Abstract
Infection with varicella zoster virus (VZV) causes varicella (chickenpox), which can be severe in immunocompromised individuals, infants and adults. Primary infection is followed by latency in ganglionic neurons. During this period, no virus particles are produced and no obvious neuronal damage occurs. Reactivation of the virus leads to virus replication, which causes zoster (shingles) in tissues innervated by the involved neurons, inflammation and cell death — a process that can lead to persistent radicular pain (postherpetic neuralgia). The pathogenesis of postherpetic neuralgia is unknown and it is difficult to treat. Furthermore, other zoster complications can develop, including myelitis, cranial nerve palsies, meningitis, stroke (vasculopathy), retinitis, and gastroenterological infections such as ulcers, pancreatitis and hepatitis. VZV is the only human herpesvirus for which highly effective vaccines are available. After varicella or vaccination, both wild-type and vaccine-type VZV establish latency, and long-term immunity to varicella develops. However, immunity does not protect against reactivation. Thus, two vaccines are used: one to prevent varicella and one to prevent zoster. In this Primer we discuss the pathogenesis, diagnosis, treatment, and prevention of VZV infections, with an emphasis on the molecular events that regulate these diseases. For an illustrated summary of this Primer, visit: http://go.nature.com/14×VI1
Varicella zoster virus (VZV, also known as human herpesvirus 3) is a ubiquitous alphaherpesvirus with a double-stranded DNA genome. VZV only naturally infects humans, with no animal reservoir; its main targets are T lymphocytes, epithelial cells and ganglia. Primary infection causes varicella (chickenpox), during which VZV becomes latent in ganglionic neurons. As cellular immunity to VZV wanes with advancing age or in immunocompromised individuals, VZV reactivates to cause zoster (shingles). Zoster can be complicated by chronic pain (postherpetic neuralgia (PHN)) and other serious neurological and ocular disorders (for example, meningoencephalitis, myelitis, cranial nerve palsies, vasculopathy, keratitis and retinopathy), as well as multiple visceral and gastrointestinal disorders, including ulcers, hepatitis and pancreatitis1,2 (Fig. 1). Antiviral drugs and vaccines against both varicella and zoster are available and are effective in treating and preventing VZV-induced disease2.
Figure 1. Different phases of varicella zoster virus infection.
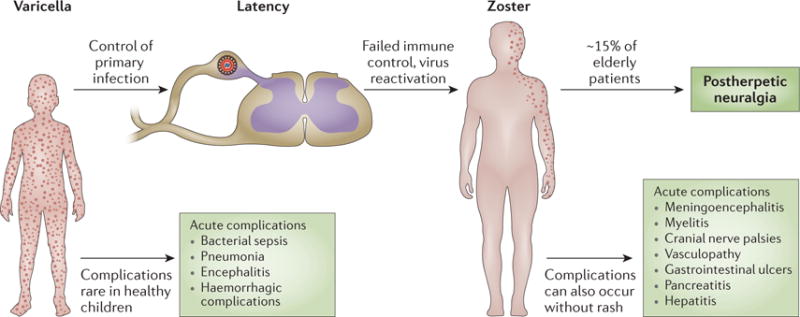
Primary infection with varicella zoster virus (VZV) in susceptible individuals causes varicella, which usually is harmless in healthy children whose immune system controls the infection. VZV establishes latency in ganglionic neurons, and reactivation of viral replication and spread of the virus to the skin innervated by these neurons causes zoster. Increasing age and compromised immune function are risk factors for complications of VZV infections. However, some of these complications, such as postherpetic neuralgia, can also occur without these predisposing factors.
VZV is highly communicable and spreads by the airborne route, with an extraordinarily high transmission rate3 in temperate countries. Traditionally, the virus was thought to spread to others from the respiratory tract, but such evidence is scant. Instead, most virus comes from skin where it is highly concentrated in vesicles; skin cells and cell-free VZV are frequently shed and are probably the major source of infectious cell-free airborne virus4,5. Infected children without skin lesions are not contagious to others4.
The highly efficient transmission of VZV assured that, before the introduction of the varicella vaccine, most children would contract varicella before 10 years of age. Varicella in children is usually self-limiting, although complications can be unpredictable, and long-lasting immunity follows once the patient recovers. Epidemics are also self-limiting because the high rate of transmission and disease-induced immunity deplete the pool of susceptible individuals6. Most older children and adults harbour latent wild-type VZV or vaccinetype VZV (vOka)2. Sporadic reactivation of VZV causes zoster and provides an evolutionary advantage for the pathogen by providing a source of infection in new, susceptible birth cohorts.
VZV occurs worldwide, but in some developed countries there is less concern for VZV than for other infectious agents, such as influenza virus, Ebola virus and multidrug-resistant staphylococci. However, even in countries where varicella vaccination is routine there has not been an eradication of VZV disease. Import of varicella from countries that do not vaccinate and zoster caused by reactivation of latent wild-type VZV or vOka can occur. Given the increasing number of immunocompromised individuals worldwide, it is important to maintain substantial levels of herd immunity against varicella in developed countries and to extend vaccination to developing countries. Recently, the WHO recommended “routine immunization of children against varicella in countries where varicella has an important public health impact”7. In addition, because of the current anti-vaccine movement in some countries, it is also wise to maintain interest in VZV research and to improve current methods to prevent and treat varicella and zoster. In this Primer article, we summarize the diseases and complications of VZV infection, how latency develops, how and when to vaccinate and treat patients, and we highlight open research questions (BOX 1).
Box 1. Questions to ponder while reading this Primer.
Which molecular mechanisms of immunity control varicella zoster virus (VZV) in the human host?
How does VZV become latent, and why only in neurons?
What explains postherpetic neuralgia?
How does stress and immunosuppression lead to virus reactivation?
How do VZV vaccines confer host control of the virus?
Why is VZV readily controlled by vaccination whereas other human herpesviruses are not?
Will new vaccines and antivirals improve the control of VZV? If yes, by what means?
Epidemiology
Varicella
Varicella occurs worldwide and is endemic in populations of sufficient size to sustain year-round transmission, with epidemics occurring every 2–3 years3. Viral genomic studies have identified five viral clades and their geographical distribution: clades 1, 3 and 5 are of European origin; clade 2 includes Asian strains such as the parental Oka strain, from which varicella and zoster vaccines were derived; and clade 4 contains African strains8. Varicella epidemiology and disease burden have been studied primarily in developed countries. Although VZV seroprevalance data are becoming more widely available, additional data are needed on severe disease outcomes and deaths to better characterize the global health burden due to varicella, particularly in regions with high HIV prevalence, such as Africa and India7.
Varicella incidence ranges from 13 to 16 cases per 1,000 persons per year, with substantial yearly variation3. In temperate climates, age-specific varicella incidence is highest in preschool aged children (1–4 years of age) or children in early elementary school (5–9 years of age) with an annual incidence of greater than 100 per 1,000 children; as a result, >90% of people become infected before adolescence and only a small proportion (<5–10%) of adults remain susceptible3,9. In tropical climates, acquisition of varicella occurs at a higher overall mean age (for example, at 14.5 years in Sri Lanka), with a higher proportion of cases in adults3,10. Differences in varicella epidemiology between temperate and tropical climates might be related to the properties of VZV, for example, inactivation by heat and/or humidity, or factors affecting the risk of exposure3.
Varicella shows a strong seasonal pattern, with peak incidence during winter and spring or during the cool, dry season3,11. Outbreaks occur commonly in settings where children congregate, such as childcare centres and schools, but can also occur in other age groups and settings, including hospitals, facilities for institutionalized people, refugee camps and military and correctional facilities12–14.
Although varicella is usually a self-limiting disease, it can result in serious complications and death. In developed countries, ~5 out of 1,000 people with varicella are hospitalized and 2–3 per 100,000 patients die15,16. Serious varicella complications include bacterial sepsis, pneumonia, encephalitis and haemorrhage3. Adults, infants and individuals who are severely immunocompromised are at higher risk of severe complications and death. Varicella acquired in the first two trimesters of pregnancy causes severe congenital defects in the newborn in ~1% of affected pregnancies17.
In countries where varicella vaccination is routinely recommended in childhood, varicella epidemiology has changed dramatically (FIG. 2). In the United States, where a one-dose vaccination programme was implemented in 1995 and a two-dose programme in 2007, varicella incidence, hospitalizations and deaths in children have declined by >95%. Furthermore, significantly less morbidity and mortality in vaccinated and in unvaccinated age groups indicate indirect vaccination effects such as herd immunity and interruption of annual epidemics18,19.
Figure 2. Epidemiology.
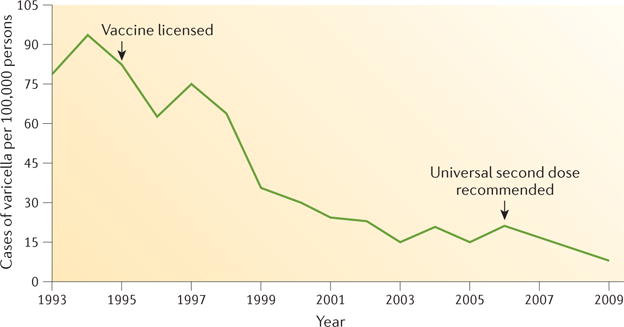
The introduction of the varicella vaccine in 1 995 reduced the number of varicella cases substantially (data shown for Illinois, Michigan, Texas and West Virginia, USA). Reprinted with permission from REF 209, Centers for Disease Control and Prevention (CDC).
Zoster
Zoster epidemiology has been described almost exclusively from developed countries with long life expectancies. The incidence and severity of zoster increase with age due to declining cell-mediated immunity to VZV3,20. PHN is the most serious complication of zoster and occurs in ~15% of cases. Age is the most important risk factor for PHN, with the risk increasing rapidly after 50 years of age3,20. The population incidence of zoster is ~3−4 per 1,000 patient-years of observation, with the incidence varying from ~1 per 1,000 patient-years of observation in children aged <10 years to >10 per 1,000 patient-years of observation in adults aged ≥60 years20–22. By the age of 85 years, >50% of the population reports at least one episode of zoster20. There are limited data on the age-specific incidence in low and middle income countries.
Before VZV vaccines were available, ~30% of adults developed zoster23. Recently, however, a higher proportion of the population has exhibited impaired immunity to VZV and develops zoster, for example, due to the growing number of elderly people, immune-suppressed organ transplant recipients, patients receiving chemotherapy for cancer or autoimmune disease, HIV-infected individuals, and patients with chronic illnesses24. Race is a well-described protective, presumably genetic, factor: black adults in the United States and the United Kingdom have a 25–50% lower incidence of zoster compared with white adults25. Exposure to exogenous VZV protects against zoster and/or boosts cellular immunity26,27. Although mathematical modelling has predicted an increase in zoster where circulating VZV is reduced by childhood vaccination programmes28, the concept of exogenous VZV exposure as the only means for immune boosting remains an area of controversy (see below). Post-licensure surveillance data provide direct evidence of the impact of the varicella vaccine on zoster epidemiology29,30. Healthy varicella vaccine recipients have a lower risk of zoster than unvaccinated individuals, and this finding is consistent with pre-licensure studies demonstrating a similar effect in children with acute leukaemia31. The effect of varicella vaccination on overall zoster epidemiology continues to be evaluated. Most studies examining the overall population rates of zoster in developed countries (the United States, the United Kingdom, Canada, Spain, Japan and Australia) show increasing incidence trends in these countries, regardless of whether they have varicella vaccination programmes3,7. In the United States, where the varicella vaccine has been used for 20 years in children, increases in zoster incidence started years before the use of the varicella vaccine and the rate of increase is similar before and after implementation of the vaccination programme3,29,30.
Mechanisms/pathophysiology
VZV infection and replication
Primary infection
Following transmission to susceptible hosts, VZV proliferates in the oral pharynx (tonsils), infects T cells that enter the circulation and disseminate virus to the skin and possibly other organs; infection is at first controlled by innate immunity32,33.
VZV can remodel diverse T cell populations to facilitate skin trafficking34. VZV DNA can be detected in T cells (viraemia) as early as 10 days prior to the occurrence of a rash and can persist for a week afterwards35. Initially, innate immunity delays viral multiplication in the skin, which provides time for adaptive immunity to develop32. Eventually, cutaneous innate immune responses are overcome by the virus, and there is substantial viral replication in the skin (and sometimes the viscera), resulting in the characteristic rash of varicella33 (FIG. 3). High titres of cell-free VZV develop in skin vesicles and transmit VZV to others5. Important and unpredictable complications of varicella in previously healthy children include encephalitis, haemorrhagic manifestations, and bacterial superinfections involving skin, blood, bones and lungs.
Figure 3. Clinical presentation of varicella withsevere rash.
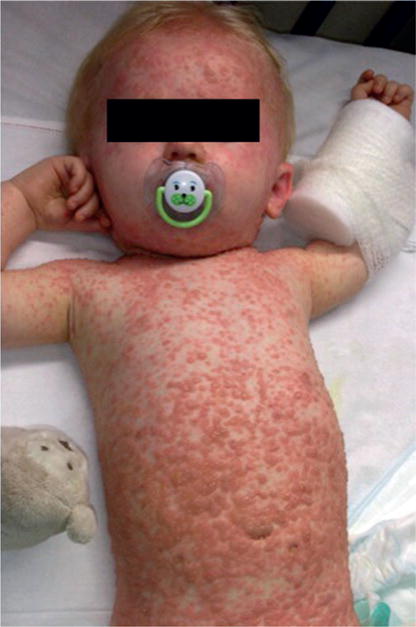
Severe varicella in an 11-month-old infant who, during the incubation period, had received dexamethasone as part of therapy for pneumococcal meningitis. Pictured on day 5 of the rash, when he was still systemically unwell and acquiring new lesions. The infant made a full recovery with intravenous acyclovir therapy.
