinical MI studies | ++ | ++ | + | +/− | + | +/− | − | +/− | − | − | − | + | +/− |
| Meta-analysis data | + | + | +/− | + | |||||||||
| Clinical outcome studies | * | * | − | * | |||||||||
Mechanism of cardioprotection known: +, known; +/−, not clear.
Pre-clinical data shows consistent cardioprotection: +, consistent cardioprotection; +/−, inconsistent cardioprotection.
Potential issues over safety: −, no known safety issues; +/−, potential safety issues.
Clinical MI studies: ++, several positive MI studies; +, only one positive MI study; +/−, inconsistent MI studies; −, neutral MI studies.
Meta-analysis data: +, positive data; +/−, inconsistent data.
Clinical outcome studies: *, outcome study ongoing; −, neutral outcome study data.
Figure 2.
Various time-windows for applying therapeutic strategies for reducing myocardial infarct size in STEMI patients undergoing PPCI.
Ischaemic post-conditioning
Zhao et al. first reported that brief episodes of ischaemia and reperfusion performed immediately after reflow can limit MI size in the dog heart.24 This novel finding was later confirmed in different experimental models.25,26 Staat et al. and Thibault et al. first demonstrated that comparable cardioprotection could be obtained in STEMI patients with four 1-min cycles alternating inflations and deflations of the angioplasty balloon applied immediately after reopening the culprit coronary artery as evidenced by a reduction in MI size, measured by cardiac enzyme release, SPECT, and cardiac magnetic resonance imaging (MRI).27,28 Several, but not all, Phase II trials have confirmed that ischaemic post-conditioning (IPost) is cardioprotective in STEMI patients admitted with a full coronary artery occlusion.29–32 Reasons for failure of some trials might be related to the absence of direct stenting and delivery of the IPost protocol within the stent with the incumbent risk of coronary micro-embolization.31–33 Specific questions remain as to whether all patients may benefit from IPost given the potential influence of risk factors (e.g. diabetes, age) and concurrent treatments (e.g. anti-platelet agents, statins).34–38
Although none of these studies have reported safety concerns, it remains uncertain whether IPost can improve clinical outcomes in STEMI patients. In this regard, the DANAMI-3 Phase III trial has completed recruitment, and the results are expected this year (NCT01435408).39
Remote ischaemic conditioning
The application of cycles of brief ischaemia and reperfusion to an organ or tissue remote from the heart has been demonstrated to reduce MI size following an episode of acute ischaemia/reperfusion injury, a phenomenon which has been termed remote ischaemic conditioning (RIC).40–44 The ability to recapitulate this cardioprotective effect by simply inflating a blood pressure cuff placed on the upper arm or thigh to induce cycles of brief ischaemia and reperfusion in the upper or lower limb, has facilitated the translation of RIC into the clinical setting, where it has been shown to reduce perioperative myocardial injury but to not improve clinical outcomes in patients undergoing coronary artery bypass graft surgery.45–49
Several clinical studies have found that RIC using transient arm or leg ischaemia/reperfusion reduced MI size by 20–30% (assessed by cardiac enzymes, SPECT or cardiac MRI) in STEMI patients reperfused by either PPCI50–54 or thrombolysis.55 Furthermore, RIC has been reported to improve LV systolic function at four weeks in a subgroup of anterior STEMI patients56 and reduce major adverse cardiac and cerebral events in a follow-up study of 251 STEMI patients.57 It has been shown to be a cost-effective intervention within the first 2 years following PPCI, an effect which was mainly driven by a reduction in hospital re-admissions for heart failure (unpublished data). Finally, post hoc analysis failed to find any major confounding effects of co-morbidities or concomitant medication on the cardioprotective efficacy of RIC in reperfused STEMI patients.58
In summary, RIC using transient limb ischaemia/reperfusion holds promise as an adjunct to PPCI in STEMI patients for reducing MI size. Whether it can improve long-term clinical outcomes is not known and is currently being investigated in the 4300 STEMI patient CONDI-2/ERIC-PPCI clinical study.59
Therapies which target the nitric oxide/cyclic guanosine monophosphate signalling pathway
There is extensive and consistent experimental evidence that nitric oxide/cyclic guanosine monophosphate (NO/cGMP) is reduced in reperfused myocardium, and pharmacological activation of this pathway at the time of reperfusion has been shown to reduce MI size.60 However, there is only one published trial testing the effect of stimulating cGMP synthesis by particulate guanylate cyclase with atrial natriuretic peptide in STEMI—it showed a modest reduction in enzymatic MI size.61 A number of other clinical trials have investigated other therapies which target the NO/cGMP signalling pathway. These include insulin, as part of glucose–insulin–potassium (GIK) therapy which has had mixed results in clinical studies, although the IMMEDIATE trial found that GIK administered in the ambulance reduced MI size in a subset of STEMI patients,62 and other insulin-mimetics such as exenatide.
Exenatide
The anti-diabetic, glucagon-like peptide-1 (GLP-1), has been demonstrated in experimental animal studies to reduce MI size when administered at the onset of reperfusion by mechanisms independent of increased insulin levels.63 As a therapeutic strategy, the GLP-1 analogue, exenatide, has also been shown to protect against myocardial reperfusion injury in small and large animal MI models.64,65 In the clinical setting, an intravenous infusion of exenatide initiated prior to PPCI has been shown to reduce MI size in patients presenting with an acute STEMI, especially in those patients presenting with short ischaemic times from symptom onset (<132 min).66–68 Another GLP-1 analogue, liraglutide, when administered prior to PPCI and continued for 7 days, has been shown in a study of 85 STEMI patients to improve LV systolic function.69
Further studies are now required to determine whether this therapeutic approach can improve clinical outcomes in reperfused STEMI patients.
Nitric oxide and nitrite
Nitric oxide is known to be an important mediator of cardioprotection in various forms of ischaemic conditioning,70 and circulatory nitrite has been demonstrated to be a potential humoral mediator of remote ischaemic preconditioning.71 Although, there have been experimental studies demonstrating cardioprotection with intravenous nitrite administered at the onset of reperfusion,72 the National Heart Lung and Blood Institute (NHLBI) Consortium for preclinicAl assESsment of cARdioprotective therapies (CESAR) Network failed to demonstrate MI size reduction with nitrite using a multi-centre approach in small and large animal MI models.73,74 Two recent clinical studies have failed to demonstrate a significant reduction in MI size with nitrite administered by either the intravenous75 or intracoronary76 routes in STEMI patients treated by PPCI. However, there was a borderline increase in myocardial salvage index and reduced MI size in a subgroup of patients presenting with a fully occluded coronary artery.76
The recent 250 patient NOMI study (NCT01398384) has investigated the role of inhaled nitric oxide (vasoKINOX 450) as an adjunct to PPCI to target myocardial reperfusion injury in STEMI patients. Although no beneficial effect on MI size (Day 3 cardiac MRI) was demonstrated, post hoc subgroup analysis revealed that there was a significant reduction in MI size in those patients who had not received nitrates in the ambulance.
