Abstract
Caspases were originally identified as important mediators of inflammatory response and apoptosis. Recent discoveries, however, have unveiled their roles in mediating and suppressing two regulated forms of necrotic cell death, respectively termed pyroptosis and necroptosis. These recent advances have significantly expanded our understanding of the roles of caspases in regulating development, adult homeostasis and host defense response.
Caspase-1 was first reported in two seminal studies as the processing enzyme for pro-interleukin-1β [named interleukin-1β converting enzyme (ICE) at the time] that mediates the production of mature IL-1β, an important pro-inflammatory cytokine involved in multitude of human diseases (Cerretti et al., 1992; Thornberry et al., 1992). The amino acid sequence homology of caspase-1 with Ced-3, encoded by a gene critically involved in regulating developmental cell death in the nematode C. elegans, provided the first hint for a potential role of caspases in apoptosis in mammalian cells (Yuan et al., 1993). This prediction was directly verified by the expression of caspase-1 or Ced-3 in cultured fibroblast cells that lead to cell death with apoptotic morphology and which was inhibited upon the expression of Bcl-2 or CrmA, a viral inhibitor of caspases (Miura et al., 1993). Furthermore, the expression of CrmA and Bcl-2 blocked neuronal cell death mediated by trophic factor deprivation, a paradigm mimicking developmental neuronal cell death (Gagliardini et al., 1994). These findings led to tremendous interests in the molecular mechanisms of apoptosis and the discovery of a cascade of activated caspases in the execution of this regulated death sentence. The cleavage of different substrates by caspases is mechanistically responsible for the morphological and biochemical characteristics of apoptotic cell death, including membrane blebbing, exposure of phosphatidylserine on the outer cytoplasmic membrane, DNA fragmentation, detachment from extracellular matrix, formation of pyknotic nuclei and apoptotic vesicles (Degterev et al., 2003; Green, 1998). These studies defined apoptosis as programmed cell death mediated by caspases. Thus, regulation of apoptosis and the production of inflammatory cytokines such as IL-1β then came to be known as the predominant functions of caspases. However, research in the past decade has led to the new revelations that caspases also play previously unexpected roles in regulating necrotic cell death.
Caspases are an ancient family of cysteine proteases with homologues present in all metazoans. Caspases are first synthesized in cells as zymogens and their activation requires either allosteric conformational change, specific cleavage after a selective aspartate residue, or both, to lead to the formation of tetrameric active enzymes. Unlike that of C. elegans, where the activation of Ced-3 is singlehandedly responsible for the execution of programmed cell death, multiple caspases are present in more complex organisms, such as mammals [10 caspases in mouse (caspase-1/-2/-3/-6/-7/-8/-9/-11/-12/-14); 11 caspases in human (caspase-1/-2/-3/-4/-5/-6/-7/-8/-9/-10/-14)]. Members of the caspase family can be grouped into those encoding long N-terminal prodomains with the CARD or DED motifs, which mediate the formation of protein complexes by providing the molecular platforms for the activation and inhibition of caspases (in mammals, caspase-1, -2, -4, -5, -8, -9, -10, -11, and -12), and those with short prodomains that require the cleavage by other caspases to be activated (in mammals, caspase-3, -6, -7, and -14). Such an expansion of the caspase family during evolution may have arisen to serve multiple purposes such as providing additional means of regulation and diversifying their roles.
This review will focus on two recently uncovered roles of caspases in regulating necrotic cell death mechanisms: the activation of pyroptosis mediated by caspases-1, caspase-4, caspase-5 and caspase-11, and the suppression of necroptosis mediated by RIPK1/RIPK3 by caspase-8.
Pyroptosis – necrotic cell death mediated by inflammatory caspases
The pro-inflammatory subfamily of caspases, including caspase-1 in both human and mice, caspase-4 and -5 in humans and caspase-11 in mice, are now known to mediate a form of necrotic cell death, termed pyroptosis (Greek roots pyro, relating to fire or fever) which is characterized by cell swelling, lysis and release of proinflammatory cytokines and intracellular content (Fink and Cookson, 2006). In a fashion similar to apoptosis, the key execution mechanism of pyroptosis involves cleavage events mediated by caspases (Figure 1). The ability of inflammatory caspases to promote cell lysis involves the cleavage of gasdermin D (GSDMD) to promote the formation of membrane pores (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015). Pyroptosis occurs predominantly in professional phagocytes — macrophages, monocytes, and dendritic cells (DCs), as well as various other cell types such as T cells — upon stimulation by pathogens such as bacteria or virus, or products from pathogens such as lipopolysaccharide (LPS) and viral DNA.
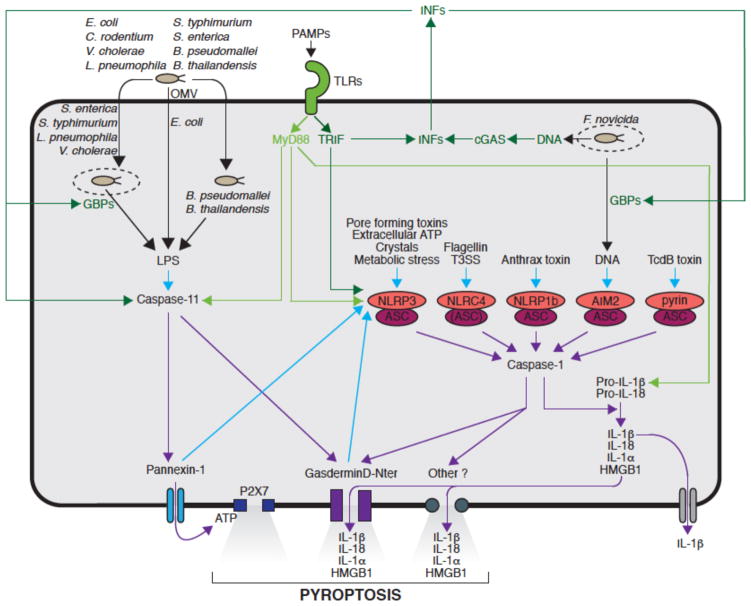
Figure 1. Pyroptosis induction by noncanonical and canonical inflammasomes.
Toll-like receptors (TLRs) and/or interferons (IFNs)-mediated priming upregulate the expression of guanylate binding proteins (GBPs) critical for bacterial vacuole lysis and/or pattern-associated molecular patterns (PAMPs, including LPS and bacterial DNA) exposure, sensors (NLRP3, caspase-11) and cytokine precursor (pro-IL-1β). Of note, MyD88 and TRIF-dependent signaling downstream of TLRs can alternatively prime NLRP3 in a transcription-independent manner. Upon detection of their respective agonists, NLRP3, NLRC4, NLRP1b, AIM2 and pyrin assemble canonical inflammasomes containing adaptor ASC and leading to the activation of caspase-1 that controls the maturation of pro-cytokines (pro-IL-1β and pro-IL-18) and pyroptosis through the cleavage of GSDMD among other mechanisms. LPS of some Gram-negative bacteria enter the cytosol through outer membrane vesicles (OMVs) or at the surface of cytosolic bacteria. Cytosolic LPS binds caspase-11 and triggers the oligomerization of the noncanonical inflammasome; Caspase-11 controls pyroptosis through the cleavage of GSDMD and pannexin-1. Pannexin-1-mediated release of ATP triggers the opening of P2X7-dependent pores, while GSDMD N-terminal fragments directly assemble to form pores. Membrane disruption leads to pyroptosis lytic cell death releasing alarmins and proinflammatory cytokines. It should be noted that IL-1β secretion can occur independently of cell lysis. Non-canonical inflammasome also controls caspase-1 activation mediated by the NLRP3 canonical inflammasome through pannexin-1 and GSDMD cleavages in a cell intrinsic manner involving K+ efflux. (Green: priming; blue: activation; purple: effector mechanisms.)
