Abstract
Background
Living kidney donors (LKDs) experience reduction in kidney function, however serum phosphate (sPi) levels are lower compared to patients with chronic kidney disease matched for reduced kidney function. Mineral metabolism adaptations that occur in LKDs have not been adequately investigated. To evaluate the effect of nephrectomy on markers of mineral metabolism in LKDs compared to healthy volunteers (HV) over 12 months.
Methods
Mineral parameters were evaluated in twenty-one adult LKDs and twenty HVs. Parameters included sPi, intact parathyroid hormone, fibroblast growth factor-23 (FGF23), soluble Klotho (sKl) and urinary phosphate, measured prior to donation (T0), 1 month (T1), 6 months (T6) and 12 months (T12) post-kidney donation. Statistical analyses were conducted on normalized variables and changes were assessed using 2-way analysis of variance.
Results
Mean ages of LKDs and HVs were 54.1 ± 14.7 and 52.6 ± 8.0 years, respectively. There were no baseline clinical or biochemical differences between LKDs and HVs. In LKDs at T1, serum creatinine increased (from 75 ± 12 to 114 ± 22 μmol/L), FGF23 increased (52 ± 15 to 70 ± 19 pg/mL) and sKl decreased (564 [469-662] to 424 [375-523] pg/mL), all P less than 0.001. Changes were sustained at T12. After donation, LKDs consistently demonstrated lower sPi compared with T0, with the maximal sPi change at T6 (−0.19 mmol/L difference, P < 0.001). Other markers of mineral metabolism were unchanged in LKDs. There were no mineral differences in HVs over 12 months.
Conclusions
Prospective evaluation of mineral metabolism parameters in LKDs provides valuable insight into compensatory mechanisms after reduction in kidney function. Further reduction of sPi at T6 despite early alterations in FGF23 and sKl suggest adaptation of mineral metabolism continues long-term in LKDs.
Biochemical aberrations in the chronic kidney disease (CKD) population, namely a reduction in soluble Klotho (sKl) and elevation in both fibroblast growth factor-23 (FGF23) and parathyroid hormone (PTH) levels, have been reported prior to a rise in serum phosphate (sPi) levels, highlighting that hyperphosphatemia is a relatively late complication of CKD.1 Both sKl and FGF23 have been independently linked to mortality; sKl levels are inversely associated with poor outcomes2,3 whereas FGF23 has been linked to increased morbidity in numerous observational studies.4-8 Considering renal function is intricately linked to these markers of mineral metabolism, a change in glomerular filtration rate (GFR) is postulated to affect sKl, FGF23 and ultimately renal phosphate handling.
Living kidney donation provides a unique clinical setting to evaluate the effects of nephrectomy (ie, a reduction in GFR) in otherwise healthy individuals. In spite of recent meta-analyses that suggest increased morbidity long-term after kidney donation, with a specific increase in risk of hypertension, proteinuria and development of end stage kidney disease in living kidney donors (LKDs) compared to healthy controls,9-12 no differences in risk of cardiovascular disease and death have been noted when comparing LKDs to well-matched control cohorts selected from large databases or national registries.13-15
Survival outcomes in LKDs after donor nephrectomy seem counterintuitive to the postulated effects of reduced GFR on mineral markers, which have otherwise been reported to portend poorer cardiovascular health and reduced longevity. This study aimed to identify the longitudinal relationship between mineral parameters, namely sKl, FGF23 and sPi, in LKDs compared to healthy volunteers (HV) over the first 12 months after living kidney donation. The following specific hypotheses were evaluated: a) sKl declines after living kidney donation, b) FGF23 increases after kidney donation, and c) urinary phosphate excretion (UPE) increases in response to elevated FGF23 levels to compensate for reduction in GFR.
MATERIALS AND METHODS
Study Design and Participants
This single-center, prospective observational study followed cohorts of LKDs and HVs for 12 months. The study received ethics approval from the Melbourne Health Human Research Ethics Committee, Melbourne, Australia and was performed in accordance with the Declaration of Helsinki. All study participants provided informed consent.
LKDs scheduled for donor nephrectomy at The Royal Melbourne Hospital were identified and approached. Patients who resided and/or had follow-up care interstate or internationally were excluded from recruitment. HVs were recruited using Melbourne Health Human Research Ethics Committee–approved flyers. HVs aged over 18 years, able to self-consent and without significant medical illness that would otherwise preclude them from kidney donation were considered. Single agent–treated stable hypertension and hypercholestrolemia were not strict exclusion criteria for the HV cohort, because otherwise healthy spouses with these stable conditions were considered suitable LKDs.
