Abstract
Release of neuroactive substances by exocytosis from dendrites is surprisingly widespread and is not confined to a particular class of transmitters: it occurs in multiple brain regions, and includes a range of neuropeptides, classical neurotransmitters and signaling molecules such as nitric oxide, carbon monoxide, ATP and arachidonic acid. This review is focused on hypothalamic neuroendocrine cells that release vasopressin and oxytocin and midbrain neurons that release dopamine. For these two model systems, the stimuli, mechanisms and physiological functions of dendritic release have been explored in greater detail than is yet available for other neurons and neuroactive substances.
Introduction
The dendrites of many neural populations transmit information back to their synaptic inputs by releasing neuroactive substances (89, 99, 128). Indeed, modulation of neuronal function by dendritic transmitter release is a widespread phenomenon and is specific neither to a localized part of the brain nor to a particular subtype of signalling molecule. In addition to membrane-permeant substances such as carbon monoxide, arachidonic acid and nitric oxide, classical transmitters can be released from dendrites to signal in a retrograde fashion. For example, somatodendritic release of dopamine, which is the exemplar small molecule transmitter emphasized in this review, modulates the firing rate and excitability of midbrain dopamine neurons. In addition, the amino acids GABA and glutamate act as retrograde transmitters in the olfactory bulb, hippocampus, cortex and cerebellum (90, 119, 226, 266). However, the most numerous class of signalling molecules in the brain is the neuropeptides and there is ample evidence for their dendritic release. There is convincing evidence for somatodendritic release of the neurohypophysial peptides oxytocin and vasopressin in the hypothalamus (111, 116, 120, 127), which are the exemplar peptides covered in this this review. Notably, there are also reports for this mode of release for other peptides, including dynorphin, encephalin, and cholecystokinin (19, 46, 216).
The hypothalamo-neurohypophysial peptide system
Oxytocin and vasopressin (antidiuretic hormone) enter the circulation following exocytotic release from magnocellular neurosecretory cells (MCNs), components of hypothalamic supraoptic (SON) and paraventricular nuclei (PVN) that project into the posterior pituitary gland. Oxytocin is required for milk ejection, and produces uterine contractions, and so has a role in parturition and lactation (4, 19, 81, 114, 202), whereas vasopressin is involved in the regulation of water excretion and blood pressure. In addition, both peptides have effects on behavior (see later). Both are released at axonal synapses, but also from the somata and dendrites of MCNs (99, 111, 116, 120, 127, 128, 155, 168). The cell bodies and dendrites of MCNs form densely packed and homogeneous nuclei, whereas their axons project into the posterior pituitary gland. As there is no blood-brain barrier in the posterior pituitary, peptide secretion from axons swiftly enters the bloodstream. The dendrites of adult rat MCNs are characteristically smooth (aspiny), thick and varicose, with little branching, and are often associated in bundles (147, 221). Most of the neuropeptides expressed in the SON and PVN are stored within MCN dendrites. Dendritic release can be studied in these regions by push-pull perfusion or microdialysis (150, 256). Importantly, these methods can be used to study dendritic release independently of axonal release, because re-entry of peripherally released peptide into the brain is prevented by the blood-brain barrier.
Although the SON contains only MCNs, the PVN contains MCNs as well as many other morphologically and functionally distinct cell types. Parvocellular neurosecretory neurons make axonal contact with the median eminence and release hypophysiotropic hormones that regulate functions of the anterior pituitary and the major hypothalamo-pituitary axes. Parvocellular preautonomic neurons modulate sympathetic and parasympathetic outflow to several organs, including the heart, the peripheral vasculature and the kidneys (31, 223, 262), through long descending projections into sympathetic and parasympathetic centers in the brainstem and spinal cord. Some neurons within the PVN also project into other limbic areas, including the central amygdala, and have recently been shown to modulate fear conditioning (103).
Because of these features, the PVN is a useful system for studying communication within and between different neuronal populations in the brain (218, 220), and particularly the role of neuropeptides in this process.
The nigrostriatal and mesolimbic dopamine systems
Another transmitter system that relies on somatodendritic release is dopamine, which is released from midbrain dopamine neurons. Dopamine neurons of the substantia nigra pars compacta (SNc) give rise to the nigrostriatal dopamine pathway, which is essential for motor learning and motor control. Indeed, loss of dopamine in this system impairs neuronal output from the basal ganglia (76), leading to the motor impairments that characterize Parkinson’s disease (1, 24, 134, 251). In addition, dopamine from this pathway, and from the ventral tegmental area (VTA), also in midbrain, influences a number of other brain functions including reward, emotion, cognition and memory (25, 181, 193).
Dopamine neurons of the SNc and VTA send axon projections that densely innervate the striatal complex in the forebrain (139); the nigrostriatal dopamine pathway projects from the SNc preferentially to the dorsal striatum (caudate-putamen, CPu), whereas the mesolimbic dopamine pathway projects from the VTA preferentially to the ventral striatum (nucleus accumbens, NAc). In addition, VTA dopamine neurons project via the mesocortical pathway to the prefrontal cortex, hippocampus, and amygdala (80, 246).
Like that of all catecholamines, the synthesis of dopamine originates from the amino acid precursor L-tyrosine, which is transported across the blood brain barrier into dopamine neurons. Tyrosine is converted to L-dihydroxyphenylalanine (L-DOPA) by the rate-limiting enzyme tyrosine hydroxylase (TH) and then to dopamine by L-aromatic amino acid decarboxylase (Fig. 1B). Notably, unlike many transmitters/neuromodulators that are synthesized in the cell body and transported to distant release sites in axons, TH protein expression in dopamine neurons can be seen throughout the soma, dendrites, and axons (254). Moreover, regulation of TH activity by phosphorylation occurs in both somatodendritic compartments and terminal fields, indicating that dopamine is synthesized locally for either somatodendritic or axonal release (209).
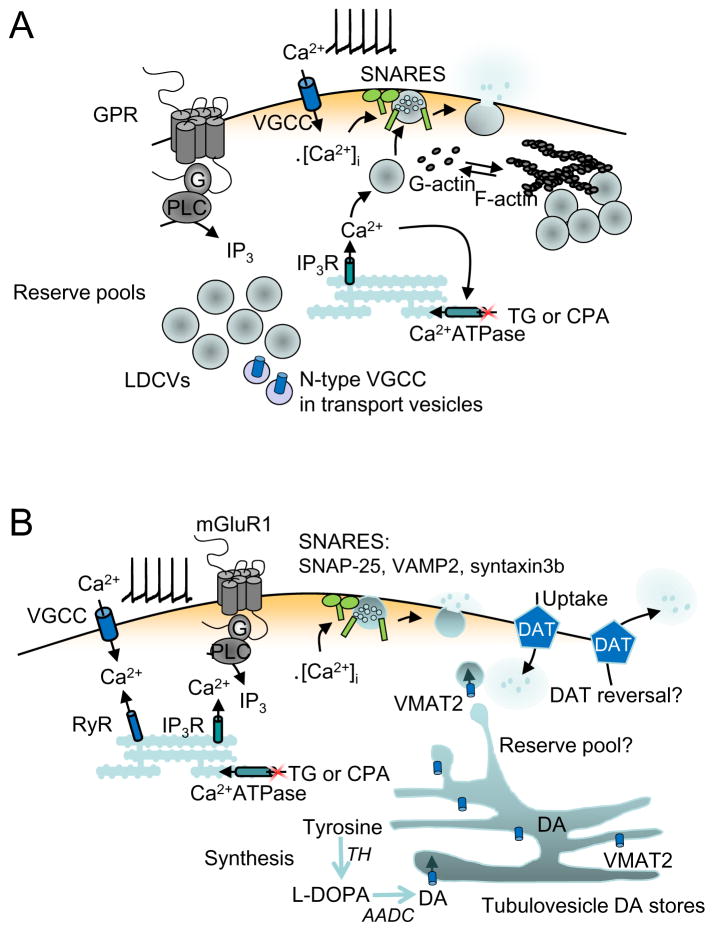
Figure 1. Comparison of the mechanisms of somatodendritic release of oxytocin and vasopressin in the hypothalamus (A) and dopamine in the substantia nigra (B).
A) Neuropeptides are synthesized and packaged in the soma and stored in dendrites in a reserve pool (RP) containing large numbers of LDCVs in dendrites. Depolarization-induced Ca2+ entry through voltage-gated calcium channels (VGCCs) stimulates peptide release by exocytosis of LDCVs. This requires the depolymerization of F-actin to G-actin. Furthermore, the stimulation of G-protein coupled receptors, such as the oxytocin receptor, stimulates the mobilization of Ca2+ from IP3-dependent intracellular stores and an increase in both the number of LDCVs and N-type VGCCs at the plasma membrane, thus priming the exocytosis machinery for subsequent activity-dependent release. Although some members of the SNARE family are detectable by immunocytochemistry, there appears to be a lack of VAMP, SNAP-25 and synaptotagmin-1 in the somata-dendrites, with their function presumably being replaced by other SNARE proteins. TG, thapsigargin; CPA, cyclopiazonic acid. (B). Features of somatodendritic dopamine release. Dopamine is synthesized in the intracellular compartment from tyrosine via TH. This process generates L-DOPA which is converted to dopamine by aromatic amino acid decarboxylase (AADC). Synthesized dopamine is stored in tubulovesicular structures that are part of the ER; these structures are the primary site of VMAT2, the vesicular monoamine transporter expressed in dopamine soma and proximal dendrites. Dopamine dendrites contain few vesicles, but those present appear to bud from tubulovesicles. Somatodendritic dopamine release is action potential dependent. Release also requires Ca2+ entry via VGCCs, but is amplified by both ryanodine receptors RyRs and metabotropic glutamate receptor (mGluR)-dependent activation of IP3Rs that release Ca2+ from intracellular ER stores. Immunohistochemical evidence suggests that a novel constellation of SNARE proteins may be involved in the release, including SNAP-25, VAMP2, and syntaxin3b. Release from dendrites has also been suggested to involve reversal of the dopamine transporter (DAT). Released dopamine is taken up and recycled via the DAT.
The release of dopamine from axonal sites is fairly well characterized. However, dopamine release from the somata and dendrites of midbrain dopamine neurons in the SNc and VTA remains incompletely understood, despite extensive research over decades (9, 10, 17, 26, 29, 32, 40, 69, 74, 169, 184, 196, 198). Because the somata and dendrites are intermingled in these regions, their individual contributions to dopamine release cannot be distinguished easily, so the term “somatodendritic” is used to describe non-axonal evoked dopamine release in SNc and VTA. Mechanistic studies of somatodendritic dopamine release have been conducted primarily in the SNc, in which dopamine release is exclusively somatodendritic (95, 249). In contrast, the VTA has collaterals from its own axons, as well as from those that arise in the SNc (7, 56).
Experimental methods used to study somatodendritic release
Oxytocin and vasopressin
Ideally, experimental methods to study somatodendritic release of oxytocin and vasopressin should have sufficient resolution to define the location and time course of release. Although the number of techniques that meet these criteria is limited, the use of hypothalamic explants containing the SON, sometimes with the pituitary gland attached (217), has provided insights into the regulation of somatodendritic release by steroids, changes in intracellular Ca2+ concentrations, the activation of autoreceptors, and second messenger pathways (33, 105, 121, 129, 204, 206, 252).
The most widely used approach is to monitor changes in neuropeptide concentrations in the plasma, through the use of sensitive chemical assay techniques such as radioimmunoassay (RIA) and enzyme-linked immunosorbent assay. RIA, in particular, offers the high sensitivity required for the quantification of neuropeptides collected in vivo using push-pull perfusion (110, 150, 160) and microdialysis (98, 256). With these methods, samples can be collected from the extracellular space of defined brain structures in unrestrained animals, with timescales of minutes to days (62, 110, 255). Other in vivo sampling techniques have also been employed, including the simultaneous detection of hormones secreted into the blood, using specialized microdialysis probes (166) or chronically implanted jugular venous catheters (257). In anesthetized animals, simultaneous electrophysiological recording has been combined with microdialysis (126).
Other approaches for measuring neuropeptides in microdialysates include immunosensing with microdialysis probes containing antibody-based electrodes and capillary liquid chromatography combined with electrospray ionization-mass spectrometry (37, 131). Although these techniques have comparable sensitivity to that of RIA they have not yet been widely applied.
Dopamine
The first studies of somatodendritic dopamine release involved either the measurement of 3H-dopamine overflow from in vitro midbrain slices (74) or in vivo measurements in midbrain using push-pull perfusion (32, 169). Subsequently, microdialysis coupled with electrochemical detection was used for in vivo studies of dopamine release (11, 13, 61, 83, 97, 199, 211). The use of microdialysis offered the advantage of a separation step, enabling dopamine, its metabolites, and in some cases other neurotransmitters to be assayed simultaneously. A problem with this or any in vivo method, however, is that systemically or locally applied drugs may affect regulatory processes, which could be mediated by extended pathways; because of this, in vitro cell culture preparations and midbrain slices have been used in most recent mechanistic studies of somatodendritic dopamine release, with dopamine overflow in culture typically measured by high-performance liquid chromatography (HLPC) or RIA (70, 143).