In this process of lytic infection (FIG. 4a), VZV expresses its 71 annotated genes and possibly additional genes that have not yet been identified. As described for other herpesviruses, gene expression is thought to proceed in an orderly cascade, beginning with immediate early genes, then early genes, followed by late genes. In latency, however, gene expression is restricted and possibly blocked (FIG. 4b). In reactivation, all VZV genes are expressed, again resulting in lytic infection2.
Figure 4. Latent and lytic infection.
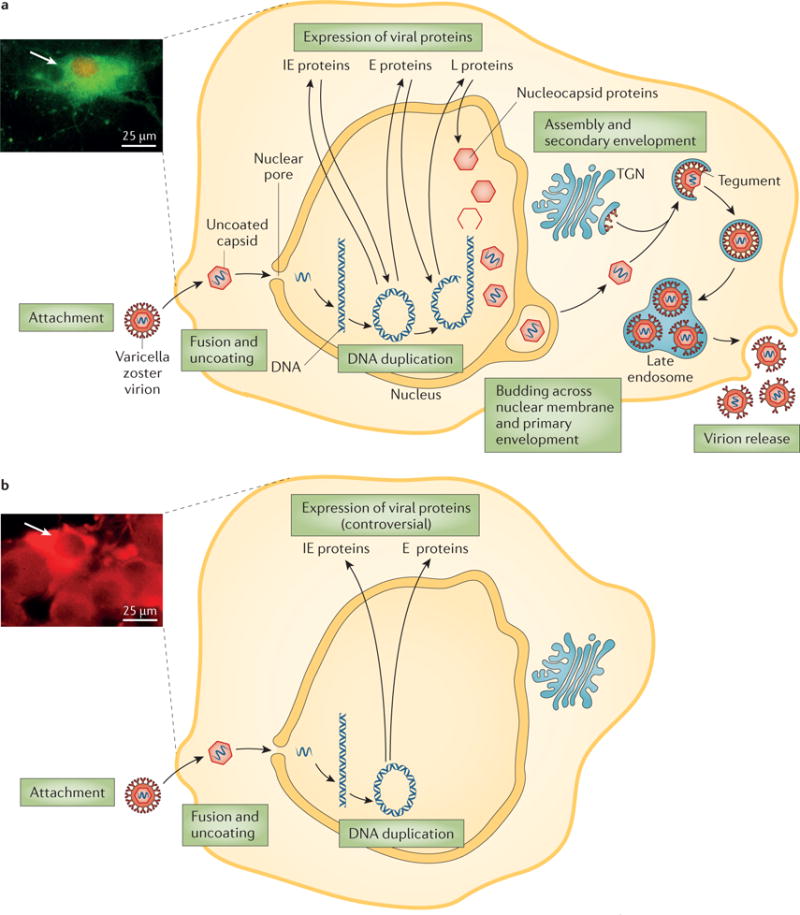
a | Lytic infection with varicella zoster virus (VZV) starts with attachment, fusion and uncoating of the virion. The virus capsid is then transported to the cell nucleus, where the viral DNA becomes circular. The full set of viral proteins, including immediate early (IE), early (E) and late (L) proteins, are expressed and enter the nucleus. New virions then bud in a two-step process. This full cycle of viral replication leads to substantial cell damage and eventually lysis; the acidic environmental in the endosome damages the virus particles and reduces their infectiousness. The micrograph shows VZV infection of guinea pig enteric neurons showing lytic infection47. Isolated neurons were cultured in vitro and infected with cell-free VZV to induce infection. The cultures were fixed and immunostained with antibodies against VZV ORF29p (red) and glycoprotein E (green). The neurons were analysed 48 h after infection with cell-associated virus; after lytic infection, neurons die with 48–72 h. The neuron is filled with cytoplasmic glycoprotein E immunoreactivity and the immunoreactivity of ORF29p has almost entirely translocated to the nucleus (arrow). b | The exact mechanisms of latent infection are unclear but viral replication is thought to stop at the circular DNA stage and no or only limited protein expression occurs. Furthermore, no viral proteins are found in the nucleus. Latent infection causes no easily observable changes of cell morphology (see micrograph). The micrograph shows VZV infection of guinea pig enteric neurons showing latent infection47. Isolated neurons were cultured in vitro and infected with cell-free VZV to induce infection. The cultures were fixed and immunostained with antibodies against VZV ORF29p (red) and glycoprotein E (green). The neurons were analysed 2 weeks after infection with cell-free VZV; after latent infection, neurons survive in vitro for as long as cultures can be maintained. Note that ORF29p immunoreactivity is confined to the cytoplasm; there is no nuclear immunoreactivity (arrow). TGN, trans-Golgi network. Adapted from REF. 210, Nature Publishing Group.
Latency
VZV is latent in neurons of cranial nerve ganglia, dorsal root ganglia, and enteric and autonomic ganglia2,36. Because sites of zoster often correspond to those most involved in varicella (face and trunk), VZV has long been thought to enter epidermal nerve endings during varicella and undergo retrograde axonal transport to reach neuronal cell bodies in the ganglia. Varicella-associated viraemia, however, is an even more likely means by which neurons may be latently infected, particularly enteric or other neurons that lack cutaneous projections. For example, latent VZV has been found in the dorsal root ganglia of children with no history of varicella and no epidermal involvement37. Moreover, when vOka is injected at a single location, it can establish latency not only in ganglia that innervate the injection site, but also bilaterally and at multiple levels of the nervous system37. Experimentally, viral DNA has been found in dorsal root ganglia days before a rash occurs in monkeys infected with simian varicella virus38, and VZV becomes latent in dorsal root ganglia and enteric neurons after intravenous injection of guinea pigs with VZV-infected lymphocytes39. However, it is unclear how VZV is transferred from infected lymphocytes to neurons, and the mechanism by which neurons become latently infected is also unclear.
Latent VZV was found in 34 (1.5%) out of 2,226 neurons and in none of the 20,700 satellite cells from the trigeminal ganglia of 18 deceased individuals with a history of varicella40. Latent VZV DNA is circular and non-replicating36; moreover, histones containing posttranslational modifications indicative of euchromatin are associated with immediate early genes, perhaps facilitating the expression of these genes, but not with late VZV genes41. Immediate early protein 63 (IE63) interacts with anti-silencing function protein 1 (ASF1) to increase its histone binding42. ASF1 mediates the deposition and eviction of histones from DNA during transcription, and IE63 through its interaction with ASF1 may regulate transcription of viral and/or cellular genes, a possibility that requires verification.
VZV gene expression during latency remains unresolved and highly controversial. Latency-associated VZV gene expression has been investigated in cadaver ganglia obtained at autopsy because human dorsal root ganglia and cranial nerve ganglia are rarely, if ever, resected during life. However, stress associated with terminal illness and the interval between death and autopsy might influence results by disrupting the regulation of VZV gene expression and permitting limited expression in cadaver ganglia; alternatively, death may transiently silence ongoing gene expression. Post-mortem reactivation and virus replication probably does not occur because infectious VZV cannot be recovered in cultures of explanted ganglia43, and VZV DNA also fails to increase as a function of time after death44. Multiple laboratories have reported the presence of transcripts encoding at least six immediate early and early VZV genes (open reading frame 4 (ORF4), ORF21, ORF29, ORF62, ORF63 and ORF66) in latently infected neurons of cadaver ganglia (reviewed in REF 2). More recently, however, only transcripts of ORF63 were found to be expressed in ganglia obtained <9 h post-mortem44. By contrast, transcripts encoding at least 12 VZV genes were detected when the ganglia were obtained later (>9 h post-mortem)44.
Enteric ganglia, however, can be examined immediately after surgical removal of bowel37,45. Transcripts encoding ORF4, ORF21, ORF29, ORF62, ORF63 and ORF66 were detected in surgical specimens of gut from 12 out of13 healthy children not in 7 negative controls (infants <1 year old with no history of varicella or vaccination)45. Transcripts were also found in 26 out of 30 surgically removed specimens of adult intestine46. However, surgery and the disorder that necessitated it are undoubtedly stressful; thus, these transcripts could reflect stress-induced gene expression rather than latency-associated transcription. Interestingly, the same genes (ORF4, ORF21, ORF29, ORF62, ORF63 and ORF66) are expressed during latency in guinea pig dorsal root ganglia and enteric neurons39,45–47 that were obtained without stress to the animals or post-mortem delay.
When VZV reactivates, ganglia become necrotic and haemorrhagic48. VZV proteins are found in neurons and non-neuronal cells, and ganglionitis is marked by the upregulation of MHC class I and II proteins and the infiltration of CD4+ and CD8+ T cells49–51. Ganglionitis and CD8+ T cell infiltration can persist after zoster51. Expression of late viral proteins, such as envelope glycoprotein E, shows that lytic VZV infection has occurred2. However, the meaning of the immunocytochemi-cal detection of immediate early and early proteins in neurons during latency is controversial2; these proteins have been detected in latently infected neurons52–54. All latency-associated proteins are cytoplasmic, but become nuclear during productive infection53–55. Immediate early and early proteins are also cytoplasmic in latently infected guinea pig neurons and translocate to the nucleus when reactivation is induced42,46,47,56.
Concerns have been raised over the specificity of VZV immunostaining in adult human neurons because of pigments in neurons57 and because immunocytochemical reagents may contain antibodies that cross react with blood group A antigens in neurons of patients with type A blood58. Neuronal pigments, however, are present in controls in which primary antibodies are omitted as well as in immunostained sections allowing pigment and reaction product to be distinguished37,53. Furthermore, cytoplasmic immunostaining of latency-associated proteins has been observed in neurons of patients who were determined to have blood type B39,53. Future studies are needed to resolve controversies of VZV transcription and translation during latency.
Neurological complications of zoster
Zoster paresis
Manifestations of zoster paresis include arm or diaphragmatic weakness after cervical zoster59, leg weakness after lumbar or sacral zoster and urinary retention after sacral zoster60. MRI reveals involvement of both dorsal and ventral roots of spinal nerves61. Rarely, cervical zoster paresis extends to the brachial plexus62. The prognosis varies, but ~50% of patients recover completely63.
Neuralgia
PHN, the most common complication of zoster, is defined as pain that persists for at least 3 months after rash onset. Three non-mutually exclusive theories have been proposed to explain the cause and pathogenesis of PHN. One is that the excitability of ganglionic or spinal cord neurons is altered during recovery64. The second is that persistent productive VZV infection exists in ganglia, a notion that is supported by possible chronic ganglionic inflammation in PHN65. A third theory is that PHN might be due to gene expression and protein production without virus replication but with disturbance of neuronal physiology.
Recently, certain strains of VZV were postulated to produce PHN by altering voltage-gated sodium channels, leading to altered excitability66,67. Isolated and then cultured VZV strains from patients with PHN and those with zoster but without PHN were applied to neuroblastoma cells that express fast and slow sodium channels. Voltage-clamp recordings from infected neuroblastoma cells revealed that the amplitude of the sodium current was greater in cells infected with VZV isolated from those with PHN than in cells from those without PHN. Increased sodium current is associated with neuropathic pain; thus, VZV-induced increases in sodium currents could have a role in PHN. These experiments suggest that PHN might be partly attributable to the particular strain causing zoster. This intriguing possibility clearly needs confirmation and further research.
Virological analyses of ganglia from patients with PHN are lacking. One study reported the detection of VZV DNA in peripheral blood mononuclear cells (PBMCs) for up to 8 years after zoster in 11 out of 51 patients with PHN but not in controls68. A case report described a correlation between pain and VZV DNA detection in PBMCs in an immunocompetent elderly woman with PHN69. After treatment with famciclovir (a guanosine analogue antiviral drug), pain resolved and the PBMCs no longer contained VZV DNA.
If PHN is caused by persistent VZV replication in neurons, antiviral treatment might decrease its severity. Treatment with oral antiviral agents reduces pain associated with acute zoster; however, this acute treatment has not reduced the incidence, severity or duration of chronic pain of immunocompetent patients with PHN70–73. Acyclovir improved symptoms in 1 out of 10 patients with PHN72, whereas valaciclovir improved symptoms in 8 out of 15 patients73. Proof of a positive effect of antivirals on PHN would require a randomized controlled study of patients with PHN. Most studies, however, have not found antiviral therapy to be effective in the treatment of PHN74 and regulatory authorities do not recommend antivirals to treat this condition75.
VZV meningoencephalitis
Acute VZV infection may present as meningitis or meningoencephalitis with or without rash. Detection of VZV DNA and antibodies in cerebrospinal fluid has confirmed VZV as a cause of aseptic meningitis76, meningoradiculitis77 and cerebellitis78.
