Abstract
Quantification of the association between the intake of vegetables and fruit and risk of nasopharyngeal cancer (NPC) is controversial. Thus, we conducted a meta-analysis to assess the relationship between vegetables and fruit and NPC risk. Pertinent studies were identified by a search in PubMed, Web of Knowledge and Wan Fang Med Online. Random-effects models were used to calculate summary relative risks (RRs) and the corresponding 95% confidence intervals (CIs). Publication bias was estimated using Egger's regression asymmetry test. Finally, 15 articles comprising 8208 NPC cases were included in this meta-analysis. The combined results showed that there was significant association between vegetables and fruit intake and NPC risk. The pooled RRs were 0.60 (95% CI = 0.47–0.76) for vegetables and 0.63 (95% CI = 0.56–0.70) for fruit. No publication bias was detected. Our analysis indicated that intake of vegetables and fruit may have a protective effect on NPC. Since the potential biases and confounders could not be ruled out completely in this meta-analysis, further studies are needed.
Nasopharyngeal cancer (NPC) is rare in most parts of the world, where incidences of age standardized rates are generally below 1 per 100,000 person-years1. But it is a common malignancy in southern China2. The incidence rate for males is more than 20 per 100,000 person-years and is as high as 25 to 40 per 100,000 person-years in some areas bordering the Xijiang River and the Pearl River2,3,4. The distinctive geographic and ethnic distribution of NPC worldwide suggests that genetic predisposition, dietary and environmental factors, and Epstein-Barr virus (EBV) all have been associated with the pathogenesis of this tumor5.
The intake of fruit and vegetables has long been associated with a decreased risk of various cancers, including NPC. The suggested mechanisms for the major role of vegetables and fruit in the prevention of cancer include: modulation of DNA methylation; protection from and repair of DNA damage; promotion of apoptosis and induction of detoxifying phase-II enzymes6. Up to date, a number of epidemiologic studies have been published to explore the relationship between vegetables and fruit intake and NPC risk. However, the results are not consistent. Therefore, we performed a comprehensive meta-analysis to test the hypothesis that vegetables and fruit intake may be a protective effect on NPC risk.
Methods
Search strategy
A comprehensive search was conducted for available articles published in English or Chinese using the databases of PubMed, Web of Knowledge and Wan Fang Med Online (http://www.wanfangdata.com.cn/) up to November 2013 and by hand-searching the reference lists of the computer retrieved articles. The following search terms were used: ‘nasopharyngeal’ AND (neoplasm OR carcinoma OR cancer) combined with “nutrition OR diet OR lifestyle OR fruit OR vegetable.” Two investigators (JJ and ZO) searched articles and reviewed of all retrieved studies independently. Disagreements between the two investigators were resolved by consensus with a third reviewer (ZW).
Inclusion criteria
All relevant studies reporting the association of vegetables and fruit and NPC risk were considered for inclusion. The inclusion criteria were as follows: (1) use a case-control, nested case-control or cohort design; (2) the exposure of interest were vegetables and fruit or total vegetables or total fruit; (3) the outcome of interest was NPC; (4) report associations in the form of RR with the 95% confidence intervals (CI) for total vegetables or total fruit or providing us with sufficient information to calculate them. Accordingly, the following exclusion criteria were also used: (1) reviews and (2) repeated or overlapped publications. In the present meta-analysis, we included the studies evaluating fruit or vegetable groups classified as “all” or “total.” Exposures presented as cooked vegetables, raw vegetables, other vegetables, citrus fruit or other fruits were not considered as equivalent to “all” or “total” and thus were not included. Studies that reported “fresh vegetables” or “fresh fruit” were included according to the hypothesis that fresh vegetables or fruit accounts for a very high proportion of the total consumption7.
Data extraction
Two researchers (JJ and ZO) independently extracted the following information: the name of the first author, study design, publication year, geographic locations, the number of cases and controls or participants, type of controls, the methods used for collection of data on exposure, exposure classification, confounders adjusted for and the RR estimates with corresponding 95% CI for the highest versus lowest level (Every study has one group for the highest versus the lowest amount). From each study, we extracted the risk estimates adjusted for the greatest number of potential confounders. If there was disagreement between the two investigators about eligibility of the data, it was resolved by consensus with a third reviewer (ZW).
