Abstract
Background
Clostridium difficile infection (CDI) is a frequent cause of diarrhea among allogeneic hematopoietic cell transplant (HCT) recipients. It is unknown whether risk factors for CDI vary by time posttransplant.
Methods
We performed a 3-year prospective cohort study of CDI in allogeneic HCT recipients. Participants were enrolled during their transplant hospitalizations. Clinical assessments were performed weekly during hospitalizations and for 12 weeks posttransplant, and monthly for 30 months thereafter. Data were collected through patient interviews and chart review, and included CDI diagnosis, demographics, transplant characteristics, medications, infections, and outcomes. CDI cases were included if they occurred within 1 year of HCT and were stratified by time from transplant. Multivariable logistic regression was used to determine risk factors for CDI.
Results
One hundred eighty-seven allogeneic HCT recipients were enrolled, including 63 (34%) patients who developed CDI. 38 (60%) CDI cases occurred during the preengraftment period (days 0-30 post-HCT) and 25 (40%) postengraftment (day >30). Lack of any preexisting comorbid disease was significantly associated with lower risk of CDI preengraftment (odds ratio [OR], 0.3; 95% confidence interval [CI], 0.1-0.9). Relapsed underlying disease (OR, 6.7; 95% CI, 1.3-33.1), receipt of any high-risk antimicrobials (OR, 11.8; 95% CI, 2.9-47.8), and graft-versus-host disease (OR, 7.8; 95% CI, 2.0-30.2) were significant independent risk factors for CDI postengraftment.
Conclusions
A large portion of CDI cases occurred during the postengraftment period in allogeneic HCT recipients, suggesting that surveillance for CDI should continue beyond the transplant hospitalization and preengraftment period. Patients with continued high underlying severity of illness were at increased risk of CDI postengraftment.
Clostridium difficile infection (CDI) is a common infectious complication of allogeneic hematopoietic cell transplantation (HCT), but the epidemiology, risk factors, and outcomes of CDI in these patients are poorly understood. Estimates of CDI incidence among allogeneic HCT recipients vary widely, with an upper range of approximately 30%.1-9 Many studies of CDI in this patient population are limited to autologous HCT recipients10-13; other studies combine allogeneic and autologous HCT recipients1,4,14-16 and such combined study results may not be applicable to allogeneic HCT recipients alone. Incidence rates and time from transplant to CDI may be different between autologous and allogeneic transplant recipients,1,17,18 possibly because of differences in immunosuppression, underlying severity of illness, or antimicrobial exposures between these 2 transplant populations.
Risk factors for CDI specific to HCT patients have proven difficult to identify, likely because of study design limitations and the ubiquity of traditional CDI risk factors among allogeneic HCT recipients. Several prior studies have evaluated CDI risk factors specifically in allogeneic HCT recipients.3,5-7,9,18,19 All of these studies were retrospective and most were limited to risk factor data collected during inpatient hospitalizations. We previously performed a retrospective study of CDI in allogeneic HCT at Barnes-Jewish Hospital (BJH), and identified third-/fourth-generation cephalosporins, diabetes, and preengraftment state as risk factors for CDI.19 Risk factors for CDI identified in other studies of HCT recipients include carbapenem use, myeloablative conditioning, and T-cell depletion.2,6,18
Most previous studies of CDI in allogeneic HCT patients have focused on the preengraftment period, but some studies have reported a median time from transplant to CDI longer than 30 days; thus, focusing on the preengraftment period may miss a significant portion of CDI cases.1,3 Furthermore, the ubiquity of traditional CDI risk factors among HCT patients during the preengraftment period might have limited identification of risk factors in previously published studies, and specific risk factors for CDI may differ by time from transplant. No studies have examined the characteristics of and risk factors for CDI among allogeneic HCT recipients stratified by time from transplant. A better understanding of the epidemiology of CDI is needed to prevent CDI in this highly susceptible population. The purpose of this study was to evaluate risk factors for and outcomes of CDI in allogeneic HCT recipients, using a prospective study design that included outpatient assessments, stratified by time from transplant.
MATERIALS AND METHODS
Study Design
This cohort study was conducted at Siteman Cancer Center, the NCI designated comprehensive Cancer Center of BJH, a 1250-bed, tertiary care facility in St. Louis, Missouri. The study was performed in conjunction with the Organ Transplant Infection Prevention and Detection Project (OTIP) of the Centers for Disease Control and Prevention. OTIP was a prospective cohort study of infections in patients undergoing allogeneic HCT or lung transplant. At BJH, only allogeneic HCT recipients were enrolled in the OTIP study, and specific additional data related to CDI were collected at BJH, as described below. The study dates were April 2007 to March 2010. During the study period, the HCT ward at BJH did not have a required neutropenic fever prophylaxis protocol, and cefepime was the preferred agent for neutropenic fever. Study participants were approached to participate after admission for their allogeneic HCT hospitalization. An assessment of each participant was performed at enrollment and weekly during each hospitalization. After discharge, participants were contacted by phone weekly for up to 12 weeks posttransplant. After 12 weeks, participants were contacted monthly for 30 months post-HCT. If participants were readmitted to the hospital, they were followed up weekly until discharge. Seven participants had >1 HCT during the study period; for these participants, only the first HCT was included in analyses. The Washington University Human Research Protection Office approved this study and written informed consent was obtained from all participants.
