Abstract
Mitochondrial Ca2+ uptake regulates diverse endothelial cell functions and has also been related to nitric oxide (NO•) production. However, it is not entirely clear if the organelles support or counteract NO• biosynthesis by taking up Ca2+. The objective of this study was to verify whether or not mitochondrial Ca2+ uptake influences Ca2+-triggered NO• generation by endothelial NO• synthase (eNOS) in an immortalized endothelial cell line (EA.hy926), respective primary human umbilical vein endothelial cells (HUVECs) and eNOS-RFP (red fluorescent protein) expressing human embryonic kidney (HEK293) cells. We used novel genetically encoded fluorescent NO• probes, the geNOps, and Ca2+ sensors to monitor single cell NO• and Ca2+ dynamics upon cell treatment with ATP, an inositol 1,4,5-trisphosphate (IP3)-generating agonist. Mitochondrial Ca2+ uptake was specifically manipulated by siRNA-mediated knock-down of recently identified key components of the mitochondrial Ca2+ uniporter machinery. In endothelial cells and the eNOS-RFP expressing HEK293 cells we show that reduced mitochondrial Ca2+ uptake upon the knock-down of the mitochondrial calcium uniporter (MCU) protein and the essential MCU regulator (EMRE) yield considerable attenuation of the Ca2+-triggered NO• increase independently of global cytosolic Ca2+ signals. The knock-down of mitochondrial calcium uptake 1 (MICU1), a gatekeeper of the MCU, increased both mitochondrial Ca2+ sequestration and Ca2+-induced NO• signals. The positive correlation between mitochondrial Ca2+ elevation and NO• production was independent of eNOS phosphorylation at serine1177. Our findings emphasize that manipulating mitochondrial Ca2+ uptake may represent a novel strategy to control eNOS-mediated NO• production.
Keywords: Calcium, Endothelial nitric oxide production, ENOS, GeNOps, Mitochondria
1. Introduction
The transfer of Ca2+ into mitochondria influences cell signaling and functions by various means. The organelles themselves immediately respond to Ca2+ elevations as they house Ca2+-sensitive enzymes and transporters [1]. Ca2+ within mitochondria is known to elevate their metabolic activity, but can also trigger cell death [2,3]. In addition, mitochondrial Ca2+ uptake and release shapes local and global cellular Ca2+ signals, which control a variety of cell signaling events [4]. Particularly, in vascular endothelial cells mitochondrial Ca2+ signals have been debated to directly or indirectly control key functions such as the synthesis and release of vasoactive compounds [5,6]. However, the exact role of mitochondrial Ca2+ uptake for endothelial nitric oxide (NO•) production, which is accomplished predominately by the Ca2+ calmodulin-dependent endothelial nitric oxide synthase (eNOS) [7], is unknown. While mitochondria-derived NO• has been detected in isolated mitochondria and permeabilized endothelial cells [5,8], the impact of mitochondrial Ca2+ uptake on Ca2+-evoked NO• production in intact cells has not been investigated so far.
In recent years the key components of mitochondrial Ca2+ channels have been identified [9,10]. These studies unveiled that the mitochondrial Ca2+ uniporter basically consists of the pore forming mitochondrial calcium uniporter (MCU) [9], the essential MCU regulator (EMRE) [11], and the mitochondrial calcium uptake 1 (MICU1) [10]. Interestingly, MICU1 shields the MCU/EMRE-dependent mitochondrial Ca2+ channel and thereby impedes mitochondrial Ca2+ uptake under basal conditions [11]. A very recent study unveiled that MICU1 methylation by protein arginine methyltransferase-1 (PRMT1) hampers MCU activation by Ca2+, indicating that mitochondrial Ca2+ uptake is tightly controlled by posttranslational modifications [12]. However, the knock-down of these key components of the mitochondrial Ca2+ uniporter allows to efficiently manipulate mitochondrial Ca2+ uptake [13]. Based on these findings the role of mitochondrial Ca2+ uptake for endothelial NO• production in intact cells has not been reinvestigated so far. Here we used novel genetically encoded fluorescent NO• probes, the geNOps, to monitor NO• signals on the level of individual cells. Recently, geNOps were used to specifically visualize the biotransformation of nitroglycerine to NO• by aldehyde dehydrogenase-2 (ALDH2) in single vascular smooth muscle cells [14]. Herein we combined the geNOp technology with Ca2+ sensors and siRNA-mediated knock-down of mitochondrial Ca2+ channel proteins [15]. This approach allowed us to demonstrate that Ca2+-triggered NO• production by eNOS is positively controlled by mitochondrial Ca2+ uniport independently of global cytosolic Ca2+ signals. Hence, this study unveiled a clear link between mitochondrial Ca2+ uptake and eNOS-mediated NO• generation which might have several implications in health and diseases.
2. Materials and methods
2.1. Chemicals
Dulbecco's modified Eagle's medium (DMEM), antimycin, oligomycin, and L-NG-nitro arginine were purchased from Sigma-Aldrich (Vienna, Austria). Fura-2-acetoxymethyl ester (fura-2/am), tetramethylrhodamine methyl ester perchlorate (TMRM) and cell culture supplements were obtained from Invitrogen (San Diego, USA). TransFast™ transfection reagent was obtained from Promega (Mannheim, Germany). Antibodies against total and phosphorylated eNOS (pS1177) were from BD Transduction Laboratories™, alpha-tubulin was from Cell Signaling Technology®. Adenosine-5´-triphosphate (ATP) and all other chemicals were purchased from Roth (Karlsruhe, Germany) unless otherwise indicated.
2.2. Cell culture, transfection and adenoviral transduction
The human umbilical vein endothelial cell line EA.hy92 (passage 35–45) [16] was cultured in DMEM medium containing 1% HAT (5 mM hypoxanthine, 20 μM aminopterin, 0.8 mM thymidine), 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator (37 °C, 5% CO2). Human embryonic kidney cells HEK293 were grown in a culture medium without HAT. Prior to transfection or adenoviral transduction cells were plated on 30 mm glass cover slips. At 50–60% confluence, cells were co-transfected with 100 μM of the respective siRNA(s) and 1.5 μg of a plasmid coding either for the NO•-sensitive probe G-geNOp, its mutated NO•-insensitive negative control (G-geNOpmut) (NGFI Next Generation Fluorescence Imaging GmbH, Graz, Austria), the mitochondrial cameleon 4mtD3cpv or the endothelial nitric oxide synthase C-terminally fused to a red fluorescent protein (eNOS-RFP) as described previously [13]. All siRNAs were purchased from Microsynth (Balgach, Switzerland) and their (5´–3´) sequences were: GCAGCUCAAGAAGCACUUCAA (hMICU1-si1), GCAAUGGCGAACUGAGCAAUA (hMICU1-si2), AGAAGUCUGUGAUGAUAAA (hMICU1-si3), GCCAGAGACAGACAAUACU (hMCU-si1), GGAAAGGGAGCUUAUUGAA (hMCU-si2), GAACUUUGCUGCUCUACUU (hEMRE-si) and a scrambled negative control siRNA UUCUCCGAACGUGUCACGU (Scrambled siRNA). Human umbilical vein endothelial cells (HUVECs) (CC-2519; Lonza, Basel, Switzerland) were grown at 37 °C and 5% CO2 on tissue culture dishes coated with 1% Gelatin (#49391; Sigma) in endothelial basal medium (EBM) culture medium (CC-3121; Lonza) supplemented with endothelial growth medium (EGM) SingleQuots and growth factors (CC-4143; Lonza). HUVECs were cultured maximally 14 days before experiments. For imaging experiments HUVECs were seeded on 1% Gelatin coated 30 mm glass cover slips. At a confluence of 50–60% siRNA transfections and infection of BacMam 4mtD3cpv virus (Life Technologies, Vienna, Austria) encoding the mitochondrial cameleon was performed as recently described [12]. The G-geNOp expression in endothelial cells was achieved by adenoviral transduction at a multiplicity of infection (MOI) of 10 for HUVECs or MOI 1000 for EA.hy926 cells. All experiments were performed 42–54 h after transfection or infection, respectively.
