Abstract
A 23-year-old teacher presented to hospital with a mild case of varicella. VZV vaccine strain vOka that resembles Varilrix but not Varivax or Biken strains was isolated from the skin lesion of the patient and was identified by single nucleotide polymorphism (SNP) analysis. The teacher denied having varicella vaccine before. Retrospective analysis suggests the transmission came from a pupil who developed zoster 13 months after varicella vaccine Varilrix. This is the first report in China in which an adult with varicella was attributable to vaccine virus and the 6th report of transmission of vaccine virus to a susceptible adult around the world.
Keywords: VZV, Secondary transmission, Varilrix
Varicella-zoster virus (VZV) vaccine (Oka strain, vOka) has been approved in many few countries and is recommended for routine childhood immunization as well as the vaccination of susceptible older children and adults. Following one dose in children, the vaccine offers 85% protection from chickenpox and close to 100% protection from severe disease [1]. In rare cases, zoster may develop in a vaccinee, caused either by the vaccine virus or by wild-type virus following breakthrough VZV infection. In vaccinated children who develop breakthrough varicalla or zoster, VZV can be transmitted to a susceptible individual to cause varicella [2–4]. There were a few documented cases of secondary transmission from immunocompetent vaccinee with herpes zoster, they happened between siblings [3,4] or in hospital [11]. We now report an unusual case of vOka-associated varicella in China, involving an exposure in a school setting.
A 23-year-old Chinese female kindergarten teacher (LG) presented to the first affiliated hospital of Anhui Medical University (Hefei, China) with a mild case of varicella. She had a fever of 37.8 °C, and next day she developed 50–100 vesicular rashes in a generalized distribution on the trunk. She did not seem to be very ill and had no complications. The diagnosis of varicella was made mainly based on the patient history and the features of the rash. Both her parents denied that she had varicella or varicella vaccine before, and she had no known exposure to varicella or zoster except for the direct contact with a 5-year-old boy with zoster in her class 17 days earlier. The boy received one dose of Varilrix®. Swelling of less than 1 cm; there was no vaccine-associated rash. Thirteen months later the boy developed a zosteriform rash in his right arm near the site of the vaccine injection. Neither individual had evidence of immunodeficiency.
Vesicular fluid from the teacher’s rash was aspirated 3 days after the onset of symptoms, and DNA was extracted for molecular typing. Restriction fragment length polymorphism (RFLP) analysis of the VZV genome open reading frames (ORFs) 38 and 62 were carried out as described [5]. In contrast to both the US wild-type MLS strain and the Chinese wild-type strain that showed the presence of a Pst I site in the ORF38 (SNP69349), the LG strain along with vOka and pOka strains were not cleaved by the Pst I (data not shown). When the Sma I site in ORF62 (SNP106262), a better marker to differentiate vaccine strain from wild-type strains [6,7], was analyzed, the LG again showed pattern as that of vOka and not wild-type VZV analyzed (Fig. 1).
Fig. 1.
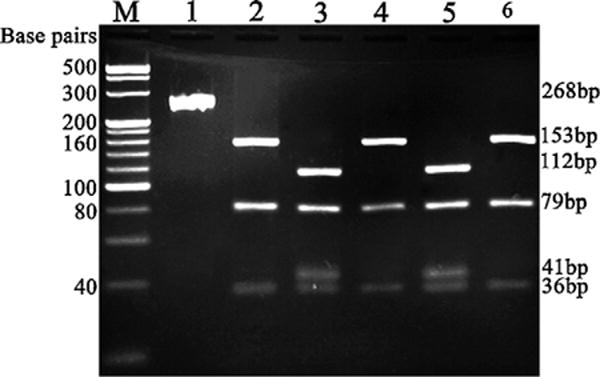
RFLP analysis of PCR product of ORF62 with Sma I digestion. Lanes M: 100 bp ladder. 1: PCR product after amplification for ORF62 without Sma I digestion. 2–6: Sma I digestion of PCR product with DNA from pOka (lane 2), vOka (lane 3), MLS (lane 4), the teacher’s strain (lane 5) and the Chinese wild-type strain 18 (lane 6).