Inflammatory caspases mediating pyroptosis may be activated by inflammasomes (de Zoete et al., 2014). The inflammasome pathways include the canonical inflammasomes that mediate the activation of caspase-1 and the non-canonical inflammasome that promotes the activation of caspase-11 in mice and caspase-4 and -5 in humans. Murine caspase-1, caspase-11 and caspase-12 are located in a gene cluster on chromosome 9; whereas human caspase-1, caspase-4 and caspase-5 are located in a gene cluster on chromosome 11. Human caspase-4 is most homologous to murine caspase-11 with ~60% identity in its amino acid sequence. Caspase-1, the enzyme directly involved in processing of pro-IL-1β, is subject to the regulation by caspase-11 and caspase-4/-5 (Vigano et al., 2015; Wang et al., 1998). Caspase-11 expression is lost in two Casp1−/− mouse mutant lines due to a germline mutation in mouse 129 background (Kang et al., 2000; Kayagaki et al., 2011).
In contrast to apoptosis, which is primarily anti-inflammatory, the activation of pyroptosis is pro-inflammatory not only because of the rapid loss of cell membrane integrity and release of cytosolic contents, but also due to the processing and release of mature IL-1β and IL-18, which have strong pro-inflammatory activity in promoting vasodilation and extravasation of cells of the immune response, the generation of IL-17-producing helper T cells-mediated (Th17) response and the production of interferon-γ (IFN-γ) by NK and Th1 cells.
A dual alarm system for activating pyroptosis
Activation of pyroptosis in macrophages and DCs may involve a dual alarm system. This alarm system includes intracellular and extracellular germline-encoded pattern-recognition receptors (PRRs) that can detect a multitude of pathogen- and danger-associated molecular patterns (PAMPs and DAMPs) exposed upon invasion of pathogens. The activation of Toll-like receptors (TLRs) by their cognate extracellular ligands primes the host defense responses by inducing the transcription and translation of critical mediators of intracellular sensors such as NLRP3 and caspase-11 (also known as Ich-3) as well as important pro-inflammatory cytokines, such as pro-IL-1β (Bauernfeind et al., 2009; Hiscott et al., 1993; Wang et al., 1996). When primed with a TLR3 agonist, Tlr4−/− and wild-type mice are equally susceptible to LPS-induced sepsis. Given that Casp11−/− mice are resistant to lethal dose of LPS, with or without priming, the induction of caspase-11 by different priming signals likely constitutes a prerequisite to alert the system for the incoming pathogenic attack (Kayagaki et al., 2011; Wang et al., 1998). Indeed, the priming role of TLR4 in detecting extracellular LPS can be bypassed by TLR3 signaling as the first alarm to promote the induction of caspase-11 transcription as it can be up-regulated downstream of both receptors through the MyD88- and/or the TRIF/type I IFN-dependent pathways (Hagar et al., 2013; Kayagaki et al., 2013). This first alarm may also be activated in a TLRs-independent manner, e.g. the activation of cGAS (cyclic GMP-AMP synthase) by cytosolic DNA (Storek et al., 2015).
The second alarm system relies on intracellular PRRs such as members of NLR and TRIM families that detect various DAMPs and PAMPs including bacterial proteins, and the Pyrin and HIN domain (PYHIN; also known as AIM2-like receptors, ALRs) family that detect cytoplasmic or nuclear DNA either from pathogens or misplaced host nucleic acids. When activated by the second signal, these PRRs may induce the assembly of inflammasomes to recruit and activate caspase-1. Noteworthy, since the direct binding of murine caspase-11 and human caspase-4/5 to cytosolic LPS can trigger their oligomerization and activation without involving another PRR in noncanonical inflammasome pathway (Shi et al., 2014), caspase-11/-4/-5 themselves may act as the second alarm system.
The canonical pathway of pyroptosis
The canonical pyroptosis is mediated by caspase-1 activated by inflammasomes, the supramolecular filamentous assemblies prototypically composed of a PRR protein, such as NLRP3, NLRP1, NAIPs–NLRC4, AIM2 and Pyrin, the adaptor ASC and caspase-1 (Rathinam and Fitzgerald, 2016). The interaction of PRR and caspase-1 is mediated by adaptor protein ASC through its pyrin domain (PYD) interacting with PRRs and its CARD interacting with caspase-1. Interestingly, both PYD and CARD form filaments. Activated PRRs, such as AIM2 and NLRP3, induce the formation of PYD filaments with ASC, which, in turn, clusters the CARD of ASC to nucleate the CARD filaments of caspase-1 forming structures known as “specks” and inducing the activation and the cleavage of caspase-1. NLRC4 and murine NLRP1b lack a PYD domain and may recruit a bridging ASC molecule directly through their CARD domains in order to form specks.
Inflammasome activation is regulated in both PRR and stimulus-dependent manner (Rathinam and Fitzgerald, 2016). Some PRRs sense indirect cytosolic changes linked to damage or infection. The NLRP3 inflammasome may be activated by exposure to intact pathogens, as well as a number of structurally diverse PAMPs, DAMPs, endogenous and environmental irritants such as uric acid crystals, amyloid-β, asbestos and alum. The exact mechanism by which NLRP3 detects such a diverse array of molecular patterns is still unclear; however, the structural diversity of NLRP3 agonists argues against direct interaction between NLRP3 and all of its activators. Similarly, Pyrin, a member of the TRIM family, also indirectly senses inhibition of Rho by bacterial toxins such as TcdB of Clostridium difficile. On the other hand, PRRs may be subject to direct modification by pathogenic agents, such as lethal toxin of Bacillus Anthracis which can activate NLRP1b by direct cleavage. Finally, direct binding of PAMPs activates some PRRs. Bacterial flagellin and type3 secretion system (T3SS) rod and needle proteins engage specific NAIPs to trigger the oligomerization of NLRC4. The PYHIN, or ALR, family members recognize and bind nucleic acids.
The noncanonical pathway of pyroptosis
The expression of murine caspase-11 is very low in un-stimulated cells and highly inducible by multiple pro-inflammatory stimuli such as TLR ligands, LPS, poly(I:C), and Pam3CSK4 and by IFNs. In contrast, human caspase-4/-5 are constitutively expressed in macrophages, monocytes and various additional cell types (Kayagaki et al., 2013; Rathinam et al., 2012; Wang et al., 1996; Wang et al., 1998). Caspase-4, -5, and -11 can be directly activated by Gram-negative bacteria in the cytoplasm within macromolecular signaling complexes called “noncanonical inflammasomes” (Hagar et al., 2013; Kayagaki et al., 2011). Oligomerized caspase-11, caspase-4 or caspase-5 is a critical component of this “noncanonical inflammasome”; however, its full composition is not yet clear. The binding of the lipid-A portion of LPS to the CARD domains of these inflammatory caspases promotes their oligomerization and activation. Furthermore, the induction of caspase-11 expression might be sufficient for auto-activation (Kang et al., 2000; Rathinam et al., 2012). In addition, activated caspase-11 can modulate the dynamics of actin cytoskeleton which may be important in restricting the growth of intracellular pathogens such as Legionella pneumophila by promoting bacteria-containing vacuoles to fuse with lysosomes (Akhter et al., 2012; Li et al., 2007).
