Summary
Cancer immunotherapy utilizing T cell checkpoint inhibitors has shown tremendous clinical success. Yet this mode of treatment is effective in only a subset of patients. Unresponsive patients tend to have non-T cell inflamed tumors that lack markers associated with the activation of adaptive anti-tumor immune responses. Notably, elimination of cancer cells by T cells is critically dependent on the optimal activity of innate immune cells. Therefore, identifying new targets that regulate innate immune cell function and promote the engagement of adaptive tumoricidal responses is likely to lead to the development of improved therapies against cancer. Here, we review the TAM receptor tyrosine kinases—TYRO3, AXL, and MERTK—as an emerging class of innate immune checkpoints that participate in key steps of anti-tumoral immunity. Namely, TAM-mediated efferocytosis, negative regulation of dendritic cell activity, and dysregulated production of chemokines collectively favor the escape of malignant cells. Hence, disabling TAM signaling may promote engagement of adaptive immunity and complement T cell checkpoint blockade.
Keywords: TYRO3, AXL, MERTK, Innate immune checkpoints
Introduction
The concept of exploiting the immune system to fight against cancer, termed immunotherapy, can be traced back to at least the 1890s and the use of Coley’s Toxins. William B. Coley was motivated to find a therapy for sarcoma patients. Based on a survey of case reports, Coley recognized a documented association between high temperature fever induced by accidental attack of erysipela or inoculations with Streptococcus erysipelatis, and the regression of sarcomas. This led him to inoculate his patients with Streptococcus erysipelatis (1). Heat or an increase in temperature is one of four classical symptoms of inflammation catalogued by Aulus Cornelius Celsus (also called the Celsus tetrad) in approximately 1st century CE. Growing knowledge of the role of the immune system, including its function during tumor progression, has enabled improved manipulation of immune cell functions for therapeutic benefit. Following Coley’s work, the immunotherapy arsenal has broadened to include cancer vaccines and genetically engineered T cells that express chimeric antigen receptors (CARs). Most recently, monoclonal antibodies (mAbs) have been used to block adaptive immune checkpoints. Specifically, administration of anti-PD1 mAbs has revealed durable tumor regression and prolonged survival in a fraction of patients with various solid tumors (2–5). A logical corollary of the success of anti-PD1 therapy has been the effort to identify and overcome the tolerance-promoting signals of novel inhibitory receptors. Accordingly, T cell checkpoints such as LAG-3 and TIGIT are being evaluated as targets of immunotherapy (Reviewed [refer to article in the same issue]). Along with this, work is underway to delineate biomarkers that can predict responsiveness to current immunotherapies.
Despite the spectacular success of anti-PD1 therapy, its efficacy is limited to only a subset of patients (4, 6, 7). Multiple studies indicate that treatment is effective in patients in which a pre-existing CD8+ T cell response is suppressed by the PD1-PDL1 axis (8–10). Hence, tumor microenvironments (TMEs) that activate, attract, and maintain CD8+ T cells favor clinical response to checkpoint inhibitors (11, 12). Notably, innate immune cells mediate the activation and recruitment of T cells. Coupled with their prominent presence in the TME, the indispensable function of innate cells in the induction and maintenance of adaptive immunity provides an impetus for investigating their role in shaping the response against cancer. Like T cells, innate immune cells also express checkpoint molecules that inhibit their activity and limit engagement of adaptive immunity. This review will focus on a family of innate immune inhibitory receptors, comprised of TYRO3, AXL and MERTK (TAMs), and will discuss their emerging role as novel candidates for checkpoint blockade.
