Abstract
Most childhood infections occur via the mucosal surfaces, however, parenterally delivered vaccines are unable to induce protective immunity at these surfaces. In contrast, delivery of vaccines via the mucosal routes can allow antigens to interact with the mucosa-associated lymphoid tissue (MALT) to induce both mucosal and systemic immunity. The induced mucosal immunity can neutralize the pathogen on the mucosal surface before it can cause infection. In addition to reinforcing the defense at mucosal surfaces, mucosal vaccination is also expected to be needle-free, which can eliminate pain and the fear of vaccination. Thus, mucosal vaccination is highly appealing, especially for the pediatric population. However, vaccine delivery across mucosal surfaces is challenging because of the different barriers that naturally exist at the various mucosal surfaces to keep the pathogens out. There have been significant developments in delivery systems for mucosal vaccination. In this review we provide an introduction to the MALT, highlight barriers to vaccine delivery at different mucosal surfaces, discuss different approaches that have been investigated for vaccine delivery across mucosal surfaces, and concludes with an assessment of perspectives for mucosal vaccination in the context of the pediatric population.
Keywords: intranasal vaccination, mucosal vaccination, nasal vaccination, oral vaccination, oral cavity vaccination, pollen grains, skin vaccination
Graphical abstract
1. INTRODUCTION - THE NEED FOR MUCOSAL VACCINATION
Majority of the pathogens invade via the mucosal surfaces such as those of the respiratory, reproductive and the gastrointestinal tracts. This is because these surfaces come in direct contact with the air, water, food, and the environment, and thus form an opportunistic portal for bacterial and viral infections. For example, infectious diseases resulted in the death of about 6.3 million children who were under the age of 5, worldwide in 2013, and the leading causes of death were pneumonia, diarrhoea and malaria each contributing 14.9%, 9.2%, and 7.3%, respectively [1]. Sub-Saharan Africa contributed roughly half of these under-5 deaths, and southern Asia almost a third. The delivery of vaccines across mucosal surfaces has the potential to stimulate synthesis of pathogen-specific mucosal immune responses [2–4], but the conventional systemic delivery of vaccines against infectious diseases using a needle and syringe is unable to induce a strong mucosal immune response. Mucosal immune responses are important because the pathogen-specific antibodies that are stimulated by mucosal vaccination get secreted into the mucus where they can neutralize the pathogens even before they can cause infection. Thus, success in generating this first-line of defense on the mucosal surfaces will represent a major advance in vaccinology, and has the potential to improve childhood vaccinations and reduce mortality. Furthermore, delivery of vaccines to mucosal surfaces can also induce systemic immunity similar to that induced by the conventional needle and syringe based vaccination.
In addition to extending the body’s immune protection to mucosal surfaces, mucosal vaccine delivery has other advantages. Importantly, mucosal vaccine delivery does not require needles and syringes, and is therefore inherently needle-free. Being needle-free, mucosal vaccinations can result in a pain free approach of vaccine delivery. Because pain from needle-injections and the ensuing fear is a significant challenge in pediatric vaccinations, mucosal vaccinations offer a very appealing alternative for the children and the parents alike [3]. Mucosal vaccines could also be self-administered, and therefore, could be provided in the comfort of one’s home, which would also reduce the burden on the healthcare professionals. Being a needle-free delivery approach, mucosal vaccination should also be able to address another major problem associated with needle-based injections, i.e., of needle reuse. In the year 2000, an estimated 40% of the 16 billion injections administered worldwide were from reused needles, which led to an estimated 21 million, 2 million, and 260,000 new cases of hepatitis B, hepatitis C, and HIV infections, respectively [5]. While not all these injections were vaccine-related, mucosal vaccination can still help to reduce this burden. Furthermore, mucosal vaccination can also reduce incidents of needle-stick injuries amongst the health care workers, and reduce sharp waste.
Mucosal vaccination is however, challenging. The numerous natural defense mechanisms of the host at mucosal surfaces, such as the acid and enzyme-rich environment of the stomach, and the mucus layer that coats all mucosal surfaces, actually work against successful delivery of vaccines across these surfaces. In this review we provide a brief discussion of the mucosa-associated lymphoid tissue (MALT) to help familiarize the readers regarding the immune system that processes vaccine antigens upon mucosal delivery, different barriers to mucosal vaccine delivery, and the different approaches that have been investigated to deliver vaccines across different mucosal surfaces. Different adjuvants that have been investigated in the context of mucosal vaccination are also discussed. The review concludes with a perspective on pediatric mucosal vaccination. While the vaginal and rectal routes for mucosal vaccination are important, especially with respect to sexually transmitted diseases and for diseases that typically affect females, however, these routes of mucosal vaccination are not included in this review. This is because the vaginal route is only applicable to females, and the rectal route for vaccination has poor acceptability due to resistance against it’s use in some ethnic groups and cultures. As a result, these routes offer a more specialized vaccine development program. In this review, we have focused on more widely applicable mucosal vaccination routes.
2. LICENSED MUCOSAL VACCINES APPROVED FOR HUMAN USE
Out of more than 25 diseases that have preventable vaccines, just five have mucosal vaccines, while the rest are delivered using needle and syringe. The diseases with mucosal vaccines are listed in Table 1. Out of the five, four are delivered via the oral route and one is delivered via the intranasal route. These five vaccines are discussed below especially with respect to safety considerations.
Table 1.
Mucosal vaccines licensed for human use
| Disease (example of licensed vaccine) | Delivery route | Live or Inactivated |
|---|---|---|
| Cholera (Dukoral®, Shanchol™, and mORC-Vax™) | Oral | Inactivated |
| Influenza (FluMist™) | Intranasal | Live attenuated |
| Poliomyelitis (Biopolio™ B1/3, and other oral polio vaccines - OPVs) | Oral | Live attenuated |
| Rotavirus (Rotarix® and RotaTeq®) | Oral | Live attenuated |
| Typhoid (Vivotif®) | Oral | Live attenuated |
The cholera vaccine Dukoral® is a mixture of inactivated Vibrio cholera and cholera toxin B subunit (CTB). Presence of CTB allows the vaccine to provide short term protection against enterotoxigenic Escherichia coli (ETEC), however, to preserve CTB in the stomach’s acidic environment the vaccine is mixed with a basic solution (sodium bicarbonate) just prior to oral uptake. The other two cholera vaccines do not contain CTB and thus can be ingested without mixing with sodium bicarbonate. Cholera vaccines are considered to be safe [6].
Mucosal influenza vaccine has been used in the Russian Federation for more than 50 years, and a variant of the formulation was first approved for use in the US in 2003. The vaccine comprises of live attenuated influenza virus (LAIV), and is delivered by spraying as a mist in the nasal cavity. The virus is capable of replicating in the cooler environment of the nasal cavity, but not in the warmer temperature of the body in the deeper parts of the respiratory tract. This vaccine is not recommended for children under the age of 2 years due to increased risk of wheezing. The virus is known to shed from the nasal cavity of children after vaccination for up to 21 days (mean 7–8 days), however, this shed–virus has not been found to be of concern [7]. Asthma is also a contraindication for this vaccine.
Oral polio vaccine (OPV) comprises of live attenuated polioviruses obtained by the passage of wild-type strains in non-human cells. These attenuated virus strains have significantly reduced neurovirulence and transmissibility. OPV is administered as two drops (about 0.1 ml) into the mouth. From a safety perspective, OPV is associated with rare vaccine-associated paralytic poliomyelitis (VAPP) and the emergence of vaccine derived polioviruses (VDPVs). VAPP occurrence rate is about 2–4 cases per million birth cohort per year. VDPVs can actually arise due to prolonged incubation and replication of the vaccine strain in a vaccinee, and could lead to transmission of the disease in the community [8].
Rotavirus vaccine consists of human or human-bovine live attenuated rotavirus strains. The vaccine is administered orally. A previous rhesus rotavirus reassortant vaccine (RotaShield®) was found to have high (1:10,000) risk of intussusception, which is a serious and potentially lethal condition arising from intestinal invagination, leading to blockage, bleeding, vomiting, and pain. Even the Rotarix® and RotaTeq® vaccines have a risk of causing intussusception, however it is lower than that of RotaShield®, and the benefit of the two vaccines outweighs their risk [9]. As reported in the product inserts of Rotarix® and RotaTeq®, it has also been found that vaccine-rotavirus is shed from the vaccinee’s stools, and can cause infection, especially in immunocompromised individuals or those on immunosuppressants.
The typhoid vaccine comprises of attenuated strain Salmonella typhi. The formulation comprises of an enteric-coated capsule that contains lyophilized bacteria. The vaccine is very well tolerated. Vaccine organisms are shed from vaccinees, however secondary infection has not been documented [10].
In general, the approved mucosal vaccines are not recommended for use in infants, except the rotavirus vaccine, which can be administered at the age of 6 weeks, and the oral polio vaccine, which can be given at birth. Other vaccines are recommended for humans above the age of 2 years. A typical reason for this age limit is the lack of safety data of the attenuated strains in infants. Live attenuated virus can replicate in mucosal epithelia at the site of delivery, and to create attenuated strains that are also safe for use in infants is often challenging. As seen from OPV, potential to regain virulence by the vaccine strain can be a safety hazard. Furthermore, choice of strain used to create the vaccine can have unforeseen effects as was seen in the case of rhesus rotavirus strain used in RotaShield®, which caused high incidence of intussusception. Clearly, the ability to use mucosal vaccines in infants is a high priority to help reduce childhood deaths, which are predominantly caused by pathogens associated with mucosal entry [1]. The use of non-viral vaccines can offer a safer alternative, however, delivery of non-viral vaccines is more challenging [2, 11], because unlike attenuated viruses, they cannot simply infect the mucosal epithelial cells to produce an immune response.
3. THE IMMUNOLOGICAL DEFENSE AT THE MUCOSAL SURFACES AND THE MUCOSA-ASSOCIATED LYMPHOID TISSUE (MALT)
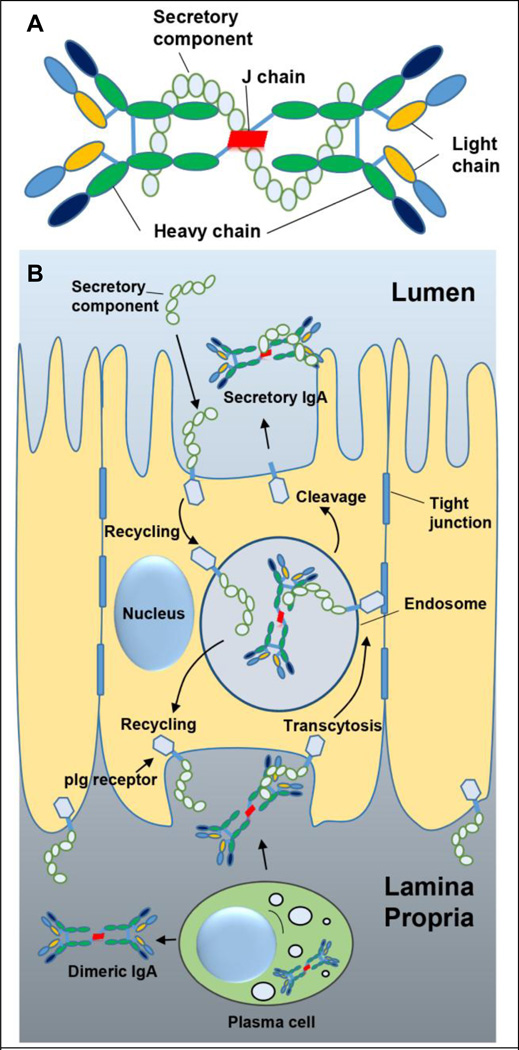
To combat infection, mucosal surfaces are equipped with physical, chemical and immunological defense mechanisms [12]. In particular, mucosal tissues comprise of a highly compartmentalized and specialized immune system in the form of MALT. MALT helps to induce pathogen-specific immune responses, and in the secretion of immunoglobulin A (IgA) at mucosal surfaces to protect against infection [2, 12]. IgA is the predominant immunoglobulin isotype in most mucosal secretions except the urogenital secretions in which IgG is found in a higher proportion. IgA can exist in monomeric or polymeric forms. IgA found in the serum is typically monomeric while that in the mucosal secretions is dimeric. IgA, which is secreted into the mucus is produced locally by the plasma cells at the mucosal surfaces. After release from the plasma cells, the dimeric form of IgA attaches to the polymeric immunoglobulin receptor (pIgR) located on the basolateral surface of mucosal epithelial cells, and is then transcytosed to the apical surface and secreted in to the mucus [13, 14]. During transcytosis a portion of pIgR is cleaved while the remaining portion stays attached to IgA and is called the secretory component (SC). SC is a distinctive feature of mucosal secretory IgA (sIgA), and is not found in systemically circulating IgA (monomeric or polymeric). The protective role of sIgA is mediated by the binding of sIgA to the pathogen or toxin. Attachment of sIgA to the pathogen or toxin can either form a shell around it, preventing its interaction with the mucosal epithelial cells, or can form a partial barrier-shell, in which case the pathogen may bind to the epithelial cell surface but its uptake is inhibited. Multiple mechanisms including steric hindrance, agglutination, neutralization and mucus trapping are believed to be involved in the protective role of sIgA. Furthermore, the sIgA-antigen complex can be ‘reverse-transcytosed’ by the microfold (M) cells for presentation and processing by the antigen presenting cells (APCs) that reside in the space just beneath the M cells, leading to an adaptive immune response against the antigen. Figure 1A shows a cartoon representation of the sIgA complex, and its secretion into the mucus is depicted in Figure 1B.
Figure 1.
Secretory immunoglobulin A (sIgA). (A) Cartoon representation of sIgA. sIgA is a complex consisting of two IgA molecules, the J-chain, and the secretory complex. The J-chain helps to form a stable dimer of the two IgA molecules, while the secretory component is attached to the dimer during secretion of sIgA into the mucus. (B) Cartoon representation of the secretion process of IgA into the mucus as sIgA. Plasma cells located in the lamina propria secrete dimeric IgA, which then binds to polymeric immunoglobulin receptor (pIg receptor) located on the basolateral side of the epithelial cells. The pIg receptor-dimeric IgA complex gets transcytosed towards the apical side where the extra cellular domain of the pIg receptor is cleaved to form the secretory component of the sIgA complex. The secretory component from the mucus can also get recycled back. Figure 1B adapted with permission from publisher of [13].
sIgA inhibits microbial colonization and invasion by direct neutralization [15]. It can also capture microbial toxins or viruses in the lamina propria, subsequently leading to their excretion by intracellular cycling [16]. Induction of IgA is dependent on activated Th cells, which secrete the transforming growth factor (TGF-β), interleukin −10 (IL-10) and IL-4 cytokines to promote the switching of B cells to produce IgA, along with differentiation into IgA producing B cells [17]. Additionally, secreted cytokines from mucosal T cells (IL-4, IL-10 & TGF-β) and epithelial cells (IL-10 & TGF-β) cooperate to promote maturation of IgA producing B cells [18, 19]. Oral mucosal immunization also induces systemic IgG response by activating IgG-producing B cells in the mucosa, which can migrate to bone marrow due to surface expression of L-selectin [20]. Drainage of activated dendritic cells (DCs) from mucosal site to lymph nodes and the spleen can also lead to stimulation of a systemic immune response. Cytotoxic T lymphocyte (CTL) response is also decisive for the clearance of microbes from the mucosa, and it has been observed that live or attenuated microbes are able to induce this response at mucosal sites. Some microbe-based adjuvants such as cholera toxin (CT) or heat labile enterotoxin (LT) are also able to induce CTL responses when administered orally with antigens [21].
To successfully design a mucosal vaccine delivery system, it is important to understand how antigens are naturally processed at the mucosal surfaces. The MALT is a secondary lymphoid tissue distributed across the mucosal surfaces. A functional unit of the MALT is considered to be a lymphoid follicle, which often comprises of: (i) a top covering of epithelial cells with a population of specialized cells to uptake antigen from the environment, (ii) a sub-epithelial region consisting of DCs to process the antigen brought in from the environment, and (iii) a zone rich in immune cells such as follicular dendritic cells (FoDCs), B and T cells to induce an antigen-specific immune response, including IgA-secreting B cells [12]. The MALT at different mucosal surfaces is briefly discussed below.
3.1 Gut-associated lymphoid tissue (GALT)
GALT is known to typically consist of organized lymphoid tissues in the form of Peyer’s patches (PPs) and solitary lymph nodules, where most of the IgA responses are initiated.
3.1.1 Peyer’s patches (PPs)
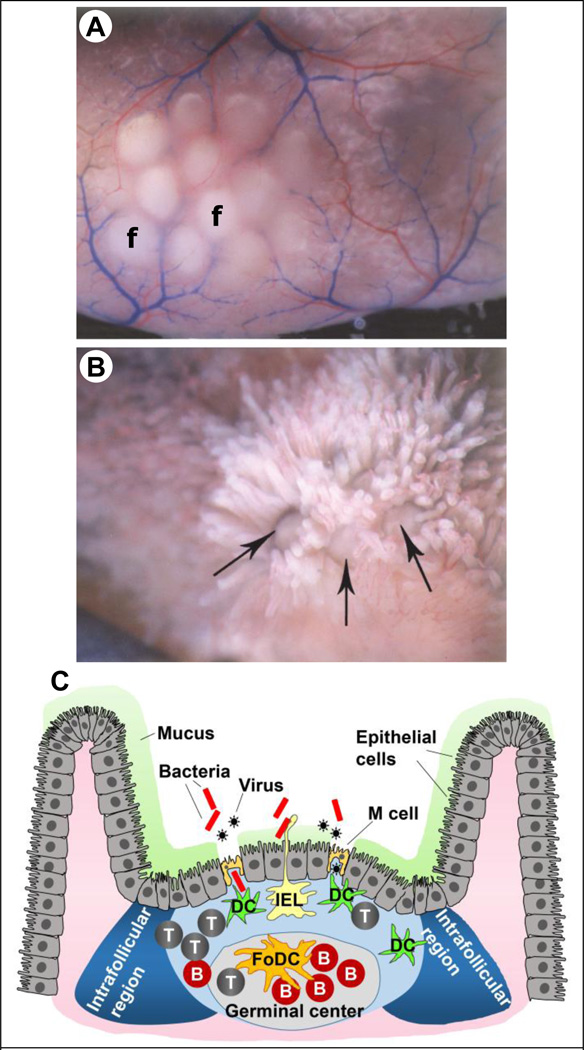
PPs are located on the anti-mesenteric side of the small intestine, and are often arranged at regular intervals in the duodenum, jejunum, and ileum [22]. Figure 2A shows a PP comprising of 12 lymphoid follicles located on the anti-mesenteric side, while Figure 2B shows the lymphoid follicle domes as seen from the luminal side of a gerbil intestine. The individual lymphoid follicles are seen surrounded by intestinal villus. There are more than 200 PPs in an average human adult [23].
