Abstract
Objective
Perioperative hypothermia is a common complication of anesthesia that can result in negative outcomes. The purpose of this review is to answer the question: Does the type of warming intervention influence the frequency or severity of inadvertent perioperative hypothermia (IPH) in surgical patients receiving neuraxial anesthesia?
Design
Systematic review and meta-analysis.
Setting
Perioperative care areas.
Patients
Adults undergoing surgery with neuraxial anesthesia.
Intervention
Perioperative active warming (AW) or passive warming (PW).
Measurements
PubMed, CINAHL, Embase, and Cochrane Central Register of Controlled Trials were searched. Inclusion criteria were: randomized controlled trials; adults undergoing surgery with neuraxial anesthesia; comparison(s) of AW and PW; and temperature measured at end of surgery/upon arrival in the Postanesthesia Care Unit. Exclusion criteria were: no full-text available; not published in English; studies of: combined neuraxial and general anesthesia, warm intravenous or irrigation fluids without using AW, and rewarming after hypothermia. Two independent reviewers screened abstracts and titles, and selected records following full-text review. The Cochrane Collaboration’s tool for assessing risk of bias was used to evaluate study quality. A random-effects model was used to calculate risk ratios for dichotomous data and mean differences for continuous data.
Main Results
Of 1587 records, 25 studies (2048 patients) were included in the qualitative synthesis. Eleven studies (1189 patients) comparing AW versus PW were included in the quantitative analysis. Meta-analysis found that intraoperative AW is more effective than PW in reducing the incidence of IPH during neuraxial anesthesia (RR = 0.71; 95% CI 0.61–0.83; P <0.0001; I2 = 32%). The qualitative synthesis revealed that IPH continues despite current AW technologies.
Conclusions
During neuraxial anesthesia, AW reduces IPH more effectively than PW. Even with AW, IPH persists in some patients. Continued innovation in AW technology and additional comparative effectiveness research studying different AW methods are needed.
Keywords: Anesthesia, Body Temperature, Heating, Hypothermia, Intraoperative Complications, Perioperative Care
1. Introduction
Hypothermia is well recognized as a common complication of surgery with anesthesia. In a recent study, 52% of total joint arthroplasty patients receiving neuraxial anesthesia (i.e. spinal, epidural) became hypothermic [1]. This inadvertent perioperative hypothermia (IPH) increases the risk of harmful patient outcomes, including: surgical site infection, morbid cardiac events, and bleeding; [2] and results in an increased length of hospital stay [3, 4].
Neuraxial anesthesia causes IPH by profoundly impairing thermoregulatory control in three ways. First, patients do not experience the magnitude of thermal discomfort that might be reasonably anticipated. Therefore, they do not complain of being cold even when they are hypothermic. Secondly, neuraxial anesthesia impairs central thermoregulatory control, reducing the vasoconstriction and shivering threshold by 0.5°C and elevating the sweating threshold by 0.3°C. The combined effect triples the interthreshold range triggering a physiologic response to cold [5]. And lastly, neuraxial anesthesia blocks efferent nerves that regulate autonomic thermoregulatory defenses, dramatically impairing vasoconstriction and shivering [6]. Shortly after administration of the neuraxial block, vasodilation shifts the warm blood from the core to the cooler peripheral tissues, resulting in a drop in core temperature and redistribution hypothermia. Because of impaired thermoregulatory control, this drop in temperature may be sustained during anesthesia.
The influence of neuraxial anesthesia on thermoregulation appears to be somewhat different than general anesthesia. In a 2016 study of total joint arthroplasty patients, those receiving neuraxial anesthesia were more likely to be hypothermic than those receiving general anesthesia (52% versus 48%, p<0.001) [1]. Therefore, the effectiveness of interventions to prevent IPH in patients receiving neuraxial anesthesia warrants separate evaluation.
A variety of warming interventions are available for prevention of IPH, including passive warming (PW) and active warming (AW). Passive warming includes interventions to promote heat retention (e.g. cotton blankets, reflective blankets). Active warming involves the application of external heat to skin and peripheral tissues (e.g. forced air warming (FAW), underbody conductive heat mat, circulating water mattress, and radiant warmer). The effectiveness of these interventions for patients receiving neuraxial anesthesia is unclear.
Previous systematic reviews have focused on the effectiveness of thermal insulation [7], warming of peritoneal gases during laparoscopy [8], using warmed intravenous or irrigation fluids [9], warming methods during Cesarean sections [10], rewarming after hypothermia [11], and prevention of shivering [12]. Issues encountered in these reviews include: heterogeneity, lack of control over covariates (e.g. fluid warming), and different types of outcome variables (temperature, temperature change, hypothermia). To date, no systematic reviews have compared the effectiveness of interventions for prevention of IPH specifically during neuraxial anesthesia.
1.1. Purpose
The purpose of this systematic review and meta-analysis was to answer the following PICO question: Does the type of warming intervention influence the frequency or severity of IPH in surgical patients receiving neuraxial anesthesia? The population is adult patients undergoing surgery with neuraxial anesthesia (spinal, epidural, or combined spinal-epidural). The interventions and comparisons are: intraoperative or pre- and intraoperative AW (FAW, conductive underbody warming, radiant heat warming, circulating water mattress), and PW (cotton blanket, prewarmed cotton blanket, reflective blanket/suit). The outcome is hypothermia or temperature change at the end of surgery or upon arrival in the Postanesthesia Care Unit (PACU). In accordance with multiple practice guidelines, we defined hypothermia as <36°C [13–15].
2. Methods
2.1. Systematic search
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) to conduct this systematic review and meta-analysis [16]. The PRISMA Checklist is included as Appendix A. The inclusion criteria were: 1) population - adult patients receiving neuraxial anesthesia for a surgical procedure; 2) intervention - AW or PW interventions administered intraoperatively or pre- and intraoperatively; 3) comparison - AW or PW interventions administered intraoperatively or pre- and intraoperatively; 4) outcome - temperature measured at the end of surgery or upon arrival in the PACU; 5) design - randomized controlled trials; and 6) published between database inception and April 2016. Exclusion criteria were: 1) conference abstracts without full-text articles; 2) not published in English; and 3) studies of: combined neuraxial with general anesthesia, distal nerve blocks or local anesthesia, warm IV and/or irrigation fluids as the primary warming intervention without AW, or rewarming after hypothermia.
We developed search strategies with the assistance of a health sciences librarian with expertise in searching for systematic reviews. Comprehensive strategies, including both index and keyword methods, were devised for the following databases: PubMed, CINAHL (Cumulative Index for Nursing Allied Health Literature, EBSCO platform), Embase (Elsevier platform), and the Cochrane Central Register of Controlled Trials (Wiley platform). No database preset limits were utilized in order to maximize sensitivity. Search filters previously validated for locating experimental studies were identified and utilized for PubMed, CINAHL, and Embase. [17, 18].
Searches were conducted during September and October 2015, and then updated in April 2016 to capture new records that became available during the screening and review process. The CINAHL search strategy, detailed in Box 1, was adapted for use with the other electronic databases. Complete search strategies including search filters are available upon request. We also searched the reference lists of relevant studies. We exported search results to EndNote® X7 (Clarivate Analytics, Philadelphia, PA) and removed duplicates electronically.
Box 1.
