SUMMARY
Background
Multidrug-resistant tuberculosis (MDR-TB) burden estimates are based on incomplete, infrequently updated data among a limited pool of cases: notified or incident, pulmonary TB patients.
Methods
Using WHO data reported by 217 countries/territories in 2014, we calculated MDR-TB burdens among prevalent TB cases and compared these with estimates among incident and notified TB patients. We also compared treatment coverage across estimates.
Findings
Among prevalent TB patients globally in 2014, we estimate that 555,545 (95% credible bounds: 499,340–617,391) MDR-TB cases occurred. This is 85% more than the 300,000 estimated among notified cases, and 16% more than the 480,000 among incident cases. Only 20% of MDR-TB cases among prevalent—compared to 37% of MDR-TB among notified—TB patients had access to MDR-TB treatment. Applying prior estimates, only 10% of MDR-TB cases will have successful outcomes.
Interpretation
Estimates based on likely-to-be-diagnosed cases of MDR-TB overlook a significant proportion of morbidity, mortality, and transmission: that occur in undiagnosed, untreated, prevalent TB patients. Still likely underestimating the true disease burden, MDR-TB among patients with prevalent TB represents a closer approximation of disease burden than currently reported indicators. Progress toward elimination—or control—depends on policies guided by a more complete representation of the disease burden.
Keywords: drug-resistant TB, epidemiology, prevalence
INTRODUCTION
Tuberculosis (TB), a curable disease, occurs in nearly 10 million people annually; less than two-thirds of cases are reported to the World Health Organization (WHO).1 Many people suffering from TB are poor, vulnerable, and disenfranchised. Their status impedes the ability to garner resources necessary to assure lifesaving treatment.2 The gap in resources for TB treatment and research was estimated at a staggering $2.7 billion for 2015.1
Multidrug-resistant (MDR) TB, caused by Mycobacterium tuberculosis resistant to both rifampicin and isoniazid, was estimated to have occurred in 3.3% (95%CI: 2.2–4.4%) of new and 20% (95%CI: 14–27%) of previously treated TB cases in 2014.1 This corresponds to MDR-TB in 300,000 (range: 220,000–370,000) of 5.16 million notified pulmonary TB cases. A total of 480,000 (range: 360,000–600,000) incident cases of MDR-TB were estimated.
MDR-TB among notified TB cases has been promoted as the indicator of choice because it: “is directly measureable at the national level and can be monitored annually with an established continuous DR surveillance system; provides a pragmatic denominator for national TB programs to monitor their progress towards diagnosing and treating MDR-TB cases among all notified TB cases; and provides a reliable number to use when forecasting drug procurement needs.”3 Country and regional estimates are therefore published annually. Notified, pulmonary TB patients, however, represent only 54% of all TB cases.1 A slightly broader indicator, incident (new, relapse and retreated) cases, accounts for more, but only 65% of all TB cases.4 Estimating MDR-TB among both, therefore, likely profoundly under-represents the global MDR-TB burden.3 And, both ignore the chronic nature of TB effectively assuming that only patients diagnosed with new disease in a year need to be considered.
Prevalence is generally considered the preferred epidemiologic indicator for infectious diseases of longer duration. Moreover, period (estimated cases, new and extant, accumulating over an interval), rather than point (estimated cases at a single point in time) prevalence would be a more inclusive indicator for estimation of risk of transmission, morbidity and mortality, and resources required to prevent and treat MDR-TB.5,6 Period prevalence will consistently provide a higher estimate of the burden of disease than will incidence or point prevalence (Figure 1).
Figure 1. Simulated cohort of 10 individuals to illustrate difference in calculated incidence, point prevalence, and period prevalence.
