Abstract
The aim of this study was to determine the effectiveness of corneal collagen cross-linking (CXL) for the treatment of progressive keratoconus (KC). Some of the published literature, including a few small, randomized controlled trials (RCTs), demonstrated good results after CXL, but large RCTs with long-term follow-up to establish a cause-effect relationship are lacking. Using PubMed, EMBASE, and the Cochrane Library database, we searched for relevant studies published between October 2007 and March 2014. A comprehensive literature search was performed using the Cochrane Collaboration methodology to identify the effectiveness of CXL for treating KC. The primary outcome parameters included uncorrected visual acuity (UCVA), best-corrected visual acuity (BCVA), refraction, corneal topography, and corneal thickness at baseline and at 1, 3, 6, 12, and 18 months after CXL. A total of 1171 participants (1557 eyes) were enrolled in this meta-analysis. CXL may be effective in halting the progress of KC for at least 12 months under certain conditions. However, further research from randomized trials is needed to confirm our findings.
Keratoconus (KC) is a disease characterized by progressive thinning and ectasia of the cornea that induces irregular astigmatism, resulting in impaired vision quality1. KC is usually diagnosed during the second and third decades of life. Because of the young age of KC patients, this disease often has a dramatic effect on quality of life and life planning2. Unfortunately, the treatment options available to date have not been encouraging3. The management of KC has mainly consisted of visual rehabilitation using glasses, contact lenses, and intracorneal ring segment (ICRS) implantation4 for early to moderate stages and lamellar or penetrating keratoplasty for advanced stages characterized by contact lens intolerance and/or corneal scarring5. Recently, corneal collagen cross-linking (CXL) has been introduced. CXL is the first treatment to address the pathophysiology of ectasia, with the goal of retarding or halting disease progression6,7,8. Several studies focusing on the successful treatment of KC with CXL have been performed and published in the peer-reviewed literature, including 4 randomized controlled trials (RCTs)8,9,10,11 and a few multicentre clinical trials12.
Although the published literature demonstrates the efficacy of CXL, prospective RCTs that establish a cause-effect relationship are lacking. Furthermore, questions have also been raised concerning the RCT studies of KC. Jain noted that because KC is a bilateral, asymmetrical disease, it is not appropriate to use the contralateral eye as a control, which is a common characteristic of all published RCTs13.
The main objectives of this systematic review and meta-analysis were to determine the safety and efficacy of CXL for the treatment of KC.
Results
Trial Characteristics
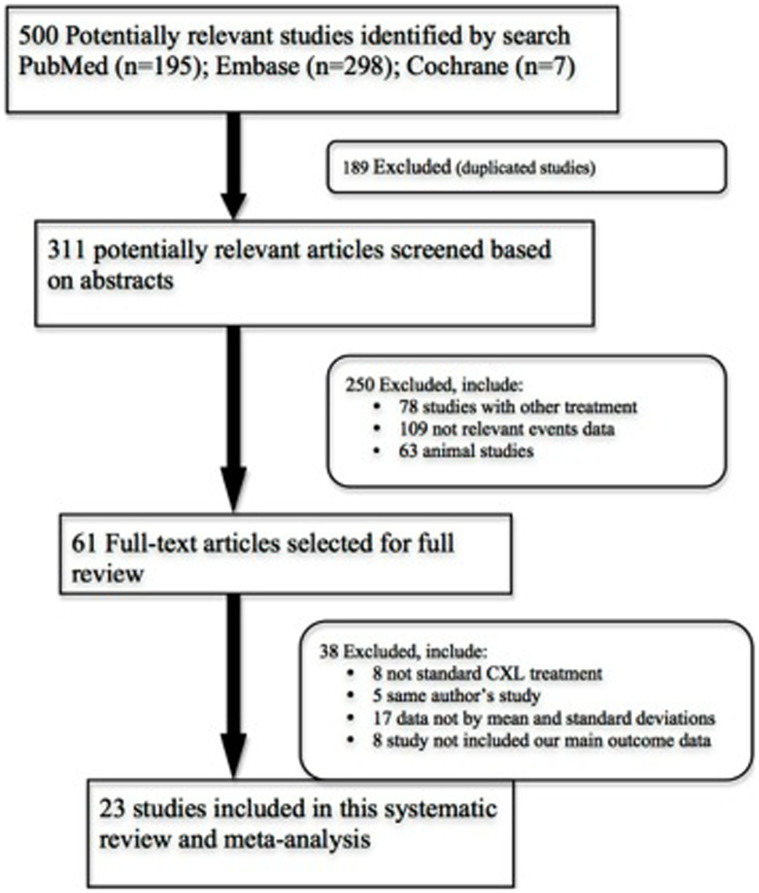
Our initial search yielded 500 studies (Fig. 1). After excluding 189 duplicate publications, we considered the abstracts of 311 studies. After evaluating the abstract of each study, 250 studies were excluded because they did not meet the inclusion criteria. Subsequently, based on the full text of each of the remaining 61 trials, we excluded 38 trials that did not use the standard CXL treatment (n = 8), enrolled duplicate patients (n = 5), tested parameters not related to our main outcome, or included data not expressed as the mean and standard deviation (n = 25). Therefore, 23 studies were included in this review.
Figure 1. Flow diagram of study selection.