CINAHL search.
| MH ("Anesthesia, Conduction+" OR "Anesthetics, Local+" OR "Transurethral Resection of Prostate" OR "Prostatectomy+" OR "Arthroplasty+" OR "Anesthetics, Local+" OR "Cesarean Section+") OR TX (cesarean or caesarean or arthroplasty or prostatectomy or turp OR "transurethral resection") OR TX ((epidural OR spinal OR regional OR local) AND TX (anesthesia OR anaesthesia)) |
| AND |
| MH ("Warming Techniques" OR MH "Heating/MT") OR TX (“carbon fiber” OR “forced air” |
| OR “circulating water garment*” OR vitaheat OR vitalheat OR “bair hugger*” OR “hot dog” |
| OR hotdog OR “bair paw*” OR heat OR heated OR heating OR normothermia OR normothermic OR warm OR warming OR warmed OR warmth OR hot OR rewarming) |
| AND |
| PT clinical trial OR TX random* OR MH "Treatment Outcomes+" MH "Experimental Studies+" OR MH "Quantitative Studies" |
Reference for search filter: Wong S, Wilczynski N, Haynes R. Optimal CINAHL search strategies for identifying therapy studies and review records. J Nurs Scholarsh 2006;38:194-9. doi:10.1111/j.1547-5069.2006.00100.x
Two investigators independently evaluated the search results manually. Following the initial title and abstract screening, potentially eligible records were evaluated through full-text review. Discrepancies between the reviewers were resolved through discussion, and when necessary a third reviewer was consulted.
2.2. Data extraction
One investigator extracted data from eligible studies and a second investigator verified the accuracy of the extraction. Discrepancies were resolved through discussion. Extracted data included: sample size, anesthesia type, surgery type, warming intervention and comparator, temperature measurement device, hypothermia definition, and outcomes (mean temperature, mean temperature change, and incidence of hypothermia). When methodologies or results were unclear from manuscripts, investigators contacted the study authors for clarification.
2.3. Statistical analysis
We performed statistical analyses using the Review Manager Version 5.3 software (RevMan 5.3; The Cochrane Collaboration, Copenhagen, Denmark). We calculated risk ratios (RR) for dichotomous data and mean differences in continuous data with 95% confidence intervals (CI) using a random-effects model. This model was selected because although studies were similar, there were unique differences (surgical procedures, temperature measurement). P values of less than or equal to .05 were considered statistically significant. Statistical analyses comparing the effectiveness of interventions were only performed if three or more RCTs were present. Heterogeneity was evaluated by I2 calculation. I2 values were interpreted using the Cochrane criteria for measuring heterogeneity: 0% to 40% represents low heterogeneity; 30% to 60% represents moderate heterogeneity; 50% to 90% represents substantial heterogeneity; and 75% to 100% represents considerable heterogeneity [19].
2.4. Appraising quality and risk of bias
The Cochrane Collaboration’s tool for assessing risk of bias was used to evaluate study quality of the included RCTs [20]. One investigator extracted information on randomization, allocation concealment, blinding, attrition, selective reporting, and other biases (manufacturer funding, temperature site/device, control of fluid warming, and statistical power) for each included study. A second investigator verified the extracted data. Through discussion, each category for all included studies were graded as having low, unclear, or high risk of bias.
3. Results
3.1. Study selection
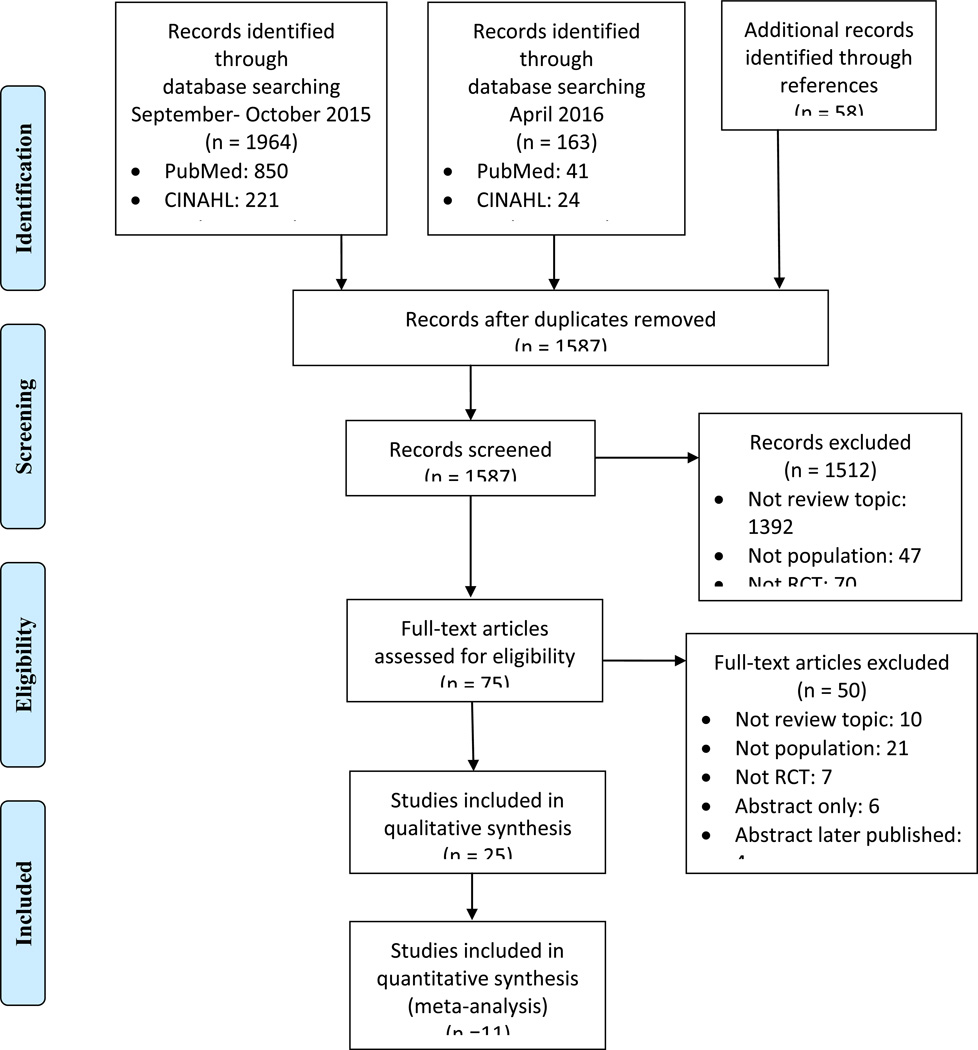
The initial systematic search yielded 1,964 records (Figure 1). The search was repeated for new publications six months following the initial search, yielding an additional 163 records. We identified 58 records through reference list searching. From these 2,185 records, we removed 598 duplicates and screened the titles and abstracts of the remaining 1,587 records. We excluded records that did not match the PICO question or were not randomized controlled trials. Next, we appraised the full-texts of 75 records. Fifty of these records were excluded because they did not match the PICO question, were not RCTs, no full-text was available, or the article was not in English. A total of 25 studies with 2,048 patients were included in the qualitative systematic review. We grouped studies for statistical analyses based on intervention, comparator, and outcome measure. Fourteen studies were excluded from the quantitative analysis because they were unable to be grouped with at least two other studies. A total of 11 studies were included in the quantitative meta-analysis with 1,189 patients.
Fig. 1.
PRISMA flow diagram
3.2. Study characteristics
Spinal anesthesia was used at least once in 20 studies, combined spinal-epidural was used in five studies, and epidural anesthesia was used in two studies (Table 1). Six surgery types were performed: C-section (n=9), total-hip arthroplasty (n=6), total-knee arthroplasty (n=5), transurethral resection of the prostate (n=4), lower abdominal (n=2), and unspecified lower limb surgeries (n=1). Twelve studies evaluated AW vs. PW interventions; eight studies evaluated AW vs. AW; three studies evaluated PW vs. PW; and two studies utilized a three-arm design and evaluated AW vs. AW vs. PW. Studies including emergent operations were not an a priori exclusion; however, all studies in the final analysis included patients undergoing non-emergent procedures that allowed for standard preoperative preparation.
Table 1.