Two prior studies have suggested up to 300 pg/mL reduction in sKl after donor nephrectomy.16,17 Thus, in order to detect a change of approximately 300 pg/mL in sKl with 80% statistical power, a sample size of 16 in each group was estimated. This size was inflated for loss to follow-up by 10%, and a further 10% for the nonparametric distribution of sKl. Therefore, a total of 20 recruited participants per group were targeted.
All participants were evaluated at 4 timepoints—baseline or prekidney donation (T0), 1 month (T1), 6 months (T6), and 12 months (T12) after the baseline visit or kidney donation. Basic demographic information was collected at baseline. At all study visits, the average (of 2) blood pressure readings and weight were recorded, blood was collected for routine biochemistry along with 2 extra tubes, one K3-EDTA tube (Vacuette; Greiner Bio-One International, Kremsmunster, Austria) and 1 serum separator tube (SST; BD Biosciences, Franklin Lakes, NJ). At T0, T1, and T12, all study participants (LKDs and HVs) were requested to provide 24-hour urine collections and spot urine collections.
Biochemical Measurements
Basic serum biochemistry, including sPi, serum calcium (sCa) and serum creatinine (sCr), was performed at a central on-site pathology laboratory. Estimated GFR (eGFR) for all study participants was calculated using the CKD Epidemiology Collaboration (CKD-Epi) equation.18 Intact PTH was measured using the ARCHITECT assay (Abbott Diagnostics, Wiesbaden, Germany) and performed on the ARCHITECT i system (Abbott). The lower and upper limits of detection were 0.3 and 315 pmol/L, respectively, with coefficient of variations (CVs) of 6.3% at 1.7 pmol/L and 5.0% at 70.7 pmol/L.
Urine samples collected in clean plastic containers were acidified promptly upon receipt by the central laboratory. Urine phosphate (uPi) and urine creatinine (uCr) concentrations were measured in both the 24-hour and spot urine collection. Total daily UPE was recorded. The formulae used to calculate urinary phosphate creatinine ratio (uPiCr), fractional excretion of phosphate (FePi), tubular reabsorption of phosphate (TRP) and maximal tubular reabsorption corrected for GFR (TmP/GFR) are as follows: uPiCr = uPi/uCr; FEPi = ([uPi x (sCr)]/[sPi x uCr] x 100%); and TRP = 1 − ((uPi/sPi) × (sCr/uCr)). TmP/GFR was estimated from a previously validated algorithm (where TmP/GFR = 0.3 × sPi × (TRP/[1 − (0.8 × TPR)]) where TRP > 86% or = TRP × sPi when TRP was ≤ 86%).19-21
Microalbuminuria was measured in 24-hour collections using the MULTIGENT Microalbumin assay (Abbott), a turbidimetric immunoassay using polyclonal antibodies targeting human albumin where degree of turbidity (measured optically) is proportional to albumin concentration, on the ARCHITECT c system (Abbott). The reportable range for this assay was 5 to 500 μg/L (5-500 mg/L) with CVs of 2.8% at 25 μg/L and 3.5% at 73 μg/L. Microalbuminuria > 30 mg/day was considered abnormal.22
The additional serum and plasma tubes were stored at −80°C until batched analysis for serum sKl and intact FGF23, performed in duplicate using the IBL sKl ELISA kit (Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) and the Kainos intact FGF23 ELISA kit (Kainos Laboratories Inc., Tokyo, Japan) respectively. The intra-assay and inter-assay CVs for this sKl were 4.6% and 9.8%, and 4.5% and 8.6% for intact FGF23.
Statistical Analysis
Data is represented as mean (±SD) for parametric variables and median (interquartile range [IQR]) for nonparametric variables. Data normality was tested using Shapiro-Wilk tests and Q-Q plots. Baseline differences between LKDs and HVs were determined using either Student t test or Mann-Whitney U test for continuous variables and chi-square test for categorical variables. Temporal changes in individual parameters were analyzed using either repeated-measures analysis of variance with Tukey test for multiple comparisons, or Friedman test with Dunn test for multiple comparisons as appropriate.
sKl and PTH were natural log-transformed (Ln) for further analyses. Mixed effects models were used to evaluate the associations over time after kidney donation. Each individual variable of interest (sKl, iFGF23, sPi, and TmP/GFR) was defined as the dependent variable, and evaluated against change observed in eGFR as well as change in the other three variables, included as independent variables in mixed model constructs. Donation status (LKD or HV) was used as a fixed independent categorical variable and timepoint was entered into the model to allow for random effects. Change detected in Ln-PTH during the study period was also included as a fixed independent variable in all models given the intimate relationship of PTH with the mineral parameters of interest. All statistical analyses were performed with SPSS Statistics Version 24.0 (IBM Corp., Armonk, NY), whereas graphics were created with GraphPad Prism 6 for Macintosh (La Jolla, CA). A P-value of <0.05 was considered significant unless otherwise stated.