Figure 2.
Peyer’s patches (PPs). (A) A PP consisting of 12 lymphoid follicles (f) as seen in a gerbil intestine. (B) Lymphoid follicles in a gerbil intestine lined by intestinal villi. The large arrow points to the lymphoid follicle dome. (C) Schematic of a PP showing M cells and the different immune cell populations. Note the absence of mucus on top of M cells.
T: T cells, B: B cells, DC: dendritic cells, IEL: intraepithelial lymphocyte, FoDC: follicular dendritic cell, IFR: intra-follicular region. Figures 2A and 2B adapted with permission from publisher of [23]
The lymphoid follicle can be divided into three main parts: the epithelium at the top, the sub epithelium dome, and the germinal center in the basal part. The epithelium covering the dome of lymphoid follicles is called the follicle associated epithelium (FAE), which like the gut epithelium is single cell thick. FAE largely comprises of columnar epithelial cells, specialized cells called the microfold (M) cells, and intraepithelial lymphocytes (IELs) [24]. M cells constitute about 5–10 % of the FAE in humans and mouse [25]. M cells use transcytosis to transport pathogens and foreign molecules from their apical lumen-facing side to their basal part. It is important to note that M cells do not have a mucus layer on their apical side. This feature allows M cells to more efficiently sample antigens from the luminal space. As seen in Figure 2C, the basal side of M cells has pockets formed from invaginated membranes, which house DCs. These DCs take up transported pathogens and molecules, and help to orchestrate the adaptive immune response. This close proximity of DCs to M cells is especially noteworthy, because it allows the DCs to rapidly process the transcytosed antigens, and to present the antigenic peptides to B and T cells to induce antigen-specific immune responses. The germinal center contains a network of FoDCs, and is also rich in B cells. In the germinal center class switching of B cells into IgA-producing cells occurs [24]. These antigen-specific IgA producing B cells can then migrate into the intestinal lamina propria to secrete IgA, which is then transported by epithelial cells into the mucosal secretions as sIgA (see Figure 1B). The space between adjacent follicles in the PPs is called intrafollicular region (IFR). This space is rich in T cells and DCs, and helps to orchestrate the overall adaptive immune response in the PPs.
In between epithelial cells and/or M cells are found IELs, which are predominantly T cells. IELs extend their appendages out into the intestinal lumen to directly sample antigens. They do not form tight junctions with epithelial cells or M cells. In normal human jejunum the average number of IELs is about 20 IELs/100 epithelial cells [26]. The exact function of IELs is not known, however they are thought to be involved in repair and turnover of epithelium, IgA class switching and secretion, and cytotoxicity against pathogens [27].
3.1.2 Solitary lymph nodes
In addition to PPs, an average human contains about 30,000 individual lymph nodes distributed along the gastrointestinal tract [23]. Similar to a PP lymph node, each solitary lymph node can uptake the antigen from the luminal side through M cells, and the DCs residing on the basal side of M cells can process it to induce an antigen specific immune response. In contrast to PPs, the solitary lymph nodes are rich in DCs and B cells but have very few T cells.
3.2 Nasopharynx-associated lymphoid tissues (NALT)
In certain species such as rodents, NALT is found as a pair of lymphoid aggregates at the entrance of the nasopharyngeal duct [29]. NALT is also found in non-human primates but they are more numerous and are located on both the lateral and septal walls of the nasopharynx. Similar to PPs, NALT is present as an organized structure consisting of a lymphoid epithelium with M cells [22]. The germinal centers are more diffuse as compared to PPs, and unlike PPs NALT has an equal proportion of B and T cells.
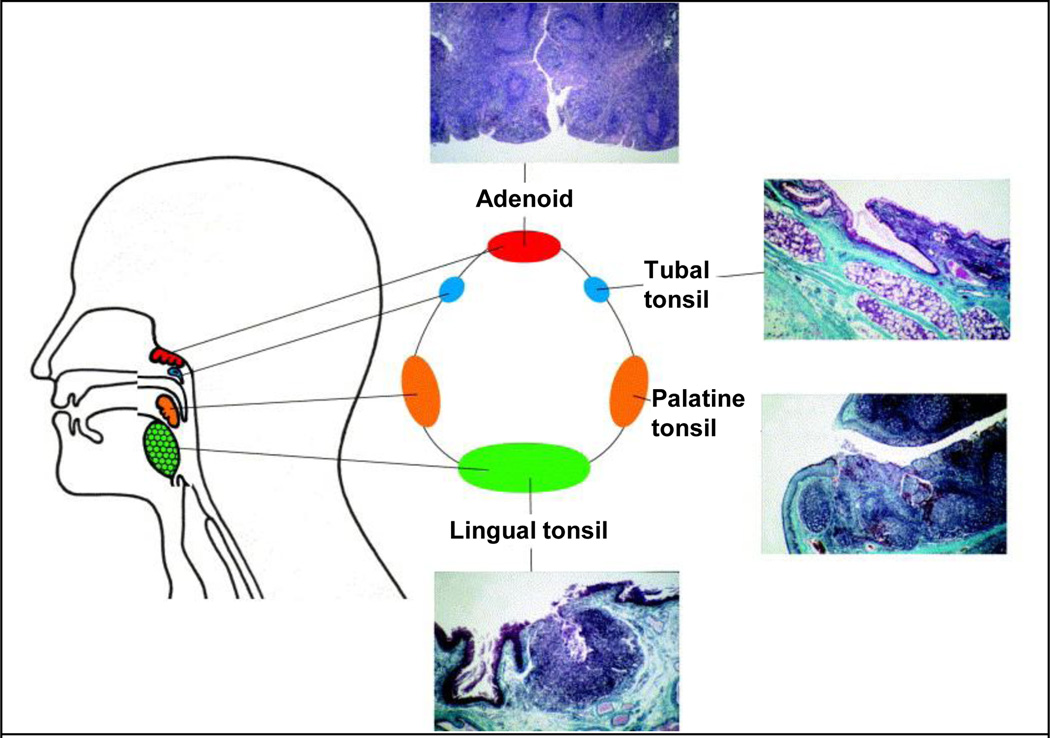
In humans however, an organized NALT like that of PPs is not seen. Instead, several lymphoid tissues including two palatine tonsils, two tubal tonsils, an adenoid and a lingual tonsil are anatomically arranged to form the Waldeyer’s ring (Figure 3) [28]. Because of their anatomical positioning, these lymphoid tissues sample antigens from food, water and air and help to maintain immunity and tolerance [28, 29]. The tonsils are covered by a squamous epithelium. To increase surface area for antigen interaction, the surface invaginates into valleys called crypts where M cells are located [22, 30]. The crypts have an estimated epithelial surface area of about 295 cm2, which is in addition to the approximately 45 cm2 of epithelium covering the oropharyngeal surfaces. The M cells from human tonsils after adenectomy have been found to be similar to M cells in PPs. M cells transport the antigen to DCs, macrophages and B cells for processing and presentation. Consequently, activated antigen-specific CD4+ T cells, interact with B cells, which develop into IgA secreting cells. IgA then forms into sIgA and is transported to the effector sites [31, 32]. sIgA is able to neutralize toxins and can eliminate bacteria or viruses, thus preventing their entry into the body and from reaching the internal organs. Studies have shown that, excretion of sIgA is dependent on these areas and tonsillectomy has been associated with decreased immunity [32, 33].
Figure 3.
Lymphoid tissues of the Waldeyer’s ring and their histological sections. Sections show crypts (white indentations in histology sections) that increase surface area available for direct antigenic uptake and stimulation. Adapted with permission from publisher of [28]
3.3 Bronchus-associated lymphoid tissues (BALT)
Presence of BALT in animals varies by species. For example cats and dogs normally don’t have BALT while rabbits, rats and guinea pigs possess BALT [22]. BALT is a cluster of lymphocytes entangled in a reticular network of stromal cells and lies under the epithelium that lacks cilia. BALT may also be found in airway bifurcations, where it is placed to trap inhaled antigens [34, 35]. Since, BALT requires additional architectural changes to facilitate leukocyte entry and exit, and lymphocytes in BALT cannot infiltrate the airway epithelium, BALT is not completely analogous to PPs or NALT. It is also not clear that antigen gets to BALT due to transfer across the epithelium by M cells. However, some studies reveal that DCs either directly migrate to the epithelium or enter afferent lymphatics that lead to BALT [34, 36, 37]. Unlike GALT, BALT is not universally present in all humans [29]. It was documented that healthy adult humans without pulmonary disease do not have BALT [38]. However BALT was found in children who had died of trauma, and in lung tissues from human fetuses [39]. It has been seen that BALT can be induced by infection in lungs. For example BALT has been seen with high-frequency in lung samples from choriomeningitis infected fetuses [40], and in lung cancer patients [41].
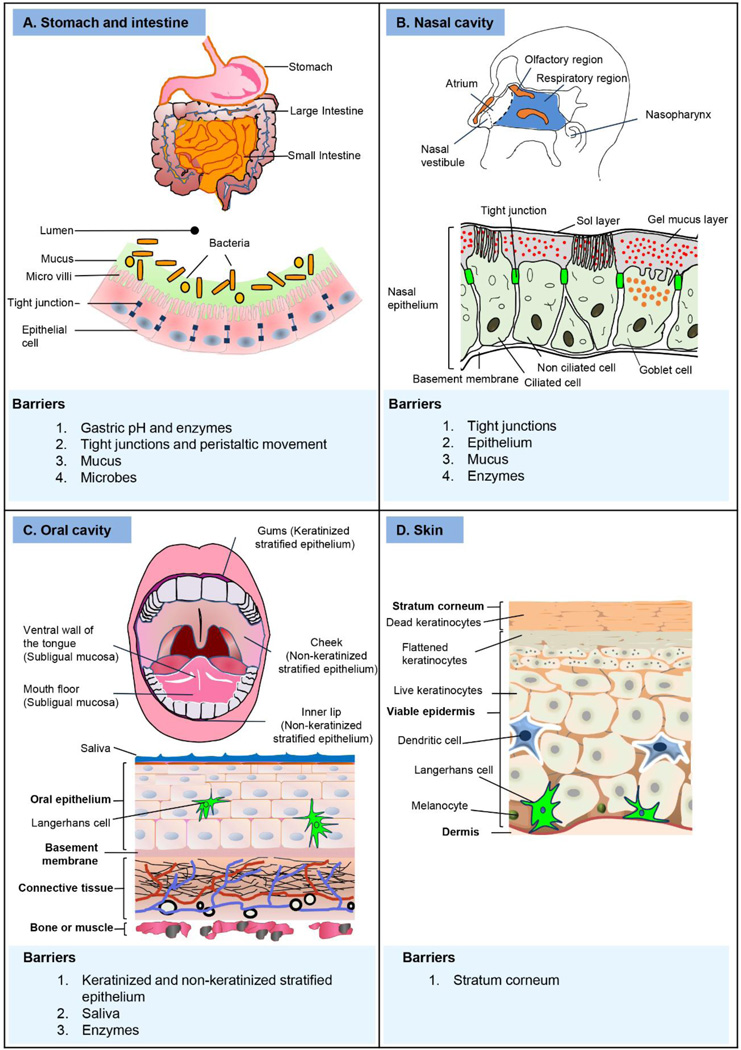
4. MUCOSAL VACCINE DELIVERY - ROUTES AND BARRIERS
4.1 ORAL VACCINATION
The oral route of vaccine delivery is highly preferred because it is painless, safe and does not need trained personnel for administration. However oral vaccination is expected to be less effective because vaccines degrade as they pass through the harsh environment of the gastrointestinal tract prior to reaching the intestinal epithelium.
4.1.1 Barriers to oral vaccination
For oral vaccination, the acid- and enzyme-rich conditions in the stomach form the major physiological barrier to vaccine delivery because exposure to such harsh conditions can result in antigen breakdown. Further, mucus, a viscous and sticky layer of glycoproteins called mucins, coats the epithelial cells. This mucus layer offers a protective function by acting as a physical barrier between epithelial cells and infectious pathogens. It can also contain competitive binding sites to entrap microbes, and can contain sIgA that can bind to and neutralize pathogens [12, 42]. However structurally, the intestinal epithelium, which is single-cell thick, represents the largest and most crucial barrier against the entry of foreign molecules. The epithelium acts as a selective barrier and allows only absorption of nutrients and electrolytes but stays impermeable to large molecules, intra-luminal toxins or microbes. The epithelium maintains its selectivity through formation of tight junctions, which mechanically connect the adjacent cells at their membranes, and seal the intracellular spaces [43]. The tight junction maybe thought of as a ‘stitch’ created between cell membranes of two adjacent cells through the interaction of membrane proteins of the two cells. The tight junctions are the rate limiting barrier for intestinal permeability. Multiple enzymes secreted by the cells of the mucosal epithelium can also degrade antigens. Indigenous microbes that colonize the mucosal surfaces establish a strong foot hold to either the mucosal epithelial cells or the mucus, and therefore, they offer resistance against colonization. These indigenous microbes also offer resistance to direct interaction of the antigen or its delivery system with the epithelial cells. Figure 4A provides a cartoon representation of the oral route of vaccine delivery and summarizes the major delivery barriers.
Figure 4.
Mucosal routes of vaccination and the major delivery barriers.
Foreign molecules are either pinocytosed by the epithelial absorptive cells, or as discussed above, phagocytosed or transcytosed by M cells [44]. It is important to note that M cells are not covered by a mucus layer, and this facilitates their antigen uptake function. Alternatively, columnar epithelial cells also have the potential to present antigens, and have been shown to induce stimulation of suppressor cells [45]. DCs of the epithelial layer can also extend dendritic cell-like processes to capture delivered antigens directly [46]. A receptor CX3CR1 is involved in the dendrite formation in DCs [47]. Following antigen uptake, B and T cells help in induction of mucosal immune responses. Sensitized B and T lymphocytes at the mucosa, leave the site and drain to other selected mucosal sites through lymph circulation. Secreted chemokines in the local microenvironment direct the expression of site specific integrins (homing receptors) and addressins (complementary mucosal tissue receptors) of the mucosal lymphocytes, which in turn control their migration to other mucosal sites [48]. Overall, this suggests that there is a common mucosal immune system where activated lymphocytes at one site can extend immunity to other remote mucosal tissues. Indeed, it has been found that immunization through a mucosal route can stimulate mucosal immune responses both locally and at other mucosal surfaces; and also that different mucosal routes preferentially induce stronger responses at some distant mucosae than at others [49–51].
Oral vaccines face similar intestinal milieu as do infecting microbes; such as getting diluted before absorption at the mucosa, getting degraded by proteases or nucleases present in the stomach and mucus, or getting excluded by the biological epithelial cell tight-junction barrier. Therefore, a comparatively larger amount of vaccine is needed for oral vaccination to produce an effective immune response that is comparable to other routes. However, it is hard to quantify the exact amount of vaccine dose that crosses the mucosa. In general, oral mucosal vaccines are likely to be effective if they are given repeatedly in higher doses or if they are designed to structurally mimic the microbes [52]. Ideally, an oral mucosal vaccine should be able to adhere to the intestinal mucosa or selectively target the M cells. For example, a microbe-based vaccine such as the live attenuated polio vaccine selectively binds to the M cells and crosses the mucosa to interact with B cells of the lymphoid tissues [53]. However, as discussed above, vaccines based on live-attenuated pathogens can have limitations related to safety in infants, and thus either non-infectious vectors, or non-living or recombinant subunit vaccines are more attractive, albeit, difficult to deliver. Multiple approaches can improve delivery of vaccines orally, such as by delivering them through microbial or plant based carrier systems, or encapsulating them in microparticles [54], nanoparticles [55], or liposomes [56]. Some of these systems are discussed in the later sections.
4.2 INTRANASAL VACCINATION
The nasal route is a major site of pathogen entry and due to its unique physiological characteristics and immunological features it is a promising alternative form of mucosal vaccination. The nasal cavity consists of four areas front to back: the nasal vestibule, atrium, respiratory region, and the nasophaynx [57]. The immune response to nasally-delivered vaccines is mediated by the nasal associated lymphoid tissue (NALT) in some animals like rodents, or the Waldeyer’s ring in humans and some other animals like horses [58]. Nasal vaccination has several interesting properties, such as ease of accessibility, highly vascular mucosa, larger surface area for absorption and antigen uptake, and potential for self-administration.
4.2.1 Barriers to intranasal vaccination
The efficiency of transport and uptake of most soluble antigens is limited in the nasal cavity due to it’s anatomical features and physiological characteristics. Different regions of the nasal cavity are shown in Figure 4B. The most anterior part of the nasal passage, the vestibule contains nasal hair, and comprises of stratified, squamous and keratinized epithelium. This region is least preferable for vaccine delivery. Just posterior to the vestibule is the atrium, which serves as the transition region. The anterior portion of the atrium consists of stratified squamous epithelium while its posterior part is made of pseudostratified columnar epithelial cells with microvilli. This region leads into the respiratory region, which consists of pseudostratified columnar epithelial cells that can be either ciliated or non-ciliated. The epithelial cells of the respiratory region possess tight junctions [57], which dramatically reduce the permeability of macromolecules across the epithelium. A thin layer of mucus also coats the nasal epithelium. Subunit antigens with little to no affinity for the nasal epithelium are generally poorly immunogenic and can be cleared rapidly by mucociliary clearance [59]. Furthermore, the stability of soluble antigens is affected by nasal enzymes and local pH [60]. Altogether, approaches have to be taken into consideration to increase the nasal residence time and to improve the stability of nasally administered vaccine formulations. Properties of the delivery system co-administered with the antigen have been utilized to exert one or more functions, such as mucoadhesiveness, antigen protection, permeation and penetration enhancement, specific inductive-site targeting, and adjuvant effect. Figure 4B provides a cartoon representation of the nasal route of vaccine delivery and summarizes the major delivery barriers.
4.3 ORAL CAVITY VACCINATION
As compared to the traditional mucosal routes (oral & nasal), oral cavity has been less explored for the delivery of mucosal vaccines, however it has been widely investigated for delivery of small molecular weight drugs in to the bloodstream [61, 62]. For mucosal vaccination, the oral cavity is of great interest as a novel route for vaccine delivery because it is easily accessible, and has a milder environment that does not significantly degrade the vaccine antigen as compared to the gastrointestinal tract milieu.