2.3. Buffer solutions for imaging experiments
For imaging of cytosolic Ca2+ or mitochondrial membrane potential, cells were loaded at room temperature with either 3.3 μM fura2/am or 100 nM TMRM, respectively supplemented in a storage buffer composed of 138 mM NaCl, 2 mM CaCl2, 5 mM KCl, 1 mM MgCl2, 1 mM HEPES, 2.6 mM NaHCO3, 0.44 mM KH2PO4, 0.34 mM Na2HPO4, 10 mM d-glucose, 0.1% vitamins, 0.2% essential amino acids, and 1% penicillin/streptomycin, pH 7.4 for 40 min. Prior to NO• measurements G-geNOp-expressing EA.hy926 cells were incubated at room temperature in a ferrous fumarate/ascorbic acid buffer for 20 min and maintained in storage buffer for at least 1 h. For dual recordings of NO• and cytosolic Ca2+, Fura2/am loading was done after ferrous fumarate incubation as recently described [15]. All imaging experiments were performed by perfusing cells in a HEPES-buffered solution (HBS) containing 138 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 MgCl2, 10 mM D-glucose, 10 mM HEPES, pH 7.4. For Ca2+-free conditions HBS containing 1 mM EGTA instead of CaCl2 was used.
2.4. Fluorescence microscopy
For fluorescent recordings an advanced wide-field fluorescent microscope (Till Photonics, Graefling, Germany) equipped with a motorized sample stage, a polychrome V (Till Photonics), a 40x objective (alpha Plan Fluar 40x, Zeiss, Göttingen, Germany) and a charge-coupled device camera (AVT Stingray F145B, Allied Vision Technologies, Stadtroda, Germany) was used. The FRET-based mitochondrial Ca2+ sensor 4mtD3cpV was excited at 430 nm and emission was collected using the dichrotome dual emission filter set (dichroic 535dcxr, CFP emitter 482/18 nm and YFP emitter 535/3 nm). TMRM was visualized at an excitation of 550 nm and emission of 575 nm. For monitoring NO• dynamics G-geNOp expressing cells were exposed to 480 nm and emitted at 515 nm. For simultaneous measurements of cytosolic Ca2+and NO•, fura-2/am loaded and G-geNOp expressing cells were alternately excited at 340, 380 and 480 nm, respectively and emission was recorded at 515 nm (515dcxr). Data acquisition and control was carried out by the Live Acquisition 2.0.0.12 software (Till Photonics). Confocal imaging of adenoviral infected G-geNOp expressing EA.hy926 or transient transfected eNOS-RFP expressing HEK293 cells was performed as described previously by using a 40× objective (Plan-Neofluar 40×/1.3 oil, Zeiss) or a 100× objective (Plan-Fluar 100×/1.45 oil, Zeiss), respectively [17].
2.5. Immunoblotting
Transfected EA.hy926 cells (siControl, siMCU/EMRE or siMICU1) were incubated with vehicle (water) or 100 μM ATP at 37 °C for 3 or 5 min. Cells were then harvested and homogenized by sonication (3×5 s) in ice-cold RIPA lysis buffer (Sigma, Vienna, Austria) containing 2 mM EDTA, protease and phosphatase inhibitors (CompleteTM, PhosSTOPTM, Roche, Vienna, Austria). Protein concentration was determined with the PierceTM BCA Protein Assay Kit using bovine serum albumin as standard (Fisher Scientific Austria GmbH, Vienna, Austria). Denatured samples (30 μg) were separated by SDS-PAGE on 10% gels and transferred electrophoretically to nitrocellulose membranes. After blocking with 5% non- fat dry milk in Tris-buffered saline containing 0.1% (v/v) TWEEN-20 for 1 h, membranes were incubated overnight at 4 °C with a primary antibody against eNOS (1:2000; BD Transduction Laboratories), Ser1177 phospho-eNOS (1:1000; BD Transduction Laboratories) or β-actin (1:200,000; Sigma). Thereafter, membranes were washed 3 times and incubated for 1 h with a horseradish peroxidase-conjugated anti-mouse IgG secondary antibody (1:5000). Immunoreactive bands were visualized by chemiluminescence using ECL detection reagent (Biozym, Germany) and quantified densitometrically using the Fusion SL system (Peqlab, Erlangen, Germany).
2.6. Statistical analyses
The acquired data were analyzed by the GraphPad Prism software version 5.04 (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± standard error of mean (SEM) of independent experiments (N) throughout the whole manuscript. For comparisons between two groups, two-tailed Student t-test was used for evaluation of statistical significance and a p value between 0.01 and 0.05 (pStudent´s t-test) was considered significant and indicated with “*”, p between 0.001 and 0.01 as very significant with “**” and p < 0.001 as highly significant with “***”. For comparisons across multiple groups, one-way ANOVA with Barlett's test for equal variances and Tukey's Multiple Comparision test were used for evaluating statistical significance expressed as described above. Data shown are either average or representative curves of at least three independent experiments, including analyses from imaging and western blot experiments.
3. Results
3.1. Real-time monitoring of NO• production in single endothelial cells
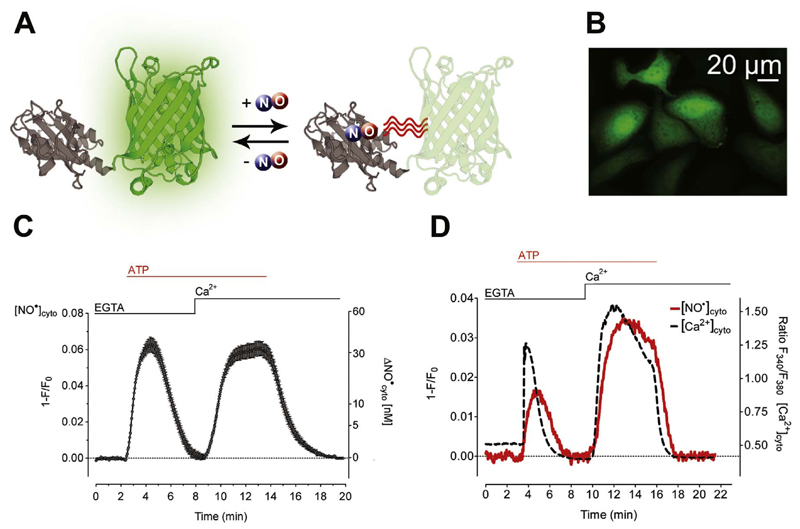
To monitor single cell NO• dynamics in EA.hy926 cells we used the novel geNOp technology [15]. The geNOps are protein-based fluorescent NO• reporters that immediately and specifically respond to physiological cellular NO• elevations by fluorescence quenching in a reversible manner (Fig. 1A). Adenoviral transduction resulted in very high numbers of geNOp-positive EA.hy926 cells (Fig. 1B). To elucidate NO• production in response to Ca2+ release from the endoplasmic reticulum (ER), endothelial cells expressing G-geNOp (green-geNOp) were first stimulated with the inositol 1,4,5-trisphosphate (IP3)-generating agonist ATP in the absence of extracellular Ca2+. Under these conditions ATP evoked a transient NO• signal in endothelial cells (Fig. 1C-D). Cytosolic NO• levels recovered rapidly upon the subsequent addition of Ca2+ to ATP stimulated cells and remained elevated in the presence of the IP3-generating agonist (Fig. 1C-D). These findings demonstrate the importance of Ca2+ entry for sustained eNOS activation in EA.hy926 cells. However, within a few minutes elevated NO• signals gradually decreased back to basal levels upon the washout of ATP (Fig. 1C-D).
Fig. 1. Live-cell imaging of Ca2+-triggered NO• formation in endothelial cells expressing G-geNOp.
(A) Schematic illustration of G-geNOp consisting of the enhanced green fluorescent protein (EGFP) fused to the bacteria-derived NO• binding domain GAF (grey). Binding of NO• in the vicinity of EGFP in G-geNOp induces fluorescence quenching. (B) Representative image showing EA.hy926 cells expressing G-geNOp. Notably, the NO• probe localizes to the nucleus and cytosol. (C) Average curve ( ± SEM) showing NO• signal over time obtained from single EA.hy926 cells obtained from independent experiments (N =40) upon treatment with 100 μM ATP in the absence of Ca2+(1 mM EGTA) and upon Ca2+(2 mM) addition. (D) Representative simultaneous recordings of NO• (solid grey line) and Ca2+(dashed black line) signals over time in a single fura-2/am loaded EA.hy926 cell expressing G-geNOp. As indicated the cell was treated with 100 μM ATP in the absence of Ca2+(1 mM EGTA) and upon Ca2+ addition (2 mM) for 14 min.
The geNOp signals were abolished in the presence of L-NG-nitro arginine, a potent eNOS inhibitor [18], which did not affect respective cytosolic Ca2+ elevations (Supplementary data – Fig. 1). To further confirm the specificity of the G-geNOp signals, endothelial cells expressing the NO• insensitive mutated version of G-geNOp, referred to as G-geNOpmut, were investigated. Cell treatment with the IP3-generating agonist ATP did not evoke significant fluorescence signals in cells expressing G-geNOpmut (Supplementary data – Fig. 2), demonstrating that geNOps do not report intracellular pH changes under these experimental conditions [15]. As the NO• probe did not respond to NO• related species such as nitrite, nitrate [14], H2S (Supplementary data – Fig. 3) and other NO• related compounds [15], these experiments confirm that geNOp-expressing EA.hy926 cells represent a suitable model to specifically study Ca2+-triggered eNOS-dependent generation of NO• on the level of individual cells. The simultaneous measurement of NO• and Ca2+ in the same single cell unveiled that cellular NO• dynamics strictly follow respective Ca2+ signals (Fig. 1D), indicating that NO• production in EA.hy926 cells is primarily controlled by the free cytosolic Ca2+ concentration ([Ca2+]cyto).
