Abstract
As an essential nutrient, Selenium (Se) is involved in many metabolic activities including mimicking insulin function. Data on Se in various biological samples and insulin resistance are contradictory, moreover there is no large study available regarding the relationship of dietary Se intake with insulin resistance in the general population. To investigate the association between dietary Se intake and variation of insulin resistance in a large population based study, a total of 2420 subjects without diabetes from the CODING (Complex Diseases in the Newfoundland Population: Environment and Genetics) study were assessed. Dietary Se intake was evaluated from the Willett Food Frequency questionnaire. Fasting blood samples were used for the measurement of glucose and insulin. Insulin resistance was determined with the homeostasis model assessment (HOMA-IR). Body composition was measured using dual energy X-ray absorptiometry. Analysis of covariance showed that high HOMA-IR groups in both males and females had the lowest dietary Se intake (μg/kg/day) (p < 0.01), being 18% and 11% lower than low HOMA-IR groups respectively. Insulin resistance decreased with the increase of dietary Se intake in females but not in males after controlling for age, total calorie intake, physical activity level, serum calcium, serum magnesium, and body fat percentage (p < 0.01). Partial correlation analysis showed that dietary Se intake was negatively correlated with HOMA-IR after adjusting for the Se confounding factors in subjects whose dietary Se intake was below 1.6 μg/kg/day (r = -0.121 for males and -0.153 for females, p < 0.05). However, the negative correlation was no longer significant when dietary Se intake was above 1.6 μg/kg/day. Our findings suggest that higher dietary Se intake is beneficially correlated with lower insulin resistance when total dietary Se intake was below 1.6 μg/kg/day. Above this cutoff, this beneficial effect disappears.
Introduction
Selenium (Se) is an essential micronutrient element, and a key component of several selenoproteins with essential enzymatic functions that include redox homeostasis [1], thyroid hormone metabolism [2], protection from oxidative stress [3] and inflammation [1,3,4]. Data from a good number of studies suggested Se was associated with the development of type 2 diabetes (T2DM) [5,6], but the findings are contradictory.
As early as 1990s, data from isolated rat adipocytes suggest that Se (as selenate) can mimic the effects of insulin, including stimulating glucose transport activity and enhancing insulin receptor kinase activity [7]. Since then, a number of cell and animal studies have provided evidence that Se has an important role in regulating glucose homeostasis [8–12]. Based on these findings and the potential of selenoproteins to protect against oxidative stress [13], the expectation that Se might be protective against T2DM arose. However, results from human observational studies using a variety of biological samples were inconsistent. Some studies identified positive association between levels of Se in serum/plasma/dietary/fingernails and increased risk of T2DM [14–19], conversely, others found negative association between toenail Se and prevalence of T2DM [20,21], or no significant association [22,23]. Moreover, results from randomized clinical Se supplementation trials have raised concern that high Se exposure might increase the development of T2DM [24–27], but the results were still contradictory [6].
The association between Se nutritional status and diabetes is very complicated and intriguing. Insulin resistance is not only a hallmark but also a pathogenic factor of T2DM. However, the quantitative relationship between dietary Se intake and insulin resistance has been only reported in studies with very small sample size [28,29] or special groups such as metabolic syndrome (MS) [30], obesity [31,32] and polycystic ovary syndrome (PCOS) patients [33]. A study with large sample size and a wide range of insulin resistance and dietary Se intake in a general population is required in order to understand the association between the two factors. More importantly, many major confounding factors that could potentially affect the results were poorly controlled in these reported studies. Although dietary Se intake is the major source of Se for the body, Se nutritional status was overwhelmingly evaluated using hair or serum Se rather than dietary Se intake in reported studies. Consequently, the evidence linking dietary Se intake and insulin resistance is lacking.
Therefore, we designed the present study to investigate the association between dietary Se intake and insulin resistance in a large general population with systematic control of major confounding factors.
Subjects and methods
Subjects
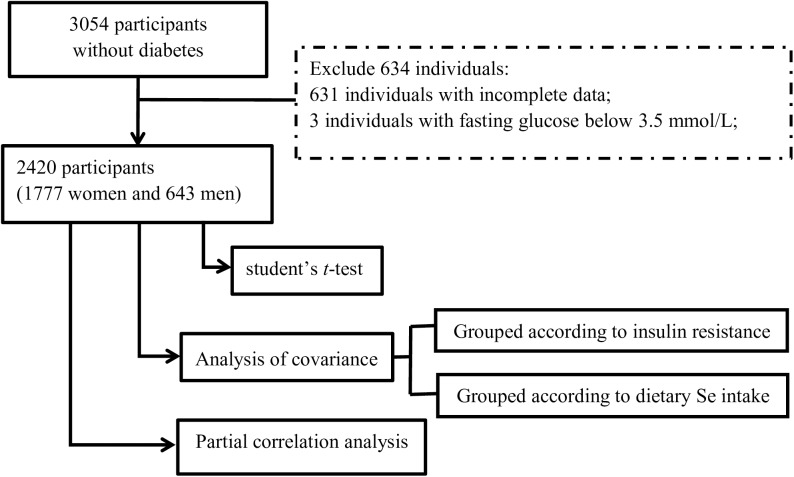
All participants were from the ongoing CODING (Complex Diseases in the Newfoundland Population: Environment and Genetics) study. Eligibility of participants for the CODING study was based upon the following inclusion criteria: 1) ≥19 years of age; 2) at least a third generation Newfoundlander; 3) without serious metabolic, cardiovascular or endocrine diseases; 4) women were not pregnant at the time of the study [34–38]. Ethics approval was obtained from the Health Research Ethics Authority (HREA), Memorial University, St. John’s, Newfoundland, Canada, with Project Identification Code #10.33 (latest date of approval: February 14, 2016). All subjects provided written and informed consent before participation in this study. Detailed information regarding the CODING Study was reported in our previously published papers [34–38]. Subjects with diabetes were excluded to focus on the non-diabetic general population and to avoid the effect of diabetic state on the results. Diabetes mellitus (DM) was defined as fasting blood glucose (FBG) ≥ 7.0 mmol/L or clinically diagnosed DM. A total of 3054 participants without diabetes were initially identified. Among them 631 individuals with incomplete data were excluded, and another 3 were excluded because their fasting glucose were lower than 3.5 mmol/L that resulted in negative values for Homeostatic Model Assessment of β-cell function (HOMA-β). Therefore, 2420 individuals (1777 females and 643 males) were included in the present study (Fig 1).