RESULTS
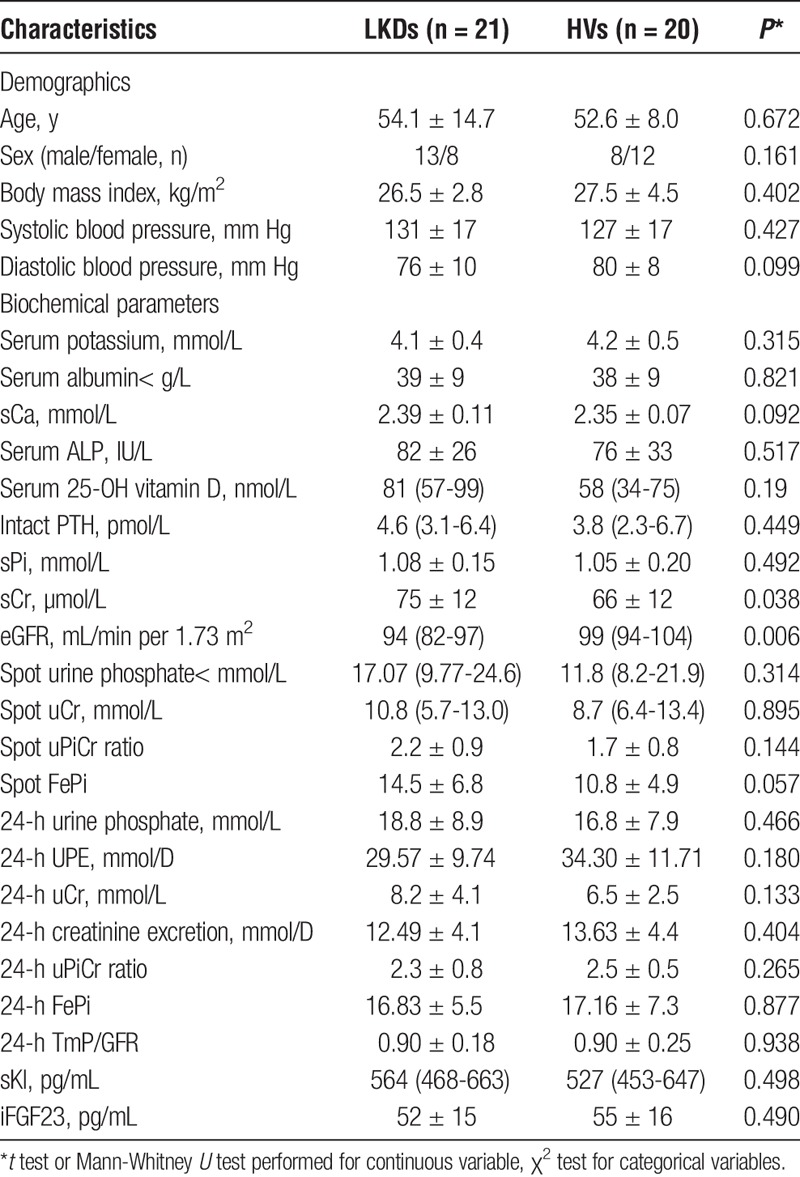
Twenty-one LKDs and 20 HVs were recruited to the study. Baseline clinical and biochemical information have been tabulated in Table 1. There were no statistical differences in age, gender, body mass index or blood pressure between the 2 groups at T0. Mean sCr in LKDs was slightly higher than in HVs at T0, 75 ± 12 versus 66 ± 12 μmol/L, P = 0.038, whereas eGFR was also marginally lower in LKDs (94 [82-97] vs 99 [94-104] mL/min per 1.73 m2, P = 0.006). Although LKDs had marginally higher sCr/lower eGFR compared with HVs, no mineral differences were detected between groups, specifically sPi, sCa, PTH, iFGF23 and sKl (Table 1). No differences were seen in either spot or 24-hour uPi (or uCr) measurements between the 2 groups at T0. All LKDs underwent donor nephrectomies with no postoperative complications.
TABLE 1.
Characteristics of study participants at baseline (T0)
In both LKDs and HVs, systolic and diastolic blood pressure measurements did not change significantly over the evaluated time points, all P > 0.5 compared to T0 (Figure 1). A trend towards a ~5 mm Hg increase in blood pressure at T12 compared to T0 was observed. Transient microalbuminuria (40 mg/d) was detected in one LKD at T1 which was not present when measured again at T12. This episode was not related to other clinical findings and was not associated with hypertension. Microalbuminuria was not seen in any other participant.
FIGURE 1.

Blood pressure in LKDs and HVs. (A) Systolic and (B) diastolic blood pressure did not change significantly in LKDs or HVs during the study period. Mean (±SD) presented.