Consistent with the role of cytosolic LPS in mediating the activation of caspase-11, the activation of caspase-11 in response to intracellular vacuolar Gram-negative bacterial pathogens such as Salmonella relies on IFN-inducible small GTPases of the guanylate-binding protein family (GBPs). GBPs mediate the lysis of the vacuole to allow the release of LPS to the cytosol to activate caspase-11 (Meunier et al., 2014; Pilla et al., 2014). Depending on bacterial species-specific LPS structures, GBPs can also be required for caspase-11 recognition of cytosolic LPS such as long fatty acid chain of Legionella pneumophila.
The downstream mechanisms of pyroptosis
The cleavage of GSDMD by inflammatory caspases including caspase-1, -4, -5 and -11 is a key execution event in pyroptosis (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015). The gasdermin-N domain in GSDMD, as well as other members of the gasdermin family such as GSDMA and GSDM3, have an intrinsic ability to bind to multiple kinds of membrane lipids, such as phosphatidylinositol phosphates and phosphatidylserine that are restricted to the inner leaflet, which allows them to form pores on plasma membrane and induce cell lysis (Shi et al., 2015). The pores formed by gasdermin-N have an inner diameter of 10–143nm and contain 16 symmetric protomers (Ding et al., 2016; Liu et al., 2016). The resistance of Gsdmd−/− mice to LPS induced sepsis supports the involvement of pyroptosis in vivo (Kayagaki et al., 2015). However, since the release of proinflammatory cytokines is also blocked by GSDMD deficiency (Shi et al., 2015), and mice deficient for IL-1R type I, the receptor for both IL-1α and IL-1β are highly resistant to LPS (Joosten et al., 2010), a major role of pyropotosis in sepsis in vivo might still be related to the release of proinflammatory cytokines. The cleavage of GSDMD by caspase-1 may play a role in the release of IL-1β by forming an ion-permeable conduit that can be inhibited by broadly acting channel inhibitors such as lanthanides (La3+ and Gd3+), before pyroptotic cell death (Russo et al., 2016). Caspase-11 mediated cleavage of pannexin-1 can also trigger the release of intracellular ATP through this non-selective large-pore channel, and the subsequent activation of the purinergic receptor P2X ligand-gated ion channel (P2X7), both critical for caspase-11-mediated pyroptosis (Yang et al., 2015). On the other hand, the activation of caspase-11 by a combination of microbial products and oxidized phospholipids released from dying cells can trigger the release of mature IL-1β from DCs without inducing pyroptosis (Zanoni et al., 2016). In addition, the stimulation of primary human monocytes by TLR2 or TLR4 ligands alone without a second signal can promote IL-1β release, a process that requires NLRP3, caspase-1/-4 and -5 but occurs in the absence of cell death (Netea et al., 2009; Vigano et al., 2015). Since activated caspase-11 is also known to cleave a number of channel proteins, such as the cationic channel subunit transient receptor potential channel 1 (TRPC1) to promote the secretion of IL-1β without modulating caspase-1 cleavage or cell death in macrophages (Py et al., 2014), these studies suggest the possibility that “leadless” cytokines such as IL-1α/β may be released from DCs and monocytes through a secretory mechanism without inducing cell lysis.
In vivo injection of LPS can induce the activation of caspase-3 in wild-type but not Casp11−/− mice, suggesting that caspase-11 can also mediate the activation of apoptosis in LPS stimulated cells (Kang et al., 2002). The cleavage specificity of caspase-11 is similar to that of caspase-9 so it can effectively cleave pro-caspase-3 and pro-caspase-7 and to promote their activation. It is possible that caspase-11 may activate pyroptosis or apoptosis depending on the expression levels of GSDMD in the target cells. In cells with high levels of GSDMD, the formation of pores, which leads to rapid disruption of cytoplasmic membrane integrity, may preempt the activation of downstream caspases. Accordingly, stimulation of LPS-primed Gsdmd−/− macrophages with nigericin or Salmonella typhimurium leads to significant activation of caspase activity which is absent in Casp-1−/− or Nlrp3−/− macrophages (He et al., 2015).
The interaction of the canonical and non-canonical pathways
Casp11−/− mice are protected against lethal doses of LPS and are unable to mediate the release of mature IL-1β/α (Wang et al., 1998). Thus, caspase-11 controls the activation of caspase-1 at least under certain conditions. Given that mice with caspase-1 deficiency alone are not as resistant to a lethal dose of LPS as that of Casp11−/− mice, inhibiting the activation of the canonical inflammasome pathway per se might not be sufficient to block sepsis induced by Gram-negative bacteria (Kayagaki et al., 2011; Wang et al., 1998). Accordingly, the induction of caspase-11 expression upon the activation of TLR4 in a TRIF-dependent pathway may be sufficient to promote its own activation as well as to license the activation of NLRP3 inflammasome to mediate the activation of caspase-1 under certain conditions (Rathinam et al., 2012; Wang et al., 1996).
Caspase-11 may be able to mediate the activation of caspase-1 directly or indirectly. Endogenous pro-caspase-11 and activated caspase-11 can interact with caspase-1, suggesting that caspase-11 might directly promote the catalytic activity of caspase-11 by forming a physical complex (Kayagaki et al., 2011; Wang et al., 1998). On the other hand, the activation of caspase-1 in macrophages infected by Gram-negative bacteria or LPS requires NLRP3 and ASC in addition to caspase-11. Similarly, in human cells, cytosolic LPS triggers IL-1β secretion in a caspase-4, NLRP3, ASC and caspase-1 dependent manner (Schmid-Burgk et al., 2015). Thus, the canonical inflammasomes might still be required for caspase-11, caspase-4 and caspase-5 to mediate the activation of caspase-1 under certain circumstances. However, caspase-11 is dispensable for the activation of caspase-1 in response to other NLRP3 agonists such as ATP, the lysomotropic agent HLLOMe and silica crystals, as well as other canonical inflammasomes such as the NLRC4 or AIM2 inflammasomes, which can promote the activation of caspase-1 directly. In addition, canonical inflammasomes can control the activation of noncanonical inflammasome in a cell extrinsic manner. For example, in a Burkholderia thailandensis in vivo infection model, NLRP3 and NLRC4-mediated IL-18 secretion and subsequent indirect IFN-γ production provides critical priming of the caspase-11 noncanonical inflammasome (Aachoui et al., 2015).