In slices, the approaches primarily used to detect somatodendritic dopamine release are fast-scan cyclic voltammetry (FCV) and amperometry with carbon-fiber microelectrodes. These methods permit the quantification of changes in extracellular dopamine concentration ([DA]o) with high temporal and spatial resolution, on scales of milliseconds and micrometers, respectively (146, 183). This is crucial for the rapid detection of release evoked in small discrete regions of the brain, including the SNc and VTA ((29, 40, 69, 184, 196, 198). In these methods, the target molecule (dopamine) is oxidized at the surface of the carbon-fiber microelectrode with a suitable applied potential, and the resulting current, which is proportional to the concentration of oxidized molecules, is recorded. With FCV, the applied potential is ramped up and down, enabling identification of released dopamine from its characteristic voltammogram (26, 196). The release of dopamine can also be verified by the effects of pharmacological agents; for example the response is increased by inhibitors of the plasma membrane dopamine transporter (DAT) (29, 42) and decreased by inhibitors of the vesicular monoamine transporter (VMAT2) (198).
In amperometry, a potential is applied at a constant value that is sufficient to oxidize dopamine. This method has been used to examine quantal release of dopamine from neuronal somata (91, 101). One drawback of voltammetric and amperometric methods is the possibility of signal contamination by contributions from other endogenous electroactive substances. For example, in FCV studies of somatodendritic dopamine release in the substantia nigra of some rodent species, including rats and mice, the voltammetric signal is heavily contaminated by locally released serotonin (5-hydroxytryptamine, 5-HT). Given that microdialysis studies include a chemical separation step, that technique avoids this lack of specificity. Interestingly, this is not a concern when detecting dopamine release in the VTA because the VTA of mice, rats, and guinea pigs lack significant 5-HT innervation and thus the voltammetric signal arises exclusively from dopamine (26, 38, 42, 93).
Most recently, Williams and colleagues applied electrophysiological techniques to detect somatodendritic dopamine release. By using whole-cell voltage-clamp recording from midbrain dopamine neurons, it is possible to record D2 dopamine autoreceptor-dependent inhibitory currents (D2ICs) to monitor evoked dopamine release in the SNc and VTA (9, 10, 38, 68, 69).
Vasopressin and oxytocin are released by exocytosis
Vasopressin and oxytocin are stored in and released from large dense-core vesicles (LDCVs). Classical morphological evidence for somatodendritic release was provided by electron-microscopic studies on hypothalamic neurons, which showed LDCVs within the dendrites and somata of MCNs, together with omega-shaped fusion profiles at the plasma membrane (189). Exocytosis from the dendrites of oxytocin and vasopressin neurons was also demonstrated by treatment of hypothalamic tissue with tannic acid to fix exocytosed peptide granules (155, 157, 189). Since peptide release from MCNs is not restricted to any particular region of the plasma membrane (157, 189), regulation of exocytosis may occur simply by controlling the access of vesicles to the sites of fusion (151). In classical neuroendocrine cells, control of this type is exerted by cytoskeletal elements, and such control may also occur in MCNs, as their cell bodies contain an actin network proximal to the plasma membrane, usually referred to as cortical F-actin. In neuroendocrine cells, this network surrounds the secretory vesicles.
The actin network undergoes rapid, transient and reversible depolymerization during exocytosis, and F-actin is depleted close to fusion zones. The cortical F-actin network has long been proposed to restrict the movement of secretory vesicles to fusion zones on the plasma membrane (57, 245). The subcortical regions of somata and dendrites in MCNs contain polymerized F-actin (238, 248), which is rapidly and reversibly depolymerized to G-actin when secretion is stimulated, and drugs that depolymerize F-actin stimulate dendritic peptide release. Thus, evoked release of peptides from the dendrites requires the depolymerization of F-actin (Fig. 1A) (238).
However, although cortical F-actin has historically been viewed as a barrier that restricts the movement of LDCVs to the plasma membrane, it might also have an enabling role in exocytosis, either by providing “tracks” for LDCV movement to fusion zones, or by constraining some components of the fusion machinery. This would suggest that during secretion the F-actin network is not simply disassembled, but reorganized to allow the access of LDCVs to fusion sites and to provide or assemble the molecular machinery necessary for membrane fusion and exocytosis (57). In MCNs, F-actin remodeling appears to be involved in the trafficking of functionally mature, release-competent vesicles to fusion sites, and it may therefore be critical in the differential control of release from different parts of the cell. However, in contrast to release from neuronal synapses, vesicle fusion in both the somata/dendrites and axon terminals in MCNs does not appear to occur at morphologically distinct fusion zones (157). In summary, actin filaments may have several roles in exocytosis, including functions in LDCV trafficking and tethering, acting as a barrier to fusion, and transporting membrane fusion machinery (238).
Exocytosis of vesicles is a multi-step process, involving a network of interacting proteins at various locations (Fig. 1A), including on the LDCVs themselves and at the active zones of the plasma membrane, where membrane fusion occurs (229). Exocytosis of both LDCVs and synaptic vesicles involves the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) complex, which opens the fusion pore and catalyzes the fusion of the vesicle membrane with the plasma membrane, resulting in the release of its cargo into the extracellular space. There is also evidence for the involvement of SNARE proteins in dendritic release, much of the data coming from work on dopamine cells in the substantia nigra (12, 180, 254) (see later). Studies of other brain regions, including the hippocampus (132, 133), olfactory bulb (140), cerebellum (59) and neocortex (266) also indicate the requirement for SNARE variants in dendritic transmitter release.
Clostridial neurotoxins, after binding to peripheral neurons and undergoing reverse axonal transport, are the precursors of zinc proteinases that specifically cleave components of the SNARE complex. Tetanus toxin (TeTX) cleaves VAMP-2 (synaptobrevin 2, an intrinsic protein component of LDCV membranes and part of the SNARE complex). Sensitivity of somatodendritic release to TeTX has been described in isolated MCNs (53), implying involvement of VAMP-2 in dendritic release of oxytocin and vasopressin, as well as in transmitter release at synapses. Although many SNARE proteins have already been identified in the terminals of the posterior pituitary (96, 263), immunofluorescence studies have failed to detect core proteins, including VAMP-2 and SNAP-25, in the somata and dendrites of the SON. Somatodendritic peptide release from MCNs thus appears to occur without all of the machinery that is needed for regulated exocytosis in other cell types (235), but it is probable that the functions of the missing protein components are fulfilled by other variants.
Does somatodendritic dopamine release occur by exocytosis?
Studies of the subcellular anatomical characteristics of dopamine neurons have raised questions about the mechanism and regulation of somatodendritic dopamine release.
First, dopamine axons in the striatum contain abundant clusters of vesicles near the plasma membrane at presumed release sites (171, 185). Although early anatomical studies also observed vesicle clusters in dopamine somata and dendrites within the SNc (253), subsequent studies, including those using immunogold labeling of VMAT2 profiles, concluded that SNc dopamine somata generally lack such clusters (170), as do dopamine dendrites that extend into the substantia nigra pars reticulata (SNr) (79, 249, 253). Nonetheless, evidence for exocytosis is provided by reports of quantal dopamine release from somata in the SNc, the estimated quantal size being 14,000 molecules per vesicle (91), which is of similar magnitude to that seen in adrenal chromaffin granules (117) and axonal varicosities of dopamine neurons in culture (188, 219).
Second, although some dopamine is stored in small electron-lucent vesicles (ELVs) and in LDCVs (170), somatic dopamine in both SNc and VTA appears to be mainly stored in “tubulovesicles,” saccules of smooth endoplasmic reticulum (SER) that express VMAT2 (Fig. 1B) (170, 253). In addition, VMAT2 can be visualized on the outer membranes of organelles that may be involved in recycling vesicular membrane proteins (170). Whether dopamine is released directly from tubulovesicles or whether they can provide a rapid on-demand source of vesicles for release is not currently known. Inhibitors of VMAT2 prevent dopamine release, confirming the importance of dopamine uptake and storage, but not necessarily the involvement of exocytosis per se. Third, although dendro-dendritic dopamine synapses are present in both SNc and VTA, they are also rare (79, 170, 249, 253), and are virtually absent from the dopamine-dendrite rich SNr (79).
Studies using botulinum toxins to target specific SNARE proteins suggest that somatodendritic dopamine release occurs primarily by exocytosis (13, 70, 180). However, immunohistochemical studies indicate that TH-positive somata and dendrites in the substantia nigra lack some typical intrinsic vesicle membrane proteins, including synaptophysin, the sv2a and sv2b isoforms of synaptic vesicle protein 2, and the Ca2+-sensors synaptotagmin 1 and 2 (254). These findings are consistent with the relative absence of conventional synaptic vesicles in dopamine neurons. Moreover, several of the conventional SNARE proteins involved in vesicle docking, including the vesicle membrane protein VAMP-1 (synaptobrevin 1) and the plasma membrane protein syntaxin 1a, are also absent. On the other hand, SNAP-25 and some non-conventional SNARE protein isoforms, including VAMP-2 and the plasma membrane protein syntaxin 3b, are expressed throughout dopamine somata (143, 254).
Overall, the molecular organization involved in dopamine release in the SNc appears to differ markedly from that of conventional synaptic vesicular release, with the SNARE triad having the unusual composition of VAMP-2, SNAP25 and syntaxin 3b (Fig. 1B). Importantly, although dopamine somata and dendrites in the SNc lack synaptotagmin 1 and 2 (143, 254), which are low-affinity isoforms of the vesicular Ca2+-sensors found at fast synapses (259), they do possess two isoforms with higher Ca2+-affinity, namely synaptotagmin 4 and 7 (143), which may play a role in the high Ca2+ sensitivity of somatodendritic release (28). It should be noted, however, that the dopamine neurons express mRNA for synaptotagmin 1, presumably because synaptotagmin 1 is involved in axonal dopamine release in the striatum. Indeed, consistent with the notion that synaptotagmin 4 and 7, but not synaptotagmin 1, are involved in somatodendritic dopamine release, down-regulation with siRNA of synaptotagmin 4 and 7, but not of synaptotagmin 1, decreases dopamine release from cultured midbrain neurons (143).
Despite the presence of SNARE complex components (syntaxin 3b, SNAP-25 and VAMP2) throughout dopamine somata and dendrites, levels of VMAT2 and V-ATPase, the vacuolar-type H+-translocating ATPase that generates the transmembrane electrochemical proton potential gradient required for active amine uptake through VMAT2, decrease from somata to distal dendrites of dopamine neurons (254). This suggests that release and storage mechanisms for dopamine may differ between cell bodies and proximal dendrites in the SNc and those in distal dendrites in the SNr. As discussed further later, the regulation of dopamine release by intracellular Ca2+ stores may also differ between these locations: dopamine dendrites in the SNr have lower levels of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), which translocates cytosolic Ca2+ into the ER, and also lower levels of regulatory inositol 1,4,5-triphosphate receptors (IP3Rs) and ryanodine receptors (RyRs) in the ER (184).
These data, together with the limited number of dendro-dendritic synapses and the small population of “classical” LDCVs (79, 145, 170, 249, 253), suggest that some somatodendritic dopamine release might occur by mechanisms other than exocytosis, including release from the cytoplasmic pool by reverse transport through the plasma membrane DAT (60, 63, 79, 170, 177). Cytoplasmic dopamine concentrations are normally too low for DAT reversal, unless enhanced by displacement of dopamine from vesicles by pharmacological agents such as amphetamine (118, 230). However, dopamine storage in tubulovesicles introduces the possibility that dopamine leakage from these organelles might raise the cytoplasmic dopamine concentration sufficiently for DAT reversal and release into the extracellular space (Fig. 1B). Importantly, given that evoked increases in [DA]o are usually enhanced, rather than abolished, by DAT inhibitors (10, 29, 40), this cannot be the primary release mechanism in the SNc. Furthermore, release by direct transport through the plasma membrane cannot account for the observed quantal transmitter release in the SNc recorded by amperometry (91). In contrast, release from distal dendrites in the SNr is abolished by inhibition of DAT when stimulated by glutamate released from subthalamic nucleus afferents (63), and has been proposed to occur by activation of metabotropic glutamate receptors (mGluR1), with subsequent PKC-induced reversal of the DAT (63, 177).
Ca2+ dependence of somatodendritic release
Somatodendritic oxytocin and vasopressin release
As described above, release of neurotransmitters from presynaptic terminals and of neuropeptides from neuroendocrine cells occurs by the ubiquitous process of Ca2+-dependent exocytosis. Like the release of oxytocin and vasopressin from axonal terminals in the neurohypophysis (65), dendritic release of these neuropeptides depends on a local increase in intracellular free Ca2+ concentration ([Ca2+]i) (53, 167, 215).
In classical synapses the patterns of neurotransmitter release depend critically on the spatio-dynamics of the [Ca2+]i transients (142), which are themselves determined by the origin of Ca2+ and its proximity to the release machinery, as well as by the various intracellular Ca2+ buffering mechanisms that control the amplitude and duration of [Ca2+]i transients. The Ca2+ that triggers the dendritic release of oxytocin and vasopressin from MCNs can originate from a number of extracellular and intracellular sources (Fig. 1A).