4.3.1 Barriers to oral cavity vaccination
Oral cavity vaccination can be performed either via the buccal (cheek lining) or sublingual (ventral surface of tongue or floor of mouth below the tongue) or gingival (gums) mucosae of the mouth or the inner surfaces of the lips. The buccal, inner lip surfaces, and sublingual mucosae have a non-keratinized epithelium while the gum mucosa is covered with keratinized epithelium similar to that of the skin [63]. The tongue mucosa is both keratinized and non-keratinized. Keratinized surfaces offer greater resistance to molecular uptake than the non-keratinized surfaces. Generally, the epithelium consists of superficial, intermediate and basal layers of cells. The cells of the superficial and intermediate layers are compact and flattened and provide transport resistance, while the intermediate layer also contains cellular lipids, which play a crucial role in controlling permeability of molecules across the mucosae. Unlike the intestinal epithelium, oral cavity mucosa does not have M-like cells, nevertheless there are many lymphoid tissues in the naso-oro-pharyngeal cavities. As discussed above, several lymphoid tissues including two palatine tonsils, two tubal tonsils, an adenoid and a lingual tonsil are anatomically arranged to form the Waldeyer’s ring (Figure 3). These lymphoid tissues, especially the lingual (tongue) tonsil can sample vaccine antigens delivered to the oral cavity mucosae to induce an immune response. Furthermore, the oral cavity epithelium is rich in dendritic cells, especially Langerhans cells (LCs), which are considered to be potent APCs, perhaps even more potent than the cutaneous LCs [64–67]. Therefore efficacy of vaccination through oral cavity mucosae depends on permeability of the mucosa, which is influenced by thickness and degree of keratinization of the epithelium. With regard to permeability, sublingual mucosa is more permeable than the buccal and gum mucosa, and therefore the sublingual method has been more widely studied for vaccine administration [68]. Antigen-loaded DCs drain from the sublingual mucosa to local lymph nodes where they prime both B and T lymphocytes. The activated lymphocytes can then leave the site of antigen presentation, enter blood circulation and seed other selected mucosal sites where they can regulate differentiation and maturation of B and T (Th1 & Th2) cells [69]. Figure 4C provides a cartoon representation of the oral cavity as a route of vaccine delivery and summarizes the major delivery barriers.
4.4 CUTANEOUS VACCINATION
Skin is not a typical mucosal surface, and yet it is a unique route to achieve both systemic and mucosal immune responses.
4.4.1 Barriers to vaccination through the skin
Anatomically, skin is composed of three major layers; the epidermis, the dermis and the hypodermis. The epidermis comprises of the outermost stratum corneum layer and the underlying viable epidermis. The stratum corneum is packed with dead keratinocytes and lipid molecules, and is the principal barrier for delivery of large vaccine molecules into the skin. The stratum corneum layer is typically 10–15 µm thick. Stratum corneum can be disrupted or ruptured by several mechanical and chemical methods [70, 71]. The viable epidermis and dermis layers contain the immune responsive cells such as skin residing DCs, LCs, T lymphocytes, NK cells, macrophages, and the mast cells [72]. To get an effective immune response, an antigen must be delivered into the viable epidermis so that it can interact with the resident immune responsive cells. Thus, unlike systemic delivery of drugs through the skin where the drug molecules must diffuse across the epidermis for absorption into blood circulation, for vaccine delivery, it is sufficient to introduce the antigens just within the viable epidermis layer.
The mechanism by which skin immunization induces mucosal immune response together with systemic response is not well known, although migration of DCs and LCs from the skin to mucosal lymphoid tissues can help explain the mucosal response observed in different mucosal samples such as the saliva, stools, intestinal washes and vaginal washes [73]. Furthermore, Chang et al., have shown that immunization via the skin leads to emergence of LCs in the mesenteric lymph node (MLN) of the mice, and depletion of langerin+ cells (langerin is a marker for LCs) reduces intestinal IgA response. They also showed that skin-based vaccination induces antigen-specific IgA antibody-secreting cells that express CCR9 and CCR10 in the small intestine in a retinoic acid-dependent manner. The intestinal IgA antibody response was found to be impaired in PP and MLN null mice, but not in just PP null mice. Their findings thus suggest that MLN is important for cross-talk between the skin and the oral mucosa, and that LCs play a key role in this process [74]. Figure 4D provides a cartoon representation of the skin as a route of vaccine delivery and summarizes the major delivery barriers.
5. MUCOSAL VACCINE DELIVERY SYSTEMS
Because each mucosal surface possess its own unique set of delivery-barriers, different delivery strategies have been designed to deliver vaccines across them. However, a common theme behind the design of the different delivery systems is to increase delivery of the antigens across the mucosal surface while protecting it from degradation and/or clearance in the local environment of the mucosa. Often, the delivery system is designed to exploit a unique characteristic of the mucosal surface to improve vaccine delivery. For example, for vaccine delivery using the gastrointestinal route, the natural ability of M cells to uptake antigens has been exploited to increase delivery efficiency. As with the conventional parenteral vaccination using needle and syringe, different adjuvants (substances added to the formulation to enhance the immune response) have also been studied in the context of mucosal vaccine delivery. In the following sections, different mucosal vaccine delivery systems are summarized. A more detailed summary of mucosal adjuvants is given later.
5.1. Live bacteria and viruses
Certain bacteria and viruses can naturally infect mucosal epithelial cells, and therefore, they are exploited as a vehicle to deliver vaccines at different mucosal surfaces. Different strategies exist by which live bacteria or viruses are used for vaccine delivery: (i) the bacteria or virus responsible for a particular disease can be attenuated (live but with limited infectious ability) and used as a vaccine, (ii) a bacteria or virus generally regarded as safe can be recombinantly engineered to express the antigenic protein of a pathogen and used as a vaccine, or (iii) a live bacteria or viruse generally regarded as safe can be genetically engineered to harbor and deliver the DNA expressing the vaccine antigen in to the body for in situ expression. Live attenuated vaccines have the advantage of mimicking natural infection and presenting antigens to APCs in their native structure, and can lead to induction of efficient and long-lasting immune responses [75, 76].
Live attenuated or recombinant bacterial strains such as E. Coli, Salmonella, and Lactobacilli, or viruses like adenoviruses have been utilized as a carrier for antigen delivery to gut associated lymphoid tissues (GALT) [77, 78]. However, the induced immune response is often predominantly against the carrier vectors and not the delivered antigen [78]. This phenomenon was recently observed in a human clinical trial that used salmonella typhoid vector to deliver an HIV gene expressing the gag protein [79]. In this study, although antibodies against the bacteria were seen, but they were not detected against gag.
There are only two intranasal vaccines approved for human use, both of which are based on live attenuated influenza virus (LAIV) with reduced virulence. The FDA-approved FluMist™ and European Medicines Agency-approved Fluenz™ contains live attenuated influenza strains H3N2, H1N1 and two influenza B strains [80], which confer better cross-protection and enhanced efficacy with increased level of nasal sIgA antibodies and cell-mediated T cell responses [75, 81]. However, safety concerns are associated with improper attenuation, which can cause infection after vaccine administration, and LAIV also poses risk to the immunocompromised population and children [82]. Many studies have also focused on increasing the immunogenicity of subunit vaccines through the intranasal route using live intracellular bacterial vectors such as Shigella spp. [83], Lactobacillus spp. [84, 85], Salmonella spp. [86], and Listeria spp. [87].
The oral cavity has also been targeted using live vectors. A live attenuated vaccinia virus expressing HIV antigen was recently used via the sublingual route in macaques [88]. In another study influenza A/PR/8/34 virus (H1N1) virus was applied sublingually and was shown to impart protection against lethal influenza virus challenge in a mouse model [89].
As noted above, the safety of live vectors is a critical design feature and a constraint in the design and development of vaccine candidates.
5.2 Polymeric particles
Micro- and nano-particles encapsulated or decorated with the vaccine antigen can be used to deliver vaccines to mucosal surfaces. The particles can offer protection to the encapsulated antigens and control their release, their surfaces can be functionalized with ligands to help target them to specific cells to enhance uptake, or they can enhance mucoadhesion. Both synthetic and natural polymers have been exploited to make particles for mucosal vaccine delivery. While in depth reviews of particles for mucosal vaccination can be found elsewhere [90–92], a brief overview is provided below.
5.2.1 Synthetic polymers
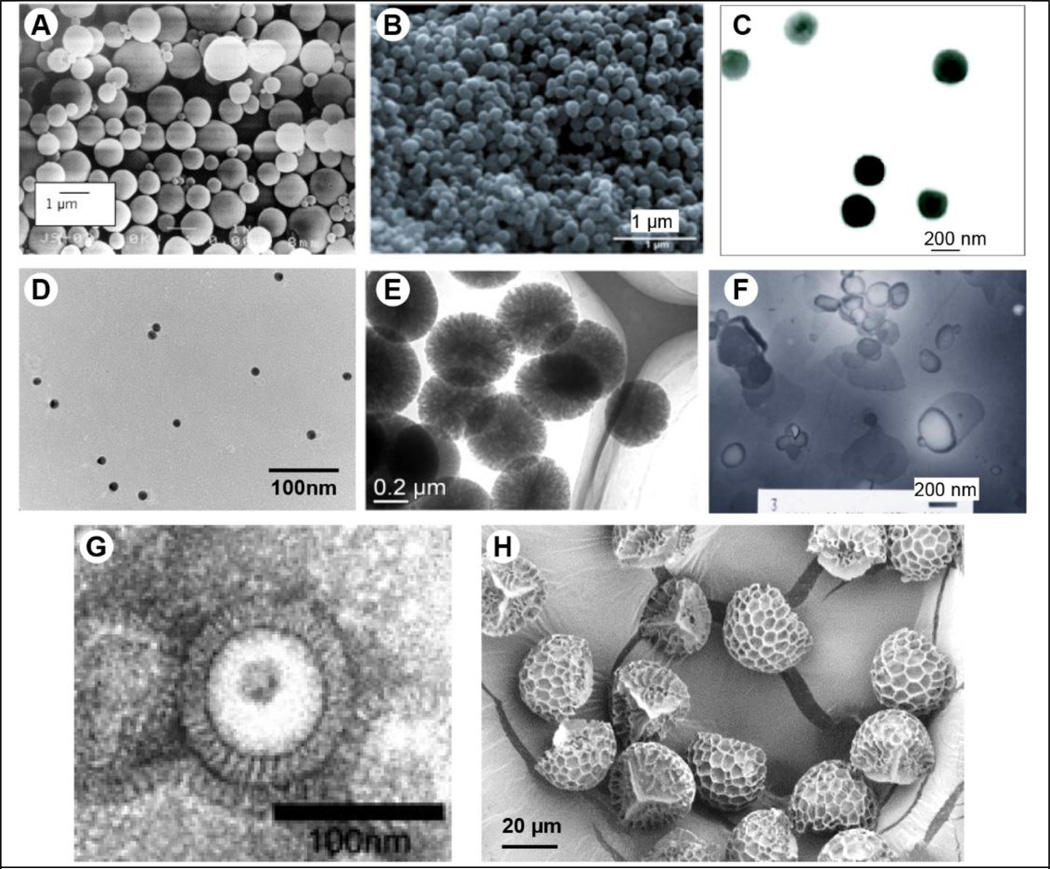
For oral vaccination, particle size affects the immune response, and it has been observed that smaller particles induce higher antibody titers than larger particles [55]. Particles (1–2 µm) have been shown to be effective in inducing an immune response because the particles can be selectively taken up by the M cells and can be actively transported across the epithelium [93]. They can also selectively target either M cells or other intestinal cells such as enterocytes by use of targeting moieties or by incorporating mucoadhesive polymers [55]. Different lectins, bacterial adhesins, and RGD analogs have been used to target M cells and the oral epithelial cells [55]. Nanoparticles based on polylactic acid (PLA) or poly(lactic-co-glycolic acid) (PLGA) polymers have been extensively explored for oral vaccination with a variety of antigens like bovine serum albumin [94], inactivated bacteria [95], their toxoids [96], and DNA [97]. PLGA and PLA are known to be biocompatible and biodegradable. Vaccine loaded PLGA nanoparticles effectively induce systemic and mucosal immune responses. For example, oral vaccination with poly(vinyl alcohol)-co-PLGA based nanaoparticles encapsulating tetanus toxoid induced serum IgG and IgA immune responses at a considerably higher level than the intraperitoneal route [96]. However, the possibility of antigen denaturation during synthesis of PLGA particles (due to high temperature and exposure to organic solvent) should be taken into consideration during design of PLGA-based vaccines. Larger particles (20 µm) are also taken up by the villous of epithelium, but with reduced frequency [98]. Figure 5A provides an illustration of PLGA particles used for vaccination.
Fig 5.
Different mucosal vaccine delivery systems. (A) Scanning electron micrograph of poly(lactic-co-glycolic acid) microparticles [94], (B) scanning electron micrograph of polyanhydride nanoparticles [111], (C) transmission electron micrograph of chitosan : poly-γ-glutamic acid nanoparticles [129], (D) transmission electron micrograph of gold nanoparticles [136], (E) transmission electron micrograph of silica nanoparticles [135], (F) transmission electron micrograph of mannose and monophosphoryl lipid A decorated cationic liposomes [138], (G) transmission electron micrograph of influenza virus-like particles [139], (H) scanning electron micrograph of lycopodium spores (pollens) [140]. Images reproduced with permission of the respective publishers.
In rodent studies it has been shown that polymeric particles can be taken up by NALT resulting in immune responses against the encapsulated antigens [99, 100]. The effect of particle size has not been systematically studied for nasal vaccination. Carr et al. showed that polystyrene microparticles with a diameter of 1 µm can adhere to NALT and improve particle uptake by intranasal administration [99]. Lemoine et al. noted that uptake of polymeric particles by NALT appeared to be crucial for enhancement in the immune response [101]. In their study, nasally administered PLGA particles with a diameter of 220 nm or 8 µm did not reach the NALT in mice, and consequently the nasal wash IgA and serum IgG levels were not enhanced compared to soluble antigen. For nasal vaccination, several studies have pointed that nano-sized particles were more rapidly taken up by nasal M cells [102, 103]. Mansoor et al. showed that calves administered intranasally with PLGA nanoparticles encapsulating bovine parainfluenza 3 virus (BPI3V) antigen in the presence of pre-existing anti-BPI3V antibodies generated greater mucosal IgA antibodies compared to commercial LAIV vaccine [104]. This finding further suggests that nanoparticles with negative zeta potential minimize nonspecific cellular uptake resulting in enhanced local mucosal immune response. Nevertheless, intranasal delivery of the cationic PLGA particles encapsulating foot and mouth disease (FMDV) capsid protein have also been shown to enhance protective immunity against FMDV [105]. Thus, it seems that charge around polymeric particles is not critical to assess its ability to enhance the immunogenicity of the antigen. Intranasal immunization with PLA microspheres encapsulating F1 and V subunit antigens of Yersinia pestis along with adjuvant CTB induced superior systemic and mucosal immunity in comparison with free F1 and V subunit antigen, and resulted in 80% protection from lethal challenge [106]. Particle carriers can be modified with molecules to increase their stability in vivo. Copolymer of PLA attached with hydrophilic polyethylene glycol (PLA-PEG) has been evaluated as a potential carrier for vaccine antigen. The PEG covering on PLA overcomes its hydrophobic nature to improve intranasal delivery. Vila et al. compared the behavior of PLA-PEG nanoparticles encapsulating tetanus toxoid protein with PLA nanoparticles [107]. It was illustrated that PLA-PEG particles are more stable and able to cross the rat nasal epithelium, resulting in significantly higher antibody levels and a long-lasting immune response. This finding is in agreement with the concept of mucus penetrating particles [108, 109] that have an inert surface formed by PEG. The mucous penetrating PEG-coated particles are more stable in mucosal fluids and can facilitate transport of encapsulated antigen to induce stronger immune responses.
Polyanydride-based particles are biodegradable and biocompatible, and have recently been used for mucosal vaccination in addition to their use for vaccination via other parenteral routes [110]. Ulrey et al. showed that a single intranasal dose of recombinant protein F1-V encapsulated in polyanydride particles could induce long-lived protective immunity in mice against Yersinia pestis, which can causes pneumonic plague, a severe respiratory disease [111]. Figure 5B provides an illustration of polyanhydride nanoparticles used for vaccination.
5.2.2 Natural polymers
Chitosan is a mucopolysaccharide, which is closely related to cellulose. It is obtained by deacetylation of chitin, the major compound of exoskeletons in crustaceans. Chitosan is biodegradable and biocompatible. After oral administration, chitosan is digested by chitinases and is safe even at high doses (mouse LD50: 16 g/kg), while exposure of rat nasal mucosa to 0.5% (w/v) chitosan solutions for over 1 h caused no significant changes in mucosal cell morphology [112]. The characteristic absorption-promoting effect of chitosan can improve the mucosal immune responses by enabling uptake of the antigen. It is found to activate the macrophages and lymphoid tissues, thereby it is capable of inducing strong systemic and mucosal immune responses against the antigens [90]. Chitosan and its derivatives have been explored for delivery via the oral, intranasal and pulmonary routes [113]. With ovalbumin as a model antigen, Kobayashi et al. studied the safety and effectiveness of chitosan and its derivatives as mucosal adjuvants via the intranasal route in BALB/c mice, polymeric Ig receptor knockout (pIgR-KO) mice, and cynomolgus monkeys (Macaca fascicularis) [114]. Vicente et al. also showed that chitosan nanocapsule co-delivery system with hepatitis B surface antigen induced balanced humoral and cellular immune responses in mice after intranasal vaccination [115]. Lubben et al. used chitosan microparticles for oral delivery of diphtheria toxin (DT) and demonstrated induction of serum IgG equivalent to the intramuscular injection, and additionally, sIgA in fecal matter [116].
Alginate is an anionic polysaccharide. It is a biocompatible, biodegradable and a mucoadhesive polymer [117]. Although many polymers have been attempted, the characteristically stronger binding affinity of alginate with chitosan makes it an attractive adjuvant and vaccine delivery system [118]. Alginate (with or without chitosan) nanoparticles have been demonstrated to be an efficient mucosal delivery system for successful induction of systemic and mucosal immunity in different animal models with a variety of antigens [119–121].