Summary of Characteristics of Studies (n=25 included in qualitative review).
|
ACTIVE WARMING VS. PASSIVE WARMING (n=11 included in meta-analysis; *excluded from meta-analysis due to outcome reporting) | |||||||||
|
Author/Ye ar |
Sampl e Size |
Anesthes ia |
Surgery |
Intraoperative Comparisons (+ prewarming if specified) |
Outcome Measurement |
||||
|
Temp site |
Hypother mia |
||||||||
|
| |||||||||
| Benson et al. (2012)[35] |
30 | Spinal | TKA | FAW with pre-op warming |
Warm cotton blanket |
Oral | <36°C | ||
|
| |||||||||
| Butwick et al. (2007)[22] |
30 | Spinal | C- section |
FAW on lower extremities |
FAW blanket turned off |
Oral | <35.5°C | ||
|
| |||||||||
| Casati et al. (1999a)[36 ] |
50 | CSE | THA | FAW on upper body |
Reflective blank on upper body & non-operative lower extremity |
Bladder | <36°C | ||
|
| |||||||||
| Chakladar et al. (2014)[37] |
116 | Spinal, epidural, general: n=1 |
C- section |
Underbody conductive heat mat + warm IV fluids if >500ml administered |
Cotton sheet + warm IV fluids if >500ml administered |
Temporal artery |
<36°C | ||
|
| |||||||||
| *Chung et al. (2012)[40] |
45 | Spinal | C- section |
FAW on upper body with 15- minute pre-op warming |
Warm IV fluids |
FAW blanket turned off |
Tympani c & skin |
||
|
| |||||||||
| Cobb et al. (2016)[33] |
44 | Spinal | C- section |
FAW on lower extremities + warm IV fluids |
Cotton blankets | Temporal artery & bladder |
<36 °C | ||
|
| |||||||||
| Fallis et al. (2006)[24] |
62 | Spinal | C- section |
FAW on upper body + warm IV fluids |
Warm cotton blankets + warm IV fluids |
Oral | |||
|
| |||||||||
| Grant et al. (2015)[25] |
484 | Spinal, CSE, general: n=17 |
C- section |
Underbody conductive heat mat + warm blanket + reflective cap + warm IV & irrigation fluids |
Warm blanket + reflective cap + warm IV & irrigation fluids |
Oral & bladder |
<36°C | ||
|
| |||||||||
| Horn et al. (2002)[27] |
30 | Epidural | C- section |
FAW on upper body with 15- minute pre-op warming on full body + IV warm fluids |
Cotton blanket + IV warm fluids |
Tympani c & skin |
|||
|
| |||||||||
| Horn et al. (2014)[28] |
40 | Spinal | C- section |
FAW on upper body |
Warm blankets | Oral & skin |
<36°C | ||
|
| |||||||||
| Paris et al. (2014)[34] |
226 | Spinal | C- section |
Underbody conductive heat mat with pre-op warming |
Warm IV fluids |
Warm Blanket s |
Oral & bladder |
<36°C | |
|
| |||||||||
| Salazar et al. (2011)[23] |
150 | Spinal | TKA | FAW with 30- minute pre-op warming + warm IV fluids |
Cotton sheet | Tympani c & axillary |
<36°C, <35°C, <34°C |
||
|
| |||||||||
| PASSIVE WARMING VS. PASSIVE WARMING (n=3 not included in meta-analysis) | |||||||||
|
Author/Ye ar |
Sampl e Size |
Anesthes ia |
Surgery |
Intraoperative Comparisons (+ prewarming if specified) |
Outcome Measurement |
||||
| Temp Site |
Hypothermi a |
||||||||
|
| |||||||||
| Dyer & Heathcote (1986)[41] |
94 | Spinal | TURP | Reflecti ve blanket + warm irrigatio n solution |
Reflecti ve blanket on full body pre-op & upper body intra-op |
Warm irrigatio n solution |
Cotton blanke t |
Oral | |
|
| |||||||||
| Hindsholm et al. (1992)[42] |
30 | CSE | THA | Reflective blanket + 3 cotton blankets + warm IV fluids |
3 cotton blankets + warm IV fluids |
Tympani c, skin, & rectal |
|||
|
| |||||||||
| Hirvonen & Niskanen (2011)[21] |
39 | Spinal | TURP | Reflective suit with 60-minute pre-op warming + warm IV & irrigation fluids |
Cotton clothing + IV & irrigation warm fluids |
Oral | <35°C or report of feeling cold |
||
|
| |||||||||
| ACTIVE WARMING VS. ACTIVE WARMING (n=8 not included in meta-analysis) | |||||||||
|
Author/Ye ar |
Sampl e Size |
Anesthes ia |
Surgery |
Intraoperative Comparisons (+ prewarming if specified) |
Outcome Measurement |
||||
| Temp Site |
Hypothermi a |
||||||||
|
| |||||||||
| Casati et al. (1999b)[32] |
48 | CSE | THA | FAW on upper body | FAW on non- operative leg |
Bladder | <36°C | ||
|
| |||||||||
| Fanelli et al. (2009)[26] |
56 | Spinal | THA | FAW on upper body + warm IV fluids |
Underbody conductive heat mat + warm IV fluids |
Tympani c |
|||
|
| |||||||||
| Jo et al. (2015)[43] |
49 | Spinal | TURP | Circulating water mattress with 20- minute pre-op FAW |
Circulating water mattress |
Tympani c |
<36°C | ||
|
| |||||||||
| Kim et al. (2014)[44] |
46 | Spinal | TKA | FAW on upper body + warm IV fluids |
Circulating water mattress + warm IV fluids |
Tympani c & rectal |
|||
|
| |||||||||
| Koeter et al. (2013)[45] |
58 | Spinal, general: n=18 |
THA or TKA |
FAW on upper body + reflective blanket during transport |
FAW on upper body |
Tympani c |
<36°C | ||
|
| |||||||||
| Ng et al. (2006)[30] |
60 | CSE | TKA | FAW on upper body + warm IV fluids |
Underbody conductive heat mat + warm IV fluids |
Tympani c & rectal |
<36°C | ||
|
| |||||||||
| Torrie et al. (2005)[31] |
60 | Spinal | TURP | FAW on upper body + warm IV & irrigation fluids |
Radiant warmer directed at palm + warm IV & irrigation fluids |
Oral & rectal |
<36°C | ||
|
| |||||||||
| Winkler et al. (2000)[46] |
150 | Spinal | THA | Conventional FAW on full body + warm IV fluids |
Titrated FAW on full body + warm IV fluids |
Tympani c, skin, & bladder |
<36°C | ||
|
| |||||||||
|
ACTIVE WARMING VS. ACTIVE WARMING VS. PASSIVE WARMING (n=2 not included in meta- analysis) | |||||||||
|
Author/Ye ar |
Sampl e Size |
Anesthes ia |
Surgery |
Intraoperative Comparisons (+ prewarming if specified) |
Outcome Measurement |
||||
| Temp Site |
Hypothermi a |
||||||||
|
| |||||||||
| Vanni et al. (2007)[38] |
30 | Spinal | Lower abdomin al |
FAW on upper body + 45- minute pre-op FAW on full body |
FAW on upper body |
2 Cotton blankets |
Tympani c & skin |
<36°C | |
|
| |||||||||
| Yamakage et al. (1995)[29] |
21 | Spinal | Lower abdomin al or lower limb |
FAW on upper body + warm IV fluids |
FAW on lower body + warm IV fluids |
Cotton blanket + warm IV fluids |
Tympani c |
||
CSE: Combined Spinal-Epidural; FAW: Forced Air Warming; THA: Total Hip Arthroplasty; TKA: Total Knee Arthroplasty; TURP: Transurethral Resection of the Prostate
Outcome reporting of the included studies were heterogeneous. Outcomes were reported in one of three measures: 1) mean temperature at end of surgery or upon admission to PACU; 2) mean temperature change intraoperatively, at the end of surgery, upon PACU admission, unspecified, or the greatest change at any point; 3) percent/ratio of hypothermia intraoperatively, at the end of surgery, upon PACU admission, or unspecified during study period. Included studies defined hypothermia as temperatures <36°C (n=14), <35.5°C (n=1), and <35°C (n=1). See Appendix B for a complete description of outcomes.
