Abstract
Background
Dabigatran is a novel oral anti-coagulant (NOAC) that reduces risk of stroke in patients with non-valvular atrial fibrillation (NVAF). It does not require routine monitoring with laboratory testing which may have an adverse impact on adherence. We aimed to describe adherence to dabigatran in the first year after initiation and assess the association between non-adherence to dabigatran and clinical outcomes in a large integrated healthcare system.
Methods
We studied a national cohort of 5,376 patients with NVAF, initiated on dabigatran between October-2010 and September-2012 at all Veterans Affairs hospitals. Adherence to dabigatran was calculated as proportion of days covered (PDC) and association between PDC and outcomes was assessed using standard regression techniques.
Results
Mean age of the study cohort was 71.3 ± 9.7 years; 98.3% were men and mean CHADS2 score was 2.4 ± 1.2 (mean CHA2DS2VASc score 3.2 ± 1.4). Median PDC was 94% (IQR 76%-100%; mean PDC 84% ± 22%) over a median follow-up of 244 days (IQR 140-351). A total of 1,494 (27.8%) patients had a PDC <80% and were classified as non-adherent. After multivariable adjustment, lower adherence was associated with increased risk for combined all-cause mortality and stroke (HR 1.13, 95% CI 1.07–1.19 per 10% decrease in PDC). Adherence to dabigatran was not associated with non-fatal bleeding or myocardial infarction.
Conclusions
In the year after initiation, adherence to dabigatran for a majority of patients is very good. However, 28% of patients in our cohort had poor adherence. Furthermore, lower adherence to dabigatran was associated with increased adverse outcomes. Concerted efforts are needed to optimize adherence to NOACs.
As the most common cardiac arrhythmia, atrial fibrillation affects more than 3 million patients and its prevalence is increasing.1 Stroke is a major sequel of atrial fibrillation and treatment to reduce this risk often requires oral anticoagulation therapy. Until recently, the primary option for oral anticoagulation was warfarin, which requires laboratory monitoring and dose adjustment. Further, safety and effectiveness of warfarin depends on the quality of International Normalized Ratio (INR) control. Dabigatran etexilate is the first in a line of new oral anticoagulants that provides an alternative to warfarin, which does not require routine laboratory monitoring and has fewer drug-drug interactions.2 Currently, little is known about how dabigatran is being adopted in routine clinical practice.
In the RE-LY trial, dabigatran was shown to be superior in efficacy to warfarin with a comparable safety profile.2 Over 95% of patients were adherent to dabigatran in the trial, which is significantly higher than adherence rates observed to other cardiovascular medications in routine clinical practice.3,4 While laboratory monitoring of INR is one way to assess adherence to warfarin, similar laboratory testing is not required with dabigatran nor any of the new oral anticoagulants.5 Accordingly, concerns have been raised about the potential for non-adherence with dabigatran and the impact of non-adherence on clinical outcomes.6 The objective of this study was to describe utilization patterns and clinical outcomes in a national cohort of Veterans treated with dabigatran in the Veterans Administration (VA), the largest integrated health care delivery system in the United States.7 Specifically, we describe the clinical characteristics of patients being initiated on dabigatran. Then, we describe adherence to dabigatran therapy in the first year of treatment using pharmacy refill data. Finally, we assess the association between non-adherence to dabigatran with effectiveness (stroke and all-cause mortality) and safety (non-fatal bleeding and myocardial infarction) outcomes.
Methods
Study design, setting and population
This is a retrospective cohort study of patients receiving health care in the VA between October 1, 2010 and September 30, 2012. We included all patients who filled a dabigatran prescription of at least 30 days duration at a VA pharmacy and had at least 30 days of follow-up. During this time, the criteria for dabigatran use were based on national, standardized VA Pharmacy Benefits Management criteria8 which included patients with non-valvular atrial fibrillation and a CHADS2 or CHA2DS2-VASc score ≥1, consistent with the inclusion criteria of the RE-LY trial.