VZV vasculopathy
A serious complication of VZV reactivation is infection of the cerebral arteries (VZV vasculopathy), which causes ischaemic and haemorrhagic stroke. The incidence of VZV vasculopathy is unknown. In children, up to one-third of ischaemic arteriopathies are associated with varicella79. In adults, the risk of stroke is increased by 30% within 1 year of zoster80 and by 4.5-fold after zoster in the ophthalmic branch of the trigeminal nerve81. One large population-based analysis showed that the risk was even higher: stroke was observed within a 1-year of follow-up in 8.1% of people with zoster ophthalmicus compared with only 1.7% in a matched control group82. However, no cases of vasculopathy or stroke were observed among 984 patients with documented zoster followed up for >6 months after rash onset, although 86% received antiviral therapy22. Indeed, stroke following zoster ophthalmicus is of high clinical importance83,84. VZV that reactivates in the trigeminal nerve can travel via the ophthalmic sensory nerves to the face and via afferent sensory fibres to the internal carotid artery and its intracranial branches85,86. Thereafter, the virus establishes infection in the arterial wall, which leads to inflammation, arterial weakening, aneurysm formation, occlusion and stroke87. Infected cerebral arteries contain multinucleated giant cells, Cowdry A inclusions (accumulations of viral DNA and protein in the cell nucleus) and herpesvirus particles, as well as VZV DNA and VZV antigens81,85.
VZV vasculopathy presents with headache, mental status changes and focal neurological deficits. Large and small vessels are involved81. Brain MRI frequently reveals lesions at grey-white matter junctions. In more than two-thirds of patients, angiography reveals focal arterial stenosis and occlusion, aneurysm or haemorrhage81.
VZV and giant cell arteritis
One of the most exciting recent developments is the detection of VZV antigen, VZV DNA and virus particles in the temporal arteries of patients with giant cell arteritis (inflammatory vasculopathy, most often involving the temporal arteries)88. Analysis of temporal artery biopsies from healthy individuals aged >50 years and from patients with arteritis revealed VZV antigen in the temporal arteries of 61 out of 82 (74%) of patients with arteritis compared with 1 out of13 (8%) in normal temporal arteries88. This discovery, if confirmed, suggests that antiviral treatment might confer additional benefit to corticosteroids in patients with giant cell arteritis.
VZV-induced diseases of the eye
VZV can cause stromal keratitis with corneal anaesthesia, acute retinal necrosis and progressive outer retinal necrosis, particularly in immunocompromised individuals. Patients complain of eye pain and loss of vision. Retinal haemorrhages and whitening with bilateral macular involvement may occur. Zoster, aseptic meningitis, vasculitis or myelitis can precede retinal necrosis89. As with neurological disease, VZV-associated ocular disorders can also occur without rash.
Diagnosis, screening and prevention
Diagnosis
Diagnosis of varicella and zoster is most often made clinically on the basis of the characteristic generalized or unilateral dermatomal vesicular rashes, respectively. Notable exceptions include the following characteristics: atypical rashes, such as disseminated zoster or a minimal or absent dermatomal rash; zosteriform herpes simplex; modified (breakthrough) varicella in vaccinated individuals; and rashes caused by enteroviruses, poxviruses, rickettsia, drug reactions or contact dermatitis; and VZV infection in the absence of a rash. The latter includes, for example, zoster without rash (known as zoster sine herpete, sometimes with or without facial palsy90), meningitis1,91,92, stroke81,93–97, myelitis98 and enteric (gastrointestinal) infections45,99–102. In these settings, rapid diagnosis is necessary to plan appropriate therapy and public health measures. Diagnosis by measurement of VZV-specific antibodies in serum samples is accurate but does not yield results rapidly enough to be clinically useful because of the time it takes for patients to develop antibodies. Serum antibodies are generally of no help unless anti-VZV IgM is detected and even its presence can be nonspecific.
For laboratory diagnosis of VZV infection, the following approaches are currently most useful: PCR on material from skin vesicles (submitted as swabs, fluid or scabs102–104), saliva90,102,103,105–107 and cerebrospinal fluid if neurological symptoms or signs are present91,92,108,109. Detection of VZV antigens by direct immunofluorescence from vesicles is also rapid and specific, although less sensitive than PCR110. During varicella and zoster, viral DNA can be detected in saliva, and this method is diagnostically useful and specific in symptomatic patients with or without rash102,104,106. PCR along with restriction enzyme digest and sequencing of specific segments of the viral genome can be used to determine whether VZV is resistant to acyclovir111.
When symptoms develop in vaccinated individuals that suggest VZV infection, such as rash or meningitis (even without rash), it is important to identify VZV by PCR, and it can be useful to determine whether the virus is wild-type VZV or vOka104,112–114. In the United States, testing can be performed free of charge at either the National Virus Laboratory at the Centers for Disease Control and Prevention (CDC) or the Columbia University VZV Identification Program of the Worldwide Adverse Experience System of Merck & Co. The FDA is notified of these results if they are related to complications of vaccination, and clinical information on these vaccinated patients is often published2. Other countries have similar testing facilities (for example, the VZV Reference Laboratory in the United Kingdom)115.
Notably, VZV DNA can be detected transiently in the saliva of severely stressed adults116 and children117 in the absence of specific symptoms, indicating subclinical reactivation of the virus. Nevertheless, testing of saliva is remarkably specific because VZV DNA is rarely detected in asymptomatic human volunteers aged <60 years40,102,118. The presence of VZV DNA does not necessarily equate to the presence of infectious virus. During zoster, a single infected cell can contain thousands of copies of VZV DNA and that DNA can persist long after infectious VZV has been cleared. ‘Contained reversions’ (silent reactivation of latent VZV) (FIG. 5) may also be a source of VZV DNA, which makes it problematic to demonstrate that vasculopathy without rash is a complication of zoster or that PHN is associated with continuing VZV replication. Furthermore, despite evidence of asymptomatic shedding of VZV DNA, there is little epidemiological evidence to indicate that asymptomatic individuals transmit VZV infection3,4. By contrast, most infections with herpes simplex virus result from asymptomatic shedding. Nevertheless, detection of VZV DNA in saliva or blood in immunocompetent individuals with minimal symptoms may permit diagnostic suspicion of early zoster before rash onset and the initiation of antiviral therapy sufficiently early to halt ganglionic infection before the damage responsible for PHN has occurred.
Figure 5. Natural history and pathogenesis of zoster.
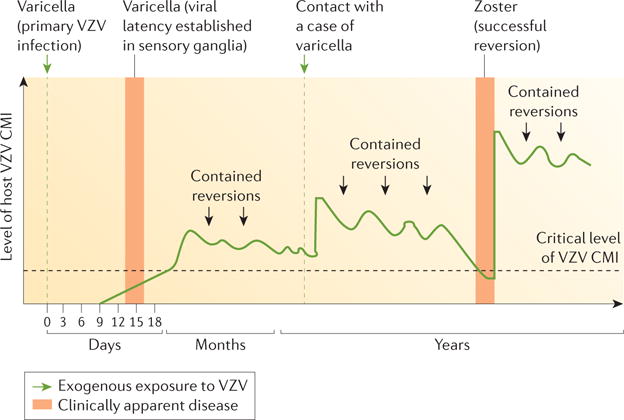
The classic mod el of zoster pathogenesis first described by Hope-Simpson20 links viral replication and clinical disease with the levels of cell-mediated immunity (CMI). In primary infection, no immunity to varicella zoster virus (VZV) exists and infection becomes apparent as varicella. CMI then controls replication, and reactivation of the virus remains subclinical and asymptomatic (‘contained reversions’). Exogenous contact with VZV can boost CMI; nevertheless, CMI levels decrease over time and when they fall below a critical level, zoster can develop. Adapted with permission from REF 20, The Royal Society of Medicine.
Routine screening of asymptomatic individuals for VZV infection is currently not recommended.
Prevention
Varicella vaccine
A live attenuated varicella vaccine was developed in Japan by Takahashi and colleagues in 1974 (REF 119). VZV was isolated from a boy with varicella, and the virus was attenuated by cultivation for 33 serial passages in human and guinea pig fibroblasts, with some passages at low temperature (34°C)119. Live, attenuated vOka consists of a mixture of distinct VZV genotypes, with 42 single nucleotide polymorphisms distinguishing vOka from the wild-type Oka parent strain120,121. The exact mechanisms for the attenuation of VZV in live vaccines remain unknown; mutations in ORF62 were identified in vOka122 and mutations at positions 106262, 107252 and 108111 in ORF62 are important112, as is a stop codon mutation at position 560 in ORF0 (REF 123). These mutations, particularly the unique mutations at positions 106262 and 108111 in ORF62, can be used to differentiate parental Oka and other wild-type strains of VZV from vOka104,112.
Initially, the vaccine was highly controversial because of the fear of latent vOka, the possibility that the virus might be oncogenic and the possibility that immunity from vaccination would not be long-lasting. The vaccine was tested in Japan for ~5 years before reaching the rest of the world124,125. By 1979, there was much interest in the vaccine in the United States because many young children who had been cured of leukaemia died of varicella infection. In a large collaborative trial, >500 children with leukaemia in remission who were still receiving maintenance chemotherapy were vaccinated with vOka. The vaccine was considerably safer than infection with wild-type VZV and protected ~85% of recipients from varicella126. Studies in healthy children also demonstrated a high degree of safety and protection against varicella127–129. In 1995, this live attenuated varicella vaccine was licensed in the United States and routine immunization recommended for healthy American children at 1 year of age9. In 2007, when a second dose was shown to offer even greater protection than one dose130, a two-dose schedule, with the second dose given at aged 4–6 years or at least 3 months after the first dose, was recommended by the CDC9. Two doses of the varicella vaccine are also recommended for older children, adolescents and adults without evidence of varicella immunity, including health-care workers. Currently, the varicella vaccine, using one or two doses, is also licensed in Australia, Brazil, Canada, China, Germany, Greece, Israel, Italy, Japan, Qatar, South Korea, Spain, Taiwan and Uruguay.
In countries without varicella vaccination, prevention by passive immunization (injection of VZV-specific antibodies) might be available, which provides temporary protection for several weeks131. Antiviral therapy has been used for the prevention of varicella132,133 and zoster134,135 in immunocompromised patients, but is not uniformly accepted as useful. Acyclovir-resistant VZV rarely develops in a small number of treated immunocompromised patients136–138.
Immunity after vaccination
The varicella vaccine is highly effective in preventing varicella, with little decline in immunity over time139,140. Between 1995 and 2003 the incidence of varicella decreased by 80% in some areas of the United States141, and the number of hospitalizations and deaths decreased19. In a study of >7,000 vaccine recipients, long-lasting (15 years) immunity was demonstrated, with 90% efficacy in preventing varicella despite 70% of children having received only one dose of the vaccine139. At present, there seems to be no need for booster doses of the varicella vaccine because significant waning immunity has not been observed139,140.
Silent reactivation (contained reversions) of latent VZV (FIG. 5) can boost immunity endogenously2 and is likely to contribute to long-lasting immunity to varicella and zoster20. Exogenous boosting due to exposure to individuals with VZV infections also occurs27,142 (FIG. 5). Not appreciating the potentially important role of asymptomatic reactivation in maintaining immunity to VZV, epidemiologists predicted that widespread vaccination and the resulting lack of exogenous immune stimulation would result in increased incidence of zoster143,144. This has been the justification for not using the varicella vaccine universally in children in several countries in Europe. The incidence of zoster has been increasing in the United States since the 1950s, long before the varicella vaccine was available, and increases are also reported in other countries without vaccination programmes145. Thus, it seems that the varicella vaccine is unlikely to contribute substantially to an increase in the incidence of zoster. The role of subclinical (or mild) endogenous reactivation of latent VZV in maintaining immunity is evidenced by a study performed in France: the rate of zoster (15–16%) in isolated populations over ~30 years was no higher than it was in the general public146. Accordingly, latency (with the possibility of silent or minimal viral reactivation) might be an asset rather than a liability for the varicella vaccine146.
Vaccine safety
The varicella vaccine is safe and well tolerated. Serious adverse events after vaccination are unusual; ~100 million children and adults have been vaccinated and vaccine-related serious adverse event reactions have been very rarely reported and have occurred almost exclusively in recipients who were immunocompromised but not recognized to be so3,138. The development of varicella in vaccine recipients (breakthrough varicella) is unusual, and when it occurs it is almost invariably mild3.
Vaccinated children who develop a rash in the first month after immunization rarely transmit vOka to close contacts; those who develop varicella due to wild-type VZV are more likely to transmit the virus to others3. Transmission is positively correlated with the number of skin lesions4,147. Rash caused by vOka is unusual following vaccination in children and even if it occurs, vaccinated children usually develop few skin lesions. When wild-type VZV infects vaccinated children, they rarely develop an extensive rash. Transmission of vaccine-type type VZV from vaccinated individuals is rare. The risk of developing zoster after vaccination is lower than after varicella caused by wild-type VZV; at present, 30–50% of cases of zoster in vaccinated children are attributable to wild-type VZV148,149.
Zoster vaccine
Development of the varicella vaccine paved the way for the development of a vaccine to prevent zoster and its complications119. Clinical observations by Hope-Simpson, reported in a landmark publication in 1965 (REF 20), provided the rationale for the development of a therapeutic zoster vaccine. Hope-Simpson postulated that primary VZV infection establishes lifelong latency of VZV in sensory ganglia and induces immunity to VZV that prevents the reactivation, replication and spread of latent VZV and, therefore, zoster. He proposed that this immunity gradually decreases, eventually permitting latent VZV to reactivate, multiply and re-emerge as zoster. He further proposed that both exogenous and endogenous exposure to VZV stimulate the host’s immunity (FIG. 5). Noting that second episodes of zoster were uncommon, he proposed that an episode of zoster also stimulates immunity to VZV, essentially immunizing the host against another episode of zoster. Subsequent investigations have validated every component of Hope-Simpson’s prescient hypothesis3,150.