Ca2+ channels
Voltage-gated Ca2+ channels (VGCCs) (65, 247) are responsible for the entry of much of the extracellular Ca2+ that triggers dendritic neuropeptide release. MCNs express several types of VGCCs (67). Despite the fact that the Ca2+ current carried by N-type channels is small compared to those of the other types of VGCCs or to the whole-cell Ca2+ current in the somata of MCNs (94, 236), N-type channels appear to be particularly important for dendritic release, as release of oxytocin from SONs is most sensitive to blockade of N-type channels. Additionally, N-type channels in both somatodendritic and axonal compartments can be opened in response to the depolarization evoked by action potentials (65). However, some signaling molecules, including oxytocin and vasopressin, can trigger dendritic peptide release without increasing the electrical activity of the neurons, via their receptors on oxytocin and vasopressin neurons (71). These peptides act at their respective receptors to produce a cell-type specific rise in [Ca2+]i that can promote transmitter release. Vasopressin-dependent vasopressin secretion depends on Ca2+ influx through VGCCs, particularly of the L-, N- and T-types (205). Similarly, Ca2+ entry, mainly through L- and N-type channels, is also the trigger for somatodendritic release of other transmitters, including dynorphin (216), dopamine (101, 143), serotonin (241) and pituitary adenylate cyclase activating polypeptide (PACAP) (215).
NMDA receptors
Extracellular Ca2+ can also enter neurons through Ca2+-permeable ionotropic glutamate receptors, including N-methyl-D-aspartate receptors (NMDARs). In MCNs NMDARs influence overall MCN excitability and are also involved in the control of burst-firing of these cells, which optimizes hormonal release from neurohypophysial terminals (66, 86, 162, 173). Opening of NMDARs results in Ca2+ entry and in large increases in dendritic [Ca2+]i in MCNs (218, 222), with consequent dendritic release of both oxytocin (51) and vasopressin (218). Consistent with this, functional NMDARs with unique molecular and functional properties have been found at extrasynaptic, as well postsynaptic sites (113, 207) in MCNs. Extrasynaptic NMDARs are also coupled to other Ca2+-dependent signaling mechanisms, including voltage-gated K+ channels and ionotropic gamma-aminobutyric acid (GABAA) receptors (162, 186, 187), unlike synaptic NMDARs. However it is not yet known whether dendritic release of neuropeptides is differentially regulated by synaptic versus extrasynaptic NMDARs.
Intracellular Ca2+ stores
As well as being triggered by the entry of extracellular Ca2+, dendritic neuropeptide release can be evoked by Ca2+ release from intracellular stores (Fig. 1A). An example of this is the autocrine release of oxytocin, in which binding of oxytocin to its receptors on oxytocin neurons causes Ca2+ to be released from its major intracellular store in the endoplasmic reticulum (ER) (107). The increase in [Ca2+]i triggers the release of oxytocin from dendrites, without inducing release from nerve terminals or affecting the firing of neurons (129). Through this autocrine mechanism, dendritic peptide release, once triggered, can become self-sustaining and thus long-lasting (129). Other agents that mobilize intracellular Ca2+ can also evoke dendritic release of neuropeptides. One example is thapsigargin, an inhibitor of SERCA, which can inhibit uptake of Ca2+ into the ER, raising cytoplasmic [Ca2+] and thereby triggering Ca2+-induced Ca2+ release from the ER, through (RyRs) (121, 129, 237).
Ca2+ buffering mechanisms
Intracellular Ca2+-buffering mechanisms influence the amplitude and duration of cytoplasmic Ca2+ transients. Various mechanisms of Ca2+ buffering and clearance mechanisms operate in MCNs, including Ca2+-translocating ATPases both in the ER and in plasma membranes, the mitochondrial Ca2+-uniporter (221) and Ca2+-binding proteins such as calbindin and calretinin (50, 148). The operation of these buffering and transport systems dampens [Ca2+]i transients in MCNs (50, 105, 214, 222), whereas their blockade prolongs the transient rise in [Ca2+]i that accompanies depolarization by K+, and thereby enhances somatodendritic vasopressin release (105). Interestingly, the repertoire of Ca2+-homeostatic systems differs between the somatodendritic and axonal compartments of MCNs (50, 105), providing further evidence to support independent regulation of neuropeptide release by these two compartments.
Ca2+-dependent priming of dendritic release
As well as triggering the final membrane-fusion step in exocytosis, elevation of intracellular free [Ca2+] also primes vesicular stores of peptides within the dendrites, rendering them “release-ready” and available for subsequent Ca2+-dependent fusion (Fig. 1A) (129). Spike activity in oxytocin or vasopressin neurons in vivo is not itself sufficient to trigger dendritic peptide release unless the stores have been primed, but agents that deplete intracellular Ca2+-stores, such as thapsigargin or cyclopiazonic acid (SERCA inhibitors), or some peptides, including oxytocin itself and alpha melanocyte-stimulating hormone (α-MSH), consistently induce dendritic release directly (129, 204). It is possible that any signal that mobilizes Ca2+ from intracellular stores might prime dendritic secretion. Moreover, exposure to agents that mobilize Ca2+ from intracellular stores greatly stimulates the peptide release evoked by many stimuli such as electrical or osmotic stimulation and K+-induced depolarization. In vitro, such priming persists for at least 90 min. During priming, neuropeptide-storing vesicles become competent to respond to the fusion trigger that will arrive at some point in the future, in other words, priming increases the size of the secretory pool available for rapid release in response to a future trigger of the target cell. One mechanism by which vesicles in MCNs leave the reserve pool and enter the release-ready pool (237) may be the remodeling of actin. Priming also involves the recruitment of VGCCs to the plasma membrane, suggesting that stimuli that increase secretory responsiveness on a relatively slow time scale (30–90 min) may act by stimulating the recruitment of N-type Ca2+ channels to release sites, where they potentiate the secretory response subsequent depolarizations (236). This priming of exocytosis appears not to require either gene transcription or de novo protein synthesis (234).
Somatodendritic dopamine release
In the very first report of somatodendritic dopamine release, Geffen and colleagues proposed that, in a manner similar to axonal dopamine release, dopamine release in the SNc occurs by exocytosis of storage vesicles (74). However, details of the precise release mechanism remain incomplete. Although the somata and dendrites of SNc dopamine neurons lack conventional synaptic structures, dopamine release from the somatodendritic compartment occurs by Ca2+-dependent exocytosis. Moreover, somatodendritic dopamine release requires Na+-dependent action potentials (29, 210), and is prevented by inhibitors of VMAT2 (10, 83, 198). However as mentioned above, the effect of VMAT2 inhibitors alone does not confirm that dopamine release occurs from conventional storage vesicles, as VMAT2 is also expressed by other subcellular organelles in dopamine neurons (170).
Ca2+ entry
The requirement for extracellular Ca2+ in somatodendritic dopamine release has become a matter of debate. Although somatodendritic release is prevented by the removal of extracellular Ca2+ (in Ca2+-free media containing the Ca2+-chelator EGTA) or by blocking Ca2+-channels in the plasma membrane with Cd2+ (28, 184, 196, 198), release can still occur at low (submillimolar) extracellular Ca2+ concentrations ([Ca2+]o) that do not trigger detectable axonal dopamine release, at least in guinea-pig SNc. Indeed, in studies using FCV to detect single-pulse evoked increases in [DA]o in guinea pig midbrain and striatal slices (28), the [Ca2+]o required for half-maximal release (EC50) is markedly lower in the SNc (0.3 mM) than in the CPu (2.3 mM); similar differences were observed in the VTA versus NAc shell. It should be noted that although these studies were done in the presence of glutamate and GABA receptor antagonists, it is possible that EC50 values for the Ca2+ dependence for striatal dopamine release could be influenced by powerful regulation through ACh acting at nAChRs. However, when nAChRs are blocked the EC50 for calcium dependence in the CPu is remarkably similar (1.9 mM) (18). Furthermore, unlike in the striatum where axonal dopamine release is abolished by a cocktail of VGCC blockers, somatodendritic dopamine release in the SNc persists in the presence of these blockers (11, 14, 27, 29, 60, 70, 85). The resistance of release to VGCC blockers presumably reflects the incomplete blockade of these channels, along with the minimal cytoplasmic Ca2+ concentration required to trigger exocytosis via the high Ca2+-sensitivity synaptotagmin isoforms involved in fusion. Somatodendritic dopamine release is therefore only weakly dependent on [Ca2+]o and Ca2+ entry. Interestingly, the dependence on [Ca2+]o for somatodendritic dopamine release has been reported to be stronger in rat and mouse than in guinea pig (38, 69).
Which VGCCs are required for Ca2+ entry in somatodendritic dopamine release? N- and P/Q-type, but not L-type, channels appear to be involved in basal somatodendritic dopamine release measured by RIA in mesencephalic cultures (143). L- and T-type channels, but not N- or P/Q-type channels, are involved in K+-evoked dopamine release in the same preparation (60), as well as in K+-induced dopamine release detected by amperometry in dissociated dopamine cells (101). Therefore, the VGCC types involved in providing Ca2+ entry to trigger somatodendritic dopamine release appear to depend on the experimental conditions, including the species studied, the type of preparation and the stimulation procedure employed. These factors all contribute to the complexity of elucidating the precise mechanisms involved in somatodendritic dopamine release and its regulation.
Intracellular Ca2+ stores
The ability to detect evoked somatodendritic dopamine release with nominally zero [Ca2+]o raises the possibility that there may be mechanisms by which small increases in [Ca2+]i are amplified. An obvious mechanism is Ca2+-induced Ca2+ release from intracellular ER stores (129, 184, 242), as discussed earlier for oxytocin and vasopressin. Within neurons, the ER forms a large network extending from the soma to dendrites and dendritic spines, and to axons and presynaptic release sites (244). In SNc dopamine neurons, this system propagates Ca2+ release from somatic ER stores to dendrites (34).
Immunohistochemical studies have identified ER membrane proteins associated with Ca2+ mobilization from ER stores in SNc somata and proximal dendrites, including SERCA-2 and inositol tris-phosphate receptors (IP3R) and RyRs (184), which are ligand-gated Ca2+-channels. Each of these facilitate somatodendritic dopamine release evoked in the SNc by local pulse-train stimulation and detected by FCV (184). In dopamine neurons, RyRs assemble in clusters that are closely apposed to the plasma membrane, a location that maximizes their activation with the entry of extracellular Ca2+ through VGCCs (Fig. 1B). This enables somatodendritic dopamine release to be amplified at physiological [Ca2+]o. This amplification, however, is not necessary when Ca2+ entry is sufficiently large, as occurs with higher [Ca2+]o. In contrast, facilitation of SNc dopamine release by IP3Rs does not necessarily require Ca2+ entry through VGCCs but can occur downstream from metabotropic receptors, including mGluR1 (Fig. 1B) (184).
The role of intracellular Ca2+ stores in amplifying somatodendritic dopamine release is complex, and also can vary with the experimental conditions and rodent species used (38, 69, 143). Moreover, whether intracellular Ca2+ stores contribute to release in the VTA is not yet known. Exocytosis involves several Ca2+-dependent steps with proteins that exhibit different Ca2+ sensitivities. The final fusion event may require a very rapid elevation in [Ca2+]i close to the secretory vesicle to trigger release, whereas a slower, less localized increase in [Ca2+]i could enhance priming of secretory vesicles, as seen in the release of oxytocin from the dendrites of hypothalamic neurons (129), discussed earlier. Although most evidence suggests that dopamine release in the SN or the VTA is not primed by the SERCA inhibitor thapsigargin, the possible involvement of other intracellular Ca2+ stores remains unknown (12). Whether the differential expression of RyR at sites close to the plasma membrane and the cytoplasmic location of IP3R reflect these different functions also remains unresolved.
Is somatodendritic release triggered by action potentials?
Action potentials are usually initiated at the initial segment, just beyond the junction between cell body and axon (axon hillock). In the classical model of synaptic neurotransmitter release, the action potential is propagated down the axon to its terminal where it opens VGCCs, resulting in the fusion of synaptic vesicles with the pre-synaptic plasma membrane. However, an action potential can travel in any direction from its point of initiation, and into dendrites if the electrical properties of the dendrite support this, as is the case in many neurons (39, 227).
Exocytotic release of vasopressin and oxytocin from axonal terminals in the posterior pituitary gland is linked to electrical activity in the somata and is produced by the opening of VGCCs following depolarization of the terminals by invading action potentials (65). At classical fast, glutamatergic synapses, the available stores of small ELVs are maintained by endocytotic membrane recycling and are quickly reacidified by the V-type H+-ATPase and refilled with neurotransmitter by secondary active transport mediated by H+-linked antiporters (190). However, unlike small neurotransmitters, neuropeptides are not taken up and repackaged after release – they must be synthesized in the rough ER and concentrated in LDCVs in the soma. Compared to ELVs, LDCVs require a more sustained increase in [Ca2+]i to trigger exocytosis. Consequently, LDCVs have longer latencies to release and require stronger stimulation for exocytosis, for example bursts of electrical activity. LDCVs also differ from ELVs in that the associated variants of synaptotagmin, the Ca2+-sensor that triggers release, have a higher affinity for Ca2+, as discussed above for dopamine. Consequently it is not necessary for LDCVs to be located close to membrane Ca2+ channels to receive a Ca2+ pulse sufficient to produce exocytosis, and synaptic specializations are not necessary (2, 6, 135, 136, 208).