Data Collection
Demographic data collected at study enrollment included age, sex, race, underlying disease status at the time of transplant, comorbid diseases, type of allogeneic HCT conditioning, prior chemotherapy/immunosuppressive therapies, and transplantation history. Comorbid diseases were defined as one for which the patient was receiving treatment or medical consultation. Other data collected at the time of transplantation included transplant date and time, ongoing immunosuppressive medications received, and laboratory culture and/or test results. The weekly inpatient and outpatient assessments included patient status (home, inpatient, ICU, deceased), mechanical ventilation, current medications, and symptoms of infection. All infections were reviewed by an infectious diseases physician (E.R.D.) to determine whether the infection was probable, confirmed, or neither. Infections were defined according to National Nosocomial Infections Surveillance (NNIS) criteria (now National Healthcare Safety Network).20 Graft-versus-host disease (GVHD) was scored according to the Glucksberg criteria.21 In addition to interviews, clinical data were collected prospectively from medical records when participants were hospitalized and as available from outpatient clinic records.
CDI-Specific Data Collection
CDI was defined as a positive toxin assay for C. difficile plus clinical symptoms consistent with CDI. Positive C. difficile toxin assay results from the BJH laboratory were collected as part of the ongoing assessments. The BJH laboratory used a toxin CDI test for CDI diagnosis during the study period (Remel Xpect C. difficile Toxin A/B, Lenexa, KS). CDI-specific data included: CDI onset date, method of diagnosis (toxin, endoscopy, CT scan), presence of CDI symptoms (diarrhea, abdominal pain or distension, ileus, peritoneal signs, fever, hypothermia, blood in stool, toxic megacolon), outcomes (duration of illness, colectomy or other surgery for CDI, death due to CDI), and type of, duration of, and response to CDI therapy. Antimicrobial exposures before, during, and after CDI were collected. Prospectively collected medication data were supplemented with data collected electronically from the hospital’s Medical Informatics database. CDI cases were classified by severity (mild, moderate, severe) according to modified Common Terminology Criteria for Adverse Events (CTCAE) criteria; details of this classification system have been published elsewhere.19,22
Data Analysis
Participants were excluded from analyses if they had a history of CDI within the previous 60 days and/or were still receiving antimicrobial treatment for CDI at the time of their allogeneic HCT (n = 9). For CDI cases, the CDI diagnosis date was considered the index date. For controls (all allogeneic HCT recipients who did not develop CDI), an index date was randomly selected such that the distribution of time from allogeneic HCT to the index date was comparable between cases and controls. Data analyses were stratified by time from transplant to index date: 0 to 30 days posttransplant (preengraftment), and 31 to 365 days posttransplant (postengraftment). Only 2 CDI cases occurred more than 365 days posttransplant; these were considered outliers and were excluded from analyses. Antimicrobials were classified into high risk and low risk categories based on risk of causing CDI and our prior analysis. High risk antimicrobials included aminopenicillins/penicillins, cephalosporins, 8-methoxyfluoroquinolones, and clindamycin23; all other antimicrobials were considered low risk. Data such as medications were included if they occurred within the 30 days before index date, including pretransplant exposures when applicable. Neutropenia within 48 hours before index date was included. Risk factors for CDI were evaluated using chi-square/Fischer exact tests or univariate logistic regression, and logistic regression was used for multivariable analyses. Because of small sample sizes, priority for inclusion of variables into the models was based on clinical/biological plausibility, sufficient sample size within the variable, and univariate analyses. Variables with zero cells on univariate analyses were excluded from multivariable models. Due to the small sample size in the postengraftment analyses, at most 3 variables could be included at a time in multivariable models to avoid over specification. Death within 180 days of CDI was compared by CDI severity and time from transplant using the log-rank test. Analyses were performed with SPSS, version 21.0 (IBM Corp, Armonk, NY) and SAS version 9.2 (SAS, Cary, NC).