Fig 1. Flow-chart of subjects selection for analyse.
Anthropometric measurements
Anthropometrics were performed following a 12-hour overnight fast. Trained personnel obtained these measurements for each subject using standard procedures. Standing height was measured using a fixed stadiometer (to the nearest 0.1 cm). After fully emptying their bladders, subjects wore standard hospital gowns for all weight measurements using a platform manual scale balance (Health O Meter, Bridgeview, IL; nearest 0.1 kg). Body mass index (BMI) (kg/m2) was calculated as weight in kilograms divided by height squared in meters. Waist circumference (WC) was defined as the midway point between the iliac crest and the lowest rib, and hip by the maximum circumference over the buttocks below the iliac crest. Waist-hip ratio (WHR) was the division of WC by hip circumference.
Body composition measurements
Body compositions including total body fat percentage (BF%), trunk fat percentage (TF%), android fat percentage (AF%) and gynoid fat percentage (GF%) were measured, in a supine position, utilizing dual energy X-ray absorptiometry (DXA: Lunar Prodigy; GE Medical Systems, Madison, WI) with the Lunar Prodigy software system. The Lunar Prodigy software system has the capacity to distinguish each of these regions. Trunk fat region is from the top of the shoulders to the top of the iliac crest, while the android fat region is the top of the second lumbar vertebra to the top of the iliac crest and the gynoid fat region extends down the iliac crest twice the height of the android area. The enCORE (Ver 12.2, 2008, GE Medical Systems, Madison, WI) software package was used for DXA data acquisition. Daily quality assurance was performed on the DXA scanner and the typical coefficient of variation was 1.3% during the study period [34–38].
Dietary assessment
Dietary intake of each participant was assessed using a 124 item semi- quantitative Willett food frequency questionnaire (FFQ) [39,40]. The Willett FFQ obtains from subjects, the number of weekly servings consumed of common food items over the past 12 months. The NutriBase Clinical Nutrition Manager (version 8.2.0; Cybersoft Inc, Phoenix, AZ) software package was used to convert weekly serving values into mean daily serving values. This information was then used to calculate the total daily calorie, macro- and micro-nutrients intakes including Se for each individual [34]. Then dietary Se intake (μg/kg/day) was calculated by dividing body weight [34].
Physical activity assessment and other information
All participants completed a self-administered screening questionnaire, which was used to collect information of personal health history. Physical activity patterns were measured using the ARIC Baecke Questionnaire, which consists of a Work Index, Sports Index, and Leisure Time Activity Index [34, 41].
Biochemical measurements
Venous blood samples were collected in the morning after an overnight fast (12 hours). Serum samples were isolated from whole blood and stored at −80°C for subsequent analysis. FBG were measured on an Lx20 analyzer (Beckman Coulter Inc., Fullerton, CA) using Synchron reagents. Fasting insulin (FINS) was measured on an Immulite Immunoassay analyzer. Insulin resistance and β cell function were determined with the homeostasis model assessment (HOMA-IR and HOMA-β), as described by Matthews et al [42].
HOMA-IR = (Fasting Insulin [mU/L]×Fasting Glucose [mmol/L])/22.5
HOMA-β = (20×Fasting Insulin [mU/L])/(Fasting Glucose [mmol/L]—3.5)
Data analyses
All data are presented as mean ± standard error of the mean(SEM). FINS, HOMA-IR, HOMA-β, calorie intake and dietary Se intake were log-transformed in order to normalize data distributions to perform effective statistical analysis. Anthropometrics, body composition, dietary intake and biochemical measurements were compared between females and males with independent Student's t-test.
In order to analyze the variation of dietary Se intake in different status of insulin resistance, participants were divided into tertiles (low, medium, and high) of insulin resistance based upon HOMA-IR. Dietary Se intake was compared among the three groups with analyses of variance and covariates (ANCOVA) controlling for age, calorie intake and physical activity. The variations of insulin resistance in different dietary Se intakes were analyzed after participants were divided into tertiles (low, medium, and high dietary Se intake). FBG, FINS, HOMA-IR, and HOMA-β were compared among groups using ANCOVA controlling for age, calorie intake, physical activity, serum calcium, serum magnesium, and BF%. Serum calcium and magnesium were taken into consideration as well because our previous studies have shown that they were associated with insulin resistance [35, 43].
Dietary Se intake below 0.4 μg/kg/day is considered as Se deficiency [44]. The relationships between dietary Se intake and HOMA-IR, HOMA-β were further analyzed, after subjects were divided into 10 groups by an interval of 0.4 μg/kg/day (≤0.4, 0.4–0.8, 0.8–1.2, 1.2–1.6, 1.6–2.0, 2.0–2.4, 2.4–2.8, 2.8–3.2, 3.2–3.6, 3.6–4.0 μg/kg/day). The number of subjects with dietary Se intake > 4.0 μg/kg/day was too small (n = 38, female/male = 27/11) to perform an effective statistical analysis. Therefore, they were excluded from the analysis.
Partial correlation analysis, controlling for age, calorie intake, physical activity, serum calcium, serum magnesium, and BF%, was subsequently applied to further confirm the findings from ANCOVA. To control for possible influence of smoking, alcohol drinking, disease status medication use and menopausal, analyses were performed in participants with and without these confounding factors.
All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL). All tests were two sided and p < 0.05 was considered to be statistically significant.
Results
Clinical and dietary Se characteristics of the study subjects
Clinical and dietary characteristics of the study subjects are presented in Table 1. Female subjects were on average 3.6 years older than male subjects. Weight, BMI, WC, WHR, FBG, FINS, HOMA-IR, physical activity, serum calcium, and serum magnesium in males were significantly greater and HOMA-β, TF%, AF%, GF%, BF% were significantly lower than females (p < 0.01 for all). In terms of dietary intake, male participants had a significantly higher calorie and Se intake (μg/day) than female participants (p < 0.01), because of the large body size and weight in males. However, after body weight was adjusted (μg/kg/day), there was no significant difference between males and females (p = 0.19).