Statistical analysis
The pooled measure was calculated as the inverse variance-weighted mean of the natural logarithm of multivariate adjusted RR with 95% CI for the highest vs. lowest levels to assess the association of vegetables and fruit intake with the risk of NPC. The DerSimo-nian and Laird random effect model was adopted as the pooling method if substantial heterogeneity is present (I2> 50%); otherwise, the fixed effect model (I2<50%) was used as the pooling method8. The Q test and I2 of Higgins and Thompson9 were used to assess heterogeneity among included studies. I2 describes the proportion of total variation attributable to between-study heterogeneity as opposed to random error or chance. Meta-regression with restricted maximum likelihood estimation was performed to describe the potentially important covariates10. If no significant covariates were found to be heterogeneous, the “leave-one-out” sensitive analysis11 was carried out to evaluate the key studies with substantial impact on between-study heterogeneity. Publication bias was estimated using Begg' funnel plot12 and Egger's regression asymmetry test13. A study of influence analysis14 was conducted to describe how robust the pooled estimator is to removal of individual studies. An individual study is suspected of excessive influence if the point estimate of its omitted analysis lies outside the 95% CI of the combined analysis. Study quality was assessed using the 9-star NewcastleeOttawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed 10/14/2013). All analyses were conducted using STATA software, version 10.0 (StataCorp LP, College Station, Texas). Two-tailed P ≤ 0.05 was accepted as statistically significant. For testing the heterogeneity and publication bias, two-tailed P ≤ 0.1 was accepted as statistically significant.
Results
Search results and study characteristics
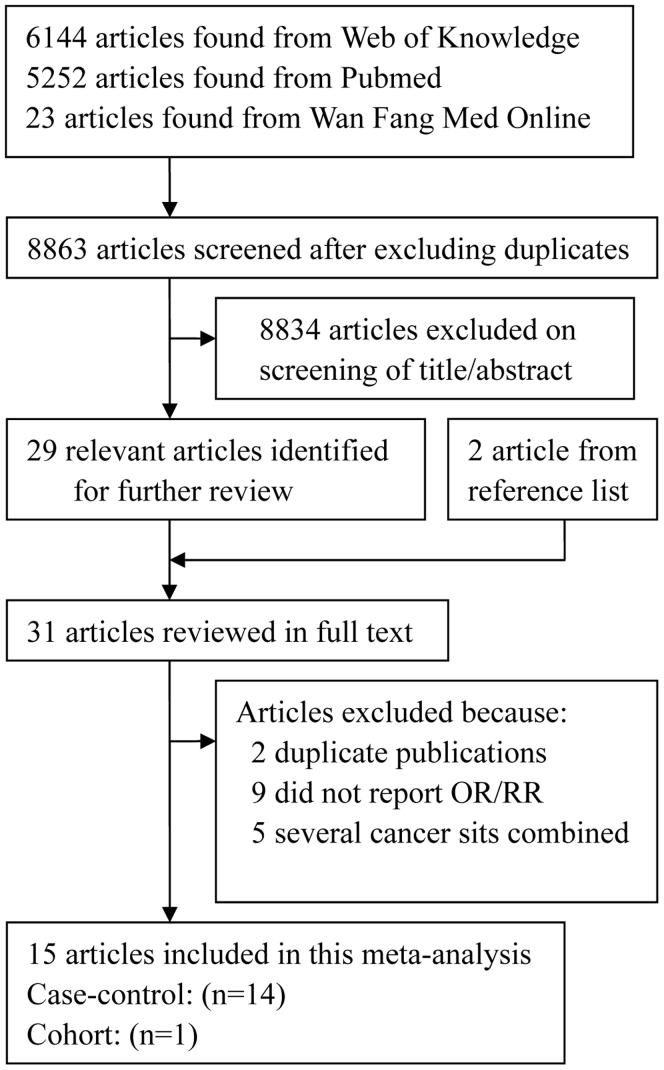
The search strategy identified 5252 articles from Pubmed, 23 articles from Wan Fang Med Online and 6144 articles from the Web of Knowledge, and 29 articles were reviewed in full after reviewing the title/abstract. By studying reference lists, we identified 2 additional articles. Sixteen of these 31 articles were subsequently excluded from the meta-analysis for various reasons. Hence, 15 articles15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 (one prospective and 14 case–control articles) involving 8208 NPC cases were used in this meta-analysis. The detailed steps of our literature search are shown in Figure 1. The characteristics of these studies are presented in Table 1. Twelve articles were from China, one from America, one from Italy and one from Africa.
Figure 1. The flow diagram of screened, excluded, and analyzed publications.
Table 1. Characteristics of studies on vegetables and fruit and NPC risk.