Since there were no adverse events in these trials, further studies on nitrite and nitric oxide appear worthwhile, to test whether this therapeutic approach may yield benefit in a selected patient group.
Cyclosporin A
As a potent inhibitor of MPTP opening, cyclosporin A (CsA) has been shown to significantly reduce MI size in a number of experimental studies,77–79 but not all.80,81 Some, but not all, Phase II clinical trials have suggested that CsA might also protect the heart and brain following a prolonged ischaemic insult.82–86 The recently completed CYCLE trial of 410 STEMI patients failed to demonstrate any benefit with CsA administered prior to PPCI in terms of ST-segment resolution and enzymatic MI size.87 Finally, in the CsA in Reperfused Acute Myocardial Infarction (CIRCUS) 970 patient trial, the administration of CsA immediately prior to PPCI failed to improve clinical outcomes at 1 year (all-cause death, heart failure hospitalization, and adverse LV remodelling) in anterior STEMI patients.1 Why these larger clinical trials failed to confirm the benefit of CsA in reducing MI size from initial Phase II trials is unclear.88,89
Apart from a classical type I error frequently observed in small-size clinical studies, several different causes may have attributed to the neutral results of the CIRCUS trial: (i) CsA is a non-specific inhibitor of cyclophilin D and its other actions (e.g. cyclophilin A and calcineurin inhibition) might have counteracted the benefit of inhibiting MPTP opening.90 (ii) Important changes in STEMI patients since the initial Phase II trial might have played a role, including a greater use of the new P2Y12 platelet inhibitors (prasugrel, ticagrelor), which are known to reduce MI size per se.91 (iii) The concentration of CsA required to inhibit MPTP opening in STEMI patients is not known. The blood concentration of CsA at 4 h after IV bolus administration averaged 533 ± 189 ng/mL in a subset of CIRCUS patients—this was comparable to that observed in the original positive Phase II trial.1,82 (iv) Whether it is enough to inhibit MPTP opening to prevent myocardial reperfusion injury in STEMI patients may be questioned. One may wonder whether much longer ischaemia times observed in humans (when compared with animal models) might alter the binding site or the function of cyclophilin D and render it inaccessible to CsA. This last point may be pertinent in the CIRCUS trial in which total ischaemic times were relatively prolonged at 4.5 h.1
In any case, the failure of CsA to improve clinical outcomes in STEMI patients by no means questions the concept of protection against myocardial reperfusion injury.
MTP-131
The mitochondria-targeting peptide, MTP-131, optimizes mitochondrial energetics and attenuates the production of ROS by selectively targeting cardiolipin in the inner mitochondrial membrane. It has been reported in small and large animal experimental studies to reduce MI size when administered at the onset of reperfusion and prevent adverse LV remodelling following MI.92,93 However, in the 117 patient EMBRACE STEMI clinical trial,94 intravenous MTP-131 administered prior to PPCI failed to reduce enzymatic MI size in a carefully selected population of anterior STEMI patients with ischaemic time <4 h, no collaterals, and fully occluded coronary artery. The reasons for the neutral results of this study are not known, but may include reasons similar to those of other MPTP-targeted interventions (as discussed previously) as well as pharmacokinetic or pharmacodynamic difficulties to target mitochondria in STEMI patients. Clinical trials are currently underway to investigate whether this agent can benefit patients with chronic heart failure.
TRO40303
The mitochondrial targeting drug, TRO40303, which binds to the translocator protein TSPO in the outer mitochondrial membrane and aims to inhibit MPTP opening by attenuating ROS production, has been reported in small animal experimental studies to reduce MI size when administered at time of reperfusion.95 However, in a clinically-relevant large animal MI model, it failed to reduce MI size in the porcine heart.96 In the 163 STEMI patient MITOCARE study,97 this agent failed to reduce MI size despite careful patient selection (completely occluded infarct-related artery, large area-at-risk). Prior experimental studies had revealed ambiguous cardioprotective capacity, and the formulation and dosage of TRO40303 used in the clinical study differed from experimental studies, which may in part explain the neutral findings of the MITOCARE study. Finally, more adverse events were reported in patients receiving TRO40303 when compared with the placebo arm,97 thereby limiting the clinical application of this therapeutic approach.
Protein kinase C-δ inhibition
After Downey et al. first identified protein kinase C (PKC) to be a cytosolic mediator of ischaemic preconditioning protection, the role of the PKC-δ isoform in cardioprotection has been contentious,70 with some studies reporting its genetic98 or pharmacological99 inhibition to be cardioprotective, while other studies finding it to be a mediator of ischaemic preconditioning and opioid cardioprotection.100,101 An initial clinical study (DELTA-MI)102 had suggested that intracoronary delcasertib administered prior to PPCI may be cardioprotective in STEMI patients. However, in the follow-up PROTECT-MI trial, delcasertib was given as an intravenous instead of intracoronary infusion and it failed to reduce MI size in acute anterior STEMI patients.103 A number of factors may have contributed to the neutral results of the PROTECT AMI trial including inconsistent experimental data, inadequate dosing with the intravenous route of administration, and inclusion of patients who had spontaneously reperfused prior to PPCI. Therefore, as a therapeutic strategy, PKC-δ inhibition appears to be limited in its clinical application.
Adenosine
The role of adenosine as a mediator of cardioprotection is well-established,70 with experimental studies demonstrating that adenosine administered prior to index ischaemia can reduce MI size; however, whether it can also reduce MI size when administered at the time of reperfusion has been very contentious.104,105 Unsurprisingly then, the results of clinical studies investigating adenosine as an adjunct to PPCI have also been inconsistent, and this may, in part, relate to patient selection, the variable doses used, and the route of administration (intravenous vs. intracoronary).106 Some studies have reported reductions in MI size with high-dose intravenous adenosine administered as a 3 h infusion initiated prior to reperfusion in STEMI patients presenting within 3 h of chest pain onset,107–109 with other studies using lower doses of IV adenosine or boluses of intracoronary adenosine being less successful at reducing MI size.110 A recent meta-analysis has shown a positive effect of adenosine treatment on heart failure outcomes in reperfused STEMI patients.106 Therefore, larger clinical trials are needed to test whether this therapeutic approach is effective in STEMI patients presenting with shorter ischaemic times.
