Abstract
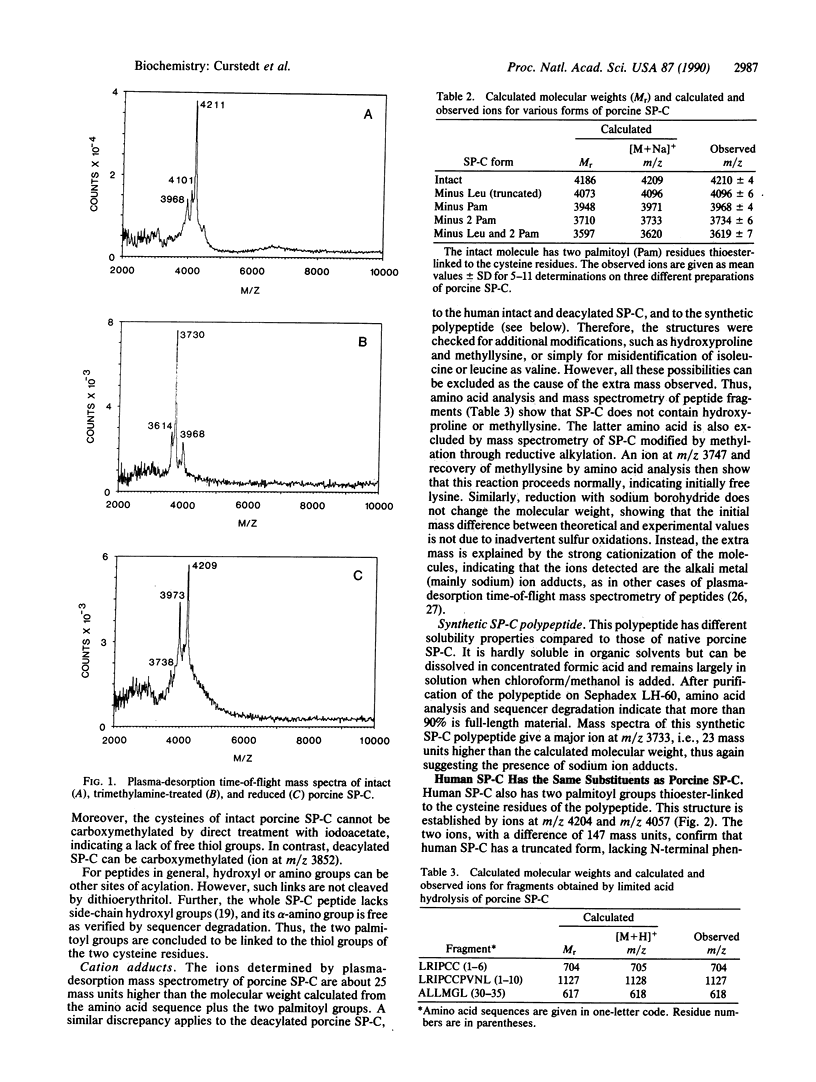
Pulmonary surfactant contains two hydrophobic polypeptides, SP-B and SP-C, with known amino acid sequences and with truncated subforms lacking the N-terminal residues. Treatment of SP-C with KOH releases fatty acids (palmitic acid to more than 85%) in molar ratios of 1.8-2.0 relative to the polypeptide. Furthermore, plasma-desorption mass spectrometry shows native SP-C of both the intact and truncated types to be monomers with masses about 500 units higher than those expected for the polypeptide chains. After treatment with KOH, trimethylamine, or dithioerythritol, the polypeptide masses are obtained. These results prove that native SP-C is a lipopeptide with two palmitoyl groups covalently linked to the polypeptide chain. The deacylation conditions, the presence of two cysteine residues in the polypeptide, and the absence of other possible attachment sites establish that the palmitoyl groups are thioester-linked to the two adjacent cysteine residues. In contrast, the major form of porcine SP-B is a dimer without fatty acid components. That SP-C is a true lipopeptide with covalently bound palmitoyl groups suggests possibilities for functional interactions. It gives a direct physical link between SP-C and surfactant phospholipid components. Long-chain acylation may constitute a means for association of proteins with membranes and could conceivably modulate the stability and biological activity of surfactant films.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bach R., Konigsberg W. H., Nemerson Y. Human tissue factor contains thioester-linked palmitate and stearate on the cytoplasmic half-cystine. Biochemistry. 1988 Jun 14;27(12):4227–4231. doi: 10.1021/bi00412a004. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Johansson J., Barros-Söderling J., Robertson B., Nilsson G., Westberg M., Jörnvall H. Low-molecular-mass surfactant protein type 1. The primary structure of a hydrophobic 8-kDa polypeptide with eight half-cystine residues. Eur J Biochem. 1988 Mar 15;172(3):521–525. doi: 10.1111/j.1432-1033.1988.tb13918.x. [DOI] [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Emrie P. A., Shannon J. M., Mason R. J., Fisher J. H. cDNA and deduced amino acid sequence for the rat hydrophobic pulmonary surfactant-associated protein, SP-B. Biochim Biophys Acta. 1989 Feb 23;994(3):215–221. doi: 10.1016/0167-4838(89)90296-3. [DOI] [PubMed] [Google Scholar]
- Enhorning G., Shennan A., Possmayer F., Dunn M., Chen C. P., Milligan J. Prevention of neonatal respiratory distress syndrome by tracheal instillation of surfactant: a randomized clinical trial. Pediatrics. 1985 Aug;76(2):145–153. [PubMed] [Google Scholar]