The pathophysiological significance of pyroptosis
Recent studies suggest that pyroptosis is a host defense mechanism. Activation of pyroptosis leads to the release of both inflammatory cytokines and DAMPs as the result of cell rupture that can act in synergy to maximize protective immunity against invading pathogens by promoting both innate as well as adaptive immunity. Concordantly, Casp1−/−; Casp11−/− double mutant mice are more susceptible to intracellular Gram-negative bacteria such as Shigella, Francisella, Salmonella and Legionella, as well as Gram-positive Listeria. On the other hand, Casp11−/− macrophages are significantly more resistant to death induced by Salmonella infection compared to WT, yet Casp11−/− mice infected with Salmonella show no difference in the load of bacteria compared to that of WT mice. However, Casp1−/−; Casp11−/− double knockout mice show lower bacterial burden than Casp1−/− mice (Broz et al., 2012). These results highlight the importance of the caspase-1 mediated canonical inflammasome pathway and suggest not only that blocking noncanonical pyroptosis by caspase-11 deficiency alone might be insufficient to change the susceptibility, and that noncanonical pyroptosis may favor extracellular release and dissemination of Salmonella, a predominantly intra-vacuolar pathogen. Conversely, Burkholderia pseudomallei, a Gram-negative bacterium that causes melioidosis that can naturally invade the cytosol, can activate caspase-11 to protect mice from lethal challenge with B. thailandensis and B. pseudomallei (Aachoui et al., 2013).
Necroptosis
Caspase-8 is an upstream caspase mediating apoptotic cell death induced by the cognate ligands of death receptor family, such as Fas and TNFR1 (Fernandes-Alnemri et al., 1996; Muzio et al., 1996). Its classical function consists of cleaving and activating downstream caspases, such as caspase-3 and caspase-7, and pro-apoptotic proteins, such as BID, to promote mitochondrial damage and apoptosis (Li et al., 1998). Caspase-8 is also involved in suppressing a necrotic cell death pathway, named necroptosis (Degterev et al., 2005; Holler et al., 2000). Necroptosis has been recently linked to multiple human diseases characterized by cell death and inflammation, such as ischemic brain/heart/kidney/eye injuries, multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) (Ito et al., 2016; Ofengeim et al., 2015; Zhou and Yuan, 2014). Therefore, caspase-8 is now viewed as having a role in suppressing necrotic cell death in multiple disease contexts.
Necroptosis can be activated upon stimulation by ligands of death receptor family including TNFα or FasL, when cells are deficient for caspase-8 or its adaptor protein FADD, or in the presence of a caspase inhibitor zVAD.fmk. The activation of RIPK1 is a critical and chemically-targetable upstream event in necroptosis (Degterev et al., 2008; Degterev et al., 2005; Ofengeim and Yuan, 2013). The development of Nec-1s, a highly specific small molecule inhibitor of RIPK1, has played a critical role in demonstrating the involvement of necroptosis in a variety of cellular models and animal models of human diseases (Zhou and Yuan, 2014). Activated RIPK1 promotes necroptosis by interacting with RIPK3, which in turn mediates the phosphorylation of MLKL, a pseudokinase (Cho et al., 2009; He et al., 2009; Zhang et al., 2009). Phosphorylation of MLKL at Ser358 leads to its oligomerization and the interaction of charged amino acids in its N-terminal four helical bundle (4HB) domain of phosphorylated MLKL with phosphatidylinositol phosphates (PIPs) allows the recruitment of MLKL to the plasma membrane, where it forms pores to promote cell lysis (Dondelinger et al., 2014; Hildebrand et al., 2014; Murphy et al., 2013; Sun et al., 2012; Wang et al., 2014).
The suppression of necroptosis by caspase-8 is critical for normal embryonic development as both Casp8−/− and Fadd−/− mutant mice have an early embryonic lethal phenotype that can be completely suppressed by RIPK3 knockout (Kaiser et al., 2011; Oberst et al., 2011). These findings support the notion that the activation of caspases might not necessarily be incompatible with life; and on the contrary, certain levels and kinds of caspase activity might be critical for cell and organismal survival. In this section, we will discuss the molecular mechanisms that control the activation of caspase-8 to suppress RIPK1/RIPK3–mediated necroptosis.
Orchestration of TNFR1 signaling
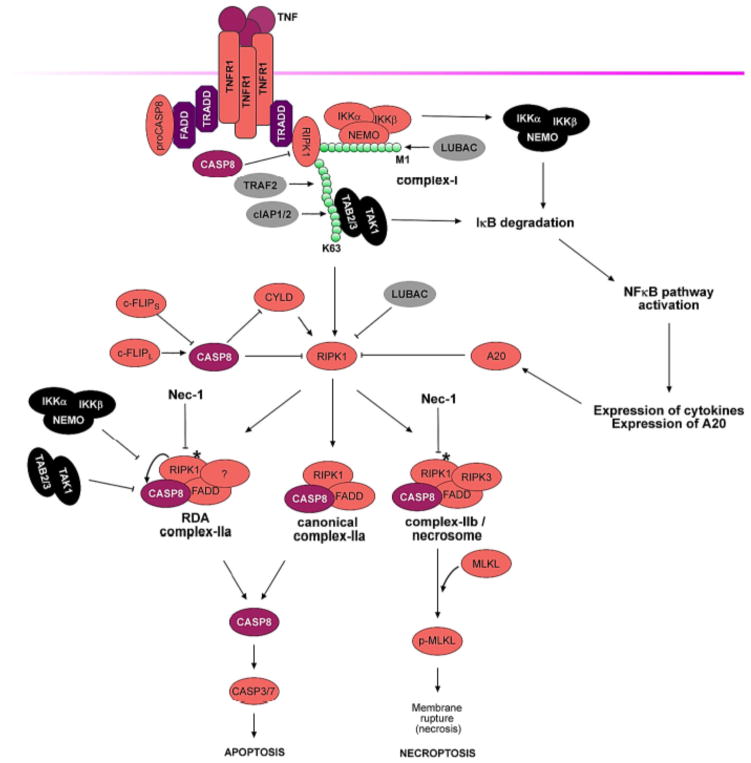
Necroptosis activated by the proinflammatory cytokine TNF has been extensively characterized. TNFα-induced trimerization of TNFR1 leads to the rapid formation of a transient intracellular multi-protein complex, called the TNF-R1 signaling complex - TNF-RSC (also called complex I) (Figure 2). TNF-RSC orchestrates a complex pattern of modification including M1, K48, K63 and K11 ubiquitination linkages in a specific spatiotemporal manner that collectively decides within minutes of TNFα stimulation if a cell and, ultimately, an organism may live or die. The intracellular DD (death domain) motif of trimerized TNFR1 complex recruits TRADD, an adaptor protein, and RIPK1 via homotypic interactions with their respective DD motifs. TRADD interacts with FADD, a critical adaptor for the recruitment and activation of caspase-8, to mediate apoptosis. TRADD also mediates the recruitment of TRAF2/TRAF5, which function as redundant adaptors to recruit cIAP1/2, two important E3 ubiquitin ligases that mediate the ubiquitination of RIPK1 by K63- and K11-linked chains (Pobezinskaya et al., 2008).
Figure 2. Caspase-8 regulates cell survival, apoptosis, and necroptosis.