Innate Immune Checkpoints as Targets of Cancer Immunotherapy
A specific, protective and potentially long-lasting immune response requires the effective integration of both the innate and adaptive arms of the immune system. Cells of the innate immune system play an indispensable role in the initiation and subsequent direction of the adaptive immune response. It has long been recognized that activation of a T cell mediated adaptive immune response requires integration of three signals: antigen recognition by the T cell receptor (TCR), costimulation and cytokine-induced differentiation and expansion. Cognate ligands that initiate these signals are provided by antigen presenting cells (APCs) of the innate immune system. Furthermore, inhibitory ligands present on the surface of APCs attenuate costimulation. When optimally operational, this system of checks and balances supports efficient mounting of adaptive responses. A measured immune response is dependent on appropriate engagement of inhibitory receptors, without which, tolerance to self would be lost and damage to host tissue would be sustained. Conversely, chronically sustained expression of checkpoint molecules leads to functional exhaustion and impairment of T cell responses (13, 14). During cancer progression, innate immune cells have multifarious functions in each of the rate limiting steps involved in immune targeting of tumor cells. Namely, they mediate antigen recognition, acquisition, processing and presentation to prime antigen-specific T cells as well as recruitment of T cells and production of various effector molecules. As such, any dominant inhibitory pathways that interfere with their function at any of these junctions can conceivably arrest an effective anti-tumoral response from being launched.
Malignant cells can express many antigens that can be recognized by tumor infiltrating T cells (TILs) (15–17). Neoantigens generated by somatic point mutations are thought to be ideal targets for initiating TIL activity (18, 19). Since they are not subject to central tolerance, neoantigens can be “seen” by T cells. This recognition is of course reliant on the acquisition and presentation of said antigens by major histocompatibility complexes (MHCs) on the surface of APCs. Yet, even malignancies that have a high estimated frequency of neoantigens evade killing and grow unhampered. Consideration should, however, be given to how somatic mutations in amino acids affect the ability of peptides to bind to the MHC groove and access the TCR (20). Assessing immunogenic mutation load instead of the total count of somatic mutations may be a better indicator of enhanced TIL activity and tumor killing. The generation of a spontaneous intratumoral CD8+ T cell response in some fraction of patients also supports this idea that there are indeed some immunogenic antigens present in some tumors. Bridging acquisition, processing and presentation of these antigens with TIL function are cells of the innate immune system.
Regardless of the nature of the antigens initiating innate immune cell activation, the fact remains that only subsets of patients are able to spontaneously develop CD8+ T cell responses against cancer cells. The innate immune mechanisms that promote the activation of dendritic cells (DCs) and give rise to this CD8+ T cell response largely remain enigmatic. Type I interferons (IFNs) are well-established, potent inducers of DC activity. Gene expression profiling studies conducted on human tumor biopsies have shown an association between increased type I IFN gene signature and T cell infiltration (21–24). A type I IFN signature was also generally found to be predictive of favorable clinical outcome. Ifnar1−/− (lacking type I IFN receptor signaling) or Stat1−/− mice (deficient for the downstream transcription factor required for interferon signaling) both showed significantly reduced T cell responses as well as increased tumor incidence in various ectopic models of cancer in vivo (21, 25, 26). Importantly, the source of type I IFN signaling was distilled down to APCs, specifically BATF3-dependent CD103+ or CD8α+ DCs (21, 25, 27). It was also demonstrated that IFN-β production is triggered during the acquisition of tumor-derived DNA that activates the cytosolic DNA sensing STING pathway in DCs (28). Hence, mice lacking either STING or IRF3 failed to activate their DCs, which in turn impaired T cell priming against tumors. Further support for innate immune cells’ ability to dictate anti-tumoral responses in mice comes from our knowledge of CD8α+ DC’s superior ability to acquire antigen from dying tumor cells and cross-present it to cytotoxic T cells. Work from the Reis e Sousa group has shown that this CD8α+ DC function is partly mediated by cell surface expression of a C-type lectin known as Clec9a (DNGR-1) (29, 30). DNGR-1 recognizes filamentous actin, a ligand that is readily generated by necrotic cells, including necrotic tumor cells (31).