Mineral Metabolism Parameters Change After Unilateral Nephrectomy
All biochemical changes over the study period have been tabulated in Table 2. LKDs showed an increase in sCr and reduction in eGFR (both P < 0.001 at T1 compared to T0) while no change was noted in HVs (Figure 2A). Renal function was stable for the remainder of the study. No change in mean sCa was observed in either group (Figure 2B). sPi levels were consistently lower in LKDs, decreased at T1 (nonsignificant) and with a maximal reduction at T6 compared with T0 (P < 0.001). At T12, mean sPi in LKDs remained lower than T0 levels (P < 0.005) and was also lower than T12 levels in HVs (P < 0.05, Figure 2B).
TABLE 2.
Changes in study participants over study period
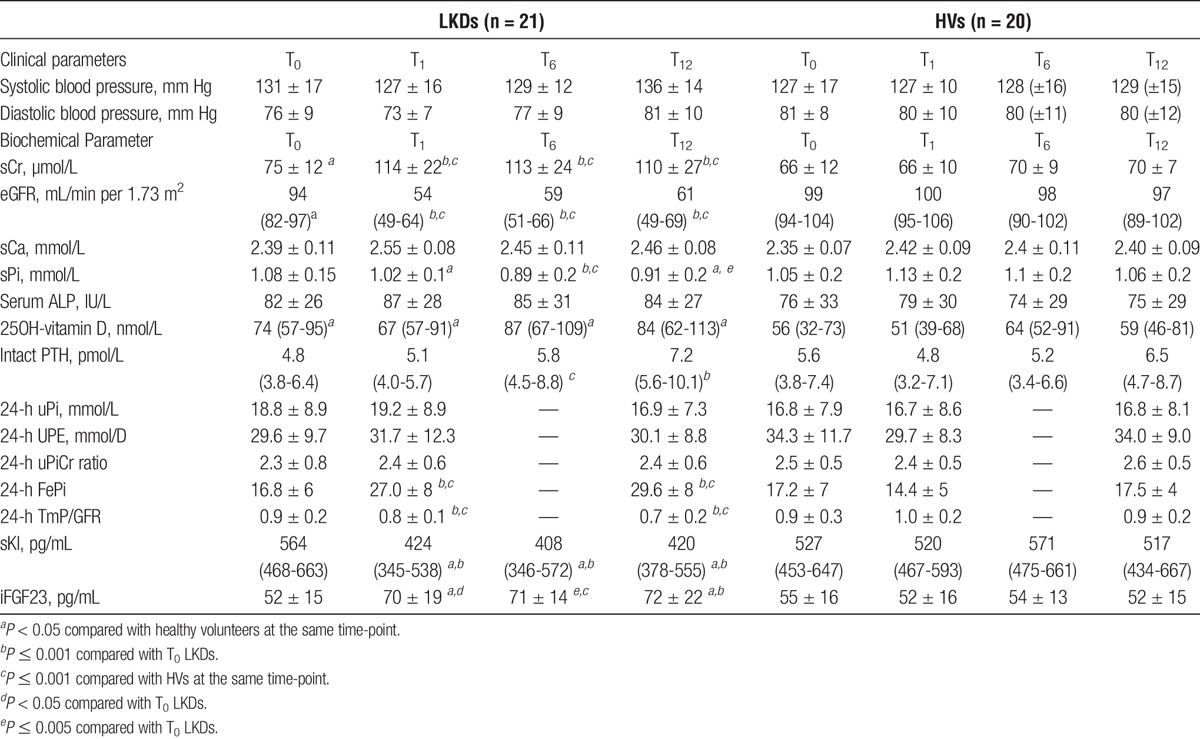
FIGURE 2.