In the Shingles Prevention Study (SPS), a doubleblind, placebo-controlled trial, 38,546 healthy adults aged ≥60 years (median 69 years) were randomly assigned to receive a single dose of high-potency vOka (14 times greater potency than the varicella vaccine) or placebo22,151,152. Two co-primary end points were investigated: the burden of illness owing to zoster (a severity-by-duration measure representing the total pain and discomfort) and the incidence of clinically significant PHN. The incidence of zoster was also determined. The higher-potency vaccine was required to increase VZV-specific cell-mediated immunity in latently infected older adults22,151.
A total of 19,270 people who received the zoster vaccine and 19,276 who received placebo were followed up on average for 3.13 years22,152. There were 957 confirmed evaluable cases of zoster (315 in vaccine recipients and 642 in placebo recipients). In both groups, >93% of the subjects with zoster were positive for wild-type VZV DNA by PCR and none had vOka DNA22,104.
The vaccine reduced burden of disease by 61.1% (65.5% in people aged 60–69 years and 55.4% in people aged ≥70 years). The duration of pain and discomfort among subjects with zoster was shorter in vaccinated candidates compared with placebo recipients22,152. The vaccine reduced the incidence of zoster by 51.3% (63.9% in people aged 60–69 years but only 37.6% in people aged ≥70 years). The incidence of clinically significant PHN was reduced by more than 65% for both age groups. Furthermore, the zoster vaccine reduced the percentage of people with zoster who developed PHN by >31%, with most benefit in the group aged ≥70 years, which had the highest risk of developing this complication22,152. The SPS also demonstrated that the vaccine reduced the adverse impact of zoster on patients’ capacity to perform activities of daily living and health-related quality of life153.
The zoster vaccine is safe. Rates of serious adverse events, systemic adverse events, hospitalizations and deaths in the SPS were low in vaccine recipients and comparable with those in placebo recipients22,151,154. During the first 42 days after vaccination, 24 cases of zoster in placebo recipients were reported and 7 cases in the vaccination group, none of which were caused by vOka104. In contrast to prophylactic vaccines, such as those against varicella and measles, the zoster vaccine is a therapeutic vaccine intended to prevent reactivation and replication of latent VZV with which the recipient is already infected before vaccination and to which the recipient already has substantial immunity.
The zoster vaccine was licensed by the FDA in 2006 and recommended by the CDC in 2008 for the routine immunization of healthy adults aged ≥60 years for prevention of zoster and its complications, primarily PHN155. Post-licensure studies have confirmed the vaccine’s safety and efficacy151,156,157. Unfortunately, uptake of the zoster vaccine has been low, which is probably due to the high cost and general lack of appreciation of the importance of preventing infectious diseases in older adults121. The vaccine has now been shown to be safe and effective in healthy individuals aged 50–59 years158. At present, the zoster vaccine can be used in this age group but it is not yet officially recommended.
The SPS demonstrated persistent efficacy 4 years after vaccination. In additional substudies159,160, efficacy for burden of disease persisted for 10 years after vaccination but efficacy for the incidence of zoster persisted only for 8 years160. The CDC currently does not recommend booster doses of the zoster vaccine, but it might do in the future.
New subunit vaccine
The development by GlaxoSmithKline of a liposome-based subunit vaccine (HZ/su), which contains the VZV glycoprotein E and the adjuvant ASO1B, promises to change prospects for immunization against zoster and its complications. Phase I and II studies established that two doses of HZ/su containing 50 μg of recombinant VZV glycoprotein E administered 1 or 2 months apart were well tolerated and induced much more robust VZV-specific and VZV glycoprotein E-specific CD4+T cell and antibody responses than vOka161,162. Two large Phase III placebocontrolled efficacy trials have been recently completed in individuals aged ≥50 years and in individuals aged ≥70 years163,164. The efficacy against zoster in the younger study group was ~97%. HZ/su has not been tested for its efficacy in preventing varicella, partly because vOka is extremely effective for this purpose. The adjuvanted glycoprotein E vaccine is non-replicating, and can, therefore, be used in immunosuppressed patients who are currently precluded from receiving the live attenuated vOka zoster vaccine155.
Management
Varicella and its complications
Most varicella in healthy children is mild, self-limiting and uncomplicated. Accordingly, and even though early antiviral therapy can reduce the duration of illness165, treatment of uncomplicated varicella in children is usually confined to symptomatic relief. Acetaminophen (paracetamol) is the preferred antipyretic agent because of the association between aspirin and Reye syndrome (life-threatening sudden onset encephalopathy and liver dysfunction)166 and because of an epidemiological link between ibuprofen and an increased risk of invasive group A streptococcal disease in the context of varicella, although not necessarily a causal one167. Topical anti-pruritic agents are of anecdotal benefit. Antiviral therapy is reserved for those with severe varicella or who are considered at greater risk of developing complications owing to age, compromised immunity or chronic diseases of the skin or lungs9,168 (FIG. 6a).
Figure 6. Antiviral treatment in VZV disease.
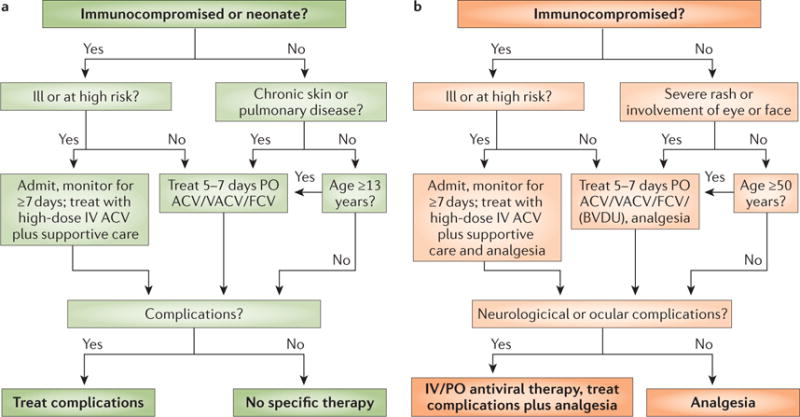
a | Antiviral treatment of varicella is indicated in immunocompromised individuals, neonates, patients with chronic skin or lung diseases and in individuals aged >13 years. Patients receive oral acyclovir (ACV), valaciclovir (VACV) or famciclovir (FCV; not approved by the FDA for use in children) unless they are clinically ill or at high risk (most immunocompromised patients are considered to be at high risk, except those who receive long-term, effective immunoglobulin replacement therapy or those who received only mildly immunosuppressive drugs a long time ago). Ill and high risk patients receive intravenous (IV) ACV or foscarnet if the infection is caused by ACV-resistant VZV. Intravenous treatment always needs careful consideration of kidney function. b | The treatment of zoster follows a similar algorithm; here compromised immunity, illness, severe rash, involvement of eyes or face, and other complications are indications for antiviral treatment. In addition to ACV, VACV and FCV, brivudin (BVDU; not approved by the FDA) might be used. Patients who develop varicella or zoster in hospital will generally receive antiviral therapy as part of an infection control strategy. PO, per os (oral administration).
Severe varicella is characterized by extensive and prolonged viral replication, often associated with fever, continued development of new skin vesicles for >5 days, and/ or involvement of the lungs, liver and/or brain (FIG. 3 illustrates a dense vesicular rash in a febrile infant on day 5). Severe varicella is a feared complication that was a major impetus towards vaccine development. Severe varicella has caused many deaths in individuals with congenital or acquired impairment of cellular immunity, even after the development of antiviral therapy169. Children with impaired innate immunity, for example, those with natural killer cell abnormalities, are also at risk for severe varicella, including that caused by vOka170. Thus, strenuous measures should be taken to prevent VZV infection in this group, including post-exposure prophylaxis9,171. Since the 1980s, the main treatment has been antiviral therapy with high-dose intravenous acyclovir172 together with supportive intensive care. Acyclovir is a guanosine analogue that inhibits the synthesis of viral DNA (FIG. 7) and treatment with acyclovir reduces visceral dissemination of the virus. It is typically given for 7–10 days and can be switched to oral therapy 48 h after the last lesions appear or continued until all lesions are crusted. Oral acyclovir has poor bioavailability; thus, the related drugs valaciclovir and famciclovir, which have excellent absorption from the intestinal tract, produce high blood antiviral activity and have a long half-life, should be used for oral therapy instead. Valaciclovir and famciclovir are prodrugs, which are converted to active guanosine analogues in vivo. As both prodrugs need less frequent administration than acyclovir, patient compliance is improved and they are frequently used in children aged >2 years and in adults. Immunocompromised patients with severe VZV infections still receive intravenous acyclovir, which results in higher blood levels than oral therapy. Alternatively, intravenous foscarnet and cidofovir can be used. However, because of toxicity, these drugs should be only used in immunocompromised individuals with acyclovir-resistant VZV. Notably, intravenous antivirals, including acyclovir, can be nephrotoxic; both acyclovir and foscarnet require dose adjustment in patients with renal impairment.
Figure 7. Mode of action of acyclovir.
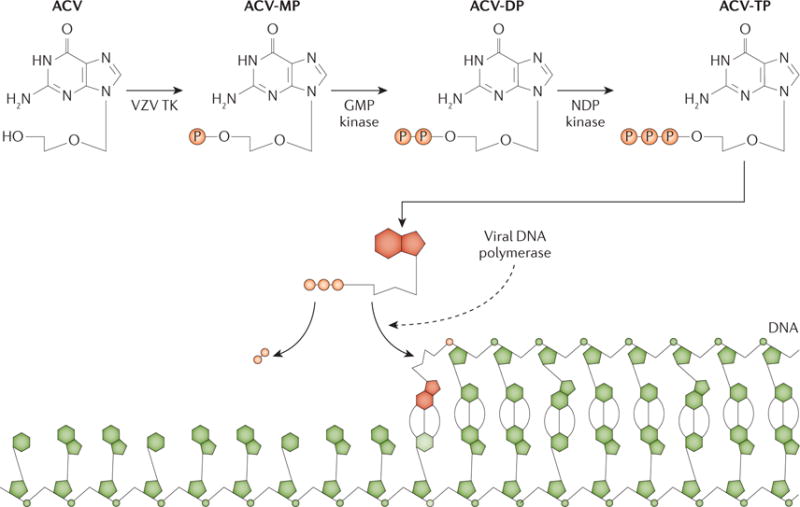
In cells infected with varicella zoster virus (VZV), acyclovir (ACV) is converted by the viral thymidine kinase (TK) to ACV-monophosphate (ACV-MP). The cellular enzymes guanylate (GMP) kinase and nucleoside-diphosphate (NDP) kinase further catalyse the production of ACV-diphosphate (ACV-DP) and ACV-triphosphate (ACV-TP). When the viral DNA polymerase uses ACV-TP, elongation of the DNA chain is terminated. Adapted from REF 211, Nature Publishing Group.
VZV infection in individuals aged >13 years is associated with an increased risk of severe or fatal outcome, and oral antiviral therapy is recommended, even in otherwise healthy adolescents and adults3 (FIG. 6). In immunocompetent patients, oral acyclovir, or preferably famciclovir, or valaciclovir need to be started within 24 h of the first skin lesions to shorten the duration of fever and rash173; 5 days and 7 days of treatment have comparable efficacy174.
Varicella in pregnant women is also problematic: pregnancy increases the risk of severe disease in the mother and VZV can harm the unborn child and lead to congenital abnormalities (congenital varicella syndrome)17,175. Thus, pregnant women with varicella are usually treated with intravenous acyclovir, even though this is a category B drug (that is, animal studies have failed to demonstrate a risk to the fetus but no wellcontrolled studies have been conducted in pregnant women) and not licensed in pregnancy. The effect of treatment on the development of congenital varicella syndrome is unknown. Maternal onset of varicella between 5 days before and 2 days after delivery is associated with a high risk of severe varicella in the newborn, who should receive prophylaxis with VZV-specific immunoglobulin175. Newborns with congenital varicella syndrome should receive high-dose intravenous acyclovir every 8 h owing to increased clearance of the drug in this age group. Oral acyclovir is poorly absorbed and should be used cautiously, if at all.
Bacterial superinfection is the most common severe complication of varicella; varicella is a major risk factor for invasive group A streptococcal disease176. Superinfection presents with recurrence of fever with or without localized signs of cellulitis (infection of the skin and subcutaneous fat tissue), bone and joint infection or pneumonia. Cellulitic features accompanied by disproportionate pain, fatigue and systemic signs and symptoms can indicate necrotizing fasciitis, even in the absence of fever, and should prompt immediate antibacterial therapy together with resuscitative measures, analgesia and urgent consideration of surgical debridement. Other possible complications of varicella are cerebellar ataxia, ischaemic stroke and acquired protein S deficiency with purpura fulminans and venous thrombosis3,177,178. The effect of antiviral therapy on individual complications is unclear but a pragmatic approach is to treat if there is evidence of ongoing viral replication (such as continuing new vesicle formation and/or persistent PCR positivity in blood or cerebrospinal fluid in a symptomatic patient).