RESULTS
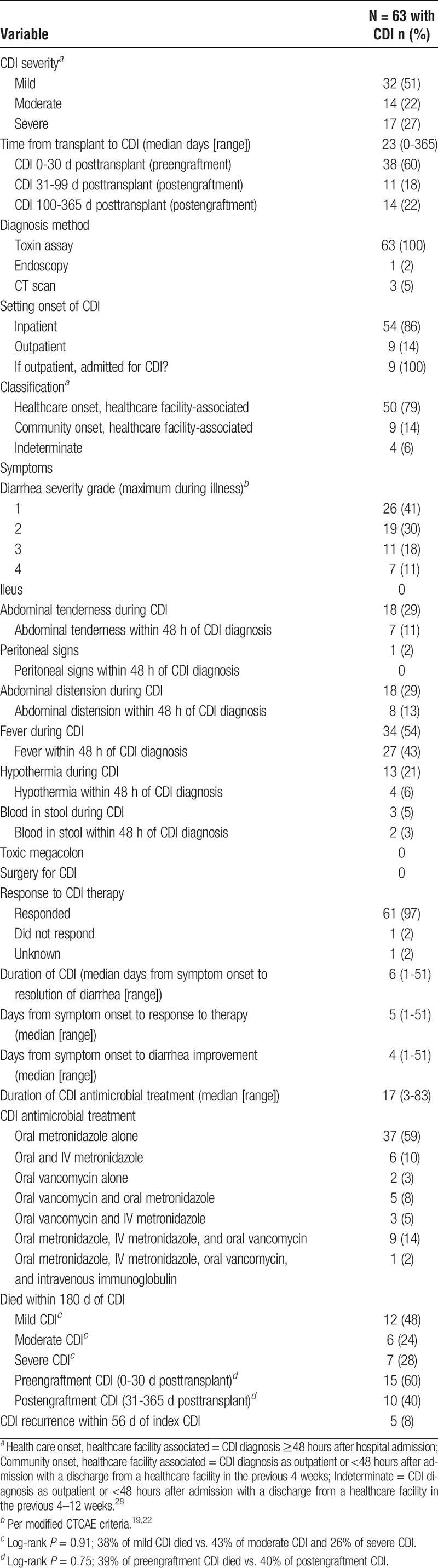
Two hundred fifty-four allogeneic HCT patients were approached to participate in the study; 199 consented to participate (78%), and 187 (74%) were included in analyses). Of the 187 patients, 63 (34%) developed CDI within 1 year of HCT and 124 (66%) did not. CDI symptoms and antimicrobial treatment are described in Table 1. Nine (14%) cases were diagnosed as outpatients. No participants required surgery for CDI. Ninety-seven percent of participants responded to antimicrobial therapy, and the median time to resolution of symptoms was 6 days. The majority of the CDI cases were classified as mild (51%), followed by moderate (22%) and severe (27%) according to the modified CTCAE criteria. There were no significant differences in death within 180 days post-CDI between mild, moderate, and severe CDI or between preengraftment (n = 38) and postengraftment CDI (n = 25) (Table 1; P > 0.05 for all).
TABLE 1.
Symptoms, treatment, and outcomes of CDI in allogeneic HCT recipients
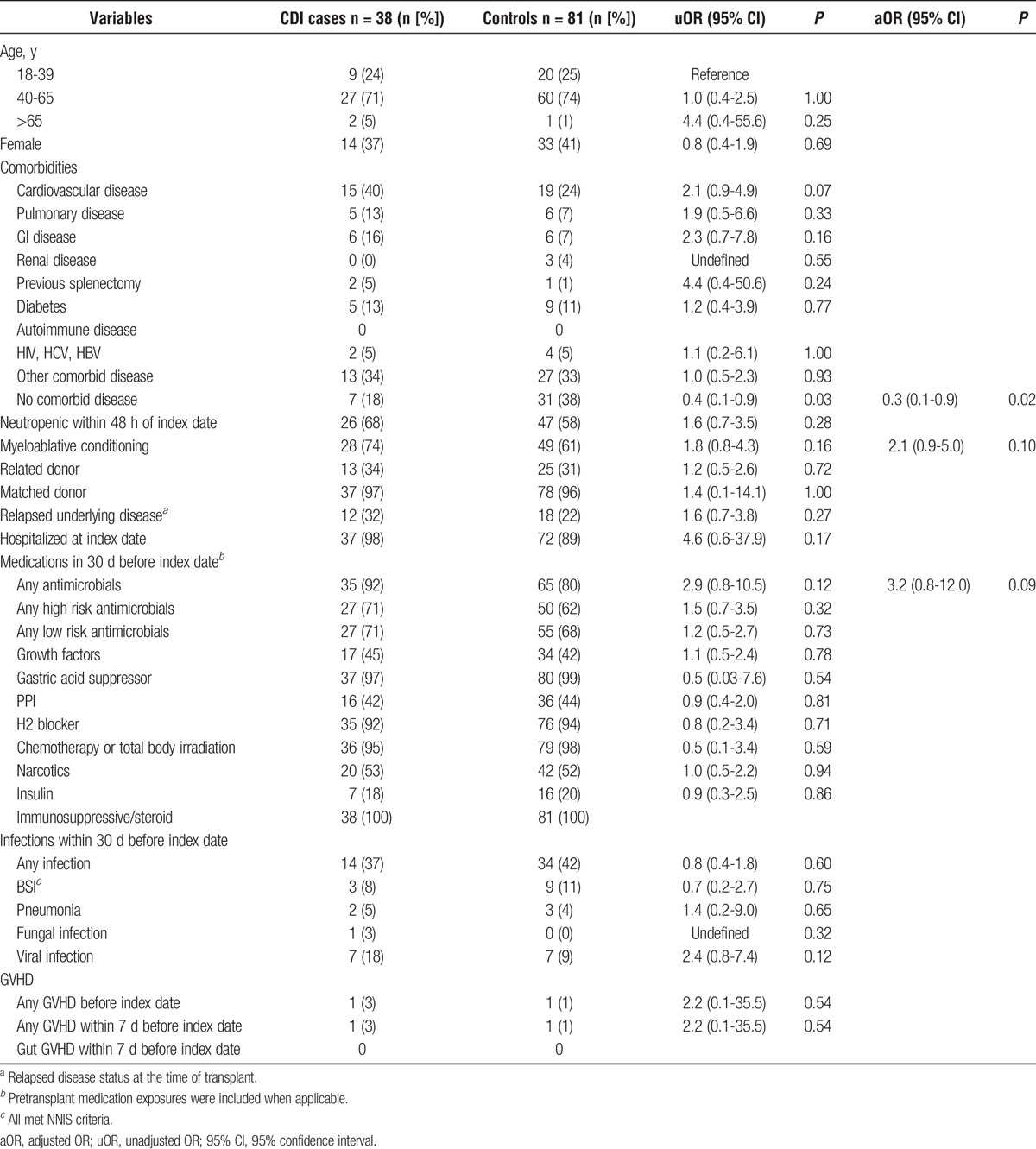
Thirty-eight (60% of total) cases of CDI were diagnosed in the preengraftment period and 81 controls had their randomly selected index date in this time period. Only lack of any underlying comorbid disease was significantly associated with CDI in univariable analysis (protective effect: odds ratio [OR], 0.4; Table 2). Cardiovascular disease was marginally associated with increased risk of CDI in univariable analysis (Table 2). In multivariable analysis, only lack of any underlying comorbid