Another promising mucosal delivery system that has been recently reviewed by Wang et al. is the polyacryl starch microparticles [122]. It not only protects the protein antigens from degradation, but also helps to facilitate the release of the encapsulated antigen and to boost the immune response upon oral and intranasal administration in mice [123]. Studies showed that, oral delivery of polyacryl starch microparticles with different antigens could induce stronger systemic and mucosal antibody responses in mice [124–126]. Other starch derivatives including diethyl aminoethyl dextran [127] have also been reported as mucosal vaccine delivery particles.
Poly-gamma-glutamate (γ-PGA) is another delivery vehicle for recombinant protein antigens. Gamma-PGA is a capsular polymer secreted by Bacillus subtilis, is generally regarded as a safe, edible substance, and is naturally degraded by γ-glutamyl transpeptidase, which is widely distributed throughout the body. It has an ability to self-assemble and can form biodegradable nanoparticles that have been reported to have an adjuvant effect when delivered orally through stimulation of TNF-α [128]. It has also been demonstrated that upon mixing low-molecular weight chitosan (polycationic) with high-molecular weight γ-PGA (anionic), γ-PGA/chitosan nanoparticles are formed that are effective in delivering peptides, proteins, and other large molecules [129, 130]. Figure 5C provides an illustration of γ-PGA/chitosan nanoparticles used for vaccination. Recently Noh et al. manufactured a mucoadhesive containing γ-PGA, 3-O-desacyl-49-monophosphoryl lipid A (lipidated-MPL-A molecule) and QS21 and showed enhanced immune responses, and broad protection against divergent influenza subtypes [131].
Hyaluronic acid is another natural polymer that has been reported as a safe, biocompatible, biodegradable, and a hydrophilic mucosal delivery vehicle [132]. This mucoadhesive polymer is a natural component of the cartilage and comprises of repeating disaccharide units of D-glucoronic acid and N-acetyl-D-glucosamine. It has been reported that influenza hemagglutinin (HA) adjuvanted with LT and delivered using an esterified hyaluronic acid microsphere system, induced potent immune response in mice, rabbits and small-pigs [133].
5.3 Inorganic particles
Inorganic materials such as silica, platinum, silver and gold are also known to form particles. These particles have also been assessed as vaccine delivery candidates [134]. For example, Wang et al. [135] have used silica nanoparticles formulated with bovine serum albumin for oral vaccination and they demonstrate that the formulation could induce mucosal and systemic antigen-specific antibody response. Gold nanoparticles (AuNPs) have attracted much research interest in the medical field because of their controlled shape and optical properties. In our laboratory, we have developed a candidate intranasal universal influenza vaccine comprising of AuNPs conjugated with a 23 amino acid long ectodomain of the membrane ion channel protein (M2e) of the influenza virus. Our studies show that attachment of M2e to the AuNP surface increased immunogenicity of M2e, however, addition of CpG as an adjuvant to the formulation was critical in conferring full protection to the mice against lethal influenza challenge [136]. Our continuing studies further found a critical role of excess soluble M2e antigen in generating the desired levels of antibody response for protection against influenza virus challenge [137]. This finding indicates that in addition to M2e immobilized on AuNPs, presence of free soluble antigen in the formulation somehow induces a stronger antigen-specific antibody response. Figure 5D provides an illustration of gold nanoparticles, and Figure 5E provides an illustration of silica nanoparticles.
5.4 Liposomes
Liposomes are micron- or nano-sized vesicles comprising of a lipid bilayer and an aqueous core, and they have been extensively explored for mucosal vaccine delivery. The vaccine antigen if hydrophilic can be incorporated in to the aqueous core or it can be incorporated in the lipid bilayer through a lipophilic anchor and oriented inwards or outwards. Alternatively, a hydrophobic antigen could be entrapped in the lipid bilayer. In addition, multi-lamellar vesicles comprising of multiple lipid bilayers can also be designed to encapsulate both hydrophilic and hydrophobic biomolecules [141]. Liposomes can be designed to have different sizes, surface charges, and permeability, to carry a wide spectrum of biomolecules. Their surface can also be modified through attachment of polymers to improve their in vivo stability and for slow release of the antigen. For example, surface modification through attachment of a mucoadhesive polymer such as chitosan can increase their stability as well as mucoadhesive property for their absorption at the intestinal surface [142].
An interesting variation of the liposomal delivery system is the viral-liposome fusion structure, which utilizes viral proteins to enhance transport of liposomes. For example, Kaneda reported the development of a novel liposome-sendai virus fusion nanoparticle by fusing inactivated sendai virus with liposomes containing DNA [143]. This fusogenic structure exhibited enhanced DNA delivery than the liposome alone. Later, they fused sendai virus to a liposome containing HIV gp160 protein. Intranasal vaccination of mice with this fusogenic nanoparticle induced systemic IgG response and sIgA in saliva, nasal wash, fecal matter, and vaginal wash [144].
Liposomes have also been used for oral vaccination. Liposomes for oral vaccination must be relatively more stable so as to survive the hostile environment of the stomach. Minato et al. showed that multi-lamellar vesicles stabilized with PEG are more effective than the liposomes without PEG in inducing mucosal sIgA against ovalbumin encapsulated in the liposomes [145]. Ligands can also be attached on their surface to provide them the ability to target different cells. In another study, mannose, a lectin binding molecule that was linked to PEG was incorporated into liposomes encapsulating ovalbumin as an antigen and MPL-A as an adjuvant, and were delivered orally [138]. Figure 5F provides an illustration of these liposomes used for vaccination. These liposomes were found to be stable and could significantly induce systemic IgG and mucosal sIgA antibodies against ovalbumin. Liposomes with PEG have also been used for sublingual vaccination [146]. Detailed reviews on liposomes for vaccine delivery including the challenges they face for scale-up are discussed elsewhere [91, 147, 148].
5.5 Virus like particles
A major challenge that needs to be addressed for a successful mucosal vaccine is to improve the poor and insufficient immunogenicity of soluble protein antigens. Virus like particles (VLPs) are self-assembled viral envelopes albeit without the genetic material of the virus, and thus do not exhibit viral replication and infection [149]. These self-assembled particles can have a protein or lipid-based envelope, and can be genetically engineered to display a high density of vaccine subunit molecules on their surface. Because VLPs are particulate in nature, they have been shown to be advantageous in the presentation of soluble epitope proteins on their surface, and in eliciting potent immune responses. Successful oral mucosal vaccination through VLPs or small vesicles that structurally mimic the pathogens and therefore can be easily phagocytosed by M cells or other APCs has also been shown [150, 151].
For nasal vaccination against pandemic influenza virus, VLPs can be engineered to contain various subtypes of influenza antigen to confer heterosubtypic protection. Kim et al. designed a tandem repeat of influenza A universal antigen peptide M2e (M2e5x) derived from human, swine, and avian influenza A viruses and incorporated it into VLPs [152]. Schwartzman et al. reported that a pre-pandemic influenza VLP vaccine including four VLPs individually displaying 1918 H1 subtype or low pathogenicity avian influenza H3, H5, or H7 HA subtypes [153], could induce cross-protection without the requirement of antigenic matching to the influenza virus challenge-strain. Hepatitis B virus core protein (HBc) is a non-infectious carrier protein with high immunogenicity, and can self-assemble into VLPs. In the pioneering work of Neirynck et al., fusion of a single copy of M2e, a potential universal influenza antigen but with poor immunogenicity, to HBc protein provided 90–100% protection against a lethal virus challenge [154]. They compared intraperitoneal and intranasal routes and concluded that the intranasal route of immunization was more promising than the intraperitoneal injection. These M2e–HBc VLPs were further investigated and continuing studies confirmed potential of using HBc as a protein carrier to induce efficient protective immunity against influenza virus in mice [155, 156]. Up to three copies of the M2e peptide were attached at the N-terminus of HBc, which further improved the immune response and provided complete protection. Bessa et al. assessed another intranasal M2e–VLP vaccine derived from incorporation of M2e into RNA phage Qβ [157]. They showed that intranasal administration of VLPs can induce efficient B cell responses (antibody-based response) as evidenced by the presence of high numbers of germinal centers and memory B cells in the spleen, and plasma cells in the bone marrow. Kang et al. have prepared VLPs containing HA of the highly pathogenic H5N1 influenza virus. They have shown that intranasally immunized mice could be protected against a lethal challenge and that the immunization could induce systemic immune response and IgA response in nasal and lung washes [139]. Figure 5G provides an illustration of these influenza VLPs used for vaccination. Other VLPs have also been evaluated for intranasal delivery, such as Norwalk virus (NV) [158]. NV VLPs have been shown to induce both systemic and mucosal immunity against Norwalk virus infection in the presence of an adjuvant.
When given sublingually, a human papillomavirus type 16 L1 virus like particle induced considerable systemic IgG and mucosal IgA response similar to the nasal route, but superior to other routes (intravaginal, intramuscular and transdermal) [44]. In another study, sublingual vaccination with an influenza H5N1 vaccine based on virosomes with 3’, 5’-cyclic diguanylic acid (c-di-GMP) as an adjuvant induced systemic IgG and nasal IgA response lower than the nasal route, but the systemic IgG response was comparable to the intramuscular route [159]. An in depth review of VLPs for mucosal vaccination can be found elsewhere [160].
5.6 Pollen grain shells for oral vaccination
Pollen grains are hollow microcapsules with an outer shell, which houses the male gamete. The outer shell is tough and can withstand the harsh environment of the stomach [161, 162], and despite having a relatively large size (tens of µm in diameter) it has been found that Lycopodium clavatum (clubmoss) spores and Secale cereale (rye) pollen grains can cross the intestinal barrier as intact particles [163, 164]. Recently, we have shown that lycopodium spores can be chemically cleaned to remove the native plant proteins and other biomolecules, and the resulting empty shell can be filled with proteins of interest [140]. We have subsequently shown that a formulation comprising of ovalbumin-filled lycopodium spores can induce efficient anti-ovalbumin systemic and mucosal antibody responses. The study also demonstrated that lycopodium spores could be seen within the intestinal wall of the mice, suggesting a mechanism by which even larger particles can cross the mucosal epithelial cells. Structurally, lycopodium spores have surface features that can help them to adhere to the intestinal mucosa (unpublished data), following which they can potentially either release antigen for uptake by M cells, or the IELs can directly sample surface-adsorbed antigen. Figure 5H provides an illustration of lycopodium spores used for vaccination.
5.7 Terrestrial plants and algae for oral vaccination
Plant cells are an attractive platform to deliver vaccines through the oral route because their tough cell wall can protect the encapsulated antigen from the harsh conditions in the stomach, but upon digestion in the intestine by microbes the encapsulated antigen can be released to stimulate an immune response [165]. Thus, in an effort to simplify production and delivery of vaccines orally, there has been a significant effort to produce transgenic terrestrial plants that can express vaccine antigens and can be directly eaten to deliver the vaccines [166]. Crops such as rice, maize and soybean have been used as expression vectors and as an edible delivery system because they offer advantages of low cost of production, easy scale up, and can prevent chances of microbial contamination. For instance, a rice-based oral vaccine expressing CTB subunit in the rice seed was shown to have long term stability including protection of the expressed antigen from pepsin digestion under in vitro conditions. Moreover, CTB was readily taken up by the M cells when rice was fed to mice, resulting in induction of antigen specific mucosal immune response with neutralizing activity [167]. With a development history of more than twenty years, the hepatitis B vaccine is perhaps the longest-studied terrestrial plant-based vaccine [168].
Unicellular green algae also possess a tough cell wall, and have other attractive features over terrestrial plants that make them attractive for oral vaccine delivery. Unicellular algae have a simpler process to perform genetic modification, they can be grown in bioreactors and thus do not require extensive land and cultivation infrastructure like the terrestrial plants do, they can be grown all year round as compared to crops that are often seasonal, and algae can be cultured at a much faster rate with better control over growth conditions as opposed to plants [169]. Dreesen et al. have shown that a unicellular green algae Chlamydomonas reinhardtii can stably express the D2 fibronectin-binding domain of Staphylococcus aureus fused with CTB, and upon oral delivery of a lyophilized form of this algae to mice, serum IgG and fecal matter IgA could be induced against D2 and CTB [170]. The oral dose also protected 80% of the vaccinated mice against a lethal dose of S. aureus. The lyophilized vaccine, which was stored at room temperature for 1.5 y performed equally well as the freshly lyophilized vaccine.
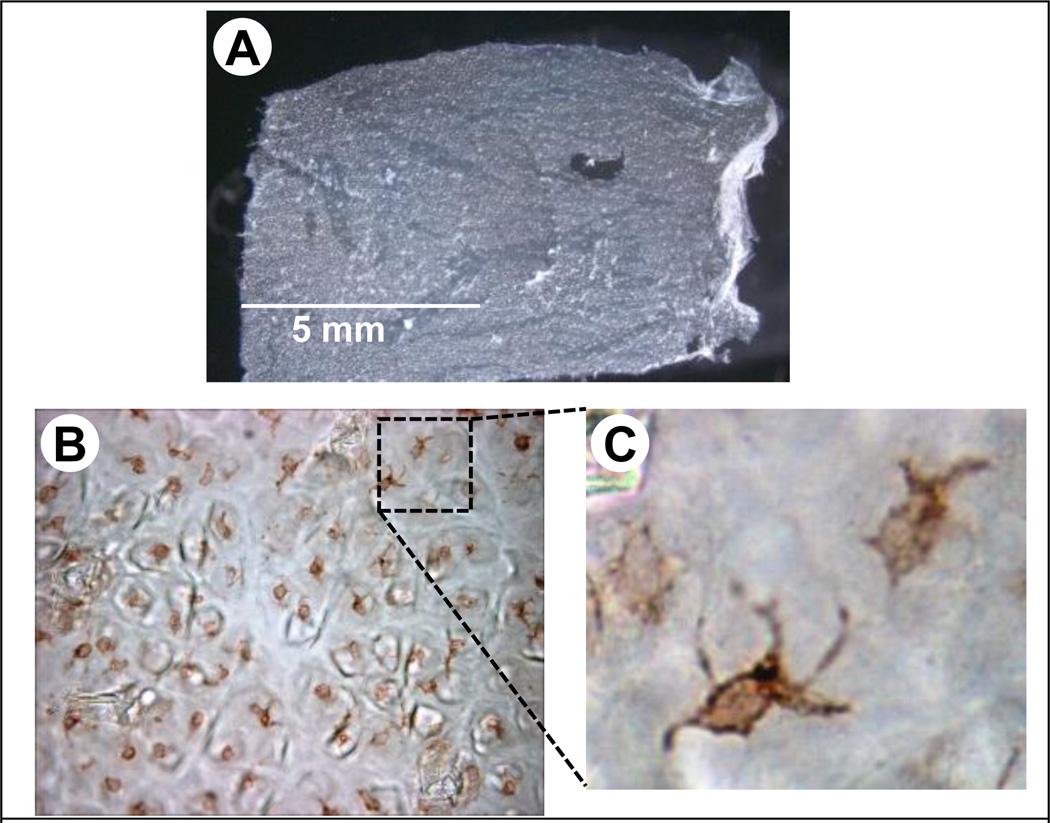
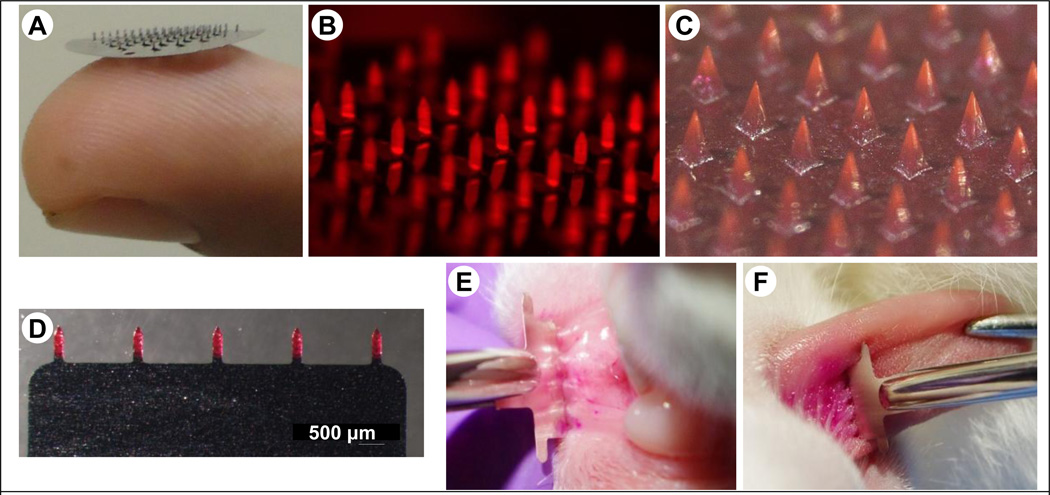
5.8 Microneedles for skin and oral cavity vaccination
Microneedles are micron-sized needles, which can penetrate the stratum corneum layer of the skin to directly deliver the antigen into the viable epidermis and dermis regions [171–175]. The viable epidermis is rich in LCs, which are potent APCs. LCs form an extensive network in the top layers of the epidermis, and act as sentries to process antigens that penetrate the skin. Figure 6 shows LCs forming a network in the epidermal sheet of a mouse. Microneedles are able to directly target LCs for antigen delivery. Because of their small size, microneedles are painless when inserted in to the skin, and have potential for self-administration [176]. Microneedles can be made of metals such as stainless steel or polymers or silicon, and can be fabricated in the form of patches containing tens or hundreds of microneedles. Figure 7A shows a solid microneedle patch made of stainless steel. Antigens can be coated on microneedles (Figure 7B), encapsulated in the polymer matrix (Figure 7C), or delivered via hollow microneedles [177]. Microneedle-based immunization via the skin can induce systemic and mucosal immune responses against the antigen. For instance, mice immunized via the skin with HIVgp140 and MPL-A as an adjuvant using dissolving microneedles elicited antigen specific systemic IgG and high level of IgA in vaginal washes, which were responsible for protection against HIV infection [178]. In another study, mice vaccinated via the skin with microneedles coated with recombinant trimeric soluble hemagglutinin (HA3) induced considerably higher immune response than the control mice, and vaccinated mice were protected from lethal challenge with influenza virus. Moreover, a greater IgA response in serum and vaginal washes was observed in microneedle-vaccinated mice [179]. Wang et al. showed that delivery of an M2e–flagellin fusion protein into mice skin using coated microneedles induced robust serum IgG antibodies and sIgA in lung washes [180].
Figure 6.