3.2. Genetic ablation of mitochondrial Ca2+ uptake reduces eNOS-mediated NO• formation
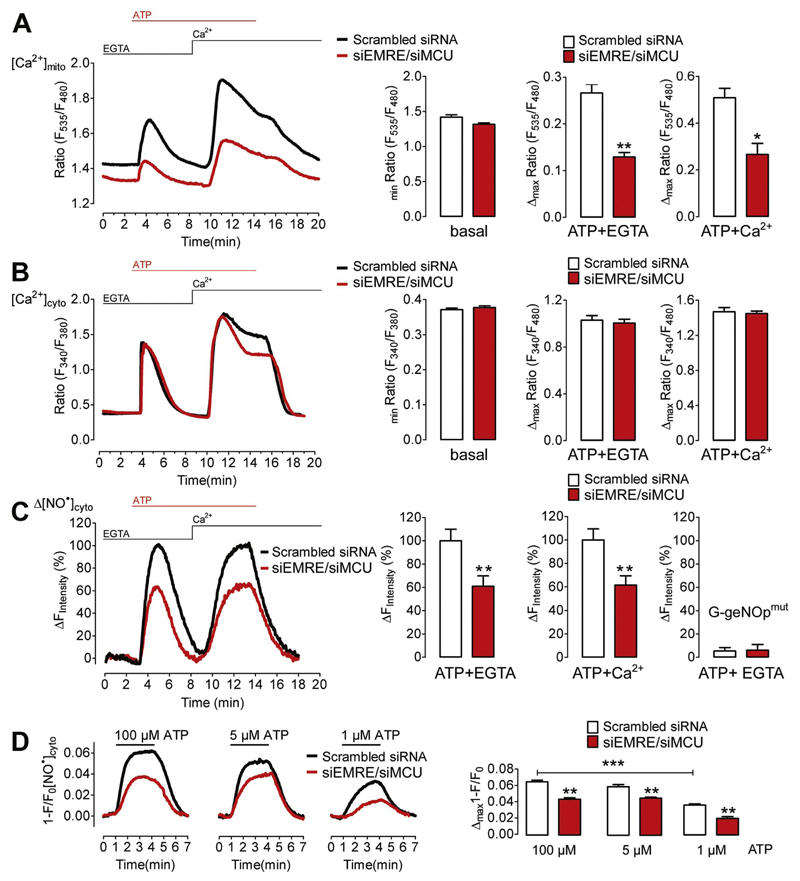
Next we set out to investigate how mitochondrial Ca2+ uptake affects NO• production in endothelial cells. For this purpose EA.hy926 cells were treated with siRNA against both MCU and EMRE, two recently identified key components of the mitochondrial Ca2+ uniporter pore [9,19]. In line with other reports [13,20–22], the siRNA-mediated knock-down of these proteins substantially reduced mitochondrial Ca2+ signals in response to ER Ca2+ release and Ca2+ entry, while the basal mitochondrial Ca2+ levels were only slightly reduced (Fig. 2A). Under the same experimental conditions global cytosolic Ca2+ signals were only diminished at the plateau phase upon Ca2+ addition (Fig. 2B), confirming that mitochondrial Ca2+ uptake facilitates store operated Ca2+ entry (SOCE) in endothelial cells [20,23,24]. However, cells ablated from MCU and EMRE showed considerably reduced NO• production upon Ca2+ mobilization in the absence and presence of Ca2+ entry (Fig. 2C). As expected both control cells and cells treated with siRNA against MCU and EMRE did not show significant changes of the fluorescence signal of the NO•-insensitive G-geNOpmut in response to ATP (Fig. 2C, right panel). Compared to respective control cells, the Ca2+-triggered NO• signals were significantly reduced in MCU- and EMRE silenced EA.hy926 cells that were stimulated with either 1 μM, 5 μM or 100 μM ATP (Fig. 2D), indicating that mitochondrial Ca2+ uptake is relevant for eNOS activation over a broad range of physiological and supra-physiological levels of Ca2+ mobilization.
Fig. 2. Knock-down of EMRE and MCU reduces Ca2+-triggered NO• production in EA.hy926 cells.
(A) Average curves of [Ca2+]mito (left panel) in EA.hy926 cells treated with either scrambled siRNA (black curve and white bars, N=3) or siRNAs against EMRE and MCU (siEMRE/siMCU, red curve and red bars, N=3) upon stimulation with 100 μM ATP in the absence of extracellular Ca2+(1 mM EGTA) followed by addition of Ca2+(2 mM). Bar graphs showing average basal Ca2+ level (basal, p=0.0581) and Ca2+ transients in response to 100 μM ATP-stimulated ER Ca2+ release (ATP + EGTA, **p=0.0029) and store-operated Ca2+ entry (ATP + Ca2+, *p=0.0169). (B) Curves representing average of [Ca2+]cyto in control (Scrambled siRNA, black curve, N=6) versus EMRE/MCU ablated (siEMRE/siMCU, red curve, N=7) EA.hy926 cells (left panel) stimulated with 100 μM ATP in the absence (1 mM EGTA) and addition of Ca2+(2 mM) as indicated. Respective statistical analyses of basal levels (basal, p=0.397), maximum cytosolic Ca2+ release (ATP + EGTA, p=0.6373) and maximum cytosolic Ca2+ entry (ATP + Ca2+, p=0.6988). (C) Representative curve of [NO•]cyto in response to ATP stimulation. Statistical analyses of average [NO•]cyto are shown in bar graphs (right panels). Maximal signals (amplitude) of scrambled siRNA- or siMCU/siEMRE-treated cells are defined as 100%. Cells were first stimulated with 100 μM ATP in the absence of extracellular Ca2+ in control cells (Scrambled siRNA, white column, ATP + EGTA, N =17) and cells reduced of EMRE and MCU (siEMRE/siMCU, red column, ATP + EGTA, N =17) and upon Ca2+( 2 mM) addition (right middle column pair). **p=0.0068 (ATP+EGTA) and **p=0.0042 (ATP+Ca2+) vs Scrambled siRNA. Right columns represent maximal fluorescence changes of G-geNOpmut in response to 100 μM ATP in EGTA (1 mM) in control cells (white column, N =11) and cells treated with siRNA against EMRE and MCU (red column, N =10). (D) Average curves of Ca2+-dependent NO formation upon stimulation with 1 μM (upper left panel) 5 μM (upper middle panel) or 100 μM ATP (upper right panel) in the absence of extracellular Ca2+ in EA.hy926 cells treated with either negative control siRNA (Scrambled siRNA, black curves) or siRNA against EMRE and MCU (siEMRE/siMCU, red curves). Statistical analysis of maximum NO• formation in cells stimulated with either 1 μM ATP (lower left panel) representing Scrambled siRNA (white bar, N=3) and siEMRE/siMCU (red bar, N=3); **p=0.0045, with 5 μM ATP (lower middle panel) representing Scrambled siRNA (white bar, N=3) and siEMRE/siMCU (red bar, N=3); **p=0.0087, or with 100 μM ATP (lower right panel) representing Scrambled siRNA (white bar, N=3) and siEMRE/siMCU (red bar, N=3); **p=0.0012.