| First author, year | Country | Study design | Cases, age | Quality score | RR (95% CI) for highest versus lowest category | Adjustment or matched for |
|---|---|---|---|---|---|---|
| Polesel et al. 2013 | Italy | Case-control (HCC) | 198,Cases: 52Controls: 52 | 8 | 0.51(0.29–0.90) for vegetable0.68(0.40–1.16) for fruit | Age, sex, place of living, year of interview, education, tobacco, smoking, alcohol drinking, and non-alcohol energy |
| Li et al. 2012 | China | Case-control (HCC) | 100,Cases: 48.2Controls: 48.6 | 7 | 0.19(0.05–0.68) for vegetable and fruit combined | Age, sex |
| Shen et al. 2012 | China | Prospective | 1533,46.1 | 7 | 0.78(0.53–1.14) for fruit | Age, BMI, spouse, education, clinical stage, smoking status, alcohol intake |
| Liu et al. 2012 | China | Case-control (HCC) | 600,Cases: 47.39Controls: 47.34 | 7 | 0.37(0.25–0.55) for vegetable and fruit combined0.33(0.22–0.50) for vegetable0.70(0.47–1.04) for fruit | BMI, educational level, marital status, occupation, household income, occupational and domestic exposure to potential toxic substances, chronic rhinitis history, smoking status, passive smoking, daily energy intake (log-transformed), and energy-adjusted intakes of other food groups (including preserved vegetables, cereals, soybeans, fresh meats, preserved meats, roasted meats, dairy products, nuts and vegetables or fruits) by stepwise forward method |
| Turkoz et al. 2011 | Turkey | Case-control (HCC) | 183,Cases: 44.9Controls: 43.9 | 8 | 0.59(0.38–0.94) for fruit | Age and sex |
| Xu et al. 2010 | China | Case-control (HCC) | 184,Cases: 45.9Controls: 47.7 | 7 | 0.30(0.18–0.50) for vegetable and fruit combined0.44(0.27–0.72) for vegetable0.56(0.34–0.92) for fruit | Age, sex, place of living, occupation, educational level, income, smoking status, daily energy intake |
| Jia et al. 2010 | China | Case-control (HCC) | 1387,Cases: 46.92Controls: 47.34 | 6 | 0.63(0.51–0.77) for fruit | Age, sex, education, dialect and household type |
| Luo et al. 2009 | China | Case-control (PCC) | 1256,Cases: 47Controls: 47.23 | 7 | 0.56(0.45–0.70) for fruit | Age, sex, place of living |
| Feng et al. 2007 | Africa | Case-control (HCC) | 636,15–81 | 7 | 0.6(0.4–0.8) for vegetable | Age, sex, socio-economic status variables and exposure to toxic substances |
| Yuan et al. 2000 | China | Case-control (PCC) | 935,15–74 | 8 | 0.85(0.65–1.10) for vegetable | Age, gender, level of education, cigarette smoking, exposure to smoke from heated rapeseed oil and burning coal during cooking, occupational exposure to chemical fumes and history of chronic ear and nose condition (see text for more detailed description of confounding variables) |
| Ward et al. 2000 | China | Case-control (PCC) | 375,≤75 | 7 | 0.9(0.3–2.6) for vegetable0.9(0.3–2.3) for fruit | Age, gender and ethnicity |
| Armstrong et al. 1998 | China | Case-control (PCC) | 282,Cases: 45.29Controls: 44.82 | 7 | 0.50(0.23–1.07) for vegetable | Age, sex, residence and marital status |
| Farrow et al. 1998 | United States | Case-control (PCC) | 133,18–74 | 8 | 0.99(0.51–1.94) for green vegetable0.59(0.29–1.22) for yellow vegetable0.87(0.41–1.83) for fruit | Age, alcohol consumption (0–6, 7–13, 14–20, or 21+ drinks per week), cigarette smoking (never, former, current with history of 1–34 pack years, current with history of 35–59 pack years or current with history of 60+ pack years), total caloric intake, broccoli, cauliflower, spinach, mustard or turnip greens, coleslaw, winter squash, carrots, yams |
| Ning et al. 1990 | China | Case-control (PCC) | 100,Cases: 44.9Controls: 45.2 | 7 | 0.8(0.3–1.9) for vegetable | Age (yr of birth within 5 yr), sex, and race (Han) |
| Yu et al. 1989 | China | Case-control (PCC) | 306,≤50 | 6 | 0.77(0.20–3.33) for vegetable0.3(0.1–1.1) for fruit | Age, sex |
Abbreviations: PCC = population-based case–control study; HCC: hospital-based case–control study; BMI: Body Mass Index.