Patients are excluded from receiving dabigatran within the VA if they have any of the following contra-indications: (a) stroke in the preceding 14 days (b) history of valvular heart disease (c) active infective endocarditis (d) active liver disease (e) concurrent indication for anticoagulant therapy such as deep venous thrombosis/pulmonary embolism or prosthetic heart valve (f) pregnancy (g) known hypersensitivity to dabigatran (h) active pathological bleeding (i) concurrent therapy with P-glycoprotein inducers (j) severe renal impairment (creatinine clearance <30 mL/min) or (k) moderate renal impairment (creati-nine clearance 30-50mL/min) and concomitant dronedarone or ketoconazole.
Data Sources
Data for this study were obtained from the VA Corporate Data Warehouse (CDW), which is a national data repository from several VA clinical and administrative systems.9 Data sources for the CDW have been previously validated and described.10–12 It has also been previously used to measure anticoagulation usage in atrial fibrillation.13 The CDW data includes patient demographics, vital status, the date, time and location of service, diagnoses and procedures of all inpatient and outpatient visits provided at the VA or performed at non-VA facilities that were paid for by the VA. In addition, we obtained data on medications dispensed at VA pharmacies. We extracted data on dabigatran utilization including fill dates for prescriptions, prescription cancel dates and amount dispensed. Since the VA has a closed pharmacy system with a fixed, non-tiered copayment, patients have a strong financial incentive to fill prescriptions within the system, particularly for newer therapies that have higher copayments in the private sector.14
Variables
Exposure variable
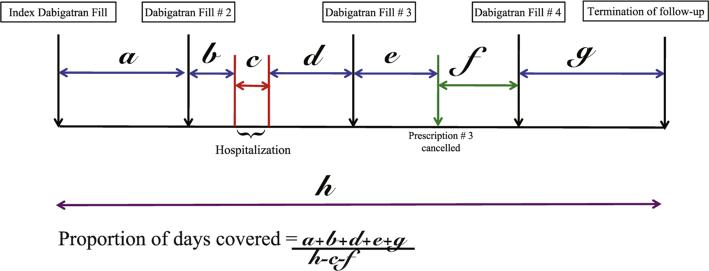
The primary exposure variable was dabigatran adherence calculated in the first year of therapy. We measured adherence using the proportion of days covered (PDC), defined as the total number of non-hospitalized days in which dabigatran was supplied divided by the observation time interval.15,16 The number of outpatient days supplied for dabigatran was determined from prescription fill dates and number of pills dispensed. If outpatient dabigatran supply was interrupted secondary to hospitalization for any cause, duration of inpatient stay was excluded from the denominator but resumed after discharge. We also accounted for any provider prescription cancellation orders by excluding all days after the cancel date from our PDC calculation (Figure 1). Pre-determined endpoints for calculation of PDC included death, transition to warfarin or end of study period. Consistent with prior literature, patients with PDC ≥80% were classified as adherent.16
Figure 1.
Pictorial representation of Proportion of Days covered calculation. Time periods labeled a, b, d, e and g represent time during which patient had dabigatran supply. Time period c represents duration of hospitalization and time period f represents duration following physician ordered prescription cancellation. Time period h represents total follow-up duration.
Further, among non-adherent patients (PDC<80%) gaps in dabigatran therapy were identified as any time duration for which a patient did not have any dabigatran supply available and the patient had not refilled the medication. The total number of gaps and their duration was assessed for every non-adherent patient during follow-up.
Outcome measures. We assessed a composite of all-cause mortality and stroke as our primary outcome for this analysis. The VA vital status file used to assess mortality outcome has a 98.3% sensitivity and 97.6% exact agreement with dates when compared with the National Death Index.12 All-cause mortality was included to ensure complete capture of all events given the high sensitivity and specificity of VA vital status index file. Stroke was ascertained using previously validated primary or secondary International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes (433-437).17 We also looked at non-fatal bleeding events and myocardial infarction (MI) as our other outcome measures. Non-fatal bleeding events were identified with previously validated ICD-9-CM codes for intra-cranial hemorrhage (430-432), gastrointestinal hemorrhage (456-580), hemarthrosis (719), hemopericardium (423), hematuria (599), vaginal bleeding (626-627), hemoptysis (786), epistaxis (784), and hemorrhage not otherwise specified (459).18 The assessment of MI as an outcome following initiation of dabigatran was based on primary discharge ICD-9-CM diagnosis code of 410.xx for any hospitalization within the VA.19
Other covariates. We included other covariates based on prior literature and/or clinical rationale. Demographic covariates included age, sex and race (white vs. other). Clinical covariates included history of hypertension, diabetes mellitus, congestive heart failure, prior MI, prior stroke, chronic kidney disease, chronic liver disease, peripheral arterial disease, bleeding requiring hospitalization in the year prior to dabigatran initiation, depression, alcohol abuse and drug abuse. Treatment covariates included warfarin use in the 100 days preceding dabigatran initiation and concomitant clopidogrel use.