TNF ligation with the TNFR1 receptor induces pro-caspase-8 recruitment to the activated TNFR1 signaling complex-I via FADD. Pro-caspase-8 cleavage and activation results in an active caspase-8, which limits complex-I activity by cleaving RIPK1. c-FLIPS inhibits caspase-8 activity, whereas c-FLIPL can partially promote it. In complex-I, RIPK1 is ubiquitinated by cIAP1/2, TRAF2 and the LUBAC complex, resulting in K63-linked and M1-linked chains, respectively. IKKα/β/NEMO and TAK1/TAB2/3 kinase complexes are recruited to ubiquitinated RIPK1 and activated, inducing degradation of IκB and subsequent NFκB pathway activation, which results in expression of cytokines and A20. The latter inhibits RIPK1 and forms a negative feedback. RIPK1 is deubiquitinated by CYLD and A20 in complex-I to promote formation of complex-II. Caspase-8 cleaves CYLD and RIPK1 to inhibit complex-II formation. Canonical complex-IIa is formed independent of RIPK1 kinase activity, where FADD and caspase-8 are recruited to RIPK1. RIPK1-dependent apoptosis (RDA) complex-IIa forms downstream of RIPK1 kinase activity and is negatively influenced by IKKα/β/NEMO and TAK1/TAB2/3 kinase complexes. Both the canonical and RDA complex-IIa promote activation of caspase-8/caspase3/7 cascade and execution of apoptotic cell death. Under the conditions of caspase-8 inhibition, complex-IIb/necrosome is formed, to which RIPK3 is recruited downstream of RIPK1 kinase activity. Complex-IIb/necrosome results in phosphorylation of MLKL and execution of necrotic cell death. RIPK1 inhibitor Nec-1 inhibits both RDA complex-IIa and complex-IIb/necrosome. Activated RIPK1 is marked with *.
A major downstream event of TNF-RSC mediated by TRADD/TRAF2/cIAP1 complex is the recruitment of the LUBAC complex to mediate M1 (also known as Met1-linked or linear ubiquitin chain)-linked ubiquitin modification of TNF-RSC (Gerlach et al., 2011; Haas et al., 2009; Ikeda et al., 2011). The LUBAC complex includes heme-oxidized IRP2 ubiquitin ligase 1 (HOIL-1), shank-associated RH domain-interacting protein (SHARPIN) and HOIL-1-interacting protein (HOIP), which is the catalytic subunit of the complex. The ubiquitination substrates of LUBAC in TNF-RSC include NEMO (Tokunaga et al., 2009), RIPK1 (Gerlach et al., 2011), TNFR1 and TRADD (Draber et al., 2015). M1-linked ubiquitination of TNF-RSC has been shown to be important for blocking TNFα-mediated apoptosis.
CYLD, a deubiquitinating enzyme that targets both M1- and K63-linked ubiquitin chains (Komander et al., 2009), is recruited to TNF-RSC to negatively regulate ubiquitinations of RIPK1, TNFR1, NEMO and TRADD to attenuate the NF-κB pathway and promote both apoptosis and necroptosis (Draber et al., 2015; Hitomi et al., 2008). CYLD is recruited to TNF-RSC by its interaction with the LUBAC complex in association with SPATA2 (Schlicher et al., 2016; Wagner et al., 2016). Thus, LUBAC and CYLD might be simultaneously recruited to provide a dynamic mechanism to edit the patterns of ubiquitination on key mediators of TNF-RSC.
A20, encoded by the gene TNFAIP3, is an important ubiquitin-editing enzyme recruited to TNF-RSC to terminate multiple downstream events including NF-κB-mediated transcriptional response, RIPK1-mediated signaling and ultimately, the disassembly of the TNFR1 signaling complex (Newton et al., 2016; Song et al., 1996; Wertz et al., 2004). The expression of A20 can be induced in multiple cell types in response to a variety of stimuli that activate NF-κB, including TNFα, IL-1 and LPS. A20 can also inhibit IKK non-catalytically via ubiquitin-binding activity of its seventh zinc-finger (ZnF7) (Skaug et al., 2011).
The tight regulation of caspase-8
A major goal of ubiquitination modification of TNF-RSC is to tightly regulate the activation of caspase-8, allowing its activation only when apoptosis is the correct choice for the cell/organism. In cells stimulated by TNFα and CHX, RIPK1 and TRADD in TNF-RSC rapidly transitions into a cytosolic complex, known as complex-IIa, where caspase-8 is activated through its interaction with its adaptor protein FADD in a RIPK1 kinase-activity-independent manner (Figure 2). Induction of apoptosis by TNFα in most cell types requires addition of CHX, which blocks the synthesis of anti-death proteins mediated by the transcriptional response of NF-κB. During classical apoptosis induced by TNFα and CHX, the binding of TRADD with TNFR1 is essential for recruiting FADD to mediate the activation of caspase-8 independently of RIPK1 (Pobezinskaya et al., 2008). Furthermore, RIPK1-deficient MEFs are hyper-sensitive to apoptosis induced by TNFα. In comparison, A20 deficiency highly sensitizes cells to both apoptosis and necroptosis, despite hyper-activated NF-κB (Lee et al., 2000; Newton et al., 2016; Onizawa et al., 2015; Vereecke et al., 2010). Inhibition of RIPK1 and RIPK3 deficiency can reduce the systemic inflammation of A20−/− mice and prolong the survival of cells stimulated by TNFα (Newton et al., 2016; Onizawa et al., 2015). Thus, promoting RIPK1-mediated cell death is a major consequence of A20 deficiency.
The activation of caspase-8 by TNF-RSC is critically regulated by both K63 and M1-linked ubiquitination. The loss of A20 leads to increased K63 ubiquitination and decreased M1 ubiquitination, resulting in sensitization of cells to TNFα-induced cell death (Draber et al., 2015). Conversely, CYLD deficiency leads to increased M1 and K63 ubiquitination and resistance to cell death. Overexpression of LUBAC components HOIL-1/HOIP substantially increases the M1 ubiquitin modification of RIPK1 and stabilizes the association of RIPK1, TRAF2 and TAK1 in the TNF-RSC, suggesting that M1 ubiquitin modification has a dominant role in maintaining TNF-RSC and downstream signaling (Haas et al., 2009). On the other hand, dysregulated M1 ubiquitination might also be detrimental for cell survival as the loss of OTULIN, an M1-linkage specific DUB, leads to accumulation of cytosolic M1 ubiquitin chains, which also promotes cell death and systemic inflammation in human and mice (Damgaard et al., 2016; Draber et al., 2015). A spatially- and temporally-appropriate M1 ubiquitination pattern may be critical to support cell survival.
Activation of caspase-8 in RIPK1-dependent apoptosis
The activation of caspase-8 can also occur in RIPK1 kinase activity-dependent manner to lead to RIPK1-dependent apoptosis (RDA). In this pathway, the recruitment of TAK1 and NEMO by K63- and M1-linked ubiquitin chains into TNF-RSC is important for suppressing the ability of RIPK1 to mediate the activation of caspase-8 and apoptosis. Similarly, TNFα stimulation of cells with deficiency in TAK1, TRAF2, UBC13, NEMO, cIAP1/2 or IKKα/IKKβ, by TNFα can lead to RDA, independent of NF-κB activation (Arslan and Scheidereit, 2011; Dondelinger et al., 2015; Legarda-Addison et al., 2009; Wang et al., 2008). RDA can be effectively induced by the co-treatment of TNFα with small molecule compounds that can promote degradation of cIAP1/2, or TAK1 inhibitor. Since the activation of TAK1 by TNF-RSC is mediated through the recruitment of TAK1/TAB2/3 complex by K63 ubiquitin chain modification on RIPK1 conjugated by cIAP1/2, loss of cIAP1/2 would lead to inhibition of TAK1.