Appendix B.
Summary of study outcomes & limitations (n=25 included in qualitative review).
|
ACTIVE WARMING VS. PASSIVE WARMING (n=11 included in meta-analysis; *excluded from meta-analysis due to outcome reporting) | |||||
|
AUTHOR/ YEAR |
RESULTS | CONCLUSIONS | LIMITATIONS | ||
|
Mean temperature (°C) |
Mean temperature change (°C) |
Hypothermia <36 °C or specified (#; %) |
|||
|
| |||||
| Benson et al. (2012)[35] |
PACU admission (p<0.001):
|
PACU admission:
|
Preoperative with intraoperative FAW is more effective than warmed cotton blankets. |
|
|
|
| |||||
| Butwick et al. (2007)[22] |
(p=0.8):
|
<35.5 (p=0.5):
|
Intraoperative FAW on lower extremities is more effective than no FAW. |
|
|
|
| |||||
| Casati et al. (1999a)[36] |
End of surgery (p<0.0005):
|
PACU admission (p<0.01):
|
Intraoperative FAW is more effective than reflective blanket. |
||
|
| |||||
| Chakladar et al. (2014)[37] |
End of Surgery (p=0.079):
admission (p=0.046):
|
PACU admission (p=0.043):
|
Intraoperative conductive heat mat warming more effective than a cotton sheet. |
|
|
|
| |||||
| *Chung et al. (2012)[40] |
Temp change at 45 min (p=0.004):
|
Preoperative with intraoperative FAW is as effective as warmed IV fluids, and both are more effective than passive warming. |
|
||
|
| |||||
| Cobb et al. (2016)[33] |
PACU admission (p=0.006):
|
PACU admission (p=0.031):
|
Intraoperative FAW in combination with warmed IV fluids is more effective than cotton blankets. |
|
|
|
| |||||
| Fallis et al. (2006)[24] |
End of surgery:
|
decrease in temps for both groups (p<0.001), but not between groups |
When IV fluids are warmed, there is no difference in effectiveness between intraoperative FAW and cotton blankets. |
|
|
|
| |||||
| Grant et al. (2015)[25] |
PACU admission (p=0.56):
|
PACU admission (p=0.169):
|
Conductive heat mat warming is more effective than cotton blankets. |
|
|
|
| |||||
| Horn et al. (2002)[27] |
End of surgery (p<0.01):
|
FAW is more effective than cotton blankets. |
|
||
|
| |||||
| Horn et al. (2014)[28] |
End of surgery (p=0.0007):
|
End of surgery:
|
FAW is more effective than warmed cotton blankets. |
||
|
| |||||
| Paris et al. (2014)[34] |
Intra-op (p<0.05):
|
Intra-op:
|
Conductive heat mat warming and warmed IV fluids are more effective than cotton blankets. Conductive heat mat warming is more effective than warmed IV fluids at maintaining normothermia outside of the intraoperative setting. |
|
|
|
| |||||
| Salazar et al. (2011)[23] |
|
During study period:
|
Preoperative and intraoperative FAW in combination with IV fluid warming is more effective than cotton blankets. |
|
|
|
| |||||
| ACTIVE VS. ACTIVE WARMING (n=8 not included in meta-analysis) | |||||
|
AUTHOR/ YEAR |
RESULTS | CONCLUSIONS | LIMITATIONS | ||
|
Mean temperature (°C) |
Mean temperature change (°C) |
Hypothermia <36 °C or specified (#; %) |
|||
|
| |||||
| Casati et al. (1999b)[32] |
End of surgery (p>0.05):
|
PACU arrival (p>0.05):
|
Upper body FAW is more effective than the lower body FAW. |
||
|
| |||||
| Fanelli et al. (2009)[26] |
End of surgery (p>0.05):
|
FAW and conductive heat mat warming are equally ineffective. |
|
||
|
| |||||
| Jo et al. (2015)[43] |
Core temperature significantly decreased in both groups (p<0.001), but changes were not significant between groups (p=0.763) |
Intra-op <35.5 (p=0.02):
admission <36 (p=0.32):
|
Prewarming with FAW significantly reduces the severity of hypothermia (<35.5), but does not maintain normothermia (>36) in combination with the circulating water mattress |
|
|
|
| |||||
| Kim et al. (2014)[44] |
Changes in core temperature were not statistically significant (p>0.05) between groups |
No difference in effectiveness between circulating water mattress and FAW. |
|
||
|
| |||||
| Koeter et al. (2013)[45] |
Lowest core (p>0.05):
|
No difference in effectiveness between FAW and combination of FAW and reflective blanket. |
|
||
|
| |||||
| Ng et al. (2006)[30] |
End of surgery (p>0.05):
|
No patients were <36 °C in either group at the end of surgery |
Intraoperative FAW and conductive heat warming are equally effective. |
|
|
|
| |||||
| Torrie et al. (2005)[31] |
End of surgery (p=0.03):
|
On PACU arrival (p=0.3):
|
FAW is more effective than radiant warming. |
|
|
|
| |||||
| Winkler et al. (2000)[46] |
Average intra-op (p<0.001):
|
Intra-op:
|
Titrating FAW based on patient temperature is more effective than conventional FAW. |
|
|
|
| |||||
|
ACTIVE WARMING VS. ACTIVE WARMING VS. PASSIVE WARMING (n=2 not included in meta- analysis) | |||||
|
AUTHOR/ YEAR |
RESULTS | CONCLUSIONS | LIMITATIONS | ||
|
Mean temperature (°C) |
Mean temperature change (°C) |
Hypothermia <36 °C or specified (#; %) |
|||
|
| |||||
| Vanni et al. (2007)[38] |
End of prewarming (p<0.05):
induction (p>0.05):
(p<0.05):
|
End of surgery:
admission:
|
Intraoperative FAW is more effective than cotton blankets. Preoperative FAW does not increase the effectiveness of intraoperative FAW. |
||
|
| |||||
| Yamakage et al. (1995)[29] |
At 40 min intraop (p<0.05):
|
Lower body FAW blanket is more effective than upper body FAW blanket or cotton blanket. |
|
||
|
| |||||
| PASSIVE VS. PASSIVE WARMING (n=3 not included in meta-analysis) | |||||
|
AUTHOR/ YEAR |
RESULTS | CONCLUSIONS | LIMITATIONS | ||
|
Mean temperature (°C) |
Mean temperature change (°C) |
Hypothermia <36 °C or specified (#; %) |
|||
|
| |||||
| Dyer & Heathcote (1986)[41] |
Greatest mean:
(p<0.01–0.05) I & II & III: (p>0.05) |
Reflective blankets are more effective than cotton blankets. |
|
||
|
| |||||
| Hindsholm et al. (1992)[42] |
|
Reflective blankets are more effective than cotton blankets. |
|
||
|
| |||||
| Hirvonen & Niskanen (2011)[21] |
End of surgery (p=0.077):
(p=0.03):
|
PACU admission (p<0.001):
|
PACU admission (<35°C):
|
Reflective blankets are more effective than cotton blankets. |
|
3.3. Risk of bias evaluation
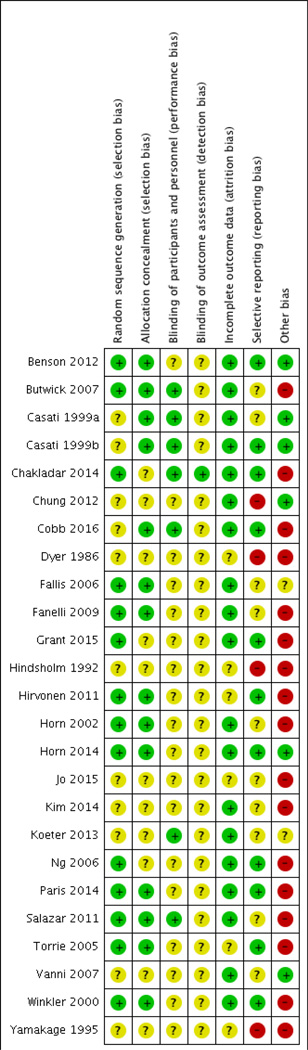
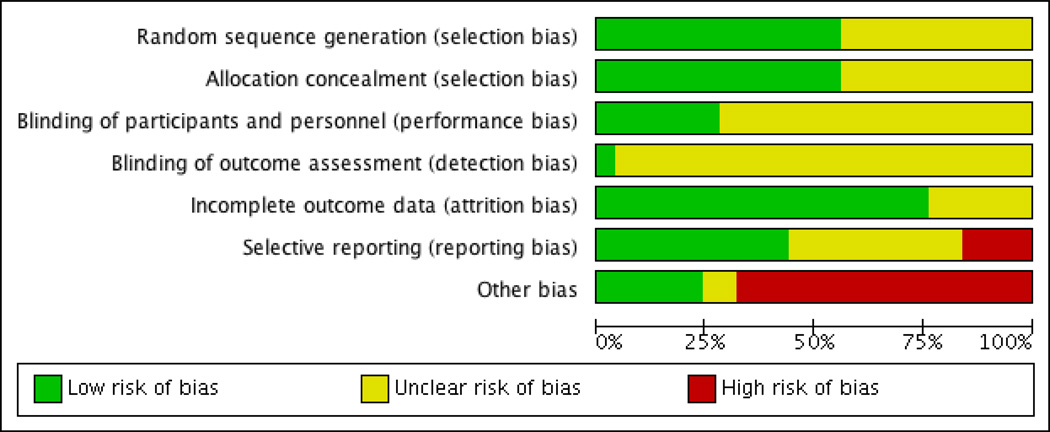