Analysis plan
Comparisons of patient characteristics were made between adherent (PDC ≥80%) and non-adherent (PDC<80%) patients using χ2 test for categorical variables, independent samples t tests for normally distributed continuous variables and Wilcoxon rank-sum test for non-normally distributed continuous variables. Next, Cox proportional hazards regression models were used to assess the association between dabigatran adherence and each of our four outcomes adjusted for the aforementioned covariates. Adherence based on PDC was recalculated at every unique event time and treated as a continuous, time-varying covariate to account for survival bias. For this analysis, predetermined censoring events included death, transition to warfarin or end of study period (September 30, 2012). Hazard ratios with 95% confidence intervals were obtained per 10% increase in PDC. The proportional hazards assumption was evaluated and found to be met.
Sensitivity analyses. First, as adherence to dabigatran may vary by time since therapy initiation, we conducted stratified analyses for adherence based on duration of follow-up after initiation (stratified into ≥30 days, ≥60 days, ≥90 days and ≥180 days). Second, because the effect of adherence on outcomes may vary by time since therapy initiation, we conducted separate Cox regression analyses assessing the adjusted association between PDC and effectiveness outcomes for stratified time periods (less than 90 days, 90-180 days, ≥180 days). Lastly, as adherence to dabigatran might differ among patients transitioned from warfarin to dabigatran, we assessed if prior warfarin use was a predictor of adherence. Accordingly, we constructed a logistic regression model with prior warfarin use as the independent variable and dabigatran adherence as the dependent variable, adjusted for the covariates described above. All analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC). The Colorado Multiple Institutional Review Board approved this study, and waiver of informed consent was granted.
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study all study analyses, the drafting and editing of the paper and its final contents.
Results
Baseline characteristics
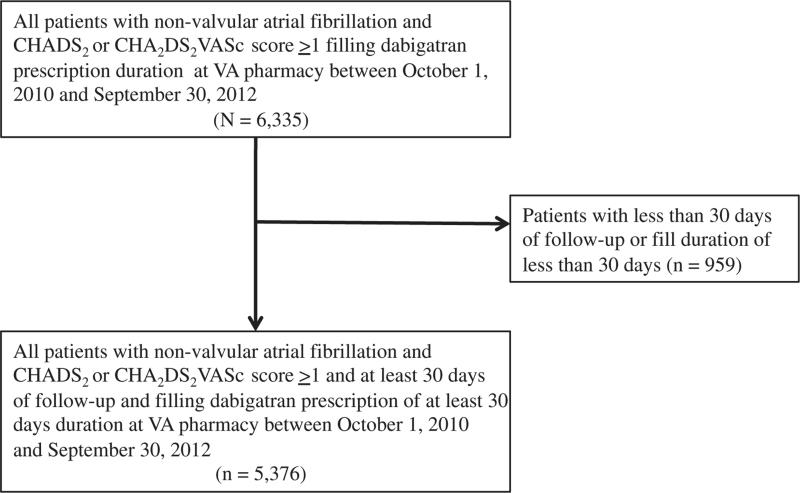
We initially identified 6,335 patients receiving at least one new dabigatran prescription between October 1, 2010 and September 30, 2012 (Figure 2). After applying our eligibility criteria, 959 (15.1%) patients with less than 30 days of follow-up and/or less than 30 days of medication fill were excluded. A total of 5,376 (84.9%) patients were included in the final study cohort with a median follow-up period of 244 days (IQR 140 - 351) and median of 5 refills (IQR 2-8) in the first year of therapy. Overall, mean age of the cohort was 71.3 ± 9.7 years, 98.3% were male and 81.5% were white. The population had a substantial co-morbidity burden, including congestive heart failure (38.3%), diabetes mellitus (42.3%), hypertension (88.1%) and prior stroke (12%). However, other risk factors associated with bleeding were relatively uncommon including chronic kidney disease (12.5%), alcohol abuse (12.8%), liver disease (3.3%) and prior bleeding events (8.2%). The mean CHADS2 score was 2.4 ± 1.2 and mean CHA2DS2VASc score was 3.2 ± 1.4. In addition, prior warfarin use was common (n = 2,821, 52.5%) but concomitant clopidogrel use (5.5%) was infrequent.