Table 1. Clinical characteristics and dietary Se intake according to gender.
| Entire cohort (n = 2420) | Females (n = 1777) | Males (n = 643) | p value | |
|---|---|---|---|---|
| Age (yr) | 42.40 ± 0.27 | 43.42 ± 0.27 | 39.80 ± 0.47 | < 0.001 |
| Weight (kg) | 74.04 ± 0.34 | 69.16 ± 0.29 | 86.64 ± 0.53 | < 0.001 |
| BMI (kg/m2) | 26.56 ± 0.10 | 26.14 ± 0.11 | 27.67 ± 0.16 | < 0.001 |
| WC (cm) | 91.57 ± 0.29 | 89.35 ± 0.29 | 97.32 ± 0.45 | < 0.001 |
| WHR | 0.91 ± 0.001 | 0.89 ± 0.001 | 0.97 ± 0.002 | < 0.001 |
| TF% | 36.11 ± 0.19 | 38.43 ± 0.19 | 30.11 ± 0.33 | < 0.001 |
| AF% | 41.15 ± 0.23 | 43.20 ± 0.23 | 35.87 ± 0.40 | < 0.001 |
| GF% | 40.08 ± 0.20 | 44.51 ± 0.14 | 28.59 ± 0.27 | < 0.001 |
| BF% | 33.82 ± 0.19 | 37.18 ± 0.17 | 25.14 ± 0.28 | < 0.001 |
| FBG (mmol/L) | 5.02 ± 0.01 | 4.96 ± 0.01 | 5.17 ± 0.02 | < 0.001 |
| FINS (pmol/L) | 67.61 ± 0.89 | 65.77 ± 0.99 | 72.6 1± 1.88 | 0.008 |
| HOMA-IR | 2.24 ± 0.03 | 2.17 ± 0.04 | 2.43 ± 0.07 | < 0.001 |
| HOMA-β | 133.84 ± 1.98 | 136.54 ± 2.56 | 126.39 ± 3.72 | 0.002 |
| Serum calcium (mmol/L) | 2.36 ± 0.002 | 2.35 ± 0.002 | 2.38 ± 0.003 | < 0.001 |
| Serum magnesium (mmol/L) | 0.88 ± 0.002 | 0.88 ± 0.001 | 0.89 ± 0.002 | < 0.001 |
| Physical activity | 8.28 ± 0.03 | 8.18 ± 0.03 | 8.53 ± 0.06 | < 0.001 |
| calorie intake (kcal/day) | 1991.55 ± 18.49 | 1873.63 ± 17.62 | 2317.87 ± 38.32 | < 0.001 |
| Se (μg/day) | 109.22 ± 1.18 | 102.34 ± 1.11 | 128.23 ± 2.78 | < 0.001 |
| Se (μg/kg/day) | 1.53 ± 0.02 | 1.53 ± 0.02 | 1.53 ± 0.03 | 0.19 |
All data presented as mean ± SEM. BMI, Body mass index; WC, Waist circumference; WHR, Waist hip rate; TF%, trunk fat percentage; AF%, android fat percentage; GF%, gynoid fat percentage; BF%, total body fat percentage; FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function.
Comparison of dietary Se intake among groups with low, medium and high insulin resistance
Significant differences of dietary Se intake were revealed between the three groups with different insulin resistance after controlling for age, total calorie intake and physical activity (Table 2). Compared with low HOMA-IR group, dietary Se intakes in medium and high HOMA-IR groups were 6%, 11% lower in males, and 8%, 18% lower correspondingly in females. The significant differences were confirmed in pairwise comparisons (p < 0.05), except between male low and medium HOMA-IR group (p = 0.24).
Table 2. Comparison of Se intake based on the levels of insulin resistance.
| Low | Medium | High | p trend | ||
|---|---|---|---|---|---|
| Females | 592 | 592 | 592 | - | |
| HOMA-IR | 0.40~1.42 | 1.42~2.32 | 2.32~24.07 | - | |
| Se (μg/day) | 102.19 ± 1.34 | 99.84 ± 1.32 | 101.23 ± 1.35 | 0.21 | |
| Se (μg/kg/day) | 1.62 ± 0.02 | 1.49 ± 0.02 | 1.34 ±0.02 | < 0.001 | |
| Males | 214 | 214 | 214 | ||
| HOMA-IR | 0.40~1.60 | 1.60~2.74 | 2.74~16.37 | - | |
| Se (μg/day) | 117.70 ± 3.15 | 117.83 ± 3.02 | 121.83 ± 3.10 | 0.62 | |
| Se (μg/kg/day) | 1.47 ± 0.04 | 1.38 ± 0.04 | 1.30 ±0.04 | < 0.001 |
Data were assessed with Covariance controlling for age, total calorie intake, and physical activity. All values are presented as means ± SEMs. HOMA-IR, homeostasis model assessment of insulin resistance.
Comparison of insulin resistance in subjects with different dietary Se intake groups
When subjects were grouped into tertiles according to dietary Se intake (low, medium and high), levels of FINS, HOMA-IR, HOMA-β presented a dose-dependent decline (high, medium and low) with the increase of dietary Se intake after controlling for age, total calorie intake, physical activity, serum calcium, serum magnesium and BF% in females (p < 0.05) not in males (Table 3).