In many neurons, the properties of the dendritic membranes support the propagation of action potentials (227). However, dendritic release of vasopressin and oxytocin in MCNs can occur independently of action potentials (121, 129), even though action potentials can propagate into the dendrites (5). Accordingly, neuropeptide release from dendrites is not linked to release from terminals within the same neurons, and this uncoupling seems to be both stimulus-dependent and peptide-specific (127). An example of this is provided by the effects of alpha melanocyte-stimulating hormone (α-MSH): binding of α-MSH to melanocortin 4 receptors on oxytocin cells releases Ca2+ from intracellular stores, stimulating dendritic oxytocin release, but inhibits the electrical activity of the cell and so inhibits oxytocin release into the periphery (204). Dissociation of dendritic and axonal release patterns is also seen in the effects of increased plasma osmolality. Systemic hypertonic saline injection immediately increases vasopressin release from axon terminals, but dendritic release of vasopressin in the SON starts an hour later, when peripheral release is subsiding. In this case there is a separation in time between transmitter release from dendrites and terminals within the same neurons (124).
In SNc dopamine neurons, the axon initial segment arises from a proximal dendrite rather than from the cell body; action potentials originate in the dendritic tree and single action potentials back-propagate into dendrites (82). However, during burst-firing of these neurons, and particularly if dopamine D2 receptors are activated, back-propagation of action potentials may not occur (75), suggesting that dopamine can influence the spread of action potentials, thereby down-regulating its own release from dendrites. Thus, burst-patterned firing, which promotes dopamine release from axon terminals, does not necessarily promote dendritic release (78). Consequently, dopamine release from distal dendrites is likely to be semi-independent of the electrical activity of the neuron. As mentioned above, dopamine release from distal dendrites has been proposed to be triggered through local glutamatergic activation of mGluRs (177). Interestingly, serotonin neurons in the dorsal raphe nucleus also exhibit somatodendritic release. Action potentials in these neurons also do not back-propagate very far; however, local activation of NMDARs can lead to dendritic release in the absence of action potentials (52). This is yet another example of the dissociation of dendritic release of monoamines from action-potential-dependent axonal release.
Modulation of somatodendritic release by synaptic inputs
Vasopressin and oxytocin
Vasopressin (and in the rat, oxytocin) is involved in the control of plasma electrolyte balance. Systemic injection of hypertonic saline stimulates vasopressin and oxytocin release from axon terminals in the neural lobe and from dendrites within the SON. Vasopressin neurons respond directly to the osmotic pressure of their environment (137, 176), but systemic osmotic stimuli also activate central receptors on cells that project, directly or indirectly, to the SON and PVN, including afferent neural pathways from the rostral forebrain anterior third ventricle region (AV3V). The response to systemic osmotic stimulation is blocked by tetrodotoxin, which blocks voltage-gated Na+-channels, and by lesions of the AV3V, suggesting that somatodendritic release is part of a cascade of events initiated by osmotic activation of synaptic pathways, rather than by the direct effect of hyperosmolarity on the MCNs (122, 123).
The afferent pathways from the AV3V include an inhibitory GABA component (174) and excitatory components mediated by amino acids and several peptides, including angiotensin II (92). Intracerebroventricular (icv) administration of angiotensin increases both systemic and somatodendritic vasopressin release within the SON and PVN (152). While somatodendritic release is unaffected by retrodialysis of the GABA agonist muscimol into the SON, the GABAAR antagonist bicuculline increases somatodendritic release (109), suggesting tonic inhibition by endogenous GABA. Furthermore glutamate stimulates dose-dependent release of angiotensin II from isolated fragments of magnocellular dendrites (158) and also increases somatodendritic release when retrodialysed into the SON, whereas kynurenic acid, a glutamate antagonist, is inhibitory (109).
Somatodendritic release of vasopressin following acute osmotic stimulation is inhibited by salt loading but not by water deprivation, while the systemic response is unaffected (130), suggesting that somatodendritic release is regulated by afferent inputs from both osmo- and baro-receptors. The control of central peptide release by volume and pressor stimuli is also revealed by studies of hemorrhage and baroreceptor denervation; concentrations of vasopressin both in the plasma and in the PVN increase markedly in response to hemorrhage (179). Hemorrhage-induced decrease of arterial blood pressure stimulates vasopressin secretion through inhibition of baroreceptors and activation of chemoreceptors in the aortic arch and carotid body, while sinoaortic baroreceptor denervation increases the osmotically induced release of vasopressin and oxytocin from the posterior pituitary (159) and also increases somatodendritic vasopressin release stimulated by direct or peripheral hypertonic saline stimulation (22).
Systemic oxytocin release increases during parturition and lactation, in parallel with increased electrical activity involving the synchronous burst firing of oxytocin neurons. That oxytocin, but not vasopressin, is released within the SON and PVN in response to suckling has been demonstrated in push-pull perfusion studies in anaesthetized lactating rats (19) and microdialysis studies in conscious parturient and lactating animals (22). Somatodendritic suckling- or parturition-induced oxytocin release is inhibited by oxytocin antagonists infused into the SON, suggesting the receptor-mediated autoregulation of oxytocin release (163, 164).
The systemic secretion of oxytocin during suckling is also subject to noradrenergic regulation, arising mainly from the brainstem. Suckling increases both noradrenaline turnover and local oxytocin levels within the SON (47), and phentolamine, an α-adrenergic antagonist, blocks oxytocin release into the SON and PVN during suckling, indicating that suckling stimulates noradrenaline release, with subsequent stimulation of somatodendritic oxytocin release through the action of α-adrenergic receptors (8).
Systemic and somatodendritic peptide release are also controlled by other neurotransmitters released by nerve fibers terminating in the hypothalamic nuclei. GABA and glutamate have already been discussed. Acetylcholine is also involved in regulating systemic vasopressin release (217), and also stimulates somatodendritic vasopressin release in hypothalamic explants (excluding axon terminals) (138, 178). Both somatodendritic and systemic release of oxytocin appear to be regulated by noradrenaline (165), since systemic administration of cholecystokinin (CCK), which acts via the vagus nerve to excite the A2 noradrenergic brainstem projection to magnocellular oxytocin neurons (243), stimulates release. CCK may also directly regulate somatodendritic peptide release; intra-SON administration of CCK increases somatodendritic oxytocin and vasopressin levels (165), and also the expression of Fos in SON neurons (125).
PACAP receptor mRNA and PACAP-like immunoreactivity are present within the SON and PVN (161) and PACAP appears to participate in the regulation of the MCNs by opening VGCCs and increasing [Ca2+]i thereby stimulating the somatodendritic release of vasopressin in vitro (215).
Dendritic oxytocin release from MCNs is inhibited by endogenous opioids in ovariectomized (87) and late-pregnant rats, although this inhibition does not occur during parturition (58, 163). In addition, in morphine-dependent rats, the opioid antagonist naloxone, introduced into the SON either by systemic injection or by retrodialysis, increases somatodendritic oxytocin release (20, 203). This “morphine withdrawal excitation” also involves somatodendritic oxytocin, the release of which is increased under these conditions, while icv administration of an oxytocin antagonist reduces this increase (20).
During late pregnancy, sex steroids promote oxytocin synthesis and storage within the dendrites of the MCNs (156), and steroids have also been proposed to exert direct, rapid, non-genomic effects on neurons (232).
Dopamine
Synaptic input to midbrain dopamine neurons comes predominantly from glutamate and GABA, with GABAergic input dominating in the SNc and glutamatergic input dominating in the VTA (154, 197). Consequently, the net influence of excitatory versus inhibitory regulation of dopamine neurons differs between the SNc and VTA. In ex vivo midbrain slices, somatodendritic dopamine release evoked by a single stimulus pulse is unaltered by ionotropic glutamate or GABA receptor antagonists (27), suggesting the absence of tonic regulation by these transmitters in slices. However, during stimulation by multiple pulses, regulation by concurrently released glutamate and GABA is seen in both the SNc and VTA (30). In the SNc, dopamine release evoked by local pulse-train stimulation is inhibited by concurrently released glutamate acting on AMPA- and NMDA-receptors. This inhibition is prevented by GABA receptor antagonists (30), which is consistent with anatomical data showing AMPARs on inhibitory input to the SNc (182, 260). In contrast, the increase in dopamine release produced by NMDA-receptor antagonists during pulse-train stimulation is unaffected by a cocktail of GABA receptor antagonists, suggesting the involvement of another inhibitory mediator. One possible candidate is endogenously generated H2O2, which has been shown to inhibit dopamine release in the SNc (26) and may be generated downstream from NMDAR activation.
In the VTA, pulse-train evoked dopamine release is unaffected by blocking GABAA-, GABAB- or AMPA-receptors. By contrast, NMDA-receptor blockade suppresses evoked [DA]o, consistent with a glutamate-dependent facilitation of dopamine release (30). Importantly, however, blockers of either AMPA- or NMDA-receptors decrease evoked dopamine release when applied in the presence of GABA-receptor antagonists, thereby unmasking the conventional direct excitatory effect of glutamate input to VTA dopamine neurons.
Regulation by glutamate of somatodendritic dopamine release in the SNc also occurs through the metabotropic, G-protein coupled receptor mGluR1 (184). mGluR1α is highly expressed in dopamine neurons and, as described above, mGluR1 activation produces IP3R-mediated Ca2+ release from ER stores, which facilitates evoked dopamine release. However, with large increases in intracellular Ca2+, inhibition of dopamine neuron excitability and consequently dopamine release may occur from activation of Ca2+-activated K+ channels (64, 153). Therefore, the net effect of dopamine release regulation by mGluR1s will depend on exogenous mGluR1 agonist concentration or on stimulus intensity for endogenous glutamate release (48, 184). As already noted, activation of mGluR1s can also facilitate dendritic dopamine release in the SNr, possibly from elevated dopamine levels in the cytoplasm and subsequent DAT reversal (177).
Possible role of co-released glutamate and GABA
The increasing recognition that dopamine neurons co-release multiple transmitters including glutamate and GABA provides a novel concept in the idea of “autoreceptor” regulation. Initial studies using cultured dopamine neurons established that they can both synthesize and release glutamate (36, 55). In support of this, subsequent optogenetic methods using selective expression of channelrhodopsin (ChR2) in dopamine neurons have demonstrated that glutamate released from dopamine axons produces glutamate receptor-dependent excitatory post-synaptic currents in striatal neurons in slices (35, 106, 225, 228, 231, 240) and mediates behavioral effects in vivo (16). Similar approaches have been used to show co-release of GABA from dopamine axons; although these neurons synthesize GABA using a non-conventional enzyme, aldehyde dehydrogenase 1a1 (100), and they can also obtain GABA by uptake from the extracellular space (225, 240). Thus, if also co-released with dopamine from somata or dendrites, glutamate and/or GABA could autoregulate somatodendritic dopamine release. This possibility is supported by the presence of vesicular glutamate transporters (vGluT2) in VTA dopamine neurons (55, 84), and co-released GABA appears to be accumulated in secretory vesicles through VMAT2 (240), which is present in all midbrain dopamine cell bodies and proximal dendrites (170).
Actions of dendritically released oxytocin, vasopressin, and dopamine
Autocrine effects
Dendritically released neurotransmitters exhibit autocrine effects on the neurons from which they are released, and also affect surrounding neurons and glia. These effects can change both the inputs to cells and the cellular response to these inputs. A good example of this occurs in oxytocin cells, in which dendritically released oxytocin enhances the milk ejection reflex, as described later.
More commonly, somatodendritic release is auto-inhibitory and thus self-limiting. Vasopressin neurons discharge in a characteristic phasic pattern that maximizes stimulus-secretion coupling at the nerve terminals, but this activity is modified by the inhibitory autocrine effects of vasopressin released from dendrites. Like oxytocin, vasopressin can also facilitate its own dendritic release (258), which may explain the temporal separation of peripheral and central release of vasopressin following a hyperosmotic stimulus. Osmotic stimulation is followed immediately by systemic secretion of vasopressin, whereas dendritic release is delayed and the response prolonged (124). Increase of extraneuronal vasopressin concentrations by retrodialysis inhibits vasopressin neurons, reducing their firing rate (126). Thus, dendritic vasopressin release may stimulate vasopressin release from adjacent dendrites until the local external concentration reaches a threshold sufficient to hyperpolarize the neuron or else to modulate inhibitory inputs, thereby limiting the extent of systemic vasopressin secretion following osmotic stimuli or volume depletion.
Understanding of these autocrine effects is further complicated by the fact that within a single LDCV, several other peptides can be co-localized with either vasopressin or oxytocin. For example, the endogenous opioid dynorphin is co-localized with vasopressin in the same vesicles (250) and dendritic vasopressin release will therefore be accompanied by the release of dynorphin to provide feedback inhibition of vasopressin cell activity (21). In addition many other neuropeptides, such as galanin, apelin, PACAP and secretin, are synthesized in MCNs (54, 72, 77, 112). Accompanying receptor expression for these peptides on MCNs provides mechanisms for autocrine feedback regulation by these co-released neuropeptides (19).