- Fisher J. H., Shannon J. M., Hofmann T., Mason R. J. Nucleotide and deduced amino acid sequence of the hydrophobic surfactant protein SP-C from rat: expression in alveolar type II cells and homology with SP-C from other species. Biochim Biophys Acta. 1989 May 1;995(3):225–230. doi: 10.1016/0167-4838(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Fohlman J., Peterson P. A., Roepstorff P., Höjrup P., Kamensky I., Säwe G., Håkansson P., Sundquist B. Comparison of 252californium plasma desorption and fast atom bombardment mass spectrometry for analysis of small peptides. Biomed Mass Spectrom. 1985 Aug;12(8):380–387. doi: 10.1002/bms.1200120805. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Maeta H., Chida S., Morita T., Watabe Y., Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980 Jan 12;1(8159):55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Gitlin J. D., Soll R. F., Parad R. B., Horbar J. D., Feldman H. A., Lucey J. F., Taeusch H. W. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987 Jan;79(1):31–37. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T. E., Clark J. C., Pilot-Matias T., Meuth J., Fox J. L., Whitsett J. A. cDNA, deduced polypeptide structure and chromosomal assignment of human pulmonary surfactant proteolipid, SPL(pVal). J Biol Chem. 1988 Jan 5;263(1):9–12. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T., Pilot-Matias T., Fox J. L., Whitsett J. A. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci U S A. 1987 Jun;84(12):4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Hallman M., Merritt T. A., Jarvenpaa A. L., Boynton B., Mannino F., Gluck L., Moore T., Edwards D. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr. 1985 Jun;106(6):963–969. doi: 10.1016/s0022-3476(85)80253-5. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. S., James G., Olson E. N. Protein fatty acylation: a novel mechanism for association of proteins with membranes and its role in transmembrane regulatory pathways. Biofactors. 1988 Oct;1(3):219–226. [PubMed] [Google Scholar]
- Huang G., Lee D. M., Singh S. Identification of the thiol ester linked lipids in apolipoprotein B. Biochemistry. 1988 Mar 8;27(5):1395–1400. doi: 10.1021/bi00405a001. [DOI] [PubMed] [Google Scholar]
- Jacobs K. A., Phelps D. S., Steinbrink R., Fisch J., Kriz R., Mitsock L., Dougherty J. P., Taeusch H. W., Floros J. Isolation of a cDNA clone encoding a high molecular weight precursor to a 6-kDa pulmonary surfactant-associated protein. J Biol Chem. 1987 Jul 15;262(20):9808–9811. [PubMed] [Google Scholar]
- Johansson J., Curstedt T., Robertson B., Jörnvall H. Size and structure of the hydrophobic low molecular weight surfactant-associated polypeptide. Biochemistry. 1988 May 17;27(10):3544–3547. doi: 10.1021/bi00410a002. [DOI] [PubMed] [Google Scholar]
- Johansson J., Jörnvall H., Eklund A., Christensen N., Robertson B., Curstedt T. Hydrophobic 3.7 kDa surfactant polypeptide: structural characterization of the human and bovine forms. FEBS Lett. 1988 May 9;232(1):61–64. doi: 10.1016/0014-5793(88)80386-7. [DOI] [PubMed] [Google Scholar]