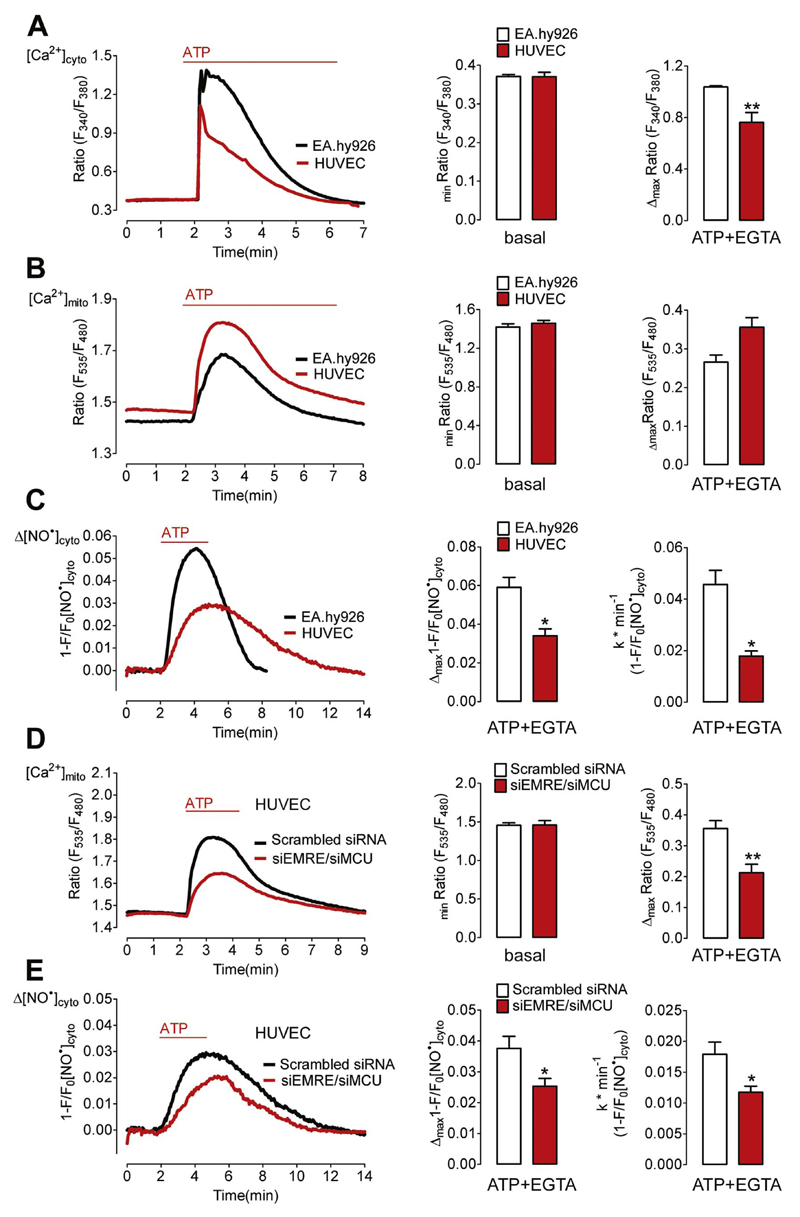
In order to investigate if silencing of MCU and EMRE also impacts on Ca2+-triggered NO• production in non-immortalized endothelial cells, primary human umbilical vein endothelial cells (HUVEC) were tested. HUVECs pooled from different donors showed more transient cytosolic Ca2+ signals in response to ATP compared to EA.hy926 cells, while the basal cytosolic Ca2+ levels were identical in both cell types (Fig. 3A). Respective mitochondrial Ca2+ elevations tended to be stronger in the primary endothelial cells (Fig. 3B). In average the ATP-evoked NO• signals in HUVECs expressing G-geNOp (Fig. 3C) were less pronounced and occurred with rather slow kinetics compared to the faster and stronger NO• elevations in EA.hy926 cells (Fig. 3C). Nevertheless, the knock down of MCU and EMRE, which reduced mitochondrial Ca2+ signals in HUVECs (Fig. 3D), also resulted in diminished ATP-evoked NO• formation in the primary endothelial cells (Fig. 3E). The siRNA-mediated knock-down of MCU and EMRE significantly reduced both the amplitude and slope of ATP-triggered NO• signals in HUVECs (Fig. 3E).
Fig. 3. Knock-down of EMRE/MCU reduces Ca2+-evoked NO• formation in HUVECs.
(A) Cytosolic Ca2+ signals in EA.hy926 cells (black curve and white bars) and primary HUVECs (red curve and red bars) in response to 100 μM ATP. [Ca2+]cyto was imaged using fura-2/am loaded cells. Middle bars represent basal fura-2 ratio (F340/F380) values of EA.hy926 cells (white bars, N =6, p=0.9708) and HUVECs (red bars, N =6). Right bars show maximal fura-2 ratio amplitudes in EA.hy926 (white bars, N =6) and HUVEC cells (red bars, N =6). **p=0.005 vs. EA.hy926 cells. (B) Mitochondrial Ca2+ signals in EA.hy926 cells (black curve and white bars) and primary HUVECs (red curve and red bars) in response to 100 μM ATP. [Ca2+]mito was imaged using cells expressing 4mtD3cpV. Middle bars represent basal 4mtD3cpV ratio (F535/F480) values of EA.hy926 cells (white bars, N =3, p=0.4753) and HUVECs (red bars, N =6). Right bars show maximal 4mtD3cpV ratio amplitudes in EA.hy926 (white bars, N =3) and HUVEC cells (red bars, N =6). (C) Cytosolic NO• signals in EA.hy926 cells (black curve and white bars) and primary HUVECs (red curve and red bars) in response to 100 μM ATP. [NO•]cyto was imaged using cells expressing G-geNOp. Middle bars represent maximal fluorescence changes of G-geNOp (1-F/F0) expressed in EA.hy926 cells (white bars, N =10) and HUVECs (red bars, N =8); *p=0.0366 vs EA.hy926. Right bars show maximal slopes of G-geNOp fluorescence changes in response to 100 mM ATP in EA.hy926 (white bars, N =10) and HUVEC cells (red bars, N =8). *p=0.015 vs. EA.hy926 cells. (D) Mitochondrial Ca2+ signals in HUVECs under control conditions (Scrambled siRNA, black curve, white bars, N =6) and in cells treated with siRNA against EMRE and MCU (siEMRE/siMCU, red curve, red columns, N =6). Middle bars represent basal 4mtD3cpV ratio values (p=0.1502). Cells were stimulated with 100 μM ATP in the absence of extracellular Ca2+. Right bars show maximal 4mtD3cpV ratio changes upon cell stimulation.**p=0.0032 vs Scrambled siRNA. (E) Cytosolic NO• signals ([NO•]cyto) in control HUVECs (Scrambled siRNA, black curve and white bars, N =8) and cells treated with siRNA against EMRE and MCU (siEMRE/siMCU, red curve and bars, N =8) in response to 100 μM ATP in EGTA (1 mM). Middle columns represent mean of maximal ATP-evoked G-geNOp responses; *p=0.0193. Right columns show respective maximal slopes of G-geNOp signals; *p=0.0206.
3.3. Mitochondrial depolarization reduces Ca2+-triggered NO• formation in EA.hy926 cells
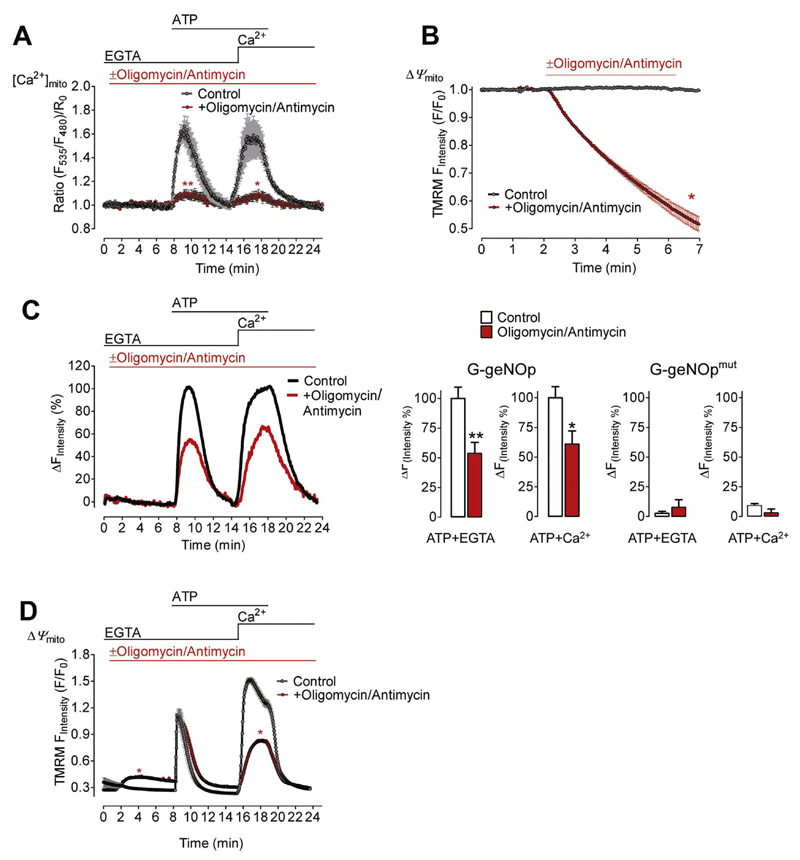
The impact of impaired mitochondrial Ca2+ uptake was studied by depolarization of the mitochondrial membrane potential with a combination of oligomycin and antimycin (Fig. 4A and B). In the presence of these compounds the Ca2+-triggered NO• signal was significantly reduced in response to Ca2+ release and entry (Fig. 4C). The fluorescence signal of the NO•-insensitive G-geNOpmut remained unaffected by oligomycin and antimycin independently of the presence of Ca2+(Fig. 4C, right panel). Under the same experimental conditions cytosolic Ca2+ signals were, however, only partially reduced during Ca2+ addition (Fig. 4D). These findings again indicate that mitochondrial Ca2+ signals positively regulate eNOS activity.