Langerhans cells in mouse skin. (A) Stereomicrograph of a mouse epidermal sheet that was separated from the whole mouse skin by incubating in ethylenediaminetetraacetic acid, and separating the sheet under a dissection microscope. (B) Micrograph of a mouse epidermal sheet showing Langerhans cells after performing standard immunohistochemistry with anti-CD1a antibody and diaminobenzidine stain to identify Langerhans cells. (C) Zoom-in showing typical Langerhans cell morphology.
Figure 7.
Microneedle devices. (A) Photograph of a stainless steel microneedle patch resting on a fingertip, (B) Stereomicrograph of a stainless microneedle patch with microneedles coated with fluorescent ovalbumin, (C) Stereomicrograph of a polymer microneedle patch (image courtesy of Georgia Institute of Technology, (D) Stereomicrograph of a planar stainless steel microneedle patch coated with sulforhodamine, (E) A planar microneedle patch being inserted into the inner surface of the lower lip of a rabbit, (F) A planar microneedle patch being inserted into the dorsal surface of a rabbit tongue.
We have recently developed a new oral cavity vaccine delivery approach that uses minimally invasive and painless microneedles [181]. Three different antigens, ovalbumin (as model antigen), E2V3 (nanoparticles with repeats of variable region 3 of HIV envelope), and gp160 DNA were coated on micron-size needles and inserted into mucosal tissues (lip and tongue) of rabbits. Figure 7D illustrates a solid stainless planar device with five microneedles coated with sulforhodamine, a fluorescent dye. Figure 7E and 7F illustrate insertion of these arrays into the lower inner lip mucosa of a rabbit and the dorsal surface of rabbit tongue, respectively. Coated microneedles have the ability to deliver the vaccine antigens directly in to the mucosa with high efficiency. With microneedles, antigen specific systemic IgG and secretory IgA in saliva was stimulated, and in contrast only systemic response was seen with intramuscular injections of the same antigens. No significant difference was observed in antibody response when microneedles were inserted only in the lips or the tongue region [181]. Recently microneedles composed of liposomes were also inserted in to the oral cavity mucosa of mice and was shown to induce robust serum IgG, and sIgA in the saliva, intestinal wash and vaginal wash [182].
5.9 Other vaccine delivery systems for the oral cavity
Mucosal vaccines delivered through the oral cavity can be classified into live attenuated microbes (wild type virus, recombinant virus & bacteria) [183–185], inactivated viruses [186], and subunit vaccines [187]. Microbe-based vaccines are able to induce broad-spectrum immunity sublingually unlike the subunit vaccines, for which poor antigen-specific immune responses are observed. For sublingual vaccination, adjuvants, especially microbe-based toxins such as CT and LT enterotoxins have been found necessary to induce strong antigen-specific systemic and mucosal antibodies [68]. For instance, HA, a subunit influenza vaccine induced low systemic IgG and HA titers with undetectable IgA response after three sublingual doses, however when LTK3 (inactivated mutant of heat labile enterotoxin of E. coli) was added as a mucosal adjuvant, considerably improved systemic IgG and mucosal IgA in nasal wash and saliva was observed as compared to the intramuscular route [188]. Other adjuvants such as CpG [189], cyclic diguanylate [159], poly (inosinic:cytidylic) (poly (I:C)) [190], MPL-A [190], vitamin D3 [191], peptidoglycans [191], chitosan [190], and γ-PGA [191] have also been explored in mice for oral cavity vaccination.
Typically, sublingual vaccines are administered in the form of droplets by placing the vaccine formulations under the tongue for a short time to enable absorption or uptake by the mucosa. Often, as is the case with sublingual allergy immunotherapy (SLIT), glycerol is added into the formulation to increase viscosity, which helps to increase allergen retention time under the tongue [192, 193]. Another delivery technology based on temperature responsive polymer gels has been studied for sublingual vaccination. This formulation is a liquid at room temperature, but gels upon contact with the warmer oral mucosa, resulting in higher retention time (>20 min) of the vaccine, and preventing rapid clearance and degradation of the vaccine from the oral cavity mucosal site [194]. Sublingual vaccination with an inactivated polio vaccine formulated with a temperature responsive gel elicited both serum IgG and mucosal IgA, while intramuscular vaccination yielded only serum IgG, and non-detectable IgA in mucosal secretions of saliva and fecal matter [194]. Presence of saliva needs to be accounted in oral cavity mucosal vaccine delivery because saliva can destabilize and digest the antigen. Excessive saliva can also dilute the antigen dose or cause it to be swallowed before absorption at the mucosa, a phenomenon termed as “saliva washout” [61]. Therefore, drying of oral cavity is recommended in some vaccine formulations before applying to the oral cavity. Extended release films and tablets have also been explored to improve sublingual or buccal vaccination by improving contact time of the antigen with the mucosa [195]. For instance, a mucoadhesive two-layer tablet with different protein-releasing rates was tested in mice for sublingual immunization. The tablet with faster antigen release rate was more favorable in inducing an immune response as compared to the extended release formulation, but was comparable to the control group (ovalbumin in solution with CT) [196].
5.10 Other vaccine delivery systems for the skin
Use of enterotoxins as adjuvants to induce effective systemic and mucosal responses towards vaccine antigens applied to the skin is well established. For example, Glenn et al. have shown that application of an aqueous solution containing CT or LT with or without DT as an antigen on intact shaved skin in mice for 2 h induced IgG serum response towards the adjuvant and the antigen. Noticeably, IgA antibodies against DT were not detected in lung wash and fecal matter when CT was not used as an adjuvant [197]. Presence of CT in the vaccine formulation acts as an adjuvant to induce strong IgG and IgA response in the serum as well as in other mucosal secretions such as the vaginal wash and saliva [198].
Gene gun is another approach that has been widely used for skin vaccination, and is an effective route for DNA vaccines. It induces protective antibody and CTL responses [199]. For example, gold nanoparticles coated with a DNA vaccine expressing gag-pol-env of the simian immunodeficiency virus (SIV) upon delivery into the epidermis of rhesus macaques using a gene gun activated serum IgG, and IgA in rectal washes and the intestinal secretions. CTL response was also seen, and the vaccination provided significant protection against mucosal challenge with SIV [200]. Gene gun based skin vaccination has also shown promising results at a clinical level in terms of requirement of a low dose of DNA in contrast to the intramuscular route. One to four microgram of an influenza DNA vaccine delivered through the skin was sufficient to induce high antibody levels with 100% incidence in the human subjects [201]. In a clinical study, human subjects vaccinated with influenza and challenged with a dose of H3 influenza virus gave around 53% protection against the respiratory infection [202].
Other approaches such as tape stripping, lasers, thermal ablation, chemical enhancers, and microdermabrasion have been developed for drug delivery in to the skin. These methods disrupt the stratum corneum layer and help to deliver the antigen into the skin, and they have also been investigated for delivery of antigens across the skin [203–205].
6 ADJUVANTS FOR MUCOSAL ROUTES
Adjuvants are commonly used in a vaccine formulation to solve the problem of low immunogenicity of recombinant protein antigens [206]. Mucosal vaccines typically require adjuvants to help stimulate stronger systemic and mucosal immune responses [3, 207]. When formulated with the antigen, adjuvants are an important factor that help to boost the immune response towards the antigen and can thus improve the efficacy of the vaccine [208, 209]. The various delivery systems discussed above, especially particulate systems offer an inherent adjuvant effect. This is because particles can non-specifically stimulate the APCs, such as macrophages and DCs. Furthermore, the particulate systems that slowly release antigen, and the mucoadhesive formulations that allow increased contact time of the antigen with mucosal surfaces could both act as an adjuvant through ‘antigen persistence’ or the ‘depot effect’.
As discussed above, the MALT system consists of immune cells including macrophages, DCs, B-cells, and T-cells underlying the epithelial cells. Stimulation of these immune cells at the same time as antigen delivery across the mucosal surfaces has the potential to increase the immune responses produced against the antigen. Evidence also suggests that the epithelial cells are not passive, and they can also be stimulated to secrete factors such as pro-inflammatory cytokines at their basolateral surface to activate the underlying immune cells [210]. Furthermore, IELs that sample antigens in the mucosal space could also be potentially directly stimulated by adjuvant molecules. Different molecules have been evaluated as mucosal adjuvants in this context, and a brief overview is given below.
The most well-studied and potent mucosal adjuvants are CT, LT, and DT. These bacterial toxins exert an adjuvant effect and are considered to be a ‘gold standard’ for mucosal adjuvants. However, due to their harmful toxic effect, they are not suitable for clinical applications. To avoid the toxicity and to explore their adjuvanticity to co-administered antigens, different approaches have been studied. Examples include, site directed mutagenesis like generation of mutants that are less toxic, and linking of other proteins or peptides to disrupt the active site of the toxin [211, 212].
Another group of adjuvants often used for mucosal vaccination are those that bind to pattern-recognition receptors (PRRs). PRRs are receptors found on (or within) cells that can detect presence of microbes by recognizing features that are associated with microbes and are conserved. These conserved features are called pathogen-associated molecular patterns (PAMPs). Few examples of PAMPs include flagellin and CpG DNA. Activation of PRRs is followed by an initial innate response including release of inflammatory cytokines, interferons and chemokines. The most familiar PRRs are the toll-like receptors (TLRs), which are involved in the recognition of viruses and bacteria [213]. TLRs are transmembrane signaling proteins expressed by mammalian cells and have the capability to bind ligands of varied molecular nature [210]. Till now, 10 TLRs in humans and 13 in mice have been identified [213]. A secondary benefit of using a TLR antagonist as an adjuvant is that the immune response can also be skewed towards either Th2 or Th1 dominance.
The epithelial cells of the gut have been shown to express TLR-2, 4, 5, and 9 in mice [214], and TLR-2, 3, 4, and 5 in humans [215]. Chabot et al. have shown in mice that TLR2 and TLR4 agonists can enhance particle uptake, and the TLR2 agonist can also induce migration of DCs in to the FAE [214]. Thus, TLR-based adjuvants could help to enhance effectiveness of mucosal vaccines. Some of the TLR agonists that have been investigated as mucosal vaccine adjuvants are summarized below.
MPL-A, a TLR4 agonist is the first and only FDA-approved adjuvant other than alum. MPL-A and alum are combined to form the adjuvant system AS04, and it is currently used for Cervarix™ (a human papillomavirus vaccine) and Fendrix™ (a hepatitis-B vaccine) vaccines by Glaxo-SmithKline. MPL-A is isolated from the lipopolysaccharide of Salmonella Minnesota R595. MPL-A has most of the immunostimulatory properties of the parent lipopolysaccharide but without any toxicity [210]. Generally, AS04 boosts the antibody titers by about 10 to 20 fold when compared to the vaccine alone, and induces the production of IgG2a Abs (Th1 dominance) in mice [216, 217]. Vaccine trials in humans have found that the level of safety profile of AS04 is similar to that of alum [217, 218].
TLR9 antagonist, CpG has been relatively well studied among the ligands of the 13 TLRs because of its generally high efficacy and synthetic nature. In general, CpG up-regulates the expression of MHC class II molecules, directly activates monocytes, macrophages and DCs and it is capable of inducing B cell proliferation and differentiation. Moreover, it can stimulate Th1-biased cytokine profile of type 1 interleukin-12 (IL-12), TNF-α, and IFN-γ, with a predominant IgG2a titer in serum, and CTLs [219–223]. In our studies, co-administration of CpG as an adjuvant with AuNPs conjugated with M2e peptide led to generation of a balanced Th1/Th2 immune response, as compared to Th2-dominant immunity in the no-adjuvant group [136]. A recent study showed that sublingual vaccination with clade C gp140 HIV-1 envelope protein adjuvanted with α-galactosylceramide (potent stimulator of natural killer T cells) and CpG, significantly induces neutralization activity against Tier 1 and Tier 2 SHIVs [224].
TLR3 ligand poly(I:C) is a synthetic analog of dsRNA, which is found in many viruses. It not only interacts with TLR3 but also with other receptors like retinoic acid inducible gene I, melanoma differentiation-associated gene 5, and double-stranded RNA-dependent protein kinase. Thus, the adjuvanticity of poly(I:C) cannot be uniquely ascribed to TLR3 activation [225, 226]. However, poly(I:C) induces the production of inflammatory cytokines and type I IFNs through the activation of NF-κB, MAP kinases, and IRF3. TLR3 agonists stimulate DC maturation and antigen cross-presentation in the context of MHC class I molecules. As a result poly(I:C) improves the generation of CTLs [227, 228]. Sloat et al. reported that, by using poly(I:C) as an adjuvant for intranasal vaccination with anthrax biantigen and triantigen, a strong immune response against all antigens could be induced [229, 230].
TLR5 ligand, flagellin is also a mucosal adjuvant. Flagellin is a structural component of the flagellar filament of bacteria, and possesses unique signaling characteristics. While other TLRs use multiple adaptor molecules and redundant signaling pathways, flagellin is the only reported TLR5 ligand which uses only MyD88 as the cytoplasmic adaptor molecule for TLR5. Furthermore, it is important to note that TLR5 is located only on the basolateral side of the epithelial cells. This prevents stimulation of TLR5 by commensal bacteria, and TLR5-activation occurs only from invading bacteria [231–233]. The adjuvant effect of flagellin has been tested intranasally for many vaccines such as against influenza virus [234, 235], tetanus toxin [236], malaria [237], Schistosoma mansoni [238], and HIV [239].
Even TLR7/8 agonists, the imidazoquinolines, such as imiquimod or gardiquimod, have been shown to induce mucosal immune responses when co-delivered with hepatitis B surface antigen in chitosan nanocapsules upon intranasal vaccination in mice [115].
Other adjuvants that have been shown to work mucosally are cytokines and chemokines. The most important of these are from the IL-1 superfamily including IL-1α/β, IL-18, and IL-33. A study showed that intranasal administration of influenza subunit vaccine with IL-1 family cytokines increased IgA and IgG antibodies [240]. Other pro-inflammatory cytokines, like IL-6, IL-12, and IL-15, have also been found to act as mucosal adjuvants, inducing CTL responses and vaccine-specific IgA antibodies [241]. Furthermore, chemokines, like MCP-1, MIP-1α, MIP-1β, SLC/CCL19, SLC/CCL21 and MIP-2 can also induce mucosal IgA secretion and CTL responses [241–244] when delivered with antigens.
Besides bacterial enterotoxins, TLR ligands, and cytokines/chemokines, other molecules have been recently reported as mucosal adjuvants. Among them are γ-PGA, α-galactosylceramide - a CD1d ligand, and Iscomatrix (colloidal, spherical structures, comprising of saponin such as Quil A, cholesterol and a phospholipid), which have been used in different vaccine studies [131, 245–247]. Furthermore, a mast cell activating compound C48/80 has been shown to be a safe and effective nasal adjuvant in mice [248, 249]. Water soluble vitamin E derivative (d-α-tocopheryl polyethylene glycol 1000 succinate) is also suggested as a promising nasal vaccination potentiator [250]. Lipid-based and archaeal lipid mucosal vaccine adjuvants, and several fungal or bacterially-derived small molecules are also being actively researched and have been recently used to enhance sIgA induction [251].
7. PERSPECTIVES FOR PEDIATRIC MUCOSAL VACCINATION AND CONCLUSION
The current needle-based delivery of vaccines is effective but the protection kicks in after the pathogen has gained a foothold by infecting the mucosal host cells. The mucosal route of vaccination has the potential to take the vaccine-induced protection to a new level by developing an active front line of defense at the mucosal surfaces. This line of defense could neutralize pathogens even before they can cause infection. As discussed above, a significant amount of work has been done to develop mucosal vaccines, however, the FDA-approved mucosal vaccines are few. In the following section we provide a perspective on the development of mucosal vaccines with a focus on the pediatric population.
7.1 Competence of the mucosal immune system of humans at birth – is it ready for mucosal vaccination?
At birth in humans, the essential components of the mucosal system are developed and can respond to antigenic stimulation. The development of the GALT occurs in utero. Figure 8 summarizes the development of GALT during gestation. The PPs can be seen as early as week 11 of gestational age, and at the time of birth there are about 80–120 PPs with at least five lymphoid follicles with germinal centers. By day 200 of gestation, SC is expressed in the gastrointestinal and respiratory epithelium. At birth, a normal full-term infant does not have IgA antibodies. This is a result of the unresponsiveness of the infant’s mucosal immune system due to hormonal influences during birth, immaturity of APCs, and immunosuppressive-effect of maternal IgG antibodies. However, the immune system is capable of mounting a rapid immune response as demonstrated by studies of fetuses getting intrauterine infections and prematurely-born infants who get pulmonary infections [252, 253]. The Waldeyer’s ring, which is the human equivalent of the NALT is also present at the time of birth, and it’s formation occurs during organogenesis in a fetus. The primary follicles are seen in tonsils at 16 weeks of gestation. The formation of germinal centers in tonsils, indicative of B cell activation by exogenous antigens is seen shortly after birth, and terminal differentiation of activated B cells into extrafollicular plasma cells can be seen 2 weeks post birth [254]. Thus, the human infant is born with a functional mucosal immune system, and can be stimulated by vaccine antigens delivered via the mucosal surfaces.
Figure 8.
In utero developmental timeline of the human mucosal immune system. At birth, for a full-term neonate, all components of the mucosal immune system are mature. (1) M-cells, (2) Peyer’s patches that contain lymphoid aggregates, (3) interstitial lymphocytes, and (4) intraepithelial lymphocytes. Adapted with permission from [256]
7.2 Considerations for pediatric mucosal vaccination
In the pediatric population, the oral, the nasal, the oral cavity, and the skin appear to be the most practical routes of vaccination. Certainly, the vaccination systems applied to each route must also be minimally invasive and easy to administer. In the context of the oral route, it must be kept in mind that in the period immediately after birth the gastrointestinal permeability in infants is higher than that in adults [255]. At birth, the infant’s mucosal immune system is rapidly stimulated and the mucosal epithelium tarts to close. Ingestion of colostrum is seen to promote maturation of the intestinal membrane. The intestinal membrane closes in about 48 h [252]. Other mucosal membranes also close rapidly. This early window when the mucosal membranes are more permeable could be an opportunistic moment to deliver the vaccines orally, however, care needs to be taken so as to not induce autoimmunity, hypersensitivity, or food enteropathies. Additional studies in animal models could help to understand this aspect further by delivering vaccines right at birth, and then evaluating the immune responses and assessing long term safety. More neonatal mucosal vaccination studies are anyway needed because most of the mucosal vaccination studies have been performed in adult animals, and fewer studies have been performed in neonatal mice [257, 258].