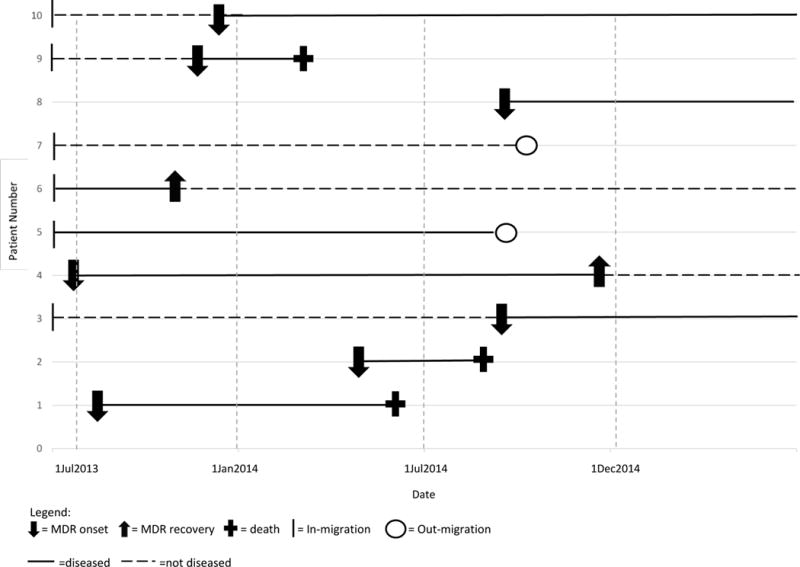
2014 Point prevalence =3; 42,857/100,000 population (number of patients with disease on 1 July 2014/number of individuals in the population on 1 July 2014)
2014 Period prevalence= 7; 70,000/100,000 population (number of patients with disease between 1 January 2014 and 31 December 2014/number of individuals in the population during any portion of that period)
2014 Incidence = 3; 44,444/100,000 person-years (number of patients with disease between 1 January 2014 and 31 December 2014/person-years of exposure between 1 January 2014 and 31 December 2014
Other factors leading to underestimated burden of MDR-TB are described by Cohen and colleagues and highlighted here.7 Routine drug susceptibility testing (DST) occurs in only a limited number of countries.3 Since 1994, 153 countries have estimated MDR-TB burden. In 80, disproportionately low-burden and high-income countries, this is from continuous surveillance of anti-TB drug resistance based on routine DST.1 The others, where a greater proportion of the global MDR-TB cases occur, relied on periodic (or a single8) surveys of national or sub-national TB patient samples1, which have possible biases.9 Many date from before the year 2000; since MDR-TB generally increases as a proportion of cases over time10, reliance on 15–20 year-old survey results likely under-represents MDR-TB burden. This effect is amplified by incorporating these outdated values in regression models, fitted with country-level characteristics of TB programs and epidemiology, to estimate MDR-TB burden in countries without surveys or surveillance.1
The resulting under-representation of the burden of MDR-TB has serious implications for disease elimination or control. Planning by Ministries of Health and Finance is based on these lower estimates, limiting allocation of resources to MDR-TB prevention, diagnosis, and treatment.11,12 Forecasting of demand for drugs, diagnostics and other consumables is affected and the incentive to manufacture products for these patients is dampened.13 Transmission, morbidity, and mortality continue to occur, even among patients who are not counted or projected. To curtail the epidemic, a more inclusive estimate of the MDR-TB burden is necessary.14
We offer an alternative appraisal of the global burden of MDR-TB, capturing some of the chronicity by estimating MDR-TB among prevalent TB cases. We interpret estimates of access to MDR-TB treatment and reports of treatment success relative to disease burden.
METHODS
This study used publicly available country-level data reported to WHO (2014): TB notifications among new and previously treated patients, TB prevalence, and percent of new and previously treated TB cases with MDR-TB.2 Data were imported into Microsoft Excel for Mac 2011 (version 14.3.9) for analysis.