- Kent S. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1988;57:957–989. doi: 10.1146/annurev.bi.57.070188.004521. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Protein modification: new clue to Ras lipid glue. Nature. 1989 Oct 5;341(6241):384–385. doi: 10.1038/341384a0. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Magee T., Hanley M. Protein modification. Sticky fingers and CAAX boxes. Nature. 1988 Sep 8;335(6186):114–115. doi: 10.1038/335114a0. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J., St Jules R. S., Reddy T. S., Bazan N. G., Zatz M. Acylation of disc membrane rhodopsin may be nonenzymatic. J Biol Chem. 1987 Apr 15;262(11):5210–5215. [PubMed] [Google Scholar]
- Olafson R. W., Rink U., Kielland S., Yu S. H., Chung J., Harding P. G., Possmayer F. Protein sequence analysis studies on the low molecular weight hydrophobic proteins associated with bovine pulmonary surfactant. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1406–1411. doi: 10.1016/s0006-291x(87)80288-7. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Persson B., Flinta C., von Heijne G., Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985 Nov 4;152(3):523–527. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- Possmayer F. A proposed nomenclature for pulmonary surfactant-associated proteins. Am Rev Respir Dis. 1988 Oct;138(4):990–998. doi: 10.1164/ajrccm/138.4.990. [DOI] [PubMed] [Google Scholar]
- Raju T. N., Vidyasagar D., Bhat R., Sobel D., McCulloch K. M., Anderson M., Maeta H., Levy P. S., Furner S. Double-blind controlled trial of single-dose treatment with bovine surfactant in severe hyaline membrane disease. Lancet. 1987 Mar 21;1(8534):651–656. doi: 10.1016/s0140-6736(87)90414-4. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Schmidt M. F., Rott R. Chemical identification of cysteine as palmitoylation site in a transmembrane protein (Semliki Forest virus E1). J Biol Chem. 1988 Dec 15;263(35):18635–18639. [PubMed] [Google Scholar]
- Sottrup-Jensen L., Hansen H. F., Mortensen S. B., Petersen T. E., Magnusson S. Sequence location of the reactive thiol ester in human alpha 2-macroglobulin. FEBS Lett. 1981 Jan 12;123(1):145–148. doi: 10.1016/0014-5793(81)80039-7. [DOI] [PubMed] [Google Scholar]
- Tack B. F., Harrison R. A., Janatova J., Thomas M. L., Prahl J. W. Evidence for presence of an internal thiolester bond in third component of human complement. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5764–5768. doi: 10.1073/pnas.77.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treston A. M., Mulshine J. L. Peptide structure. Beyond transcriptional events. Nature. 1989 Feb 2;337(6206):406–406. doi: 10.1038/337406a0. [DOI] [PubMed] [Google Scholar]
- Warr R. G., Hawgood S., Buckley D. I., Crisp T. M., Schilling J., Benson B. J., Ballard P. L., Clements J. A., White R. T. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. J., Richardson C., Ford C., Spencer T., Yao L. J., Mackie G., Hammond G., Possmayer F. Isolation and characterization of the cDNA for pulmonary surfactant-associated protein-B (SP-B) in the rabbit. Biochem Biophys Res Commun. 1989 Apr 14;160(1):325–332. doi: 10.1016/0006-291x(89)91659-8. [DOI] [PubMed] [Google Scholar]