Tumor antigen recognition, acquisition and APC activation are not the only stages in which changes in innate immune cell function can promote or blunt productive anti-tumoral responses. Poor chemokine-mediated trafficking of T cells is a key factor that favors tumor growth (32, 33). Effector T cells, guided by adhesion molecules as well as chemotactic cytokines, need to migrate into the TME to mediate tumor destruction. Local production of the chemokines CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10 has been positively correlated with the presence of CD8+ T cells (11). Effector CD8+ T cells were also found to upregulate the corresponding chemokine receptors. Since these gene expression profile analyses were performed on whole lesions, the source of chemokine production is unclear. Tumor cells, stromal cells as well as infiltrating innate immune cells could be sources of the T cell-recruiting chemokines. Therefore, failed production of specific chemokines by innate immune cells will not only be a barrier to recruiting activated cytotoxic T cells into the tumor niche, but it can also limit recruitment of other types of innate immune cells, such as CD103+ DCs that are catalysts of tumoricidal responses (34).
In addition to having chemokine profiles that do not support T cell recruitment and/or retention, innate immune cells such as macrophages (MΦ) can also produce other tumor promoting factors. These include matrix-remodeling proteins and cytokines that collectively nurture tumor cell growth (35). They can also maintain an immunosuppressive environment by metabolically dysregulating activity of T cells (36, 37).
Another interface at which innate cells have a critical input is during presentation of processed tumor antigens. Although a heterogeneous mix, myeloid cells make up the largest fraction of immune infiltrates. As an aggregate group, the myeloid fraction comprised of monocytes, tumor-associated MΦs, and DCs, presents ample machinery with potential for sufficient antigen presentation to T cells. Yet, intratumoral immature monocytes, MΦs and subsets of DCs have been shown to either inhibit or minimally stimulate CD8+ T cells that they engage (38–41). An exception to this rule has been the population of sparse, but highly potent, Batf3-dependent CD103+ DCs, described above (42, 43). In mice, these cells are able to cross-present tumor-derived antigen to stimulate both naïve and activated T cells. CD103+ DCs also maintain cytotoxic T cell function by adopting an enhanced expression of costimulatory molecules. Finally, in agreement with early studies showing the indispensable role of Batf3+ DCs in efficiently priming T cells and impeding tumor growth, recent studies in mice have confirmed that the expansion of CD103+ DCs can improve anti-PDL1 and BRAF inhibition therapies (44). Whether a similar expansion of DCs will render the same benefits in humans remains to be seen.
An alternate innate immune axis that thwarts tumor growth is the activity of natural killer (NK) cells. The ability of NK cells to kill malignant cells, in both mouse models and patient derived samples, has been well documented. NK cells distinguish malignant cells from healthy cells by detecting changes in self-molecules displayed on the surface of cells. Accordingly, tumor cells with absent or low expression of MHC-I molecules are selectively killed by NK cells (45). Similarly, ligands for the NKG2D stimulatory receptor are rarely detectable on the surface of normal tissues, but are frequently upregulated on tumor cells. This renders the latter sensitive to NK cell recognition and targeted killing. The contribution of NK cells in tumor surveillance has been highlighted in studies in which loss of NKG2D expression abrogated NK-mediated sensing and immunoediting of ligand expressing tumor cells (46, 47). In line with this, various groups have engineered and adoptively transferred chimeric NKG2D-expressing T cells to better stimulate T cell activity (48–50).
Given all the aforementioned stages at which innate immune cells play pivotal roles in initiating and maintaining responses against cancer cells, it is worthwhile to consider their utility as platforms for targeted immunotherapy. Previously, ineffective killing of transformed cells has been primarily linked to chronic engagement of adaptive checkpoints. Apart from releasing these adaptive brakes, tumor cells could be targeted by promoting activation of innate immune responses. Though recent immunotherapeutic advances have focused on reversing the exhausted state of TILs by targeting adaptive immune checkpoints, the benefit can be further enhanced by also blocking inhibitory innate immune checkpoints. There are a plethora of innate receptors that function at different phases of immunity that are worth considering as potential targets for immunotherapy. Among these innate immune checkpoints is a family of receptor tyrosine kinases (RTKs), known as the TAMs, which this review will mainly focus on. We will discuss findings in preclinical tumor models that posit these receptors as potential therapeutic targets of checkpoint inhibition.