Among these 23 trials, 4 were RCTs9,10,11,14, 11 were prospective controlled studies7,15,16,17,18,19,20,21,22,23,24, and 8 were retrospective studies12,25,26,27,28,29,30,31 (Table 1). A total of 1171 participants with 1557 affected eyes were included in the meta-analysis. The sample sizes in these studies ranged from 9 to 272. These studies were performed in 15 countries (3 each in Australia, Germany, the United States, and Italy; 1 each in Norway, Denmark, France, the United Kingdom, Switzerland, the Netherlands, Israel, Iran, Oman, India, and Egypt). Thirteen trials reported the follow-up results for participants 6 months post-CXL, and 8 trials provided follow-up results after 1 and 3 months post-CXL. Eighteen trials reported follow-up results after more than 1 year, and 6 of the trials included a control group. The inclusion criteria were consistent. All of the patients were reported to have progressive KC, although the definition of progressive KC varied slightly and it was not defined in some cases.
Table 1. Trial characteristics. Among the 23 trials included, 4 were RCTs, 11 were prospective controlled studies, and 8 were retrospective studies.
| A. Prospective and retrospective studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Year of Publication | Study Quality(NOS) | Country of Origin | No. of Patients | No. of Eyes | Mean Age (years) | Main Results | Mean follow up |
| Stojanovic | 2012 | 3 | Norway | 53 | 61 | 32 ± 10 | 27.4% of the eyes gained 2 or more lines | 12 |
| Anders Ivarsen | 2013 | 4 | Denmark | 22 | 28 | 22 | 14 eyes showed a Kmax decrease of more than 2.0D | 22 |
| Cosimo Mazzotta | 2012 | 5 | Italy | 44 | 44 | 10–40 | Corneal micro-morphological changes observed with in vivo confocal microscopy related with function | 12 |
| Dalal Asri | 2011 | 4 | France | 142 | 142 | 24.12 ± 7.58 | Keratoconus progression had stopped in 42 eyes (68.8%) | 12 |
| Deepa Viswanathan | 2013 | 7 | Australia | 35 | 51 | 24.25 ± 8.08 | Kmax decreased 0.96 ± 2.23D | 14.38 ± 9.36 |
| Eberhand Spoerl | 2011 | 7 | Germany | 46 | 50 | 29.4 ± 9.3 | CH and CRF did not change significantly after CXL | 12 |
| Efekan Coskunseven | 2009 | 5 | Switzerland | 19 | 19 | 22 ± 5 | Kmax decreased 1.57 ± 1.14D | 9 ± 2 |
| Frea Sloot | 2013 | 5 | Netherlands | 42 | 53 | 21.5 | Progression was halted in 48 eyes (91%) | 12 |
| Frederik Raiskup-Wolf | 2008 | 4 | Germany | 272 | 480 | 30.04 ± 10.46 | Kmax decreased 2.68D in the first year; 53% of 142 improved BCVA more than 1 line | 26.7 ± 16.2 |
| Hassan Hashemi | 2013 | 6 | Iran | 32 | 40 | 22.45 ± 5.48 | CXL could stop disease progression, based on 5-year studies | 60 |
| Kinga Kranitz | 2012 | 7 | Germany | 22 | 40 | 29.92 | Posterior elevation is a sensitive parameter for monitoring corneal remodelling after CXL | 12 |
| Ladan Saffarian | 2010 | 4 | Iran | 53 | 92 | 21.5 ± 3.4 | CXL halted the progression of keratoconus in Iranian patients | 12 |
| Maria A. Henriquez | 2011 | 5 | CA, USA | 10 | 10 | 29.7 | Eight (80%) and 6 (60%) of 10 eyes showed a decrease in the anterior and posterior elevation values, respectively | 12 |
| Maria Clara Arbelaez | 2009 | 3 | Oman | 19 | 20 | 24.4 | BCVA increased 1.65 lines, and Kave decreased 1.36D | 6 |
| Paolo Vinciguerra | 2009 | 6 | Italy | 28 | 28 | 18–60 | AK reduced from 58.94D to 55.18D | 12 |