Fig. 4. Cell treatment with oligomycin/antimycin reduces Ca2+-triggered NO• formation.
(A) Average [Ca2+]mito signals ± SEM of EA.hy926 cells expressing 4mtD3cpv under control conditions (black curve N =8) and in the presence of 1 μM oligomycin and 5 μM antimycin (red curve, N =12) in response to 100 μM ATP first in the absence of extracellular Ca2+(1 mM EGTA) and upon addition of Ca2+(2 mM).**p=0.0096 (in EGTA) and *p=0.0159 (in Ca2+) vs. Control. (B) TMRM signals of EA.hy926 cells over time to estimate changes in Ψmito (mitochondrial membrane potential) under control conditions (black curve N =6) and upon addition of 1 μM oligomycin and 5 μM antimycin (red curve, N =6). *p=0.0286. (C) NO• signals of EA.hy926 cells over time expressing G-geNOp in the absence (control, black curve, white bars, N =11) or presence of 1 μM oligomycin and 5 μM antimycin (red curve and bars, N =9). Maximal signals under control conditions in response to 100 μM ATP were defined as 100% (middle right bars). **p=0.0074 (in EGTA) and *p=0.0200 (in Ca2+) vs. Control. Right bars represent maximal fluorescence changes of the NO•-insensitive G-geNOpmut in control cells (white bars N =6) and in the presence of the mitochondria toxins (red bars, N =6). p=0.8016 (in EGTA) and p=0.1508 (in Ca2+). (D) Fura-2 ratio signals of EA.hy926 cells over time under control conditions (black curve, N =6) and in the presence of 1 μM oligomycin and 5 μM antimycin (red curve, N =6). *p=0.0286 (in EGTA) and *p=0.0283 (in Ca2+) vs. Control.
3.4. Genetic augmentation of mitochondrial Ca2+ uptake increases eNOS-mediated NO• formation
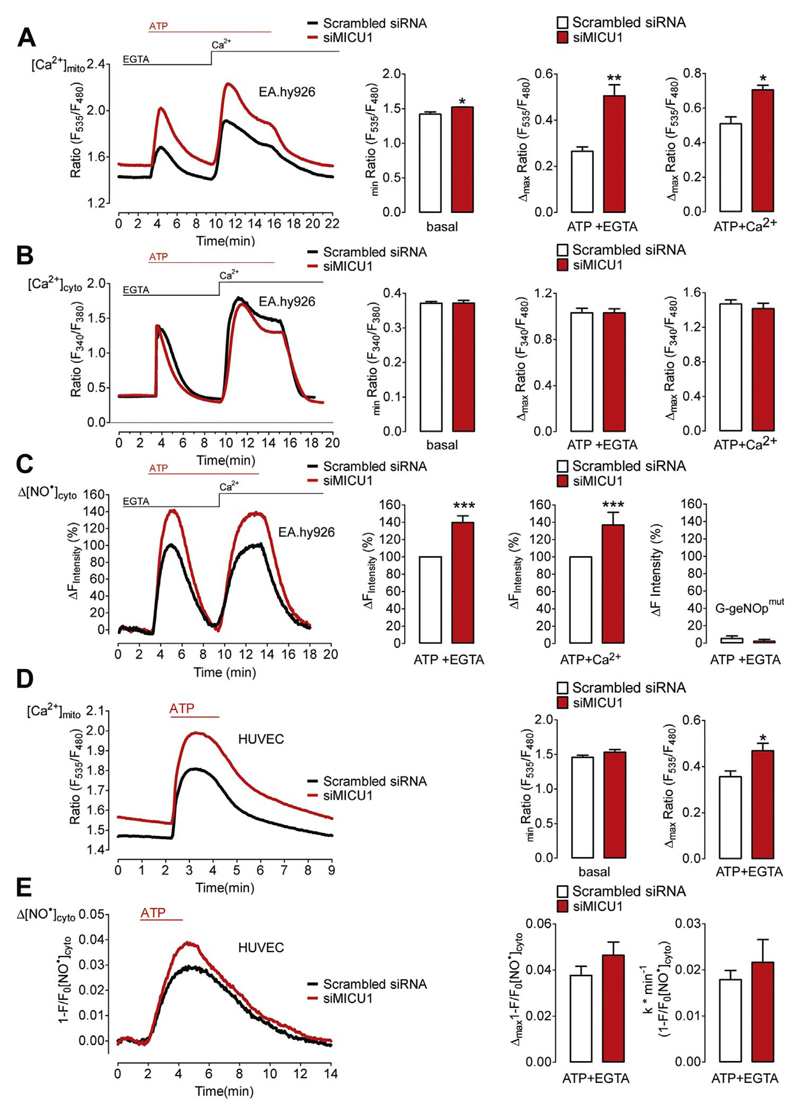
We next investigated whether Ca2+-evoked NO• production is gradable by increasing mitochondrial Ca2+ uptake. For this purpose the expression level of MICU1, a negative regulator of mitochondrial Ca2+ uptake [10], was silenced by siRNA in EA.hy926 cells. As expected, cells treated with siRNA against MICU1 showed increased basal mitochondrial Ca2+ levels and ATP-evoked Ca2+ uptake in the absence and presence of extracellular Ca2+(Fig. 5A). Respective global cytosolic Ca2+ elevations remained almost unaffected by the knock-down of MICU1 (Fig. 5B). However, the Ca2+-triggered NO• production was significantly higher in the MICU1-depleted EA.hy926 cells upon both Ca2+ release and entry (Fig. 5C), emphasizing that increasing mitochondrial Ca2+ uptake augments NO• production. The fluorescence signal of the NO•-insensitive G-geNOpmut remained unaffected by cell treatment with the IP3-generating agonist independently of MICU1 expression (Fig. 5C, right panel). In primary HUVECs siRNA-mediated silencing of MICU1 also increased mitochondrial Ca2+ signals in response to ATP (Fig. 5D). However, the ATP-induced NO• formation (Fig. 5D) was only slightly increased in HUVECs that had been treated with siRNA against MICU1 compared to respective control cells (Fig. 5E).
Fig. 5. Silencing of MICU1 increases Ca2+-triggered NO• production in endothelial cells.
(A) Average curves of [Ca2+]mito (left panel) in EA.hy926 cells treated with either scrambled siRNA (black curve and white bars, N=3) or siRNAs against MICU1 (siMICU1, red curve and red bars, N=3) upon stimulation with 100 μM ATP in the absence of extracellular Ca2+(1 mM EGTA) followed by addition of Ca2+(2 mM). Bar graphs showing average basal Ca2+ level (basal, *p=0.0356) and Ca2+ transients in response to 100 μM ATP in 1 mM EGTA (ATP + EGTA, **p=0.0098) and upon Ca2+ addition (ATP + Ca2+, *p=0.0151). (B) Curves representing average of [Ca2+]cyto in control (Scrambled siRNA, black curve, N=6) versus MICU1 ablated (siMICU1, red curve, N=6) EA.hy926 cells (left panel) stimulated with 100 μM ATP in the absence (1 mM EGTA) and addition of Ca2+(2 mM) as indicated. Respective statistical analyses of basal levels (basal, p=0.9621), maximum cytosolic Ca2+ release (ATP + EGTA, p=0.9911) and maximum cytosolic Ca2+ entry (ATP + Ca2+, p=0.4977). (C) Representative curve of [NO•]cyto in response to ATP stimulation. Statistical analyses of average [NO•]cyto are shown in bar graphs (right panels). Maximal signals (amplitude) of scrambled siRNA- or siMICU1-treated cells are defined as 100%. Cells were first stimulated with 100 μM ATP in the absence of extracellular Ca2+ in control cells (Scrambled siRNA, white column, ATP + EGTA, N =17) and cells reduced of MICU1 (siMICU1, red column, ATP + EGTA, N =16) and upon Ca2+(2 mM) addition (right middle column pair). **p=0.0083 (ATP + EGTA) and **p=0.0079 (ATP+Ca2+) vs Scrambled siRNA. Right columns represent maximal fluorescence changes of G-geNOpmut in response to 100 μM ATP in EGTA (1 mM) in control cells (white column, N =11) and cells treated with siRNA against MICU1 (siMICU1, red column, N =8); p=0.4424 vs Scrambled siRNA. (D) Mitochondrial Ca2+ signals in HUVECs under control conditions (Scrambled siRNA, black curve, white bars, N =6) and in cells treated with siRNA against MICU1 (siMICU1, red curve, red columns, N =6). Middle bars represent basal 4mtD3cpV ratio values (p=0.1502). Cells were stimulated with 100 μM ATP in the absence of extracellular Ca2+. Right bars show maximal 4mtD3cpV ratio changes upon cell stimulation.*p=0.0211 vs Scrambled siRNA. (E) Cytosolic NO• signals ([NO•]cyto) in control HUVECs (Scrambled siRNA, black curve and white bars, N =8) and cells treated with siRNA against MICU1 (siMICU1, red curve and bars, N =10) in response to 100 μM ATP in EGTA (1 mM). Middle columns represent mean of maximal ATP-evoked G-geNOp responses; p=0.2532. Right columns show respective maximal slopes of G-geNOp signals; p=0.4567.