Figure 2.
Cohort Creation. CHADS2 score components include congestive heart failure (1 point), hypertension (1 point), age ≥75 years (1 point), diabetes mellitus (1 point), prior stroke (2 points), CHA2DS2VASc score components include congestive heart failure (1 point), hypertension (1 point), age ≥75 years (2 points), diabetes mellitus (1 point), prior stroke (2 points), vascular disease (1 point), age 65-74 years (1 point), female sex (1 point).
Table I demonstrates baseline characteristics of the entire study cohort stratified by adherence to dabigatran. Overall, 3,882 (72.2%) patients were adherent to dabigatran (PDC ≥80%). Compared to non-adherent patients, adherent patients were more likely to be older (71.6 ± 9.4 vs. 70.6 ± 10.6 years P = .001) and white (83% vs. 77.6%, P < 0.001). Adherent patients also had a lower burden of depression (25.8% vs. 29.3%, P = .01), drug abuse (4.7% vs. 7.2%, P < .001), alcohol abuse (11.8% vs. 15.5%, P < .001) and were less likely to be on concomitant clopidogrel (4.8% vs. 7.3%, P < .001) compared to non-adherent patients.
Table I.
Baseline characteristics of study cohort
| Entire study cohort |
Patients adherent* to dabigatran |
Patients non-adherent* to dabigatran |
||
|---|---|---|---|---|
| Variable | (N = 5376) | (n = 3882) | (n = 1494) | P |
| Age, years (mean ± SD) | 71.3 ± 9.7 | 71.6 ± 9.4 | 70.6 ± 10.6 | .001 |
| Males (%) | 5284 (98.3) | 3820 (98.4) | 1464 (98.0) | .32 |
| White Race (%) | 4383 (81.5) | 3224 (83.0) | 1159 (77.6) | <.001 |
| Hypertension (%) | 4726 (87.9) | 3415 (88.0) | 1311 (87.8) | .86 |
| Congestive heart failure (%) | 2039 (37.9) | 1430 (36.8) | 609 (40.8) | .009 |
| Diabetes mellitus (%) | 2273 (42.3) | 1648 (42.5) | 625 (41.8) | .70 |
| Prior stroke (%) | 634 (11.8) | 476 (12.3) | 158 (10.6) | .10 |
| Prior MI (%) | 693 (12.9) | 508 (13.1) | 185 (12.4) | .52 |
| CHADS2 score (mean ± SD) | 2.38 ± 1.23 | 2.39 ± 1.22 | 2.36 ± 1.24 | .47 |
| CHADS2VASc score (mean ± SD) | 3.22 ± 1.37 | 3.24 ± 1.36 | 3.17 ± 1.40 | .10 |
| Chronic kidney disease (%) | 672 (12.5) | 480 (12.4) | 192 (12.9) | .66 |
| Chronic liver disease (%) | 178 (3.3) | 123 (3.2) | 55 (3.7) | .39 |
| Peripheral arterial disease (%) | 937 (17.4) | 671 (17.3) | 266 (17.8) | .68 |
| Depression (%) | 1440 (26.8) | 1002 (25.8) | 438 (29.3) | .01 |
| Alcohol abuse (%) | 688 (12.8) | 457 (11.8) | 231 (15.5) | <.001 |
| Drug abuse (%) | 289 (5.4) | 182 (4.7) | 107 (7.2) | <.001 |
| Concomitant clopidogrel use (%) | 294 (5.5) | 185 (4.8) | 109 (7.3) | <.001 |
| Warfarin use in preceding 100 days (%) | 2821 (52.5%) | 2082 (53.6) | 739 (49.5) | .007 |
MI, Myocardial infarction; SD, Standard deviation; CHADS2 score components include congestive heart failure (1 point), hypertension (1 point), age ≥75 years (1 point), diabetes mellitus (1 point), prior stroke (2 points).