Zoster and PHN
Antiviral therapy is recommended for patients with zoster, particularly those who are immunocompromised, aged ≥50 years, have lesions involving the face or eye, severe rash or other complications of zoster (reviewed in REFS 179,180) (FIG. 6b). Oral acyclovir, valaciclovir or famciclovir are used to treat immunocompetent patients in the United States. Oral brivudin is available in some European countries and VZV is more sensitive to brivudin than to other antivirals. As mentioned above, valaciclovir or famciclovir, rather than acyclovir, are preferred due to their higher bioavailability and easier dosing regimen; furthermore, they may be more effective than acyclovir for reducing acute zoster pain181,182. Treatment is usually given for 7–10 days and reduces the time to cessation of new lesion formation, to lesion crusting and to cessation of acute pain183. Immunocompromised patients, hospitalized patients or those with neurological complications are treated for 7–10 days with intravenous acyclovir, which has been shown to reduce the risk of visceral disease and skin dissemination3. Foscarnet is used for patients with acyclovir-resistant VZV. Antiviral therapy should be initiated within 3 days of the onset of rash if possible, but should still be initiated if patients are seen later with continuing new lesion formation. Although antiviral therapy reduces acute pain associated with zoster, it has not been shown to reliably reduce the risk of PHN, nor is it recommended to treat established PHN74. Prednisone reduces acute pain and improves the ability of patients with zoster to perform activities of daily living184; however, prednisone does not reduce the risk of PHN185 and many elderly patients have conditions such as hypertension, diabetes mellitus or osteoporosis, which may preclude the use of this drug. Antiviral therapy should be given whenever prednisone is used.
Mild pain associated with zoster can be treated with NSAIDs or acetaminophen. Lidocaine patches can reduce local pain, although they should only be used on intact skin and can cause local irritation. For more-severe pain, opioids or opioid agonists (oxycodone or tramadol), anticonvulsants (gabapentin or pregabalin) or tricyclic antidepressants (nortriptyline) have been used180,186. Oxycodone was more effective than gabapentin in a randomized controlled trial186. Systemic therapies need to be gradually increased as tolerated, and titrated specifically for each patient. Systemic therapies are often associated with drowsiness and/or dizziness; these adverse effects may be problematic, particularly in the elderly. Gabapentin and pregabalin can cause ataxia and peripheral oedema whereas nortriptyline has been associated with dry mouth, urinary retention and weight gain.
Immunocompetent patients with Ramsay Hunt syndrome (peripheral facial weakness, zoster rash and pain inside the ear, and loss of taste in the anterior part of the tongue) should be treated with oral antivirals and prednisone. Patients with zoster involving the eye should be evaluated by an ophthalmologist; additional therapy might be needed to reduce intraocular pressure, to reduce the risk of scar formation in the eye, or to treat keratitis, iritis or episcleritis. Early antiviral therapy has been correlated with a better outcome in patients with zoster ophthalmicus187. Patients with vasculopathy should be treated with intravenous acyclovir and prednisone86.
PHN is often very difficult to treat and fewer than half of patients have a substantial reduction in pain. Moreover, the currently available medications only treat symptoms and not the underlying cause of the pain. Treatments include topical lidocaine (often considered first-line therapy), topical capsaicin, gabapentin, pregabalin and tricyclic antidepressants75. Topical capsaicin is often difficult to tolerate owing to pain and erythema. Combined treatments, such as gabapentin and nortriptyline188, can be more effective, but adverse effects are often increased. As noted above, these systemic drugs all have adverse effects that can be challenging for elderly patients and the dosage must be titrated for each patient. Although opioids are sometimes used, they are considered third-line drugs because of the potential for abuse and uncertainty about their long-term benefits189. Referral to a pain specialist is often helpful. Other therapies, including acupuncture, intrathecal injection of corticosteroids, injection of local anaesthetics and nerve blocks have not shown consistent benefits.
Quality of life
In the early twentieth century, varicella and zoster seemed to be diseases of little consequence compared with other common infections such as influenza, measles and poliomyelitis, which frequently were fatal. There was little demand for treatment because both infections were usually mild and self-limiting. Life expectancy was shorter than it is today, which limited the number of zoster cases. Varicella illness lasted only a few days, and meant a few days off from school. School curricula permitted missing a few days without consequence and most families had one parent at home who could care for their sick children.
Then technological developments, new procedures, such as transplantation and drugs to treat cancer, improved medical care and increased life expectancy, but this also increased the number of people susceptible to severe VZV infection. For example, curing children with leukaemia became possible but, at the same time, damage to the immune system by chemotherapy and other curative drugs resulted in severe varicella169 and subsequent bacterial infections176 becoming threats. Now, healthy children and those with leukaemia can be protected from varicella by vaccination, the latter by herd immunity. The varicella vaccine also somewhat protects children from zoster31,148,190,191.
Today, the varicella and zoster vaccines have dramatically improved quality of life in the United States. Children rarely miss school due to varicella, and fewer working parents need to stay home to care for their sick children. As lifespans have increased, zoster vaccines have become increasingly important. Antiviral therapy and improved diagnostic methods have also decreased the damage VZV can inflict. Basic research has made a tremendous difference in controlling VZV infections and improving quality of life (BOX 2).
Box 2. Vaccine and antiviral therapy benefits.
Fewer cases of varicella and zoster in children and adults
Fewer complications of varicella such as pneumonia, encephalitis, congenital infections, zoster and deaths
Possibly fewer complications of zoster such as vasculopathy and stroke, postherpetic neuralgia, meningitis and paralysis
Convenience for families (fewer school absences, easier for single or working parents)
Decreased transmission of varicella in hospital settings
PHN is a feared consequence of zoster, particularly in the elderly and immunocompromised. Pain from PHN can be so severe it can lead to suicide, and often is a scourge of the retirement years. Zoster impairs activities of daily living and decreases the quality of life192. PHN is difficult to treat and thus prevention, for example, by preventing zoster by vaccination with vOka and, in the future, with the new subunit vaccine, remains crucial for the preservation of quality of life in the elderly.
Outlook
Given the considerable success of the VZV vaccines, it might be assumed that further research on this virus is unnecessary. This has been the attitude for other viruses such as measles and rubella for which vaccines are available. In fact, the opposite is true: much remains to be learnt about VZV, which, because of latency, is a complicated and persistent human pathogen.
Latency and reactivation
How VZV latency is achieved and maintained is a challenging question. It remains unclear whether VZV latency is a period of relative viral quiescence due to a transient block in complete gene expression or whether the virus is often or constantly in a state of abortive reactivation. Studies of surgical removal of enteric ganglia suggest a block in gene expression43,44, although recent autopsy studies support the hypothesis of abortive reactivation, but further research is needed42. Asymptomatic VZV reactivation is also of great interest. For example, whether some viral genes are translated with or without synthesis of virus particles, and whether these are delivered to skin by axonal transport with or without spread to satellite cells surrounding the neuron, remain unclear. Delivery of virions to skin may not necessarily cause lesions if replication is controlled rapidly by innate immunity and by adaptive immune responses. Further research in this area is important.
The recognition that VZV is a pathogen in the human gut was an unanticipated finding because VZV infections were long associated with cutaneous manifestations, which may not occur when VZV reactivates in enteric neurons. The nature of enteric zoster is, therefore, another issue that requires further study.
Autophagy and herpesviruses
Autophagy is one of the basic mechanisms required for cellular homeostasis and survival. An important role of autophagy is the removal of organelles through autophagosomes and lysosomes. Autophagy, however, is also linked to degenerative processes and cell death. Viruses can also be affected by autophagy. Many herpesviruses, such as herpes simplex virus, encode inhibitors of autophagy, including ICP34.5 and US11 (REFS 193–195). By contrast, VZV — the human herpesvirus with the smallest genome — encodes no such inhibitors196–198.
Perhaps the most intriguing question is why VZV lacks ICP34.5, a homologue of human PPP1R15A (protein phosphatase 1 regulatory subunit 15A; a protein that is involved in recovery from cell stress)199. Because ICP34.5 is present in herpes simplex virus 1 and 2, but lacking in VZV and betaherpesviruses, the gene may have been acquired by herpes simplex viruses more recently than 30 million years ago (before the common ancestor of all these viruses)200,201. Alternatively, VZV could have lost the gene at a later time. In either case, therefore, in an evolutionary sense, VZV is a minimalist herpesvirus that adopted an entirely different intracellular survival strategy than herpes simplex virus. Rather than inhibiting autophagy, VZV might actually depend on autophagy to prolong the life of the infected cell during both primary infection and reactivation194,195 (FIG. 8). However, this dependence on autophagy needs confirmation.
Figure 8. Autophagosomes in VZV-infected cells.
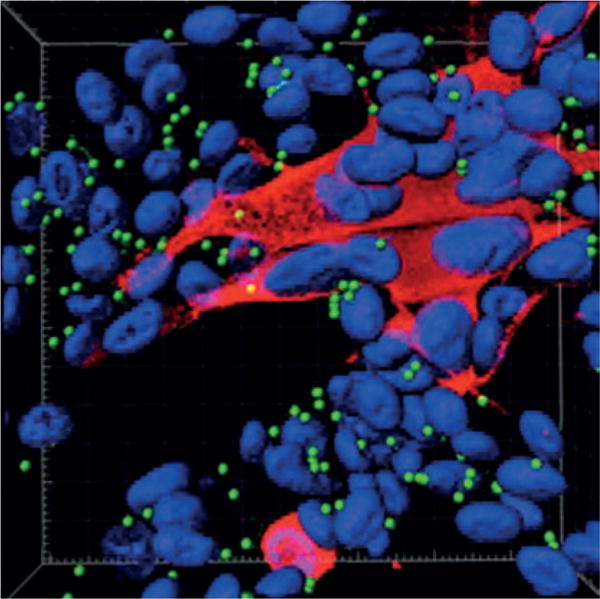
Autophagosomes are present in the skin vesicles during both varicella and zoster. Autophagy is required for the replication of varicella zoster virus (VZV), and replication is enhanced when autophagy is induced. Autophagy can be quantitated by enumeration of autophagosomes194. The figure illustrates a monolayer of VZV-infected cells labelled with antibodies against a VZV protein (IE62 protein; red) and autophagosomes (LC3–II protein; green); nuclei were stained blue with Hoechst 33342. The monolayer was imaged by confocal microscopy, after which the confocal images were converted into a 3D animation by Imaris software. A single frame from the animation is shown in the figure195. Autophagosomes (~100) appear as green dots against the background of blue nuclei (~70). Many of the nuclei are clustered within a syncytium of VZV-infected cells (red cytoplasm). By contrast, only the nuclei of uninfected cells are visible in this image, as the cytoplasm of uninfected cells is not stained.
Asthma and zoster
Zoster occurs in children but much less often than in adults20. VZV reactivation is more common in highly immunosuppressed children who are receiving therapy for cancer or rheumatological diseases202. Asthma, a common illness in children, might also increase the rate of zoster203. Asthma is an inflammatory disorder of the airways with a skewed T cell response, increased IgE production and alterations in innate immunity, including cytokine dysregulation203. A population-based case–control study in Minnesota, USA, showed a higher incidence of zoster in children with asthma than in those without asthma; inhaled or systemic corticosteroids were excluded as a cause for this difference204. Subsequently, a similar analysis was carried out in a much larger adult study cohort in Spain, with similar results205. Asthma might, therefore, be a contributing cause to VZV reactivation in both children and adults, suggesting that intact innate immunity, a normal balance of T helper 1 and 2 responses or the absence of chronic inflammation from asthma may be involved in maintenance of latency. Further research on this subject is needed.
Research questions and needs
Although there is progress, eradication of VZV remains a challenge. Reactivation of VZV after natural infection or vaccination is a worldwide problem for elderly and immunosuppressed individuals. Zoster is considered to be a consequence of diminished adaptive cellular immunity2,20. A recent publication on VZV-specific antibody titres after zoster vaccination, however, raises a decades’ old argument of whether higher adaptive humoral immunity can predict protection against zoster. Further exploration of this concept could be worthwhile because the development of an antibody test to predict the susceptibility to varicella and zoster would be extremely useful clinically.
A remaining question regarding VZV includes how similar VZV is to its close alphaherpesvirus relatives herpes simplex virus 1 and 2. For years it was claimed that all three viruses are highly similar, but with modern molecular research this concept has been challenged206. For example, reactivation of herpes simplex viruses has been claimed to be common, whereas reactivation of VZV is uncommon. More recently, it has been recognized that VZV frequently reactivates asymptomatically. One obvious and so far unexplained difference between VZV and herpes simplex viruses is why developing preventive and therapeutic VZV vaccines has been possible, whereas successful vaccines against herpes simplex viruses remain elusive. Further comparative research between these viruses is needed.
A challenge for VZV vaccination is how to broaden acceptance of the varicella vaccine worldwide and possibly to improve it by developing a more stable vaccine. The WHO now recommends the use of vOka in countries where varicella has a substantial impact on public health. Countries should consider vaccination of potentially susceptible health-care workers (that is, unvaccinated individuals with no history of varicella) with two doses of the varicella vaccine, even if it is not included in the routine immunization schedule, in settings where the risk of severe varicella in the population in direct contact with the health-care workers is high. In the future, the subunit zoster vaccine seems particularly useful for wide distribution because of its stability, probable relatively low cost, and the possibility of using it in immunocompromised individuals who cannot receive live, replicating vOka. However, whether the subunit vaccine can also be used to induce stable primary immunity to varicella is unclear. The broad immune responses stimulated by the live attenuated vaccine may be necessary to produce robust immunity to varicella. In fact, long-lasting immunity, which is essential, might depend on the ability of vOka to establish latency and reactivate subclinically. Thus, the inability of the subunit vaccine to establish latent infection might prove to be a disadvantage. Despite the current successes of VZV vaccines, further research in this area is likely to be productive.