Studies reporting randomization and allocation without a description of procedures were rated as having unclear risk of bias per the Cochrane Collaboration standards (Figure 2)[19]. Seven of the 25 studies attempted to blind the study staff measuring and recording temperatures. The other studies cited difficulty in concealing the warming intervention from hospital staff; these studies were categorized as having an unclear risk of bias. Most studies reported all data on patients consented, due to the short duration of the trial. Other biases include: analysis not controlling for administration of warm IV/irrigation fluids, multiple temperature sites with multiple devices, lack of statistical power, and manufacturer funding. The overall assessment indicates a moderate level of bias (Figure 3). Individual study limitations are included in Appendix B.
Fig. 2.
Risk of Bias within the included studies (n=25).
Fig. 3.
Risk of Bias across the included studies (n=25).
3.4. Qualitative results: Systematic review
Twenty-five studies were included in the qualitative review and synthesis. The primary interventions compared included PW (cotton blankets, reflective blankets) and AW (FAW, conductive heat mat).
3.4.1. Passive warming
Fourteen studies utilized cotton blankets reporting temperatures as low as 35.2±0.5°C upon arrival in the PACU [21] and temperature changes as substantial as −1.3±0.3°C [22]. In one study of older adults, all subjects receiving cotton blankets were hypothermic with a temperature less than 36°C and 88% with a temperature less than 35°C [23]. Four studies evaluated reflective blankets or suits. All studies reported low temperatures, large temperature decreases, or a high percent of subjects with IPH with the use of reflective blankets/suits, cotton blankets, and FAW covers alone without warm forced-air. Even in studies reporting no significant difference between PW and AW outcomes, PW did not consistently prevent IPH [22, 24, 25].
3.4.2 Active warming: Forced air warming
The impact of FAW varied tremendously among the 19 studies evaluating its effectiveness. The lowest and highest reported mean temperatures when patients received FAW were 35.3±0.5 [26] and 37.1±0.4 [27], respectively. One study of patients undergoing C-sections reported that 53% of temperatures dropped below 35.5°C with FAW [22]. In contrast, another study of patients undergoing C-sections reported that only 5% of temperatures dropped below 36°C at the end of surgery with FAW [28]. Of the five studies reporting mean temperature change with FAW use, the greatest temperature drop was 1.3±0.4°C [22], while another study reported no change in temperature from baseline with use of intraoperative lower body FAW [29].
Since the full body surface cannot be exposed to FAW during some surgeries, 12 studies clarified if FAW was utilized on the upper or lower body. Nine studies evaluated FAW use on the upper body with the highest mean temperature reported as 37.1±0.4°C [27] and the lowest percent hypothermia reported was 0% of patients [30]; the lowest mean temperature reported was 35.3±0.5°C [26], and the highest percent hypothermia was 33% of patients [31]. Within the three studies that evaluated FAW on the lower body, the highest mean temperature reported was 36.3±0.5°C with 12.5% of patients hypothermic[32]; the lowest mean temperature was 35.9±0.5°C with 64% hypothermic [33]. One study of patients undergoing total hip arthroplasty with a combined spinal-epidural anesthesia, compared the effectiveness of using FAW on the upper body versus FAW on the nonoperative lower extremity. No significant difference was found in temperature at the end of surgery or upon admission to the PACU [32].
3.4.3. Active warming: Conductive heat mat
A conductive heat mat was evaluated in five studies and results again varied. Four different brands of mats were evaluated. The lowest and highest reported mean temperatures at the end of surgery when a conductive heat mat was used were 35.1±0.6°C [26], and 36.9±0.4°C [30] respectively. Another study found that 51% of patients receiving the conductive heat mat were hypothermic upon admission to the PACU [34]. Two studies compared the conductive heat mat with FAW, and both found no significant differences in patient temperatures between the two groups [26, 30].
3.5. Quantitative results: Meta-analysis
Statistical analyses were performed on studies evaluating types of AW versus PW. Outcomes for AW versus PW were either reported as continuous—mean temperature, or dichotomous—normothermic or hypothermic. Additional subgroup analyses were identified post hoc and were performed to evaluate if there is a difference between AW device, AW application time, IV/irrigation fluid temperature, and procedure type when compared to PW. Head to head statistical analyses of PW versus PW and AW versus AW were not performed because there were fewer than three RCTs that performed the same intervention with the same outcome reporting measure. Subsequently, 14 studies were excluded, leaving 11 studies in the statistical analysis.
3.5.1. Dichotomous outcome
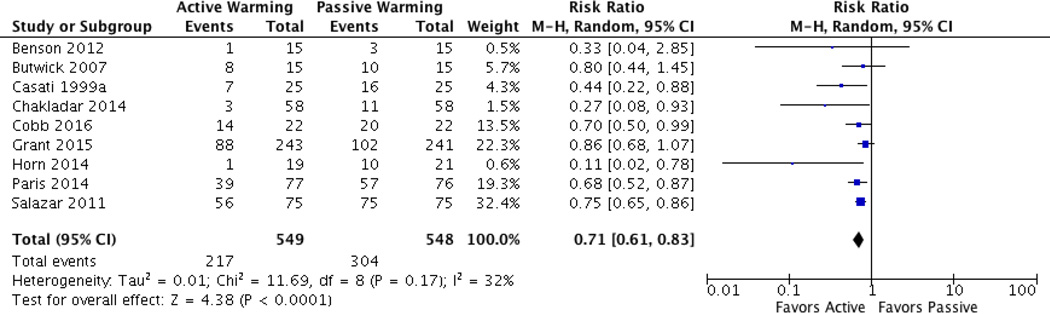
Nine studies evaluated active versus passive warming and reported dichotomous outcomes of percent/ratio of hypothermic patients at the end of surgery or admission to PACU [22, 23, 25, 28, 33–37]. One additional study met these criteria, but was ultimately excluded from analysis because the authors did not report separate results for each group in a three-arm design; rather, they reported total hypothermia present [38]. Pooled analysis of these nine studies found that intraoperative active warming significantly reduced hypothermia rates (RR = 0.71; 95% CI 0.61–0.83; P < 0.0001; I2 = 32%) (Figure 4). Subgroup analyses determined that PW is less effective than AW in preventing hypothermia using: a) FAW, b) conductive heat mat, c) intraoperative AW only, d) pre- and intraoperative AW, e) AW and warm IV/irrigation fluids, f) AW and room temperature fluids, and g) AW during C-sections. A significant difference in hypothermia rates was not found with the use of AW when compared to PW in total joint arthroplasties (Table 2).