To induce vigorous systemic and mucosal immune responses, a combination of different mucosal routes of vaccination might be needed. Typically the nasal and skin-based vaccinations provide high serum IgG titers, however, the oral route can provide lower serum IgG titers. On the other hand, oral vaccination can induce stronger sIgA titers in the intestinal secretions. Thus, a combination of different routes of vaccination might offer a synergistic effect, increasing both systemic and mucosal antibodies over the individual routes. Indeed, using CT as an adjuvant and CTB as the antigen, John et al. have shown that immunization via the skin (intact skin applied with immunization solution) induced higher serum IgG against CTB than the oral delivery of the immunization solution, however, a comparable IgA response in fecal matter was seen from both routes of vaccination. But when the mice were orally primed and boosted via the skin, a more prominent serum IgG and oral IgA response was seen [259]. In fact, initially priming the immune system via the intramuscular route and providing later boosts via the mucosal routes may also provide synergistic effects in enhancing mucosal immune responses. This strategy although not ideal, could still have potential for clinical implementation, wherein the first dose of the vaccine to the infant is given parenterally, and subsequent doses are given via a mucosal route.
Toxin-based adjuvants such as CT and LT are often used in pre-clinical mucosal vaccine formulations. While they provide proof-of-principle, however, due to their toxicity, their approval for human use is unlikely. It is therefore important to evaluate other safer but perhaps less potent adjuvants for mucosal vaccination. Different adjuvants could be combined to evaluate their synergistic effects in mucosal vaccines. Because adjuvants form a critical component of mucosal vaccine formulations, a safe yet potent adjuvant cocktail is needed.
The economic considerations to introduce mucosal vaccines in place of existing needle-based vaccines should be very carefully considered. This is because the cost of development and the clinical trials may drive the cost of the mucosal vaccine to be higher than the existing vaccine. In this regard, the cost of formulation and the delivery system will also be critical. If a mucosal vaccine delivery platform can be developed that can be simply mixed with the vaccines that are already being produced by the vaccine manufacturing companies, then the existing infrastructure for vaccine production could be utilized. Instead of packaging the vaccines in vials or syringes, the delivery system (such as a nanoparticle that hypothetically enhances intestinal permeability) could be simply added to the vaccines to form a mucosal vaccine formulation.
Furthermore, sIgA is thought to be an important effector of mucosal immunity. However, the mechanisms of mucosal immunity are not very well known. Systematic studies are therefore needed that can examine the importance of sIgA in vaccine design, and evaluate their correlate of protection for mucosal vaccines. In addition to sIgA, IgG can be found in mucus secretions, and can also play a role in the clearance of pathogens. Besides humoral immunity, the role of cellular immunity and innate immune factors needs to be investigated to better understand the mechanism of protection from mucosal vaccination.
Overall, mucosal vaccine design and delivery involves multiple complex questions, including choice of route, selection of delivery carriers, and selection of adjuvants. Although much has been learnt about the mucosal immune system in the past few decades, significant knowledge gaps still exist. For future studies, it will be important to more thoroughly evaluate the different mucosal vaccination routes. It is possible that prime-boost strategies that involve different mucosal surfaces might actually offer a superior immune response compared to a single mucosal route of vaccination. More creative synergetic use of nanotechnology and recombinant technology might also help to design the next generation of mucosal vaccines.
Acknowledgments
We acknowledge the support by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) under Award Number DP2HD075691, National Institute of Health 1R03DE021667-01A1, R21AI099575 to HSG, and Defense Advanced Research Projects Agency (DARPA) - Young Faculty Award under grant number N66001-12-1-4251 to HSG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
A patent has been filed by the authors regarding use of pollen grains for oral vaccination through Texas Tech University. This potential conflict of interest has been disclosed and is managed by Texas Tech University.
REFERENCES
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. global regional national causes of child mortality in 2000-13 with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 3.Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012;12:592–605. doi: 10.1038/nri3251. [DOI] [PubMed] [Google Scholar]
- 4.Russell MW, Mestecky J. Chapter 55 - Mucosal Vaccines: An Overview. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. Fourth. Boston: Academic Press; 2015. pp. 1039–1046. [Google Scholar]
- 5.Hauri AM, Armstrong GL, Hutin YJF. The global burden of disease attributable to contaminated injections given in health care settings. International Journal of STD & AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Cholera vaccines: WHO position paper. Weekly Epidemiological Record. 2010;85:117–128. [Google Scholar]
- 7.World Health Organization. Vaccines against influenza WHO position paper - November 2012. Weekly Epidemiological Record. 2012;87:461–476. [Google Scholar]
- 8.World Health Organization Polio vaccines: WHO position paper. January 2014. Weekly Epidemiological Record. 2014;89:73–92. [Google Scholar]
- 9.World Health Organization. Rotavirus vaccines WHO position paper - January 2013. Weekly Epidemiological Record. 2013;88:49–64. [Google Scholar]
- 10.World Health Organization. Typhoid vaccines: WHO position paper. Weekly Epidemiological Record. 2008;83:49–60. [Google Scholar]
- 11.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 12.Russell M, Mestecky J, Strober W, Lambrecht BN, Kelsall BL, Cheroutre H. Chapter 1 -Overview: The Mucosal Immune System. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. Fourth. Boston: Academic Press; 2015. pp. 3–8. [Google Scholar]
- 13.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nature reviews. Microbiology. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 14.Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3:944–955. doi: 10.1038/nrm972. [DOI] [PubMed] [Google Scholar]
- 15.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 16.Kaetzel CS, Robinson JK, Chintalacharuvu KR, Vaerman JP, Lamm ME. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci U S A. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornquist CE, Ekman L, Grdic KD, Schon K, Lycke NY. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J Immunol. 1995;155:2877–2887. [PubMed] [Google Scholar]
- 18.Goodrich ME, McGee DW. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine. 1998;10:948–955. doi: 10.1006/cyto.1998.0385. [DOI] [PubMed] [Google Scholar]
- 19.Asano T, Kaneko H, Terada T, Kasahara Y, Fukao T, Kasahara K, Kondo N. Molecular analysis of B-cell differentiation in selective or partial IgA deficiency. Clin Exp Immunol. 2004;136:284–290. doi: 10.1111/j.1365-2249.2004.02440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 21.Simmons CP, Hussell T, Sparer T, Walzl G, Openshaw P, Dougan G. Mucosal delivery of a respiratory syncytial virus CTL peptide with enterotoxin-based adjuvants elicits protective immunopathogenic and immunoregulatory antiviral CD8+ T cell responses. J Immunol. 2001;166:1106–1113. doi: 10.4049/jimmunol.166.2.1106. [DOI] [PubMed] [Google Scholar]
- 22.Cesta MF. Normal Structure function and Histology of Mucosa-Associated Lymphoid Tissue. Toxicologic Pathology. 2006;34:599–608. doi: 10.1080/01926230600865531. [DOI] [PubMed] [Google Scholar]
- 23.Azzali G. Structure lymphatic vascularization and lymphocyte migration in mucosa-associated lymphoid tissue. Immunol Rev. 2003;195:178–189. doi: 10.1034/j.1600-065x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 24.Flach M, Diefenbach A. Chapter 3 - Development of Gut-Associated Lymphoid Tissues. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. Fourth. Boston: Academic Press; 2015. pp. 31–42. [Google Scholar]
- 25.Kunisawa J, Kurashima Y, Kiyono H. Gut-associated lymphoid tissues for the development of oral vaccines. Adv Drug Deliv Rev. 2012;64:523–530. doi: 10.1016/j.addr.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Dobbins WO., III Human intestinal intraepithelial lymphocytes. Gut. 1986;27:972–985. doi: 10.1136/gut.27.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry M, Whyte A. Immunology of the tonsils. Immunology Today. 1998;19:414–421. doi: 10.1016/s0167-5699(98)01307-3. [DOI] [PubMed] [Google Scholar]
- 29.Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev. 2005;206:22–31. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Howie AJ. Scanning and transmission electron microscopy on the epithelium of human palatine tonsils. J Pathol. 1980;130:91–98. doi: 10.1002/path.1711300205. [DOI] [PubMed] [Google Scholar]
- 31.Chadwick S, Kriegel C, Amiji M. Delivery strategies to enhance mucosal vaccination. Expert Opin Biol Ther. 2009;9:427–440. doi: 10.1517/14712590902849224. [DOI] [PubMed] [Google Scholar]
- 32.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer’s patches are required for oral tolerance to proteins. Proc Natl Acad Sci U S A. 2001;98:3310–3315. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morag A, Morag B, Bernstein JM, Beutner K, Ogra PL. In vitro correlates of cell-mediated immunity in human tonsils after natural or induced Rubella virus infection. J Infect Dis. 1975;131:409–416. doi: 10.1093/infdis/131.4.409. [DOI] [PubMed] [Google Scholar]
- 34.Sminia T, van der Brugge-Gamelkoorn GJ, Jeurissen SH. Structure and function of bronchus-associated lymphoid tissue (BALT) Crit Rev Immunol. 1989;9:119–150. [PubMed] [Google Scholar]
- 35.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, Worbs T, Forster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tango M, Suzuki E, Gejyo F, Ushiki T. The presence of specialized epithelial cells on the bronchus-associated lymphoid tissue (BALT) in the mouse. Arch Histol Cytol. 2000;63:81–89. doi: 10.1679/aohc.63.81. [DOI] [PubMed] [Google Scholar]
- 37.Gregson RL, Edmondson NA, Plesch B. Preferential uptake of soluble antigen by respiratory tract epithelium overlying bronchus-associated lymphoid tissue in the rat. Adv Exp Med Biol. 1982;149:499–505. doi: 10.1007/978-1-4684-9066-4_70. [DOI] [PubMed] [Google Scholar]
- 38.Pabst R, Gehrke I. Is the Bronchus-associated Lymphoid Tissue (BALT) an Integral Structure of the Lung in Normal Mammals Including Humans? American Journal of Respiratory Cell and Molecular Biology. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- 39.Pabst R, Tschernig T. Bronchus-Associated Lymphoid Tissue. American Journal of Respiratory Cell and Molecular Biology. 2010;43:137–141. doi: 10.1165/rcmb.2010-0152RT. [DOI] [PubMed] [Google Scholar]
- 40.Ersch J, Tschernig T, Stallmach T. Frequency and potential cause of bronchus-associated lymphoid tissue in fetal lungs. Pediatr Allergy Immunol. 2005;16:295–298. doi: 10.1111/j.1399-3038.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawamata N, Xu B, Nishijima H, Aoyama K, Kusumoto M, Takeuchi T, Tei C, Michie SA, Matsuyama T. Expression of endothelia and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lung. Respir Res. 2009;10:97. doi: 10.1186/1465-9921-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGuckin MA, Thornton DJ, Whitsett JA. Chapter 14 - Mucins and Mucus. In: Mestecky J, Strober W, Russell MW, Kelsall BL, Cheroutre H, Lambrecht BN, editors. Mucosal Immunology. Fourth. Boston: Academic Press; 2015. pp. 231–250. [Google Scholar]
- 43.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark MA, Jepson MA, Hirst BH. Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev. 2001;50:81–106. doi: 10.1016/s0169-409x(01)00149-1. [DOI] [PubMed] [Google Scholar]
- 45.Bland PW, Warren LG. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology. 1986;58:9–14. [PMC free article] [PubMed] [Google Scholar]
- 46.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 47.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 48.Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–286. doi: 10.1016/j.smim.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson LA, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-gamma production and evokes local immunological memory. J Clin Invest. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral rectal and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A. Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun. 2001;69:7481–7486. doi: 10.1128/IAI.69.12.7481-7486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell-Jones GJ. Oral vaccine delivery. J Control Release. 2000;65:49–54. doi: 10.1016/s0168-3659(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 53.Kim SH, Jang YS. Antigen targeting to M cells for enhancing the efficacy of mucosal vaccines. Exp Mol Med. 2014;46:e85. doi: 10.1038/emm.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh B, Maharjan S, Jiang T, Kang SK, Choi YJ, Cho CS. Combinatorial Approach of Antigen Delivery Using M Cell-Homing Peptide and Mucoadhesive Vehicle to Enhance the Efficacy of Oral Vaccine. Mol Pharm. 2015 doi: 10.1021/acs.molpharmaceut.5b00265. [DOI] [PubMed] [Google Scholar]
- 55.des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Liau JJ, Hook S, Prestidge CA, Barnes TJ. A lipid based multi-compartmental system: Liposomes-in-double emulsion for oral vaccine delivery. Eur J Pharm Biopharm. 2015 doi: 10.1016/j.ejpb.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Ugwoke MI, Verbeke N, Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. The Journal of pharmacy and pharmacology. 2001;53:3–21. doi: 10.1211/0022357011775145. [DOI] [PubMed] [Google Scholar]
- 58.Brandtzaeg P. Immune functions of nasopharyngeal lymphoid tissue. 2011 doi: 10.1159/000324588. [DOI] [PubMed] [Google Scholar]
- 59.Mall MA. Role of cilia mucus and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- 60.Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how. why and what for? 2009 doi: 10.18433/j3nc79. [DOI] [PubMed] [Google Scholar]
- 61.Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153:106–116. doi: 10.1016/j.jconrel.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 62.Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, Kerr AR, Lockhart PB, Patton LL, Porter S, Thornhill MH. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliv Rev. 2012;64:16–28. doi: 10.1016/j.addr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Pather SI, Rathbone MJ, Senel S. Current status and the future of buccal drug delivery systems. Expert Opin Drug Deliv. 2008;5:531–542. doi: 10.1517/17425247.5.5.531. [DOI] [PubMed] [Google Scholar]
- 64.Barrett AW, Cruchley AT, Williams DM. Oral mucosal Langerhans’ cells. Crit Rev Oral Biol Med. 1996;7:36–58. doi: 10.1177/10454411960070010301. [DOI] [PubMed] [Google Scholar]
- 65.Hasseus B, Jontell M, Bergenholtz G, Dahlgren UI. Langerhans cells from human oral epithelium are more effective at stimulating allogeneic T cells in vitro than Langerhans cells from skin. Clin Exp Immunol. 2004;136:483–489. doi: 10.1111/j.1365-2249.2004.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Desvignes C, Esteves F, Etchart N, Bella C, Czerkinsky C, Kaiserlian D. The murine buccal mucosa is an inductive site for priming class I-restricted CD8+ effector T cells in vivo. Clin Exp Immunol. 1998;113:386–393. doi: 10.1046/j.1365-2249.1998.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu FX, Jacobson RS. Oral mucosal immunity and HIV/SIV infection. J Dent Res. 2007;86:216–226. doi: 10.1177/154405910708600305. [DOI] [PubMed] [Google Scholar]
- 68.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–592. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hervouet C, Luci C, Bekri S, Juhel T, Bihl F, Braud VM, Czerkinsky C, Anjuere F. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunol. 2014;7:280–291. doi: 10.1038/mi.2013.45. [DOI] [PubMed] [Google Scholar]
- 70.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. International Journal of Pharmaceutics. 2008;364:227–236. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karande P, Arora A, Pham TK, Stevens D, Wojicki A, Mitragotri S. Transcutaneous immunization using common chemicals. Journal of Controlled Release. 2009;138:134–140. doi: 10.1016/j.jconrel.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Nicolas J-F, Guy B. Intradermal epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Review of Vaccines. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 73.Belyakov IM, Hammond SA, Ahlers JD, Glenn GM, Berzofsky JA. Transcutaneous immunization induces mucosal CTLs and protective immunity by migration of primed skin dendritic cells. J Clin Invest. 2004;113:998–1007. doi: 10.1172/JCI20261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chang SY, Cha HR, Igarashi O, Rennert PD, Kissenpfennig A, Malissen B, Nanno M, Kiyono H, Kweon MN. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180:4361–4365. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]
- 75.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 76.Sridhar S, Brokstad KA, Cox RJ. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines. 2015;3:373–389. doi: 10.3390/vaccines3020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6:e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tucker SN, Tingley DW, Scallan CD. Oral adenoviral-based vaccines: historical perspective and future opportunity. Expert Rev Vaccines. 2008;7:25–31. doi: 10.1586/14760584.7.1.25. [DOI] [PubMed] [Google Scholar]
- 79.Kotton CN, Lankowski AJ, Scott N, Sisul D, Chen LM, Raschke K, Borders G, Boaz M, Spentzou A, Galan JE, Hohmann EL. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella Type III secretion system. Vaccine. 2006;24:6216–6224. doi: 10.1016/j.vaccine.2006.05.094. [DOI] [PubMed] [Google Scholar]