TAM RTKs
The TAM receptor tyrosine kinases, TYRO3, AXL and MERTK have a pivotal role in homeostatic regulation of the immune system. These receptors were first identified as a distinct RTK subfamily, in a screen for RTKs expressed in Schwann cells (51). The extracellular, N-termini of the TAMs have a characteristic arrangement of two immunoglobulin-like (Ig-like) domains and two fibronectin type III domains. A hydrophobic, single pass transmembrane domain and a tyrosine kinase domain at the cytoplasmic tail follow these. The two Ig-like domains serve as the ligand-binding regions of the TAMs.
Two closely related proteins— protein S (PROS1) and growth-arrest-specific 6 (GAS6)— serve as cognate ligands that bind and activate the TAM receptors (52). Both ligands contain gamma-carboxylated glutamic acid (Gla) residues near their N-terminal domains. Gamma-carboxylation of the Gla domain enables binding to phosphatidylserine (PtdSer) (53). Maximal bioactivity of both GAS6 and PROS1 is dependent on gamma-carboxylation and PtdSer binding (54–57). Hence, these ligands function as bridges in a tripartite arrangement involving a PtdSer-exposing cell and a TAM receptor-bearing cell. Although these ligands share the same structural motifs, they differ in some of their functions and receptor specificities. PROS1 was isolated as the active component of adult bovine aortic endothelial (ABAE) cell line-conditioned media. PROS1 phosphorylated TYRO3, while ABAE-derived GAS6 was identified as an Axl agonist (52). Albeit with different affinities, GAS6 was later found to bind and activate all three TAM receptors (AXL≥TYRO3≫MER) (58). Although PROS1 was initially described as a specific agonist of TYRO3 (52), it was shown to also potently activate MERTK (59). Nevertheless, since all these studies have looked at individual receptor-agonist interactions, it remains to be discovered whether these receptors and ligands function as heterodimers. If they do, the ligand binding and activation profiles will likely differ from homodimeric settings.
TAM receptors have a broad expression pattern spanning cells of the nervous, reproductive, vascular and immune systems [see (60) for a comprehensive survey]. In both mice and humans, the TAMs are primarily expressed on APCs (such as MΦs and DCs) as well as NK cells of the immune system. Their wide expression allows the TAM receptors to undertake many different functions, including stabilization of platelet aggregates during the clotting cascade (61–63), phagocytosis of apoptotic cells (ACs) and negative regulation of inflammation. The latter two functions will be discussed in detail in subsequent sections. The first indication of an immune function for the TAMs came from receptor knock-out (KO) mice. In an effort to identify the function of MERTK, Camenisch et al. generated Mertk−/− mice (64). Compared to cultures of peritoneal WT MΦs, Mertk−/− MΦs had significantly higher production of TNF-α (64). Subsequent injection of Mertk−/− and WT mice with LPS confirmed a hyper-responsive reaction to the endotoxin in the Mertk−/− mice. Not only were the levels of pro-inflammatory cytokines higher in the KO mice, but the LD50 of LPS for Mertk−/− mice was half of that of WT mice (64). Lu et al. then generated triple KO mice (Tyro3−/−Axl−/−Mertk−/−) (65). Remarkably, these triple KOs were viable and did not appear to have any obvious defects at birth (66). However, starting as early as three weeks after birth, the germ cells in the testis and the photoreceptors of the eyes were observed to die in increasing numbers. Most strikingly, these triple KOs developed chronic inflammation and systemic autoimmunity (66). By six-eight months of age, these mice had splenomegaly, lymphadenopathy, thrombosis & hemorrhages in multiple tissues, swollen joints, skin lesions and glomerulonephritis (66). The authors also showed that the lymphoid hyperproliferation observed in the triple KO mice was due to non-cell autonomous function of the TAM RTKs. The autoimmune syndrome in these mice was a result of the loss of TAM regulation in APCs. Triple KO mice-derived APCs were, in fact, hyper-responsive to various toll-like receptor (TLR) agonists (67). Loss of TAM expression in APCs led to enhanced type I IFN production and increased CD86 and MHCII expression (67). This hyperactivation of APCs promotes engagement and subsequent hyperproliferation of B and T cells in triple KO mice. Thus, upon activation of APCs, induction of TAM RTK expression and activation results in the inhibition of inflammation (67, 68).