Fig. 4.
Dichotomous data (hypothermia vs. normothermia) forest plot for AW vs. PW.
Table 2.
Meta-analysis of dichotomous data (normothermia vs. hypothermia).
| Comparison | No. of Studies |
RR (95% CI) |
P Value |
I2 | |
|---|---|---|---|---|---|
| AW vs. PW (overall) | 9 | 0.71 [0.61, 0.83] |
<0.0001 | 32% | |
|
| |||||
| AW device vs. PW | Forced-air | 6 | 0.66 [0.49, 0.88] |
0.004 | 47% |
| Conductive heat mat |
3 | 0.72 [0.53, 0.98] |
0.04 | 57% | |
|
| |||||
| AW application time vs. PW | Intraoperative | 6 | 0.65 [0.47, 0.89] |
0.008 | 52% |
| Pre- and intraoperative |
3 | 0.73 [0.65, 0.82] |
<0.0001 | 0% | |
|
| |||||
| IV/irrigation fluid temperature vs. PW |
Warmed | 4 | 0.76 [0.65, 0.88] |
0.0004 | 27% |
| Room temperature | 5 | 0.59 [0.40, 0.87] |
0.007 | 34% | |
|
| |||||
| Procedure type with AW vs. PW |
Total joint arthroplasty |
3 | 0.61 [0.36, 1.03] |
0.06 | 48% |
| C-section | 6 | 0.71 [0.57, 0.89] |
0.003 | 42% | |
AW = Active Warming; PW = Passive Warming
3.5.2. Continuous outcome
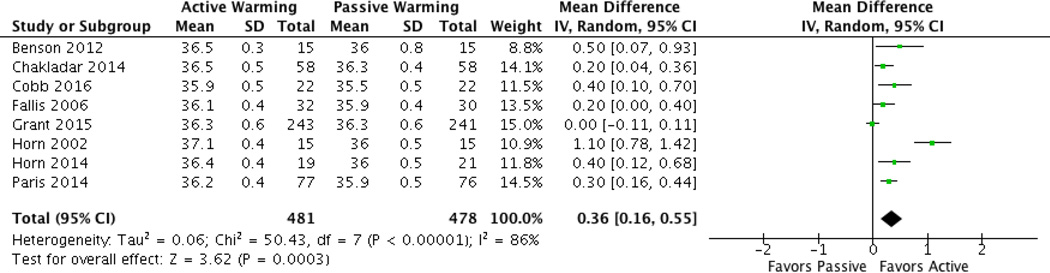
Eight studies—including six from the dichotomous analyses—reported mean temperatures at the end of surgery or admission to PACU [24, 25, 27, 28, 33–35, 37]. Pooled analysis found that temperatures were significantly different between the intraoperative active and passive warming groups (Mean Difference = 0.36; 95% CI 0.16–0.55; P =0.0003; I2 = 86%) (Figure 5). However, heterogeneity for mean temperature as a continuous variable was considerable at 86%. This significant heterogeneity is similar to a previous meta-analysis on perioperative warming during C-sections that used continuous outcome variables for statistical tests [10].
Fig. 5.
Continuous data (mean temperature) forest plot for AW vs. PW.
Subgroup analyses for continuous data concluded that mean temperatures were significantly lower when PW was used compared to a) FAW, b) intraoperative AW only, c) pre- and intraoperative AW, d) AW and warm IV/irrigation fluids, e) AW and room temperature IV/irrigation fluids, and f) AW in C-sections. No significant difference in mean temperature was found between AW with the conductive heat mat and PW. Considerable heterogeneity was maintained with subgroup analyses, except in the room temperature fluid subgroup where I2 = 0% (See Table 3).
Table 3.
Meta-analysis for continuous data (mean temperature).
| Comparison | No. of Studies |
Mean Difference (95% CI) |
P Value | I2 | |
|---|---|---|---|---|---|
| AW vs. PW (overall) | 8 | 0.36 [0.16, 0.55] | 0.0003 | 86% | |
|
| |||||
| AW device vs. PW | Forced-air | 5 | 0.51 [0.20, 0.81] | 0.001 | 82% |
| Conductive heat mat | 3 | 0.16 [−0.03, 0.35] | 0.10 | 83% | |
|
| |||||
| AW application time vs. PW |
Intraoperative | 5 | 0.21 [0.05, 0.37] | 0.01 | 71% |
| Pre- + intraoperative | 3 | 0.62 [0.65, 1.15] | 0.02 | 90% | |
|
| |||||
| IV/irrigation fluid temperature vs. PW |
Warm | 5 | 0.35 [0.07, 0.64] | 0.02 | 91% |
| Room temperature | 3 | 0.34 [0.21, 0.46] | <0.00001 | 0% | |
|
| |||||
| Procedure type with AW vs. PW |
C-section | 7 | 0.34 [0.14, 0.54] | 0.001 | 88% |
AW = Active Warming; PW = Passive Warming
4. DISCUSSION
4.1. Summary of evidence
This is the first systematic review and meta-analysis comparing the effectiveness of warming interventions for the prevention of IPH in patients receiving neuraxial anesthesia. We included 25 studies (n= 2,048 patients) in the qualitative synthesis and 11 studies (n = 1,189 patients) in the meta-analysis. The results of this systematic review and meta-analysis provide key findings. First, PW does not maintain normothermia in surgical procedures with neuraxial anesthesia. Although cotton blankets are very commonly used in clinical practice, this is an ineffective intervention for preventing hypothermia. In the 14 studies evaluating cotton blankets, mean temperatures were as low as 35.2±0.5°C upon arrival in the PACU [21] and in one study, 88% of elderly patients had a temperature less than 35°C [23]. This was supported by our meta-analysis of 11 studies evaluating outcomes of PW versus AW. When PW was used, temperatures at the end of surgery or upon admission to PACU were significantly lower (P = 0.0003) and a significantly greater proportion of patients were hypothermic (P < 0.0001) when compared to AW.
Secondly, we found that intraoperative AW is more effective than PW at reducing the incidence of IPH in patients receiving neuraxial anesthesia. In five studies (n = 206 patients), mean temperatures were significantly lower when PW was used compared to AW (P = 0.001); and in six studies (n = 344 patients) more patients were hypothermic (P = 0.004). This is clinically relevant, because over 55% of the patients receiving PW intraoperatively were hypothermic, whereas less than 40% were hypothermic when intraoperative AW was utilized (P < 0.0001); reflecting a 29% decreased risk of IPH with the use of intraoperative AW during neuraxial anesthesia.
Third, although intraoperative AW reduces the incidence of IPH when compared to PW, our systematic review found that AW did not consistently prevent the IPH with neuraxial anesthesia. Our meta-analysis of three studies (n = 213 patients) found that using AW pre- and intraoperatively resulted in the greatest mean temperature difference between AW and PW (P = 0.02). Preoperative AW decreases the temperature gradient between the core and peripheral tissues when anesthesia is initiated, thus minimizing redistribution [6]. Despite these influential findings, studies evaluating prewarming with neuraxial anesthesia cited difficulties in maintaining active warming interventions during anesthesia induction [38].