Total vegetables
High versus low analyses
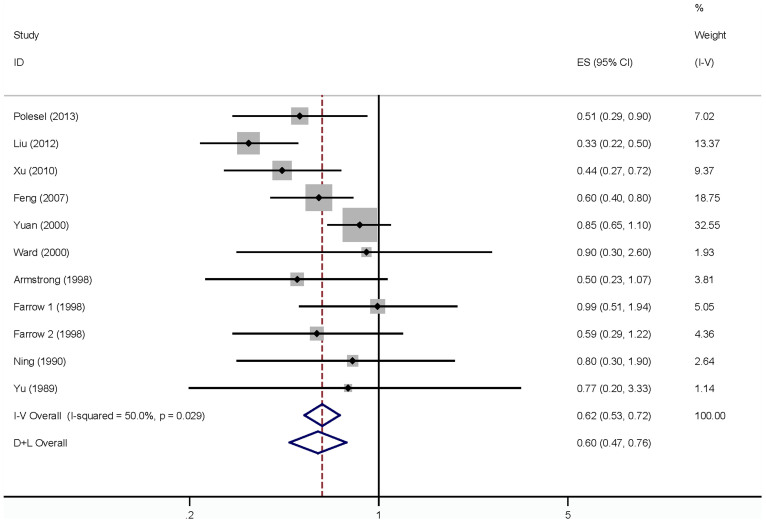
For vegetable intake and NPC, data from 10 articles15,18,20,23,24,25,26,27,28,29 with 11 case-control studies were used including 3749 NPC cases and 4452 controls. Inverse association of vegetable intake with risk of NPC was reported in 4 studies, and no significant association of vegetable intake with risk of NPC was reported in 7 studies. Pooled results suggested that highest vegetable intake versus lowest level was significantly associated with the risk of NPC [summary RR = 0.60, 95% CI = 0.47–0.76, I2 = 50.0%, Pheterogeneity = 0.03] (Figure 2). The power of effect estimates is 0.86 for vegetables while α = 0.05 in this study.
Figure 2. The forest plot between highest versus lowest categories of vegetables intake and NPC risk.
Sources of heterogeneity and subgroup analyses
As seen in Figure 2, evidence of heterogeneity (I2 = 50.0%, Pheterogeneity = 0.03) was found in the pooled results. However, univariate meta-regression analysis, with the covariates of study region, number of cases, and sources of controls showed no covariate having a significant impact on between-study heterogeneity, respectively. The key contributor to this high between-study heterogeneity assessed by the leave-one-out analysis was one study conducted by Liu et al (2012). After excluding this study, heterogeneity was reduced to I2 = 6.9%, and the summary RR for NPC was 0.67 (95% CI = 0.56–0.80; Pheterogeneity = 0.14).
In subgroup analyses for ethnicity, when we restricted the analysis to Asia and Caucasian, the pooled RR of NPC for the highest category of vegetable intake versus the lowest category were 0.58 (95% CI = 0.39–0.85) and 0.65(0.45–0.94), respectively. When we conducted the subgroup analysis by sources of control, number of cases (<200 or ≥200)7, adjustment for smoking or alcohol, the significant associations were found between vegetable intake and NPC in all strata. The main results are summarized in Table 2.
Table 2. Summary risk estimates of the association between vegetables and fruit and NPC risk.
| Sub-groups | Vegetables | Fruit | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n | RR(95%CI)a | Pb | Q value | I2 (%) | Pc | Pd | Studies, n | RR(95%CI)a | Pb | Q value | I2 (%) | Pc | Pd | |
| All | 11 | 0.60(0.47–0.76) | 0.00 | 20.0 | 50.0 | 0.03 | 0.81 | 10 | 0.67(0.58–0.70) | 0.00 | 9.0 | 0.0 | 0.78 | 0.60 |