While this negative regulatory system is decidedly important to avoid the aberrant effects of unreined inflammation, there also needs to be a mechanism that triggers it only after innate immune cells have sufficiently activated adaptive immunity. Indeed, our group demonstrated that such an elegant feedback loop mechanism exists (69). The TAM ligand PROS1 is expressed in both mouse and human activated, but not resting, T cells (69, 70). Selective deletion of T cell-derived PROS1 enhanced APC activation and cytokine production in an antigen-specific, TAM RTK-dependent manner (69). Furthermore, in a model of T cell induced colitis, the transfer of PROS1 deficient T cells into Rag1−/− recipients accelerated colitis (69). Importantly, this mechanism is conserved in humans. In mixed lymphocyte reactions, antibody-mediated blockade of human PROS1 resulted in increased activation of human DCs (69). Overall, these data indicated that the PROS1-TAM signaling axis functions at the interface of innate and adaptive immunity to restrain the activity of innate cells and ultimately curb unwanted consequences of inflammation.
Yet another subset of cells regulated by the TAMs are NK cells (71). An NK cell’s ability to recognize and kill target cells relies on its expression of activating and inhibitory receptors. These receptors are acquired as an NK cell transitions through various stages of differentiation (72). Bone marrow-derived stromal cells release PROS1 and GAS6 to activate the TAM RTKs present on immature NK cells. In the absence of TAM receptor signaling, these immature NK cells do not acquire expression of the activating and inhibitory receptors required for late NK cell differentiation (71). Thus, while TAM receptor signaling is required for NK cell maturation, the function of the TAMs in fully differentiated NK cells is unclear. A report implicated TAM receptor signaling in NK cells in the promotion of cancer metastasis (73). Administration of a TAM kinase inhibitor significantly reduced murine melanoma and mammary cancer metastasis in an NK cell dependent fashion. This suggests that acute inhibition of TAM signaling on NK cells can boost anti-tumoral activity.
Finally, the TAM RTKs are overexpressed or ectopically expressed in many types of human cancers, including breast, colon, renal, skin, lung, liver, brain, ovarian, prostate, and thyroid malignancies (74, 75). Elevated expression of one or more TAM receptors is often associated with cancer progression, resistance to targeted therapies, and metastasis (76–78). In fact, the human orthologs of AXL and MERTK were cloned from neoplastic cells (79–81). Several studies indicate that AXL and MERTK promote the proliferation, motility and chemotaxis of tumor cells [reviewed extensively in (74)]. Currently, literature on TAM RTK signaling in cancer predominantly focuses on the function of AXL and MERTK in cancer cells. TYRO3 is understudied and as a result, much less is known about it. In a genome-wide gain-of-function cDNA screen, Zhu and colleagues identified TYRO3, as an upstream regulator of MITF, a master regulator of oncogenic survival and proliferation in malignant melanoma (82). In vitro overexpression of TYRO3 in non-tumorigenic, BRAF(V600E)-bearing, senescent primary melanocytes induced transformation of cells. Additionally, the knockdown of TYRO3 in human melanoma cells inhibited tumorigenesis when the cells were injected into nude mice (82). A very recent publication has also implicated overexpression of TYRO3 as a driving signal for increased anchorage-independent growth and motility of HCT116 colon cancer cells, in vitro (83). Enhanced TYRO3 expression was observed to induce expression of SNAI1, a master regulator of epithelial-mesenchymal transition (EMT) (83). Knocking down SNAI1 reversed the TYRO3-induced EMT signature in HCT116 cells. Addition of anti-TYRO3 antibody significantly improved drug sensitivity in primary colon cancer cells (83). Treatment of NOD/SCID mice that were xenografted with HCT116 cells with the anti-TYRO3 antibody inhibited tumor growth. Since both of these studies used immunodeficient mice to demonstrate the efficacy of inhibiting TYRO3 signaling to halt tumor growth, more experiments are needed to verify that this remains true in immune competent mice.