Caspase-8 suppresses the activation of necroptosis
Two key upstream mediators of necroptosis, RIPK1 and CYLD, are both proteolytic cleavage substrates of caspase-8 (Lin et al., 1999; O’Donnell et al., 2011). The cleavage of RIPK1 at D324 by caspase-8 separates its N-terminal kinase domain from the C-terminal RHIM domain and DD motif involved in mediating the interaction of RIPK1 and TNFR1; whereas the cleavage of CYLD by caspase-8 occurs at D215 in the middle of second GAP-Gly domain. By mediating the cleavage of RIPK1, a major target of ubiquitination in TNF-RSC, and CYLD, a deubiquitinating enzyme critically involved in TNFα signaling, caspase-8 plays a critical role in controlling the ubiquitination of TNF-RSC and negatively regulating the activation of RIPK1. Furthermore, since CYLD is also a negative regulator of NF-κB, increased levels of CYLD in the absence of caspase-8 might reduce the activation of NF-κB which is critical for the development of embryonic vasculature (Hou et al., 2008).
The knockout of caspase-8 or its adaptor FADD in mice leads to embryonic lethality around embryonic day E10.5, demonstrating the pro-survival role of caspase-8. Caspase-8 might also be required to regulate the ubiquitination status of TNF-RSC, RIPK1 and RIPK3 during embryonic development to allow the development of normal vasculature (Dillon et al., 2012; Kaiser et al., 2011; Oberst et al., 2011). Consistently, cIAP1/cIAP2 double knockout mice die around E10.5 whose survival can be extended to later embryonic development stage or to birth in double mutant with Ripk3−/− or Tnfr1−/− (Moulin et al., 2012).
Oligodendrocytes, the cell type responsible for mediating myelination of axons in the central nervous system (CNS), undergo necroptosis when treated with TNFα alone which can be blocked by genetic or pharmacological inhibition of RIPK1 and RIPK3 deficiency (Ofengeim et al., 2015). Thus, necroptosis might be the preferred cell death pathway when oligodendrocytes are stimulated with TNFα. Furthermore, null mutation in Optineurin, which encodes a ubiquitin-binding protein, can further sensitize oligodendrocytes to TNFα-mediated necroptosis (Ito et al., 2016). Since mutations in Optineurin (OPTN) gene have been found in both familial and sporadic ALS cases (Cirulli et al., 2015; Maruyama et al., 2010), RIPK1/RIPK3-mediated necroptotic pathway might play a critical role in mediating progressive axonal degeneration in ALS and other human degenerative diseases characterized by axonal degeneration.
The activation of caspase-8 is negatively regulated by cellular FLICE-inhibitory protein cFLIPL which can form heterodimers with pro-caspase-8 that allows the initial processing of pro-caspase-8 to lead to an intermediary p43 fragment, but prevents the full maturation of caspase-8 (Krueger et al., 2001). In the CNS, cFLIPL is expressed in many types of cells including microglia and oligodendrocytes, but not neurons (Zhang et al., 2014). The expression of cFLIPL in cortical lesions of multiple sclerosis patients is elevated which corresponds to a reduction in the levels of activated caspase-8. Since cFLIPL is permissive for the initial processing of pro-caspase-8 into the p43 subunit but blocks the full activation of caspase-8 and the production of p18, the elevated levels of cFLIPL suggest a possible mechanism by which caspase-8 activation may be inhibited in MS.
Regulation of caspase-8 activation by RIPK3
Although RIPK3 was originally isolated as a specific mediator of necroptosis, recent studies suggest that RIPK3 might also be involved in regulating the activation of caspase-8 and apoptosis. RIPK3 kinase-dead knock-in mice die prematurely from caspase-8-mediated apoptosis (Newton et al., 2014). Likewise, pharmacological inhibition of RIPK3 kinase activity can also promote the activation of caspase-8 and apoptosis (Mandal et al., 2014). Thus, RIPK3 might be involved in restraining apoptosis by a yet unknown mechanism. In addition, infection of influenza A virus might be able to activate RIPK3 to mediate MLKL-driven necroptosis and caspase-8 activation, both independent of RIPK1 (Nogusa et al., 2016). Therefore, RIPK3 might be activated without the involvement of RIPK1 by pathogens.
Caspase-8-regulated apoptosis and necroptosis in human autoinflammatory diseases
Dysregulation of TNF-RSC in humans is also known to promote autoinflammatory diseases, for instance in cryopyrin (NLRP3)-associated periodic syndrome (CAPS) (Kastner et al., 2010). A prototypic example are patients with TNFR1-associated periodic syndromes (TRAPS), caused by missense mutations in TNFR1 that lead to impaired down-regulation of membrane-localized TNFR1 and diminished receptor shedding (McDermott et al., 1999). TRAPS is characterized by unexplained episodes of prolonged fever and severe localized inflammation and in some cases, development of renal failure. Similarly, mutations in TNFAIP3 in humans that lead to the expression of a truncated A20 missing its DUB domain promote an early-onset autoinflammatory disease (Zhou et al., 2016). Although cells expressing this patient-derived truncated form of A20 have increased activation of the NF-κB pathway (similar to that of A20-null cells), the contribution of uninhibited RDA and/or necroptosis to development of autoimmunity in these patients should be considered as well. A20-regulated caspase-8 activity and inflammatory response mediated in RIPK1-dependent manner may play an important role in autoinflammatory diseases characterized by misregulation of TNF-RSC signaling.
In addition, a failure in the recruitment of A20 to TNF-RSC has been suggested to be involved in a distinct group of patients carrying a NEMO C-terminal deletion (ΔCT-NEMO) mutation. The disease symptoms of these patients, such as arthritis, colitis and dermatitis, are similar to those observed in the A20−/− mice (Zilberman-Rudenko et al., 2016). Furthermore, some patients with ΔCT-NEMO or A20 mutations have been diagnosed with Behçet disease, a polygenic inflammatory disorder. Similar to that of A20−/− cells, ΔCT-NEMO cells exhibit increased IKK activity in response to TNFα and stabilization of K63-ubiquitinated RIPK1 in the TNFR1 signaling complex, suggesting possible involvement of RDA and necroptosis.
Concluding remarks
Although originally discovered and subsequently characterized extensively as a proinflammatory caspase involved mediating the maturation of cytokines such as IL-1β and IL-18, caspase-1 is capable of mediating proteolytic cleavage of protein substrates similar to all of the canonical caspases. In comparison, caspase-11 was identified as a proinflammatory caspase highly inducible by LPS and critical for mediating sepsis but also capable of mediating the cleavage of downstream caspases such as caspase-3/-7 (Kang et al., 2002; Wang et al., 1996; Wang et al., 1998). The ability of proinflammatory caspases to cleave GSDMD to promote pyroptosis adds a new dimension to the repertoire of biological mechanisms mediated by caspases (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015). Pyroptosis might represent a specific host defense mechanism against pathogens. Whereas the loss of caspase-8 has detrimental consequence to mouse embryonic development, caspase-8-mediated inactivation of RIPK1/RIPK3 likely represents critical developmental checkpoints. Mutations that inactivate caspase-8 sensitize cells to RIPK1/RIPK3 mediated cell death lead to embryonic lethality and systemic inflammatory diseases in mice. Thus, activating RIPK1/RIPK3 has deleterious consequences incompatible with normal development and tissue homeostasis. In contrast, blocking necroptosis, as in RIPK1 kinase dead mutant mice, Ripk3−/− mice and Mlkl−/− mice, does not have major impact on development or adult life.