disease was significantly associated with lower risk of CDI (OR, 0.3), although there was a trend for myeloablative conditioning and receipt of any antimicrobials in the previous 30 days to be associated with increased risk of CDI (Table 2).
TABLE 2.
Risk factors for CDI preengraftment (0-30 days after transplant) (N = 119): univariate analysis and multivariable logistic regression model
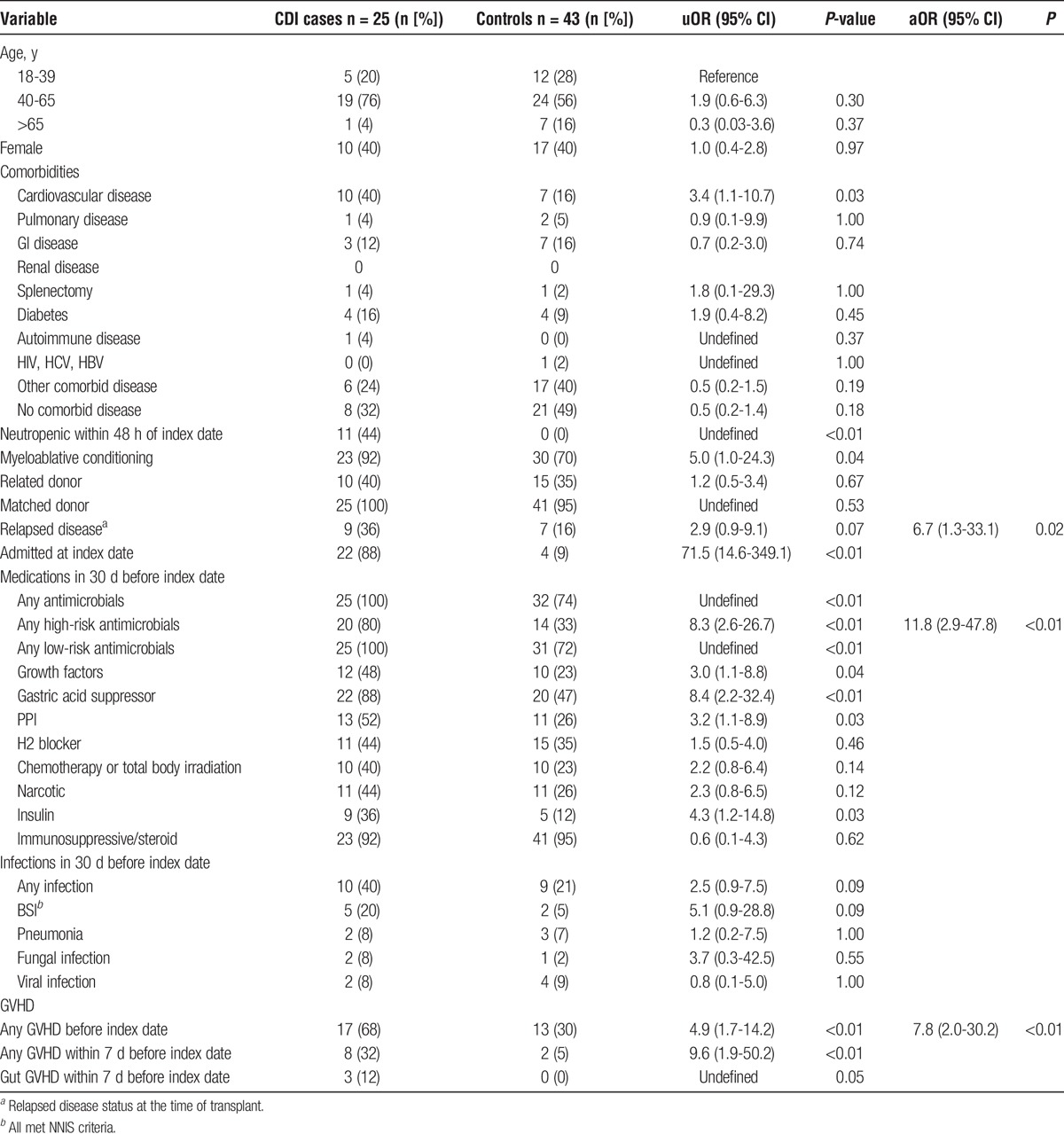
There were 25 CDI cases in the postengraftment period, and 43 controls had their randomly selected index date during that time (Table 3). Numerous risk factors for CDI were identified in univariable analysis: neutropenia within 48 hours before CDI/index, myeloablative conditioning, admitted at index date, receipt of any antimicrobials, high risk antimicrobials, low risk antimicrobials, gastric acid suppressor, proton pump inhibitor (PPI), or insulin therapy in the previous 30 days, any GVHD before the index date, any GVHD in the 7 days before CDI/index date, and gut GVHD in the 7 days before CDI/index date. Relapsed disease was associated with marginally increased risk of CDI. The multivariable model with best fit (Hosmer-Lemeshow P = 0.70) and strongest clinical plausibility was receipt of high risk antimicrobials (OR, 11.8), any GVHD before the index date (OR, 7.8), and relapsed disease status at transplant (OR, 6.7).
TABLE 3.
Risk factors for CDI postengraftment (>30 days posttransplant) (N = 68): univariate analysis and multivariable logistic regression model
DISCUSSION