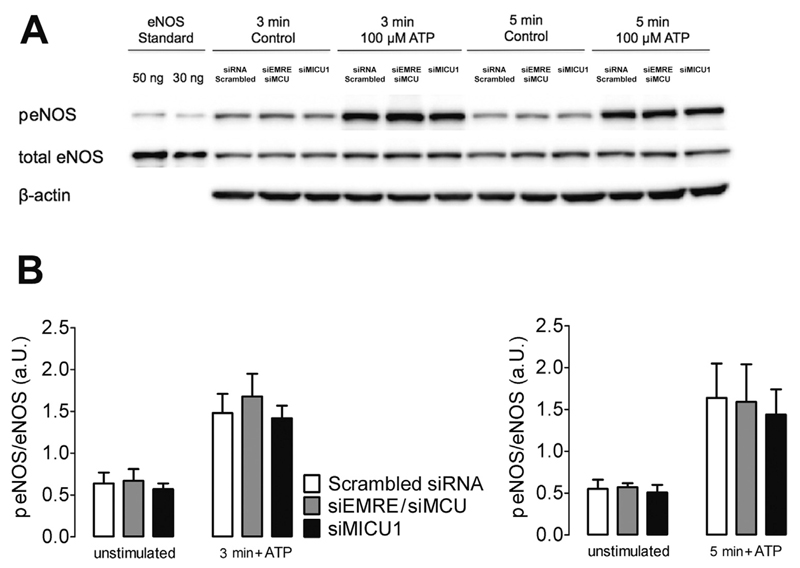
3.5. The eNOS expression levels as well as basal and ATP-stimulated eNOS phosphorylation remain unaffected by silencing MCU, EMRE or MICU1
Phosphorylation of several sites within the eNOS protein significantly modulates its activity [25]. Particularly, eNOS phosphorylation at serine1177 is known to enhance its activity [25,26]. Accordingly, we performed western blot analysis using EA.hy926 cells to investigate whether or not the knock-down of MCU/EMRE and MICU1 affects eNOS phosphorylation at serine1177. As shown in Fig. 6 the knock-down of neither MCU/EMRE nor MICU1 affected eNOS phosphorylation under basal (unstimulated) conditions. Cell treatment with ATP raised eNOS phosphorylation levels within 3 and 5 min (Fig. 6), while the ATP-induced increase of phosphorylated eNOS (peNOS) was not altered by MCU/EMRE and MICU1 silencing (Fig. 6). In line with these findings the expression of eNOS in EA.hy926 cells was not affected by knock-down of MCU/EMRE or MICU1 (total eNOS, Fig. 6A). These findings again confirm that mitochondrial Ca2+ uptake supports NO• production in endothelial cells without affecting the global cytosolic Ca2+ concentration, eNOS-expression levels and phosphorylation at serine1177.
Fig. 6. The expression level and phosphorylation status of eNOS remain unaffected by transient down-regulation of MCU, EMRE and MICU1.
(A) Representative Western blot of total homogenates of transfected EA.hy926 cells (30 μg of protein) showing phosphorylated eNOS (peNOS; 140 kDa), total eNOS (140 kDa) and β-actin (43 kDa) under basal conditions (unstimulated) or stimulated with 100 μM ATP for 3 or 5 min. Purified human eNOS (30 and 50 ng) was used as standard. Bands of control cells (Scrambled siRNA) and cells treated with siRNA against both EMRE and MCU (siEMRE/siMCU) or against MICU1 (siMICU1) are shown. (B) Bars show the ratio of phosphorylated to total eNOS protein in transfected EA.hy926 cells (Scrambled siRNA, siEMRE/siMCU and siMICU1) under basal conditions (unstimulated), after 3 (left panel) and 5 (right panel) minutes of incubation with 100 μM ATP. N =3 for all conditions.
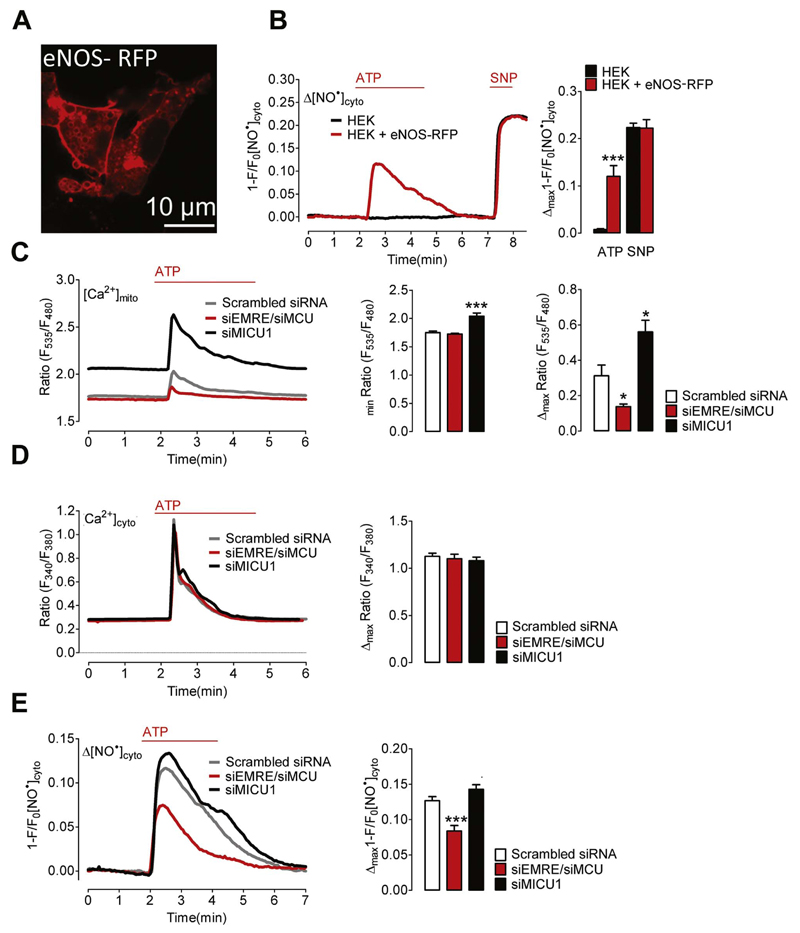
3.6. Mitochondrial Ca2+ uptake significantly contributes to Ca2+-triggered NO• formation in HEK293 cells transiently expressing eNOS-RFP
There is general agreement that eNOS is mainly localized at the plasma membrane [27]. However, in a previous study it has been suggested that mitochondrial Ca2+ uptake stimulates NO• formation by a mitochondrial NOS isoform in bovine endothelial cells [5]. Therefore, we transiently expressed wild-type eNOS-RFP in HEK293 cells, which do not express any NOS isoforms. Confocal microscopy of eNOS-RFP expressing HEK293 cells confirmed that the NO•-generating enzyme is located predominately at the plasma membrane and intracellular vesicular structures but not within mitochondria of cells (Fig. 7A). HEK293 cells expressing eNOS-RFP showed a considerable increase of cellular NO• levels in response to the IP3-generating agonist ATP (Fig. 7B). Control cells which were not transfected with eNOS-RFP were unable to produce NO• upon the addition of the agonist (Fig. 7B). However, the response of the NO• probe to the NO•-donor sodium nitroprusside (SNP) was the same in both control (HEK) and eNOS-RFP expressing HEK293 cells (HEK + eNOS-RFP) (Fig. 7B). Knock-down of MCU/EMRE in HEK293 cells significantly reduced mitochondrial Ca2+ signals in response to ATP (Fig. 7C), while the respective cytosolic Ca2+ elevation remained unaffected by silencing these MCU components (Fig. 7D). As expected, knock-down of MICU1 resulted in increased mitochondrial Ca2+- levels and uptake without affecting respective cytosolic Ca2+ signals (Fig. 7C and D). In line with our results using primary endothelial cells ATP-evoked NO•-signals were significantly reduced in cells silenced of MCU and EMRE (Fig. 7E), while MICU1 silencing only marginally increased respective NO• signals in HEK293 cells expressing eNOS-RFP.