- 80.Chowdhury MY, Seo SK, Moon HJ, Talactac MR, Kim JH, Park ME, Son HY, Lee JS, Kim CJ. Heterosubtypic protective immunity against widely divergent influenza subtypes induced by fusion protein 4sM2 in BALB/c mice. Virol J. 2014;11:21. doi: 10.1186/1743-422X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He X-S, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, Dekker CL, Greenberg HB, Arvin AM. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M, Investigators SS. Safety of live attenuated influenza vaccine in atopic children with egg allergy. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2014.12.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shata MT, Hone DM. Vaccination with a Shigella DNA vaccine vector induces antigen-specific CD8(+) T cells and antiviral protective immunity. J Virol. 2001;75:9665–9670. doi: 10.1128/JVI.75.20.9665-9670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chowdhury MY, Li R, Kim JH, Park ME, Kim TH, Pathinayake P, Weeratunga P, Song MK, Son HY, Hong SP, Sung MH, Lee JS, Kim CJ. Mucosal vaccination with recombinant Lactobacillus casei-displayed CTA1-conjugated consensus matrix protein-2 (sM2) induces broad protection against divergent influenza subtypes in BALB/c mice. PLoS One. 2014;9:e94051. doi: 10.1371/journal.pone.0094051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li R, Chowdhury MY, Kim JH, Kim TH, Pathinayake P, Koo WS, Park ME, Yoon JE, Roh JB, Hong SP, Sung MH, Lee JS, Kim CJ. Mucosally administered Lactobacillus surface-displayed influenza antigens (sM2 and HA2) with cholera toxin subunit A1 (CTA1) Induce broadly protective immune responses against divergent influenza subtypes. Vet Microbiol. 2015;179:250–263. doi: 10.1016/j.vetmic.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 86.Hopkins S, Kraehenbuhl JP, Schodel F, Potts A, Peterson D, de Grandi P, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Friedman RS, Frankel FR, Xu Z, Lieberman J. Induction of human immunodeficiency virus (HIV)-specific CD8 T-cell responses by Listeria monocytogenes and a hyperattenuated Listeria strain engineered to express HIV antigens. J Virol. 2000;74:9987–9993. doi: 10.1128/jvi.74.21.9987-9993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thippeshappa R, Tian B, Cleveland B, Guo W, Polacino P, Hu S-L. Oral Immunization with Recombinant Vaccinia Virus Prime and Intramuscular Protein Boost Provides Protection against Intra-Rectal SHIV Challenge in Macaques. Clinical and Vaccine Immunology. 2015 doi: 10.1128/CVI.00597-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Çuburu N, Kweon M-N, Song J-H, Hervouet C, Luci C, Sun J-B, Hofman P, Holmgren J, Anjuère F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–8610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 90.van der Lubben IM, Verhoef JC, Borchard G, Junginger HE. Chitosan for mucosal vaccination. Adv Drug Deliv Rev. 2001;52:139–144. doi: 10.1016/s0169-409x(01)00197-1. [DOI] [PubMed] [Google Scholar]
- 91.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chemical Reviews. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brayden DJ, Baird AW. Microparticle vaccine approaches to stimulate mucosal immunisation. Microbes and Infection. 2001;3:867–876. doi: 10.1016/s1286-4579(01)01445-9. [DOI] [PubMed] [Google Scholar]
- 93.Carr KE, Hazzard RA, Reid S, Hodges GM. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm Res. 1996;13:1205–1209. doi: 10.1023/a:1016064320334. [DOI] [PubMed] [Google Scholar]
- 94.Gutierro I, Hernandez RM, Igartua M, Gascon AR, Pedraz JL. Influence of dose and immunization route on the serum Ig G antibody response to BSA loaded PLGA microspheres. Vaccine. 2002;20:2181–2190. doi: 10.1016/s0264-410x(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 95.Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori-loaded poly(DL-lactide-co-glycolide) nanoparticles. Helicobacter. 1999;4:33–39. doi: 10.1046/j.1523-5378.1999.09046.x. [DOI] [PubMed] [Google Scholar]
- 96.Jung T, Kamm W, Breitenbach A, Hungerer KD, Hundt E, Kissel T. Tetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): evaluation of antibody response after oral and nasal application in mice. Pharm Res. 2001;18:352–360. doi: 10.1023/a:1011063232257. [DOI] [PubMed] [Google Scholar]
- 97.Adomako M, St-Hilaire S, Zheng Y, Eley J, Marcum RD, Sealey W, Donahower BC, Lapatra S, Sheridan PP. Oral DNA vaccination of rainbow trout Oncorhynchus mykiss (Walbaum) against infectious haematopoietic necrosis virus using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] nanoparticles. J Fish Dis. 2012;35:203–214. doi: 10.1111/j.1365-2761.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 98.Smyth SH, Feldhaus S, Schumacher U, Carr KE. Uptake of inert microparticles in normal and immune deficient mice. Int J Pharm. 2008;346:109–118. doi: 10.1016/j.ijpharm.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 99.Carr RM, Lolachi CM, Albaran RG, Ridley DM, Montgomery PC, O’sullivan NL. Nasal-associated lymphoid tissue is an inductive site for rat tear IgA antibody responses. Immunol Invest. 1996;25:387–396. doi: 10.3109/08820139609055728. [DOI] [PubMed] [Google Scholar]
- 100.Lemoine D, Wauters F, Bouchend’Homme S, Préat V. Preparation and characterization of alginate microspheres containing a model antigen. Int J Pharm. 1998;176:9–19. [Google Scholar]
- 101.Lemoine D, Deschuyteneer M, Hogge F, Préat V. Intranasal immunization against influenza virus using polymeric particles. J Biomater Sci Polym Ed. 1999;10:805–825. doi: 10.1163/156856299x00892. [DOI] [PubMed] [Google Scholar]
- 102.Köping-Höggård M, Sánchez A. M.J. Alonso. Nanoparticles as carriers for nasal vaccine delivery. 2005 doi: 10.1586/14760584.4.2.185. [DOI] [PubMed] [Google Scholar]
- 103.Vila A, Sanchez A, Evora C, Soriano I, McCallion O, Alonso M. PLA-PEG particles as nasal protein carriers: the influence of the particle size. Int J Pharm. 2005;292:43–52. doi: 10.1016/j.ijpharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Mansoor F, Earley B, Cassidy JP, Markey B, Doherty S, Welsh MD. Comparing the immune response to a novel intranasal nanoparticle PLGA vaccine and a commercial BPI3V vaccine in dairy calves. BMC Vet Res. 2015;11:220. doi: 10.1186/s12917-015-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang G, Pan L, Zhang Y, Wang Y, Zhang Z, Lü J, Zhou P, Fang Y, Jiang S. Intranasal delivery of cationic PLGA nano/microparticles-loaded FMDV DNA vaccine encoding IL-6 elicited protective immunity against FMDV challenge. PloS one. 2011;6:e27605. doi: 10.1371/journal.pone.0027605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eyles JE, Sharp GJ, Williamson ED, Spiers ID, Alpar HO. Intra nasal administration of poly-lactic acid microsphere co-encapsulated Yersinia pestis subunits confers protection from pneumonic plague in the mouse. Vaccine. 1998;16:698–707. doi: 10.1016/s0264-410x(97)00249-1. [DOI] [PubMed] [Google Scholar]
- 107.Vila A, Sanchez A, Evora C, Soriano I, Vila Jato J, Alonso M. PEG-PLA nanoparticles as carriers for nasal vaccine delivery. J Aerosol Med. 2004;17:174–185. doi: 10.1089/0894268041457183. [DOI] [PubMed] [Google Scholar]
- 108.Ensign LM, Tang BC, Wang YY, Tse TA, Hoen T, Cone R, Hanes J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med. 2012;4:138ra179. doi: 10.1126/scitranslmed.3003453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Renukaradhya GJ, Narasimhan B, Mallapragada SK. Respiratory nanoparticle-based vaccines and challenges associated with animal models and translation. Journal of Controlled Release. 2015;219:622–631. doi: 10.1016/j.jconrel.2015.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a Protective Single-Dose Intranasal Nanoparticle-Based Vaccine Platform for Respiratory Infectious Diseases. PLoS ONE. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kean T, Thanou M. Biodegradation biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi T, Fukushima K, Sannan T, Saito N, Takiguchi Y, Sato Y, Hasegawa H, Ishikawa K. Evaluation of the effectiveness and safety of chitosan derivatives as adjuvants for intranasal vaccines. Viral Immunol. 26:133–142. doi: 10.1089/vim.2012.0057. [DOI] [PubMed] [Google Scholar]
- 115.Vicente S, Peleteiro M, Diaz-Freitas B, Sanchez A, Gonzalez-Fernandez A, Alonso MJ. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: a needle-free vaccination strategy. J Control Release. 172:773–781. doi: 10.1016/j.jconrel.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 116.van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Coos Verhoef J, Junginger HE. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine. 2003;21:1400–1408. doi: 10.1016/s0264-410x(02)00686-2. [DOI] [PubMed] [Google Scholar]
- 117.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 118.De S, Robinson D. Polymer relationships during preparation of chitosan-alginate and poly-l-lysine-alginate nanospheres. J Control Release. 2003;89:101–112. doi: 10.1016/s0168-3659(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 119.Rebelatto MC, Guimond P, Bowersock TL, HogenEsch H. Induction of systemic and mucosal immune response in cattle by intranasal administration of pig serum albumin in alginate microparticles. Vet Immunol Immunopathol. 2001;83:93–105. doi: 10.1016/s0165-2427(01)00370-1. [DOI] [PubMed] [Google Scholar]
- 120.Malik B, Goyal AK, Markandeywar TS, Rath G, Zakir F, Vyas SP. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J Drug Target. 20:76–84. doi: 10.3109/1061186X.2011.611516. [DOI] [PubMed] [Google Scholar]
- 121.Tafaghodi M, Rastegar S. Preparation and in vivo study of dry powder microspheres for nasal immunization. J Drug Target. 18:235–242. doi: 10.3109/10611860903434035. [DOI] [PubMed] [Google Scholar]
- 122.Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens: advantages challenges and formulation strategies. Protein Cell. 6:480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McDermott MR, Heritage PL, Bartzoka V, Brook MA. Polymer-grafted starch microparticles for oral and nasal immunization. Immunol Cell Biol. 1998;76:256–262. doi: 10.1046/j.1440-1711.1998.00743.x. [DOI] [PubMed] [Google Scholar]
- 124.Rydell N, Sjoholm I. Oral vaccination against diphtheria using polyacryl starch microparticles as adjuvant. Vaccine. 2004;22:1265–1274. doi: 10.1016/j.vaccine.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 125.Rydell N, Sjoholm I. Mucosal vaccination against diphtheria using starch microparticles as adjuvant for cross-reacting material (CRM197) of diphtheria toxin. Vaccine. 2005;23:2775–2783. doi: 10.1016/j.vaccine.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 126.Wikingsson L, Sjoholm I. Polyacryl starch microparticles as adjuvant in oral immunisation inducing mucosal and systemic immune responses in mice. Vaccine. 2002;20:3355–3363. doi: 10.1016/s0264-410x(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 127.Illum L, Fisher AN, Jabbal-Gill I, Davis SS. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int J Pharm. 2001;222:109–119. doi: 10.1016/s0378-5173(01)00708-6. [DOI] [PubMed] [Google Scholar]
- 128.Lee TY, Kim YH, Yoon SW, Choi JC, Yang JM, Kim CJ, Schiller JT, Sung MH, Poo H. Oral administration of poly-gamma-glutamate induces TLR4- dendritic cell-dependent antitumor effect Cancer immunology. immunotherapy : CII. 2009;58:1781–1794. doi: 10.1007/s00262-009-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin YH, Chung CK, Chen CT, Liang HF, Chen SC, Sung HW. Preparation of nanoparticles composed of chitosan/poly-gamma-glutamic acid and evaluation of their permeability through Caco-2 cells. Biomacromolecules. 2005;6:1104–1112. doi: 10.1021/bm049312a. [DOI] [PubMed] [Google Scholar]
- 130.Moon HJ, Lee JS, Talactac MR, Chowdhury MY, Kim JH, Park ME, Choi YK, Sung MH, Kim CJ. Mucosal immunization with recombinant influenza hemagglutinin protein and poly gamma-glutamate/chitosan nanoparticles induces protection against highly pathogenic influenza A virus. Vet Microbiol. 160:277–289. doi: 10.1016/j.vetmic.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 131.Noh HJ, Chowdhury MY, Cho S, Kim JH, Park HS, Kim CJ, Poo H, Sung MH, Lee JS, Lim YT. Programming of Influenza Vaccine Broadness and Persistence by Mucoadhesive Polymer-Based Adjuvant Systems. J Immunol. 195:2472–2482. doi: 10.4049/jimmunol.1500492. [DOI] [PubMed] [Google Scholar]
- 132.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 31:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh M, Briones M, O’Hagan DT. A novel bioadhesive intranasal delivery system for inactivated influenza vaccines. J Control Release. 2001;70:267–276. doi: 10.1016/s0168-3659(00)00330-8. [DOI] [PubMed] [Google Scholar]
- 134.Saupe A, McBurney W, Rades T. S. Hook. Immunostimulatory colloidal delivery systems for cancer vaccines. 2006 doi: 10.1517/17425247.3.3.345. [DOI] [PubMed] [Google Scholar]
- 135.Wang T, Jiang H, Zhao Q, Wang S, Zou M, Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. International Journal of Pharmaceutics. 2012;436:351–358. doi: 10.1016/j.ijpharm.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 136.Tao W, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 2014;9:237–251. doi: 10.2217/nnm.13.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tao W, Gill HS. M2e–immobilized gold nanoparticles as influenza A vaccine: Role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307–2315. doi: 10.1016/j.vaccine.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang N, Wang T, Zhang M, Chen R, Niu R, Deng Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. European Journal of Pharmaceutics and Biopharmaceutics. 2014;88:194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 139.Kang S-M, Yoo D-G, Lipatov AS, Song J-M, Davis CT, Quan F-S, Chen L-M, Donis RO, Compans RW. Induction of Long-Term Protective Immune Responses by Influenza H5N1 VirusLike Particles. PLoS ONE. 2009;4:e4667. doi: 10.1371/journal.pone.0004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atwe SU, Ma Y, Gill HS. Pollen grains for oral vaccination. J Control Release. 2014;194:45–52. doi: 10.1016/j.jconrel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baca-Estrada ME, Foldvari M, Babiuk SL, Babiuk LA. Vaccine delivery: lipid-based delivery systems. J Biotechnol. 2000;83:91–104. doi: 10.1016/s0168-1656(00)00313-8. [DOI] [PubMed] [Google Scholar]
- 142.Filipovic-Grcic J, Skalko-Basnet N, Jalsenjak I. Mucoadhesive chitosan-coated liposomes: characteristics and stability. J Microencapsul. 2001;18:3–12. doi: 10.1080/026520401750038557. [DOI] [PubMed] [Google Scholar]
- 143.Kaneda Y. Development of a novel fusogenic viral liposome system (HVJ-liposomes) and its applications to the treatment of acquired diseases. Molecular Membrane Biology. 1999;16:119–122. doi: 10.1080/096876899294841. [DOI] [PubMed] [Google Scholar]
- 144.Sakaue G, Hiroi T, Nakagawa Y, Someya K, Iwatani K, Sawa Y, Takahashi H, Honda M, Kunisawa J, Kiyono H. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J Immunol. 2003;170:495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 145.Minato S, Iwanaga K, Kakemi M, Yamashita S, Oku N. Application of polyethyleneglycol (PEG)-modified liposomes for oral vaccine: effect of lipid dose on systemic and mucosal immunity. Journal of Controlled Release. 2003;89:189–197. doi: 10.1016/s0168-3659(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 146.Oberoi HS, Yorgensen YM, Morasse A, Evans JT, Burkhart DJ. PEG modified liposomes containing CRX-601 adjuvant in combination with methylglycol chitosan enhance the murine sublingual immune response to influenza vaccination. Journal of Controlled Release. 2016;223:64–74. doi: 10.1016/j.jconrel.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Advanced Drug Delivery Reviews. doi: 10.1016/j.addr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 148.Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014;2:159–182. doi: 10.1177/2051013614541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zeltins A. Construction and characterization of virus-like particles: a review. Mol Biotechnol. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takamura S, Niikura M, Li TC, Takeda N, Kusagawa S, Takebe Y, Miyamura T, Yasutomi Y. DNA vaccine-encapsulated virus-like particles derived from an orally transmissible virus stimulate mucosal and systemic immune responses by oral administration. Gene Ther. 2004;11:628–635. doi: 10.1038/sj.gt.3302193. [DOI] [PubMed] [Google Scholar]
- 151.Mann JF, Scales HE, Shakir E, Alexander J, Carter KC, Mullen AB, Ferro VA. Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods. 2006;38:90–95. doi: 10.1016/j.ymeth.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 152.Kim M-C, Song J-M, Eunju O, Kwon Y-M, Lee Y-J, Compans RW, Kang S-M. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–492. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK. An intranasal virus-like particle vaccine broadly protects mice from multiple subtypes of influenza A virus. mBio. 2015;6:e01044–e01015. doi: 10.1128/mBio.01044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 155.De Filette M, Fiers W, Martens W, Birkett A, Ramne A, Löwenadler B, Lycke N, Jou WM, Saelens X. Improved design and intranasal delivery of an M2e–based human influenza A vaccine. Vaccine. 2006;24:6597–6601. doi: 10.1016/j.vaccine.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 156.De Filette M, Jou WM, Birkett A, Lyons K, Schultz B, Tonkyro A, Resch S, Fiers W. Universal influenza A vaccine: optimization of M2-based constructs. Virology. 2005;337:149–161. doi: 10.1016/j.virol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 157.Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008;38:114–126. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 158.Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. Journal of virology. 2001;75:9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pedersen GK, Ebensen T, Gjeraker IH, Svindland S, Bredholt G, Guzman CA, Cox RJ. Evaluation of the sublingual route for administration of influenza H5N1 virosomes in combination with the bacterial second messenger c-di-GMP. PLoS One. 2011;6:e26973. doi: 10.1371/journal.pone.0026973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vacher G, Kaeser MD, Moser C, Gurny R, Borchard G. Recent Advances in Mucosal Immunization Using Virus-like Particles. Molecular Pharmaceutics. 2013;10:1596–1609. doi: 10.1021/mp300597g. [DOI] [PubMed] [Google Scholar]
- 161.Shivanna KR. Pollen Biology and Biotechnology Science Publishers. Enfield, NH, USA: 2003. [Google Scholar]
- 162.Roulston TH, Cane JH. Pollen nutritional content and digestibility for animals. Pl Syst Evol. 2000;222:187–209. [Google Scholar]
- 163.Barrier S. Physical and chemical properties of sporopollenin exine particles. The University of Hull, Hull: 2008. [Google Scholar]
- 164.Jorde W, Linskens HF. Zur persorption von pollen und spoken durch die intake darmschleimhaut. Acta Allergologica. 1974;29:165–175. [PubMed] [Google Scholar]
- 165.Kwon K-C, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals autoantigens and vaccine antigens bioencapsulated in plant cells. Advanced Drug Delivery Reviews. 2013;65:782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tacket CO. Plant-based oral vaccines: results of human trials. Curr Top Microbiol Immunol. 2009;332:103–117. doi: 10.1007/978-3-540-70868-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H. Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci U S A. 2007;104:10986–10991. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pniewski T. The twenty-year story of a plant-based vaccine against hepatitis B: stagnation or promising prospects? Int J Mol Sci. 2013;14:1978–1998. doi: 10.3390/ijms14011978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Specht EA, Mayfield SP. Algae-based oral recombinant vaccines. Frontiers in Microbiology. 2014;5:60. doi: 10.3389/fmicb.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dreesen IAJ, Hamri GC-E, Fussenegger M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. Journal of Biotechnology. 2010;145:273–280. doi: 10.1016/j.jbiotec.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 171.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shakya AK, Gill HS. A comparative study of microneedle-based cutaneous immunization with other conventional routes to assess feasibility of microneedles for allergy immunotherapy. Vaccine. 2015;33:4060–4064. doi: 10.1016/j.vaccine.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 174.Pearson FE, McNeilly CL, Crichton ML, Primiero CA, Yukiko SR, Fernando GJ, Chen X, Gilbert SC, Hill AV, Kendall MA. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific T cell responses in mice. PLoS One. 2013;8:e67888. doi: 10.1371/journal.pone.0067888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fernando GJ, Chen X, Primiero CA, Yukiko SR, Fairmaid EJ, Corbett HJ, Frazer IH, Brown LE, Kendall MA. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J Control Release. 2012;159:215–221. doi: 10.1016/j.jconrel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 176.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. The Clinical journal of pain. 2008;24:585–594. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-Based Vaccines. In: Compans RW, Orenstein WA, editors. Vaccines for Pandemic Influenza. Springer Berlin Heidelberg; 2009. pp. 369–393. [Google Scholar]