Adherence to dabigatran defined as proportion of days covered ≥80%, non-adherence to dabigatran defined as proportion of days covered <80%.
Adherence patterns
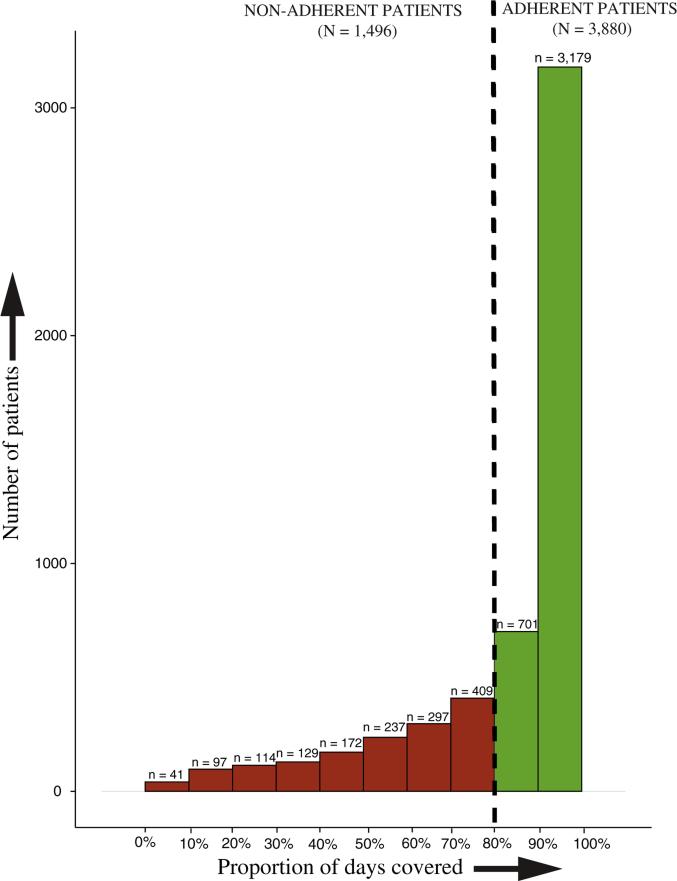
Median PDC in the 12 months following initiation of dabigatran was 94% (IQR 76-100%) and mean PDC was 84% ± 22%. Figure 3 displays the overall PDC distribution. Among non-adherent patients, the median number of gaps was 2 (IQR 1-3) with a median gap length of 24 days (IQR 10-53 days). Furthermore, 1,247 (23%) patients had a gap longer than 30 days in duration.
Figure 3.
Distribution of Proportion of days covered across study cohort. x-axis displays proportion of days covered in increments of 10%, y-axis displays number of patients. Patients with PDC <80% were classified as non-adherent. Patients with PDC ≥80% were classified as adherent.
Association between dabigatran adherence and outcomes
During follow-up, we observed 247 (5%) death and stroke events in the entire study cohort. Following multivariable adjustment, a decrease in PDC by 10% was associated with a 13% increased hazards of the combined outcomes of all-cause mortality and stroke (HR 1.13, 95% CI 1.08-1.19 per 10% decrease in PDC; Table II). These findings were consistent for the stroke only outcome (HR 1.13 per 10% decrease in PDC, 95% CI 0.97-1.33), but not statistically significant due to the small number of strokes (n = 31, 0.6%). Adherence was not associated with non-fatal bleeding events (HR 1.04 per 10% increase in PDC, 95% CI 0.94-1.14) or MI (HR 0.97 per 10% increase in PDC, 95% CI 0.78-1.21).
Table II.