| Case-control | 11 | 0.60(0.47–0.76) | 0.00 | 20.0 | 50.0 | 0.03 | 0.81 | 9 | 0.61(0.54–0.69) | 0.00 | 8.0 | 0.0 | 0.84 | 0.68 |
| Sources of control | ||||||||||||||
| Population-based | 7 | 0.80(0.65–0.99) | 0.04 | 6.0 | 0.0 | 0.84 | 0.43 | 4 | 0.58(0.47–0.71) | 0.00 | 3.1 | 3.3 | 0.38 | 0.75 |
| Hospital-based | 4 | 0.47(0.380.58) | 0.00 | 4.9 | 38.9 | 0.18 | 0.51 | 5 | 0.63(0.54–0.74) | 0.00 | 4.0 | 0.0 | 0.96 | 0.47 |
| Ethnicity | ||||||||||||||
| Asia | 7 | 0.58(0.39–0.85) | 0.00 | 17.6 | 65.9 | 0.01 | 0.67 | 7 | 0.62(0.55–0.70) | 0.00 | 6.1 | 0.0 | 0.59 | 0.69 |
| Caucasian | 3 | 0.65(0.45–0.94) | 0.03 | 2.9 | 13.0 | 0.32 | 0.24 | 3 | 0.66(0.49–0.91) | 0.01 | 2.0 | 0.0 | 0.68 | 0.42 |
| Publication language | ||||||||||||||
| Chinese | 1 | 0.44(0.27–0.72) | -- | -- | -- | -- | -- | 2 | 0.63(0.56–0.70) | 0.00 | 1.1 | 0.0 | 1.00 | 0.52 |
| English | 10 | 0.64(0.55–0.75) | 0.00 | 17.9 | 49.9 | 0.04 | 0.73 | 8 | 0.66(0.57–0.77) | 0.00 | 7.0 | 0.0 | 0.80 | 0.79 |
| Number of cases | ||||||||||||||
| <200 | 5 | 0.58(0.44–0.77) | 0.00 | 4.3 | 8.0 | 0.36 | 0.46 | 4 | 0.63(0.49–0.82) | 0.00 | 3.0 | 0.0 | 0.78 | 0.51 |
| ≥200 | 6 | 0.59(0.41–0.86) | 0.01 | 15.4 | 67.6 | 0.01 | 0.52 | 6 | 0.63(0.55–0.71) | 0.00 | 4.9 | 0.0 | 0.48 | 0.69 |
| Adjustments | ||||||||||||||
| Smoking, yes | 6 | 0.57(0.39–0.84) | 0.00 | 18.8 | 73.4 | 0.00 | 0.63 | 4 | 0.69(0.56–0.86) | 0.00 | 3.0 | 0.0 | 0.78 | 0.40 |
| no | 5 | 0.63(0.47–0.83) | 0.00 | 4.0 | 0.0 | 0.88 | 0.49 | 6 | 0.60(0.53–0.69) | 0.00 | 5.0 | 0.0 | 0.63 | 0.58 |
| Alcohol, yes | 3 | 0.65(0.45–0.94) | 0.03 | 2.9 | 13.0 | 0.32 | 0.16 | 2 | 0.74(0.55–1.02) | 0.06 | 1.1 | 0.0 | 0.68 | 0.73 |
| no | 8 | 0.58(0.43–0.79) | 0.00 | 17.6 | 60.3 | 0.01 | 0.65 | 8 | 0.61(0.54–0.69) | 0.00 | 7.1 | 0.0 | 0.78 | 0.49 |
aThe random effect model was adopted the pooling method if I2>50%; otherwise, the fixed effect model (I2<50%) was used as the pooling method.
bthe P value for RR; cthe p value for heterogeneity; dthe p value for publication bias.
Influence analysis and publication bias
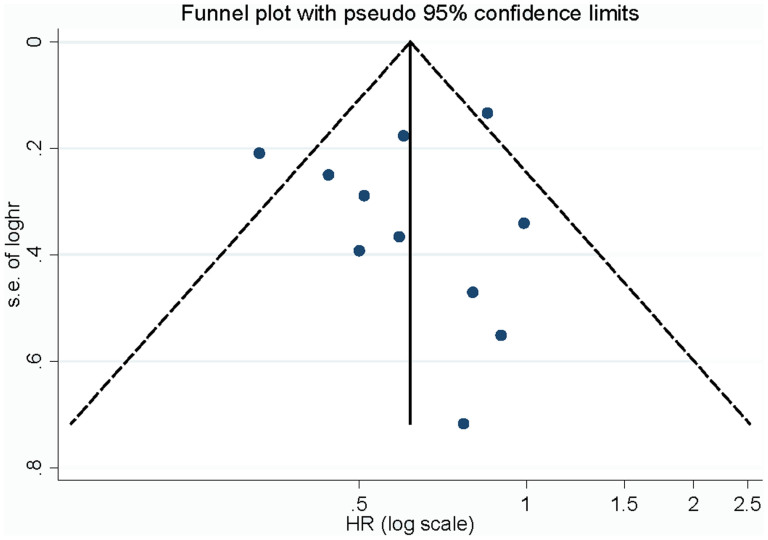
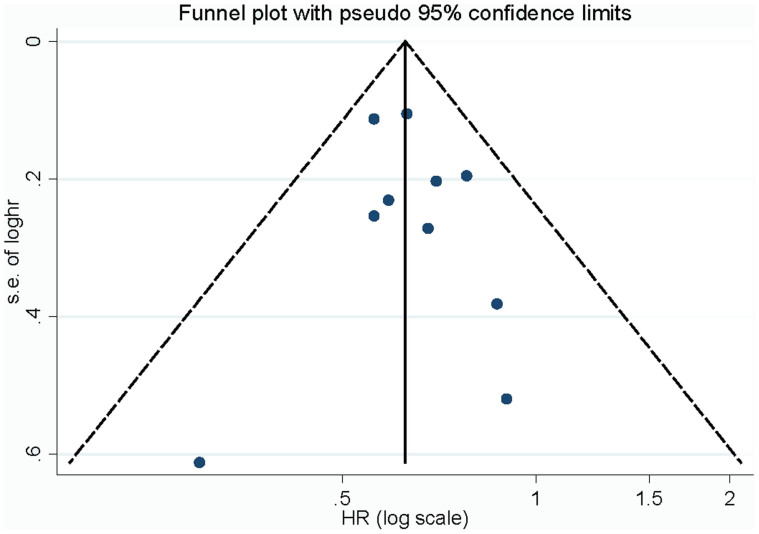
Influence analysis showed that no individual study had excessive influence on the association of vegetable intake and NPC. Begg's funnel plot (Figure 3) and Egger's test showed no evidence of significant publication bias between vegetable intake and NPC (Table 2).