This study is the first to compare risk factors for CDI in allogeneic HCT recipients during both the preengraftment and postengraftment periods. This was done because these are well defined periods of risk for infections post-HCT. The results of this study are consistent with this observation, as risk factors for CDI varied by time from HCT. Previously published studies of CDI in allogeneic and autologous HCT patients include a variety of follow-up periods, ranging from 30 days to 1 year.1,3,5,6,9-15,17,18,24 Kinnebrew et al6 found that the majority of CDI cases occurred within a few days before or after HCT; however, the maximum follow-up time was 35 days after transplant. Alonso et al10 found 81% of CDI cases occurred within 30 days of autologous HCT. By contrast, Willems et al,9 who evaluated CDI up to a year post-HCT, found that the median time to CDI in their cohort of allogeneic transplant patients was 25 days after HCT. Chakrabarti and Alonso1,3 found median times to CDI of 38 and 33 days in their allogeneic HCT populations, respectively. Similarly, we found that 60% of the CDI cases occurred within 30 days of HCT, 18% occurred between days 31 and 99, and 22% of CDI cases occurred 100 or more days after transplant. These results, along with those of previous investigators, indicate CDI may be more common during the late posttransplant period than has previously been recognized.1,3,9 Post-HCT surveillance for CDI should continue beyond 30 days to fully understand the epidemiology of CDI in HCT patients, and to facilitate interventions to prevent CDI at different times posttransplant.