| Ritu Arora | 2013 | 6 | India | 15 | 15 | 21.73 ± 9.5 | Progression was halted in 11 of 15 patients | 16 |
| Tamer M. EL-Raggal | 2009 | 3 | Egypt | 9 | 15 | No progression within 6 months follow-up | 6 | |
| Tobias Koller | 2009 | 4 | Italy | 99 | 117 | 27–40 | In 39 (37.1%) eyes, Kmax decreased more than 1D | 12 |
| Yakov Goldich | 2012 | 5 | Israel | 14 | 14 | 28.2 ± 5.9 | Two years after CXL, improved BCVA and reduced Kmax were observed | 24 |
Primary Outcome
Visual acuity
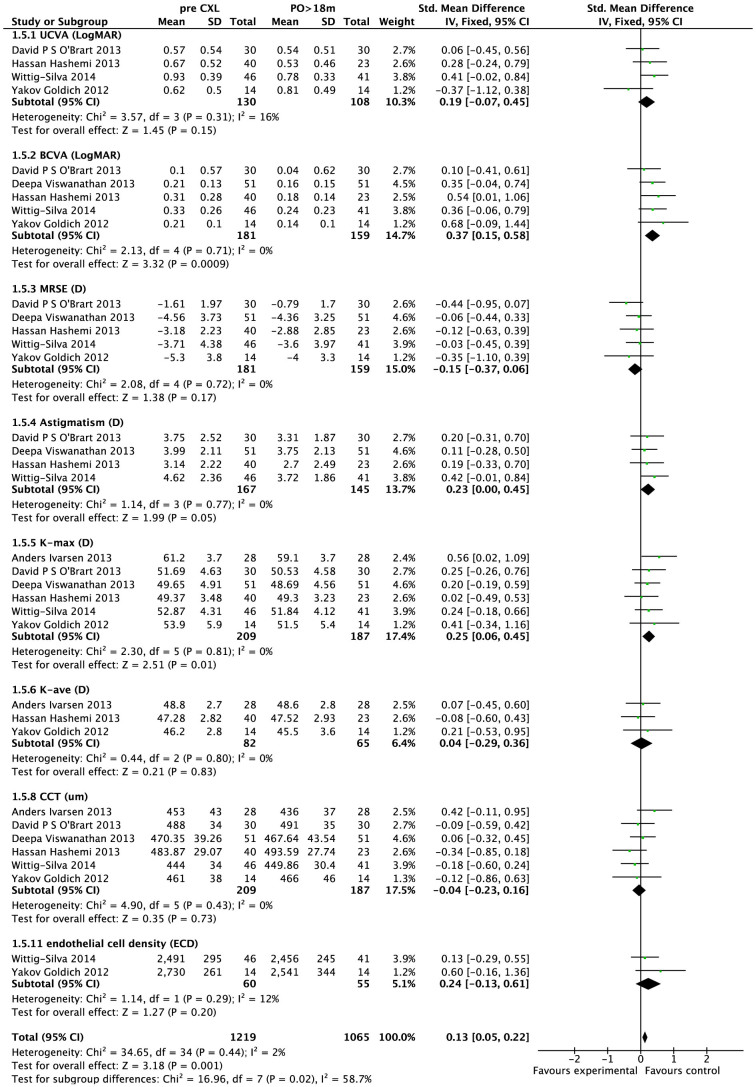
Table 2 shows the primary outcome results at 1, 3, 6, and 12 months post-CXL. Seven studies11,12,15,17,19,21,25 reported the mean UCVA and BCVA at 1 and 3 months post-CXL. There was no statistically significant difference in UCVA or BCVA pre- and 1 month post-CXL; however, at 3 months post-CXL, both UCVA (SMD, 0.46; 95% CI, 0.17 to 0.76; p < 0.01) and BCVA (SMD 0.55; 95%CI, 0.19 to 0.92; p < 0.01) improved (Table 2). Eleven studies reported 6-month post-CXL follow-up data; they also showed improvements in UCVA (SMD, 0.49; 95% CI, 0.18 to 0.8; p < 0.01) and BCVA (SMD, 0.72; 95%CI, 0.17 to 1.28; p < 0.05, Table 2). In 14 studies, UCVA (SMD, 0.52; 95%CI, 0.25 to 0.78; p < 0.01) and BCVA (SMD, 0.81; 95%CI, 0.43 to 1.18; p < 0.01) improvements were evident 12 months post-CXL (Table 2). However, after 18 months post-CXL, only BCVA (SMD, 0.37; 95%CI, 0.15 to 0.58; p < 0.001) still showed significant improvement in our systematic analysis, which was based on 5 studies that included 181 participants (Fig. 2)9,16,17,24.
Table 2. The primary outcomes in subgroups after CXL treatment. The 1-, 3-, 6- and 12-month CXL results are shown. UCVA = uncorrected visual acuity, BCVA = best-corrected visual acuity, MRSE = mean refractive spherical equivalent, Kmax = steepest simulated keratometry, Kave = average simulated keratometry, CCT = central corneal thickness.
| Group | Main Outcome | No. of Studies | No. of Eyes | Effect Size SMD (95% CI) | Heterogeneity (I2; p-value) | Test for Overall Effect (Z, p-value) |
|---|---|---|---|---|---|---|
| Post-CXL-1m | UCVA | 7 | 374 | 0.13 (−0.09, 0.36) | 51%; 0.06 | 1.14; 0.26 |
| BCVA | 7 | 374 | −0.05 (−0.32, 0.22) | 66%; 0.007 | 1.35; 0.18 | |
| MRSE | 3 | 150 | −0.14 (−0.37, 0.09) | 37%; 0.23 | 0.89; 0.37 | |
| Astigmatism | 4 | 292 | −0.11 (−0.28, 0.05) | 13%; 0.33 | 1.34; 0.18 | |
| Kmax | 3 | 231 | 0.04 (−0.15, 0.22) | 0%; 0.47 | 0.38; 0.7 | |
| Kave | 4 | 275 | 0.04 (−0.13, 0.21) | 0%; 0.72 | 0.48; 0.63 | |
| CCT | 4 | 297 | 0.24 (0.08, 0.41) | 84%; 0.0003 | 2.93; 0.003* | |
| Post-CXL-3m | UCVA | 7 | 374 | 0.37 (0.22, 0.52) | 68%; 0.005 | 4.80; <0.00001* |
| BCVA | 7 | 374 | 0.41 (0.26, 0.56) | 79%; <0.0001 | 2.53; <0.00001* | |
| MRSE | 3 | 150 | −0.16 (−0.41, 0.09) | 0%; 0.76 | 1.24; 0.22 | |
| Astigmatism | 4 | 292 | 0.00 (−0.17, 0.17) | 6%; 0.37 | 0.05; 0.96 | |
| Kmax | 3 | 231 | 0.14 (−0.04, 0.33) | 15%; 0.31 | 1.54; 0.12 | |
| Kave | 4 | 275 | 0.22 (0.05, 0.39) | 6%; 0.36 | 2.54; 0.01* | |
| CCT | 4 | 297 | 0.16 (−0.01, 0.33) | 81%; 0.001 | 1.43; 015 | |
| Post-CXL-6m | UCVA | 11 | 442 | 0.49 (0.18, 0.8) | 75%; <0.0001 | 3.07; 0.002* |
| BCVA | 11 | 442 | 0.72 (0.17, 1.28) | 91%; <0.00001 | 2.56; 0.01* | |
| MRSE | 6 | 203 | −0.55 (−1.09, −0.02) | 83%; <0.0001 | 2.02; 0.04* | |
| Astigmatism | 7 | 346 | −0.04 (−0.25, 0.17) | 31%; 0.19 | 0.37; 0.71 | |
| Kmax | 7 | 321 | 0.15 (−0.01, 0.32) | 0%; 0.98 | 1.84; 0.07 | |