First, we estimated the percent of prevalent TB cases that were new (Pn) and previously treated (Pr) by multiplying the total number of prevalent TB cases (P) by the percentage of each type, new (Nn) and previously treated (Nr), reported among notified cases. Then, we multiplied Pn and Pr respectively, by the percentage of new (Mn) and previously treated (Mr) TB patients who had MDR-TB. The sum of these products was the total number of estimated MDR-TB cases among prevalent TB patients in 2014:
-
(1)
MDR-TB among prevalent TB cases = ((P × Nn)× Mn) + ((P × Nr)× Mr)
We performed calculations for every country/territory separately. We used bootstrapping to generate each estimate and a 95% credible bound (CB) around that value. These bounds, reflecting the degree of uncertainty around our estimates, contain 95% of the 1000 estimates simulated. For each input parameter used from WHO reports, we drew 1000 different potential values based on the point estimate and its confidence interval, and on assumptions about the distribution: normal for P, Mn and Mr. When the confidence intervals around Mn or Mr were particularly wide, non-symmetric, and bounded at the lower end by 0, we assumed a uniform distribution. We assumed that Nn and Nr were binomially distributed.
We produced 1000 iterations of the number of MDR-TB cases for each country/territory: the median value was selected as the number of MDR-TB cases among prevalent TB cases and the 2.5th and 97.5th percentiles, respectively as the lower and upper CB. We then aggregated these over WHO regions and the world and calculated the following, all as a percent of (1):
-
(2)
MDR-TB cases reported to WHO;
-
(3)
MDR-TB patients reported to WHO who started treatment in 2014; and
-
(4)
MDR-TB patients successfully treated, using percent treatment success reported in 2015.
Finally, we compared measures 1–4 with analogous WHO estimates.1
Ethics approval was not required because this analysis used publicly available, de-identified data.
RESULTS
Data were available for all 217 countries included in the 2015 WHO report. We calculated 555,545 (CB: 499,340–617,391) cases of MDR-TB among prevalent TB patients globally. This is 16% higher than the figure of 480,000 (95% CI: 360,000–600,000) cases of MDR-TB among incident TB cases and 85% higher than the estimate of 300,000 (95% CI: 220,000–370,000) cases of MDR-TB among notified TB cases. Regional differences between our figures and those among notified cases ranged from 44% to 190% (Table 1); the largest difference was observed in the Eastern Mediterranean Region.
Table 1.
MDR-TB estimates by WHO region among prevalent and notified cases (2014)
| Region | TB Prevalence* (95% CI) |
MDR-TB among prevalent cases (95% CI) |
Notified cases of TB* | MDR-TB cases among notified cases* (95% CI) |
% increase MDR among notified to MDR among prevalent |
|---|---|---|---|---|---|
| AFR | 3,200,000 (2,800,000, 3,600,000) |
83,210 (69,261, 101,034) |
1,342,400 | 32,000 (15,000, 49,000) |
160% |
| AMR | 350,000 (270,000, 440,000) |
14,201 (11,392, 17,418) |
228,476 | 7,000 (4,700, 9,300) |
103% |
| EMR | 1,000,000 (880,000, 1,200,000) |
43,525 (34,051, 52,998) |
465,677 | 15,000 (12,000, 19,000) |
190% |
| EUR | 440,000 (330,000, 560,000) |
103,955 (77,579, 133,776) |
321,421 | 72,000 (62,000, 81,000) |
44% |
| SEAR | 5,400,000 (4,400,000, 6,500,000) |
197,236 (157,087, 237,931) |
2,580,605 | 99,000 (90,000, 110,000) |
99% |
| WPR | 2,100,000 (1,900,000, 2,400,000) |
111,908 (91,891, 134,494) |
1,335,816 | 71,000 (47,000, 94,000) |
58% |
| Global | 13,000,000 (11,000,000, 14 000,000) |
555,545 (499,340, 617,391) |
6,314,151 | 300,000 (220,000, 370,000) |
85% |
CI: Confidence Interval; AFR: African Region; AMR: Region of the Americas; EMR: Eastern Mediterranean Region; EUR: European Region; SEAR: South-East Asia Region; WPR: Western Pacific Region.