Table 3. Insulin resistance according to dietary Se intake.
| Low | Medium | High | p | ||
|---|---|---|---|---|---|
| Female | Number | 592 | 592 | 592 | - |
| Se (μg/kg/day) | 0.16 ~1.12 | 1.22 ~ 1.66 | 1.66 ~ 8.89 | - | |
| FBG (mmol/L) | 5.05 ± 0.02 | 4.99 ± 0.02 | 4.99 ± 0.02 | 0.13 | |
| FINS (pmol/L) | 73.02 ± 1.86 | 62.47 ± 1.59 | 61.23 ± 1.96 | <0.001 | |
| HOMA-IR | 2.41 ± 0.07 | 2.02 ± 0.06 | 2.04 ± 0.08 | <0.001 | |
| HOMA-β | 142.22 ± 4.36 | 131.40 ± 3.72 | 126.77 ± 4.59 | 0.01 | |
| Male | Number | 214 | 214 | 214 | - |
| Se (μg/kg/day) | 0.22 ~ 1.05 | 1.05 ~ 1.61 | 1.61 ~ 7.19 | - | |
| FBG (mmol/L) | 5.25 ± 0.04 | 5.23 ± 0.03 | 5.27 ± 0.04 | 0.69 | |
| FINS (pmol/L) | 78.24 ± 3.88 | 69.76 ± 3.34 | 73.61 ± 4.62 | 0.30 | |
| HOMA-IR | 2.63 ± 0.14 | 2.36 ± 0.12 | 2.51 ± 0.17 | 0.28 | |
| HOMA-β | 129.75 ± 8.57 | 122.21 ± 7.37 | 135.42 ± 10.19 | 0.41 |
Data were assessed with covariates controlling for age, calorie intake, physical activity, serum calcium, serum magnesium, and BF%. Data presented as mean ± SEM. FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function.
For each 1 μg/kg/day increase in dietary Se intake, average weight, BMI, WC, and WHR decreased by 8.39 kg, 2.98 kg/m2, 8.03 cm, and 0.02 in women, and by 8.85 kg, 2.34 kg/m2, 7.39 cm, and 0.02 in men, respectively. Likewise, TF%, AF%, GF% and BF% were reduced by 4.58%, 5.56%, 3.05% and 4.16% in women, and by 5.43%, 5.94%, 4.19% and 4.45% in men, respectively (Table 3). Dietary Se intake (μg/kg/day) alone accounted for 9–27% of the variations in body fat.
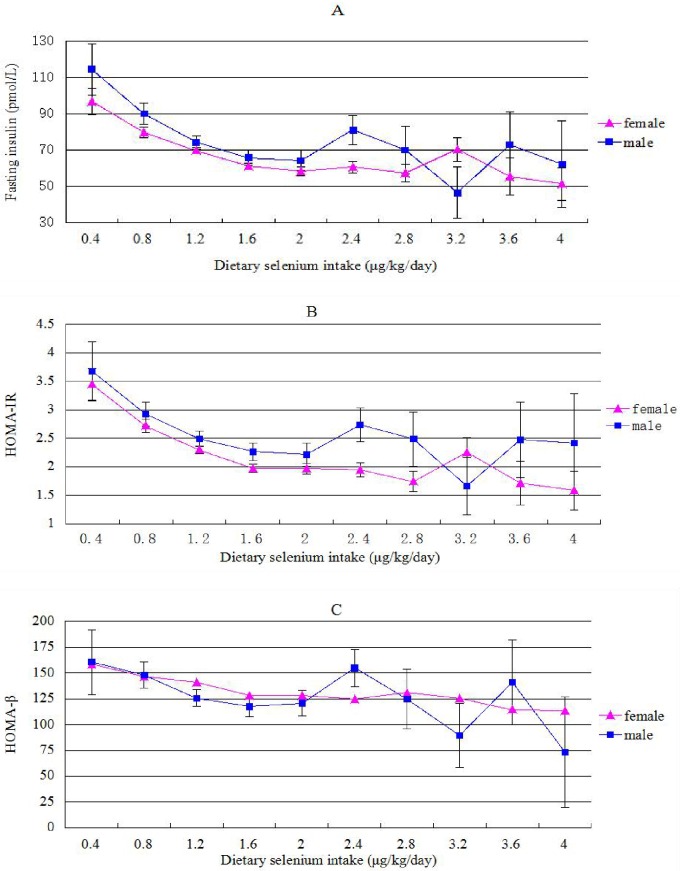
Subjects were divided into 10 groups based on dietary Se intake with an interval of 0.4 μg/kg/day, and the variations of FINS, HOMA-IR and HOMA-β with dietary Se intake were analyzed with covariates (Fig 2). As dietary Se intake increased from 0.4 to 1.6 μg/kg/day, FINS, HOMA-IR, HOMA-β decreased correspondingly in a pattern close to linear relationship for both genders. However this linear pattern came to a plateau, and disappeared from the level of 1.6 to 4.0 μg/kg/day in both genders.
Fig 2. Variations in fasting insulin, HOMA-IR and HOMA-β with increasing dietary Se intake.
HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function.
Correlations between dietary Se intake and insulin resistance by sex
The correlations between dietary Se intake and FINS, HOMA-IR and HOMA-β are presented in Table 4. When dietary Se intake was ≤ 1.6 μg/kg/day, in both male and female subjects, dietary Se intake (μg/kg/day) was significantly negatively correlated with FINS, HOMA-IR and HOMA-β (r0 was from -0.107 to -0.227, p < 0.001). The correlations remained significant (r1 was from -0.102 to -0.153, p < 0.05) after adjusting for age, total calorie intake, physical activity, serum calcium, serum magnesium and BF% in both genders. When dietary Se intake was greater than 1.6 μg/kg/day, the negative correlations disappeared after controlling these confounding factors in both genders.
Table 4. Correlation of dietary Se intake with insulin resistance.
| Dietary Se intake (μg/kg/day) |
≤ 1.6 | > 1.6 | |||
|---|---|---|---|---|---|
| r0 (p) | r1(p) | r0 (p) | r1(p) | ||
| Females | FBG | -0.157 (0.000) | -0.103 (0.001) | -0.042 (0.303) | -0.048 (0.240) |
| FINS | -0.183 (0.000) | -0.148 (0.000) | -0.028 (0.492) | -0.049 (0.238) | |
| HOMA-IR | -0.191 (0.000) | -0.153 (0.000) | -0.036 (0.375) | -0.064 (0.121) | |
| HOMA-β | -0.107 (0.000) | -0.102 (0.001) | -0.001 (0.981) | -0.003 (0.939) | |
| Males | FBG | -0.165 (0.001) | 0.022 (0.664) | -0.112 (0.150) | -0.043 (0.586) |
| FINS | -0.216 (0.000) | -0.124 (0.014) | -0.177 (0.023) | -0.004 (0.958) | |
| HOMA-IR | -0.227 (0.000) | -0.121 (0.017) | -0.186 (0.016) | -0.015 (0.853) | |
| HOMA-β | -0.156 (0.002) | -0.121 (0.017) | -0.111 (0.154) | 0.041 (0.606) | |
Partial correlations between dietary Se intake (μg/kg/day) and insulin resistance were controlling for age, total caloric intake, physical activity, serum calcium, serum magnesium, and body fat percentage. FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function. r0: correlation coefficient; r1: partial correlation coefficient.