Few specific risk factors for CDI after allogeneic HCT have been identified previously. This may be due to the universality of common risk factors for CDI in the preengraftment period, as suggested by our risk factor analysis in the preengraftment period. Antimicrobial exposure is widely considered the primary risk factor for CDI, but antimicrobial use in the preengraftment period was ubiquitous in our allogeneic HCT population; 92% of CDI cases and 80% of noncases received an antimicrobial in the previous 30 days. Other medications or procedures that could predispose to CDI due to immune disruption, such as chemotherapy and immunosuppressive or steroid use, were also nearly universal in this allogeneic HCT population. It is likely these variables do increase patients’ risk of CDI, but all allogeneic HCT patients are exposed to these types of medications. Instead, our results indicate patients’ underlying health status pretransplant may be the primary determinant of individual risk for CDI in the preengraftment period. The only independent predictor of CDI in the preengraftment period we identified was having no underlying comorbid disease, which was protective of CDI. Although underlying health status is not a modifiable risk factor, the presence of any comorbidity in allogeneic HCT recipients could be used to target CDI prevention efforts. Receipt of myeloablative conditioning pretransplant was marginally associated with increased risk of CDI during the preengraftment period. Kinnebrew et al6 also found that myeloablative conditioning increased the risk of CDI. Myeloablative conditioning leads to greater neutropenia and damage to the mucosa than nonmyeloablative conditioning,25 resulting in increased susceptibility to other infections (leading to antimicrobial exposures) and thus increased risk of CDI. Conversely, the mucositis caused by myeloablative conditioning with resultant diarrhea may lead to increased testing for C. difficile and detection of asymptomatic carriage in people with diarrhea from other causes.26
In contrast, we identified numerous risk factors for CDI during the postengraftment period. The results of univariable and multivariable analyses indicate the patients at highest risk for CDI in the postengraftment period were those patients with prolonged immune disruption, as indicated by prior infections, antimicrobial use, GVHD, and neutropenia. Reducing these patients’ exposure to the inpatient healthcare environment may decrease their risk of CDI.27 When this approach is impossible, careful assessment of the need for and selection of antimicrobials and gastric acid suppressants should be performed. That a much larger proportion of patients received antimicrobials than had an infection does indicate it may be possible to safely reduce or narrow the spectrum of antimicrobial prescriptions for these patients. These approaches have been somewhat successful at reducing rates of CDI in the general hospital population,28 although CDI remains a significant problem overall.29
The relationship between GVHD and CDI is complex and deserves additional study. Available data suggest GVHD may be both a risk factor and/or an outcome of CDI. In our study, GVHD of any kind was associated with significantly increased risk of CDI in the postengraftment population. Gut GVHD was marginally associated with increased risk of CDI in univariable analysis, but the number of individuals with gut GVHD was too small for the variable to be included in the multivariable model. We have noted this relationship between GVHD and increased risk of CDI previously at our institution,19 as have Alonso et al and Chakrabarti et al.1,3 Alonso found that CDI preceded GVHD in 86% of patients, suggesting that GVHD may be an outcome of CDI. In our analyses, we specifically examined GVHD with onset before CDI and found GVHD to be a risk factor for CDI in the postengraftment period. This apparent contradiction can be explained by the high degree of colinearity between the conditions, particularly in the case of gut GVHD and CDI.25 Both gut GVHD and CDI may arise from loss of a healthy gut microbiome. Jenq et al30 reported the loss of gastrointestinal species diversity post-HCT in patients with GVHD, both in a mouse model and in humans. This loss of the normal gut microbiome has serious consequences for patients. Taur et al31 reported increased post-HCT mortality in patients with low bacterial diversity in the gut microbiome. The growing list of conditions and/or negative patient outcomes arising from the disrupted microbiome should give further impetus to efforts aimed at protecting patients’ normal flora, particularly through the judicious and responsible use of antimicrobials.