- 178.Pattani A, McKay PF, Garland MJ, Curran RM, Migalska K, Cassidy CM, Malcolm RK, Shattock RJ, McCarthy HO, Donnelly RF. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J Control Release. 2012;162:529–537. doi: 10.1016/j.jconrel.2012.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weldon WC, Martin MP, Zarnitsyn V, Wang B, Koutsonanos D, Skountzou I, Prausnitz MR, Compans RW. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol. 2011;18:647–654. doi: 10.1128/CVI.00435-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang B-Z, Gill HS, He C, Ou C, Wang L, Wang Y-C, Feng H, Zhang H, Prausnitz MR, Compans RW. Microneedle delivery of an M2e–TLR5 ligand fusion protein to skin confers broadly cross-protective influenza immunity. Journal of Controlled Release. 2014;178:1–7. doi: 10.1016/j.jconrel.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ma Y, Tao W, Krebs SJ, Sutton WF, Haigwood NL, Gill HS. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res. 2014;31:2393–2403. doi: 10.1007/s11095-014-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhen Y, Wang N, Gao Z, Ma X, Wei B, Deng Y, Wang T. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine. 2015;33:4330–4340. doi: 10.1016/j.vaccine.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 183.Park HJ, Ferko B, Byun YH, Song JH, Han GY, Roethl E, Egorov A, Muster T, Seong B, Kweon MN, Song M, Czerkinsky C, Nguyen HH. Sublingual immunization with a live attenuated influenza a virus lacking the nonstructural protein 1 induces broad protective immunity in mice. PLoS One. 2012;7:e39921. doi: 10.1371/journal.pone.0039921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, Amalfitano A. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin Vaccine Immunol. 2011;18:150–160. doi: 10.1128/CVI.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Amuguni JH, Lee S, Kerstein KO, Brown DW, Belitsky BR, Herrmann JE, Keusch GT, Sonenshein AL, Tzipori S. Sublingually administered Bacillus subtilis cells expressing tetanus toxin C fragment induce protective systemic and mucosal antibodies against tetanus toxin in mice. Vaccine. 2011;29:4778–4784. doi: 10.1016/j.vaccine.2011.04.083. [DOI] [PubMed] [Google Scholar]
- 186.Murugappan S, Patil HP, Frijlink HW, Huckriede A, Hinrichs WL. Simplifying influenza vaccination during pandemics: sublingual priming and intramuscular boosting of immune responses with heterologous whole inactivated influenza vaccine. AAPS J. 2014;16:342–349. doi: 10.1208/s12248-014-9565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hervouet C, Luci C, Cuburu N, Cremel M, Bekri S, Vimeux L, Maranon C, Czerkinsky C, Hosmalin A, Anjuere F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine. 2010;28:5582–5590. doi: 10.1016/j.vaccine.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 188.Gallorini S, Taccone M, Bonci A, Nardelli F, Casini D, Bonificio A, Kommareddy S, Bertholet S, O’Hagan DT, Baudner BC. Sublingual immunization with a subunit influenza vaccine elicits comparable systemic immune response as intramuscular immunization but also induces local IgA and TH17 responses. Vaccine. 2014;32:2382–2388. doi: 10.1016/j.vaccine.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 189.Huang CF, Wu TC, Wu CC, Lee CC, Lo WT, Hwang KS, Hsu ML, Peng HJ. Sublingual vaccination with sonicated Salmonella proteins and mucosal adjuvant induces mucosal and systemic immunity and protects mice from lethal enteritis. APMIS. 2011;119:468–478. doi: 10.1111/j.1600-0463.2011.02761.x. [DOI] [PubMed] [Google Scholar]
- 190.Buffa V, Klein K, Fischetti L, Shattock RJ. Evaluation of TLR agonists as potential mucosal adjuvants for HIV gp140 and tetanus toxoid in mice. PLoS One. 2012;7:e50529. doi: 10.1371/journal.pone.0050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cho HJ, Kim JY, Lee Y, Kim JM, Kim YB, Chun T, Oh YK. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. 2010;28:2598–2606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 192.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, Bousquet J, Calderon M, Compalati E, Durham SR, van Wijk RG, Larenas-Linnemann D, Nelson H, Passalacqua G, Pfaar O, Rosario N, Ryan D, Rosenwasser L, Schmid-Grendelmeier P, Senna G, Valovirta E, Van Bever H, Vichyanond P, Wahn U, Yusuf O. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Grier TJ, LeFevre DM, Duncan EA, Esch RE. Stability of standardized grass dust mite, cat and short ragweed allergens after mixing with mold or cockroach extracts. Ann Allergy Asthma Immunol. 2007;99:151–160. doi: 10.1016/S1081-1206(10)60639-4. [DOI] [PubMed] [Google Scholar]
- 194.White JA, Blum JS, Hosken NA, Marshak JO, Duncan L, Zhu C, Norton EB, Clements JD, Koelle DM, Chen D, Weldon WC, Oberste MS, Lal M. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccin Immunother. 2014;10:3611–3621. doi: 10.4161/hv.32253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Narang N, Sharma J. Sublingual mucosa as a route for systemic drug delivery. Int JPharm Pharm Sci. 2011;3(Suppl 2):18–22. [Google Scholar]
- 196.Borde A, Ekman A, Holmgren J, Larsson A. Effect of protein release rates from tablet formulations on the immune response after sublingual immunization. Eur J Pharm Sci. 2012;47:695–700. doi: 10.1016/j.ejps.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 197.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999;67:1100–1106. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gockel CM, Bao S, Beagley KW. Transcutaneous immunization induces mucosal and systemic immunity: a potent method for targeting immunity to the female reproductive tract. Mol Immunol. 2000;37:537–544. doi: 10.1016/s0161-5890(00)00074-2. [DOI] [PubMed] [Google Scholar]
- 199.Loudon PT, Yager EJ, Lynch DT, Narendran A, Stagnar C, Franchini AM, Fuller JT, White PA, Nyuandi J, Wiley CA, Murphey-Corb M, Fuller DH. GM-CSF increases mucosal and systemic immunogenicity of an H1N1 influenza DNA vaccine administered into the epidermis of non-human primates. PLoS One. 2010;5:e11021. doi: 10.1371/journal.pone.0011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, Wu MS, Weis K, Rinaldo CR, Haynes JR, Murphey-Corb M, Induction of mucosal protection against primary. heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. 2002;76:3309–3317. doi: 10.1128/JVI.76.7.3309-3317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine. 2006;24:4475–4481. doi: 10.1016/j.vaccine.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 202.Jones S, Evans K, McElwaine-Johnn H, Sharpe M, Oxford J, Lambkin-Williams R, Mant T, Nolan A, Zambon M, Ellis J, Beadle J, Loudon PT. DNA vaccination protects against an influenza challenge in a double-blind randomised placebo-controlled phase 1b clinical trial. Vaccine. 2009;27:2506–2512. doi: 10.1016/j.vaccine.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 203.Engelke L, Winter G, Hook S, Engert J. Recent insights into cutaneous immunization: How to vaccinate via the skin. Vaccine. 2015;33:4663–4674. doi: 10.1016/j.vaccine.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 204.Mitragotri S. Immunization without needles. Nat Rev Immunol. 2005;5:905–916. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 205.Gill HS, Kang S-M, Quan F-S, Compans RW. Cutaneous immunization: an evolving paradigm in influenza vaccines. Expert Opinion on Drug Delivery. 2014;11:615–627. doi: 10.1517/17425247.2014.885947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.McElrath MJ. Seminars in cancer biology. Elsevier; 1995. Selection of potent immunological adjuvants for vaccine construction; pp. 375–385. [DOI] [PubMed] [Google Scholar]
- 207.Stevceva L, Ferrari MG. Mucosal adjuvants. Curr Pharm Des. 2005;11:801–811. doi: 10.2174/1381612053381846. [DOI] [PubMed] [Google Scholar]
- 208.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. Journal of pharmaceutical sciences. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jones KS. Biomaterials as vaccine adjuvants. Biotechnology progress. 2008;24:807–814. doi: 10.1002/btpr.10. [DOI] [PubMed] [Google Scholar]
- 210.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 211.Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KH, Rappuoli R, Del Giudice G. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–2541. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 212.Sanchez J, Wallerstrom G, Fredriksson M, Angstrom J, Holmgren J. Detoxification of cholera toxin without removal of its immunoadjuvanticity by the addition of (STa-related) peptides to the catalytic subunit. A potential new strategy to generate immunostimulants for vaccination. J Biol Chem. 2002;277:33369–33377. doi: 10.1074/jbc.M112337200. [DOI] [PubMed] [Google Scholar]
- 213.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 214.Chabot S, Wagner JS, Farrant S, Neutra MR. TLRs Regulate the Gatekeeping Functions of the Intestinal Follicle-Associated Epithelium. The Journal of Immunology. 2006;176:4275–4283. doi: 10.4049/jimmunol.176.7.4275. [DOI] [PubMed] [Google Scholar]
- 215.Cario E, Podolsky DK. Differential Alteration in Intestinal Epithelial Cell Expression of TollLike Receptor 3 (TLR3) and TLR4 in Inflammatory Bowel Disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moore A, McCarthy L, Mills KH. The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1. Vaccine. 1999;17:2517–2527. doi: 10.1016/s0264-410x(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 217.Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. doi: 10.1517/14712598.4.7.1129. [DOI] [PubMed] [Google Scholar]
- 218.Ulrich JT, Myers KR. Monophosphoryl lipid A as an adjuvant. Past experiences and new directions. Pharm Biotechnol. 1995;6:495–524. [PubMed] [Google Scholar]
- 219.Krieg AM, Yi A-K, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 220.Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–216. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- 221.Yi A-K, Hornbeck P, Lafrenz DE, Krieg AM. CpG DNA rescue of murine B lymphoma cells from anti-IgM-induced growth arrest and programmed cell death is associated with increased expression of c-myc and bcl-xL. J Immunol. 1996;157:4918–4925. [PubMed] [Google Scholar]
- 222.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: A new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–2344. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 223.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Singh S, Yang G, Byrareddy SN, Barry MA, Sastry KJ. Natural killer T cell and TLR9 agonists as mucosal adjuvants for sublingual vaccination with clade C HIV-1 envelope protein. Vaccine. 32:6934–6940. doi: 10.1016/j.vaccine.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 226.Stowell NC, Seideman J, Raymond HA, Smalley KA, Lamb RJ, Egenolf DD, Bugelski PJ, Murray LA, Marsters PA, Bunting RA, Flavell RA, Alexopoulou L, San Mateo LR, Griswold DE, Sarisky RT, Mbow ML, Das AM. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 2009;10:43. doi: 10.1186/1465-9921-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e, Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 228.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 229.Sloat BR, Cui Z. Nasal immunization with a dual antigen anthrax vaccine induced strong mucosal and systemic immune responses against toxins and bacilli. Vaccine. 2006;24:6405–6413. doi: 10.1016/j.vaccine.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 230.Sloat BR, Shaker DS, Le UM, Cui Z. Nasal immunization with the mixture of PA63 LF and a PGA conjugate induced strong antibody responses against all three antigens. FEMS Immunol Med Microbiol. 2008;52:169–179. doi: 10.1111/j.1574-695X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 231.Akira S. Innate immunity and adjuvants. Philos Trans R Soc Lond B Biol Sci. 2011;366:2748–2755. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Honko AN, Mizel SB. Effects of flagellin on innate and adaptive immunity. Immunol Res. 2005;33:83–101. doi: 10.1385/IR:33:1:083. [DOI] [PubMed] [Google Scholar]
- 233.Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, Tschoeke SK, Ungaro RF, Ono S, Seki S. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol. 2005;78:888–897. doi: 10.1189/jlb.0105051. [DOI] [PubMed] [Google Scholar]
- 234.Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang B-Z, Xu R, Quan F-S, Kang S-M, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. 2010 doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee J-J, Song H-C, Kim JM, Choy HE. A bacterial flagellin Vibrio vulnificus FlaB has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006;74:694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nacer A, Carapau D, Mitchell R, Meltzer A, Shaw A, Frevert U, Nardin EH. Imaging murine NALT following intranasal immunization with flagellin-modified circumsporozoite protein malaria vaccines. Mucosal Immunol. 2014;7:304–314. doi: 10.1038/mi.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ben-Yedidia T, Tarrab-Hazdai R, Schechtman D, Arnon R. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect Immun. 1999;67:4360–4366. doi: 10.1128/iai.67.9.4360-4366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Vassilieva EV, Wang B-Z, Vzorov AN, Wang L, Wang Y-C, Bozja J, Xu R, Compans RW. Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. MBio. 2011;2:e00328–e00310. doi: 10.1128/mBio.00328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kayamuro H, Yoshioka Y, Abe Y, Arita S, Katayama K, Nomura T, Yoshikawa T, Kubota-Koketsu R, Ikuta K, Okamoto S, Mori Y, Kunisawa J, Kiyono H, Itoh N, Nagano K, Kamada H, Tsutsumi Y, Tsunoda S. Interleukin-1 family cytokines as mucosal vaccine adjuvants for induction of protective immunity against influenza virus. J Virol. 84:12703–12712. doi: 10.1128/JVI.01182-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 1:50–63. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Carbonneil C, Aouba A, Burgard M, Cardinaud S, Rouzioux C, Langlade-Demoyen P, Weiss L. Dendritic cells generated in the presence of granulocyte-macrophage colony-stimulating factor and IFN-alpha are potent inducers of HIV-specific CD8 T cells. AIDS. 2003;17:1731–1740. doi: 10.1097/00002030-200308150-00002. [DOI] [PubMed] [Google Scholar]
- 243.Carbonneil C, Saidi H, Donkova-Petrini V, Weiss L. Dendritic cells generated in the presence of interferon-alpha stimulate allogeneic CD4+ T-cell proliferation: modulation by autocrine IL-10 enhanced T-cell apoptosis and T regulatory type 1 cells. Int Immunol. 2004;16:1037–1052. doi: 10.1093/intimm/dxh106. [DOI] [PubMed] [Google Scholar]
- 244.Tighe H, Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 245.Coulter A, Harris R, Davis R, Drane D, Cox J, Ryan D, Sutton P, Rockman S, Pearse M. Intranasal vaccination with ISCOMATRIX adjuvanted influenza vaccine. Vaccine. 2003;21:946–949. doi: 10.1016/s0264-410x(02)00545-5. [DOI] [PubMed] [Google Scholar]
- 246.Vujanic A, Snibson KJ, Wee JL, Edwards SJ, Pearse MJ, Scheerlinck JP, Sutton P. Long-term antibody and immune memory response induced by pulmonary delivery of the influenza Iscomatrix vaccine. Clin Vaccine Immunol. 19:79–83. doi: 10.1128/CVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, Seid R, Look J, Alderson M, Tate A, Maisonneuve JF, Robertson G, Anderson PW, Malley R. Options for inactivation adjuvant, route of topical administration of a killed unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 17:1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Meng S, Liu Z, Xu L, Li L, Mei S, Bao L, Deng W, Lei R, Xie L, Qin C, Zhang L. Intranasal immunization with recombinant HA and mast cell activator C48/80 elicits protective immunity against 2009 pandemic H1N1 influenza in mice. PLoS One. 6:e19863. doi: 10.1371/journal.pone.0019863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, Abraham SN, Pascual DW. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS One. 6:e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Somavarapu S, Pandit S, Gradassi G, Bandera M, Ravichandran E, Alpar OH. Effect of vitamin E TPGS on immune response to nasally delivered diphtheria toxoid loaded poly(caprolactone) microparticles. Int J Pharm. 2005;298:344–347. doi: 10.1016/j.ijpharm.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 251.Agrawal S, Gupta S, Agrawal A. Human dendritic cells activated via dectin-1 are efficient at priming Th17, cytotoxic CD8 T and B cell responses. PLoS One. 5:e13418. doi: 10.1371/journal.pone.0013418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gleeson M, Cripps AW. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunology & Medical Microbiology. 2004;42:21–33. doi: 10.1016/j.femsim.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 253.Teitelbaum JE, Allan Walker W. The development of mucosal immunity. Eur J Gastroenterol Hepatol. 2005;17:1273–1278. doi: 10.1097/00042737-200512000-00002. [DOI] [PubMed] [Google Scholar]
- 254.Brandtzaeg P. Potential of Nasopharynx-associated Lymphoid Tissue for Vaccine Responses in the Airways. American Journal of Respiratory and Critical Care Medicine. 2011;183:1595–1604. doi: 10.1164/rccm.201011-1783OC. [DOI] [PubMed] [Google Scholar]
- 255.Axelsson I, Jakobsson I, Lindberg T, Polberger S, Benediktsson B, Räihä N. Macromolecular Absorption in Preterm and Term Infants. Acta Pædiatrica. 1989;78:532–537. doi: 10.1111/j.1651-2227.1989.tb17932.x. [DOI] [PubMed] [Google Scholar]
- 256.Insoft RM, Sanderson IR, Walker WA. Development of immune function in the intestine and its role in neonatal diseases. Pediatric clinics of North America. 1996;43:551–571. doi: 10.1016/s0031-3955(05)70420-x. [DOI] [PubMed] [Google Scholar]
- 257.Hale C, Humphreys IR, Hussell T, Bowe F, Clare S, Pickard D, Preston A, Del Giudice G, Dougan G. Mucosal immunisation of murine neonates using whole cell and acellular Pertussis vaccines. Vaccine. 2004;22:3595–3602. doi: 10.1016/j.vaccine.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 258.Capozzo AVE, Cuberos L, Levine MM, Pasetti MF. Mucosally Delivered Salmonella Live Vector Vaccines Elicit Potent Immune Responses against a Foreign Antigen in Neonatal Mice Born to Naive and Immune Mothers. Infect Immun. 2004;72:4637–4646. doi: 10.1128/IAI.72.8.4637-4646.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.John M, Bridges EA, Miller AO, Calderwood SB, Ryan ET. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine. 2002;20:2720–2726. doi: 10.1016/s0264-410x(02)00208-6. [DOI] [PubMed] [Google Scholar]