Autocrine effects of somatodendritic dopamine release are central to the consequences of this process. Locally released dopamine binds to inhibitory D2 autoreceptors on dopamine neurons in the SNc and VTA, and thereby regulates the rate and pattern of firing of dopamine neurons (10, 73, 75, 191, 265), which ultimately influences the level and pattern of axonal dopamine release in CPu and NAc (211). Somatodendritic dopamine release in SNc and VTA is also controlled by local feedback via D2 autoreceptors (41).
Paracrine effects
Exogenously applied or endogenously released oxytocin also acts on afferent nerve endings. The SON does not contain presynaptic oxytocin receptors, suggesting that this paracrine action is indirect. One indirect mechanism involves oxytocin-dependent endocannabinoid release from oxytocin neurons (104, 175), mediated by the action of dendritically released oxytocin on oxytocin receptors, release of Ca2+ from intracellular stores and consequent “on-demand” synthesis of endocannabinoids. Endocannabinoids are arachidonate-based lipids that are hydrophobic enough to diffuse passively through plasma membranes; their binding to presynaptic cannabinoid receptors (CB1) inhibits both GABAergic and glutamatergic afferents onto MCNs. Indeed, CB1 receptors have been found using immunohistochemistry on both excitatory and inhibitory axon terminals that innervate dendrites in the SON. In SON slices, cannabinoid agonists presynaptically inhibit spontaneous excitatory and inhibitory postsynaptic currents.
The milk-ejection reflex during suckling provides an interesting example of local paracrine control by oxytocin. Oxytocin neurons are continuously active under basal conditions, but during parturition and in response to suckling in lactating animals, discharge brief, intense bursts of action potentials. These bursts release large boluses of oxytocin into the circulation, producing intense contractions of the pregnant uterus or milk ejection from the mammary glands. The bursts are blocked by administration of oxytocin antagonists into the SON, and facilitated by oxytocin agonists (108). Dendritic release of oxytocin is up-regulated during parturition and in lactation, and has an essential role in the generation of these intermittent synchronized bursts (201). Its effects are not restricted to the cell of origin, but are also exerted on the dendrites of other oxytocin cells, possibly to facilitate homotypic interactions
Dendritically-released vasopressin modulates the activity of neighboring presympathetic neurons within the PVN (218), providing another example of a long-distance, paracrine action of dendritically-released neuropeptides. Activity-dependent dendritic release of vasopressin from MCNs concomitantly increases the firing activity of neurons projecting from the PVN to the rostroventrolateral medulla. This interpopulation crosstalk involves extracellular diffusion of released vasopressin to V1a receptors in presympathetic neurons. Consequently, unlike conventional synaptic transmission, the efficiency and strength of this diffuse paracrine action of vasopressin depends on the extracellular vasopressin concentration, which in turn depends on the average activity of the entire vasopressin neuron population. It also depends on the half-life of vasopressin in the extracellular space, as well as on the ability of vasopressin to reach relatively distant targets by diffusion (e.g., the tortuosity of the extracellular space). These examples illustrate the importance of dendritic release of vasopressin in the ability of the PVN to control the activity of distinct populations of neurons, and thus, to produce a multimodal homeostatic response (218, 220).
Given the structural characteristics of midbrain SNc and VTA dopamine neurons, somatodendritic dopamine release is likely to be at least partly non-synaptic. Moreover, dopamine receptors and reuptake transporters on dopamine cell bodies and dendrites are largely extrasynaptic (171, 172, 213, 261), as are D1 receptors on non-dopaminergic terminals in these regions (23, 261). Thus, somatodendritically released dopamine must act through volume transmission via the extracellular fluid (194, 195). Extracellular dopamine concentrations are in turn regulated by diffusion, by uptake through the DAT and by the (probably negligible) effects of enzyme-catalyzed degradation (43, 195). Diffusion measurements in ex vivo guinea pig midbrain slices reveal that the extracellular volume fraction in the SNc, SNr, and VTA is ~50% larger than that in forebrain structures, including the striatum (43), which would lead to lower extracellular concentrations in midbrain than forebrain for a given number of molecules released. Of course, net [DA]o is also influenced by other regulators, especially uptake, which is greater in the striatum than in the midbrain, so that absolute concentrations in these regions do not differ as much as predicted by diffusion characteristics alone (195). Interestingly, DAT activity is greater in SNc than VTA, as well, leading to greater effects of DAT inhibitors on evoked increases in [DA]o in SNc than in VTA (40, 43).
In contrast to evidence for diffusion-based volume transmission of dopamine in the SNc and VTA from anatomical and voltammetric studies, evaluation of evoked D2ICs in dopamine neurons suggests that the actions of dopamine are not only largely diffusion-independent, but also synaptic (9, 10, 38). These conclusions are based on several observations. First, the concentration of exogenous dopamine required to induce D2ICs of similar magnitude to those produced by endogenously released dopamine is in the micromolar range. This is in contrast to the nanomolar concentrations expected for D2 autoreceptors in the high-affinity state. Given that the external dopamine concentration declines sharply with distance from a site of release (38, 44, 195), an interpretation of this result is that the dopamine detected by D2 receptors is immediately post-synaptic, or at least peri-synaptic. Synaptic dopamine release does occur in the VTA, given the presence of axon collaterals (7, 28). Second, the time-course of evoked D2ICs is relatively constant across stimulations, as might be expected for a postsynaptic response. However, the kinetics of the G-protein coupled, inwardly-rectifying K+ (GIRK) channel activated by dopamine are likely to be the rate-limiting factor in this autoreceptor-mediated process. Indeed, modeling studies of quantal dopamine release indicate that the peak concentration is reached ~10 ms after a release event, even at a distance of 5 μm from the release site (43, 44, 195), whereas the D2 IPSC peak occurs several hundred ms after stimulation (38, 68). Thus, the debate about synaptic transmission vs. volume transmission for somatodendritic release of dopamine remains open (68, 195).
Functional roles for somatodendritic oxytocin, vasopressin, and dopamine, and other diffusible messengers in the brain
Oxytocin and vasopressin exert specific behavioral effects: oxytocin is involved in maternal behavior and social bonding, while vasopressin has actions in the brain that affect social recognition and aggression (88, 168, 224). The sites at which these behavioral effects are exerted show, in some cases, high levels of receptor expression but little innervation by peptide-containing projections. Could dendritically released peptides exert long-lasting behavioral effects by acting on distant targets within the brain? Similar changes in neuropeptide concentrations often occur at widely separated sites, even though the absolute values vary between sites (127). Dendritic peptide release is not targeted specifically to synapses, but peptides may travel to their targets by bulk flow through the extracellular fluid and cerebrospinal fluid. The half-life of oxytocin in the CSF is about 20 min (144), in contrast with the typically subsecond lifetime of dopamine, which limits the range of its action after both axonal and somatodendritic release (38, 44, 195).
Somatodendritic dopamine release also plays a role in animal behavior, including motor activity. The action of dendritically released dopamine on D1 dopamine receptors in the terminals of the striatonigral direct pathway enhances GABA release from axons in the SNr, thereby amplifying inhibition of the principal cells of the SNr (149, 192, 239). Through these pathways, somatodendritic, as well as axonal dopamine release regulates motor behavior (3, 15, 45, 56, 141, 200, 212, 233).
The paracrine or hormone-like actions of neuropeptides and dopamine can enable signaling between entire populations of neurons, some of which may be relatively distant from each other. Thus these transmitters act in a more diffuse, less spatially constrained manner – and on a longer time scale – than those involved in classical fast synaptic transmission. At chemical synapses, the “secrecy” of signal transmission is maintained by the structure of the synapse and by the surrounding network of reuptake transporters. In contrast paracrine transmission has evolved so as to maximize spillover, and its specificity depends only on that of the signal/receptor interactions. In addition to the transmitters that are the focus of this review, other biogenic amines and acetylcholine are released from en passant boutons on axonal segments (264). Extending this concept further are gaseous neurotransmitters such as nitric oxide and carbon monoxide (49). It is likely that all of these molecules transmit chemical signals not simply from one cell to another, but from one population of neurons to another (115, 116, 127), while maintaining signal specificity by actions at transmitter-specific receptors.
Conclusions
Somatodendritic oxytocin and vasopressin release from hypothalamic neurons provides paracrine signals that are critical in several key physiological events, including birth, milk let-down, and social bonding. Somatodendritic release and volume transmission of neuropeptides represents a form of hormonal action. These neurohormones can act in a temporally coherent way at discrete brain sites to establish and co-ordinate complex behaviors.
Somatodendritic dopamine release from midbrain dopamine neurons provides an autocrine signal that regulates dopamine neuron activity, and thus contributes to axonal release regulation in target regions. Equally importantly, dopamine released within these cell body regions also helps regulate the release of other transmitters, including GABA and glutamate. Through these actions, somatodendritic dopamine release contributes to motor regulation, as well as to plasticity in rewards circuits, including aberrant plasticity in response to drugs of abuse.
Recent studies provide evidence about the mechanism and regulation of somatodendritic release of oxytocin, vasopressin, and dopamine, including underlying commonalities and differences. There is no reason to believe that somatodendritic release is restricted to these systems, and study of this process in other neuronal types is likely to be fruitful. How well we can understand somatodendritic release in the healthy brain necessarily influences how well we can harness these processes to treat disorders involving these pathways. The goal of future work into the somatodendritic release of peptides, dopamine and related molecules will be to provide this understanding.