To further exclude the influence of additional covariates, data of participants who were non-smokers, non-drinkers, on no medication and otherwise healthy were analyzed. In Table 5, for each subgroup, partial correlation analyses were conducted controlling for age, total calorie intake, physical activity, serum calcium, serum magnesium and BF%. Dietary Se intake remained negatively correlated with HOMA-IR.
Table 5. Partial correlations between dietary Se intake and body compositions excluding the effect of Smoking, drinking, medication use and disease.
| No Smoking | No Alcohol | No Medication | No Disease | |||||
|---|---|---|---|---|---|---|---|---|
| r1 | r2 | r1 | r2 | r1 | r2 | r1 | r2 | |
| Female | ||||||||
| FBG | -0.086** | -0.045 | -0.078 | 0.055 | -0.081* | 0.012 | -0.128** | -0.065 |
| FINS | -0.137** | -0.038 | -0.179** | -0.062 | -0.106* | -0.012 | -0.133** | -0.042 |
| HOMA-IR | -0.138** | -0.055 | -0.180** | -0.078 | -0.110* | -0.015 | -0.140** | -0.061 |
| HOMA-β | -0.101** | 0.010 | -0.139* | -0.027 | -0.075* | -0.014 | -0.082* | -0.007 |
| Male | ||||||||
| FBG | -0.063 | -0.065 | -0.154 | 0.268 | -0.027 | -0.035 | -0.010 | -0.046 |
| FINS | -0.137* | -0.024 | -0.279* | 0.220 | 0.105* | -0.061 | -0.104* | -0.014 |
| HOMA-IR | -0.136* | -0.037 | -0.280* | 0.211 | -0.096* | -0.048 | -0.098* | -0.024 |
| HOMA-β | -0.122* | 0.031 | -0.238 | 0.233 | 0.124 | -0.095 | -0.112 | 0.030 |
Partial correlations between dietary Se intake (μg/kg/day) and insulin resistance controlling for age, total caloric intake, physical activity, serum calcium, serum magnesium, and body fat percentage. FBG, fasting blood glucose; FINS, fasting insulin; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β cell function. r1: partial correlation coefficient, when dietary Se intake was below or equal to 1.6 μg/kg/day; r2: partial correlation coefficient, when dietary Se intake was greater than 1.6 μg/kg/day.
*, P<0.05
** P<0.01.
Discussion
In the present study, we analyzed the associations between dietary Se intake and insulin resistance in the large CODING study with a wide range of dietary Se intake among 2,420 adult Newfoundlanders. To the best of our knowledge, this is the first large cross-sectional study specifically designed to analyze the association between dietary Se intake and insulin resistance in the general population. The most important finding is that dietary Se intake was significantly negatively associated with insulin resistance in females and males after controlling all major confounding factors, when dietary Se intake was ≤ 1.6 μg/kg/day most subjects fell in this range. However, when dietary Se intake was > 1.6 μg/kg/day, this reverse relationship was no longer significant. The findings suggest that the beneficial negative relationship between dietary Se intake and insulin resistance may have a dose ceiling.
Se may affect insulin resistance via multiple routes including insulin-like action, inflammatory cytokines and oxidative stress. Early studies showed that sodium selenate may mimic insulin to stimulate glucose uptake [7–9]. In addition, Se may reduce insulin resistance through inhibiting the activity and the production of inflammatory cytokines including tumour necrosis factor-a (TNF-a), nuclear factor-kappa B (NF-κB) and interleukin (IL-1 and IL-18) [45–49]. On the other hand, roles of reactive oxygen species (ROS) in insulin signaling depend on the balance of ROS production and antioxidant defense [50]. Both excessive ROS [51–53] and excessive consumption of ROS [32, 33] are involved in insulin resistance. Adequate Se intake as a potent antioxidant can decrease ROS and improve insulin resistance. However higher Se intake can increase the expression of selenoproteins including GPx, which may induce insulin resistance by removing hydrogen peroxide [54–57]. The ceiling phenomenon of dietary Se intake discovered from the present study may provide further evidence demonstrating the changing effect on insulin resistance with increase the of dietary Se intake.
Insulin resistance is a complex pathophysiological condition. There are numerous factors that can potentially be involved in the development of insulin resistance. It is critical to identify and properly control or adjust major factors in a large population study because if these factors are not properly adjusted, they would potentially cause either false positive or negative results. Similarly a variety of factors may potentially affect dietary Se intake. Food choice and consumption and insulin resistance vary among different age and gender groups [58–61], making age and gender primarily important confounding factors to be adjusted. Dietary calorie intake as a general indicator of nutrient intake is positively correlated with dietary Se intake [34] and negatively with insulin sensitivity [62]. Because physical activity [63], body fat accumulation [64], Serum magnesium [35] and calcium [43] are also associated with insulin resistance, they were adjusted as potential confounding factors in our analyses. Smoking, alcohol drinking, concomitant illness and medication use (including Se supplement) are all potentially important covariates of insulin resistance. Even after these covariates were excluded, the association between dietary Se intake and insulin resistance remained significant. One of major strengths in the present study is the systematic control of these confounding factors, ensuring the findings in the present study are more accurate and reliable.
Previous studies had reported conflicting results for the relationships between Se in various biological samples and insulin resistance. It was reported that hair Se were negatively correlated with the HOMA-IR controlled for age and sex in a small Korean study [28]. A weak negative correlation between serum Se level and HOMA-IR was found in first-degree relatives of diabetic patients in Turkey [29]. However, in a Swedish study including 1024 elderly men, serum Se level was not associated with insulin resistance after adjusting for age, BMI, cigarette smoking, leisure time physical activity and education [23]. Contrarily positive correlations between serum Se level and HOMA-IR were reported in elderly Polish men with MS [30] and obese Egyptian children [31]. Administration of 200 μg/day Se supplements for 6 weeks resulted in a significant decrease in serum insulin and HOMA-IR levels among women with central obesity [32] and PCOS [33]. However, there is no large study available focusing on dietary Se intake and insulin resistance in the general population. The present study filled the knowledge gap, and revealed that dietary Se intake is indeed associated with low insulin resistance suggesting a beneficial effect of dietary Se on insulin sensitivity.