Association between adherence (PDC) and outcomes
| Outcome | Number of events | Unadjusted HR (95% CI) per 10% decrease in PDC | Adjusted HR (95% CI) per 10% decrease in PDC |
|---|---|---|---|
| Combined all-cause mortality and stroke | 247 | 1.13 (1.07-1.19) | 1.13 (1.08-1.19) |
| Stroke | 31 | 1.09 (0.94-1.27) | 1.13 (0.97-1.33) |
| Non-fatal bleeding events | 96 | 1.05 (0.95-1.15) | 1.04 (0.94-1.13) |
| Myocardial infarction | 20 | 1.03 (0.83-1.27) | 0.97 (0.78-1.21) |
Sensitivity analyses
In the sensitivity analyses, patterns of dabigatran adherence demonstrated in the primary analysis did not change with different durations of follow-up after dabigatran initiation (Table III). Further, the risk associated with death and stroke remained consistent regardless of the duration of follow-up since dabigatran initiation (Table IV). Finally, after multivariable adjustment, prior warfarin use was associated with higher odds of adherence to dabigatran (OR 1.25, 95% CI 1.12-1.37).
Table III.
Adherence measures for study cohort stratified by duration of outpatient follow-up
| Minimum duration of out-patient follow-up | Number of patients | PDC, median (IQR) | PDC, mean (SD) | Patients adherent* to dabigatran, N (%) |
|---|---|---|---|---|
| ≥30 days | 5376 | 94% (76%-100%) | 84% (22%) | 3882 (72.2) |
| ≥60 days | 4978 | 94% (76%-100%) | 84% (23%) | 3567 (71.7) |
| ≥90 days | 4598 | 93% (75%-100%) | 83% (23%) | 3273 (71.2) |
| ≥180 days | 3571 | 93% (75%-100%) | 83% (23%) | 2546 (71.3) |
IQR, Interquartile range.
Adherence to dabigatran defined as PDC ≥80%.
Table IV.
Association between adherence (PDC) and outcomes stratified by duration of outpatient follow-up
| Duration of outpatient follow-up (days) | Adjusted HR (95% CI) per 10% unit decrease in PDC |
|
|---|---|---|
| Combined all-cause mortality and stroke | Stroke only | |
| 30-90 | 1.12 (0.96-1.32) | 1.06 (0.68-1.67) |
| 90-180 | 1.16 (1.05-1.27) | 1.06 (0.81-1.41) |
| ≥180 | 1.12 (1.05-1.20) | 1.06 (0.68-1.67) |
Discussion
The objective of this study was to describe adherence and its association with clinical outcomes in a national cohort of Veterans treated with dabigatran. We found that in patients with non-valvular atrial fibrillation, adherence to dabigatran in the first year was good for a majority of patients. However, more than one-quarter of patients had sub-optimal adherence to dabigatran and poor adherence was associated with an increased risk for stroke and all-cause mortality. There was no association between dabigatran adherence and non-fatal bleeding or MI. This report is one of the first nationwide cohort study assessing patterns of adherence to dabigatran in an integrated healthcare system and its association with outcomes.
Prior studies have suggested that the differences between clinical trial efficacy and real-world effectiveness are primarily due to patient selection and an increased potential for medication non-adherence.20 In the RE-LY trial, dabigatran was associated with a lower risk of stroke and systemic embolism compared to warfarin with comparable rates of major hemorrhage.2 Subsequent analyses of real-world cohorts have shown that over a third of atrial fibrillation patients fail to receive appropriate, guideline-directed oral anti-coagulant or anti-platelet therapies.21,22 Additionally, under-treatment of eligible patients with oral anti-coagulants was found to be associated with a two-folds higher odds of a thromboembolic event underscoring the importance of appropriate therapy utilization.21,22 Nonetheless, real-world cohort studies evaluating dabigatran against warfarin have shown comparable safety and effectiveness. For example, Larssen et al examined a national cohort of Danish patients on dabigatran with atrial fibrillation and found lower rates of stroke and mortality without an increased risk of bleeding or MI with dabigatran compared to warfarin.23 Similarly, the FDA conducted a mini-sentinel study to examine rates of adverse events on dabigatran and found comparable bleeding rates with dabigatran and warfarin.24 However, real-world patient adherence to dabigatran therapy was not in any of these studies examined. While The RE-LY4 and RE-COVER trials25 reported over 95% adherence to dabigatran, only 72% of patients were noted to be adherent in our analyses highlighting critical differences between a randomized setting and the real-world scenario. Furthermore, internal national VA metrics show that 68% of patients receiving warfarin are adherent.8 Because of these reasons, the real-world effectiveness of dabigatran may be lower than that observed in the RE-LY trial.