Fig. 7. Mitochondrial Ca2+ uptake controls NO• formation in HEK293 cells expressing eNOS-RFP.
(A) Representative image showing HEK293 cells expressing eNOS-RFP. (B) Representative curves of cytosolic NO• signals ([NO•]cyto) over time in response to 100 μM ATP and 1 mM SNP in control HEK293 cells (black curve and bars, N =5) and HEK293 cells co-expressing eNOS-RFP (red curve and bars, N =3). Left Bars show maximal changes of G-geNOp fluorescence in response to ATP; ***p=0.0005 vs HEK293. Right bars show maximal G-geNOps signals in response to SNP, p=0.9386. (C) Mitochondrial Ca2+ signals in HEK293 cells under control conditions (Scrambled siRNA, grey curve and white columns, N =8), in cells treated with siRNA against EMRE and MCU (siEMRE/siMCU, red curve and red columns, N =8) or against MICU1 (siMICU1, black curve and columns, N =7). Cells were stimulated with 100 μM ATP in EGTA (1 mM). Left columns represent basal 4mtD3cpV ratio values; p=0.4088 (siEMRE/siMCU) vs Scrambled siRNA; ***p=0.0004 (siMICU1) vs Scrambled siRNA. Right columns show mean values of maximal 4mtD3cpV ratio signals; *p=0.0145 (siEMRE/siMCU) vs Scrambled siRNA; *p=0.0152 (siMICU1) vs Scrambled siRNA. (D) Representative cytosolic Ca2+ signals of fura-2/am loaded HEK293 cells treated with scrambled siRNA (Scrambled siRNA, grey curve and white columns, N =3) siRNA against EMRE and MCU (siEMRE/siMCU, red curve and red columns, N =3) or MICU1 (siMICU1, black curve and columns, N =4). Cells were stimulated with 100 μM ATP in EGTA (1 mM). Columns represent mean values of maximal fura-2 ratio signals; p=0.6842 (siEMRE/siMCU) vs Scrambled siRNA; p=0.4253 (siMICU1) vs Scrambled siRNA. (E) Representative cytosolic NO• signals eNOS-RFP expressing HEK293 cells treated with scrambled siRNA (Scrambled siRNA, grey curve and white columns, N =9) siRNA against EMRE and MCU (siEMRE/siMCU, red curve and red columns, N =11) or MICU1 (siMICU1, black curve and columns, N =8). Cells were stimulated with 100 μM ATP in EGTA (1 mM). Columns represent mean values of maximal G-geNOp signals; ***p=0.0005 (siEMRE/siMCU) vs Scrambled siRNA; p=0.0829 (siMICU1) vs Scrambled siRNA.
4. Discussion
Taking advantage of the novel genetically encoded fluorescent NO• probes [15] and the knowledge of recently identified key components of mitochondrial Ca2+ channels, we could specifically investigate the impact of mitochondrial Ca2+ uptake on eNOS-mediated NO• formation on the level of individual cells. Our data show a positive association of mitochondrial Ca2+ signals and NO• production upon cell treatment with a physiological Ca2+-mobilizing agonist and, hence, emphasize that mitochondrial Ca2+ uptake promotes the Ca2+-triggered biosynthesis of this vasoactive molecule by eNOS.
Our simultaneous recordings of Ca2+ and NO• signals confirm that the Ca2+/calmodulin-dependent activation mechanism of the eNOS [7] is under the control of the global cytosolic Ca2+ concentration [28,29]. However, genetic manipulations of the mitochondrial Ca2+ uptake capacity markedly attenuated the ATP-evoked NO• formation without affecting global cytosolic Ca2+ signals. These observations strongly indicate that mitochondrial Ca2+ uptake contributes to the regulation of eNOS activity. Several data point to a localization of eNOS or other NOS isoforms within mitochondria or at their surface [5,30–33]. It has been suggested that mitochondrial Ca2+ uptake stimulates NO• formation within mitochondria of calf pulmonary artery endothelial cells by a mitochondria-specific NOS isoform [5]. The positive association between mitochondrial Ca2+ uptake and NO• generation, found in the present study, is in line with the concept of Ca2+-dependent mitochondria targeted NOS isoforms [5,34,35]. However, because of the lack of a clear proof, the existence of mitochondria-specific NOS isoforms in endothelial cells remains an issue of debate [36]. While we did not investigate whether or not a mitochondria located NOS is present in EA.hy926 cells and HUVECs, our data with HEK293 cells expressing eNOS-RFP indicate that mitochondrial Ca2+ uptake contributes to eNOS activity even if the enzyme is outside the organelle. It is, hence, feasible, that mitochondria in the vicinity of extra-mitochondrial NOS might positively control the cellular NO• production by shaping the patterns of local Ca2+ signals [24] and/or contributing to the availability of essential NOS cofactors [37]. Further experiments are necessary to clarify by which molecular mechanisms mitochondrial Ca2+ uptake supports NO• production by eNOS. In this study we could, however, exclude that eNOS phosphorylation at serine1177, which is known to enhance eNOS activity [25,26], is under the control of mitochondrial Ca2+ uptake. Cell treatment with ATP significantly increased eNOS phosphorylation at serine1177. This finding is in line with other reports that demonstrated interrelations between the Ca2+/calmodulin- and phosphorylation-dependent activation of eNOS [25,26]. Different cellular kinases such as the AMPK [38], PKC delta [39] or the PI3K/Akt pathway [40,41] might be responsible for the ATP-dependent phosphorylation of eNOS at serine1177 in EA.hy926 cells.
Our results strongly suggest that targeting the mitochondrial Ca2+ homeostasis represents a promising approach to therapeutically intervene in endothelium-governed NO•-dependent vasoreactivity. However, the availability of pharmacological tools that specifically target the MCU machinery is very limited. We used a combination of oligomycin, an inhibitor of the ATP synthase, and antimycin, an inhibitor of complex III, in order to abolish mitochondrial Ca2+ uptake indirectly. This kind of cell treatment strongly affects the metabolic activity of the organelle and can lead to a severe cellular ATP depletion [42], which in turn affects the local and global cellular Ca2+ homeostasis [24]. While our data show that cell treatment with the mitochondria depolarizing compounds reduces eNOS-mediated NO• production in response to the IP3-generating agonist in EA.hy926 cells, the molecular mechanisms responsible for this effect on eNOS activity might be different from those in cells with reduced levels of MCU components. Due to the lack of suitable agents that specifically target the mitochondrial Ca2+ homeostasis, the impact of an acute alteration of mitochondrial Ca2+ fluxes on eNOS activity is difficult to investigate. It is possible that the siRNA-mediated knock-down of components of the MCU machinery introduces secondary effects on the cellular bioenergetics that may interfere with eNOS function. Such effects cannot be ruled out, although other studies have demonstrated that silencing or even the knock-out of MCU components have surprisingly little impact on the cellular metabolic homeostasis in situ [12] and in vivo [43,44]. Eventually, our data suggest that manipulating mitochondrial Ca2+ uptake may represent an attractive strategy for controlling the eNOS/NO•-dependent vascular reactivity. Nevertheless, the molecular mechanism linking mitochondrial Ca2+ uptake to eNOS function and/or NO• bioavailability remains to be clarified in more detail.
Supplementary Material
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.freeradbiomed.2016.11.049.
Acknowledgements
We thank Margarete Lechleitner, Anna Schreilechner and Dr. Rene Rost for their excellent technical assistance and Dr. C.J.S. Edgell (University of North Carolina, Chapel Hill, NC, USA) for providing the EA.hy926 cells. Moreover we thank Professor Roger Tsien (University of California San Diego, CA, USA) for providing 4mtD3cpv. Special thanks to the team of NGFI (Next Generation Fluorescence Imaging GmbH, Graz, Austria, http://www.ngfi.eu/) for optimizing the infection protocol with the G-geNOp adenovirus.
Sources of funding
E.E. is supported by Nikon Austria within the Nikon-Center of Excellence Graz. E.E. and M.R.D. are fellows of the PhD program Molecular Medicine (MOLMED) at the Medical University of Graz. This work was also funded by the FWF project P 28529B27 at the Medical University of Graz. C.T.M.-S. is funded by the FWF within the PhD Program Metabolic and Cardiovascular Disease (DK-W1226). S.C. holds an academic development scholarship from the University of Phayao (Phayao, Thailand) and is a student of the doctoral school Molecular Medicine and Inflammation of the Medical University of Graz. Microscopic equipment is part of the Nikon-Center of Excellence Graz that is supported by the Austrian infrastructure program 2013/2014, Nikon Austria Inc., and BioTechMed-Graz.