Previous estimates of CDI incidence in the allogeneic HCT population range from 12% to 30%.1,3-7,9 Our observed CDI incidence of 34% is slightly higher than previously published estimates, but this may be related to differences in study design. Kinnebrew et al6 reported an incidence of 17% within 35 days posttransplant; our CDI incidence within 30 days of transplant was comparable at 20%. In addition, because of our prospective study design and frequent follow-up with participants, we were able to capture outpatient CDI cases among the allogeneic transplant population that may have been missed if only inpatient data were used. It is also possible that awareness of CDI among transplant physicians has increased in recent years. In a previous analysis of CDI in allogeneic HCT recipients at our facility, 43% of CDI cases were classified as having mild to moderate CDI and 57% as having severe CDI.19 In the current study, using the same modified CTCAE criteria for grading CDI severity, 73% of cases were classified as mild/moderate and only 27% as severe. Heightened awareness of CDI may lead to increased diagnosis of mild CDI cases that would previously have been undetected or earlier detection of CDI that would have become severe if diagnosis was delayed; however, transplant patients are particularly prone to diarrhea due to multiple other causes (side effects of chemotherapy, radiation, or other medications, GVHD, and so on), and the increase in mild/moderate cases of CDI also may have led to increased false-positive rates. Throughout the duration of the current study and our previously published study, our clinical microbiology laboratory used toxin enzyme immunoassay assays for C. difficile detection. Compared to PCR-based C. difficile detection, toxin enzyme immunoassay assays are less likely to detect asymptomatic colonization, so detection of asymptomatically colonized participants with diarrhea due to unrelated causes should have been minimized.26 Analyses of CDI incidence and outcomes in allogeneic transplant patients over time should take into consideration the diagnostic tests used and how they may impact our understanding of CDI epidemiology.26
There are several limitations to this study. We did not obtain stool samples from participants, so we were unable to determine whether preexisting colonization was a risk factor for CDI or may have led to detection of asymptomatic carriage in subjects with diarrhea due to other causes. Data on asymptomatic colonization preallogeneic HCT combined with detailed, prospective clinical data could resolve the question of whether some participants with mild CDI are in fact asymptomatically colonized and experiencing diarrhea due to other causes. Finally, as with many studies of risk factors for CDI among allogeneic transplant recipients, our multivariable models were limited by small sample size. Allogeneic HCT recipients are a fairly small patient population, and larger, multicenter studies are needed to alleviate this problem. Despite these limitations, ours is one of the larger analyses of risk factors for CDI after allogeneic HCT. In addition, due to differences in patient risk factors for infection during the pre versus postengraftment period, we believe it is more appropriate to separate the pre and postengraftment periods to identify risk factors for CDI.
Although most previous studies have focused on CDI in the immediate posttransplant period, our study indicates that surveillance for CDI should continue into the postengraftment period as CDI continues to impact allogeneic HCT recipients’ health postengraftment. Clinicians should carefully weigh patients’ needs for antimicrobials with the potential long-term consequences of extensive antimicrobial use. Future studies, particularly larger, multicenter studies, will help further elucidate the epidemiology of CDI in allogeneic HCT recipients and may reveal novel strategies for CDI prevention in this challenging patient population.
ACKNOWLEDGMENT
The authors would like to thank Dustin Stwalley for his advice on statistical analyses.
Footnotes
Published online 17 March, 2017.
Funding: The Organ Transplant Infection Detection and Prevention Program (OTIP) was funded by the Centers for Disease Control and Prevention (U01CK000134). This study was also funded by a grant from the National Institutes of Health (K23AI065806).
Disclosures: E.R.D. has received grant funding from Sanofi Pasteur, Merck, and Rebiotix, and has been a consultant for Sanofi Pasteur, Merck, Rebiotix, Summit, and Valneva, all unrelated to this study. M.A.O. has received grant funding from Pfizer and Sanofi-Pasteur, and has been a consultant for Pfizer, Merck, and Sanofi-Pasteur, all unrelated to this study. V.J.F. has received funding from the CDC, unrelated to this study. K.A.R., K.M.B., S.S., F.P.S., T.M.C., and J.D. declare no conflicts of interest.
E.R.D. was responsible for obtaining funding, study design, data collection, data analysis, and article preparation. K.A.R. was responsible for data collection and management, data analysis, and article preparation. M.A.O. was responsible for data analysis and article preparation. K.M.B. was responsible for data collection and management, and article preparation. S.S. was responsible for data collection and management. F.P.S. was responsible for obtaining funding, study design, and article preparation. T.M.C. was responsible for study design and article preparation. J.D. was responsible for study design and article preparation. V.J.F. was responsible for obtaining funding, study design, and article preparation.
The findings and conclusions in this report are those of the authors and do no necessarily represent the official position of CDC.