Therapeutic hypothermia
Therapeutic hypothermia has been consistently shown to reduce MI size in pre-clinical studies.111 Large animal experiments have shown that hypothermia dose-dependently down to 32°C is cardioprotective if initiated during ischaemia but not after reperfusion.112–115 Even prolonged ischaemia to induce hypothermia has been noted to reduce MI size.116 However, early clinical studies (ICE-IT, COOL-MI) failed to demonstrate a benefit of hypothermia, possibly due to slow cooling.117,118 Combination of cold saline and endovascular cooling induced a faster temperature fall and reduced MI size in a 20-patient pilot trial (RAPID MI-ICE), while the larger CHILL-MI trial failed to demonstrate a significant reduction in MI size, although patients presenting within 4 h with an anterior STEMI had a reduction in MI infarct size and there was also a significant reduction in heart failure rate.119,120 It is thought therefore that, in order to translate this therapeutic approach into the clinical setting, new devices capable of delivering faster cooling are needed. This possibility is currently being investigated in anterior STEMI patients in the COOL AMI EU Pilot Trial (NCT02509832), and newer techniques are being developed, which allow non-invasive rapid hypothermia to <32°C in 20 min to be initiated in the ambulance.121
Metoprolol
Intravenous metoprolol administered prior to reperfusion has been shown to reduce MI size and preserve LV systolic function in the porcine heart.122 The mechanisms underlying this cardioprotective effect are currently being investigated and appear to extend beyond their effects on haemodynamics and myocardial oxygen consumption. In the 270 anterior STEMI patient METOCARD-CNIC trial, intravenous metoprolol administered in the ambulance prior to PPCI reduced MI size prevented LV adverse remodelling, preserved LV systolic function, and lowered hospital re-admissions for heart failure.123,124 Results are awaited from the EARLY BAMI trial, which has recently completed recruitment of 600 STEMI patients and which investigated the effect of IV metoprolol or placebo prior to PPCI on MI size by cardiac MRI.125 However, this therapeutic approach may not be suitable for all STEMI, and those with heart failure, hypotensive, or presenting with AV block will not qualify for this therapy.
Whether this therapeutic approach can improve clinical outcome in reperfused STEMI patients will be addressed by the MOVE ON! randomized clinical trial, which will investigate the effect of metoprolol on cardiac death and heart failure hospitalization.
Optimizing approaches to cardioprotection
More rigorous selection of cardioprotective therapy
A number of clinical trials may have failed to demonstrate benefit with some cardioprotective therapies due to inconsistent and/or insufficient experimental data (see Figure 1). In some cases, meta-analyses of experimental studies have been necessary to determine the efficacy of a particular treatment (see Figure 1). Other treatments have been tested in clinical trials without prior experimental studies in large animals. In general, most interventions have been studied only in healthy, young animals, and pre-clinical studies in adult or older animals, with co-morbidities and concomitant medication usually received by patients with STEMI have been lacking.38,126 Among concomitant medication relevant to STEMI patients, platelet inhibitors may be particularly important, as they have been shown to have cardioprotective effects,10,91 which may interfere with cardioprotective interventions.127
In general, studies on novel cardioprotective therapies should be performed only in patients after consistent demonstration of efficacy and absence of safety concerns obtained in adequate small and large animal models in different laboratories using standardized methods. Research networks may be necessary to obtain the necessary level of pre-clinical evidence. In this regard, the NHLBI CESAR network was set up in the USA with this purpose in mind,73,128 and similar networks should be created in Europe.
Optimizing clinical study design
In some instances, the failure of some clinical trials may have been predictable based on issues related to clinical study design.
Patient selection
It is important to select the patients who have been shown in clinical studies who derive the most benefit from an intervention applied as an adjunct to PPCI to reduce MI size; this includes those STEMI patients presenting with the following:
Dosing the intervention
A failure to ascertain the most efficacious dose of the cardioprotective intervention, whether it be a mechanical or pharmacological one, may have contributed to the failure to translate cardioprotection in some of the clinical STEMI studies.
Timing the intervention
The intervention is more likely to be effective at targeting myocardial reperfusion injury in the following circumstances:
There is consistent pre-clinical evidence that the intervention can reduce MI size when administered prior or at the onset of reperfusion, and it has achieved sufficient concentrations in the blood in the first few minutes of reperfusion.
It is important to note that those cardioprotective interventions that are effective only when present during the ischaemic period may act by reducing acute myocardial ischaemic injury.62,123 Limiting ischaemic injury is a very effective strategy to limit MI size, but it may be difficult to apply in STEMI because it requires very early administration, and in patients with a completely occluded artery, the treatment may not be able to reach the ischaemic myocardium. Even when drugs are administered before reperfusion, they may not reach a sufficient concentration in time to protect against the cell death, which occurs in the first few minutes of reflow.
Combination therapy for reducing myocardial infarct size
Using combination reperfusion therapy to target either the different pro-survival signalling pathways within the cardiomyocyte or different proponents of myocardial reperfusion injury (cardiomyocyte, platelets, inflammation, and microvasculature) may provide more effective cardioprotection against myocardial reperfusion injury than a single targeted approach. Alburquerque-Béjar et al.130 found an additional 26% reduction in MI size when combining RIC with insulin-like therapies (such as GIK and exenatide) in a porcine acute MI model. The COMBinAtion Therapy in Myocardial Infarction (COMBAT-MI) study (NCT02404376) will investigate the potential benefits of combined reperfusion therapy using RIC with exenatide on MI size reduction in STEMI patients treated by PPCI. Although an initial clinical study of 54 patients in reperfused STEMI patients failed to show an additive cardioprotective effect with RIC and IPost administered in combination,52 the recently published LIPSIA study of 696 patients reported increased myocardial salvage in those patients administered RIC in combination with IPost when compared with control.54
Future perspectives
Translating cardioprotective therapies for targeting myocardial reperfusion injury from experimental studies into the clinical setting for patient benefit has been extremely challenging. The failure to find an effective agent for preventing myocardial reperfusion injury thus far, however, does not question the existence of myocardial reperfusion injury as a valid target for cardioprotection. Rather it underscores the need to better understand the mechanisms underlying myocardial reperfusion injury. As such experimental studies in this area should continue, as this will allow us to better define effective therapeutic strategies for targeting reperfusion injury to reduce MI size. Currently, an incomplete understanding and lack of appreciation of the complexities of myocardial reperfusion injury has contributed, in part, to the failure to effectively target myocardial reperfusion injury in the clinical setting for patient benefit.