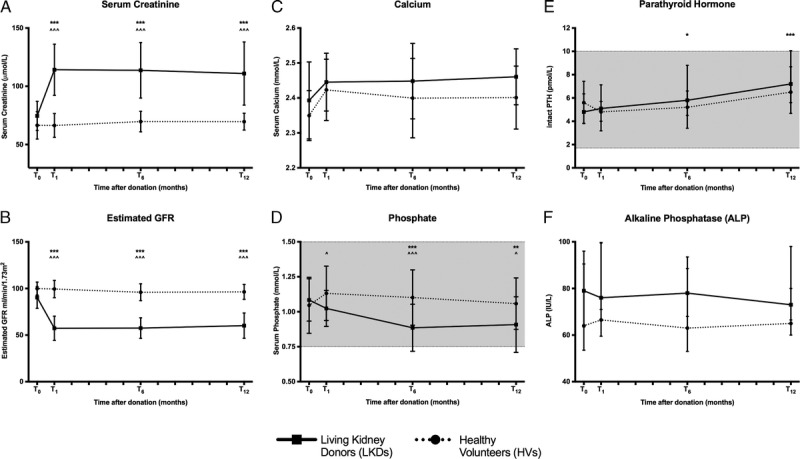
Biochemical parameters alter after donor nephrectomies. (A) Mean sCr increases while (B) eGFR declines in LKDs. Individual data-points presented. (C) sCa does not change though (D) sPi declines in LKDs (Mean (±SD) presented). (E) Intact PTH increases but no change is noted in (F) ALP activity in LKDs (Median (IQR) presented). ***P < 0.001, **P < 0.01 and *P < 0.05 compared to T0 LKDs. ^^^P ≤ 0.001 compared with HVs at the same time-point. ^P < 0.05 compared with HVs at the same time-point. Reference ranges for sCa, sPi, intact PTH and ALP are 2.1 to 2.6 mmol/L, 0.75 to 1.5 mmol/L, 1.7 to 10.0 pmol/L, and 30 to 110 IU/L respectively (shaded area).
LKDs exhibited changes in PTH at T6 (P < 0.05) and T12 (P < 0.001) but not at T1, when compared with T0 levels (Figure 2C). T12 PTH levels in LKDs were not significantly different to T12 PTH levels in HVs. Total alkaline phosphatase (ALP) levels were unchanged in both groups (Figure 2D).
At T1, sKl levels exhibited a decline after kidney donation while iFGF23 levels increased in LKDs (Figures 3A and B, respectively), as hypothesized. These changes persisted for the remainder of the follow-up period for both markers. No changes were seen in HVs.
FIGURE 3.

Markers of mineral metabolism change after donor nephrectomies. A, sKl levels decrease in LKDs (median [IQR] presented). B, iFGF23 levels increase in LKDs (mean [±SD] presented). ***P < 0.001, **P < 0.01, and *P < 0.05 compared to T0 LKDs. ^^^P < 0.001 compared with HVs at the same timepoint. ^P < 0.05 compared with HVs at the same timepoint.
Urinary Phosphate Changes
Urine collections were performed at T0, T1 and T12 but not at T6. Total UPE was not different between groups and was stable throughout the study period (Figure 4). Both FePi and TmP/GFR demonstrated appropriate reciprocal changes in keeping with changes in sCr after kidney donation (Figures 4C and D) where FePi increased and TmP/GFR decreased after nephrectomy.
FIGURE 4.
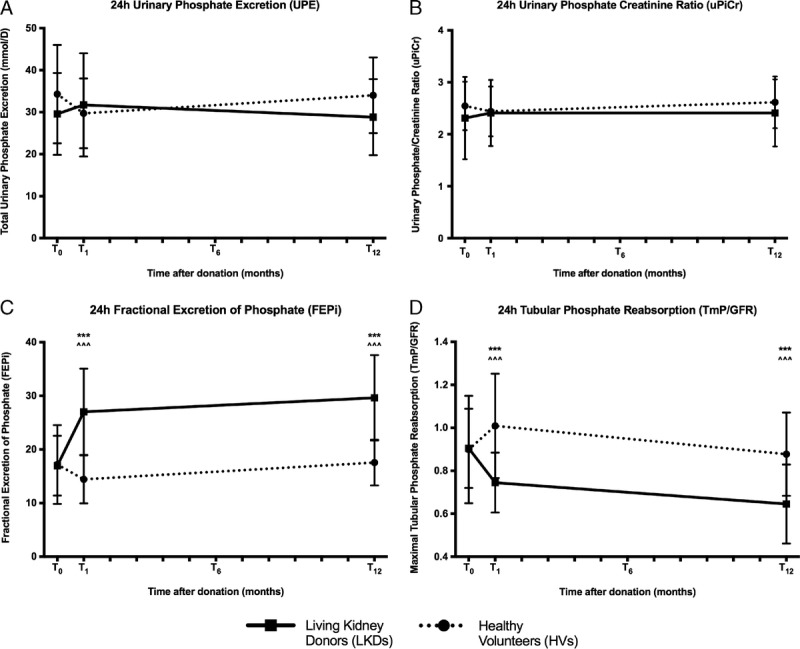
Urinary profile in LKDs. (A) 24-hour UPE, (B) 24-hour uPiCr show no change but (C) 24-hour FePi increases and (D) 24-hour TmP/GFR decreases in LKDs after donor nephrectomies. Mean (±SD) presented. ***P < 0.001 compared to T0 LKDs. ^^^P < 0.001 compared with HVs at the same timepoint.
