Abstract
The X-ray crystallographic structure of a truncated teixobactin analogue reveals hydrogen-bonding and hydrophobic interactions and a cavity that binds a chloride anion. Minimum inhibitory concentration (MIC) assays against Gram-positive bacteria correlate the observed structure with antibiotic activity.
The antibiotic teixobactin—first reported in 2015—kills Gram-positive bacteria without detectable resistance and offers promise against rising resistance in pathogens such as methicillin-resistant Staphylococcus aureus (MRSA).1,2 In reflection of this promise, the initial report has been cited more than 500 times. Teixobactin is a non-ribosomal cyclic undecadepsipeptide and contains the rare amino acid allo-enduracididine at position 10.3 Teixobactin inhibits cell wall formation in Gram-positive bacteria by binding to lipid II and related peptidoglycan precursors.
Since the initial publication, multiple research groups have worked to synthesize teixobactin and to elucidate its pharmacophore. Two reports of the total synthesis of teixobactin have been published,4,5 as well as a third describing the synthesis of the cyclic depsipeptide ring.6 A 10-step synthesis of allo-enduracididine suitable for preparing gram-quantities has also been reported.7 Several research groups have reported structure-activity relationship studies of Arg10-teixobactin (Fig. 1) and related homologues in which arginine is used as a surrogate for allo-enduracididine.8–13 Very recently, Singh et al. reported NMR-based structures and structure-activity-relationships of Arg10-teixobactin and its diastereomers at positions 1, 4, 5, and 8.14
Fig. 1.
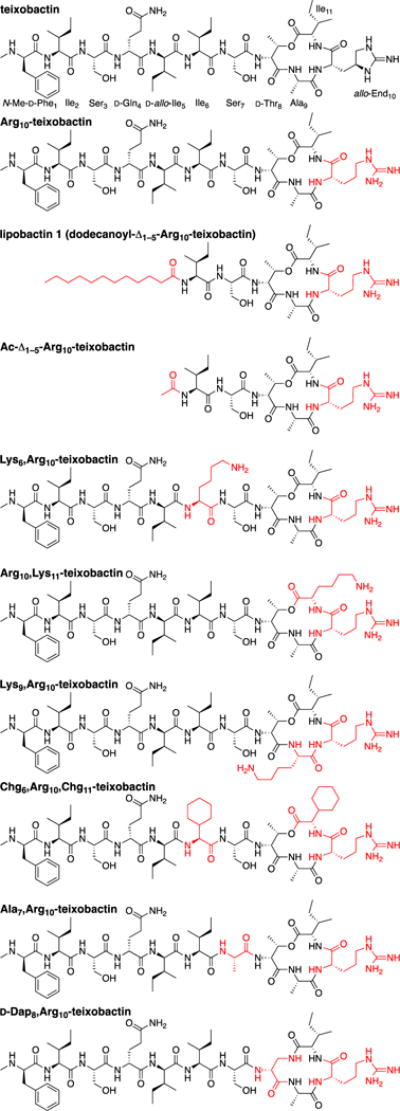
Structures of teixobactin and homologues.
We recently reported the elucidation of the teixobactin pharmacophore, describing syntheses and structure-activity studies of a variety of teixobactin homologues.10 On the basis of these data, we proposed a model in which the amide NH groups of the cyclic depsipeptide ring bind to the pyrophosphate group of lipid II through hydrogen-bonding interactions, in a fashion similar to the binding of nisin to lipid II (PDB 1WCO).15 We further proposed that the hydrophobic residues N-Me-D-Phe, Ile, and D-allo-Ile at positions 1, 2, and 5 help anchor teixobactin to the plasma membrane and demonstrated that residues 1–5 could be replaced with a lipid group. The resulting homologue lipobactin 1 (dodecanoyl-Δ1–5-Arg10-teixobactin) is only 2–4 times less active than Arg10-teixobactin (Fig. 1).
In the current study, we report the X-ray crystallographic structure of a truncated version of lipobactin 1 in which the dodecanoyl group is replaced with an acetyl group, Ac-Δ1–5-Arg10-teixobactin (Fig. 1). In attempting to crystallize this homologue with inorganic pyrophosphate anions, we instead obtained a complex with chloride anion and observe that the chloride anion coordinates to three amide NH groups of the cyclic depsipeptide ring, the amide NH group of Ser7, and the guanidinium group of Arg10. Here we describe the X-ray crystallographic structure of Ac-Δ1–5-Arg10-teixobactin as the hydrochloride salt and relate the observed structure to changes in activity upon mutation of Arg10-teixobactin.