Nonstandard Abbreviations and Acronyms
- Ψmito
mitochondrial membrane potential
- EMRE
essential mitochondrial calcium uniporter regulator
- eNOS
endothelial nitric oxide synthase
- geNOps
genetically encoded fluorescent nitric oxide probes
- EGFP
enhanced green fluorescent protein
- ER
endoplasmic reticulum
- IP3
inositol 1,4,5-trisphosphate
- MCU
mitochondrial calcium uniporter
- MICU1
mitochondrial calcium uptake 1
- NO•
nitric oxide
- P-eNOS
phosphorylated endothelial nitric oxide synthase
- RFP
red fluorescent protein
- SOCE
store operated Ca2+ entry
- TMRM
tetramethylrhodamine methyl ester perchlorate
Footnotes
Disclosures
None.
References
- [1].Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 1787;2009:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- [2].Bhosale G, Sharpe JA, Sundier SY, Duchen MR. Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann N Y Acad Sci. 2015;1350:107–116. doi: 10.1111/nyas.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 1777;2008:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Graier WF, Frieden M, Malli R. Mitochondria and Ca2+ signaling: old guests, new functions. Pflug Arch. 2007;455:375–396. doi: 10.1007/s00424-007-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dedkova EN, Ji X, Lipsius SL, Blatter LA. Mitochondrial calcium uptake stimulates nitric oxide production in mitochondria of bovine vascular endothelial cells. Am J Physiol Cell Physiol. 2004;286:406–415. doi: 10.1152/ajpcell.00155.2003. [DOI] [PubMed] [Google Scholar]
- [6].Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol. 2013;75:95–126. doi: 10.1146/annurev-physiol-030212-183804. [DOI] [PubMed] [Google Scholar]
- [7].Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflug Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- [8].Ghafourifar P, Parihar MS, Nazarewicz R, Zenebe WJ, Parihar A. Detection assays for determination of mitochondrial nitric oxide synthase activity; advantages and limitations. Meth Enzym. 2008;440:317–334. doi: 10.1016/S0076-6879(07)00821-X. [DOI] [PubMed] [Google Scholar]
- [9].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Madreiter-Sokolowski CT, Klec C, Parichatikanond W, Stryeck S, Gottschalk B, Pulido S, Rost R, Eroglu E, Hofmann NA, Bondarenko AI, Madl T, et al. PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca2+ uptake in immortalized cells. Nat Commun. 2016;7:e12897. doi: 10.1038/ncomms12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Waldeck-Weiermair M, Malli R, Parichatikanond W, Gottschalk B, Madreiter-Sokolowski CT, Klec C, Rost R, Graier WF. Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca2+ Sci Rep. 2015;5:e15602. doi: 10.1038/srep15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Opelt M, Eroglu E, Waldeck-Weiermair M, Russwurm M, Koesling D, Malli R, Graier WF, Fassett JT, Schrammel A, Mayer B. Formation of nitric oxide by aldehyde dehydrogenase-2 is necessary and sufficient for vascular bioactivation of nitroglycerin. J Biol Chem. 2016 doi: 10.1074/jbc.M116.752071. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eroglu E, Gottschalk B, Charoensin S, Blass S, Bischof H, Rost R, Madreiter-Sokolowski CT, Pelzmann B, Bernhart E, Sattler W, Hallstrom S, et al. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat Commun. 2016;7:e10623. doi: 10.1038/ncomms10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci USA. 1983;80:3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waldeck-Weiermair M, Bischof H, Blass S, Deak AT, Klec C, Graier T, Roller C, Rost R, Eroglu E, Gottschalk B, Hofmann NA, et al. Generation of red-shifted cameleons for imaging Ca2+ dynamics of the endoplasmic reticulum. Sensors. 2015;15:13052–13068. doi: 10.3390/s150613052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Griffith OW, Kilbourn RG. Nitric oxide synthase inhibitors: amino acids. Meth Enzym. 1996;268:375–392. doi: 10.1016/s0076-6879(96)68040-9. [DOI] [PubMed] [Google Scholar]
- [19].Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deak AT, Blass S, Khan MJ, Groschner LN, Waldeck-Weiermair M, Hallstrom S, Graier WF, Malli R. IP3-mediated STIM1 oligomerization requires intact mitochondrial Ca2+ uptake. J Cell Sci. 2014;127:2944–2955. doi: 10.1242/jcs.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bondarenko AI, Jean-Quartier C, Parichatikanond W, Alam MR, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial, Ca2+ uniporter (MCU)-dependent and MCU-independent Ca2+ channels coexist in the inner mitochondrial membrane. Pflug Arch. 2014;466:1411–1420. doi: 10.1007/s00424-013-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bondarenko AI, Parichatikanond W, Madreiter CT, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. UCP2 modulates single-channel properties of a MCU-dependent Ca2+ inward current in mitochondria. Pflug Arch. 2015;467:2509–2518. doi: 10.1007/s00424-015-1727-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Naghdi S, Waldeck-Weiermair M, Fertschai I, Poteser M, Graier WF, Malli R. Mitochondrial Ca2+ uptake and not mitochondrial motility is required for STIM1-Orai1-dependent store-operated Ca2+ entry. J Cell Sci. 2010;123:2553–2564. doi: 10.1242/jcs.070151. [DOI] [PubMed] [Google Scholar]
- [24].Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–44779. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- [25].Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- [26].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- [27].Villanueva C, Giulivi C. Subcellular and cellular locations of nitric oxide synthase isoforms as determinants of health and disease. Free Radic Biol Med. 2010;49:307–316. doi: 10.1016/j.freeradbiomed.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luckhoff A, Pohl U, Mulsch A, Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharm. 1988;95:189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Graier WF, Schmidt K, Kukovetz WR. Effect of sodium fluoride on cytosolic free Ca2+-concentrations and cGMP-levels in endothelial cells. Cell Signal. 1990;2:369–375. doi: 10.1016/0898-6568(90)90067-k. [DOI] [PubMed] [Google Scholar]
- [30].Zaobornyj T, Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. Am J Physiol Heart Circ Physiol. 2012;303:1283–1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- [31].Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, Sen CK, Ghafourifar P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468:114–120. doi: 10.1016/j.abb.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Valdez LB, Boveris A. Mitochondrial nitric oxide synthase, a voltage-dependent enzyme, is responsible for nitric oxide diffusion to cytosol. Front Biosci. 2007;12:1210–1219. doi: 10.2741/2139. [DOI] [PubMed] [Google Scholar]
- [33].Parihar MS, Parihar A, Villamena FA, Vaccaro PS, Ghafourifar P. Inactivation of mitochondrial respiratory chain complex I leads mitochondrial nitric oxide synthase to become pro-oxidative. Biochem Biophys Res Commun. 2008;367:761–767. doi: 10.1016/j.bbrc.2008.01.015. [DOI] [PubMed] [Google Scholar]
- [34].Traaseth N, Elfering S, Solien J, Haynes V, Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim Biophys Acta. 1658;2004:64–71. doi: 10.1016/j.bbabio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- [35].Valdez LB, Zaobornyj T, Boveris A. Mitochondrial metabolic states and membrane potential modulate mtNOS activity. Biochim Biophys Acta. 1757;2006:166–172. doi: 10.1016/j.bbabio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- [36].Lacza Z, Pankotai E, Busija DW. Mitochondrial nitric oxide synthase: current concepts and controversies. Front Biosci. 2009;14:4436–4443. doi: 10.2741/3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- [38].Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- [39].da Silva CG, Specht A, Wegiel B, Ferran C, Kaczmarek E. Mechanism of purinergic activation of endothelial nitric oxide synthase in endothelial cells. Circulation. 2009;119:871–879. doi: 10.1161/CIRCULATIONAHA.108.764571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- [41].Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- [42].Vishnu N, Jadoon Khan M, Karsten F, Groschner LN, Waldeck-Weiermair M, Rost R, Hallstrom S, Imamura H, Graier WF, Malli R. ATP increases within the lumen of the endoplasmic reticulum upon intracellular Ca2+ release. Mol Biol Cell. 2014;25:368–379. doi: 10.1091/mbc.E13-07-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pendin D, Greotti E, Pozzan T. The elusive importance of being a mitochondrial Ca2+ uniporter. Cell Calcium. 2014;55:139–145. doi: 10.1016/j.ceca.2014.02.008. [DOI] [PubMed] [Google Scholar]
- [44].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.