4.2. Limitations
A limitation of this analysis is the potential bias for authors of included studies to selectively report outcomes. In some studies, temperature was measured every five to thirty-minutes but not all temperatures were reported. This lack of standardization in outcome reporting limited our ability to include a larger number of studies in the statistical analysis of continuous outcome data. Temperature measurement sites varied between studies and although invasive temperature measures are more accurate [39], they are not feasible during neuraxial anesthesia. The control over covariates was unclear in some studies, for example, use for warm vs. room temperature IV and irrigation fluids. Many studies gave vague explanations of randomization, allocation, and blinding, leaving these bias ratings unclear. Additionally, the heterogeneity of the continuous outcome analysis was considerable. We recommend that future studies give detailed descriptions of methods and report complete outcomes including mean temperatures to ensure future comparisons of AW devices.
5. Conclusion
Perioperative hypothermia is a serious perioperative concern and can result in negative patient outcomes [2]. Understanding the effectiveness of preventive measures is essential. This review confirms that utilization of PW interventions consistently results in low temperatures, large temperature changes, and a higher incidence of hypothermic patients. Even in the studies that found no difference between AW and PW, most subjects did not maintain normothermia with the PW interventions. This is similar to findings of studies of patents under general anesthesia[7]. Passive warming is only acceptable when used for comfort in the perioperative setting, and should not be considered an intervention to prevent IPH. Active warming should be used for patients receiving neuraxial anesthesia. However, our systematic review found that perioperative hypothermia persists with current AW technology. Further research is needed to examine how to improve the technology and use of AW with a focus on head-to-head comparisons of different AW methods, controlling for covariates and when feasible, and reporting actual core body temperatures.
Highlights.
Perioperative hypothermia is a common complication of neuraxial anesthesia.
Perioperative hypothermia increases the risk of negative patient outcomes.
Active warming (AW) is superior to passive warming during neuraxial anesthesia.
Perioperative hypothermia still occurs in some patients receiving AW.
Innovation in AW technology and comparative effectiveness research are needed.
Acknowledgments
Disclosure information: This paper was funded by the Agency for Healthcare Research and Quality grant 3 IR18HS021422-01A1. Dr. Steelman is a consultant for VitaHeat Medical and 3M (St. Paul, MN).
Appendix A
PRISMA checklist
| Section/topic | # | Checklist item | Reported on page # or section # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | p. 1 |
| ABSTRACT | |||
| Structured summary |
2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. |
p. 2–3 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | Section 1 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). |
Section 1.1 |
| METHODS | |||
| Protocol and registration |
5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. |
Not published |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. |
Section 2.1 |
| Information sources |
7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. |
Section 2.1; Fig. 1 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. |
Section 2.1; Box 1 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). |
Section 2.1; Section 2.3; Fig 1 |
| Data collection process |
10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. |
Section 2.1; Section 2.2 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. |
Section 2 |
| Risk of bias in individual studies |
12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. |
Section 2.4 |
| Summary measures |
13 | State the principal summary measures (e.g., risk ratio, difference in means). | Section 2.3 |
| Synthesis of results |
14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. |
Section 2.2; Section 2.3 |
| Risk of bias across studies |
15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). |
Section 2.4 |
| Additional analyses |
16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. |
Section 3.5 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. |
Section 3.1; Fig 1 |
| Study characteristics |
18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. |
Section 3.2; Table 1; Appendix B |
| Risk of bias within studies |
19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). |
Section 3.3; Fig. 2 |
| Results of individual studies |
20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. |
Section 3.4, 3.4.1, 3.4.2, 3.4.3; Section 3.5, 3.5.1, 3.5.2; Fig. 4; Fig 5; Table 2; Table 3; Appendix B |
| Synthesis of results |
21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. |
Section 3.5.1, 3.5.2; Fig. 4; Fig 5; Table 2; Table 3; |
| Risk of bias across studies |
22 | Present results of any assessment of risk of bias across studies (see Item 15). | Section 3.3; Fig. 3 |
| Additional analysis |
23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). |
Section 3.5.1, 3.5.2; Table 2; Table 3; |
| DISCUSSION | |||
| Summary of evidence |
24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). |
Section 4.1 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). |
Section 4.2 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. |
Section 4.1; Section 5 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. |
1 |
Adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frisch NB, Pepper AM, Rooney E, Silverton C. Intraoperative Hypothermia in Total Hip and Knee Arthroplasty. Orthopedics. 2016:1–8. doi: 10.3928/01477447-20161017-04. [DOI] [PubMed] [Google Scholar]
- 2.Madrid E, Urrutia G, Roque i Figuls M, Pardo-Hernandez H, Campos JM, Paniagua P, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev. 2016;4:CD009016. doi: 10.1002/14651858.CD009016.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. The New England journal of medicine. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 4.Sun Z, Honar H, Sessler DI, Dalton JE, Yang D, Panjasawatwong K, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology. 2015;122:276–285. doi: 10.1097/ALN.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol. 2008;22:627–644. doi: 10.1016/j.bpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655–2664. doi: 10.1016/S0140-6736(15)00981-2. [DOI] [PubMed] [Google Scholar]
- 7.Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2014:CD009908. doi: 10.1002/14651858.CD009908.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch DW, Dang JT, Switzer NJ, Manouchehri N, Shi X, Hadi G, et al. Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev. 2016;10:CD007821. doi: 10.1002/14651858.CD007821.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell G, Alderson P, Smith AF, Warttig S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2015:CD009891. doi: 10.1002/14651858.CD009891.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultan P, Habib AS, Cho Y, Carvalho B. The Effect of patient warming during Caesarean delivery on maternal and neonatal outcomes: a meta-analysis. Br J Anaesth. 2015;115:500–510. doi: 10.1093/bja/aev325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warttig S, Alderson P, Campbell G, Smith AF. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database Syst Rev. 2014:CD009892. doi: 10.1002/14651858.CD009892.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis SR, Nicholson A, Smith AF, Alderson P. Alpha-2 adrenergic agonists for the prevention of shivering following general anaesthesia. Cochrane Database Syst Rev. 2015:CD011107. doi: 10.1002/14651858.CD011107.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card RSM, Degnan B, Harder K, Kemper J, Marshall M, Matteson M, Roemer R, Schuller-Bebus G, Swanson C, Stultz J, Sypura W, Terrell C, Varela N. Perioperative Protocol. Institute for Clinical Systems Improvement. 2014 [Google Scholar]