eGFR Is Strongly Associated With Levels of sKl, iFGF23, sPi, and TmP/GFR
Mixed effects models analyses were undertaken with normalized parameters to determine associations, if any, between change in eGFR, sKl, iFGF23, sPi, and/or TmP/GFR after kidney donation with sKl, iFGF23, sPi, and TmP/GFR as individual dependent variables. Reduction in eGFR was the only significant predictor (P < 0.001) of reduction in Ln-sKl after adjusting for donation status, study time-point, and changes that occur over the same time points in iFGF23, sPi, TmP/GFR and Ln-PTH. A change of 0.005 (95% confidence interval [CI], 0.003-0.008) mL/min per 1.73 m2 in eGFR was noted per unit change in sKl over time. Repeating the analysis after removing iFGF23 and Ln-PTH from the model did not change the associations between Ln-sKl with sPi or TmP/GFR. This sequential analysis did not improve Akaike Information Criterion (AIC).
eGFR remained a robust predictor of change in iFGF23 levels (P < 0.001), whereas Ln-PTH showed a possible interaction with P = 0.094, after adjusting for donation status, study time-point, and changes that occur over the same time points in sKl, sPi and TmP/GFR. A change of −0.551 (95%CI, −0.80 to −0.30) mL/min per 1.73 m2 in eGFR was noted per unit change in iFGF23 over time. The analysis was repeated adjusting for the categorical variables as mentioned with only eGFR, Ln-PTH, and sPi. The P value for Ln-PTH only improved to 0.086 while the AIC score was worse compared to the original model.
Mixed models analyses were also performed with sPi and 24-hour TmP/GFR to gauge the relationship between sKl and iFGF23 with urinary phosphate handling parameters adjusting for donation status and allowing for random effects as above. In the model assessing sPi change adjusting for the same categorical variables, only the variables eGFR, Ln-sKl, iFGF23 and Ln-PTH were assessed. eGFR showed a strong relationship, P = 0.005, while both Ln-sKl and iFGF23 demonstrated a trend towards significance, P = 0.09. A change of 0.003 (95% CI, 0.001-0.005) mL/min per 1.73 m2 in eGFR, 0.12 (95%CI −0.02 to 0.06) Ln-sKl, and 0.001 (95% CI, −0.0002 to 0.003) pg/mL iFGF23 was noted per unit change in sPi over time. Exclusion of Ln-PTH from sequential analysis did not materially affect the model. Only eGFR (P < 0.001) remained significant in the model evaluating 24-hour TmP/GFR. A change of 0.006 (95% CI, 0.003-0.009) mL/min per 1.73 m2 in eGFR was noted per unit change in 24-hour TmP/GFR over time. Ln-sKl and iFGF23 change was not associated with change seen in 24-hour TmP/GFR.
DISCUSSION
This 12-month longitudinal study confirmed a multitude of changes that occur in LKDs after donor nephrectomy with possible long-term adaptive responses that evolve, specifically (a) a decline in sKl, (b) an increase in iFGF23, (c) adaptations in phosphate reabsorption/excretion, and (d) a reduction in sPi subsequent to other changes in mineral markers without any accompanying fluctuation in total UPE at T12. There are very few studies that have investigated extended mineral parameter changes after unilateral donor nephrectomies,16,17,23–25 and only 2 have measured sKl.16,17 To our knowledge, this is the first prospective clinical study designed to evaluate sKl in LKDs with a healthy comparator group.
The biochemical variations observed in this study after kidney donation are largely concordant with prior prospective reports16,17,23,24 though most of these prior studies lacked comparator groups and investigated acute changes within the first week after surgery which our study did not. T1 was selected as an optimal study timepoint to avoid acute postoperative changes (eg, within the first week) that could add to confounding and recalibration of mineral parameters was anticipated by T1.
sPi variation has previously been detected at (the earliest) day 2 after kidney donation.17 This reduction is sustained in the longer term17,25 and validated by T12 observations in this study. Albeit a small study, Westerberg et al23 did not find any differences in sPi measured at 1-week after surgery compared with baseline. This together with nonsignificant sPi reduction observed at T1 compared with T0 implies sPi may be affected in the acute phase after major surgery although the long-term change with maximal reduction noted at T6 supports the notion of long-term adaptive responses that may occur in LKDs.
A larger cross-sectional study has also evaluated LKDs at a median time of 5.3 (3.3-8.4) years after kidney donation, where 198 kidney donors were compared to 98 nondonors identified through potential donor records previously evaluated for donation.25 Long-term reduction in sPi and elevation of iFGF23 were once again compatible with T12 findings in our study, although the study by Young et al25 was limited by lack of temporal comparison with paired baseline samples.