Clinical research in this area should also continue. However, lessons should be learned from recent clinical trials: (i) future clinical trials should be restricted to interventions with consistent experimental data and the latter should include studies in large animals; (ii) clinical study design is crucial when testing novel cardioprotective therapies in STEMI patients; and (iii) only interventions consistently found to be effective at limiting MI size in Phase II clinical trials should be investigated in large clinical outcome trials.
Therapeutic strategies that have potential to improve clinical outcomes in reperfused STEMI patients include remote ischaemic conditioning, exenatide, and metoprolol, and clinical studies are underway to test their efficacy in this regard. New approaches for limiting MI size should include combination therapy to (i) target different cardioprotective signalling pathways within the cardiomyocyte in order to provide additive cardioprotection and (ii) target the different players involved in myocardial reperfusion injury (cardiomyocyte, microvasculature, inflammatory cells, and platelets). These experimental and clinical studies are currently underway and should allow more effective targeting of myocardial reperfusion injury, thereby reducing MI size in reperfused STEMI and preventing the onset of heart failure.
Funding
D.J.H. and D.M.Y. are funded by the British Heart Foundation and the Rosetrees Trust, and are supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre of which D.M.Y. is a senior investigator. D.G.-D. is funded by the Cardiovascular Research Network of the Spanish Institute of Health Instituto de Salud Carlos III (ISCiii RETICS-RIC, RD12/0042/0021). G.H. is supported by the German Research Foundation (He 1320/18-3; SFB 1116 B8). B.I. is funded by the Carlos III Institute of Health and European Regional Development Fund (ERDF/FEDER) (PI13/01979), and the ISCiii Cardiovascular Research Network (RD12/0042/0054). Funding to pay the Open Access publication charges for this article was provided by Red de Investigación Cardiovascular del Instituto de Salud Carlos III, grupo Hospital Universitari Vall d'Hebron (RETICS 2012 RD12/0042/0021).
Conflict of interest: H.E.B. is shareholder of CellAegis Inc. M.O. was a consultant for Neurovive Pharmaceuticals. D.E. has received speaker fees from Zoll. G.H. served as a consultant to Servier. D.G.-D. served as a consultant to Neurovive Pharmaceuticals. R.A.K. serves as a consultant and receives research support from Stealth BioTherapeutics; he is a consultant to Servier, IC Therapeutics/Endothelix, Pfizer, Gilead, Neurovive; he is on the speaker bureau for AMGEN.
References
- 1. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De PF, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le BH, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, Ovize M. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–1031. [DOI] [PubMed] [Google Scholar]
- 2. Campo G, Guastaroba P, Marzocchi A, Santarelli A, Varani E, Vignali L, Sangiorgio P, Tondi S, Serenelli C, De PR, Saia F. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest 2013;144:750–757. [DOI] [PubMed] [Google Scholar]
- 3. Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res 1998;38:291–300. [DOI] [PubMed] [Google Scholar]
- 4. Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357:1121–1135. [DOI] [PubMed] [Google Scholar]
- 5. Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet 2013;381:166–175. [DOI] [PubMed] [Google Scholar]
- 6. Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart 2016;102:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heusch G, Kleinbongard P, Skyschally A, Levkau B, Schulz R, Erbel R. The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc Res 2012;94:237–245. [DOI] [PubMed] [Google Scholar]
- 9. Ruiz-Meana M, Inserte J, Fernandez-Sanz C, Hernando V, Miro-Casas E, Barba I, Garcia-Dorado D. The role of mitochondrial permeability transition in reperfusion-induced cardiomyocyte death depends on the duration of ischemia. Basic Res Cardiol 2011;106:1259–1268. [DOI] [PubMed] [Google Scholar]
- 10. Barrabes JA, Inserte J, Mirabet M, Quiroga A, Hernando V, Figueras J, Garcia-Dorado D. Antagonism of P2Y12 or GPIIb/IIIa receptors reduces platelet-mediated myocardial injury after ischaemia and reperfusion in isolated rat hearts. Thromb Haemost 2010;104:128–135. [DOI] [PubMed] [Google Scholar]
- 11. Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 2011;123:594–604. [DOI] [PubMed] [Google Scholar]
- 12. Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res 2013;99:164–174. [DOI] [PubMed] [Google Scholar]
- 13. Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging 2014;7:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 1998;97:765–772. [DOI] [PubMed] [Google Scholar]
- 15. Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: state of the art. Arch Cardiovasc Dis 2015;108:661–674. [DOI] [PubMed] [Google Scholar]
- 16. Atar D, Petzelbauer P, Schwitter J, Huber K, Rensing B, Kasprzak JD, Butter C, Grip L, Hansen PR, Suselbeck T, Clemmensen PM, Marin-Galiano M, Geudelin B, Buser PT. Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol 2009;53:720–729. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D Jr, O'Neill WW, Todaro TG, Vahanian A, Van De WF. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA 2007;297:43–51. [DOI] [PubMed] [Google Scholar]
- 18. Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol 2015;78C:23–34. [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res 2012;94:168–180. [DOI] [PubMed] [Google Scholar]
- 20. Inserte J, Hernando V, Garcia-Dorado D. Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovasc Res 2012;96:23–31. [DOI] [PubMed] [Google Scholar]
- 21. Valls-Lacalle L, Barba I, Miro-Casas E, Alburquerque-Bejar JJ, Ruiz-Meana M, Fuertes-Agudo M, Rodriguez-Sinovas A, Garcia-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res 2016;109:374–384. [DOI] [PubMed] [Google Scholar]
- 22. Inserte J, Hernando V, Vilardosa U, Abad E, Poncelas-Nozal M, Garcia-Dorado D. Activation of cGMP/protein kinase G pathway in postconditioned myocardium depends on reduced oxidative stress and preserved endothelial nitric oxide synthase coupling. J Am Heart Assoc 2013;2:e005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 2016;22:175–182. [DOI] [PubMed] [Google Scholar]