We began our efforts to crystallize the teixobactin pharmacophore by screening Arg10-teixobactin in 864 conditions in a 96-well plate format using crystallization kits from Hampton Research (PEG/Ion, Index, and Crystal Screen). Initial efforts to screen Arg10-teixobactin for crystallization were thwarted by the propensity of the peptide to form a gel at concentrations as low as 5 mg/mL used for screening. Truncation by removal of residues 1–5 (Δ1–5-Arg10-teixobactin) eliminated the propensity to form a gel but afforded no crystals. We postulated that a monocationic homologue would better crystallize than the dicationic homologue and were gratified that Ac-Δ1–5-Arg10-teixobactin afforded crystals suitable for X-ray crystallography. Only conditions containing chloride anion afforded suitable crystals. Attempts to crystallize with inorganic pyrophosphate anions, with HCl being used to vary the pH of the pyrophosphate buffer, still afforded the chloride salt. The X-ray crystallographic structure shows Ac-Δ1–5-Arg10-teixobactin as the hydrochloride salt (Fig. 2).‡
Fig. 2.
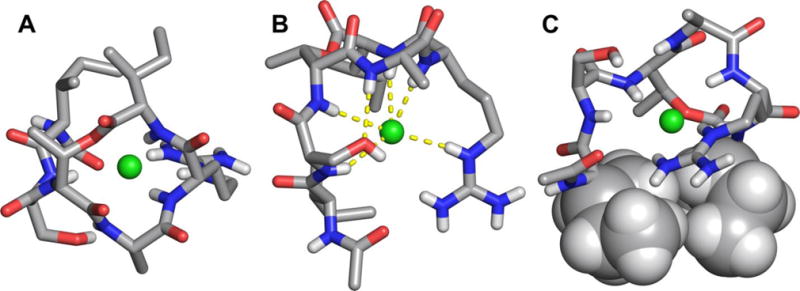
X-ray crystallographic structure of Ac-Δ1–5-Arg10-teixobactin as the hydrochloride salt. (A) Top view. (B) Side view. (C) Rotated side view, in which the side chains of Ile6 and Ile11 are shown as spheres. Hydrogens attached to carbons that are shown as sticks are omitted for clarity. Water of crystallization (1.5 H2O per molecule of peptide) is not shown.
In the X-ray crystallographic structure, the carbonyl groups of D-Thr8, Ala9, Arg10, and Ile11 in the cyclic depsipeptide ring point upward, while the amide NH groups of Ala9, Arg10, and Ile11 point downward (Fig. 2B). The α-amino group of D-Thr8 and the attached residues (Ser7 and Ile6), run downward at almost a right angle to the cyclic depsipeptide ring. The side chain of Arg10 also runs downward. The side chains of Ala9 and Ile11, as well as the methyl group of D-Thr8 point outward from the cyclic depsipeptide ring (Fig. 2A). The amide NH group of Ala9 hydrogen bonds to the oxygen atom of the hydroxy group of Ser7. The side chains of Ile6 and Ile11 are in loose contact, suggesting a hydrophobic interaction (Fig. 2C). The methyl group of D-Thr8 sits near the Ile6 and Ile11 side chains, creating a hydrophobic patch.
The amide NH groups of Arg10 and Ile11 in the cyclic depsipeptide ring, as well as the amide NH groups of Ser7 and D-Thr8 and the guanidinium group of Arg10, hydrogen bond to the chloride anion (Fig. 2B). This mode of interaction is similar to that of nisin with the pyrophosphate group of lipid II.15 We envision that the binding cavity of teixobactin and its analogues may be able to adjust to accommodate larger anions, including the pyrophosphate group of lipid II and other related peptidoglycan precursors.
To explore the roles of the hydrophobic residues at positions 6, 9, and 11, we mutated each of these residues to lysine and compared the activity of the resulting homologues to that of Arg10-teixobactin in minimum inhibitory concentration (MIC) assays in four types of Gram-positive bacteria. Mutation of either Ile6 or Ile11 to lysine results in loss of activity, while mutation of Ala9 to lysine does not (Table 1).§ These data suggest that the hydrophobicity of Ile6 and Ile11 is important in teixobactin activity, while that of Ala9 is not. The outward pointing geometry of the Ala9 side chain, coupled with the activity of Lys9,Arg10-teixobactin, suggest that the 9-position should allow functionalization to provide other modified homologous of teixobactin that are active.