The results of this study support that initiating dabigatran in atrial fibrillation patients in of itself is not adequate to reduce the stroke risk. Since dabigatran and the other new oral anticoagulation agents do not require routine laboratory monitoring, they may require closer clinical follow-up to ensure adequate adherence.26 Potential reasons for suboptimal adherence to dabigatran in clinical practice may include lack of close follow-up such as that seen in a trial-setting, twice daily dosing, gastro-intestinal side effects, poly-pharmacy given the high co-morbidity burden and financial reasons. Studies evaluating adherence to warfarin in a setting similar to ours have shown that adherence improves with attendance at high-performing anticoagulation clinics.27 Consistent with this observation, individuals transitioned from warfarin had a higher odds of being adherent to dabigatran compared to individuals started on dabigatran de novo. Thus, multi-modal interventions aiming at improving adherence to dabigatran may be beneficial and future studies should evaluate the effect of regular follow-up on dabigatran adherence.
Our study should be interpreted in light of several considerations. First, our study cohort comprises exclusively of US Veterans. This cohort is predominantly male and has a higher prevalence of factors associated with poorer adherence.28 Therefore, our results do not necessarily generalize to cohorts under-represented in this study (for example women, or non US populations). Nonetheless, the VA provides a unique opportunity to assess medication adherence since it has a closed pharmacy system and high reliability and validity of its data sources. Furthermore, as patients can obtain dabigatran with lower co-pay at VA pharmacies compared to non-VA pharmacies, it is unlikely that patients obtained dabigatran from other sources.14 Second, due to the retrospective nature of our study, there may be an under-estimation of event rates in our cohort. However, we included all hospitalizations at non-VA facilities paid for by the VA and all-cause mortality as an outcome to ensure complete capture given high sensitivity of the VA vital index file. Cause-specific mortality could not be ascertained to examine non-cardiovascular and non-bleeding causes of death in the study cohort. Third, while we utilized pharmacy databases that accurately capture medication dispensing, we do not know whether dispensed medications were actually taken. Currently, laboratory tests for dabigatran levels are not available29 and other surveillance measures such as electronic monitors recording bottle opening are not readily feasible. Moreover, refill compliance has been shown to be an accurate marker of patient adherence in closed pharmacy systems such as the VA when measured at multiple points in time.30 Further, we accounted for all in-hospital stays in our calculation of medication gaps and PDC as well as physician cancellation of the medication. Therefore our estimates of refill adherence are more specific than prior measures. Also, since we analyzed PDC as a time varying covariate, we addressed the potential for survival bias. Fourth, dabigatran was approved for use relatively recently. Accordingly, our follow-up duration is relatively short and may explain the higher PDC compared to prior reports on other cardiovascular medications. Future studies should evaluate adherence to dabigatran over a longer duration of follow-up. Lastly, due to the observational nature of our study, residual confounding in assessment of the association of adherence and outcomes cannot be completely eliminated. However, we accounted for covariates known to modify this association and used robust statistical techniques to assess the association between poor adherence and outcomes.
In conclusion, in this national cohort study, we found that the majority of patients who initiated therapy with dabigatran for atrial fibrillation were adherent. However, more than one-quarter of patients were non-adherent and lower adherence was associated with increased risk of stroke/death. These findings suggest the advantages of dabigatran relative to warfarin in terms of laboratory monitoring and reduced interactions must be weighed against the implications of non-adherence on patient outcomes. Further, these findings highlight the need for concerted efforts to bolster adherence to dabigatran to ensure optimal patient outcomes. Future studies should evaluate interventions aiming at improving dabigatran adherence.
Acknowledgments
Funding sources: No funding agencies were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not represent the United States government.