| Kave | 8 | 352 | 0.19 (0.04, 0.35) | 0%; 0.75 | 2.42; 0.02* | |
| CCT | 8 | 373 | 0.38 (0.16, 0.60) | 41%; 0.11 | 3.40; 0.0007* | |
| Post-CXL-12m | UCVA | 13 | 585 | 0.52(0.25, 0.78) | 75%; <0.0001 | 3.83; 0.0001* |
| BCVA | 14 | 638 | 0.81 (0.43, 1.18) | 89%; <0.0001 | 4.19; <0.00001* | |
| MRSE | 8 | 334 | −0.27(−0.42, −0.11) | 0%; 1 | 3.42; <0.001* | |
| Astigmatism | 9 | 487 | 0.23(0.1, 0.37) | 33%; 0.16 | 3.42; <0.001* | |
| Kmax | 12 | 487 | 0.15 (0.02, 0.28) | 0%; 0.99 | 2.21; 0.03* | |
| Kave | 13 | 598 | 0.19 (0.07, 0.31) | 0%; 0.81 | 3.06; 0.02* | |
| CCT | 11 | 536 | 0.32 (0.19, 0.44) | 68%; 0.0003 | 4.82; <0.00001* | |
| Front elevation | 4 | 98 | −0.1 (−0.39, 0.18) | 43% 0.16 | 0.72; 0.47 | |
| Back elevation | 5 | 123 | 0.31 (−0.46, 1.09) | 88%; <0.0001 | 0.79; 0.43 | |
| ECD | 3 | 72 | 0.1(−0.23, 0.43) | 0%; 0.9 | 0.6; 0.55 |
Figure 2. The clinical results at 18 months post-CXL treatment.
UCVA = uncorrected visual acuity, BCVA = best-corrected visual acuity, MRSE = mean refractive spherical equivalent, Kmax, Kave, CCT = central corneal thickness, ECD = endothelial cell density.
Corneal topography
Seven studies reported corneal topography data at 6 months post-CXL, and 3 of them also included these data at 1 and 3 months post-CXL (Table 2). The analysis of these data showed that the pre-post value differences in Kmax (p = 0.7, p = 0.12, and p > 0.07 at 1, 3, and 6 months post-CXL, respectively) and Kave (p = 0.63, p = 0.07, and p = 0.07 at 1, 3, and 6 months post-CXL, respectively) were not statistically significant (Table 2). Thirteen studies reported corneal topography data at 12 months post-CXL. Examination of the forest plot showed that both Kmax (SMD, 0.15; 95%CI, 0.02 to 0.28; p = 0.03) and Kave (SMD, 0.19; 95%CI, 0.07 to 0.31; p = 0.002) decreased significantly at 12 months post-CXL (Table 2). In a long-term follow-up of over 18-months, Kmax (SMD, 0.25; 95%CI, 0.06 to 0.45; p = 0.01) decreased significantly, according to the results of 6 studies9,16,17,24,26. However, Kave (SMD, 0.04; 95%CI, -0.29 to 0.36; p = 0.83) was not significantly different at the long-term follow-up of more than 18 months (Fig. 2).
Central corneal thickness
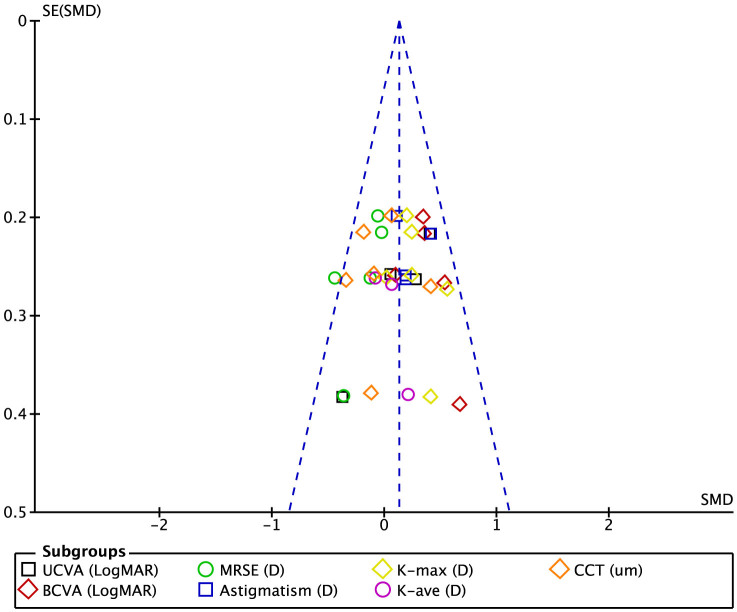
Four studies reported corneal thickness (CCT) data at 3 months post-CXL. The analysis of these data showed that the pre- and post-CXL value differences in CCT were not statistically significant (SMD, 0.16; 95% CI, -0.01 to 0.33; p = 0.07) (Table 2). At 6 months post-CXL, the CCT value decreased significantly compared with the pre-CXL value (SMD, 0.38; 95% CI, 0.16 to 0.60; p < 0.01) based on 8 studies7,12,14,17,24,25,26,31 with 373 participants (Table 2). Twelve studies mentioned CCT data at 12 months post-CXL9,12,14,17,18,21,22,24,26,28,30. The analysis of these data showed that the CCT value was significantly lower in the 12-month post-CXL group than in the pre-CXL group (SMD, 0.41; 95% CI, 0.16 to 0.65; p < 0.01; Table 2). Six long-term follow-up studies of more than 18 months reported CCT values9,16,17,24,26. The funnel plot showed that no significant between-group differences were detected in any of the studies (Fig. 3). The analysis of these data showed that the between-group differences in CCT were not statistically significant (SMD, −0.04; 95% CI, −0.23 to 0.16; p = 0.73; Fig. 2).