Table 1.
MIC values of teixobactin homologues in μg/mL.a
|
Staphylococcus epidermidis ATCC 14990 |
Streptococcus salivarius ATCC 13419 |
Enterococcus durans ATCC 6056 |
Bacillus subtilis ATCC 6051 |
Escherichia coli ATCC 10798 |
|
|---|---|---|---|---|---|
| Arg10-teixobactin | 1 | 1 | 4 | 2 | >32 |
| lipobactin 1 | 4 | 4 | 8 | 4 | >32 |
| Ac-Δ1–5-Arg10-teixobactin | >32 | >32 | >32 | >32 | >32 |
| Lys6,Arg10-teixobactin | >32 | >32 | >32 | >32 | >32 |
| Arg10,Lys11-teixobactin | >32 | >32 | >32 | >32 | >32 |
| Lys9,Arg10-teixobactin | 1 | 1 | 4 | 1 | >32 |
| Chg6,Arg10,Chg11-teixobactin | 1 | 0.5 | 2 | 1 | >32 |
| Ala7,Arg10-teixobactin | 32 | 16 | >32 | 32 | >32 |
| D-Dap8,Arg10-teixobactin | 2 | 1 | 4 | 1 | >32 |
| vancomycin | 0.5 | 0.5 | 0.5 | 1 | >32 |
| teixobactin | 0.06 | 0.03 | 0.5 | 0.06 | >32 |
All teixobactin homologues were prepared and studied as the trifluoroacetate salts. The Staphylococcus, Streptococcus, Enterococcus, and Bacillus species are non-pathogenic (BSL-1) Gram-positive bacteria. The E. coli serves as a Gram-negative control. Vancomycin and teixobactin serve as positive controls.
To further explore the role of hydrophobicity at positions 6 and 11 and the contact between the Ile6 and Ile11 side chains, we mutated both of these residues to cyclohexylglycine (Chg). Cyclohexylglycine may be thought of as a homologue of isoleucine, in which two carbons have been added to the sec-butyl side chain to form a cyclohexane ring. The resulting homologue, Chg6,Arg10,Chg11-teixobactin, has slightly greater activity than Arg10-teixobactin, with three of the four measured MIC values in the Gram-positive bacteria lower by a factor of two (Table 1). This finding suggests that hydrophobicity or hydrophobic contact at positions 6 and 11 is important in the activity of teixobactin.
To explore the hydrogen bond between the amide NH group of Ala9 and side chain of Ser7, we mutated Ser7 to alanine. The resulting homologue, Ala7,Arg10-teixobactin, shows greatly diminished activity (Table 1).§§ This finding supports the importance of this hydrogen bond in the activity of teixobactin.
The hydrogen bonding of the depsipeptide ring to the chloride anion (Fig. 2) suggests the possibility of increasing the activity of teixobactin homologues by strengthening the complexation with the pyrophosphate group of lipid II. To explore this idea, we mutated D-Thr8 to D-diaminopropionic acid (D-Dap). The mutation of D-Thr8 to D-Dap replaces the lactone oxygen atom with an amide NH group, but also results in the loss of the threonine methyl group. The resulting homologue, D-Dap8,Arg10-teixobactin, shows comparable activity to Arg10-teixobactin (Table 1). Direct comparison of these two homologues is hampered, because two factors are changed at one time in making this mutation. A reasonable interpretation of this observation is that enhanced activity from replacing the lactone oxygen atom with an NH group is offset by the increased conformational flexibility of the ring associated with removal of the D-Thr8 methyl group.
The studies described here demonstrate how the X-ray crystallographic structure of a truncated teixobactin analogue can reveal key interactions of teixobactin. The structure reveals a 13-membered cyclic depsipeptide ring in which the amide groups and ester group of residues 8–11 align. The amide NH groups of residues 10 and 11, in conjunction with those of residues 7 and 8 and the guanidinium side chain of residue 10, create a cavity that can bind an anion. The hydrophobic side chains at positions 6 and 11 are required for activity, whereas that at position 9 is not. The hydrogen bond between Ser7 and Ala9 is also important for activity. The teixobactin pharmacophore tolerates the amide substitution of lactone oxygen in the ring. Fig. 3 summarizes these findings. We are now using the X-ray crystallographic structure and structure-activity relationships that we have observed to design teixobactin homologues with better pharmacological properties.
Fig. 3.