An acute rise in PTH within 24 hours of unilateral nephrectomy has previously been reported.17,23 This was not sustained and showed normalization to baseline after 24-hours, when measured anytime between day 2 and day 7. Conflicting trajectories have been reported thereafter with 1 study detecting no change at 3 to 6 months,23 whereas another study found an absolute difference in median PTH values of 1.8 and 1.5 pmol/L to be statistically different at day 180 or day 360 respectively17 compared with baseline values. Similar significant alterations in PTH levels were observed after kidney donation in this study. However, when assessed prospectively against HVs, the T12 LKD PTH values were not different between the groups.
This study did not evaluate changes in 1,25-dihydroxyvitamin D after donor nephrectomy although an acute reduction within the first 6 months has been reported in other studies.17,23–25 This could reflect both the lack of renal activation as well as the negative feedback with increase in iFGF23 levels. With a major role in intestinal calcium and phosphate uptake, the lower levels of 1,25-dihydroxyvitamin D after nephrectomy likely contribute to the lower sPi levels seen in LKDs at T6 and thereafter.
A much larger ongoing prospective controlled study recruited 220 pairs (LKDs and HVs) designed to evaluate overall LKD health (ALTOLD study) reported similar findings to those presented here in regards to biochemical parameters (sCr, sCa, and sPi), including a small increase in PTH levels in LKDs at 6-months and 36-months postnephrectomy compared to baseline (0.9 and 0.8 pmol/L change) as well as a significant reduction in 1,25- dihydroxyvitamin D within the first six months that stabilized thereafter.24 Given the small absolute differences in PTH documented, it is not surprising that the difference is not significant in our study. Simple sample size calculations to detect a true difference of 1pmol/L with 80% power would suggest a minimum of 222 matched pairs would be required. However, the ALTOLD study did not measure sKl.
The changes seen in uPi after unilateral nephrectomies are consistent with previously published studies evaluating 24-hour urine collections.17,23 Although urine was not collected for assessment at T6 in our study, Ponte et al documented no obvious differences in FePi and TmP/GFR between day 180 and 360, the equivalent of T6 and T12 measurements in this study. The larger ALTOLD study has also reported similar uPi findings after donor nephrectomies however these assessments were limited to spot morning urine collections.24
sKl has only been measured in 2 previous studies involving LKDs.16,17 Reduction in sKl observed here is consistent with the long-term findings in the latter study. Meanwhile, discrepancies in iFGF23 levels are noted between the published studies17,23 and our findings. Changes within the first week are conflicting and may be related to the acute phase after major surgery possibly reflecting local/institution-specific peri- and postoperative management. In our study, iFGF23 levels increased at T1 and this was sustained for the duration of the study. Studies reporting longer-term outcomes in LKDs support this sustained FGF23 elevation.24,25
Mineral parameters described thus far are intricately linked. Mixed models analyses allowed further interrogation of the temporal relationships between these mineral parameters. In LKDs, reduced eGFR demonstrated the strongest association with the change seen in all 4 variables assessed—lower sKl, higher iFGF23, lower sPi, and lower 24-hour TmP/GFR.
A reduction in renal capacity undoubtedly activates compensatory mechanisms, possibly long-term, to adjust to the normal mineral burden, namely, phosphate. The lack of change in (mainly dietary) phosphate load in LKDs, evident by lack of change in total UPE, provides a constant drive to sustain the elevated FePi. While similar mineral alterations, elevated iFGF23 and sKl reduction, are likely to eventuate in those with early CKD, these individuals do not experience the reduction in sPi levels observed in LKDs. One possibility is that the remaining normal kidney in LKDs is able to compensate and adapt to the rise in iFGF23 levels, resulting in a disturbance to the sPi homeostatic set-point. On the other hand, an ongoing decline in renal capacity in patients with CKD and diseased renal parenchyma upsets the “physiological adaptive process” and will ultimately be overwhelmed by the constant iFGF23 drive.
The strengths of this study include the comprehensive, concurrent, and prospective measurement of sKl, iFGF23, sPi, and 24-hour urinary phosphate measurements as well as the inclusion of a comparator HV group. Sample size was small although this was predetermined to detect significant changes between the groups for sKl. This calculation provided the biggest sample size and would be large enough to capture differences in iFGF23 and FePi (but not PTH) as well as differences between pairs and repeat measures. Furthermore, this clinical study experienced no dropouts. Limitations of this study include the lack of urinary assessment at T6, which may have been very insightful in view of sPi changes at T6, and finally, the lack of 1,25-dihydroxyvitamin D measurement limits somewhat the data interpretation in this study. However, both have been investigated in other LKD studies with no conflicting outcomes.