Acknowledgments
Work on vasopressin and oxytocin release is supported by grants from the Biotechnology and Biological Research Council (BB/J004723/1) and Medical Research Council (MR/M022838/1) awarded to ML. Work on dopamine release regulation in the Rice laboratory is supported by NIH grants DA033811, DA038616, AG049137, and the Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.An S, Zenisek D. Regulation of exocytosis in neurons and neuroendocrine cells. Cur Opin Neurobiol. 2004;14:522–530. doi: 10.1016/j.conb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur J Neurosci. 2006;24:617–624. doi: 10.1111/j.1460-9568.2006.04953.x. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. [PubMed] [Google Scholar]
- 5.Bains JS, Ferguson AV. Activation of N-methyl-D-aspartate receptors evokes calcium spikes in the dendrites of rat hypothalamic paraventricular nucleus neurons. Neuroscience. 1999;90:885–891. doi: 10.1016/s0306-4522(98)00525-9. [DOI] [PubMed] [Google Scholar]
- 6.Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Bayer VE, Pickel VM. Ultrastructural localization of tyrosine hydroxylase in the rat ventral tegmental area: relationship between immunolabeling density and neuronal associations. J Neurosci. 1990;10:2996–3013. doi: 10.1523/JNEUROSCI.10-09-02996.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bealer SL, Armstrong WE, Crowley WR. Oxytocin release in magnocellular nuclei: neurochemical mediators and functional significance during gestation. Am J Physiol. 2010;299:R452–458. doi: 10.1152/ajpregu.00217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beckstead MJ, Ford CP, Phillips PE, Williams JT. Presynaptic regulation of dendrodendritic dopamine transmission. Eur J Neurosci. 2007;26:1479–1488. doi: 10.1111/j.1460-9568.2007.05775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Bergquist F, Jonason J, Pileblad E, Nissbrandt H. Effects of local administration of L-, N-, and P/Q-type calcium channel blockers on spontaneous dopamine release in the striatum and the substantia nigra: a microdialysis study in rat. J Neurochem. 1998;70:1532–1540. doi: 10.1046/j.1471-4159.1998.70041532.x. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist F, Ludwig M. Dendritic transmitter release: a comparison of two model systems. J Neuroendocrinol. 2008;20:677–686. doi: 10.1111/j.1365-2826.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- 13.Bergquist F, Niazi HS, Nissbrandt H. Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002;950:245–253. doi: 10.1016/s0006-8993(02)03047-0. [DOI] [PubMed] [Google Scholar]
- 14.Bergquist F, Nissbrandt H. Influence of R-type (Cav2.3) and t-type (Cav3.1-3.3) antagonists on nigral somatodendritic dopamine release measured by microdialysis. Neuroscience. 2003;120:757–764. doi: 10.1016/s0306-4522(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 15.Bergquist F, Shahabi HN, Nissbrandt H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003;973:81–91. doi: 10.1016/s0006-8993(03)02555-1. [DOI] [PubMed] [Google Scholar]
- 16.Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- 18.Brimblecombe KR, Gracie CJ, Platt NJ, Cragg SJ. Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J Physiol. 2015;593:929–946. doi: 10.1113/jphysiol.2014.285890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown CH, Bains JS, Ludwig M, Stern JE. Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol. 2013;25:678–710. doi: 10.1111/jne.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown CH, Munro G, Johnstone LE, Robson AC, Landgraf R, Russell JA. Oxytocin neurone autoexcitation during morphine withdrawal in anaesthetized rats. Neuroreport. 1997;8:951–955. doi: 10.1097/00001756-199703030-00027. [DOI] [PubMed] [Google Scholar]
- 21.Brown CH, Scott V, Ludwig M, Leng G, Bourque CW. Somatodendritic dynorphin release: orchestrating activity patterns of vasopressin neurons. Biochem Soc Trans. 2007;35:1236–1242. doi: 10.1042/BST0351236. [DOI] [PubMed] [Google Scholar]
- 22.Callahan MF, Ludwig M, Tsai KP, Sim LJ, Morris M. Baroreceptor input regulates osmotic control of central vasopressin secretion. Neuroendocrinology. 1997;65:238–245. doi: 10.1159/000127181. [DOI] [PubMed] [Google Scholar]
- 23.Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- 24.Carlsson A. Treatment of Parkinson’s with L-DOPA. The early discovery phase, and a comment on current problems. J Neural Transm (Vienna) 2002;109:777–787. doi: 10.1007/s007020200064. [DOI] [PubMed] [Google Scholar]
- 25.Carta M, Bezard E. Contribution of pre-synaptic mechanisms to L-DOPA-induced dyskinesia. Neuroscience. 2011;198:245–251. doi: 10.1016/j.neuroscience.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 26.Chen BT, Avshalumov MV, Rice ME. Modulation of somatodendritic dopamine release by endogenous H2O2: susceptibility in substantia nigra but resistance in VTA. J Neurophysiol. 2002;87:1155–1158. doi: 10.1152/jn.00629.2001. [DOI] [PubMed] [Google Scholar]
- 27.Chen BT, Moran KA, Avshalumov MV, Rice ME. Limited regulation of somatodendritic dopamine release by voltage-sensitive Ca2+ channels contrasted with strong regulation of axonal dopamine release. J Neurochem. 2006;96:645–655. doi: 10.1111/j.1471-4159.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen BT, Patel JC, Moran KA, Rice ME. Differential calcium dependence of axonal versus somatodendritic dopamine release, with characteristics of both in the ventral tegmental area. Front Syst Neurosci. 2011;5:39. doi: 10.3389/fnsys.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen BT, Rice ME. Synaptic regulation of somatodendritic dopamine release by glutamate and GABA differs between substantia nigra and ventral tegmental area. J Neurochem. 2002;81:158–169. doi: 10.1046/j.1471-4159.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience. 2003;118:797–807. doi: 10.1016/s0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 32.Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. doi: 10.1038/289537a0. [DOI] [PubMed] [Google Scholar]
- 33.Chevaleyre V, Dayanithi G, Moos FC, Desarmenien MG. Developmental regulation of a local positive autocontrol of supraoptic neurons. J Neurosci. 2000;20:5813–5819. doi: 10.1523/JNEUROSCI.20-15-05813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi YM, Kim SH, Chung S, Uhm DY, Park MK. Regional interaction of endoplasmic reticulum Ca2+ signals between soma and dendrites through rapid luminal Ca2+ diffusion. J Neurosci. 2006;26:12127–12136. doi: 10.1523/JNEUROSCI.3158-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004;24:972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook CJ, Devine CE. Antibody-based electrodes for hormonal and neurotransmitter measurements in vivo. Electronalaysis. 1998;10:1108–1111. [Google Scholar]
- 38.Courtney NA, Mamaligas AA, Ford CP. Species differences in somatodendritic dopamine transmission determine D2-autoreceptor-mediated inhibition of ventral tegmental area neuron firing. J Neurosci. 2012;32:13520–13528. doi: 10.1523/JNEUROSCI.2745-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox CL. Complex regulation of dendritic transmitter release from thalamic interneurons. Cur Opin Neurobiol. 2014;29C:126–132. doi: 10.1016/j.conb.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cragg S, Rice ME, Greenfield SA. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:863–873. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- 41.Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cragg SJ, Hawkey CR, Greenfield SA. Comparison of serotonin and dopamine release in substantia nigra and ventral tegmental area: region and species differences. J Neurochem. 1997;69:2378–2386. doi: 10.1046/j.1471-4159.1997.69062378.x. [DOI] [PubMed] [Google Scholar]
- 43.Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J Neurophysiol. 2001;85:1761–1771. doi: 10.1152/jn.2001.85.4.1761. [DOI] [PubMed] [Google Scholar]
- 44.Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Crocker AD. The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Rev Neurosci. 1997;8:55–76. doi: 10.1515/revneuro.1997.8.1.55. [DOI] [PubMed] [Google Scholar]
- 46.Crosby KM, Baimoukhametova DV, Bains JS, Pittman QJ. Postsynaptic Depolarization Enhances GABA Drive to Dorsomedial Hypothalamic Neurons through Somatodendritic Cholecystokinin Release. J Neurosci. 2015;35:13160–13170. doi: 10.1523/JNEUROSCI.3123-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley WR, Armstrong WE. Neurochemical regulation of oxytocin secretion in lactation. Endocr Rev. 1992;13:33–65. doi: 10.1210/edrv-13-1-33. [DOI] [PubMed] [Google Scholar]
- 48.Cui G, Bernier BE, Harnett MT, Morikawa H. Differential regulation of action potential- and metabotropic glutamate receptor-induced Ca2+ signals by inositol 1,4,5-trisphosphate in dopaminergic neurons. J Neurosci. 2007;27:4776–4785. doi: 10.1523/JNEUROSCI.0139-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dayanithi G, Forostyak O, Ueta Y, Verkhratsky A, Toescu EC. Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium. 2012;51:293–299. doi: 10.1016/j.ceca.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 51.de Kock CP, Burnashev N, Lodder JC, Mansvelder HD, Brussaard AB. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J Physiol. 2004;561:53–64. doi: 10.1113/jphysiol.2004.069005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Kock CP, Cornelisse LN, Burnashev N, Lodder JC, Timmerman AJ, Couey JJ, Mansvelder HD, Brussaard AB. NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing. J Physiol. 2006;577:891–905. doi: 10.1113/jphysiol.2006.115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Kock CP, Wierda KD, Bosman LW, Min R, Koksma JJ, Mansvelder HD, Verhage M, Brussaard AB. Somatodendritic secretion in oxytocin neurons is upregulated during the female reproductive cycle. J Neurosci. 2003;23:2726–2734. doi: 10.1523/JNEUROSCI.23-07-02726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Descarries L, Berube-Carriere N, Riad M, Bo GD, Mendez JA, Trudeau LE. Glutamate in dopamine neurons: synaptic versus diffuse transmission. Brain Res Rev. 2008;58:290–302. doi: 10.1016/j.brainresrev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Deutch AY, Goldstein M, Baldino F, Jr, Roth RH. Telencephalic projections of the A8 dopamine cell group. Ann New York Acad Sci. 1988;537:27–50. doi: 10.1111/j.1749-6632.1988.tb42095.x. [DOI] [PubMed] [Google Scholar]
- 57.Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Ann Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 58.Douglas AJ, Neumann I, Meeren HK, Leng G, Johnstone LE, Munro G, Russell JA. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J Neurosci. 1995;15:5049–5057. doi: 10.1523/JNEUROSCI.15-07-05049.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duguid IC, Pankratov Y, Moss GW, Smart TG. Somatodendritic release of glutamate regulates synaptic inhibition in cerebellar Purkinje cells via autocrine mGluR1 activation. J Neurosci. 2007;27:12464–12474. doi: 10.1523/JNEUROSCI.0178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elverfors A, Jonason J, Jonason G, Nissbrandt H. Effects of drugs interfering with sodium channels and calcium channels on the release of endogenous dopamine from superfused substantia nigra slices. Synapse. 1997;26:359–369. doi: 10.1002/(SICI)1098-2396(199708)26:4<359::AID-SYN4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 61.Elverfors A, Nissbrandt H. Reserpine-insensitive dopamine release in the substantia nigra? Brain Res. 1991;557:5–12. doi: 10.1016/0006-8993(91)90109-9. [DOI] [PubMed] [Google Scholar]
- 62.Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 63.Falkenburger BH, Barstow KL, Mintz IM. Dendrodendritic inhibition through reversal of dopamine transport. Science. 2001;293:2465–2470. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- 64.Fiorillo CD, Williams JT. Glutamate mediates an inhibitory postsynaptic potential in dopamine neurons. Nature. 1998;394:78–82. doi: 10.1038/27919. [DOI] [PubMed] [Google Scholar]
- 65.Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci. 1996;19:440–444. doi: 10.1016/0166-2236(96)10034-5. [DOI] [PubMed] [Google Scholar]
- 66.Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589:3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foehring RC, Armstrong WE. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol. 1996;76:977–983. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- 68.Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282C:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ford CP, Gantz SC, Phillips PE, Williams JT. Control of extracellular dopamine at dendrite and axon terminals. J Neurosci. 2010;30:6975–6983. doi: 10.1523/JNEUROSCI.1020-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau LE. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- 71.Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J Physiol. 1994;480:155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujihara H, Sasaki K, Mishiro-Sato E, Ohbuchi T, Dayanithi G, Yamasaki M, Ueta Y, Minamino N. Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology. 2012;153:1377–1386. doi: 10.1210/en.2011-1539. [DOI] [PubMed] [Google Scholar]
- 73.Gantz SC, Bunzow JR, Williams JT. Spontaneous inhibitory synaptic currents mediated by a G protein-coupled receptor. Neuron. 2013;78:807–812. doi: 10.1016/j.neuron.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- 75.Gentet LJ, Williams SR. Dopamine gates action potential backpropagation in midbrain dopaminergic neurons. J Neurosci. 2007;27:1892–1901. doi: 10.1523/JNEUROSCI.5234-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gillard ER, Leon-Olea M, Mucio-Ramirez S, Coburn CG, Sanchez-Islas E, de Leon A, Mussenden H, Bauce LG, Pittman QJ, Curras-Collazo MC. A novel role for endogenous pituitary adenylate cyclase activating polypeptide in the magnocellular neuroendocrine system. Endocrinology. 2006;147:791–803. doi: 10.1210/en.2005-1103. [DOI] [PubMed] [Google Scholar]
- 78.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 79.Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- 80.Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 82.Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- 83.Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- 84.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffman AF, Gerhardt GA. Differences in pharmacological properties of dopamine release between the substantia nigra and striatum: an in vivo electrochemical study. J Pharmacol Exp Ther. 1999;289:455–463. [PubMed] [Google Scholar]
- 86.Hu B, Bourque CW. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ingram CD, Kavadas V, Thomas MR, Threapleton JD. Endogenous opioid control of somatodendritic oxytocin release from the hypothalamic supraoptic and paraventricular nuclei in vitro. Neurosci Res. 1996;25:17–24. doi: 10.1016/0168-0102(96)01027-9. [DOI] [PubMed] [Google Scholar]