Varicella became a reportable disease in China in 2005, and the epidemiology of varicella appears to be similar to that in the pre-vaccine era in the US. Recent studies showed that most people in China are infected before adolescence and the seroprevalence reached ≥90% by 10–15 years of age. The varicella incidence is 60–70 cases/100,000 people/year in big cities such as Shanghai and Beijing [8].
Varicella vaccine was approved in China in 1998 and one dose of vaccine was recommended for susceptible individuals at ≥12 months of age. The vaccine is for use voluntarily and has not been included in the National Immunization Program in China. Although no statistical data are available for the vaccine coverage nationally in China, it is estimated to be 20–30% among the children generally and near 90% in children in kindergartens in Hefei where the teacher was born and grew up (Dr. Yong-Gang Shen, CDC of Anhui Province, China, personal communication, 2009).
Both Varilrix® (GlaxoSmithKline Biologicals, Rixensart, Belgium) and Varivax® (Merck, Rahway, NJ) vaccines have been available since 1998, while local manufacture with vOka provided by Biken, Japan, began in Shanghai in 2000. Considering that VZV vaccination is not commonly practiced in China, and Varilrix is uncommonly used, we attempted to determine if LG strain has the molecular characteristics of Varilrix®, the vaccine that the boy was received. Because vOka vaccine is composed of a mixture of genetically distinct virus strains, multiple SNPs were have to be analyzed. Based on a recent study of the complete nucleotide sequence of Varivax® and Varilrix® [9], a group of SNPs in ORFs 1, 31, 51 and 62 were selected to be compared by sequencing (Fig. 2). The C at SNP178 differentiates the teacher’s strain from Biken/Shanghai, and the A at SNP58595 may show the difference between the teacher’s strain and Varivax®. The data provided the evidence that the SNPs in those regions of LG strain were the same or similar to that of Varilrix®, but were different from those of products from Biken/Shanghai, Biken/Changchun and Merck (Varivax®).
Fig. 2.
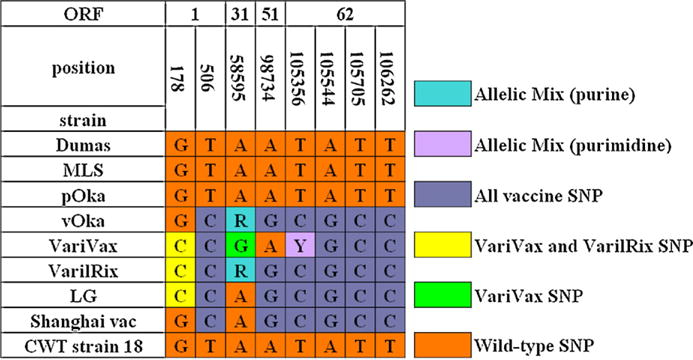
Analysis of the vaccine strain-associated SNPs of the teacher’s strain (LG strain) compared with wild-type strains and vaccine strains. All the nucleotide positions were derived from the reference sequence of Dumas strain (NC 001348). MLS is a US wild-type strain, pOka and vOka represent the parental Oka strain and vaccine Oka strain respectively. The Shanghai vac represents the Biken vaccine manufactured in Shanghai. The CWT strain 18 represents Chinese wild-type strain 18.
The occurrence of zoster in normal varicella vaccine recipients has been documented and can be caused by either vaccine strain or wild-type strain. No matter what strain of virus is involved, the patient is contagious and may rarely transmit varicella to susceptible children [3,4] or adults [10,11].
It is rare to find an adult with varicella due to a vaccine strain of VZV. In contrast to severe symptoms that commonly accompanied with adults with natural varicella, this teacher had only mild fever (<38 °C) and 50–100 skin lesions without complications. This observation is consistent with others that secondary transmission of vOka generally induces mild infection [4,11].