Disclosures
Conflicts of Interest disclosures: Drs. Bradley, Turakhia and Maddox are supported by Career Development Awards from Veterans Affairs Health Services Research & Development. All the other authors report no relevant disclosures.
References
- 1.Naccarelli GV, Varker H, Lin J, et al. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009;104(11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Maddox TM, Ho PM. Medication adherence and the patient with coronary artery disease: challenges for the practitioner. Curr Opin Cardiol. 2009;24(5):468–72. doi: 10.1097/HCO.0b013e32832ed62d. [DOI] [PubMed] [Google Scholar]
- 4.Data on file Boehringer Ingelheim, Ridgefield, CT. Clinical Trial Report 1160. 26:2010. [Google Scholar]
- 5.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–95. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell W, Bennett CL. Will newer anticoagulants improve therapy persistence? Arch Intern Med. 2012;172(21):1689–90. doi: 10.1001/2013.jamainternmed.616. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Moran E, Almenoff PL, et al. Variations in efficiency and the relationship to quality of care in the veterans health system. Health Aff. 2011;30(4):655–63. doi: 10.1377/hlthaff.2010.0435. [DOI] [PubMed] [Google Scholar]
- 8. http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. [In]
- 9. www.virec.research.va.gov/CDW/Overview.htm.
- 10.VIRec Research User Guide: FY2002 VHA Medical SAS Inpatient Datasets. Hines; IL: 2003. [Google Scholar]
- 11.Cowper DC, Kubal JD, Maynard C, et al. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462–8. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 12.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metrics. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turakhia MP, Hoang DD, Xu X, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165(1):93 e1–101 e1. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Good CB, Valentino M. Access to affordable medications: the Department of Veterans Affairs pharmacy plan as a national model. Am J Public Health. 2007;97(12):2129–31. doi: 10.2105/AJPH.2007.124008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–8. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 16.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–35. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 17.Birman-Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37(4):1070–4. doi: 10.1161/01.STR.0000208294.46968.a4. [DOI] [PubMed] [Google Scholar]
- 18.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen LA, Wright S, Normand SL, et al. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Intern Med. 1999;14(9):555–8. doi: 10.1046/j.1525-1497.1999.10198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho PM, Peterson PN, Masoudi FA. Evaluating the evidence: is there a rigid hierarchy? Circulation. 2008;118(16):1675–84. doi: 10.1161/CIRCULATIONAHA.107.721357. [DOI] [PubMed] [Google Scholar]
- 21.Gorin L, Fauchier L, Nonin E, et al. Prognosis and guideline-adherent antithrombotic treatment in patients with atrial fibrillation and atrial flutter: implications of undertreatment and overtreatment in real-life clinical practice; the Loire Valley Atrial Fibrillation Project. Chest. 2011;140(4):911–7. doi: 10.1378/chest.10-2436. [DOI] [PubMed] [Google Scholar]
- 22.Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153(6):1006–12. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Larsen TB, Rasmussen LH, Skjoth F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61(22):2264–73. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Southworth MR, Reichman ME, Unger EF. Dabigatran and post-marketing reports of bleeding. N Engl J Med. 2013;368(14):1272–4. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 26.Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154–61. doi: 10.2146/ajhp120634. [DOI] [PubMed] [Google Scholar]
- 27.Rose AJ, Miller DR, Ozonoff A, et al. Gaps in monitoring during oral anticoagulation: insights into care transitions, monitoring barriers, and medication nonadherence. Chest. 2013;143(3):751–7. doi: 10.1378/chest.12-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers WH, Kazis LE, Miller DR, et al. Comparing the health status of VA and non-VA ambulatory patients: the veterans' health and medical outcomes studies. J Ambul Care Manage. 2004;27(3):249–62. doi: 10.1097/00004479-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Gulseth MP, Wittkowsky AK, Fanikos J, et al. Dabigatran etexilate in clinical practice: confronting challenges to improve safety and effectiveness. Pharmacotherapy. 2011;31(12):1232–49. doi: 10.1592/phco.31.12.1232. [DOI] [PubMed] [Google Scholar]
- 30.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J. Clin Epidemiol. 1997;50(1):105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]