In closing, the authors would like to acknowledge and memorialize the seminal contributions to our understanding of varicella and zoster by the following late pioneers in this field: Nobel Laureate Thomas Huckle Weller, MD207,208, Robert Edgar Hope-Simpson, MD20, and Michiaki Takahashi, MD119.
Acknowledgments
J.B. receives funding from the National Institute for Health Research (NIHR) University College London (UCL)/University College London Hospitals NHS Foundation Trust (UCLH) Biomedical Research Centre, UK. J.I.C. is supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, USA. R.J.C. is supported by grants NS082228 and AG032958 from the US National Institutes of Health (NIH). D.G. is supported by grants AG006127 and AG032958 from the NIH. C.G. is supported by grant AI89716 from the NIH. S.H. receives funding from the Sir Jules Thorn Charitable Trust. M.D.G. and A.A.G. receive funding from NIH R01 Grant DK 09394. M.N.O.’s work is partially supported by the James R. and Jesse V. Scott Fund for Shingles Research in the Veterans Medical Research Foundation. P.G.E.K. receives grant funding for research from the Wellcome Trust.
Footnotes
Author contributions
Introduction (A.A.G.); Epidemiology (J.F.S. and J.B.); Mechanisms/pathophysiology (D.G., R.J.C., P.G.E.K. and M.G.); Diagnosis, screening and prevention (A.A.G., M.N.O. and K.Y.), Management (J.I.C. and S.H.); Quality of life (A.A.G.); Outlook (C.G); and overview of Primer (A.A.G.).
Competing interests
J.B., J.I.C., R.J.C., M.D.G., D.G., C.G., S.H., M.N.O. and J.F.S. declare no competing interests. A.A.G. declares service contracts from Merck to investigate the safety of VZV vaccines (identifying VZV in samples from patients with possible adverse reactions), chairs an independent data monitoring committee for GlaxoSmithKline’s Phase III subunit glycoprotein E zoster vaccine trial, consults with Pfizer when invited, and has participated in an educational programme (supported by an unrestricted educational grant) on zoster for GlaxoSmithKline. P.G.E.K. has served on a scientific advisory board on zoster vaccination for Sanofi Pasteur MSD. Y.K. is Director General of the BIKEN foundation (The Research Foundation for Microbial Diseases of Osaka University), which produces varicella vaccines.
References
- 1.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–253. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. 2013;26:728–743. doi: 10.1128/CMR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershon AA, Takahashi M, Seward JF. In: Vaccines. Plotkin S, Orenstein W, Offit P, editors. Saunders Elsevier; 2011. pp. 915–958. [Google Scholar]
- 4.Tsolia M, Gershon AA, Steinberg SP, Gelb L. Live attenuated varicella vaccine: evidence that the virus is attenuated and the importance of skin lesions in transmission of varicella-zoster virus. National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study Group. J Pediatr. 1990;116:184–189. doi: 10.1016/s0022-3476(05)82872-0. A study demonstrating that VZV spreads from skin lesions. [DOI] [PubMed] [Google Scholar]
- 5.Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–926. doi: 10.1016/j.cell.2004.11.007. This study demonstrates the importance of the skin in VZV infection and identifies the cellular receptor that the virus uses for infection. [DOI] [PubMed] [Google Scholar]
- 6.Weller TH. Varicella: historical perspective and clinical overview. J Infect Dis. 1996;174(Suppl):S306–S309. doi: 10.1093/infdis/174.supplement_3.s306. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014;89:265–287. [PubMed] [Google Scholar]
- 8.Breuer J, Grose C, Norberg P, Tipples G, Schmid DS. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008. J Gen Virol. 2010;91:821–828. doi: 10.1099/vir.0.017814-0. This paper describes the five different clades of VZV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin M, Güris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–40. [PubMed] [Google Scholar]
- 10.Liyanage NPM, et al. Seroprevalence of varicella zoster virus infections in Colombo district Sri Lanka. Indian J Med Sci. 2007;61:128–134. [PubMed] [Google Scholar]
- 11.Lolekha S, et al. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg. 2001;64:131–136. doi: 10.4269/ajtmh.2001.64.131. [DOI] [PubMed] [Google Scholar]
- 12.Izurieta HS, Strebel PM, Blake PA. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA. 1997;278:1495–1499. [PubMed] [Google Scholar]
- 13.Levy MH, et al. Pox in the docks: varicella outbreak in an Australian prison system. Publ Health. 2003;117:446–451. doi: 10.1016/S0033-3506(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 14.Longfield JN, Winn RE, Gibson RL, Juchau SV, Hoffman PV. Varicella outbreaks in army recruits from Puerto Rico. Varicella susceptibility in a population from the tropics. Arch Intern Med. 1990;150:970–973. [PubMed] [Google Scholar]
- 15.Galil K, Brown C, Lin F, Seward J. Hospitalizations for varicella in the United States 1988 to 1999. Pediatr Infect Dis J. 2002;21:931–935. doi: 10.1097/00006454-200210000-00009. This paper describes the effects of the varicella vaccine (decreased morbidity and mortality in the United States). [DOI] [PubMed] [Google Scholar]
- 16.Rawson H, Crampin A, Noah N. Deaths from chickenpox in England and Wales 1995–7: analysis of routine mortality data. BMJ. 2001;323:1091–1093. doi: 10.1136/bmj.323.7321.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enders G. Varicella-zoster virus infection in pregnancy. Prog Med Virol. 1984;29:166–196. [PubMed] [Google Scholar]
- 18.Bialek SR, et al. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics. 2013;132:e1134–e1140. doi: 10.1542/peds.2013-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011;128:214–220. doi: 10.1542/peds.2010-3385. [DOI] [PubMed] [Google Scholar]
- 20.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. This paper demonstrates the age-dependent incidence and severity of zoster and presents a hypothesis regarding the complex immune response to the virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weitzman D, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463–469. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Oxman MN, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. This article presents the first demonstration of a successful therapeutic vaccine against zoster. [DOI] [PubMed] [Google Scholar]
- 23.Yawn BP, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 24.Forbes HJ, et al. Quantification of risk factors for herpes zoster: population based case–control study. BMJ. 2014;348:g2911. doi: 10.1136/bmj.g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171:701–704. doi: 10.1093/infdis/171.3.701. [DOI] [PubMed] [Google Scholar]
- 26.Arvin AM, Koropchak CM, Wittek AE. Immunologic evidence of reinfection with varicella-zoster virus. J Infect Dis. 1983;148:200–205. doi: 10.1093/infdis/148.2.200. [DOI] [PubMed] [Google Scholar]
- 27.Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case–control study. Lancet. 2002;360:678–682. doi: 10.1016/S0140-6736(02)09837-9. [DOI] [PubMed] [Google Scholar]
- 28.Brisson M, et al. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine. 2010;28:3385–3397. doi: 10.1016/j.vaccine.2010.02.079. [DOI] [PubMed] [Google Scholar]
- 29.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159:739–745. doi: 10.7326/0003-4819-159-11-201312030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–340. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 31.Hardy I, Gershon AA, Steinberg SP, LaRussa P. The incidence of zoster after immunization with live attenuated varicella vaccine. A study in children with leukemia. Varicella Vaccine Collaborative Study Group. N Engl J Med. 1991;325:1545–1550. doi: 10.1056/NEJM199111283252204. This article provides the first solid evidence that vaccination against varicella decreases the incidence of zoster. [DOI] [PubMed] [Google Scholar]
- 32.Ku CC, Padilla JA, Grose C, Butcher EC, Arvin AM. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J Virol. 2002;76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ku CC, et al. Varicella-zoster virus transfer to skin by T cells and modulation of viral replication by epidermal cell interferon-α. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. This paper reveals how VZV infects human hosts in primary infection, how the virus spreads in the body and the early role of innate immunity in its control. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sen N, et al. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Rep. 2014;8:633–645. doi: 10.1016/j.celrep.2014.06.024. This article provides the first demonstration that VZV remodels host cells to increase its infectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levin MJ. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J Clin Virol. 2014;61:487–495. doi: 10.1016/j.jcv.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Arvin AM, Gilden DH. In: Fields Virology. Knipe D, Howley P, editors. Lippincott; 2013. pp. 2015–2057. [Google Scholar]
- 37.Gershon AA, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 123:17–33. discussion 33–35 (2012) [PMC free article] [PubMed] [Google Scholar]
- 38.Mahalingam R, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- 39.Gan L, Wang M, Chen JJ, Gershon MD, Gershon AA. Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system. J Neurovirol. 2014;20:442–456. doi: 10.1007/s13365-014-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin MJ, Cai GY, Manchak MD, Pizer LI. Varicella-zoster virus DNA in cells isolated from human trigeminal ganglia. J Virol. 2003;77:6979–6987. doi: 10.1128/JVI.77.12.6979-6987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 in latently infected human trigeminal ganglia. J Virol. 2006;80:4921–4926. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambagala AP, et al. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J Virol. 2009;83:200–209. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plotkin SA, Stein S, Snyder M, Immesoete P. Attempts to recover varicella virus from ganglia. Ann Neurol. 1977;2:249. doi: 10.1002/ana.410020313. [DOI] [PubMed] [Google Scholar]
- 44.Ouwendijk WJD, et al. Restricted varicella-zoster virus transcription in human trigeminal ganglia obtained soon after death. J Virol. 2012;86:10203–10206. doi: 10.1128/JVI.01331-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen JJ, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol. 2011;17:578–589. doi: 10.1007/s13365-011-0070-1. This study demonstrates the latency of VZV in the enteric nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis. 2008;197(Suppl):S61–S65. doi: 10.1086/522149. [DOI] [PubMed] [Google Scholar]
- 47.Chen JJ, Gershon AA, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J Med Virol. 2003;70(Suppl. 1):S71–S78. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- 48.Head H, Campbell A, Kennedy P. The pathology of herpes zoster and its bearing on sensory localisation. Rev Med Virol. 1997;7:131–143. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Schmidbauer M, Budka H, Pilz P, Kurata T, Hondo R. Presence, distribution and spread of productive varicella zoster virus infection in nervous tissues. Brain. 1992;115:383–398. doi: 10.1093/brain/115.2.383. [DOI] [PubMed] [Google Scholar]
- 50.Steain M, et al. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. J Virol. 2014;88:2704–2716. doi: 10.1128/JVI.03445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gowrishankar K, et al. Characterization of the host immune response in human ganglia after herpes zoster. J Virol. 2010;84:8861–8870. doi: 10.1128/JVI.01020-10. This paper describes the immunological events in latently infected dorsal root ganglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grinfeld E, Kennedy PGE. Translation of varicella-zoster virus genes during human ganglionic latency. Virus Genes. 2004;29:317–319. doi: 10.1007/s11262-004-7434-z. [DOI] [PubMed] [Google Scholar]
- 53.Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc Natl Acad Sci USA. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohrs RJ, Gilden DH, Kinchington PR, Grinfeld E, Kennedy PGE. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J Virol. 2003;77:6660–6665. doi: 10.1128/JVI.77.12.6660-6665.2003. A study of the VZV gene expression in the latent infection of neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azarkh Y, Gilden D, Cohrs RJ. Molecular characterization of varicella zoster virus in latently infected human ganglia: physical state and abundance of VZV DNA, quantitation of viral transcripts and detection of VZV-specific proteins. Curr Top Microbiol Immunol. 2010;342:229–241. doi: 10.1007/82_2009_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walters MS, Kyratsous CA, Wan S, Silverstein S. Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J Virol. 2008;82:8673–8686. doi: 10.1128/JVI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zerboni L, et al. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J Virol. 2010;84:3421–3430. doi: 10.1128/JVI.02416-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zerboni L, et al. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J Virol. 2012;86:578–583. doi: 10.1128/JVI.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stowasser M, Cameron J, Oliver WA. Diaphragmatic paralysis following cervical herpes zoster. Med J Aust. 1990;153:555–556. doi: 10.5694/j.1326-5377.1990.tb126199.x. [DOI] [PubMed] [Google Scholar]
- 60.Jellinek EH, Tulloch WS. Herpes zoster with dysfunction of bladder and anus. Lancet. 1976;2:1219–1222. doi: 10.1016/s0140-6736(76)91144-2. [DOI] [PubMed] [Google Scholar]
- 61.Umehara T, Sengoku R, Mitsumura H, Mochio S. Neurological picture. Findings of segmental zoster paresis on MRI. J Neurol Neurosurg Psychiatry. 2011;82:694. doi: 10.1136/jnnp.2010.235127. [DOI] [PubMed] [Google Scholar]
- 62.Choi JY, Kang CH, Kim BJ, Park KW, Yu SW. Brachial plexopathy following herpes zoster infection: two cases with MRI findings. J Neurol Sci. 2009;285:224–226. doi: 10.1016/j.jns.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Thomas JE, Howard FM. Segmental zoster paresis — a disease profile. Neurology. 1972;22:459–466. doi: 10.1212/wnl.22.5.459. [DOI] [PubMed] [Google Scholar]
- 64.Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol. 1978;10:50–53. [PubMed] [Google Scholar]