REFERENCES
- 1.Alonso CD, Treadway SB, Hanna DB, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2012;54:1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle NM, Magaret A, Stednick Z, et al. Evaluating risk factors for Clostridium difficile infection in adult and pediatric hematopoietic cell transplant recipients. Antimicrob Resist Infect Control. 2015;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti S, Lees A, Jones SG, et al. Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transplant. 2000;26:871–876. [DOI] [PubMed] [Google Scholar]
- 4.Chopra T, Chandrasekar P, Salimnia H, et al. Recent epidemiology of Clostridium difficile infection during hematopoietic stem cell transplantation. Clin Transplant. 2011;25:E82–E87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamboj M, Xiao K, Kaltsas A, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: strain diversity and outcomes associated with NAP1/027. Biol Blood Marrow Transplant. 2014;20:1626–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnebrew MA, Lee YJ, Jenq RR, et al. Early Clostridium difficile infection during allogeneic hematopoietic stem cell transplantation. PLoS One. 2014;9:e90158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung S, Metzger BS, Currie BP. Incidence of Clostridium difficile infection in patients with acute leukemia and lymphoma after allogeneic hematopoietic stem cell transplantation. Infect Control Hosp Epidemiol. 2010;31:313–315. [DOI] [PubMed] [Google Scholar]
- 8.Mani S, Rybicki L, Jagadeesh D, et al. Risk factors for recurrent Clostridium difficile infection in allogeneic hematopoietic cell transplant recipients. Bone Marrow Transplant. 2016;51:713–717. [DOI] [PubMed] [Google Scholar]
- 9.Willems L, Porcher R, Lafaurie M, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol Blood Marrow Transplant. 2012;18:1295–1301. [DOI] [PubMed] [Google Scholar]
- 10.Alonso CD, Dufresne SF, Hanna DB, et al. Clostridium difficile infection after adult autologous stem cell transplantation: a multicenter study of epidemiology and risk factors. Biol Blood Marrow Transplant. 2013;19:1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arango JI, Restrepo A, Schneider DL, et al. Incidence of Clostridium difficile-associated diarrhea before and after autologous peripheral blood stem cell transplantation for lymphoma and multiple myeloma. Bone Marrow Transplant. 2006;37:517–521. [DOI] [PubMed] [Google Scholar]
- 12.Avery R, Pohlman B, Adal K, et al. High prevalence of diarrhea but infrequency of documented Clostridium difficile in autologous peripheral blood progenitor cell transplant recipients. Bone Marrow Transplant. 2000;25:67–69. [DOI] [PubMed] [Google Scholar]
- 13.Bilgrami S, Feingold JM, Dorsky D, et al. Incidence and outcome of Clostridium difficile infection following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1999;23:1039–1042. [DOI] [PubMed] [Google Scholar]
- 14.Tomblyn M, Gordon L, Singhal S, et al. Rarity of toxigenic Clostridium difficile infections after hematopoietic stem cell transplantation: implications for symptomatic management of diarrhea. Bone Marrow Transplant. 2002;30:517–519. [DOI] [PubMed] [Google Scholar]
- 15.Trifilio SM, Pi J, Mehta J. Changing epidemiology of Clostridium difficile-associated disease during stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:405–409. [DOI] [PubMed] [Google Scholar]
- 16.van Kraaij MG, Dekker AW, Verdonck LF, et al. Infectious gastro-enteritis: an uncommon cause of diarrhoea in adult allogeneic and autologous stem cell transplant recipients. Bone Marrow Transplant. 2000;26:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso CD, Marr KA. Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr Opin Infect Dis. 2013;26:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vehreschild MJ, Weitershagen D, Biehl LM, et al. Clostridium difficile infection in patients with acute myelogenous leukemia and in patients undergoing allogeneic stem cell transplantation: epidemiology and risk factor analysis. Biol Blood Marrow Transplant. 2014;20:823–828. [DOI] [PubMed] [Google Scholar]
- 19.Dubberke ER, Reske KA, Srivastava A, et al. Clostridium difficile-associated disease in allogeneic hematopoietic stem-cell transplant recipients: risk associations, protective associations, and outcomes. Clin Transplant. 2010;24:192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. [DOI] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Dubberke ER, Sadhu J, Gatti R, et al. Severity of Clostridium difficile-associated disease (CDAD) in allogeneic stem cell transplant recipients: evaluation of a CDAD severity grading system. Infect Control Hosp Epidemiol. 2007;28:208–211. [DOI] [PubMed] [Google Scholar]
- 23.Dubberke ER, Yan Y, Reske KA, et al. Development and Validation of a Clostridium difficile Infection Risk Prediction Model. Infect Control Hosp Epidemiol. 2011;32:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobak D, Arfons LM, Creger RJ, et al. Clostridium difficile-associated disease in human stem cell transplant recipients: coming epidemic or false alarm? Bone Marrow Transplant. 2008;42:705–713. [DOI] [PubMed] [Google Scholar]
- 25.Docampo MD, Auletta JJ, Jenq RR. Emerging Influence of the Intestinal Microbiota during Allogeneic Hematopoietic Cell Transplantation: Control the Gut and the Body Will Follow. Biol Blood Marrow Transplant. 2015;21:1360–1366. [DOI] [PubMed] [Google Scholar]
- 26.Dubberke ER, Burnham CA. Diagnosis of Clostridium difficile infection: treat the patient, not the test. JAMA Intern Med. 2015;175:1801–1802. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention (CDC). Vital Signs: Preventing Clostridium difficile Infections. MMWR Morb Mortal Wkly Rep. 2012;61:157–162. [PubMed] [Google Scholar]
- 28.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455. [DOI] [PubMed] [Google Scholar]
- 29. Antibiotic Resistance Threats in the United States, 2013. 7-17-2014. Centers for Disease Control and Prevention. Accessed 1-9-2016.
- 30.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]