These numbers were taken from the WHO Global Tuberculosis Report 20151
Countries reported nearly 123,000 MDR-TB cases to WHO for 2014, or 41% of estimated MDR-TB cases among notified TB patients and 22% of MDR-TB cases among prevalent TB patients. Treatment was started in 111,000 of them: more than 90% of reported cases, 37% of the 300,000 MDR-TB cases among notified TB patients, 23% of 480,000 cases of MDR-TB among incident TB patients, and 20% of MDR-TB cases among prevalent TB patients (Figure 2). MDR-TB treatment was successful in only 50% of those who started treatment in 2012. If the same percent success is achieved among those starting treatment in 2014, only 10% of MDR-TB patients among prevalent TB cases, 11.5% of MDR-TB patients among incident TB cases, and 18.5% of MDR-TB patients among notified TB cases would be successfully treated.
Figure 2.

Estimated percentage of MDR-TB cases occurring, reported, treated, and successfully treated among the global total of notified (right-hand side of figure) and prevalent (left-hand side of figure) TB cases in 2014
DISCUSSION
The global MDR-TB burden is likely underestimated by limiting the pool of patients considered to have MDR-TB to those with notified or incident TB. Considering all prevalent cases of TB increases the estimated burden by 85% over that in notified TB cases and 15% compared to that in incident TB patients. These differences reflect the limits applied to the population pool considered at risk for MDR-TB: standard estimates exclude unreported and/or non-new TB patients.
Estimates of MDR-TB among notified cases of TB (i.e., the cases that would be detected if all notified cases of TB were tested for drug resistance using current methods) are presented by country and region in the 2015 WHO Global Tuberculosis Report1 and are the benchmark against which treatment coverage is measured. Their use has a cascade effect that results in overly optimistic assessments of progress against the epidemic, across successive indicators: the 111,000 patients who received treatment in 2014 represent only 20% of MDR-TB cases among prevalent TB patients and 37% of MDR-TB cases among notified TB patients. The poor prognosis of treated MDR-TB means that only 10% of MDR-TB cases among prevalent TB patients can expect treatment success.
Recent reports that expanded the at-risk population from notified to incident TB provided estimates for subsets of countries of epidemiologic interest.15,16 The present study provides estimates for all countries and regions, facilitating comparison, albeit to the narrower indicator used in WHO reports, MDR-TB among notified TB. This permits detection of important variability in the burden of MDR-TB and in the difference between previously published estimates and those presented here. The smallest regional differences between the two estimates of MDR-TB burden were 44% and 58%, in the European and Western Pacific regions. The greatest were 160% and 190%, in the African and Eastern Mediterranean regions. This is likely because in Europe and the Western Pacific, a much larger proportion of prevalent TB cases are notified than in Africa and the Eastern Mediterranean.1,3
More complicated is the uneven reporting of MDR-TB as a percentage of estimated MDR-TB cases. In Africa, nearly 80% of MDR-TB cases estimated to have occurred among notified TB patients were reported. This is surprising as, in 2014, only 6.4% of new notified and 33% of previously treated notified patients in Africa had DST results17 and only 42% of estimated prevalent TB cases in Africa were notified.1 This suggests that the burden of MDR-TB may be more severely underestimated in Africa than in other regions.18 Further improvements in TB reporting and surveillance or surveys for drug resistance are required there. In contrast, in Europe, 59% of estimated MDR-TB cases among notified patients and 40% of estimated MDR-TB cases among prevalent TB patients are reported.17 DST was performed in 97% of new patients and 52% of previously treated patients. For Europe, the emphasis on increasing DST coverage among previously treated patients, as well as improvements in reporting of TB cases, may close the gap between anticipated burden and reported numbers of cases.