Figure 3. Funnel plot of long-term results after CXL treatment.
In summary, vision acuity showed significant improvement 3 months post-CXL, and the effect could last for more than 18 months post-CXL. Kmax was significantly flattened after 12 months post-CXL, and this effect could remain for more than 18 months. CCT values were decreased at 6 months and 12 months post-CXL (p < 0.05); however, at the long-term follow-up of more than 18 months, the values showed no statistical difference (p > 0.05).
Secondary Outcome Parameters
Four studies reported front elevation data at 12 months post-CXL17,19,20,21. An examination of the forest plot showed that there were no statistically significant between-group differences in front elevation (SMD, −0.1; 95% CI, −0.39 to 0.18; p = 0.47; Table 2). Five studies reported back elevation data at 12 months post-CXL17,18,19,20,21. An examination of the forest plot showed that in the Kinga Kranittz et al. study18, back elevation was significantly lower in the post-CXL group compared with the pre-CXL group. In the remaining studies, there was no statistically significant difference between the pre- and post-CXL groups (Table 2). An analysis of these data showed that the between-group difference in back elevation was not statistically significant (p = 0.21, Table 2). Three studies provided ECD data at 12 months post-CXL21,22,24. The forest plot showed that none of these studies demonstrated significant differences in ECD between groups. The analysis of these data showed that there were no statistically significant pre- and post-CXL differences in ECD (SMD, 0.13; 95% CI, −0.23 to 0.50; p = 0.48; Table 2). Two studies investigated corneal biomechanics changes after CXL24,27. Both studies used an ocular response analyser (ORA, Reichert, Inc.), which is a new, non-invasive device, to measure corneal hysteresis (CH) and the corneal resistance factor (CRF) at 12 months post-CXL. None of the studies showed significant pre- and post-CXL differences in CH (SMD, 0.08; 95% CI, −0.27 to 0.43; p = 0.65) or CRF (SMD, 0.08; 95% CI, −0.27 to 0.42; p = 0.67), as assessed by the forest plot analysis.
Heterogeneity
The test of heterogeneity seeks to determine whether there are genuine differences in study results (heterogeneity) or whether the variation in findings is a result of chance alone (homogeneity). Review Manager5 heterogeneity tests were applied. If p > 0.1, a fixed model was used; a random effects model was used if p < 0.1. In the present meta-analysis, statistical heterogeneity was detected in some of the outcome measures (p < 0.1). Heterogeneity may be explained by variability in the participants (e.g., patient characteristics, sample size) or interventions (e.g., surgical skills, the CXL procedure).
Publication Bias
The funnel plot showed no correlation between study size and effect size or any other evidence of publication bias (Fig. 3). We used the Jadad scoring methods of quality literature assessment for the RCTs and the NOS scoring methods for the prospective and retrospective studies that were selected for this research.
Discussion
In this systematic review and meta-analysis, we found that corneal cross-linking could effectively stabilize the progression of KC, as assessed by key corneal topographic parameters. The effects of CXL on visual acuity improvement are also remarkable. However, CCT remained slightly decreased from baseline to 12 months post-CXL and then recovered to baseline thickness after more than 18 months.
Because the main treatment objective is to stabilize the underlying disease process, corneal topography (Kmax and Kave) was considered one of the key outcome measures. Based on our meta-analysis, Kmax and Kave decreased slightly after the CXL procedure and reached a statistically significant difference at 12 months post-CXL compared with baseline (p < 0.05, Table 2). Most of the studies reported a Kmax reduction of 1–2 dioptre (D) after 1-year post-CXL. In the United States, Henriquez et al. reported a 2.66 D reduction in Kmax based on their randomized prospective comparative study of 10 eyes19. Hersh et al. reported a reduction of 1.70 D based on their RCT of 48 eyes11. In Australia, Wittig-Silva et al. reported a mean reduction of 1.45 D at 12 months26 post-CXL based on their RCT of 49 eyes10. Individually, Kmax value decreases of 2.0D or more were reported in 22 eyes (31%) in Hersh's study11, in 13 eyes (21.3%) in Asri's study12, and in 14 eyes (50%) in Ivarsen's study26. Ivarsen also reported the most pronounced decrease in the Kmax value (7.4 D). However, the corneal topography results from long-term follow-up varied between different studies. Raiskup-Wolf et al. reported that Kmax decreased significantly, by 2.21 D in the second year and by 4.84 D in the third year29. Hashemi et al. reported that Kmax and Kave decreased slightly (by 0.16 D and 0.1 D, respectively) at 5 years after the procedure17. In our meta-analysis, a small regression of corneal topography was found after 18 months post-CXL. The Kave value (SMD, 0.04; 95%CI, −0.29 to 0.36; p = 0.83) was not significantly different at 18 months post-CXL (Fig. 2) based on the results of 3 studies, and Kmax (SMD, 0.26; 95%CI, 0.03 to 0.48; p = 0.02) decreased significantly based on the results of 5 studies (Fig. 2). These results could be attributed to the rearrangement of the corneal lamellae and surrounding matrix32,33. Considering the collagen turnover in the cornea over several years, long-term studies have yet to determine whether repeated CXL treatment is necessary. Currently, no studies have assessed repeated CXL procedures in KC patients because some authors believe that CXL yields good results in long-term follow-up.