Another important finding from the present study is the ‘threshold’ of the beneficial effect of dietary Se intake on insulin resistance. The beneficial association generally started from very low level to 1.6 μg/kg/day, approximately equivalent to 118 μg/day for person with average body weight (139 μg/day for males and 110 μg/day for females). This beneficial association was nearly linear within this range but became weaker and disappeared if dietary Se intake was above this level. This cut-off point is similar to the suggested dietary Se dose (100–150 μg/day) for tumor protection [65]. This cut-off point will be essential for determining the most appropriate dose of Se supplementation required to maximize its beneficial effect and to avoid potential adverse effects. Existing data suggest that both Se deficiency and over supplementation may be associated with insulin resistance [65, 66] and increased the risk of type 2 diabetes [6].Previous studies have reported a trend of U-shaped association between serum Se and risk of cardiovascular diseases [67], serum Se and serum triglycerides [68]. The association between dietary Se and insulin resistance revealed in the present study seems to be U-shaped as well. Se supplementation will be beneficial to insulin resistance for those with a Se level below 1.6 μg/kg/day. However, Se supplement may be unnecessary for most people without significant low dietary Se intake. Caution has to be applied in Se supplementation as excessive amount potentially have harmful effects. It must be noted that the cutoff point used in the present study was obtained from statistical analysis in the Newfoundland population. More studies in different populations are warranted.
There are several potential limitations in the present study. Although the study has identified and adjusted many major confounding factors, it is inevitably other potential factors were not included, such as zinc and copper which have been reported to have effects on insulin sensitivity [69, 70], however, the data of zinc and copper was not available at the time when analyses were performed. In addition, dietary Se cannot completely represent the Se nutrition status [71]. Other potential metrics of Se nutrition status such as serum Se level and selenoproteins will be measured and analyzed in the near future. In addition, dietary Se exists in two forms: inorganic (selenate and selenite) and organic (selenomethionine and selenocysteine). The nature of the dietary questionnaires did not allow us to evaluate further and identify Se chemical form that exist in the foods, which needs to measure in special method. It is hard to evaluate/separate the data also by predominant selenium chemical forms that exist in the foods in a large sample study. Hence, the effect of different Se chemical forms on insulin resistance cannot be distinguished either. Finally, a cross-sectional design does not allow the determination if dietary Se intake is a causal effect for reduced insulin resistance. Direct intervention study using Se supplement among patients with insulin resistance and low level of dietary Se will be warranted.
Conclusions
In conclusion, our findings revealed a significantly negative association of dietary Se intake with insulin resistance in the large CODING study with many major confounding factors adjusted, when dietary Se intake was below 1.6 μg/kg/day. Given the narrow margin between Se deficiency, adequacy, over-nutrition and toxicity, accurately determining this cutoff point is clinically important. People with an adequate or high Se status have already received the maximum benefit from Se and need not take additional Se supplementation, because of the potential risks, such as the increased risk of type 2 diabetes. Ultimately, this study aims to add to our present knowledge about the optimal constitution of an ideal diet to reduce insulin resistance and risk of type 2 diabetes in terms of specific macronutrient and micronutrient composition.
Acknowledgments
We would like to thank all of the volunteers who participated in this present study. We declare that we have no conflicts of interest.
Data Availability
All relevant data are within the paper.
Funding Statement
This project has been funded by a Canadian Institutes of Health Research (CIHR) (operating grant: MOP192552). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rayman MP. Selenium and human health. Lancet. 2012, 379, 1256–1268. 10.1016/S0140-6736(11)61452-9 [DOI] [PubMed] [Google Scholar]
- 2.Combs GF Jr, Midthune DN, Patterson KY, Canfield WK, Hill AD, Levander OA, et al. Effects of selenomethionine supplementation on selenium status and thyroid hormone concentrations in healthy adults. Am J Clin Nutr. 2009, 89, 1808–1814. 10.3945/ajcn.2008.27356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Rose AH, Hoffmann PR. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid Redox Signal. 2012, 16, 705–743. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattmiller SA, Carlson BA, Sordillo LM. Regulation of inflammation by selenium and selenoproteins: impact on eicosanoid biosynthesis. J Nutr Sci. 2013, 2, e28 10.1017/jns.2013.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med 2013, 65, 1557–1564. 10.1016/j.freeradbiomed.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Rocourt CRB and Cheng WH. Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients 2013, 5, 1349–1365. 10.3390/nu5041349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem. 1990, 265, 1124–1128. [PubMed] [Google Scholar]
- 8.Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH. Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem. 1998, 178, 367–375. [DOI] [PubMed] [Google Scholar]
- 9.Fürnsinn C, Englisch R, Ebner K, Nowotny P, Vogl C, Waldhäsl W. Insulin-like vs. non-insulin-like stimulation of glucose metabolism by vanadium, tungsten, and selenium compounds in rat muscle. Life Sci. 1996, 59, 1989–2000. [DOI] [PubMed] [Google Scholar]
- 10.Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006, 17, 548–560. 10.1016/j.jnutbio.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 11.McNeill JH, Delgatty HL, Battell ML. Insulin like effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes. 1991, 40, 1675–1678. [DOI] [PubMed] [Google Scholar]
- 12.Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia. 1996, 39, 3–11. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009, 1790, 1478–1485. 10.1016/j.bbagen.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 14.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007, 30, 829–834. 10.2337/dc06-1726 [DOI] [PubMed] [Google Scholar]