- 65.Watson CP, Watt VR, Chipman M, Birkett N, Evans RJ. The prognosis with postherpetic neuralgia. Pain. 1991;46:195–199. doi: 10.1016/0304-3959(91)90076-A. [DOI] [PubMed] [Google Scholar]
- 66.Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- 67.Kennedy PGE, et al. Varicella-zoster viruses associated with post-herpetic neuralgia induce sodium current density increases in the ND7-23 Nav-1.8 neuroblastoma cell line. PLoS ONE. 2013;8:e51570. doi: 10.1371/journal.pone.0051570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–133. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 69.Gilden DH, Cohrs RJ, Hayward AR, Wellish M, Mahalingam R. Chronic varicella-zoster virus ganglionitis — a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–407. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- 70.Beutner KR, Friedman DJ, Forszpaniak C, Andersen PL, Wood MJ. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546–1553. doi: 10.1128/aac.39.7.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Degreef H. Famciclovir, a new oral antiherpes drug: results of the first controlled clinical study demonstrating its efficacy and safety in the treatment of uncomplicated herpes zoster in immunocompetent patients. Int J Antimicrob Agents. 1994;4:241–246. doi: 10.1016/0924-8579(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 72.Acosta EP, Balfour HH. Acyclovir for treatment of postherpetic neuralgia: efficacy and pharmacokinetics. Antimicrob Agents Chemother. 2001;45:2771–2774. doi: 10.1128/AAC.45.10.2771-2774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quan D, Hammack BN, Kittelson J, Gilden DH. Improvement of postherpetic neuralgia after treatment with intravenous acyclovir followed by oral valacyclovir. Arch Neurol. 2006;63:940–942. doi: 10.1001/archneur.63.7.noc60049. [DOI] [PubMed] [Google Scholar]
- 74.Chen N, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866. doi: 10.1002/14651858.CD006866.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solomon CG, Johnson RW, Rice ASC. Postherpetic neuralgia. N Engl J Med. 2014;371:1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 76.Klein NC, McDermott B, Cunha BA. Varicella-zoster virus meningoencephalitis in an immunocompetent patient without a rash. Scand J Infect Dis. 2010;42:631–633. doi: 10.3109/00365540903510716. [DOI] [PubMed] [Google Scholar]
- 77.Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011;50:191–193. doi: 10.1016/j.jcv.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ratzka P, Schlachetzki JCM, Bähr M, Nau R. Varicella zoster virus cerebellitis in a 66-year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- 79.Ciccone S, et al. Stroke after varicella-zoster infection: report of a case and review of the literature. Pediatr Infect Dis J. 2010;29:864–867. doi: 10.1097/inf.0b013e3181ddefb6. [DOI] [PubMed] [Google Scholar]
- 80.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 81.Nagel MA, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. This paper demonstrates that VZV vasculopathies in cerebral arteries lead to strokes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 83.Hilt DC, Buchholz D, Krumholz A, Weiss H, Wolinsky JS. Herpes zoster ophthalmicus and delayed contralateral hemiparesis caused by cerebral angiitis: diagnosis and management approaches. Ann Neurol. 1983;14:543–553. doi: 10.1002/ana.410140509. [DOI] [PubMed] [Google Scholar]
- 84.Bourdette DN, Rosenberg L, Yatsu FM. Herpes zoster ophthalmicus and delayed ipsilateral cerebral infarction. Neurology. 1983;33:1428–1432. doi: 10.1212/wnl.33.11.1428. This paper shows the link between VZV infection and stroke. [DOI] [PubMed] [Google Scholar]
- 85.Nagel MA, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grose C. Stroke after varicella and zoster ophthalmicus: another indication for treatment and immunization. Pediatr Infect Dis J. 2010;29:868–869. doi: 10.1097/INF.0b013e3181ddef9e. [DOI] [PubMed] [Google Scholar]
- 88.Gilden D, et al. Prevalence and distribution of VZV in temporal arteries of patients with giant cell arteritis. Neurology. 2015;84:1948–1955. doi: 10.1212/WNL.0000000000001409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franco-Paredes C, et al. Aseptic meningitis and optic neuritis preceding varicella-zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–1049. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- 90.Furuta Y, et al. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- 91.Pahud BA, Glaser CA, Dekker CL, Arvin AM, Schmid DS. Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine. J Infect Dis. 2011;203:316–323. doi: 10.1093/infdis/jiq066. This paper describes meningitis and encephalitis caused by VZV, often without rash. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilden DH, Dueland AN, Devlin ME, Mahalingam R, Cohrs R. Varicella-zoster virus reactivation without rash. J Infect Dis. 1992;166(Suppl):S30–S34. doi: 10.1093/infdis/166.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 93.Gilden DH, et al. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5–1995 revisited. Neurology. 1996;47:1441–1446. doi: 10.1212/wnl.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 94.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 95.McKelvie PA, et al. Meningoencephalomyelitis with vasculitis due to varicella zoster virus: a case report and review of the literature. Pathology. 2002;34:88–93. doi: 10.1080/00313020120105705. [DOI] [PubMed] [Google Scholar]
- 96.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58:1497–1503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Breuer J, Pacou M, Gauthier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;82:206–212. doi: 10.1212/WNL.0000000000000038. This paper provides further recognition of the relationship between VZV and stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilden DH, et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994;44:1818–1823. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- 99.Chen JJ, et al. Latent, lytic and reactivating varicella zoster virus in the ENS of humans and guinea pigs: could intestinal shingles be a hidden cause of gastrointestinal disease? Neuroastroenterol Motil. 2003;15:196–237. abstr. [Google Scholar]
- 100.Edelman DA, et al. Ogilvie syndrome and herpes zoster: case report and review of the literature. J Emerg Med. 2010;39:696–700. doi: 10.1016/j.jemermed.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Scholl S, Hocke M, Hoffken K, Sayer HG. Acute abdomen by varicella zoster virus induced gastritis after autologous peripheral blood stem cell transplantation in a patient with non-Hodgkin’s lymphoma. Acta Haematol. 2006;116:58–61. doi: 10.1159/000092349. [DOI] [PubMed] [Google Scholar]
- 102.Gershon AA, Chen J, Gershon MD. Use of saliva to identify varicella-zoster virus (VZV) infection of the gut. Clin Infect Dis. 2015 doi: 10.1093/cid/civ320. http://dx.doi.org/10.1093/cid/civ320. This paper identifies VZV infection of the gastrointestinal tract in the absence of rash. [DOI] [PMC free article] [PubMed]
- 103.Leung J, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23–32. doi: 10.1086/653113. [DOI] [PubMed] [Google Scholar]
- 104.Harbecke R, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310–1322. doi: 10.1002/jmv.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mehta SK, et al. Rapid and sensitive detection of varicella zoster virus in saliva of patients with herpes zoster. J Virol Methods. 2013;193:128–130. doi: 10.1016/j.jviromet.2013.05.019. This paper describes the diagnosis of VZV infection by analysing VZV DNA in saliva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mehta SK, et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis. 2008;197:654–657. doi: 10.1086/527420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furuta Y, Ohtani F, Sawa H, Fukuda S, Inuyama Y. Quantitation of varicella-zoster virus DNA in patients with Ramsay Hunt syndrome and zoster sine herpete. J Clin Microbiol. 2001;39:2856–2859. doi: 10.1128/JCM.39.8.2856-2859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Puchhammer-Stöckl E, Popow-Kraupp T, Heinz FX, Mandl CW, Kunz C. Detection of varicella-zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol. 1991;29:1513–1516. doi: 10.1128/jcm.29.7.1513-1516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jääskeläinen AJ, Piiparinen H, Lappalainen M, Vaheri A. Improved multiplex-PCR and microarray for herpesvirus detection from CSF. J Clin Virol. 2008;42:172–175. doi: 10.1016/j.jcv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Vázquez M, et al. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001;344:955–960. doi: 10.1056/NEJM200103293441302. [DOI] [PubMed] [Google Scholar]
- 111.Sauerbrei A, Taut J, Zell R, Wutzler P. Resistance testing of clinical varicella-zoster virus strains. Antiviral Res. 2011;90:242–247. doi: 10.1016/j.antiviral.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 112.Quinlivan ML, Jensen NJ, Radford KW, Schmid DS. Novel genetic variation identified at fixed loci in ORF62 of the Oka varicella vaccine and in a case of vaccine-associated herpes zoster. J Clin Microbiol. 2012;50:1533–1538. doi: 10.1128/JCM.06630-11. This article describes the molecular differentiation between wild-type and vaccine-types of VZV using viral DNA from patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.LaRussa P, et al. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992;66:1016–1020. doi: 10.1128/jvi.66.2.1016-1020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ibraheem M, et al. Fatal wild-type varicella-zoster virus encephalitis without a rash in a vaccinated child. Pediatr Infect Dis J. 2013;32:183–185. doi: 10.1097/INF.0b013e318273e43d. [DOI] [PubMed] [Google Scholar]
- 115.National Health Service. Varicella Zoster Virus Reference Lab. Great Ormond Street Hospital for Children NHS Trust. 2011 [online] http://www.labs.gosh.nhs.uk/laboratory-services/microbiology-virology-and-infection-control/vz-reference-lab.
- 116.Mehta SK, et al. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. This study describes asymptomatic reactivation of VZV as demonstrated by the transient presence of VZV DNA in saliva. [DOI] [PubMed] [Google Scholar]
- 117.Papaevangelou V, et al. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect. 2013;19:E245–E251. doi: 10.1111/1469-0691.12131. [DOI] [PubMed] [Google Scholar]
- 118.Birlea M, et al. Search for varicella zoster virus DNA in saliva of healthy individuals aged 20–59 years. J Med Virol. 2014;86:360–362. doi: 10.1002/jmv.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–1290. doi: 10.1016/s0140-6736(74)90144-5. This study describes the successful attenuation of VZV and the use of this live attenuated virus to prevent varicella, thereby demonstrating the first successful vaccine against varicella. [DOI] [PubMed] [Google Scholar]
- 120.Gomi Y, et al. Comparison of the complete DNA sequences of the Oka varicella vaccine and its parental virus. J Virol. 2002;76:11447–11459. doi: 10.1128/JVI.76.22.11447-11459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197–213. doi: 10.1086/653605. [DOI] [PubMed] [Google Scholar]
- 122.Yamanishi K. Molecular analysis of the Oka vaccine strain of varicella-zoster virus. J Infect Dis. 2008;197(Suppl):S45–S48. doi: 10.1086/522122. This study identifies the molecular features of the attenuated vaccine strain of VZV compared with the wild-type strain. [DOI] [PubMed] [Google Scholar]
- 123.Peters GA, et al. The attenuated genotype of varicella-zoster virus includes an ORF0 transitional stop codon mutation. J Virol. 2012;86:10695–10703. doi: 10.1128/JVI.01067-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Asano Y, Nakayama H, Yazaki T, Kato R, Hirose S. Protection against varicella in family contacts by immediate inoculation with live varicella vaccine. Pediatrics. 1977;59:3–7. This is the first demonstration of the clinical effectiveness of the Oka strain in preventing varicella, together with immunological data. [PubMed] [Google Scholar]
- 125.Asano Y, Takahashi M. Clinical and serologic testing of a live varicella vaccine and two-year follow-up for immunity of the vaccinated children. Pediatrics. 1977;60:810–814. [PubMed] [Google Scholar]
- 126.Gershon AA, et al. Live attenuated varicella vaccine. Efficacy for children with leukemia in remission. JAMA. 1984;252:355–362. doi: 10.1001/jama.252.3.355. This paper provides the first proof that the varicella vaccine is safe and effective in preventing varicella in children with underlying leukaemia. [DOI] [PubMed] [Google Scholar]
- 127.White CJ. Clinical trials of varicella vaccine in healthy children. Infect Dis Clin North Am. 1996;10:595–608. doi: 10.1016/s0891-5520(05)70315-9. [DOI] [PubMed] [Google Scholar]
- 128.White CJ, et al. Varicella vaccine (VARIVAX) in healthy children and adolescents: results from clinical trials, 1987 to 1989. Pediatrics. 1991;87:604–610. [PubMed] [Google Scholar]
- 129.Weibel RE, et al. Live attenuated varicella virus vaccine. Efficacy trial in healthy children. N Engl J Med. 1984;310:1409–1415. doi: 10.1056/NEJM198405313102201. This study demonstrates that varicella vaccination protects healthy children from chickenpox. [DOI] [PubMed] [Google Scholar]
- 130.Shapiro ED, et al. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203:312–315. doi: 10.1093/infdis/jiq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Centers for Disease Control and Prevention (CDC) FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella. MMWR Morb Mortal Wkly Rep. 2012;61:212. [PubMed] [Google Scholar]
- 132.Lin TY, Huang YC, Ning HC, Hsueh C. Oral acyclovir prophylaxis of varicella after intimate contact. Pediatr Infect Dis J. 1997;16:1162–1165. doi: 10.1097/00006454-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 133.Huang YC, Lin TY, Chiu CH. Acyclovir prophylaxis of varicella after household exposure. Pediatr Infect Dis J. 1995;14:152–154. [PubMed] [Google Scholar]
- 134.Klein A, Miller KB, Sprague K, DesJardin JA, Snydman DR. A randomized, double-blind, placebo-controlled trial of valacyclovir prophylaxis to prevent zoster recurrence from months 4 to 24 after BMT. Bone Marrow Transplant. 2011;46:294–299. doi: 10.1038/bmt.2010.99. [DOI] [PubMed] [Google Scholar]
- 135.Ljungman P, et al. Long-term acyclovir prophylaxis in bone marrow transplant recipients and lymphocyte proliferation responses to herpes virus antigens in vitro. Bone Marrow Transplant. 1986;1:185–192. [PubMed] [Google Scholar]