Recent studies highlighted the role of caspase-8 and RIPK1 in regulating inflammation independent of the NF-κB pathway. Although many factors in the TNF-RSC were originally isolated as regulators of NF-κB, the new findings suggest important roles of these factors in regulating inflammation through caspase-8 and RIPK1 controlled apoptosis and necroptosis independent of NF-κB. On the other hand, the ability of NF-κB to induce the expression of A20 might provide an important mechanism to control the activation of RIPK1 and caspase-8. The kinase activity of RIPK1 is critical not only for necroptosis, but also RIPK1-dependent apoptosis that mediates the activation of caspase-8 and inflammation (Ofengeim and Yuan, 2013). Given its role in mediating multiple deleterious processes activated by TNFα and production of pro-inflammatory cytokines, inhibiting the kinase activity of RIPK1 is recognized as providing exciting opportunities for developing new therapeutics targeting a multitude of human diseases characterized by cell death and inflammation.
Acknowledgments
This work was supported in part by grants to JY from the NINDS-US (1R01NS082257) and the NIA-US (1R01AG047231) and to BP from the European Research Council (ERC-2013-CoG_616986). We thank Palak Amin and Albert D. Yu for comments on the manuscript.
Abbreviations
- AIM2
Absent In Melanoma-2
- ALR
AIM2-Like Receptor
- ALS
Amyotrophic Lateral Sclerosis
- ASC
Apotosis-associated Speck-like protein
- Bcl-2
B cell lymphoma-2
- BID
BH3 Interacting Domain death agonist
- CAPS
Cryopyrin-Associated Periodic Syndrome
- CARD
Caspase Activation and Recruitment Domain
- Ced-3
Cell Death gene 3
- cGAS
cyclic GMP-AMP Synthase
- cFLIPL
cellular FLICE Inhibitory Protein
- CHX
cycloheximide
- cIAP1/2
cellular Inhibitor of Apoptosis 1/2
- CNS
Central Nervous System
- CrmA
Cytokine response modifier A
- DC
Dendritic Cell
- DD
Death Domain
- DED
Death Effector Domain
- DAMPs
Danger-Associated Molecular Patterns
- FADD
Fas-Associated protein with Death Domain
- FasL
Fas Ligand
- GBP
Guanylate-Binding Protein family
- GSDM3
Gasdermin 3
- GSDMA
Gasdermin A
- GSDMD
Gasdermin D
- HOIL-1
heme-oxidized IRP2 ubiquitin ligase 1
- HOIP
HOIL-1-interacting protein
- HLLOMe
L-Leucyl-L-leucine methyl ester
- IBD
Inflammatory Bowel Disease
- ICE
Interleukin-1 Converting Enzyme
- Ich-3
ICE and Ced-3 homolog-3, original name for caspase-11
- IKK
IκB kinase
- IL
Interleukin
- INF
Interferon
- IRAK1
Interleukin-1 Receptor Associated Kinase-1
- LPS
Lipopolysaccharide
- LUBAC
Linear Ubiquitin chain Assembly Complex
- MEFs
Murine Embryonic Fibroblasts
- MLKL
Mixed Lineage Kinase domain Like
- MS
Multiple Sclerosis
- MyD88
Myeloid Differentiation primary response 88
- NAIPs
NLR family Apoptosis Inhibitory Proteins
- NEMO
Nf-Kappa B Essential Modulator
- NF-κB
Nuclear Factor κ-light-chain-enhancer of activated B cells
- NLRC4
Nucleotide-binding domain Leucine-rich Repeat and CARD domains-containing gene 4
- NLRP3
Nucleotide-binding domain Leucine-rich Repeat and Pyrin domains-containing gene 3
- NLRP1
Nucleotide-binding domain Leucine-rich Repeat and Pyrin domains-containing gene 1
- NLR
Nucleotide-binding domain and Leucine-rich Repeat-containing gene
- NK
Natural Killer
- OMVs
Outer Membrane Vesicles
- OTULIN
OTU deubiquitinase with Linear linkage specificity
- P2X7R
Purinergic receptor P2X 7
- Pam3CSK4
Synthetic triacylated lipopeptide (LP) mimetic of bacterial LPs acylated amino terminus
- PAMPs
Pathogen- Associated Molecular Patterns
- PIPs
phosphatidylinositol phosphates
- Poly(I:C)
Polyinosinic:polycytidylic, structurally similar to double-stranded RNA
- PRR
Pattern Recognition Receptor
- PYD
Pyrin Domain
- PYHIN
Pyrin and HIN domain family, also known as AIM2-like receptors
- RDA
RIPK1-dependent apoptosis
- RHIM
RIP homotypic interaction motif
- RIPK1 (also called RIP1)
Receptor Interacting Protein Kinase 1
- RIPK3 (also called RIP3)
Receptor Interacting Protein Kinase 3
- SHARPIN
shank-associated RH domain-interacting protein
- SLE
Systemic Lupus Erythematosus
- SPATA2
Spermatogenesis associated 2
- T3SS
Type-3 Secretion System
- TAB2/3
Transforming growth factor β-Activated Kinase Binding protein 2/3
- TAK1
Transforming growth factor β-Activated Kinase 1
- TcdB
Clostridium difficile toxin B
- TLR
Toll-Like Receptor
- TNFα
Tumor Necrosis Factor α
- TNFAIP3
Tumor Necrosis Factor α Induced Protein 3
- TNFR1
Tumor Necrosis Factor Receptor 1
- TNF-RSC
TNFR1 signaling complex (also called complex I)
- TNFRSF
Tumor Necrosis Factor Receptor Superfamily
- TRADD
TNFRSF1A Associated via Death Domain
- TRAF2
TNF Receptor Associated Factor 2
- TRAF5
TNF Receptor Associated Factor 5
- TRAPS
TNFR1-Associated Periodic Syndrome
- TRIF
Toll-Interleukin Receptor (TIR)-domain containing adapter inducing interferon-β
- TRIM
TRIpartite Motif–containing
- UBAN
Ubiquitin Binding In Abin and Nemo Domain
- UBCH5
alternative name of UBE2D1
- UBC13
Ubiquitin-conjugating enzyme 13
- UBE2D1
Ubiquitin-conjugating enzyme E2 D1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aachoui Y, Kajiwara Y, Leaf IA, Mao D, Ting JP, Coers J, Aderem A, Buxbaum JD, Miao EA. Canonical Inflammasomes Drive IFN-gamma to Prime Caspase-11 in Defense against a Cytosol-Invasive Bacterium. Cell Host Microbe. 2015;18:320–332. doi: 10.1016/j.chom.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, et al. Caspase-11 protects against bacteria that escape the vacuole. Science. 2013;339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, et al. Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity. 2012;37:35–47. doi: 10.1016/j.immuni.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan SC, Scheidereit C. The prevalence of TNFalpha-induced necrosis over apoptosis is determined by TAK1-RIP1 interplay. PLoS One. 2011;6:e26069. doi: 10.1371/journal.pone.0026069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, March CJ, Kronheim SR, Druck T, Cannizzaro LA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256:97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli ET, Lasseigne BN, Petrovski S, Sapp PC, Dion PA, Leblond CS, Couthouis J, Lu YF, Wang Q, Krueger BJ, et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard RB, Walker JA, Marco-Casanova P, Morgan NV, Titheradge HL, Elliott PR, McHale D, Maher ER, McKenzie AN, Komander D. The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell. 2016;166:1215–1230. e1220. doi: 10.1016/j.cell.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harbor perspectives in biology. 2014;6:a016287. doi: 10.1101/cshperspect.a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell reports. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016 doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell reports. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, et al. NF-kappaB-Independent Role of IKKalpha/IKKbeta in Preventing RIPK1 Kinase-Dependent Apoptotic and Necroptotic Cell Death during TNF Signaling. Mol Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, et al. LUBAC-Recruited CYLD and A20 Regulate Gene Activation and Cell Death by Exerting Opposing Effects on Linear Ubiquitin in Signaling Complexes. Cell reports. 