Figure 3. Begg's funnel plot for publication bias of vegetables intake and NPC risk.
Total fruit
High versus low analyses
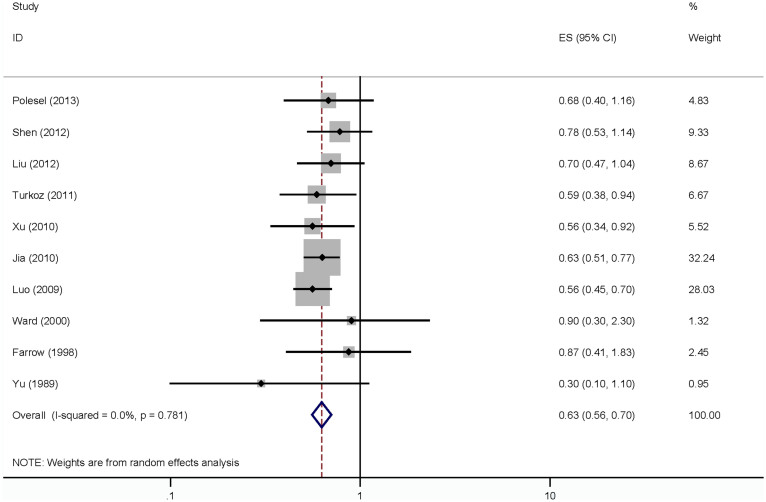
Data from 10 articles15,17,18,19,20,21,22,25,27,29 (1 prospective study and 9 case-control studies) for fruit intake and NPC risk were used including 6155 NPC cases 6654 controls. Four studies reported that fruit intake can reduce the NPC risk, while 6 studies didn't showed the significant association between fruit intake and NPC risk. The meta-analysis showed an inverse association between total fruit intake and NPC risk (summary RR = 0.63, 95% CI = 0.56–0.70) (Figure 4). There was no evidence of heterogeneity was found (I2 = 0.0%, Pheterogeneity = 0.78). The power of effect estimates is 0.91 for vegetables while α = 0.05 in this study.
Figure 4. The forest plot between highest versus lowest categories of fruit intake and NPC risk.
Subgroup analyses
Nine case-control studies were included in this meta-analysis, and the pooled RR was 0.61 (95% CI = 0.54–0.69) for the highest category of fruit intake versus the lowest category and NPC risk. For the subgroup of ethnicity, the associations were significant in the Asia (RR = 0.62, 95% CI = 0.55–0.70) and Caucasian (RR = 0.66, 95% CI = 0.49–0.91). Inverse associations of fruit intake with risk of NPC were found in all strata for the subgroups of sources of control, number of cases, adjustment for smoking or alcohol, respectively. The main results are summarized in Table 2.
Influence analysis and publication bias
No individual study had excessive influence on the association of fruit intake and NPC in the influence analysis. Begg's funnel plot (Figure 5) and Egger's test (P = 0.60) showed no evidence of significant publication bias between fruit intake and NPC risk (Table 2).
Figure 5. Begg's funnel plot for publication bias of fruit intake and NPC risk.
Total vegetables and fruit
Three studies16,18,20 were conducted to assess the association between total vegetables and fruit and NPC risk, and the summary RR for the highest versus the lowest intake was 0.33 (95% CI = 0.25–0.45, I2 = 0.0%, Pheterogeneity = 0.56) for fruits and vegetables combined.
Discussion
Finding from this meta-analysis suggested that the intake of fruit and vegetables is associated with significant reductions in the risk of NPC. The associations were also found in subgroups of Asia and Caucasian for vegetables or fruit intake and NPC risk.
One previous meta-analysis has suggested that a favorable effect was found between the intake of fruit and vegetables and risk of esophageal squamous cell carcinoma7. Although no association was found between vegetables and breast cancer risk, inverse associations of fruits intake and fruits and vegetables combined with risk of breast cancer were found30. In the lung cancer study, cruciferous vegetables intake was showed a favorable effect for female in a meta-analysis31. Furthermore, cruciferous vegetables intake has a significantly decreased risk with renal cell carcinoma32, colorectal neoplasms33 and gastric cancer34. Our meta-analysis result is consisted with most of the published studies.