Mechanisms Underlying Innate Checkpoint Function of TAMs
A. Efferocytosis
Clearance of apoptotic cells (ACs) and cellular debris is critical for maintaining organismal homeostasis. It involves phagocytic clearance of dying cells, termed efferocytosis. It has long been appreciated that the TAM RTKs are critical regulators of this process (84–87). Major, non-immune mediators of efferocytosis, such as retinal pigment epithelia (RPE), sertoli cells, and mammary epithelial cells (MECs) also express MERTK (88). In fact, MERTK-dependent removal of rod outer segments by RPE, dead germ cells by Sertoli cells and secretory epithelial cells by MECs during post-lactation remodeling, was shown to be crucial for maintaining tissue homeostasis (59, 65, 89). It remains to be determined whether expression of MERTK on these stromal phagocytes contributes to tumor progression. MERTK is also expressed on tissue-resident MΦs that are professional phagocytic cells of the immune system. MERTK expression on MΦs is crucial for their phagocytic function in both healthy and injured tissues. In a model of dexamethasone-induced death of cortical thymocytes, Mertk−/− mice showed significantly increased accumulation of apoptotic thymocytes (85). The inability to clear these ACs was reversed in chimeric mice that were reconstituted with wild-type (WT) bone marrow. Additionally, injection of fluorescently labeled ACs either intravenously or into the peritoneal cavity of Mertk−/− mice demonstrated the inability of MERTK deficient splenic and peritoneal MΦs to adequately phagocytize ACs (85). Work from our laboratory also supports this notion that the TAM receptors are involved in efferocytosis of ACs generated during an inflammatory response in the colon (90). Following administration of dextran sodium sulfate (DSS), Axl−/−Mertk−/− mice accumulated higher numbers of TUNEL+Ly6G+ neutrophils in the lamina propria of their large intestine, compared to their WT counterparts. This defect in efferocytosis was traced to an AXL+MERTK+ radioresistant population of intestinal MΦs (90).
An important feature of MΦs ingesting ACs is their subsequent propensity to downregulate the generation of proinflammatory cytokines and upregulate factors associated with immunosuppression (91–96). Consistent with this, in a model of DSS-induced colitis, lamina propria MΦs from Axl−/−Mertk−/− mice showed markedly increased production of proinflammatory mediators, such as iNOS and TNF-α, and had impaired production of tissue repair factors including Relm-α, TGF-β, and IL-10 (90). This has prompted investigation of how TAM receptor mediated efferocytosis in the TME shapes anti-tumor responses. In this regard, the Earp group did pioneering work showing that Mertk ablation resulted in remarkably reduced tumor growth and metastasis in syngeneic breast, melanoma and colon cancer models (97). Bone marrow transplantation of Mertk−/−, but not WT, cells into lethally irradiated MMTV-PyVmT mice conferred reduced tumor growth. This indicated that a hematopoietic source of MERTK signaling contributed to tumor growth and metastasis. In line with this, increased levels of IL-6 and IL-12p40 as well as reduced levels of IL-10 were detected in tumor derived Mertk−/− CD11b+ cells than in Mertk+/+ CD11b+ cells (97). Additionally, depletion of CD8+ T cells favored tumor growth in Mertk−/− mice. Therefore, it was proposed that activation of MERTK in tumor-associated MΦs leads to the generation of an immunosuppressive cytokine milieu that limits CD8+ T cell expansion.