The paltry proportion of MDR-TB cases that are treated—and even smaller proportion successfully treated—is underscored by the present exercise. With only 20% of the estimated global MDR-TB burden in 2014 enrolled in treatment and, only half expected to experience successful treatment outcomes,19 this means that 90% of cases continue to transmit to community and family members and be at high risk for mortality.20,21 Models built on under-projection of cases (and over-projection of treatment success) will not accurately predict the epidemiologic impact of interventions. The rationale for using the indicator MDR-TB among notified cases rests on the benefits for short-term programmatic planning when forecasting drug procurement and other resource needs.3,22,23 Planning for long-term impact, i.e., elimination, requires that national TB programs; Ministries of Finance; donors; policy-makers; activists; and manufacturers of diagnostics, treatment, and other consumables have access to estimates that reflect the totality of the problem.
Our estimate of 555,545 MDR-TB cases among prevalent TB patients likely still falls far short of true prevalence of MDR-TB. The remaining gap has multiple sources. First, per cent MDR among TB patients is likely underestimated. Data from drug-resistance surveys are not representative of the current, global situation: 69 countries have either never conducted drug-resistance surveys or have not done one in the last 10 years. Resistance estimates in these countries are based on models fitted with old data and data from other countries. MDR-TB as a proportion of TB cases, however, generally increases over time, especially in places with poorer surveillance;8 exceptions are some high GDP countries8 where resistance testing and appropriate treatment for all forms of TB are available at first TB diagnosis. Disproportionately represented among countries without recent surveys or surveillance are those that have experienced important disruptions in recent years (e.g., Afghanistan, Burundi, DR Congo, Haiti, Liberia, Sierra Leone, South Sudan, Sudan, and West Bank and Gaza Strip), which could increase the risk of MDR-TB.24 This limitation affects all projections of MDR-TB burden derived from global survey results.
Second, there may be important differences between notified and prevalent TB cases, linked to the distribution of patients previously treated for TB in these groups. We assumed that MDR-TB is equally common among notified and prevalent TB cases, when, in fact, MDR-TB is likely more common among prevalent TB cases, at least those that have been previously treated. We assumed that the proportion of previously treated cases was the same among prevalent and notified cases. If the proportion of previously treated cases is higher among prevalent cases than among notified cases, then our figures would be under-estimated. Prevalence of MDR-TB is consistently higher among previously treated than among new patients.
Another source of bias is the longer duration of MDR-TB compared to drug-susceptible (DS) TB: delays to diagnosis and treatment of MDR-TB25 generally exceed those for treatment of DS-TB. This is reflected in the difference in percent of cases treated: only 23% of estimated, incident MDR-TB patients in a year start treatment, while 2.6 times more, or 60% of, DS-TB (5.4 million treated1 among 9.0 million who developed TB in 201326) patients receive treatment. And, treatment of MDR-TB (18–24 months27) lasts approximately 3–4 times as long as treatment for DS-TB (6 months). The presumed difference in duration may be shortened by increased risk of death among MDR-TB patients. If, conservatively, MDR-TB lasts twice as long as DS-TB—and the proportion of MDR-TB among prevalent TB is correspondingly two times the proportion of MDR-TB among incident cases—then, the 2014 point prevalence of MDR-TB would be estimated at more than 1.1 million. As noted earlier, point prevalence ignores cases that occurred and resolved during the interval of interest, but before the measurement of the indicator (Figure 1). Period prevalence of MDR-TB would be even higher and would more closely approximate the true burden of disease. The ability to estimate this indicator is constrained by absence of robust data on duration of disease (which requires information on treatment delay, time to elimination of disease through treatment or death).