CONCLUSIONS
Our prospective evaluation of mineral metabolism parameters in LKDs provides valuable insight into compensatory mechanisms after reduction in kidney function. Comparison with HVs in this study provides a comprehensive assessment of the temporal alterations observed in LKDs. Reduced eGFR, subsequent to unilateral nephrectomy, is highly predictive and strongly associated with the early alterations in iFGF23 and sKl. Lack of change in phosphate load in LKDs provides a constant drive to sustain elevated FePi. The remaining normal kidney in LKDs responds very adequately to the elevated iFGF23 signal by continuing to excrete phosphate. It is yet unclear why such compensatory mechanisms lead to a chronic reduction in sPi, and in some LKDs below the normal reference range, beyond the early period after kidney donation. These changes collectively substantiate the likelihood of long-term mineral adaptation in LKDs, potentially offsetting any detrimental changes that may occur with loss of a kidney.
Footnotes
Published online 28 March, 2017.
Funding sources: S.J.T. is a current recipient of a National Health and Medical Research Council (NHMRC) Postgraduate Research Scholarship. N.D.T. is supported by a Jacquot Foundation Research Establishment Award. The contents of this article are solely the views of the individual authors and do not reflect the views of NHMRC or the Jacquot Foundation.
S.J.T. has received speaking honoraria from Shire. S.G.H. has received research funding or honoraria from Amgen, Baxter, Gilead, Novartis and Shire. N.D.T. has received consultancy fees, honoraria and research funding from Amgen and Shire Pharmaceuticals.
The authors declare no conflicts of interest.
S.J.T. and N.D.T. designed the study. S.J.T. recruited and conducted the study, performed the assays, undertook the analysis and prepared the article. P.D.H. and S.G.H. contributed intellectually to the critique and final draft of the article. T.D.H. and N.D.T. assisted with analysis and article preparation. All authors have critically revised and approved the article.
REFERENCES
- 1.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semba RD, Cappola AR, Sun K, et al. Plasma Klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdallah E, Mosbah O, Khalifa G, et al. Assessment of the relationship between serum soluble Klotho and carotid intima-media thickness and left ventricular dysfunction in hemodialysis patients. Kidney Res Clin Pract. 2016;35:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith ER, McMahon LP, Holt SG. Fibroblast growth factor 23. Ann Clin Biochem. 2014;51(Pt 2):203–227. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 associates with death, cardiovascular events, and initiation of chronic dialysis. J Am Soc Nephrol. 2011;22:1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano C, Hamano T, Fujii N, et al. Intact fibroblast growth factor 23 levels predict incident cardiovascular event before but not after the start of dialysis. Bone. 2012;50:1266–1274. [DOI] [PubMed] [Google Scholar]
- 9.Garg AX, Muirhead N, Knoll G, et al. Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70:1801–1810. [DOI] [PubMed] [Google Scholar]
- 10.Boudville N, Prasad GV, Knoll G, et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145:185–196. [DOI] [PubMed] [Google Scholar]
- 11.Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mjoen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int. 2014;86:162–167. [DOI] [PubMed] [Google Scholar]
- 13.Fehrman-Ekholm I, Elinder CG, Stenbeck M, et al. Kidney donors live longer. Transplantation. 1997;64:976–978. [DOI] [PubMed] [Google Scholar]
- 14.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. [DOI] [PubMed] [Google Scholar]
- 15.Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ. 2012;344:e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akimoto T, Kimura T, Watanabe Y, et al. The impact of nephrectomy and renal transplantation on serum levels of soluble Klotho protein. Transplant Proc. 2013;45:134–136. [DOI] [PubMed] [Google Scholar]
- 17.Ponte B, Trombetti A, Hadaya K, et al. Acute and long term mineral metabolism adaptation in living kidney donors: a prospective study. Bone. 2014;62:36–42. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walton RJ, Bijvoet OL. Nomogram for derivation of renal threshold phosphate concentration. Lancet. 1975;2:309–310. [DOI] [PubMed] [Google Scholar]
- 20.Kenny AP, Glen AC. Tests of phosphate reabsorption. Lancet. 1973;2:158. [DOI] [PubMed] [Google Scholar]
- 21.Barth JH, Jones RG, Payne RB. Calculation of renal tubular reabsorption of phosphate: the algorithm performs better than the nomogram. Ann Clin Biochem. 2000;37(Pt 1):79–81. [DOI] [PubMed] [Google Scholar]
- 22.KDIGO Working Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 23.Westerberg PA, Ljunggren O, Larsson TE, et al. Fibroblast growth factor-23 and mineral metabolism after unilateral nephrectomy. Nephrol Dial Transplant. 2010;25:4068–4071. [DOI] [PubMed] [Google Scholar]
- 24.Kasiske BL, Kumar R, Kimmel PL, et al. Abnormalities in biomarkers of mineral and bone metabolism in kidney donors. Kidney Int. 2016;90:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young A, Hodsman AB, Boudville N, et al. Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am J Kidney Dis. 2012;59:761–769. [DOI] [PubMed] [Google Scholar]