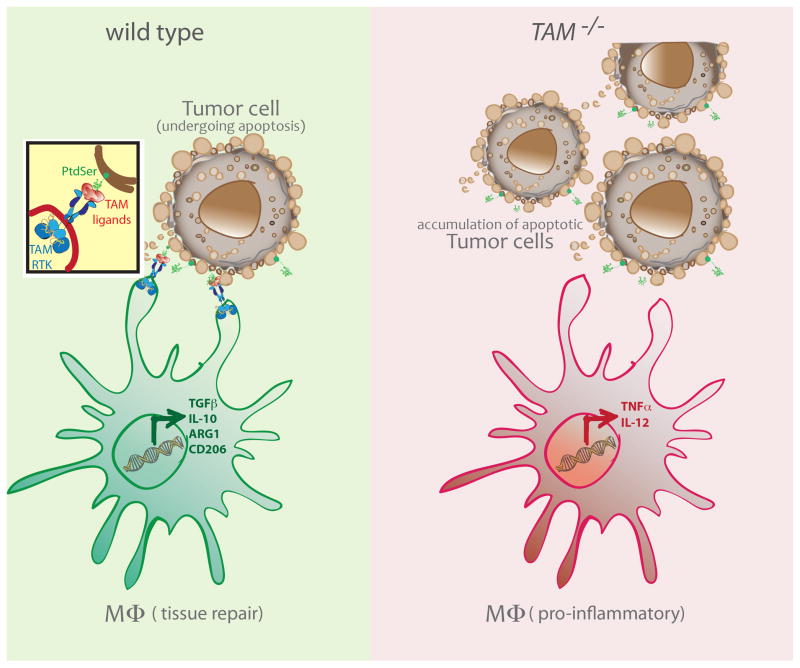
To further determine whether MERTK function nurtures tumor malignancy through efferocytosis-induced cytokine modulation, Stanford et al. utilized a postpartum cancer transplantation model (98). They found that postpartum involution increased the incidence of mammary tumor metastasis. In addition to the widespread death of milk-producing epithelial cells, tumor cells within breast tissue undergoing postpartum involution had significantly higher frequencies of death. That is, compared to tumors derived from virgin mice, the frequency of TUNEL+ tumor cells was about fourfold higher in parous mice. Yet, mice lacking MERTK expression had impaired efferocytosis and the frequency of tumor metastasis in these mice was comparable to the nulliparous group. Tumors from Mertk−/− mice also exhibited decreased levels of IL-4, IL-10 and TGFβ, which are normally enhanced in postpartum mammary tumors (98). Additionally, using spontaneous MMTV-PyVmT and allografted PyVmT tumors, it was shown that loss of MERTK resulted in substantially lower expression of arginase and CD206, which are molecular markers of MΦs that are proposed to favor tumor growth. Alternatively, tumor-bearing mice were treated daily with BMS-777607, a small molecule inhibitor targeting MERTK, for the first seven days of involution. Subsequent counts of lung tumor metastases in vehicle and BMS-77607-treated mice confirmed the remarkable sufficiency of blocking MERTK signaling to impair tumor metastasis (98). Interestingly, blocking TGF-β for the first 14 days of involution reproduced the same results. This result suggests that upstream MERTK signaling may induce TGF-β production that then interferes with the mounting of an activated CD8+ T cell response. In agreement with these findings, a very recent publication illustrated that post radiation therapy, MERTK is upregulated on tumoral CD11b+Gr1(lo)I-A+ MΦs (99). While loss of Mertk expression and radiation therapy were sufficient to limit growth of immunogenic CT26 colorectal tumors in vivo, small molecule inhibition of TGF-β (SM16) was also required to control the growth of poorly immunogenic Panc02 pancreatic tumors. In the latter context, TGF-β signaling seems to be functioning independently of the MERTK signaling pathway. Raw264.7 MΦs cultured with irradiated 4T1 cancer cells in the presence of a MER-Fc and TGF-β inhibitor induced increased TNF-α and limited IL-10 production (99). Taken together, these studies support the idea that MERTK-dependent phagocytosis of apoptotic tumor cells leads to a signaling cascade that favors tumor-promoting polarization of MΦs; these pro-tumorigenic programs augment production of immunosuppressive cytokines that aid tumor growth (Fig. 1). Whether loss of this TAM function on MΦs is sufficient to account for protection against tumor growth has yet to be determined.
Figure 1. TAM RTK-mediated efferocytosis promotes a pro-tumorigenic program in MΦs.