- 14.Hooper VD, Chard R, Clifford T, Fetzer S, Fossum S, Godden B, et al. ASPAN’s Evidence-Based Clinical Practice Guideline for the Promotion of Perioperative Normothermia: second edition. Journal of PeriAnesthesia Nursing. 2010;25:346–365. 20p. doi: 10.1016/j.jopan.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 15.NICE. Hypothermia: prevention and management in adults having surgery. National Institute for Health and Care Excellence guideline. 2008 [PubMed] [Google Scholar]
- 16.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic reviews. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR, Hedges T. Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ. 2005;330:1179. doi: 10.1136/bmj.38446.498542.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SS, Wilczynski NL, Haynes RB. Optimal CINAHL search strategies for identifying therapy studies and review articles. J Nurs Scholarsh. 2006;38:194–199. doi: 10.1111/j.1547-5069.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 19.Deeks J, Higgins J, Altman D. Analysing data and undertaking meta-analyses. Cochrane Handbook for Systemtic Reviews of Interventions: The Cochrane Collaboration. 2011 [Google Scholar]
- 20.Higgins J, Altman D, Sterne J. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions: The Cohrane Collaboration. 2011 [Google Scholar]
- 21.Hirvonen EA, Niskanen M. Thermal suits as an alternative way to keep patients warm peri-operatively: a randomised trial. European journal of anaesthesiology. 2011;28:376–381. doi: 10.1097/EJA.0b013e328340507d. [DOI] [PubMed] [Google Scholar]
- 22.Butwick AJ, Lipman SS, Carvalho B. Intraoperative forced air-warming during cesarean delivery under spinal anesthesia does not prevent maternal hypothermia. Anesthesia and analgesia. 2007;105:1413–1419. doi: 10.1213/01.ane.0000286167.96410.27. [DOI] [PubMed] [Google Scholar]
- 23.Salazar F, Donate M, Boget T, Bogdanovich A, Basora M, Torres F, et al. Intraoperative warming and post-operative cognitive dysfunction after total knee replacement. Acta anaesthesiologica Scandinavica. 2011;55:216–222. doi: 10.1111/j.1399-6576.2010.02362.x. [DOI] [PubMed] [Google Scholar]
- 24.Fallis WM, Hamelin K, Symonds J, Wang X. Maternal and newborn outcomes related to maternal warming during cesarean delivery. Journal of obstetric, gynecologic, and neonatal nursing. 2006;35:324–331. doi: 10.1111/j.1552-6909.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 25.Grant EN, Craig MG, Tao W, McIntire DD, Leveno KJ. Active Warming during Cesarean Delivery: Should We SCIP It? American journal of perinatology. 2015;32:933–938. doi: 10.1055/s-0034-1543986. [DOI] [PubMed] [Google Scholar]
- 26.Fanelli A, Danelli G, Ghisi D, Ortu A, Moschini E, Fanelli G. The efficacy of a resistive heating under-patient blanket versus a forced-air warming system: a randomized controlled trial. Anesthesia and analgesia. 2009;108:199–201. doi: 10.1213/ane.0b013e31818e6199. [DOI] [PubMed] [Google Scholar]
- 27.Horn EP, Schroeder F, Gottschalk A, Sessler DI, Hiltmeyer N, Standl T, et al. Active warming during cesarean delivery. Anesthesia and analgesia. 2002;94:409–414. doi: 10.1097/00000539-200202000-00034. [DOI] [PubMed] [Google Scholar]
- 28.Horn EP, Bein B, Steinfath M, Ramaker K, Buchloh B, Hocker J. The incidence and prevention of hypothermia in newborn bonding after cesarean delivery: a randomized controlled trial. Anesthesia and analgesia. 2014;118:997–1002. doi: 10.1213/ANE.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 29.Yamakage M, Kawana S, Yamauchi M, Kohro S, Namiki A. Evaluation of a forced-air warning system during spinal anesthesia. Journal of anesthesia. 1995;9:93–95. doi: 10.1007/BF02482048. [DOI] [PubMed] [Google Scholar]
- 30.Ng V, Lai A, Ho V. Comparison of forced-air warming and electric heating pad for maintenance of body temperature during total knee replacement. Anaesthesia. 2006;61:1100–1104. doi: 10.1111/j.1365-2044.2006.04816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torrie JJ, Yip P, Robinson E. Comparison of forced-air warming and radiant heating during transurethral prostatic resection under spinal anaesthesia. Anaesthesia and intensive care. 2005;33:733–738. doi: 10.1177/0310057X0503300605. [DOI] [PubMed] [Google Scholar]
- 32.Casati A, Baroncini S, Pattono R, Fanelli G, Bonarelli S, Musto P, et al. Effects of sympathetic blockade on the efficiency of forced-air warming during combined spinal-epidural anesthesia for total hip arthroplasty. Journal of clinical anesthesia. 1999;11:360–363. doi: 10.1016/s0952-8180(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 33.Cobb B, Cho Y, Hilton G, Ting V, Carvalho B. Active Warming Utilizing Combined IV Fluid and Forced-Air Warming Decreases Hypothermia and Improves Maternal Comfort During Cesarean Delivery: A Randomized Control Trial. Anesthesia and analgesia. 2016 doi: 10.1213/ANE.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 34.Paris LG, Seitz M, McElroy KG, Regan M. A randomized controlled trial to improve outcomes utilizing various warming techniques during cesarean birth. Journal of obstetric, gynecologic, and neonatal nursing. 2014;43:719–728. doi: 10.1111/1552-6909.12510. [DOI] [PubMed] [Google Scholar]
- 35.Benson EE, McMillan DE, Ong B. The effects of active warming on patient temperature and pain after total knee arthroplasty. The American journal of nursing. 2012;112:26–33. doi: 10.1097/01.NAJ.0000414315.41460.bf. [DOI] [PubMed] [Google Scholar]
- 36.Casati A, Fanelli G, Ricci A, Musto P, Cedrati V, Altimari G, et al. Shortening the discharging time after total hip replacement under combined spinal/epidural anesthesia by actively warming the patient during surgery. Minerva anestesiologica. 1999;65:507–514. [PubMed] [Google Scholar]
- 37.Chakladar A, Dixon MJ, Crook D, Harper CM. The effects of a resistive warming mattress during caesarean section: a randomised, controlled trial. International journal of obstetric anesthesia. 2014;23:309–316. doi: 10.1016/j.ijoa.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 38.D'Angelo Vanni SM, Castiglia YM, Ganem EM, Rodrigues Junior GR, Amorim RB, Ferrari F, et al. Preoperative warming combined with intraoperative skin-surface warming does not avoid hypothermia caused by spinal anesthesia in patients with midazolam premedication. Sao Paulo medical journal. 2007;125:144–149. doi: 10.1590/S1516-31802007000300004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niven DJ, Gaudet JE, Laupland KB, Mrklas KJ, Roberts DJ, Stelfox HT. Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Annals of internal medicine. 2015;163:768–777. doi: 10.7326/M15-1150. [DOI] [PubMed] [Google Scholar]
- 40.Chung SH, Lee BS, Yang HJ, Kweon KS, Kim HH, Song J, et al. Effect of preoperative warming during cesarean section under spinal anesthesia. Korean journal of anesthesiology. 2012;62:454–460. doi: 10.4097/kjae.2012.62.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dyer PM, Heathcote PS. Reduction of heat loss during transurethral resection of the prostate. Anaesthesia and intensive care. 1986;14:12–16. doi: 10.1177/0310057X8601400104. [DOI] [PubMed] [Google Scholar]
- 42.Hindsholm KB, Bredahl C, Herlevsen P, Kruhoffer PK. Reflective blankets used for reduction of heat loss during regional anaesthesia. British journal of anaesthesia. 1992;68:531–533. doi: 10.1093/bja/68.5.531. [DOI] [PubMed] [Google Scholar]
- 43.Jo YY, Chang YJ, Kim YB, Lee S, Kwak HJ. Effect of preoperative forced-air warming on hypothermia in elderly patients undergoing transurethral resection of the prostate. Urology journal. 2015;12:2366–2370. [PubMed] [Google Scholar]
- 44.Kim HY, Lee KC, Lee MJ, Kim MN, Kim JS, Lee WS, et al. Comparison of the efficacy of a forced-air warming system and circulating-water mattress on core temperature and post-anesthesia shivering in elderly patients undergoing total knee arthroplasty under spinal anesthesia. Korean journal of anesthesiology. 2014;66:352–357. doi: 10.4097/kjae.2014.66.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koeter M, Leijtens B, Koeter S. Effect of thermal reflective blanket placement on hypothermia in primary unilateral total hip or knee arthroplasty. Journal of perianesthesia nursing. 2013;28:347–352. doi: 10.1016/j.jopan.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Winkler M, Akca O, Birkenberg B, Hetz H, Scheck T, Arkilic CF, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesthesia and analgesia. 2000;91:978–984. doi: 10.1097/00000539-200010000-00039. [DOI] [PubMed] [Google Scholar]