The mechanisms of the anti-NPC properties of fruit and vegetables have not been thoroughly investigated. As we know, the epidemiology of NPC varies greatly between Asia (predominantly WHO type 3) and the rest of the world (predominantly WHO Type 1). Although they are difference in histology because of traditionally, the natural history and risk factors for WHO type 1 and 3 nasopharyngeal carcinoma, the association of fruits and vegetables with NPC remains consistent. A few in vitro and epidemiological studies have discovered mechanisms that allow fruit and vegetables to protect against other cancers35,36,37. Antioxidants and dietary fibers might play a key role in the prevention of NPC development. The protective effects of vegetables and fruit are thought to be mediated by multiple components, including beta-carotene, fiber, vitamins, alpha-tocopherol, retinoids, phytoestrogens and folate5. These components are involved in numerous biological processes that may alter cancer risk, including the inhibition of cell growth, the normal synthesis and methylation of DNA, and protection against oxidative stress and DNA damage.
Between-study heterogeneity is common in meta-analysis38, and exploring the potential sources of between-study heterogeneity is the essential component of meta-analysis. For vegetable intake and NPC, evidence of heterogeneity (I2 = 50.0%, Pheterogeneity = 0.03) was found in the pooled results. The between-study heterogeneity might arise from study region, number of cases and sources of controls. Thus, we used meta-regression to explore the causes of heterogeneity for covariates. No covariate having a significant impact on between-study heterogeneity for the above mentioned covariates. Then, we used “leave-one-out” sensitive analysis, which aims to reduce between-study heterogeneity and explore the potential important causes of between-study heterogeneity for both covariates and studies. The key contributor to this high between-study heterogeneity was one study conducted by Liu et al16. After excluding this study, heterogeneity was reduced to I2 = 6.9% and the association was also significant between vegetable and NPC risk. For the larger studies (≥200), high heterogeneity was found (I2 = 67.6%, Pheterogeneity = 0.01). The key contributor to this high between-study heterogeneity assessed by the leave-one-out analysis was one study conducted by Liu et al (2012). The study conducted by Liu et al. has large participants (600 cases and 600 controls), and the RR was 0.33 (95% CI = 0.22–0.50). After excluding this study, heterogeneity was reduced to I2 = 0.0%, and the summary RR for NPC was 0.73 (95% CI = 0.60–0.89).
As a meta-analysis of published studies, our findings showed some advantages. First, a major strength of this study was the large number of participants included in this analysis and this may derive a more precise estimation of the relationship between vegetables and fruit and NPC risk. Second, no significant publication bias was found. However, there were some limitations in this meta-analysis. First, a meta-analysis of observational studies is susceptible to potential bias inherent in the original studies, especially for case-control studies. Overstated association may be expected from the case-control studies because of recall or selection bias, and early symptoms in patients may have resulted in a change in dietary habits. Second, measurement errors are important in the assessment of dietary intake, which can lead to overestimation of the range of intake and underestimation of the magnitude of the relationship between dietary intake and cancer risk39,40. Third, incomparability of results between studies may also occur because definitions and categories of vegetables and fruit as well as analytical comparisons vary across studies. Studies from different regions, ethnicities and periods probably address very different exposures. However, we only decided to consider the studies that evaluated all types of fruit or vegetables to assess exposure that was as broad as possible. Finally, small sample sizes in subgroup analysis were present, more studies need to confirm the result.
In summary, results from this meta-analysis suggested that intake of fruit and vegetables may have a protective effect on NPC. Since the potential biases and confounders could not be ruled out completely in this meta-analysis, further studies are warranted to confirm this result.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.J. and Z.W. designed of the experiments; J.J., Z.O. and Z.W. collected the date; J.J. and Z.W. wrote the main manuscript text and all authors reviewed the manuscript.
References
- Sun X. et al. Can Global Variation of Nasopharynx Cancer Be Retrieved from the Combined Analyses of IARC Cancer Information (CIN) Databases? PLoS One 6, e22039 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M. C., Ho J. H., Ross R. K. & Henderson B. E. Nasopharyngeal carcinoma in Chinese---salted fish or inhaled smoke? Prev Med 10, 15–24 (1981). [DOI] [PubMed] [Google Scholar]
- Jeannel D., Bouvier G. & Huber A. [Nasopharyngeal carcinoma, an epidemiological approach to carcinogenesis]. Infections and human cancer [125–155] (1999). [Google Scholar]
- Jia W. H. et al. Trends in incidence and mortality of nasopharyngeal carcinoma over a 20–25 year period (1978/1983-2002) in Sihui and Cangwu counties in southern China. BMC cancer 6, 178 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. T. & Adami H. O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer. Cancer Epidemiol Biomarkers Prev 15, 1765–77 (2006). [DOI] [PubMed] [Google Scholar]
- McCullough M. L. & Giovannucci E. L. Diet and cancer prevention. Oncogene 23, 6349–64 (2004). [DOI] [PubMed] [Google Scholar]
- Liu J., Wang J., Leng Y. & Lv C. Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. International journal of cancer. Journal Int J Cancer 133, 473–485 (2013). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Controlling the risk of spurious findings from meta-regression. Stat Med 23, 1663–1682 (2004). [DOI] [PubMed] [Google Scholar]
- Patsopoulos N. A., Evangelou E. & Ioannidis J. P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37, 1148–1157 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey, Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias A. Assessing the in fluence of a single study in the meta-analysis estimate. Stata Tech Bull 47, 15–17 (1999). [Google Scholar]
- Polesel J. et al. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control 24, 1157–1165 (2013). [DOI] [PubMed] [Google Scholar]
- Li W. D. et al. An epidemiological exploration on risk factors of nasopharyngeal carcinoma in Zhongshan City. Chin J Dis Control Prev 16, 486–489 (2012). [Google Scholar]
- Shen G. P. et al. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS One 7, e36515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. T. et al. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. Cancer causes & control 23, 589–599 (2012). [DOI] [PubMed] [Google Scholar]
- Turkoz F. P. et al. Risk factors of nasopharyngeal carcinoma in Turkey-an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev 12, 3017–21 (2011). [PubMed] [Google Scholar]
- Xu C. H. A case-control study of the association between dietary factor and nasopharyngeal carcinoma [master]: Zhong Shan University. (2010).