Lastly, underestimation may also result from the calculation of TB prevalence;4 indirect country estimates are frequently revised upwards after representative prevalence surveys.28 In addition, prevalence surveys generally exclude culture-negative, extra-pulmonary and pediatric patients.29
In sum, this alternative, which shows a larger burden of MDR-TB, provides only a starting point for addressing the true MDR-TB burden and quantifying the gap in treatment coverage by country and region. Further improvement would derive from use of period prevalence, with more accurate estimates of the distribution of previously treated patients and MDR-TB among prevalent TB patients, and more accurate estimates of TB prevalence and resistance. Elimination depends on diagnosis and treatment of all cases of MDR-TB, which in turn relies on planning for allocation of resources based on an improved assessment of the burden. This should be considered when the scheduled review of methods to estimate MDR-TB takes place in 2016.1 If decisions—by countries, industry, and normative bodies—continue to rely on a minimalist assessment of MDR-TB burden, the impact of the epidemic will persist and grow, causing preventable transmission and mortality.
Acknowledgments
HEJ was supported by Award Number K01AI102944 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
We thank F. Cobelens for his contribution of knowledge and fruitful discussions during this research and J. Lindstrom-Vautrin for production of figures.
Footnotes
AUTHOR CONTRIBUTION
CDM, HEJ and SN designed the study. SN extracted data from the WHO global reports and HEJ performed analyses. CDM, HEJ and SN contributed substantially to interpretation of results. SN and CDM wrote the first draft of the paper. All authors revised it critically for important intellectual content and prepared the final version of the paper. All authors have approved this version for publication.
COMPETING INTERESTS
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.WHO. World Tuberculosis Report 2015. World Health Organization; 2015. [Google Scholar]
- 2.Keshavjee S, Farmer PE. Time to put boots on the ground: making universal access to MDR-TB treatment a reality. International Union against Tuberculosis and Lung Disease. 2010;14(10):1222–5. [PubMed] [Google Scholar]
- 3.Stop TB Partnership. Estimates of the burden of disease caused by multidrug-resistant TB and monitoring the programmatic response: what indicators should be used and for what purpose? MDR-TB Stakeholders Meeting (Paris, 2013) 2014 [Google Scholar]
- 4.WHO. Global TB Report 2015: Technical appendix on methods used to estimate the global burden of disease caused by TB. World Health Organization; 2015. Updated 2015. [Google Scholar]
- 5.Giesecke J. Modern infectious disease epidemiology. 2nd. London: Arnold; 2002. [Google Scholar]
- 6.Aschengrau A, Seage G. Essentials of epidemiology in public health. 2nd. Sudbury, MA: Jones and Bartlett; 2008. [Google Scholar]
- 7.Cohen T, Colijn C, Wright A, Zignol M, Pym A, Murray M. Challenges in estimating the total burden of drug-resistant tuberculosis. American journal of respiratory and critical care medicine. 2008;177(12):1302–6. doi: 10.1164/rccm.200801-175PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen T, Jenkins HE, Lu C, McLaughlin M, Floyd K, Zignol M. On the spread and control of MDR-TB epidemics: an examination of trends in anti-tuberculosis drug resistance surveillance data. Antimicrobial and anticancer chemotherapy. 2014;17(4–6):105–23. doi: 10.1016/j.drup.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen T, Hedt BL, Pagano M. Estimating the magnitude and direction of bias in tuberculosis drug resistance surveys conducted only in the public sector: a simulation study. BMC public health. 2010;10:355. doi: 10.1186/1471-2458-10-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C, Maher D, Weil D, Espinal M, Raviglione M. Targets for global tuberculosis control. International Union against Tuberculosis and Lung Disease. 2006;10(4):460–2. [PubMed] [Google Scholar]
- 11.MDR-TB Planning Toolkit. 2012 [cited 2015]; Available from: http://www.path.org/publications/files/TB_mdr-tb_toolkit.pdf.