- 24. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 2003;285:H579–H588. [DOI] [PubMed] [Google Scholar]
- 25. Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation 2005;111:194–197. [DOI] [PubMed] [Google Scholar]
- 26. Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 2009;104:15–18. [DOI] [PubMed] [Google Scholar]
- 27. Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation 2005;112:2143–2148. [DOI] [PubMed] [Google Scholar]
- 28. Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation 2008;117:1037–1044. [DOI] [PubMed] [Google Scholar]
- 29. Roubille F, Mewton N, Elbaz M, Roth O, Prunier F, Cung TT, Piot C, Roncalli J, Rioufol G, Bonnefoy-Cudraz E, Wiedemann JY, Furber A, Jacquemin L, Willoteaux S, Abi-Khallil W, Sanchez I, Finet G, Sibellas F, Ranc S, Boussaha I, Croisille P, Ovize M. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2-3 on admission. Eur Heart J 2014;35:1675–1682. [DOI] [PubMed] [Google Scholar]
- 30. Lonborg J, Kelbaek H, Vejlstrup N, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, Engstrom T. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv 2010;3:34–41. [DOI] [PubMed] [Google Scholar]
- 31. Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X, De Caralt TM, Betriu A, Masotti M. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J 2012;33:103–112. [DOI] [PubMed] [Google Scholar]
- 32. Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, Park JS, Oh JH, Kim SW, Hwang JY, Ryu JK, Park HS, Lim DS, Gwon HC. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation 2013;128:1889–1896. [DOI] [PubMed] [Google Scholar]
- 33. Heusch G. Reduction of infarct size by ischaemic post-conditioning in humans: fact or fiction? Eur Heart J 2012;33:13–15. [DOI] [PubMed] [Google Scholar]
- 34. Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2010;87:406–423. [DOI] [PubMed] [Google Scholar]
- 35. Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol 2010;105:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hausenloy DJ, Erik BH, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res 2013;98:7–27. [DOI] [PubMed] [Google Scholar]
- 37. Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015;65:1454–1471. [DOI] [PubMed] [Google Scholar]
- 38. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 39. Hofsten DE, Kelbaek H, Helqvist S, Klovgaard L, Holmvang L, Clemmensen P, Torp-Pedersen C, Tilsted HH, Botker HE, Jensen LO, Kober L, Engstrom T. The third DANish study of optimal acute treatment of patients with ST-segment elevation myocardial infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only: rationale and design of the DANAMI 3 trial program. Am Heart J 2015;169:613–621. [DOI] [PubMed] [Google Scholar]
- 40. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87:893–899. [DOI] [PubMed] [Google Scholar]
- 41. Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 1997;96:1641–1646. [DOI] [PubMed] [Google Scholar]
- 42. Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 2008;79:377–386. [DOI] [PubMed] [Google Scholar]
- 43. Sivaraman V, Pickard JM, Hausenloy DJ. Remote ischaemic conditioning: cardiac protection from afar. Anaesthesia 2015;70:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol 2015;65:177–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, Yellon DM. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370:575–579. [DOI] [PubMed] [Google Scholar]
- 46. Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 2010;105:657–664. [DOI] [PubMed] [Google Scholar]
- 47. Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med 2015;373:1408–1417. [DOI] [PubMed] [Google Scholar]
- 48. Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med 2015;373:1397–1407. [DOI] [PubMed] [Google Scholar]
- 49. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhauser M, Peters J, Jakob H, Heusch G. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013;382:597–604. [DOI] [PubMed] [Google Scholar]
- 50. Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 2010;375:727–734. [DOI] [PubMed] [Google Scholar]
- 51. Crimi G, Pica S, Raineri C, Bramucci E, De Ferrari GM, Klersy C, Ferlini M, Marinoni B, Repetto A, Romeo M, Rosti V, Massa M, Raisaro A, Leonardi S, Rubartelli P, Oltrona VL, Ferrario M. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc Interv 2013;6:1055–1063. [DOI] [PubMed] [Google Scholar]
- 52. Prunier F, Angoulvant D, Saint EC, Vermes E, Gilard M, Piot C, Roubille F, Elbaz M, Ovize M, Biere L, Jeanneteau J, Delepine S, Benard T, Abi-Khalil W, Furber A. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol 2014;109:400. [DOI] [PubMed] [Google Scholar]
- 53. White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA, Tehrani S, Flett AS, Meier P, Ariti C, Davies JR, Moon JC, Yellon DM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8(Pt B):178–188. [DOI] [PubMed] [Google Scholar]
- 54. Eitel I, Stiermaier T, Rommel KP, Fuernau G, Sandri M, Mangner N, Linke A, Erbs S, Lurz P, Boudriot E, Mende M, Desch S, Schuler G, Thiele H. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: the randomized LIPSIA CONDITIONING trial. Eur Heart J 2015;36:3049–3057. [DOI] [PubMed] [Google Scholar]
- 55. Yellon DM, Ackbarkhan AK, Balgobin V, Bulluck H, Deelchand A, Dhuny MR, Domah N, Gaoneadry D, Jagessur RK, Joonas N, Kowlessur S, Lutchoo J, Nicholas JM, Pauvaday K, Shamloll O, Walker JM, Hausenloy DJ. Remote ischemic conditioning reduces myocardial infarct size in STEMI patients treated by thrombolysis. J Am Coll Cardiol 2015;65:2764–2765. [DOI] [PubMed] [Google Scholar]
- 56. Munk K, Andersen NH, Schmidt MR, Nielsen SS, Terkelsen CJ, Sloth E, Botker HE, Nielsen TT, Poulsen SH. Remote ischemic conditioning in patients with myocardial infarction treated with primary angioplasty: impact on left ventricular function assessed by comprehensive echocardiography and gated single-photon emission CT. Circ Cardiovasc Imaging 2010;3:656–662. [DOI] [PubMed] [Google Scholar]
- 57. Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 2014;35:168–175. [DOI] [PubMed] [Google Scholar]
- 58. Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sorensen HT, Botker HE. Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open 2015;5:e006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hausenloy DJ, Kharbanda R, Rahbek SM, Moller UK, Ravkilde J, Okkels JL, Engstrom T, Garcia Ruiz JM, Radovanovic N, Christensen EF, Sorensen HT, Ramlall M, Bulluck H, Evans R, Nicholas J, Knight R, Clayton T, Yellon DM, Botker HE. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2015;36:1846–1848. [PubMed] [Google Scholar]