In the present analysis, we investigated front elevation and back elevation because they are important parameters for determining the progression of KC. An examination of the forest plot showed that there were no statistically significant between-group differences in front elevation (p = 0.47) or back elevation (p = 0.21) (Table 2) at 12 months post-CXL. Therefore, front and back corneal elevation remained stable for at least 12 months post-CXL. Four studies were included in this analysis17,19,20,21, and all of them used a Pentacam to measure front and back elevation at the thinnest point of the cornea. However, some studies yielded more positive results. Henriquez et al. reported that 8 (80%) of the eyes had a reduction in the anterior elevation value and 6 (60%) had a reduction in the posterior elevation value at 1 year post-CXL19. Hashemi et al. reported significant decreases in both front and back elevation values at 5 years post-CXL17.
Although CXL treatment is not intended to improve visual acuity, the induced changes in corneal topography may result in such improvement secondarily. Based on our systematic review and meta-analysis, the impact of CXL on visual acuity is remarkable. Significant improvement in UCVA and BCVA was observed at 3 months post-CXL (p < 0.05; Table 2) and can last at least 12 months post-CXL (p < 0.05; Table 2). UCVA and BCVA were reported in all studies but in different formats, such as Snellen VA and LogMAR. We converted all the data into LogMAR units for comparison. Among recent studies with 12-month follow-up data, O'Brart reported that BCVA increased by two lines in 6 (43%) eyes and by one line in 6 (20%) eyes9; Asri reported that BCVA improved by at least two lines in 87.6% of cases12; and Hashemi reported that BCVA improved by at least two lines in 19 (47.5%) eyes17. However, in our study, after 18 months post-CXL, UCVA (SMD, 0.19; 95%CI, -0.07 to 0.45; p = 0.15) showed no significant improvement based on a systematic analysis of 3 studies with 84 participants (Fig. 2). Only BCVA (SMD, 0.37; 95%CI 0.15 to 0.58; p = 0.0009) still showed a significant improvement based on a systematic analysis of 5 studies with 181 participants (Fig. 2). We assume that there are two possible reasons for this discrepancy. First, Goldich found a progressive change in axial length (AL) in keratoconus24. This change may contribute to the myopic shift and cause a decrease in UCVA. Second, a small regression may cause a decrease in UCVA. The regression may be explained as an effect of the rearrangement of the corneal lamellae and surrounding matrix27,32,33. However, the reason for the change in AL that resulted from the progressive myopic shift in keratoconus patients is unknown, and no clinical studies investigating the mechanism of regression have been performed. Therefore, additional studies on this subject may be needed.
We also investigated corneal biomechanical properties (namely, CH and CRF), as measured by the ORA system. None of these values showed significant differences between pre- and post-CXL according to forest plot examination. An analysis of these data showed that the pre- and post-CXL differences in CH (SMD, 0.08; 95% CI, −0.27 to 0.43; p = 0.65) and CRF (SMD, 0.08; 95% CI, −0.27 to 0.42; p = 0.67) were not statistically significant. However, there were only 2 studies on this subject that were included in the analysis. One was a prospective, controlled study consisting of 14 participants from Israel24, and the other was a retrospective study consisting of 272 participants from Germany27. Additionally, the biomechanical changes induced by CXL are too subtle to be measured by ORA. Therefore, further studies are needed.
In our study, we found that endothelial cell density (ECD) and intraocular pressure (IOP) remained unchanged during the 12 months post-CXL (p > 0.05) and long-term follow-up of more than 18 months post-CXL (p > 0.05). Almost all studies reported that after CXL, the cornea and lens were both transparent, and there were no significant pre- and post-CXL differences in ECD and IOP values. Complications included bacterial keratitis, herpes simplex keratitis, cornea burn, and Descmet folds34. The most common complication was corneal haze, which is temporary and does not disrupt vision. In our meta-analysis, most participants did not have corneal haze after the CXL procedure (RR, 0.14; 95% CI, 0.03 to 0.6; p = 0.008; six trials, n = 359).
The present study has limitations that result from the quality of the individual trials and the methods of the meta-analysis itself. First, our research was restricted to studies published in indexed journals or trial registers. We did not search for unpublished studies. Second, the included trials varied with respect to population, participant age, KC stage, clinical outcome measurement, follow-up period, and quality. Only 4 studies were randomized controlled clinical trials that used the contralateral eye as a control. However, keratoconus is a bilateral, asymmetrical disease with varying rates of progression. Thus, in the present study, we included retrospective and prospective controlled studies and used the pre-CXL value of the same eye as the baseline value. Using this method, we obtained more valuable data for our analysis.
Third, there is substantial heterogeneity between the studies. Heterogeneity is common in meta-analyses of observational studies because unmeasured confounders and methodological issues can limit the comparability of groups and of the outcome assessment. Review Manager5 heterogeneity tests were applied. If p > 0.1, a fixed effects model was used; a random effects model was used if p < 0.1.
In conclusion, in this systematic review and meta-analysis, we found that CXL may be effective in halting the progress of KC for at least 12 months under certain conditions. The effects of CXL on visual acuity improvement are also remarkable. With long-term follow-up (after 18 months post-CXL), a significant decrease in Kave and MRSE was observed, and BCVA also significantly increased compared with the pre-CXL values. However, no statistical difference in CT after CXL was found during long-term follow-up. Further research from randomized trials is necessary to confirm these findings.
Methods
This systematic review and meta-analysis was performed following a predefined protocol and using generally accepted methodological practices and recommendations35,36.
Study selection
Two reviewers independently searched the PubMed, EMBASE, and Controlled Trials Register databases for publications from October 2007 to March 2014. The following keywords were used: corneal cross-linking (CXL), corneal collagen cross-linking, collagen cross-linkage, and keratoconus.