- 15.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009, 117, 1409–1413. 10.1289/ehp.0900704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stranges S, Galletti F, Farinaro E, D'Elia L, Russo O, Iacone R, et al. Associations of selenium status with cardiometabolic risk factors: an 8-year follow-up analysis of the Olivetti Heart study. Atherosclerosis. 2011, 217, 274–278. 10.1016/j.atherosclerosis.2011.03.027 [DOI] [PubMed] [Google Scholar]
- 17.Gao S, Jin Y, Hall KS, Liang C, Unverzagt FW, Ji R, et al. Selenium level and cognitive function in rural elderly Chinese. Am. J. Epidemiol. 2007, 955–965. 10.1093/aje/kwk073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coudray C, Roussel AM, Mainard F, Arnaud J, Favier A. Lipid peroxidation level and antioxidant micronutrient status in a pre-aging population: correlation with chronic disease prevalence in a French epidemiological study (Nantes, France). J. Am. Coll. Nutr. 1997, 16:584–591. [PubMed] [Google Scholar]
- 19.Wei J, Zeng C, Gong QY, Yang HB, Li XX, Lei GH, et al. The association between dietary selenium intake and diabetes: a cross-sectional study among middle-aged and older adults. Nutrition Journal. 2015, 14:18 10.1186/s12937-015-0007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajpathak S, Rimm E, Morris JS, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am Coll Nutr. 2005, 24, 250–256. [DOI] [PubMed] [Google Scholar]
- 21.Park K, Rimm EB, Siscovick DS, Spiegelman D, Manson JE, Morris JS, et al. Toenail selenium and incidence of type 2 diabetes in U.S. Men and women. Diabetes Care. 2012, 35, 1544–1551. 10.2337/dc11-2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes K, Aw TC, Kuperan P, Choo M. Central obesity, insulin resistance, syndrome X, lipoprotein(a), and cardiovascular risk in Indians, Malays and Chinese in Singapore. J. Epidemiol. Community Health. 1997, 51, 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao H, Hägg S, Sjögren P, Lambert PC, Ingelsson E, van Dam RM. Serum selenium in relation to measures of glucose metabolism and incidence of Type 2 diabetes in an older Swedish population. Diabet Med. 2014, 31: 787–793. 10.1111/dme.12429 [DOI] [PubMed] [Google Scholar]
- 24.Stranges S, Sieri S, Vinceti M, Grioni S, Guallar E, Laclaustra M, et al. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010, 10: 564 10.1186/1471-2458-10-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007, 147: 217–223. [DOI] [PubMed] [Google Scholar]
- 26.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2011, 48: 40–45. 10.3164/jcbn.11-002FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao S, Zhang A, Huang S. Selenium supplementation and the risk of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Endocrine. 2014, 47: 758–763. 10.1007/s12020-014-0298-7 [DOI] [PubMed] [Google Scholar]
- 28.Kim HN, Song SW. Concentrations of chromium, selenium, and copper in the hair of viscerally obese adults are associated with insulin resistance. Trace Elem Res. 2014,158:152–157. [DOI] [PubMed] [Google Scholar]
- 29.Ozkaya M, Sahin M, Cakal E, Gisi K, Bilge F, Kilinc M. Selenium levels in first-degree relatives of diabetic patients. Biol Trace Elem Res. 2009. May,128:144–151. 10.1007/s12011-008-8263-z [DOI] [PubMed] [Google Scholar]
- 30.Rotter I, Kosik-Bogacka D, Dołęgowska B, Safranow K, Lubkowska A, Laszczyńska M. Relationship between the concentrations of heavy metals and bioelements in aging men with metabolic syndrome. Int J Environ Res Public Health. 2015, 12: 3944–3961. 10.3390/ijerph120403944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM. Serum trace elements in obese Egyptian children: a case-control study. Ital J Pediatr. 2014, 40: 20 10.1186/1824-7288-40-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alizadeh M, Safaeiyan A, Ostadrahimi A, Estakhri R, Daneghian S, Ghaffari A, et al. Effect of L-arginine and selenium added to a hypocaloric diet enriched with legumes on cardiovascular disease risk factors in women with central obesity: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. 2012, 60: 157–168. 10.1159/000335470 [DOI] [PubMed] [Google Scholar]
- 33.Jamilian M, Razavi M, Fakhrie Kashan Z, Ghandi Y, Bagherian T, Asemi Z. Metabolic response to selenium supplementation in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2015, 82: 885–891. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Gao X, Pedram P, Shahidi M, Du J, Yi Y, et al. Significant beneficial association of dietary selenium intake with reduced body fat. Nutrients. 2016, 8. Pii:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cahill F, Shahidi M, Shea J, Wadden D, Gulliver W, Randell E, et al. High Dietary magnesium intake is associated with low insulin resistance in the Newfoundland population. PLoS One. 2013, 8: e58278 10.1371/journal.pone.0058278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fontaine-Bisson B, Thorburn J, Gregory A, Zhang H and Sun G. Melanin-concentrating hormone receptor 1 polymorphisms are associated with components of energy balance in the Newfoundland CODING study. Am J Clin Nutr. 2014, 99:384–391. 10.3945/ajcn.113.073387 [DOI] [PubMed] [Google Scholar]
- 37.Shea JL, King MT, Yi Y, Gulliver W, Sun G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr Metab Cardiovasc Dis. 2012, 22: 741–747. 10.1016/j.numecd.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Green KK, Shea JL, Vasdev S, Randell E, Gulliver W, Sun G. Higher Dietary Protein Intake is Associated with Lower Body Fat in the Newfoundland Population. Clin Med Insights Endocrinol Diabetes. 2010, 3: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985, 122: 51–65. [DOI] [PubMed] [Google Scholar]
- 40.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001, 154: 1089–1099. [DOI] [PubMed] [Google Scholar]
- 41.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982, 36: 936–942. [DOI] [PubMed] [Google Scholar]
- 42.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985, 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 43.Sun G, Vasdev S, Martin GR, Gadag V, Zhang H. Altered calcium homeostasis is correlated with abnormalities of fasting serum glucose, insulin resistance, and beta-cell function in the Newfoundland population. Diabetes. 2005, 54: 3336–3339. [DOI] [PubMed] [Google Scholar]