- 60. Padilla F, Garcia-Dorado D, Agullo L, Barrabes JA, Inserte J, Escalona N, Meyer M, Mirabet M, Pina P, Soler-Soler J. Intravenous administration of the natriuretic peptide urodilatin at low doses during coronary reperfusion limits infarct size in anesthetized pigs. Cardiovasc Res 2001;51:592–600. [DOI] [PubMed] [Google Scholar]
- 61. Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, Nagai Y, Nanto S, Watanabe K, Fukuzawa S, Hirayama A, Nakamura N, Kimura K, Fujii K, Ishihara M, Saito Y, Tomoike H, Kitamura S. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 2007;370:1483–1493. [DOI] [PubMed] [Google Scholar]
- 62. Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D'Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, Richards ME, Aufderheide TP, Braude DA, Pirrallo RG, Doyle DD, Frascone RJ, Kosiak DJ, Leaming JM, Van Gelder CM, Walter GP, Wayne MA, Woolard RH, Opie LH, Rackley CE, Apstein CS, Udelson JE. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA 2012;307:1925–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hausenloy DJ, Yellon DM. GLP-1 therapy: beyond glucose control. Circ Heart Fail 2008;1:147–149. [DOI] [PubMed] [Google Scholar]
- 64. Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 2008;146:243–249. [DOI] [PubMed] [Google Scholar]
- 65. Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, Pasterkamp G, Hoefer IE. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 2009;53:501–510. [DOI] [PubMed] [Google Scholar]
- 66. Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 2012;33:1491–1499. [DOI] [PubMed] [Google Scholar]
- 67. Lonborg J, Kelbaek H, Vejlstrup N, Botker HE, Kim WY, Holmvang L, Jorgensen E, Helqvist S, Saunamaki K, Terkelsen CJ, Schoos MM, Kober L, Clemmensen P, Treiman M, Engstrom T. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv 2012;5:288–295. [DOI] [PubMed] [Google Scholar]
- 68. Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol 2013;33:2252–2260. [DOI] [PubMed] [Google Scholar]
- 69. Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, Yang JJ, Wang ZF, Tian F, Ning QX. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2015;170:845–854. [DOI] [PubMed] [Google Scholar]
- 70. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015;116:674–699. [DOI] [PubMed] [Google Scholar]
- 71. Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 2014;114:1601–1610. [DOI] [PubMed] [Google Scholar]
- 72. Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 2005;115:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther 2011;16:332–339. [DOI] [PubMed] [Google Scholar]
- 74. Lefer D, Jones S, Steenbergen C, Kukreja R, Guo Y, Tang X-L, Li Q, Ockaili R, Salloum F, Kong M, Polhemus D, Bhushan S, Goodchild T, Chang C, Book M, Du J, Bolli R. Sodium nitrite fails to limit myocardial infarct size: results from the CAESAR cardioprotection consortium. FASEB J 2014;28:LB645. [Google Scholar]
- 75. Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, Frenneaux M. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI). Eur Heart J 2014;35:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jones DA, Pellaton C, Velmurugan S, Rathod KS, Andiapen M, Antoniou S, van ES, Webb AJ, Westwood MA, Parmar MK, Mathur A, Ahluwalia A. Randomized phase 2 trial of intracoronary nitrite during acute myocardial infarction. Circ Res 2015;116:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res 2002;55:534–543. [DOI] [PubMed] [Google Scholar]
- 78. Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 2005;38:367–374. [DOI] [PubMed] [Google Scholar]
- 79. Skyschally A, Schulz R, Heusch G. Cyclosporine A at reperfusion reduces infarct size in pigs. Cardiovasc Drugs Ther 2010;24:85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karlsson LO, Zhou AX, Larsson E, Astrom-Olsson K, Mansson C, Akyurek LM, Grip L. Cyclosporine does not reduce myocardial infarct size in a porcine ischemia-reperfusion model. J Cardiovasc Pharmacol Ther 2010;15:182–189. [DOI] [PubMed] [Google Scholar]
- 81. Lim WY, Messow CM, Berry C. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: a systematic review and meta-analysis. Br J Pharmacol 2012;165:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–481. [DOI] [PubMed] [Google Scholar]
- 83. Ghaffari S, Kazemi B, Toluey M, Sepehrvand N. The effect of prethrombolytic cyclosporine-A injection on clinical outcome of acute anterior ST-elevation myocardial infarction. Cardiovasc Ther 2013;31:e34–e39. [DOI] [PubMed] [Google Scholar]
- 84. Chiari P, Angoulvant D, Mewton N, Desebbe O, Obadia JF, Robin J, Farhat F, Jegaden O, Bastien O, Lehot JJ, Ovize M. Cyclosporine protects the heart during aortic valve surgery. Anesthesiology 2014;121:232–238. [DOI] [PubMed] [Google Scholar]
- 85. Hausenloy D, Kunst G, Boston-Griffiths E, Kolvekar S, Chaubey S, John L, Desai J, Yellon D. The effect of cyclosporin-A on peri-operative myocardial injury in adult patients undergoing coronary artery bypass graft surgery: a randomised controlled clinical trial. Heart 2014;100:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nighoghossian N, Berthezene Y, Mechtouff L, Derex L, Cho TH, Ritzenthaler T, Rheims S, Chauveau F, Bejot Y, Jacquin A, Giroud M, Ricolfi F, Philippeau F, Lamy C, Turc G, Bodiguel E, Domigo V, Guiraud V, Mas JL, Oppenheim C, Amarenco P, Cakmak S, Sevin-Allouet M, Guillon B, Desal H, Hosseini H, Sibon I, Mahagne MH, Ong E, Mewton N, Ovize M. Cyclosporine in acute ischemic stroke. Neurology 2015;84:2216–2223. [DOI] [PubMed] [Google Scholar]
- 87. Latini R, Limbruno U, La Vecchia L, Locuratolo N, Costalunga A, Sixuro M, Lombardi M, Staszewsky L, Masson S, Milani V, Barlera S, Maggioni AP, Ottani F. Effect of cyclosporine a on infarct size reduction in reperfused acute myocardial infarction treated with primary angioplasty. Circulation 2014;130:A15211. [Google Scholar]
- 88. Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury—the search continues. N Engl J Med 2015;373:1073–1075. [DOI] [PubMed] [Google Scholar]
- 89. Heusch G. CIRCUS: a kiss of death for cardioprotection? Cardiovasc Res 2015;108:215–216. [DOI] [PubMed] [Google Scholar]