We reviewed the abstracts of related titles and retrieved the full articles if their title or abstract appeared to meet the objectives of this review. Studies were included if they met the inclusion criteria. Only studies that included human subjects and were published in the English language were included.
Study inclusion and exclusion criteria
Studies were included if they discussed the diagnosis of progressive keratoconus (Amsler-Krumeich grades I and III). Given the paucity of available evidence addressing the study question, the search was not restricted to randomized controlled trials (RCTs); prospective and retrospective controlled clinical trials and comparative cohort studies were also included. All of the articles that were found were carefully reviewed to select those that reported original clinical data pre- and post-operatively. Data from previously reported cases included in different articles were omitted to avoid duplication of data. Articles on corneal collagen cross-linking combined with other treatments, such as topography-guided photorefractive keratectomy (PRK) or intrastromal corneal ring segments, were excluded.
Quality assessment
Two reviewers (TC and FZ) separately evaluated the studies based on the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions37. The reviewers examined the following aspects of methodological quality: allocation concealment, method of treatment allocation, masking of outcome assessment, and completeness of follow-up. On the basis of their assessments and the Cochrane Collaboration guidelines, the two reviewers independently compiled a list of trials to be included in the meta-analysis. The lists were compared and found to be identical.
Data abstraction and clinical outcome
Study selection and data abstraction were performed independently by two reviewers according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement37. We extracted the raw data and converted them into a standard format. The primary outcome parameters investigated in this study were pre- and post-CXL uncorrected distance visual acuity (UCVA), best-corrected distance visual acuity (BCVA), astigmatism, spherical equivalent (SE), Kmax, Kave, and the thinnest corneal thickness (CT). The secondary outcome parameters investigated in this study were front elevation, back elevation, endothelial cell density (ECD), corneal hysteresis (CH), corneal resistance factor (CRF), intraocular pressure (IOP), and post-CXL complications.
Statistical analysis
The quantitative data associated with the outcome parameters were entered into the Cochrane Review Manager software program, version 5.1 (RevMan, computer program, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark, 2011) and were analysed. Standard mean differences (SMDs) and 95% confidence intervals (CIs) were calculated. For continuous outcome data (e.g., UCVA and BCVA), the standardized mean difference was calculated using the mean and standard deviation. For dichotomous outcomes (e.g., corneal haze post-CXL), the risk ratios (RRs) and 95% CIs were calculated.
Statistical heterogeneity between the trials included in the meta-analysis was assessed and quantified using the I2 statistic, which estimates the percentage of total variation across studies caused by heterogeneity rather than chance38. If I2 < 50%, we presented the analysis results according to a random effects model using the method of DerSimonian and Laird, which considers both within- and between-study variations39. The random effects model was chosen because it incorporates statistical heterogeneity and provides a more conservative estimate of the pooled effect size compared with the fixed model. If I2 > 50, we used the fixed model method. Two-tailed p values less than 0.05 were considered statistically significant.
Acknowledgments
We thank Mr. Li Nan of the Beijing University Associated Hospital Institute of Epidemiology for statistical support. We also thank Huang Peng of PLA Hospital for collecting and sorting the paper forms.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.C. and P.X. designed the study. T.C. and F.Z. separately evaluated the quality of each of the enrolled studies and independently formed a list of studies to include in the meta-analysis. Z.F. performed the meta-analysis and produced the tables and pictures in this manuscript. Z.X. and P.X. contributed to the raw data collection.
References
- Romero-Jimenez M., Santodomingo-Rubido J. & Wolffsohn J. S. Keratoconus: a review. Cont. Lens Anterior Eye 33, 157–166; quiz 205 (2010). [DOI] [PubMed] [Google Scholar]
- Kymes S. M., Walline J. J., Zadnik K., Sterling J. & Gordon M. O. Changes in the quality-of-life of people with keratoconus. Am. J. Ophthalmol. 145, 611–617 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno A., Naor J., Lee H. M., Hunter W. S. & Rootman D. S. Three decades of corneal transplantation: indications and patient characteristics. Cornea 19, 7–11 (2000). [DOI] [PubMed] [Google Scholar]
- Alio J. L., Pinero D. P. & Daxer A. Clinical outcomes after complete ring implantation in corneal ectasia using the femtosecond technology: a pilot study. Ophthalmology 118, 1282–1290 (2011). [DOI] [PubMed] [Google Scholar]
- Raiskup F. & Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. Part II. Clinical indications and results. Ocul. Surf. 11, 93–108 (2013). [DOI] [PubMed] [Google Scholar]
- Ashwin P. T. & McDonnell P. J. Collagen cross-linkage: a comprehensive review and directions for future research. Br. J. Ophthalmol. 94, 965–970 (2010). [DOI] [PubMed] [Google Scholar]
- Coskunseven E., Jankov M. R. 2nd & Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J. Refract. Surg. 25, 371–376 (2009). [DOI] [PubMed] [Google Scholar]