Blood samples were collected from the teacher for serological analysis using the fluorescent antibody to membrane antigen test (FAMA) [12]. It revealed a specific VZV IgG antibody titer of 1:4 in the serum collected on day 3, and 1:128 in the second serum obtained 14 days after onset of symptoms. The 32-fold increase in VZV-specific IgG antibody titer indicates that the teacher was infected with VZV and has immunity to the virus.
We reported here a secondary transmission of varicella vaccine strain from an immunocompetent vaccinated child to an immunocompetent adult. In contrast to the cases in which the secondary transmission occurred in a household, the current case happened outside a family. Because of the One Family One Child Policy, most children in China do not have siblings. The case demonstrates that secondary transmission of vOka may occur not only in household contacts and hospitals but also in other locations where close contact may occur, such as in educational institutions. It suggests that the susceptible individuals in youth educational institutions such as day care centers and kindergartens should be the important targets for VZV vaccination.
Acknowledgments
The study was supported by a grant from Natural Science Foundation of China (Grant No.30872253).
References
- 1.Gershon AA. The current status of live attenuated varicella vaccine. Arch Virol Suppl. 2001;17:1–6. doi: 10.1007/978-3-7091-6259-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Sharrar RG, LaRussa P, Galea SA, Steinberg SP, Sweet AR, Keatley RM, et al. The postmarketing safety profile of varicella vaccine. Vaccine. 2001;19:916–23. doi: 10.1016/s0264-410x(00)00297-8. [DOI] [PubMed] [Google Scholar]
- 3.Brunell P, Takele A. Chickenpox attributable to a vaccine virus contracted from a vaccinee with zoster. Pediatrics. 2000;106:1–2. doi: 10.1542/peds.106.2.e28. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka T, Gomi Y, Inoue N, Uchiyama M. Transmission of varicella vaccine virus, Japan. Emerg Infect Dis. 2009;15:1702–3. doi: 10.3201/eid1510.090597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu JJ, Wang ML, Gan L, Yang S, Chen J. Genotyping of clinical varicella-zoster virus isolates collected in China. J Clin Microbiol. 2009;47:1418–23. doi: 10.1128/JCM.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loparev VN, Argaw T, Krause PR, Takayama M, Schmid DS. Improved identification and differentiation of varicella-zoster virus (VZV) wild-type strains and an attenuated varicella vaccine strainusing a VZV open reading frame 62-based PCR. J Clin Microbiol. 2000;38:3156–60. doi: 10.1128/jcm.38.9.3156-3160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinlivan M, Gershon AA, Steinberg SP, Breuer J. An evaluation of single nucleotide polymorphisms used to differentiate vaccine and wild type strains of varicella-zoster virus. J Med Virol. 2005;75:174–80. doi: 10.1002/jmv.20253. [DOI] [PubMed] [Google Scholar]
- 8.Yin DP. Descriptive analysis of varicella of China in 2006. Prev Med Trib. 2007;13:488–9. [in Chinese] [Google Scholar]
- 9.Tillieux SL, Halsey WS, Thomas ES, Voycik JJ, Sathe GM, Vassilev V. Complete DNA sequences of two Oka strain varicella-zoster virus genomes. J Virol. 2008;82:11023–44. doi: 10.1128/JVI.00777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salzman MB, Sharrar RG, Steinberg S, LaRussa P. Transmission of varicella-vaccine from a healthy 12-month-old to his pregnant mother. J Pediatr. 1997;131:151–4. doi: 10.1016/s0022-3476(97)70140-9. [DOI] [PubMed] [Google Scholar]
- 11.Grossberg R, Harpaz R, Rubtcova E, Loparev V, Seward JF, Schmid DS. Secondary transmission of varicella vaccine virus in a chronic care facility for children. J Pediatr. 2006;148:842–4. doi: 10.1016/j.jpeds.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Gershon AA, Chen J, LaRussa P, Steinburg S. Varicella-Zoster Virus. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th. Vol. 2. ASM Press; 2007. pp. 1537–48. [Google Scholar]