- 90. Hausenloy DJ, Boston-Griffiths EA, Yellon DM. Cyclosporin A and cardioprotection: from investigative tool to therapeutic agent. Br J Pharmacol 2012;165:1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV. Platelet P2Y(1)(2) blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 2013;18:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol 2014;64:543–553. [DOI] [PubMed] [Google Scholar]
- 93. Shi J, Dai W, Hale SL, Brown DA, Wang M, Han X, Kloner RA. Bendavia restores mitochondrial energy metabolism gene expression and suppresses cardiac fibrosis in the border zone of the infarcted heart. Life Sci 2015;141:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Janosi A, Merkely B, Godlewski J, Halaby R, Korjian S, Daaboul Y, Chakrabarti AK, Spielman K, Neal BJ, Weaver WD. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J 2015;doi.org/10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]
- 95. Schaller S, Paradis S, Ngoh GA, Assaly R, Buisson B, Drouot C, Ostuni MA, Lacapere JJ, Bassissi F, Bordet T, Berdeaux A, Jones SP, Morin D, Pruss RM. TRO40303, a new cardioprotective compound, inhibits mitochondrial permeability transition. J Pharmacol Exp Ther 2010;333:696–706. [DOI] [PubMed] [Google Scholar]
- 96. Hansson MJ, Llwyd O, Morin D, De PD, Arnoux T, Gouarne C, Koul S, Engblom H, Bordet T, Tissier R, Arheden H, Erlinge D, Halestrap AP, Berdeaux A, Pruss RM, Schaller S. Differences in the profile of protection afforded by TRO40303 and mild hypothermia in models of cardiac ischemia/reperfusion injury. Eur J Pharmacol 2015;760:7–19. [DOI] [PubMed] [Google Scholar]
- 97. Atar D, Arheden H, Berdeaux A, Bonnet JL, Carlsson M, Clemmensen P, Cuvier V, Danchin N, Dubois-Rande JL, Engblom H, Erlinge D, Firat H, Halvorsen S, Hansen HS, Hauke W, Heiberg E, Koul S, Larsen AI, Le CP, Nordrehaug JE, Paganelli F, Pruss RM, Rousseau H, Schaller S, Sonou G, Tuseth V, Veys J, Vicaut E, Jensen SE. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J 2015;36:112–119. [DOI] [PubMed] [Google Scholar]
- 98. Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA 2001;98:11114–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Inagaki K, Chen L, Ikeno F, Lee FH, Imahashi K, Bouley DM, Rezaee M, Yock PG, Murphy E, Mochly-Rosen D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart in vivo. Circulation 2003;108:2304–2307. [DOI] [PubMed] [Google Scholar]
- 100. Fryer RM, Wang Y, Hsu AK, Gross GJ. Essential activation of PKC-delta in opioid-initiated cardioprotection. Am J Physiol Heart Circ Physiol 2001;280:H1346–H1353. [DOI] [PubMed] [Google Scholar]
- 101. Mayr M, Metzler B, Chung YL, McGregor E, Mayr U, Troy H, Hu Y, Leitges M, Pachinger O, Griffiths JR, Dunn MJ, Xu Q. Ischemic preconditioning exaggerates cardiac damage in PKC-delta null mice. Am J Physiol Heart Circ Physiol 2004;287:H946–H956. [DOI] [PubMed] [Google Scholar]
- 102. Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N, Mochly-Rosen D, Roe M, Sadowski Z, Solomon S, Widimsky P. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation 2008;117:886–896. [DOI] [PubMed] [Google Scholar]
- 103. Lincoff AM, Roe M, Aylward P, Galla J, Rynkiewicz A, Guetta V, Zelizko M, Kleiman N, White H, McErlean E, Erlinge D, Laine M, Dos Santos Ferreira JM, Goodman S, Mehta S, Atar D, Suryapranata H, Jensen SE, Forster T, Fernandez-Ortiz A, Schoors D, Radke P, Belli G, Brennan D, Bell G, Krucoff M. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI randomized controlled trial. Eur Heart J 2014;35:2516–2523. [DOI] [PubMed] [Google Scholar]
- 104. Olafsson B, Forman MB, Puett DW, Pou A, Cates CU, Friesinger GC, Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation 1987;76:1135–1145. [DOI] [PubMed] [Google Scholar]
- 105. Goto M, Miura T, Iliodoromitis EK, O'Leary EL, Ishimoto R, Yellon DM, Iimura O. Adenosine infusion during early reperfusion failed to limit myocardial infarct size in a collateral deficient species. Cardiovasc Res 1991;25:943–949. [DOI] [PubMed] [Google Scholar]
- 106. Bulluck H, Sirker A, Loke YK, Garcia-Dorado D, Hausenloy DJ. Clinical benefit of adenosine as an adjunct to reperfusion in ST-elevation myocardial infarction patients: an updated meta-analysis of randomized controlled trials. Int J Cardiol 2015;202:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, Orlandi C, Blevins R, Gibbons RJ, Califf RM, Granger CB. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol 1999;34:1711–1720. [DOI] [PubMed] [Google Scholar]
- 108. Ross AM, Gibbons RJ, Stone GW, Kloner RA, Alexander RW. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J Am Coll Cardiol 2005;45:1775–1780. [DOI] [PubMed] [Google Scholar]
- 109. Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J 2006;27:2400–2405. [DOI] [PubMed] [Google Scholar]
- 110. Garcia-Dorado D, Garcia-del-Blanco B, Otaegui I, Rodriguez-Palomares J, Pineda V, Gimeno F, Ruiz-Salmeron R, Elizaga J, Evangelista A, Fernandez-Aviles F, San-Roman A, Ferreira-Gonzalez I. Intracoronary injection of adenosine before reperfusion in patients with ST-segment elevation myocardial infarction: a randomized controlled clinical trial. Int J Cardiol 2014;177:935–941. [DOI] [PubMed] [Google Scholar]
- 111. Herring MJ, Hale SL, Dai W, Oskui PM, Kloner RA. Hypothermia in the setting of experimental acute myocardial infarction: a comprehensive review. Ther Hypothermia Temp Manag 2014;4:159–167. [DOI] [PubMed] [Google Scholar]
- 112. Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol 1996;270(Pt 2):H1189–H1199. [DOI] [PubMed] [Google Scholar]
- 113. Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol 2002;282:H1584–H1591. [DOI] [PubMed] [Google Scholar]
- 114. Gotberg M, Olivecrona GK, Engblom H, Ugander M, van der Pals J, Heiberg E, Arheden H, Erlinge D. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc Disord 2008;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol 2006;101:61–68. [DOI] [PubMed] [Google Scholar]
- 116. Gotberg M, van der Pals J, Gotberg M, Olivecrona GK, Kanski M, Koul S, Otto A, Engblom H, Ugander M, Arheden H, Erlinge D. Optimal timing of hypothermia in relation to myocardial reperfusion. Basic Res Cardiol 2011;106:697–708. [DOI] [PubMed] [Google Scholar]
- 117. Grines CL. Intravascular cooling adjunctive to percutaneous coronary intervention (part 1). Presentation at Transcatheter Cardiovascular Therapeutics, September 2004, Washington DC, USA.