- Brooks N. O., Greenstein S., Fry K. & Hersh P. S. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J. Cataract Refract. Surg. 38, 615–619 (2012). [DOI] [PubMed] [Google Scholar]
- O'Brart D. P., Chan E., Samaras K., Patel P. & Shah S. P. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br. J. Ophthalmol. 95, 1519–1524 (2011). [DOI] [PubMed] [Google Scholar]
- Wittig-Silva C. et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J. Refract. Surg. 24, S720–725 (2008). [DOI] [PubMed] [Google Scholar]
- Hersh P. S., Greenstein S. A. & Fry K. L. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J. Cataract Refract. Surg. 37, 149–160 (2011). [DOI] [PubMed] [Google Scholar]
- Asri D. et al. Corneal collagen crosslinking in progressive keratoconus: multicenter results from the French National Reference Center for Keratoconus. J. Cataract Refract. Surg. 37, 2137–2143 (2011). [DOI] [PubMed] [Google Scholar]
- Jain R., Basu S. & Garg P. Corneal collagen cross-linkage in keratoconus. Br. J. Ophthalmol. 97, 108–109 (2013). [DOI] [PubMed] [Google Scholar]
- Greenstein S. A., Fry K. L. & Hersh P. S. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J. Cataract Refract. Surg. 37, 1282–1290 (2011). [DOI] [PubMed] [Google Scholar]
- Mazzotta C. et al. Morphological and functional correlations in riboflavin UV A corneal collagen cross-linking for keratoconus. Acta. Ophthalmol. 90, 259–265 (2012). [DOI] [PubMed] [Google Scholar]
- Viswanathan D. & Males J. Prospective longitudinal study of corneal collagen cross-linking in progressive keratoconus. Clin. Experiment. Ophthalmol. 41, 531–536 (2013). [DOI] [PubMed] [Google Scholar]
- Hashemi H., Seyedian M. A., Miraftab M., Fotouhi A. & Asgari S. Corneal collagen cross-linking with riboflavin and ultraviolet a irradiation for keratoconus: long-term results. Ophthalmology 120, 1515–1520 (2013). [DOI] [PubMed] [Google Scholar]
- Kranitz K. et al. Corneal changes in progressive keratoconus after cross-linking assessed by Scheimpflug camera. J. Refract. Surg. 28, 645–649 (2012). [DOI] [PubMed] [Google Scholar]
- Henriquez M. A., Izquierdo L. Jr, Bernilla C., Zakrzewski P. A. & Mannis M. Riboflavin/Ultraviolet A corneal collagen cross-linking for the treatment of keratoconus: visual outcomes and Scheimpflug analysis. Cornea 30, 281–286 (2011). [DOI] [PubMed] [Google Scholar]
- Arbelaez M. C., Sekito M. B., Vidal C. & Choudhury S. R. Collagen cross-linking with riboflavin and ultraviolet-A light in keratoconus: One-year results. Oman J. Ophthalmol. 2, 33–38 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinciguerra P. et al. Refractive, topographic, tomographic, and aberrometric analysis of keratoconic eyes undergoing corneal cross-linking. Ophthalmology 116, 369–378 (2009). [DOI] [PubMed] [Google Scholar]
- Arora R., Jain P., Goyal J. L. & Gupta D. Comparative analysis of refractive and topographic changes in early and advanced keratoconic eyes undergoing corneal collagen crosslinking. Cornea Epub ahead of print (2013). [DOI] [PubMed] [Google Scholar]
- Koller T., Iseli H. P., Hafezi F., Vinciguerra P. & Seiler T. Scheimpflug imaging of corneas after collagen cross-linking. Cornea 28, 510–515 (2009). [DOI] [PubMed] [Google Scholar]
- Goldich Y. et al. Clinical and corneal biomechanical changes after collagen cross-linking with riboflavin and UV irradiation in patients with progressive keratoconus: results after 2 years of follow-up. Cornea 31, 609–614 (2012). [DOI] [PubMed] [Google Scholar]
- Stojanovic A. et al. Safety and efficacy of epithelium-on corneal collagen cross-linking using a multifactorial approach to achieve proper stromal riboflavin saturation. J. Ophthalmol. 2012, 498435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarsen A. & Hjortdal J. Collagen cross-linking for advanced progressive keratoconus. Cornea 32, 903–906 (2013). [DOI] [PubMed] [Google Scholar]
- Spoerl E., Terai N., Scholz F., Raiskup F. & Pillunat L. E. Detection of biomechanical changes after corneal cross-linking using Ocular Response Analyzer software. J. Refract. Surg. 27, 452–457 (2011). [DOI] [PubMed] [Google Scholar]
- Sloot F., Soeters N., van der Valk R. & Tahzib N. G. Effective corneal collagen crosslinking in advanced cases of progressive keratoconus. J. Cataract Refract. Surg. 39, 1141–1145 (2013). [DOI] [PubMed] [Google Scholar]
- Raiskup-Wolf F., Hoyer A., Spoerl E. & Pillunat L. E. Collagen crosslinking with riboflavin and ultraviolet-A light in keratoconus: long-term results. J. Cataract Refract. Surg. 34, 796–801 (2008). [DOI] [PubMed] [Google Scholar]
- Saffarian L., Khakshoor H., Zarei-Ghanavati M. & Esmaily H. Corneal crosslinking for keratoconus in Iranian patients: outcomes at 1 year following treatment. Middle East Afr. J. Ophthalmol. 17, 365–368 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Raggal T. M. Riboflavin-ultraviolet A corneal cross-linking for keratoconus. Middle East Afr. J. Ophthalmol. 16, 256–259 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporl E. et al. [Biomechanical condition of the cornea as a new indicator for pathological and structural changes]. Ophthalmology 106, 512–520 (2009). [DOI] [PubMed] [Google Scholar]
- Spoerl E., Huhle M. & Seiler T. Induction of cross-links in corneal tissue. Exp. Eye Res. 66, 97–103 (1998). [DOI] [PubMed] [Google Scholar]
- Bromley J. G. & Randleman J. B. Treatment strategies for corneal ectasia. Curr. Opin. Ophthalmol. 21, 255–258 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Smith G. D. & Phillips A. N. Meta-analysis: principles and procedures. BMJ 315, 1533–1537 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D. et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354, 1896–1900 (1999). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 (2010). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]