- 12.Madansein R, Parida S, Padayatchi N, Singh N, Master I, Naidu K, et al. Surgical treatment of complications of pulmonary tuberculosis, including drug-resistant tuberculosis. International Journal of Infectious Diseases. 2015;32:61–7. doi: 10.1016/j.ijid.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Hwang TJ, Keshavjee S. Global financing and long-term technical assistance for multidrug-resistant tuberculosis: scaling up access to treatment. PLoS medicine. 2014;11(9):e1001738. doi: 10.1371/journal.pmed.1001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitnick CD, Keravec J, Cohen T. Planning for the invisible: projecting resources needed to identify and treat all patients with MDR-TB. International Union against Tuberculosis and Lung Disease. 2013;17(4):427–8. doi: 10.5588/ijtld.13.0110. [DOI] [PubMed] [Google Scholar]
- 15.Falzon D, Jaramillo E, Wares F, Zignol M, Floyd K, Raviglione MC. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. The Lancet Infectious diseases. 2013;13(8):690–7. doi: 10.1016/S1473-3099(13)70130-0. [DOI] [PubMed] [Google Scholar]
- 16.Royce S, Falzon D, van Weezenbeek C, Dara M, Hyder K, Hopewell P, et al. Multidrug resistance in new tuberculosis patients: burden and implications. International Union against Tuberculosis and Lung Disease. 2013;17(4):511–3. doi: 10.5588/ijtld.12.0286. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Global TB Report Annex 4: Key TB indicators for individual countries and territories, WHO regions and the world. World Health Organization; 2015. [Google Scholar]
- 18.Lukoye D, Ssengooba W, Musisi K, Kasule GW, Cobelens FG, Joloba M, et al. Variation and risk factors of drug resistant tuberculosis in sub-Saharan Africa: a systematic review and meta-analysis. BMC public health. 2015;15:291. doi: 10.1186/s12889-015-1614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falzon D, Mirzayev F, Wares F, Baena IG, Zignol M, Linh N, et al. Multidrug-resistant tuberculosis around the world: what progress has been made? The European respiratory journal. 2015;45(1):150–60. doi: 10.1183/09031936.00101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dharmadhikari AS, Mphahlele M, Venter K, Stoltz A, Mathebula R, Masotla T, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. International Union against Tuberculosis and Lung Disease. 2014;18(9):1019–25. doi: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clinical infectious diseases. 2014;58(3):381–91. doi: 10.1093/cid/cit643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet M, Bastard M, du Cros P, Khamraev A, Kimenye K, Khurkhumal S, et al. Identification of patients who could benefit from bedaquiline or delamanid: a multisite MDR-TB cohort study. International Union against Tuberculosis and Lung Disease. 2016;20(2):177–86. doi: 10.5588/ijtld.15.0962. [DOI] [PubMed] [Google Scholar]
- 23.Mitnick C, van den Hof S. Using existing data to illustrate-and close-the gap in access to new anti-tuberculosis drugs. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2016;20(2):145. doi: 10.5588/ijtld.15.0911. Epub 2016/01/23. [DOI] [PubMed] [Google Scholar]
- 24.Umubyeyi AN, Vandebriel G, Gasana M, Basinga P, Zawadi JP, Gatabazi J, et al. Results of a national survey on drug resistance among pulmonary tuberculosis patients in Rwanda. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2007;11(2):189–94. Epub 2007/02/01. [PubMed] [Google Scholar]
- 25.Zhang X, Yin J, Li H, Li S, Walley J, Zou G, et al. Diagnostic and treatment delays of multidrug-resistant tuberculosis before initiating treatment: a cross-sectional study. Tropical medicine & international health : TM & IH. 2015;20(11):1431–7. doi: 10.1111/tmi.12566. Epub 2015/07/15. [DOI] [PubMed] [Google Scholar]
- 26.WHO. World Tuberculosis Report 2014. World Health Organization; 2014. [Google Scholar]
- 27.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS medicine. 2012;9(8):e1001300. doi: 10.1371/journal.pmed.1001300. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Tropical medicine & international health : TM & IH. 2015;20(9):1128–45. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Perez-Velez CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet (London, England) 2014;383(9928):1572–9. doi: 10.1016/S0140-6736(14)60195-1. Epub 2014/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]