- 44.Tanguy S, Grauzam S, de Leiris J, Boucher F. Impact of dietary selenium intake on cardiac health: experimental approaches and human studies. Mol Nutr Food Res. 2012, 56: 1106–1121. 10.1002/mnfr.201100766 [DOI] [PubMed] [Google Scholar]
- 45.Brigelius-Flohé R, Banning A, Kny M, Böl GF. Redox events in interleukin-1 signaling. Arch Biochem Biophys. 2004, 423: 66–73. [DOI] [PubMed] [Google Scholar]
- 46.Faure P, Ramon O, Favier A, Halimi S. Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Invest. 2004, 34: 475–481. 10.1111/j.1365-2362.2004.01362.x [DOI] [PubMed] [Google Scholar]
- 47.Dhanya BL, Swathy RP, Indira M. Selenium downregulates oxidative stress-induced activation of leukotriene pathway in experimental rats with diabetic cardiac hypertrophy. Biol Trace Elem Res. 2014, 161: 107–115. 10.1007/s12011-014-0076-7 [DOI] [PubMed] [Google Scholar]
- 48.Puchau B, Zulet MA, Hermsdorff HH, Navarro-Blasco I, Martínez JA. Nail antioxidant trace elements are inversely associated with inflammatory markers in healthy young adults. Biol Trace Elem Res. 2010, 133: 304–312. 10.1007/s12011-009-8443-5 [DOI] [PubMed] [Google Scholar]
- 49.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014, 1842: 446–462. 10.1016/j.bbadis.2013.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truong TH, Carroll KS. Redox regulation of protein kinases. Crit Rev Biochem Mol Biol. 2013, 48: 332–356. 10.3109/10409238.2013.790873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farinha JB, Steckling FM, Stefanello ST, Cardoso MS, Nunes LS, Barcelos RP, et al. Response of oxidative stress and inflammatory biomarkers to a 12-week aerobic exercise training in women with metabolic syndrome. Sports Med Open. 2015,1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razavi M, Jamilian M, Kashan ZF, Heidar Z, Mohseni M, Ghandi Y,et al. Selenium Supplementation and the Effects on Reproductive Outcomes, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome. Horm Metab Res. 2016. 48: 185–190. 10.1055/s-0035-1559604 [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Xu WM, Zhang D. Association of abdominal obesity, insulin resistance, and oxidative stress in adipose tissue in women with polycystic ovary syndrome. Fertil Steril. 2014, 102:1167–1174. 10.1016/j.fertnstert.2014.06.027 [DOI] [PubMed] [Google Scholar]
- 54.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004, 101: 8852–8857. 10.1073/pnas.0308096101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by over-production of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008, 51: 1515–1524. 10.1007/s00125-008-1055-3 [DOI] [PubMed] [Google Scholar]
- 56.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10: 260–272. 10.1016/j.cmet.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X, et al. High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett. 2014, 224: 16–23. 10.1016/j.toxlet.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 58.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009, 8: 339–348. 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- 59.Arganini C, Saba A, Comitato R, Virgili F and Turrini A. Gender Differences in Food Choice and Dietary Intake in Modern Western Societies, Public Health—Social and Behavioral Health, Prof. Jay Maddock (Ed.), 2012, ISBN: 978-953-51-0620-3, InTech, Available from: http://www.intechopen.com/books/public-health-social-and-behavioral-health/gender-differences-in-foodchoice-and-dietary-intake-in-modern-western-societies.
- 60.Honek J, Seki T, Iwamoto H, Fischer C, Li J, Lim S, et al. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc Natl Acad Sci U S A. 2014, 111: 14906–14911. 10.1073/pnas.1415825111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marucci A, Menzaghi C, Copetti M, Vinciguerra F, Baratta R, Salvemini L, et al. Strong evidence of sexual dimorphic effect of adiposity excess on insulin sensitivity. Acta Diabetol. 2015, 52:991–998. 10.1007/s00592-015-0804-2 [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, Watanabe RM, Stram DO, Buchanan TA, Xiang AH. High calorie intake is associated with worsening insulin resistance and β-cell function in Hispanic women after gestational diabetes mellitus. Diabetes Care. 2014, 37: 3294–300. 10.2337/dc14-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamasaki H, Noda M, Moriyama S, Yoshikawa R, Katsuyama H, Sako A, et al. Daily Physical Activity Assessed by a Triaxial Accelerometer Is Beneficially Associated with Waist Circumference, Serum Triglycerides, and Insulin Resistance in Japanese Patients with Prediabetes or Untreated Early Type 2 Diabetes. J Diabetes Res. 2015, 2015: 526201 10.1155/2015/526201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshimura E, Kumahara H, Tobina T, Ayabe M, Matono S, Anzai K, et al. Relationships between body fat accumulation, aerobic capacity and insulin resistance in Japanese participants. Obes Res Clin Pract. 2011, 5: e79–e156. [DOI] [PubMed] [Google Scholar]
- 65.Thomson CD. Assessment of requirements for selenium and adequacy of selenium status: a review. Eur J Clin Nutr. 2004, 58: 391–402. 10.1038/sj.ejcn.1601800 [DOI] [PubMed] [Google Scholar]
- 66.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid. Redox Signal. 2011, 14: 2327–2336. 10.1089/ars.2010.3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bleys J, Navas-Acien A, Laclaustra M, Pastor-Barriuso R, Menke A, Ordovas J, et al. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003-2004. Am J Epidemiol. 2009, 169: 996–1003. 10.1093/aje/kwn414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003-2004. Atherosclerosis. 2010, 210: 643–648. 10.1016/j.atherosclerosis.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suliburska J, Bogdański P, Pupek-Musialik D, Krejpcio Z. Dietary intake and serum and hair concentrations of minerals and their relationship with serum lipids and glucose levels in hypertensive and obese patients with insulin resistance. Biol Trace Elem Res. 2011, 139: 137–150. 10.1007/s12011-010-8650-0 [DOI] [PubMed] [Google Scholar]
- 70.Faure P, Barclay D, Joyeux-Faure M, Halimi S. Comparison of the effects of zinc alone and zinc associated with selenium and vitamin E on insulin sensitivity and oxidative stress in high-fructose-fed rats. J Trace Elem Med Biol. 2007, 21:113–119. 10.1016/j.jtemb.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 71.Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr. 2009, 89: 2025S–2039S. 10.3945/ajcn.2009.27230F [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.