- 88.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iremonger KJ, Wamsteeker Cusulin JI, Bains JS. Changing the tune: plasticity and adaptation of retrograde signals. Trends Neurosci. 2013;36:471–479. doi: 10.1016/j.tins.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jhamandas JH, Lind RW, Renaud LP. Angiotensin II may mediate excitatory neurotransmission from the subfornical organ to the hypothalamic supraoptic nucleus: an anatomical and electrophysiological study in the rat. Brain Res. 1989;487:52–61. doi: 10.1016/0006-8993(89)90939-6. [DOI] [PubMed] [Google Scholar]
- 93.John CE, Budygin EA, Mateo Y, Jones SR. Neurochemical characterization of the release and uptake of dopamine in ventral tegmental area and serotonin in substantia nigra of the mouse. J Neurochem. 2006;96:267–282. doi: 10.1111/j.1471-4159.2005.03557.x. [DOI] [PubMed] [Google Scholar]
- 94.Joux N, Chevaleyre V, Alonso G, Boissin-Agasse L, Moos FC, Desarmenien MG, Hussy N. High voltage-activated Ca2+ currents in rat supraoptic neurones: biophysical properties and expression of the various channel alpha1 subunits. J Neuroendocrinol. 2001;13:638–649. doi: 10.1046/j.1365-2826.2001.00679.x. [DOI] [PubMed] [Google Scholar]
- 95.Juraska JM, Wilson CJ, Groves PM. The substantia nigra of the rat: a Golgi study. J Comp Neurol. 1977;172:585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- 96.Jurgutis P, Shuang R, Fletcher A, Stuenkel EL. Characterization and distribution of SNARE proteins at neuroendocrine nerve endings. Neuroendocrinology. 1996;64:379–392. doi: 10.1159/000127141. [DOI] [PubMed] [Google Scholar]
- 97.Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 98.Kendrick KM. Microdialysis measurement of in vivo neuropeptide release. J Neurosci Meth. 1990;34:35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 99.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Chen L, Ding JB. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim Y, Park MK, Chung S. Voltage-operated Ca2+ channels regulate dopamine release from somata of dopamine neurons in the substantia nigra pars compacta. Biochem Biophys Res Commun. 2008;373:665–669. doi: 10.1016/j.bbrc.2008.06.099. [DOI] [PubMed] [Google Scholar]
- 102.Kim Y, Park MK, Chung S. Regulation of somatodendritic dopamine release by corticotropin-releasing factor via the inhibition of voltage-operated Ca2+ channels. Neurosci Lett. 2009;465:31–35. doi: 10.1016/j.neulet.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 103.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 104.Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 105.Komori Y, Tanaka M, Kuba M, Ishii M, Abe M, Kitamura N, Verkhratsky A, Shibuya I, Dayanithi G. Ca2+ homeostasis, Ca2+ signalling and somatodendritic vasopressin release in adult rat supraoptic nucleus neurones. Cell Calcium. 2010;48:324–332. doi: 10.1016/j.ceca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Koos T, Tecuapetla F, Tepper JM. Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies. Cur Opin Neurobiol. 2011;21:393–401. doi: 10.1016/j.conb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–287. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lambert RC, Moos FC, Richard P. Action of endogenous oxytocin within the paraventricular or supraoptic nuclei: a powerful link in the regulation of the bursting pattern of oxytocin neurons during the milk-ejection reflex in rats. Neuroscience. 1993;57:1027–1038. doi: 10.1016/0306-4522(93)90046-i. [DOI] [PubMed] [Google Scholar]
- 109.Landgraf R, Kuba M, Holsboer F, Wotjak CT. Release of vasopressin and oxytocin within the brain and into blood: microdialysis and antisense targeting. In: Saito T, Kurokawa K, Yoshida S, editors. Neurohypophysis: recent progress of vasopressin and oxytocin research. Amsterdam: Elesevier; 1995. pp. 243–256. [Google Scholar]
- 110.Landgraf R, Neumann I, Russell JA, Pittman QJ. Push-pull perfusion and microdialysis studies of central oxytocin and vasopressin release in freely moving rats during pregnancy, parturition, and lactation. Ann New York Acad Sci. 1992;652:326–339. doi: 10.1111/j.1749-6632.1992.tb34364.x. [DOI] [PubMed] [Google Scholar]
- 111.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Landry M, Vila-Porcile E, Hokfelt T, Calas A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur J Neurosci. 2003;17:579–589. [PubMed] [Google Scholar]
- 113.Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 115.Leng G, Ludwig M. Jacques Benoit Lecture. Information processing in the hypothalamus: peptides and analogue computation. J Neuroendocrinol. 2006;18:379–392. doi: 10.1111/j.1365-2826.2006.01428.x. [DOI] [PubMed] [Google Scholar]
- 116.Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–5632. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leszczyszyn DJ, Jankowski JA, Viveros OH, Diliberto EJ, Jr, Near JA, Wightman RM. Nicotinic receptor-mediated catecholamine secretion from individual chromaffin cells. Chemical evidence for exocytosis. J Biol Chem. 1990;265:14736–14737. [PubMed] [Google Scholar]
- 118.Leviel V. The reverse transport of DA, what physiological significance? Neurochem Int. 2001;38:83–106. doi: 10.1016/s0197-0186(00)00076-0. [DOI] [PubMed] [Google Scholar]
- 119.Ludwig M. Dendritic neurotransmitter release. New York: Springer; 2005. [Google Scholar]
- 120.Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 121.Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G, Leng G. Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol. 2005;564:515–522. doi: 10.1113/jphysiol.2005.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ludwig M, Callahan MF, Landgraf R, Johnson AK, Morris M. Neural input modulates osmotically stimulated release of vasopressin into the supraoptic nucleus. Am J Physiol. 1996;270:E787–792. doi: 10.1152/ajpendo.1996.270.5.E787. [DOI] [PubMed] [Google Scholar]
- 123.Ludwig M, Callahan MF, Morris M. Effects of tetrodotoxin on osmotically stimulated central and peripheral vasopressin and oxytocin release. Neuroendocrinology. 1995;62:619–627. doi: 10.1159/000127058. [DOI] [PubMed] [Google Scholar]
- 124.Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 125.Ludwig M, Johnstone LE, Neumann I, Landgraf R, Russell JA. Direct hypertonic stimulation of the rat supraoptic nucleus increases c-fos expressionin glial cells rather than magnocellular neurones. Cell Tissue Res. 1997;287:79–90. doi: 10.1007/s004410050733. [DOI] [PubMed] [Google Scholar]
- 126.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 127.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 128.Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 129.Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- 130.Ludwig M, Williams K, Callahan MF, Morris M. Salt loading abolishes osmotically stimulated vasopressin release within the supraoptic nucleus. Neurosci Lett. 1996;215:1–4. doi: 10.1016/s0304-3940(96)12956-6. [DOI] [PubMed] [Google Scholar]
- 131.Mabrouk OS, Kennedy RT. Simultaneous oxytocin and arg-vasopressin measurements in microdialysates using capillary liquid chromatography-mass spectrometry. J Neurosci Meth. 2012;209:127–133. doi: 10.1016/j.jneumeth.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maletic-Savatic M, Koothan T, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part II: mediation by calcium/calmodulin-dependent protein kinase II. J Neurosci. 1998;18:6814–6821. doi: 10.1523/JNEUROSCI.18-17-06814.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maletic-Savatic M, Malinow R. Calcium-evoked dendritic exocytosis in cultured hippocampal neurons. Part I: trans-Golgi network-derived organelles undergo regulated exocytosis. J Neurosci. 1998;18:6803–6813. doi: 10.1523/JNEUROSCI.18-17-06803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mansvelder HD, Kits KS. Calcium channels and the release of large dense core vesicles from neuroendocrine cells: spatial organization and functional coupling. Prog Neurobiol. 2000;62:427–441. doi: 10.1016/s0301-0082(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 136.Martin TF. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 137.Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- 138.Mason WT, Hatton GI, Ho YW, Chapman C, Robinson IC. Central release of oxytocin, vasopressin and neurophysin by magnocellular neurone depolarization: evidence in slices of guinea pig and rat hypothalamus. Neuroendocrinology. 1986;42:311–322. doi: 10.1159/000124457. [DOI] [PubMed] [Google Scholar]
- 139.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsutani S, Yamamoto N. Postnatal development of dendritic spines on olfactory bulb granule cells in rats. J Comp Neurol. 2004;473:553–561. doi: 10.1002/cne.20107. [DOI] [PubMed] [Google Scholar]
- 141.Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. 2012;36:2336–2346. doi: 10.1111/j.1460-9568.2012.08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meinrenken CJ, Borst JG, Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mendez JA, Bourque MJ, Fasano C, Kortleven C, Trudeau LE. Somatodendritic dopamine release requires synaptotagmin 4 and 7 and the participation of voltage-gated calcium channels. J Biol Chem. 2011;286:23928–23937. doi: 10.1074/jbc.M111.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 145.Mercer L, del Fiacco M, Cuello AC. The smooth endoplasmic reticulum as a possible storage site for dendritic dopamine in substantia nigra neurones. Experientia. 1979;35:101–103. doi: 10.1007/BF01917903. [DOI] [PubMed] [Google Scholar]
- 146.Millar J, Stamford JA, Kruk ZL, Wightman RM. Electrochemical, pharmacological and electrophysiological evidence of rapid dopamine release and removal in the rat caudate nucleus following electrical stimulation of the median forebrain bundle. Eur J Pharmacol. 1985;109:341–348. doi: 10.1016/0014-2999(85)90394-2. [DOI] [PubMed] [Google Scholar]
- 147.Miyata S, Hatton GI. Activity-related, dynamic neuron-glial interactions in the hypothalamo-neurohypophysial system. Microscopy Res Tech. 2002;56:143–157. doi: 10.1002/jemt.10012. [DOI] [PubMed] [Google Scholar]
- 148.Miyata S, Khan AM, Hatton GI. Colocalization of calretinin and calbindin-D28k with oxytocin and vasopressin in rat supraoptic nucleus neurons: a quantitative study. Brain Res. 1998;785:178–182. doi: 10.1016/s0006-8993(97)01375-9. [DOI] [PubMed] [Google Scholar]
- 149.Miyazaki T, Lacey MG. Presynaptic inhibition by dopamine of a discrete component of GABA release in rat substantia nigra pars reticulata. J Physiol. 1998;513:805–817. doi: 10.1111/j.1469-7793.1998.805ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- 151.Morgan A. Exocytosis. Essays Biochem. 1995;30:77–95. [PubMed] [Google Scholar]
- 152.Moriguchi A, Ferrario CM, Brosnihan KB, Ganten D, Morris M. Differential regulation of central vasopressin in transgenic rats harboring the mouse Ren-2 gene. Am J Physiol. 1994;267:R786–791. doi: 10.1152/ajpregu.1994.267.3.R786. [DOI] [PubMed] [Google Scholar]
- 153.Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Morikawa H, Paladini CA. Dynamic regulation of midbrain dopamine neuron activity: intrinsic, synaptic, and plasticity mechanisms. Neuroscience. 2011;198:95–111. doi: 10.1016/j.neuroscience.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morris JF, Ludwig M. Magnocellular dendrites: prototypic receiver/transmitters. J Neuroendocrinol. 2004;16:403–408. doi: 10.1111/j.0953-8194.2004.01182.x. [DOI] [PubMed] [Google Scholar]
- 156.Morris JF, Pow DV. New anatomical insights into the inputs and outputs from hypothalamic magnocellular neurons. Ann New York Acad Sci. 1993;689:16–33. doi: 10.1111/j.1749-6632.1993.tb55534.x. [DOI] [PubMed] [Google Scholar]
- 157.Morris JF, Pow DV. Widespread release of peptides in the central nervous system: quantitation of tannic acid-captured exocytoses. Anat Record. 1991;231:437–445. doi: 10.1002/ar.1092310406. [DOI] [PubMed] [Google Scholar]
- 158.Morris JF, Pow DV, Sokol HW, Ward A. Dendritic release of peptides from magnocellular neurons in normal rats, Brattleboro rats and mice with hereditary nephrogenic diabetes insipidus. In: Gross P, Richter D, Roberston GL, editors. Vasopressin. Paris: John Libbey Eurotext; 1993. pp. 171–182. [Google Scholar]
- 159.Morris M, Alexander N. Baroreceptor influences on oxytocin and vasopressin secretion. Hypertension. 1989;13:110–114. doi: 10.1161/01.hyp.13.2.110. [DOI] [PubMed] [Google Scholar]
- 160.Morris M, Barnard RR, Jr, Sain LE. Osmotic mechanisms regulating cerebrospinal fluid vasopressin and oxytocin in the conscious rat. Neuroendocrinology. 1984;39:377–383. doi: 10.1159/000124008. [DOI] [PubMed] [Google Scholar]
- 161.Murase T, Kondo K, Otake K, Oiso Y. Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology. 1993;57:1092–1096. doi: 10.1159/000126475. [DOI] [PubMed] [Google Scholar]
- 162.Naskar K, Stern JE. A functional coupling between extrasynaptic NMDA receptors and A-type K+ channels under astrocyte control regulates hypothalamic neurosecretory neuronal activity. J Physiol. 2014;592:2813–2827. doi: 10.1113/jphysiol.2014.270793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Neumann I, Douglas AJ, Pittman QJ, Russell JA, Landgraf R. Oxytocin released within the supraoptic nucleus of the rat brain by positive feedback action is involved in parturition-related events. J Neuroendocrinol. 1996;8:227–233. doi: 10.1046/j.1365-2826.1996.04557.x. [DOI] [PubMed] [Google Scholar]
- 164.Neumann I, Koehler E, Landgraf R, Summy-Long J. An oxytocin receptor antagonist infused into the supraoptic nucleus attenuates intranuclear and peripheral release of oxytocin during suckling in conscious rats. Endocrinology. 1994;134:141–148. doi: 10.1210/endo.134.1.8275928. [DOI] [PubMed] [Google Scholar]
- 165.Neumann I, Landgraf R, Takahashi Y, Pittman QJ, Russell JA. Stimulation of oxytocin release within the supraoptic nucleus and into blood by CCK-8. Am J Physiol. 1994;267:R1626–1631. doi: 10.1152/ajpregu.1994.267.6.R1626. [DOI] [PubMed] [Google Scholar]
- 166.Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–645. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- 167.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: a microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 168.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Nieoullon A, Cheramy A, Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977;266:375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- 170.Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nirenberg MJ, Chan J, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. Immunogold localization of the dopamine transporter: an ultrastructural study of the rat ventral tegmental area. J Neurosci. 1997;17:5255–5262. doi: 10.1523/JNEUROSCI.17-14-05255.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nissen R, Hu B, Renaud LP. Regulation of spontaneous phasic firing of rat supraoptic vasopressin neurones in vivo by glutamate receptors. J Physiol. 1995;484:415–424. doi: 10.1113/jphysiol.1995.sp020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nissen R, Renaud LP. GABA receptor mediation of median preoptic nucleus-evoked inhibition of supraoptic neurosecretory neurones in rat. J Physiol. 1994;479:207–216. doi: 10.1113/jphysiol.1994.sp020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–1333. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993;364:341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- 177.Opazo F, Schulz JB, Falkenburger BH. PKC links Gq-coupled receptors to DAT-mediated dopamine release. J Neurochem. 2010;114:587–596. doi: 10.1111/j.1471-4159.2010.06788.x. [DOI] [PubMed] [Google Scholar]