2015;13:2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Armstrong RC, Krebs J, Srinivasula SM, Wang L, Bullrich F, Fritz LC, Trapani JA, Tomaselli KJ, Litwack G, et al. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci U S A. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cellular microbiology. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Gagliardini V, Fernandez PA, Lee RK, Drexler HC, Rotello RJ, Fishman MC, Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994;263:826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–698. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, Pierotti C, Garnier JM, Dobson RC, Webb AI, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci U S A. 2014;111:15072–15077. doi: 10.1073/pnas.1408987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J, Marois J, Garoufalis J, D’Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G, et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231–6240. doi: 10.1128/mcb.13.10.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- Hou Y, Li F, Karin M, Ostrowski MC. Analysis of the IKKbeta/NF-kappaB signaling pathway during embryonic angiogenesis. Dev Dyn. 2008;237:2926–2935. doi: 10.1002/dvdy.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–608. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joosten LA, Van De Veerdonk FL, Vonk AG, Boerman OC, Keuter M, Fantuzzi G, Verschueren I, Van Der Poll T, Dinarello CA, Kullberg BJ, et al. Differential susceptibility to lethal endotoxaemia in mice deficient in IL-1alpha, IL-1beta or IL-1 receptor type I. APMIS. 2010;118:1000–1007. doi: 10.1111/j.1600-0463.2010.02684.x. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Hara H, Peterson EP, Namura S, Amin-Hanjani S, Huang Z, Srinivasan A, Tomaselli KJ, Thornberry NA, et al. Dual role of caspase-11 in mediating activation of caspase-1 and caspase-3 under pathological conditions. J Cell Biol. 2000;149:613–622. doi: 10.1083/jcb.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SJ, Wang S, Kuida K, Yuan J. Distinct downstream pathways of caspase-11 in regulating apoptosis and cytokine maturation during septic shock response. Cell Death Differ. 2002;9:1115–1125. doi: 10.1038/sj.cdd.4401087. [DOI] [PubMed] [Google Scholar]
- Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140:784–790. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legarda-Addison D, Hase H, O’Donnell MA, Ting AT. NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 2009;16:1279–1288. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li J, Brieher WM, Scimone ML, Kang SJ, Zhu H, Yin H, von Andrian UH, Mitchison T, Yuan J. Caspase-11 regulates cell migration by promoting Aip1-Cofilin-mediated actin depolymerization. Nat Cell Biol. 2007;9:276–286. doi: 10.1038/ncb1541. [DOI] [PubMed] [Google Scholar]
- Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- Meunier E, Dick MS, Dreier RF, Schurmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Maltzman A, Greve JM, Hedehus M, Martin-McNulty B, Carano RA, Cao TC, van Bruggen N, Bernstein L, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- Nogusa S, Thapa RJ, Dillon CP, Liedmann S, Oguin TH, 3rd, Ingram JP, Rodriguez DA, Kosoff R, Sharma S, Sturm O, et al. RIPK3 Activates Parallel Pathways of MLKL-Driven Necroptosis and FADD-Mediated Apoptosis to Protect against Influenza A Virus. Cell Host Microbe. 2016;20:13–24. doi: 10.1016/j.chom.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, et al. Activation of necroptosis in multiple sclerosis. Cell reports. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- Onizawa M, Oshima S, Schulze-Topphoff U, Oses-Prieto JA, Lu T, Tavares R, Prodhomme T, Duong B, Whang MI, Advincula R, et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol. 2015;16:618–627. doi: 10.1038/ni.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilla DM, Hagar JA, Haldar AK, Mason AK, Degrandi D, Pfeffer K, Ernst RK, Yamamoto M, Miao EA, Coers J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc Natl Acad Sci U S A. 2014;111:6046–6051. doi: 10.1073/pnas.1321700111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, Liu Z. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–1054. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py BF, Jin M, Desai BN, Penumaka A, Zhu H, Kober M, Dietrich A, Lipinski MM, Henry T, Clapham DE, et al. Caspase-11 controls interleukin-1beta release through degradation of TRPC1. Cell reports. 2014;6:1122–1128. doi: 10.1016/j.celrep.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Fitzgerald KA. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. Active Caspase-1 Induces Plasma Membrane Pores That Precede Pyroptotic Lysis and Are Blocked by Lanthanides. J Immunol. 2016;197:1353–1367. doi: 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicher L, Wissler M, Preiss F, Brauns-Schubert P, Jakob C, Dumit V, Borner C, Dengjel J, Maurer U. SPATA2 promotes CYLD activity and regulates TNF-induced NF-kappaB signaling and cell death. EMBO Rep. 2016;17:1485–1497. doi: 10.15252/embr.201642592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell. 2011;44:559–571. doi: 10.1016/j.molcel.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci U S A. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storek KM, Gertsvolf NA, Ohlson MB, Monack DM. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol. 2015;194:3236–3245. doi: 10.4049/jimmunol.1402764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano E, Diamond CE, Spreafico R, Balachander A, Sobota RM, Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nature communications. 2015;6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner SA, Satpathy S, Beli P, Choudhary C. SPATA2 links CYLD to the TNF-alpha receptor signaling complex and modulates the receptor signaling outcomes. EMBO J. 2016 doi: 10.15252/embj.201694300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung Y, Zhu H, Gagliardini V, Shi L, Greenberg AH, Yuan J. Identification and characterization of Ich-3, a member of the interleukin-1beta converting enzyme (ICE)/Ced-3 family and an upstream regulator of ICE. J Biol Chem. 1996;271:20580–20587. doi: 10.1074/jbc.271.34.20580. [DOI] [PubMed] [Google Scholar]
- Wang S, Miura M, Jung YK, Zhu H, Li E, Yuan J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell. 1998;92:501–509. doi: 10.1016/s0092-8674(00)80943-5. [DOI] [PubMed] [Google Scholar]
- Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity. 2015;43:923–932. doi: 10.1016/j.immuni.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Wang H, Schwartz DM, Stoffels M, Park YH, Zhang Y, Yang D, Demirkaya E, Takeuchi M, Tsai WL, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat Genet. 2016;48:67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Yuan J. Necroptosis in health and diseases. Seminars in cell & developmental biology. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Zilberman-Rudenko J, Shawver LM, Wessel AW, Luo Y, Pelletier M, Tsai WL, Lee Y, Vonortas S, Cheng L, Ashwell JD, et al. Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-kappaB activation and autoinflammatory disease. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1518163113. [DOI] [PMC free article] [PubMed] [Google Scholar]