- 118. O'Neill WW, Dixon SR, Grines CL. The year in interventional cardiology. J Am Coll Cardiol 2005;45:1117–1134. [DOI] [PubMed] [Google Scholar]
- 119. Gotberg M, Olivecrona GK, Koul S, Carlsson M, Engblom H, Ugander M, van der Pals J, Algotsson L, Arheden H, Erlinge D. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv 2010;3:400–407. [DOI] [PubMed] [Google Scholar]
- 120. Erlinge D, Gotberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Botker HE, Omerovic E, Engblom H, Carlsson M, Arheden H, Ostlund O, Wallentin L, Harnek J, Olivecrona GK. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol 2014;63:1857–1865. [DOI] [PubMed] [Google Scholar]
- 121. Herring MJ, Dai W, Hale SL, Kloner RA. Rapid induction of hypothermia by the thermosuit system profoundly reduces infarct size and anatomic zone of no reflow following ischemia-reperfusion in rabbit and rat hearts. J Cardiovasc Pharmacol Ther 2015;20:193–202. [DOI] [PubMed] [Google Scholar]
- 122. Ibanez B, Prat-Gonzalez S, Speidl WS, Vilahur G, Pinero A, Cimmino G, Garcia MJ, Fuster V, Sanz J, Badimon JJ. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation 2007;115:2909–2916. [DOI] [PubMed] [Google Scholar]
- 123. Ibanez B, Macaya C, Sanchez-Brunete V, Pizarro G, Fernandez-Friera L, Mateos A, Fernandez-Ortiz A, Garcia-Ruiz JM, Garcia-Alvarez A, Iniguez A, Jimenez-Borreguero J, Lopez-Romero P, Fernandez-Jimenez R, Goicolea J, Ruiz-Mateos B, Bastante T, Arias M, Iglesias-Vazquez JA, Rodriguez MD, Escalera N, Acebal C, Cabrera JA, Valenciano J, Perez de PA, Fernandez-Campos MJ, Casado I, Garcia-Rubira JC, Garcia-Prieto J, Sanz-Rosa D, Cuellas C, Hernandez-Antolin R, Albarran A, Fernandez-Vazquez F, de la Torre-Hernandez JM, Pocock S, Sanz G, Fuster V. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the effect of metoprolol in cardioprotection during an acute myocardial infarction (METOCARD-CNIC) trial. Circulation 2013;128:1495–1503. [DOI] [PubMed] [Google Scholar]
- 124. Pizarro G, Fernandez-Friera L, Fuster V, Fernandez-Jimenez R, Garcia-Ruiz JM, Garcia-Alvarez A, Mateos A, Barreiro MV, Escalera N, Rodriguez MD, de MA, Garcia-Lunar I, Parra-Fuertes JJ, Sanchez-Gonzalez J, Pardillos L, Nieto B, Jimenez A, Abejon R, Bastante T, Martinez dV V, Cabrera JA, Lopez-Melgar B, Guzman G, Garcia-Prieto J, Mirelis JG, Zamorano JL, Albarran A, Goicolea J, Escaned J, Pocock S, Iniguez A, Fernandez-Ortiz A, Sanchez-Brunete V, Macaya C, Ibanez B. Long-term benefit of early pre-reperfusion metoprolol administration in patients with acute myocardial infarction: results from the METOCARD-CNIC trial (Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction). J Am Coll Cardiol 2014;63:2356–2362. [DOI] [PubMed] [Google Scholar]
- 125. Roolvink V, Rasoul S, Ottervanger JP, Dambrink JH, Lipsic E, van der Horst IC, Bart de SB, Kedhi E, Marcel Gosselink AT, Piek JJ, Sanchez-Brunete V, Ibanez B, Fuster V, Van't Hof AW. Rationale and design of a double-blind, multicenter, randomized, placebo-controlled clinical trial of early administration of intravenous beta-blockers in patients with ST-elevation myocardial infarction before primary percutaneous coronary intervention: EARLY beta-blocker administration before primary PCI in patients with ST-elevation myocardial infarction trial. Am Heart J 2014;168:661–666. [DOI] [PubMed] [Google Scholar]
- 126. Lecour S, Botker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB, Ferdinandy P, Heusch G, Madonna R, Ovize M, Ruiz-Meana M, Schulz R, Sluijter JP, van Laake LW, Yellon DM, Hausenloy DJ. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res 2014;104:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M. Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 2012;107:275. [DOI] [PubMed] [Google Scholar]
- 128. Kloner RA, Schwartz LL. State of the Science of Cardioprotection: challenges and opportunities—proceedings of the 2010 NHLBI workshop on cardioprotection. J Cardiovasc Pharmacol Ther 2011;16:223–232. [DOI] [PubMed] [Google Scholar]
- 129. Sorensson P, Saleh N, Bouvier F, Bohm F, Settergren M, Caidahl K, Tornvall P, Arheden H, Ryden L, Pernow J. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart 2010;96:1710–1715. [DOI] [PubMed] [Google Scholar]
- 130. Alburquerque-Bejar JJ, Barba I, Inserte J, Miro-Casas E, Ruiz-Meana M, Poncelas M, Vilardosa U, Valls-Lacalle L, Rodriguez-Sinovas A, Garcia-Dorado D. Combination therapy with remote ischaemic conditioning and insulin or exenatide enhances infarct size limitation in pigs. Cardiovasc Res 2015;107:246–254. [DOI] [PubMed] [Google Scholar]