- Jia W. H. et al. Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC cancer 10, 446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. Y. EpidemioIogical Study on Risk Factors for Incident Cases of Nasopharyngeal Carcinoma in Cantonese [master]: Zhong Shan University. (2009).
- Feng B. J. et al. Dietary risk factors for nasopharyngeal carcinoma in Maghrebian countries. International journal of cancer. Int J Cancer 121, 1550–1555 (2007). [DOI] [PubMed] [Google Scholar]
- Yuan J. M. et al. Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. International journal of cancer. Int J Cance 85, 358–363 (2000). [DOI] [PubMed] [Google Scholar]
- Ward M. H. et al. Dietary exposure to nitrite and nitrosamines and risk of nasopharyngeal carcinoma in Taiwan. International journal of cancer. Int J Cancer 86, 603–609 (2000). [DOI] [PubMed] [Google Scholar]
- Armstrong R. W. et al. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. International journal of cancer. Int J Cancer 77, 228–235 (1998). [DOI] [PubMed] [Google Scholar]
- Farrow D. C. et al. Diet and nasopharyngeal cancer in a low-risk population. International journal of cancer. Int J Cancer 78, 675–679 (1998). [DOI] [PubMed] [Google Scholar]
- Ning J. P., Yu M. C., Wang Q. S. & Henderson B. E. Consumption of salted fish and other risk factors for nasopharyngeal carcinoma (NPC) in Tianjin, a low-risk region for NPC in the People's Republic of China. J Natl Cancer Inst 82, 291–296 (1990). [DOI] [PubMed] [Google Scholar]
- Yu M. C., Huang T. B. & Henderson B. E. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. International journal of cancer. Int J Cancer 43, 1077–1082 (1989). [DOI] [PubMed] [Google Scholar]
- Aune D. et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat 134, 479–493 (2012). [DOI] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol 24, 1918–1924 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. et al. Cruciferous vegetables consumption and risk of renal cell carcinoma: a meta-analysis. Nutr Cancer 65, 668–76 (2013). [DOI] [PubMed] [Google Scholar]
- Tse G. & Eslick G. D. Cruciferous vegetables and risk of colorectal neoplasms: a systematic review and meta-analysis. Nutr Cancer 66, 128–139 (2014). [DOI] [PubMed] [Google Scholar]
- Wu Q. J., Yang Y., Wang J., Han L. H. & Xiang Y. B. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci 104, 1067–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg F., Hou S. M., Pershagen G. & Lambert B. Dietary fruit and vegetables protect against somatic mutation in vivo, but low or high intake of carotenoids does not. Carcinogenesis 24, 689–696 (2003). [DOI] [PubMed] [Google Scholar]
- Wettasinghe M., Bolling B., Plhak L., Xiao H. & Parkin K. Phase II enzyme-inducing and antioxidant activities of beetroot (Beta vulgaris L.) extracts from phenotypes of different pigmentation. J Agric Food Chem 50, 6704–6709 (2002). [DOI] [PubMed] [Google Scholar]
- Xiao D., Vogel V. & Singh S. V. Benzyl isothiocyanate-induced apoptosis in human breast cancer cells is initiated by reactive oxygen species and regulated by Bax and Bak. Mol Cancer Ther 5, 2931–2945 (2006). [DOI] [PubMed] [Google Scholar]
- Munafo M. R. & Flint J. Meta-analysis of genetic association studies. Trends Genet 20, 439–44 (2004). [DOI] [PubMed] [Google Scholar]
- Prentice R. L. Dietary assessment and the reliability of nutritional epidemiology reports. Lancet 362, 182–183 (2003). [DOI] [PubMed] [Google Scholar]
- Willett W. C. et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 122, 51–65 (1985). [DOI] [PubMed] [Google Scholar]