- 136.Linnemann CC, Biron KK, Hoppenjans WG, Solinger AM. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577–579. doi: 10.1097/00002030-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 137.Levin MJ, et al. Development of resistance to acyclovir during chronic infection with the Oka vaccine strain of varicella-zoster virus, in an immunosuppressed child. J Infect Dis. 2003;188:954–959. doi: 10.1086/378502. This paper demonstrates that VZV can reactivate in immunocompromised children and that the vaccine virus can become resistant to acyclovir. [DOI] [PubMed] [Google Scholar]
- 138.Bhalla P, et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis. 2015;60:1068–1074. doi: 10.1093/cid/ciu970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baxter R, et al. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics. 2013;131:e1389–e1396. doi: 10.1542/peds.2012-3303. [DOI] [PubMed] [Google Scholar]
- 140.Vázquez M, et al. Effectiveness over time of varicella vaccine. JAMA. 2004;291:851–855. doi: 10.1001/jama.291.7.851. This study shows that immunity to varicella after vaccination does not wane substantially with time. [DOI] [PubMed] [Google Scholar]
- 141.Seward JF, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287:606–611. doi: 10.1001/jama.287.5.606. This study demonstrates personal and herd immunity as a result of vaccination in healthy children. [DOI] [PubMed] [Google Scholar]
- 142.Gershon AA, et al. The protective effect of immunologic boosting against zoster: an analysis in leukemic children who were vaccinated against chickenpox. J Infect Dis. 1996;173:450–453. doi: 10.1093/infdis/173.2.450. [DOI] [PubMed] [Google Scholar]
- 143.Brisson M, Edmunds WJ, Gay NJ. Varicella vaccination: impact of vaccine efficacy on the epidemiology of VZV. J Med Virol. 2003;70(Suppl. 1):S31–S37. doi: 10.1002/jmv.10317. [DOI] [PubMed] [Google Scholar]
- 144.Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–2507. doi: 10.1016/s0264-410x(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 145.Reynolds MA, Chaves SS, Harpaz R, Lopez AS, Seward JF. The impact of the varicella vaccination program on herpes zoster epidemiology in the United States: a review. J Infect Dis. 2008;197(Suppl):S224–S227. doi: 10.1086/522162. [DOI] [PubMed] [Google Scholar]
- 146.Gaillat J, et al. Does monastic life predispose to the risk of Saint Anthony’s fire (herpes zoster)? Clin Infect Dis. 2011;53:405–410. doi: 10.1093/cid/cir436. This paper shows that immunity to VZV does not require continued exogenous exposure to VZV and that asymptomatic episodes of reactivation of VZV are likely to have a role in maintaining long-term immunity to the virus. [DOI] [PubMed] [Google Scholar]
- 147.Seward JF, Zhang JX, Maupin TJ, Mascola L, Jumaan AO. Contagiousness of varicella in vaccinated cases: a household contact study. JAMA. 2004;292:704–708. doi: 10.1001/jama.292.6.704. [DOI] [PubMed] [Google Scholar]
- 148.Weinmann S, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis. 2013;208:1859–1868. doi: 10.1093/infdis/jit405. [DOI] [PubMed] [Google Scholar]
- 149.Galea SA, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(Suppl):S165–S169. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- 150.Oxman MN. Immunization to reduce the frequency and severity of herpes zoster and its complications. Neurology. 1995;45:S41–S46. doi: 10.1212/wnl.45.12_suppl_8.s41. [DOI] [PubMed] [Google Scholar]
- 151.Levin MJ. In: Vaccines. Plotkin S, Orenstein W, Offit P, editors. Elsevier; 2013. pp. 969–980. [Google Scholar]
- 152.Oxman MN, Levin MJ. Vaccination against herpes zoster and postherpetic neuralgia. J Infect Dis. 2008;197(Suppl):S228–S236. doi: 10.1086/522159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schmader KE, et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc. 2010;58:1634–1641. doi: 10.1111/j.1532-5415.2010.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simberkoff MS, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Intern Med. 2010;152:545–554. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 155.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. quiz CE2–CE4. [PubMed] [Google Scholar]
- 156.Tseng HF, et al. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 157.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. doi: 10.1371/journal.pmed.1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schmader KE, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schmader KE, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–1328. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morrison VA, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2014;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berkowitz EM, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211:1279–1287. doi: 10.1093/infdis/jiu606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chlibek R, et al. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis. 2013;208:1953–1961. doi: 10.1093/infdis/jit365. [DOI] [PubMed] [Google Scholar]
- 163.Lal H, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. This is the first demonstration of an adjuvanted subunit vaccine that is not infectious can prevent zoster in the elderly. [DOI] [PubMed] [Google Scholar]
- 164.Cohen JIA. A new vaccine to prevent herpes zoster. N Engl J Med. 2015;372:2149–2150. doi: 10.1056/NEJMe1505050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klassen TP, Hartling L, Wiebe N, Belseck EM. Acyclovir for treating varicella in otherwise healthy children and adolescents. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD002980.pub3. http://dx.doi.org/10.1002/14651858.CD002980.pub3. [DOI] [PMC free article] [PubMed]
- 166.Belay ED, et al. Reye’s syndrome in the United States from 1981 through 1997. N Engl J Med. 1999;340:1377–1382. doi: 10.1056/NEJM199905063401801. [DOI] [PubMed] [Google Scholar]
- 167.Souyri C, Olivier P, Grolleau S, Lapeyre-Mestre M. Severe necrotizing soft-tissue infections and nonsteroidal anti-inflammatory drugs. Clin Exp Dermatol. 2008;33:249–255. doi: 10.1111/j.1365-2230.2007.02652.x. [DOI] [PubMed] [Google Scholar]
- 168.American Academy of Pediatrics, Committee on Infectious Diseases. The use of oral acyclovir in otherwise healthy children with varicella. Pediatrics. 1993;91:674–676. [PubMed] [Google Scholar]
- 169.Feldman S, Lott L. Varicella in children with cancer: impact of antiviral therapy and prophylaxis. Pediatrics. 1987;80:465–472. [PubMed] [Google Scholar]
- 170.Etzioni A, et al. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146:423–425. doi: 10.1016/j.jpeds.2004.11.022. This study shows the importance of innate immunity in host defences against VZV. [DOI] [PubMed] [Google Scholar]
- 171.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of VariZIG — United States, 2013. MMWR Morb Mortal Wkly Rep. 2013;62:574–576. [PMC free article] [PubMed] [Google Scholar]
- 172.Prober CG, Kirk LE, Keeney RE. Acyclovir therapy of chickenpox in immunosuppressed children — a collaborative study. J Pediatr. 1982;101:622–625. doi: 10.1016/s0022-3476(82)80725-7. This is the first demonstration of successful antiviral therapy to protect immunocompromised children from varicella. [DOI] [PubMed] [Google Scholar]
- 173.Wallace MR, Bowler WA, Murray NB, Brodine SK, Oldfield EC. Treatment of adult varicella with oral acyclovir. A randomized, placebo-controlled trial. Ann Intern Med. 1992;117:358–363. doi: 10.7326/0003-4819-117-5-358. [DOI] [PubMed] [Google Scholar]
- 174.Balfour HH, et al. Controlled trial of acyclovir for chickenpox evaluating time of initiation and duration of therapy and viral resistance. Pediatr Infect Dis J. 2001;20:919–926. doi: 10.1097/00006454-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 175.Sauerbrei A, Wutzler P. Herpes simplex and varicella-zoster virus infections during pregnancy: current concepts of prevention, diagnosis and therapy. Part 2: Varicella-zoster virus infections. Med Microbiol Immunol. 2007;196:95–102. doi: 10.1007/s00430-006-0032-z. [DOI] [PubMed] [Google Scholar]
- 176.Davies HD, et al. Invasive group A streptococcal infections in Ontario, Canada. Ontario Group A Streptococcal Study Group. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 177.Phillips WG, Marsden JR, Hill FG. Purpura fulminans due to protein S deficiency following chickenpox. Br J Dermatol. 1992;127:30–32. doi: 10.1111/j.1365-2133.1992.tb14821.x. [DOI] [PubMed] [Google Scholar]
- 178.Science M, et al. Central nervous system complications of varicella-zoster virus. J Pediatr. 2014;165:779–785. doi: 10.1016/j.jpeds.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 179.Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369:255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dworkin RH, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl. 1):S1–S26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 181.McDonald EM, de Kock J, Ram FSF. Antivirals for management of herpes zoster including ophthalmicus: a systematic review of high-quality randomized controlled trials. Antivir Ther. 2012;17:255–264. doi: 10.3851/IMP2011. [DOI] [PubMed] [Google Scholar]
- 182.Tyring SK, Beutner KR, Tucker BA, Anderson WC, Crooks RJ. Antiviral therapy for herpes zoster: randomized, controlled clinical trial of valacyclovir and famciclovir therapy in immunocompetent patients 50 years and older. Arch Fam Med. 2000;9:863–869. doi: 10.1001/archfami.9.9.863. [DOI] [PubMed] [Google Scholar]
- 183.Lin WR, et al. Comparative study of the efficacy and safety of valaciclovir versus acyclovir in the treatment of herpes zoster. J Microbiol Immunol Infect. 2001;34:138–142. [PubMed] [Google Scholar]
- 184.Whitley RJ, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–383. doi: 10.7326/0003-4819-125-5-199609010-00004. [DOI] [PubMed] [Google Scholar]
- 185.Han Y, et al. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;3:CD005582. doi: 10.1002/14651858.CD005582.pub4. [DOI] [PubMed] [Google Scholar]
- 186.Dworkin RH, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain. 2009;142:209–217. doi: 10.1016/j.pain.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 187.Severson EA, Baratz KH, Hodge DO, Burke JP. Herpes zoster ophthalmicus in Olmsted County, Minnesota: have systemic antivirals made a difference? Arch Ophthalmol. 2003;121:386–390. doi: 10.1001/archopht.121.3.386. [DOI] [PubMed] [Google Scholar]
- 188.Gilron I, et al. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: a double-blind, randomised controlled crossover trial. Lancet. 2009;374:1252–1261. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 189.McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;8:CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brunell PA, Taylor-Wiedeman J, Geiser CF, Frierson L, Lydick E. Risk of herpes zoster in children with leukemia: varicella vaccine compared with history of chickenpox. Pediatrics. 1986;77:53–56. [PubMed] [Google Scholar]
- 191.Civen R, et al. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009;28:954–959. doi: 10.1097/INF.0b013e3181a90b16. [DOI] [PubMed] [Google Scholar]
- 192.Coplan PM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 193.Orvedahl A, Levine B. Viral evasion of autophagy. Autophagy. 2008;4:280–285. doi: 10.4161/auto.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carpenter JE, Jackson W, Benetti L, Grose C. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. J Virol. 2011;85:9414–9424. doi: 10.1128/JVI.00281-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Buckingham EM, et al. Autophagic flux without a block differentiates varicella-zoster virus infection from herpes simplex virus infection. Proc Natl Acad Sci USA. 2015;112:256–261. doi: 10.1073/pnas.1417878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. This paper provides the first complete DNA sequence of VZV. [DOI] [PubMed] [Google Scholar]
- 197.McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 198.Grose C. Pangaea and the Out-of-Africa model of varicella-zoster virus evolution and phylogeography. J Virol. 2012;86:9558–9565. doi: 10.1128/JVI.00357-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kojima E, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 200.Ravi V, Kennedy PG, MacLean AR. Functional analysis of the herpes simplex virus type 2 strain HG52 RL1 gene: the intron plays no role in virulence. J Gen Virol. 1998;79:1613–1617. doi: 10.1099/0022-1317-79-7-1613. [DOI] [PubMed] [Google Scholar]
- 201.Perelygina L, et al. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J Virol. 2003;77:6167–6177. doi: 10.1128/JVI.77.11.6167-6177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grose C, Giller RH. Varicella-zoster virus infection and immunization in the healthy and the immunocompromised host. Crit Rev Oncol Hematol. 1988;8:27–64. doi: 10.1016/s1040-8428(88)80004-0. [DOI] [PubMed] [Google Scholar]
- 203.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 204.Kim BS, et al. Increased risk of herpes zoster in children with asthma: a population-based case–control study. J Pediatr. 2013;163:816–821. doi: 10.1016/j.jpeds.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Esteban-Vasallo MD, Domínguez-Berjón MF, Gil-Prieto R, Astray-Mochales J, Gil de Miguel A. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain) Hum Vaccin Immunother. 2014;10:1650–1660. doi: 10.4161/hv.28620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Straus SE. Clinical and biological differences between recurrent herpes simplex virus and varicella-zoster virus infections. JAMA. 1989;262:3455–3458. [PubMed] [Google Scholar]
- 207.Weller TH, Stoddard MB. Intranuclear inclusion bodies in cultures of human tissue inoculated with varicella vesicle fluid. J Immunol. 1952;68:311–319. In this study, VZV was isolated in cell culture for the first time, which was critical for the eventual development of a vaccine. [PubMed] [Google Scholar]
- 208.Weller TH. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med. 1953;83:340–346. doi: 10.3181/00379727-83-20354. [DOI] [PubMed] [Google Scholar]
- 209.Centers for Disease Control and Prevention (CDC) Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep. 2011;58:1–100. [PubMed] [Google Scholar]
- 210.Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014;12:197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.De Clercq E. Strategies in the design of antiviral drugs. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]