- 178.Ota M, Crofton JT, Festavan G, Share L. Central carbachol stimulates vasopressin release into interstitial fluid adjacent to the paraventricular nucleus. Brain Res. 1992;592:249–254. doi: 10.1016/0006-8993(92)91682-5. [DOI] [PubMed] [Google Scholar]
- 179.Ota M, Crofton JT, Share L. Hemorrhage-induced vasopressin release in the paraventricular nucleus measured by in vivo microdialysis. Brain Res. 1994;658:49–54. doi: 10.1016/s0006-8993(09)90009-9. [DOI] [PubMed] [Google Scholar]
- 180.Ovsepian SV, Dolly JO. Dendritic SNAREs add a new twist to the old neuron theory. Proc Natl Acad Sci U S A. 2011;108:19113–19120. doi: 10.1073/pnas.1017235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neuroscience. 2011;198:213–220. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- 182.Paquet M, Tremblay M, Soghomonian JJ, Smith Y. AMPA and NMDA glutamate receptor subunits in midbrain dopaminergic neurons in the squirrel monkey: an immunohistochemical and in situ hybridization study. J Neurosci. 1997;17:1377–1396. doi: 10.1523/JNEUROSCI.17-04-01377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Patel JC, Rice ME. Monitoring axonal and somatodendritic dopamine release using fast-scan cyclic voltammetry in brain slices. Meth Mol Biol. 2013;964:243–273. doi: 10.1007/978-1-62703-251-3_15. [DOI] [PubMed] [Google Scholar]
- 184.Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pickel VM, Nirenberg MJ, Milner TA. Ultrastructural view of central catecholaminergic transmission: immunocytochemical localization of synthesizing enzymes, transporters and receptors. J Neurocytol. 1996;25:843–856. doi: 10.1007/BF02284846. [DOI] [PubMed] [Google Scholar]
- 186.Potapenko ES, Biancardi VC, Florschutz RM, Ryu PD, Stern JE. Inhibitory-excitatory synaptic balance is shifted toward increased excitation in magnocellular neurosecretory cells of heart failure rats. J Neurophysiol. 2011;106:1545–1557. doi: 10.1152/jn.00218.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Astrocytes modulate a postsynaptic NMDA-GABAA-receptor crosstalk in hypothalamic neurosecretory neurons. J Neurosci. 2013;33:631–640. doi: 10.1523/JNEUROSCI.3936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 190.Prior IA, Clague MJ. Glutamate uptake occurs at an early stage of synaptic vesicle recycling. Curr Biol. 1997;7:353–356. doi: 10.1016/s0960-9822(06)00159-x. [DOI] [PubMed] [Google Scholar]
- 191.Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J Pharmacol Exp Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- 192.Radnikow G, Misgeld U. Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata. J Neurosci. 1998;18:2009–2016. doi: 10.1523/JNEUROSCI.18-06-02009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Redgrave P, Vautrelle N, Reynolds JN. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neuroscience. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 194.Rice ME. Distinct regional differences in dopamine-mediated volume transmission. Prog Brain Res. 2000;125:277–290. doi: 10.1016/S0079-6123(00)25017-6. [DOI] [PubMed] [Google Scholar]
- 195.Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- 197.Rice ME, Patel JC, Cragg SJ. Dopamine release in the basal ganglia. Neuroscience. 2011;198:112–137. doi: 10.1016/j.neuroscience.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Exp Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- 199.Robertson GS, Damsma G, Fibiger HC. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J Neurosci. 1991;11:2209–2216. doi: 10.1523/JNEUROSCI.11-07-02209.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Robertson GS, Robertson HA. Evidence that L-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci. 1989;9:3326–3331. doi: 10.1523/JNEUROSCI.09-09-03326.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rossoni E, Feng J, Tirozzi B, Brown D, Leng G, Moos F. Emergent synchronous bursting of oxytocin neuronal network. PLoS Comp Biol. 2008;4:e1000123. doi: 10.1371/journal.pcbi.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: adaptations in pregnancy. Front Neuroendocrinol. 2003;24:27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 203.Russell JA, Neumann I, Landgraf R. Oxytocin and vasopressin release in discrete brain areas after naloxone in morphine-tolerant and -dependent anesthetized rats: push-pull perfusion study. J Neurosci. 1992;12:1024–1032. doi: 10.1523/JNEUROSCI.12-03-01024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sabatier N, Richard P, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. J Physiol. 1997;503:253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: involvement of multiple intracellular transduction signals. J Neuroendocrinol. 2004;16:221–236. doi: 10.1111/j.0953-8194.2004.01155.x. [DOI] [PubMed] [Google Scholar]
- 207.Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 208.Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- 209.Salvatore MF, Pruett BS. Dichotomy of tyrosine hydroxylase and dopamine regulation between somatodendritic and terminal field areas of nigrostriatal and mesoaccumbens pathways. PloS One. 2012;7:e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Santiago M, Machado A, Cano J. Fast sodium channel dependency of the somatodendritic release of dopamine in the rat’s brain. Neurosci Lett. 1992;148:145–147. doi: 10.1016/0304-3940(92)90825-r. [DOI] [PubMed] [Google Scholar]
- 211.Santiago M, Westerink BH. Characterization and pharmacological responsiveness of dopamine release recorded by microdialysis in the substantia nigra of conscious rats. J Neurochem. 1991;57:738–747. doi: 10.1111/j.1471-4159.1991.tb08214.x. [DOI] [PubMed] [Google Scholar]
- 212.Schoffelmeer AN, Drukarch B, De Vries TJ, Hogenboom F, Schetters D, Pattij T. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J Neurosci. 2011;31:1284–1291. doi: 10.1523/JNEUROSCI.3779-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shaw FD, Morris JF. Calcium localization in the rat neurohypophysis. Nature. 1980;287:56–58. doi: 10.1038/287056a0. [DOI] [PubMed] [Google Scholar]
- 215.Shibuya I, Noguchi J, Tanaka K, Harayama N, Inoue U, Kabashima N, Ueta Y, Hattori Y, Yamashita H. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. J Neuroendocrinol. 1998;10:31–42. doi: 10.1046/j.1365-2826.1998.00168.x. [DOI] [PubMed] [Google Scholar]
- 216.Simmons ML, Terman GW, Gibbs SM, Chavkin C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron. 1995;14:1265–1272. doi: 10.1016/0896-6273(95)90273-2. [DOI] [PubMed] [Google Scholar]
- 217.Sladek CD. Regulation of vasopressin release by neurotransmitters, neuropeptides and osmotic stimuli. Prog Brain Res. 1983;60:71–90. doi: 10.1016/S0079-6123(08)64376-9. [DOI] [PubMed] [Google Scholar]
- 218.Son SJ, Filosa JA, Potapenko ES, Biancardi VC, Zheng H, Patel KP, Tobin VA, Ludwig M, Stern JE. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron. 2013;78:1036–1049. doi: 10.1016/j.neuron.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nature Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- 220.Stern JE. Neuroendocrine-autonomic integration in the PVN: Novel roles for dendritically released neuropeptides. J Neuroendocrinol. 2015;27:487–497. doi: 10.1111/jne.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–853. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stern JE, Potapenko ES. Enhanced NMDA receptor-mediated intracellular calcium signaling in magnocellular neurosecretory neurons in heart failure rats. Am J Physiol. 2013;305:R414–422. doi: 10.1152/ajpregu.00160.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol. 2004;286:R719–725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- 224.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 225.Straub C, Tritsch NX, Hagan NA, Gu C, Sabatini BL. Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci. 2014;34:8557–8569. doi: 10.1523/JNEUROSCI.0589-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stuart G, Spruston N, Hausser M. Dendrites. Oxford: Oxford University Press; 1999. [Google Scholar]
- 227.Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- 228.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sudhof TC. The synaptic vesicle cycle. Ann Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 230.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 233.Timmerman W, Abercrombie ED. Amphetamine-induced release of dendritic dopamine in substantia nigra pars reticulata: D1-mediated behavioral and electrophysiological effects. Synapse. 1996;23:280–291. doi: 10.1002/(SICI)1098-2396(199608)23:4<280::AID-SYN6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 234.Tobin V, Leng G, Ludwig M. The involvement of actin, calcium channels and exocytosis proteins in somato-dendritic oxytocin and vasopressin release. Front Physiol. 2012;3:261. doi: 10.3389/fphys.2012.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tobin V, Schwab Y, Lelos N, Onaka T, Pittman QJ, Ludwig M. Expression of exocytosis proteins in rat supraoptic nucleus neurones. J Neuroendocrinol. 2012;24:629–641. doi: 10.1111/j.1365-2826.2011.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tobin VA, Douglas AJ, Leng G, Ludwig M. The involvement of voltage–operated calcium channels in somato-dendritic oxytocin release. PloS One. 2011;6:e25366. doi: 10.1371/journal.pone.0025366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tobin VA, Hurst G, Norrie L, Dal Rio FP, Bull PM, Ludwig M. Thapsigargin-induced mobilization of dendritic dense-cored vesicles in rat supraoptic neurons. Eur J Neurosci. 2004;19:2909–2912. doi: 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 238.Tobin VA, Ludwig M. The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurons. J Physiol. 2007;582:1337–1348. doi: 10.1113/jphysiol.2007.132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Trevitt JT, Carlson BB, Nowend K, Salamone JD. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology. 2001;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- 240.Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 2012;490:262–266. doi: 10.1038/nature11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Trueta C, De-Miguel FF. Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Front Physiol. 2012;3:319. doi: 10.3389/fphys.2012.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Trueta C, Sanchez-Armass S, Morales MA, De-Miguel FF. Calcium-induced calcium release contributes to somatic secretion of serotonin in leech Retzius neurons. J Neurobiol. 2004;61:309–316. doi: 10.1002/neu.20055. [DOI] [PubMed] [Google Scholar]
- 243.Verbalis JG, McCann MJ, McHale CM, Stricker EM. Oxytocin secretion in response to cholecystokinin and food: differentiation of nausea from satiety. Science. 1986;232:1417–1419. doi: 10.1126/science.3715453. [DOI] [PubMed] [Google Scholar]
- 244.Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- 245.Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 246.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 247.Wang D, Fisher TE. Expression of CaV 2.2 and splice variants of CaV 2.1 in oxytocin- and vasopressin-releasing supraoptic neurones. J Neuroendocrinol. 2014;26:100–110. doi: 10.1111/jne.12127. [DOI] [PubMed] [Google Scholar]
- 248.Wang YF, Hatton GI. Mechanisms underlying oxytocin-induced excitation of supraoptic neurons: prostaglandin mediation of actin polymerization. J Neurophysiol. 2006;95:3933–3947. doi: 10.1152/jn.01267.2005. [DOI] [PubMed] [Google Scholar]
- 249.Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in the pars reticulata of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neuroscience. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- 250.Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, Nilaver G, van Wimersma Greidanus TB. Dynorphin and vasopressin: common localization in magnocellular neurons. Science. 1982;216:85–87. doi: 10.1126/science.6121376. [DOI] [PubMed] [Google Scholar]
- 251.Wichmann T, Dostrovsky JO. Pathological basal ganglia activity in movement disorders. Neuroscience. 2011;198:232–244. doi: 10.1016/j.neuroscience.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Widmer H, Ludwig M, Bancel F, Leng G, Dayanithi G. Neurosteroid regulation of oxytocin and vasopressin release from the rat supraoptic nucleus. J Physiol. 2003;548:233–244. doi: 10.1113/jphysiol.2002.036863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- 254.Witkovsky P, Patel JC, Lee CR, Rice ME. Immunocytochemical identification of proteins involved in dopamine release from the somatodendritic compartment of nigral dopaminergic neurons. Neuroscience. 2009;164:488–496. doi: 10.1016/j.neuroscience.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 256.Wotjak CT, Landgraf R, Engelmann M. Listening to neuropeptides by microdialysis: echoes and new sounds? Pharmacol Biochem Behav. 2008;90:125–134. doi: 10.1016/j.pbb.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 257.Wotjak CT, Ludwig M, Ebner K, Russell JA, Singewald N, Landgraf R, Engelmann M. Vasopressin from hypothalamic magnocellular neurons has opposite actions at the adenohypophysis and in the supraoptic nucleus on ACTH secretion. Eur J Neurosci. 2002;16:477–485. doi: 10.1046/j.1460-9568.2002.02101.x. [DOI] [PubMed] [Google Scholar]
- 258.Wotjak CT, Ludwig M, Landgraf R. Vasopressin facilitates its own release within the rat supraoptic nucleus in vivo. Neuroreport. 1994;5:1181–1184. doi: 10.1097/00001756-199406020-00005. [DOI] [PubMed] [Google Scholar]
- 259.Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 260.Yung KK. Localization of ionotropic and metabotropic glutamate receptors in distinct neuronal elements of the rat substantia nigra. Neurochem Inter. 1998;33:313–326. doi: 10.1016/s0197-0186(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 261.Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- 262.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–734. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Z, Bhalla A, Dean C, Chapman ER, Jackson MB. Synaptotagmin IV: a multifunctional regulator of peptidergic nerve terminals. Nature Neurosci. 2009;12:163–171. doi: 10.1038/nn.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang ZW, Kang JI, Vaucher E. Axonal varicosity density as an index of local neuronal interactions. PloS One. 2011;6:e22543. doi: 10.1371/journal.pone.0022543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. J Neurosci. 2009;29:10424–10435. doi: 10.1523/JNEUROSCI.4402-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zilberter Y, Harkany T, Holmgren CD. Dendritic release of retrograde messengers controls synaptic transmission in local neocortical networks. Neuroscientist. 2005;11:334–344. doi: 10.1177/1073858405275827. [DOI] [PubMed] [Google Scholar]