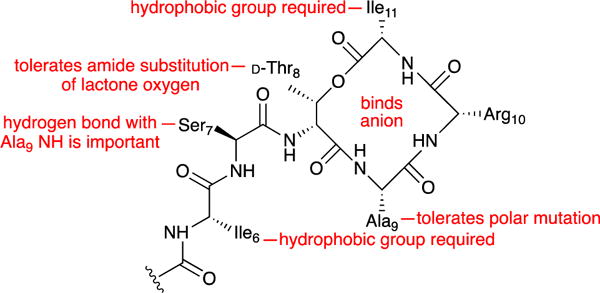
Summary of key findings.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH; grant 1R21AI121548) and a grant from the Bill & Melinda Gates Foundation. We thank NovoBiotic Pharmaceuticals, LLC for generously providing teixobactin, which we have used as a positive control in MIC assays.
Footnotes
Electronic Supplementary Information (ESI) available. See DOI: 10.1039/x0xx00000x
The crystallographic coordinates were deposited in the Cambridge Crystallographic Data Centre (CCDC), deposition number CCDC 1523518.
Albericio et al. recently reported that Lys6,Arg10-teixobactin and Arg10,Lys11-teixobactin are inactive against Gram-positive bacteria, and that Lys9,Arg10-teixobactin is less active than Arg10-teixobactin. For details, see reference 12.
Su et al. recently reported that Ala7,Arg10-teixobactin is substantially less active than Arg10-teixobactin. For details, see reference 13.
References
- 1.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. Nature. 2015;517:455. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homma T, Nuxoll A, Gandt AB, Ebner P, Engels I, Schneider T, Götz F, Lewis K, Conlon BP. Antimicrob Agents Chemother. 2016;60:6510. doi: 10.1128/AAC.01050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson DJ, Naysmith BJ, Furkert DP, Brimble MA. Beilstein J Org Chem. 2016;12:2325. doi: 10.3762/bjoc.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin K, Sam LH, Laam Po KH, Lin D, Ghazvini Zadeh EH, Chen S, Yuan Y, Li X. Nat Commun. 2016;7:12394. doi: 10.1038/ncomms12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giltrap AM, Dowman LJ, Nagalingam G, Ochoa JL, Linington RG, Britton WJ, Payne RJ. Org Lett. 2016;18:2788. doi: 10.1021/acs.orglett.6b01324. [DOI] [PubMed] [Google Scholar]
- 6.Dhara S, Gunjal VB, Handore KL, Reddy DS. Eur J Org Chem. 2016;25:4289. [Google Scholar]
- 7.Craig W, Chen J, Richardson D, Thorpe R, Yuan Y. Org Lett. 2015;17:4620. doi: 10.1021/acs.orglett.5b02362. [DOI] [PubMed] [Google Scholar]
- 8.Jad YE, Acosta GA, Naicker T, Ramtahal M, El-Faham A, Govender T, de la Torre HG, Albericio F. Org Lett. 2015;17:6182. doi: 10.1021/acs.orglett.5b03176. [DOI] [PubMed] [Google Scholar]
- 9.Parmar A, Iyer A, Vincent CS, Van Lysebetten D, Prior SH, Madder A, Taylor EJ, Singh I. Chem Commun. 2016;52:6060. doi: 10.1039/c5cc10249a. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Chen KH, Nowick JS. ACS Chem Biol. 2016;11:1823. doi: 10.1021/acschembio.6b00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Monaim SAH, Jad YE, Acosta GA, Naicker T, Ramchuran EJ, El-Faham A, Govender T, Kruger HG, de la Torre BG, Albericio F. RSC Adv. 2016;6:73827. doi: 10.1021/acsomega.6b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel Monaim SAH, Jad YE, Ramchuran EJ, El-Faham A, Govender T, Kruger HG, de la Torre BG, Albericio F. ACS Omega. 2016;1:1262. doi: 10.1021/acsomega.6b00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Pan Z, Yao G, Wang W, Fang L, Su W. RSC Adv. 2017;7:1923. [Google Scholar]
- 14.Parmar A, Prior SH, Iyer A, Vincent C, Van Lysebetten D, Breukink E, Madder A, Taylor EJ, Singh I. Chem Commun. 2017 doi: 10.1039/C6CC09490B. [DOI] [PubMed] [Google Scholar]
- 15.Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, Nuland NA. Nat Struct Mol Biol. 2004;11:963. doi: 10.1038/nsmb830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