Apoptotic tumor cells expose PtdSer on their surfaces. TAM agonists bind to PtdSer and trigger activation of TAM RTKs on the surface of phagocytes, such as MΦs. Efferocytosis by MΦs can lead to the expression of factors like ARG1, TGF-β and IL-10 (left). In the absence of TAM RTK signaling, MΦs in the TME maintain their production of pro-inflammatory factors that could contribute to the killing of tumor cells (right).
So far, this mode of initiating a polarization of MΦs downstream of efferocytosis that influences tumor progression is uniquely ascribed to the TAM family of receptors. Notably, there are other efferocytic receptors that are co-expressed with the TAMs and known to modulate the immune response against tumor cells. Two such receptors are TIM-4 and αvβ5 integrin. TIM-4 is a PtdSer receptor that is exclusively expressed on APCs to facilitate engulfment of ACs. Monoclonal antibody based targeting of TIM-4 augments the efficacy of irradiated B16 melanoma vaccines against established tumors [reviewed in (100)]. However, TIM-4’s overall role in the response against tumor development remains largely obscure. Since TIM-4 lacks an extensive intracellular domain, it requires cooperation with MERTK to facilitate phagocytosis of ACs (101); in the absence of MERTK expression, TIM-4 is only able to tether ACs without engulfing them. The TIM4 domain that facilitates this interaction with MERTK has not been mapped yet. Another efferocytic receptor that has been reported to interact with MERTK is the αvβ5 integrin. αvβ5 functions with MERTK to promote engulfment of ACs in HEK293T and RPE cells (102, 103). MΦs use αvβ5 to bind MFG-E8, which in turn recognizes PtdSer on the surface of ACs. In combination with chemotherapy, targeted blocking of MFG-E8 results in an improved DC cross-presentation of dying tumor cells, decreased infiltration with Foxp3+ T reg cells, increased TIL production of IFN-γ and enhanced generation of inflammatory cytokines (IL-12, IL-23 and TNF-α) by APCs (104).
Importantly, all the aforementioned phagocytic receptors recognize PtdSer either directly or indirectly through their ligands. Relevant to this, therapeutic antibodies that bind exposed PtdSer and block recognition of dying cells by phagocytes have been developed (105). In mice, antibodies against PtdSer significantly enhance benefits derived from radiation, chemotherapy and anti-PD1 therapy (106–108). It is thought that anti-PtdSer therapy promotes tumor killing by favoring the polarization towards inflammatory MΦs, decreasing the presence of myeloid-derived suppressor cells, and enhancing recruitment of mature DCs into the TME (109). Nevertheless, key phagocytic pathways underlying the action of the anti-PtdSer mAb remain enigmatic. PtdSer targeting would not only inhibit efferocytosis of dying tumor cells but it could also interfere with the acquisition of cargo by professional APCs. It would indiscriminately also block all the PtdSer-dependent phagocytic receptors. This sort of blanket PtdSer blocking approach is likely to cause other harmful side effects on the host. Notably, a Phase III trial evaluating the efficacy of combining the anti-PtdSer mAb, bavituximab, with chemotherapy was discontinued as, compared to the control group, the addition of bavituximab did not result in improved survival (110). Thus it is probably better to specifically disable pathways like MERTK/GAS-6/PROS1 to derive the anti-tumorigenic activity of anti-PtdSer targeting. Unlike many other efferocytic receptors, MERTK is universally expressed on tissue-resident MΦs(111), has the capacity to transduce signals that favor tumor promoting polarization of MΦs, and can complex with other phagocytic receptors to direct macrophage function.
It is also entirely possible that the TAM receptors mediate efferocytosis through PtdSer-independent mechanisms. In addition to the cognate agonists PROS1 and GAS6, the “eat-me” signals galectin-3 (Gal-3), tubby, and tubby-like protein 1 (Tulp1) were identified as ligands of MERTK that facilitate phagocytosis of ACs (112, 113). While Tulp1 binds to all three TAM RTKs, Gal-3 and tubby exclusively bind to MERTK (112, 113). Caberoy et al. also found that tubby and Tulp 1 interact with MERTK through an N-terminal domain and bind dead cells through their C-terminal phagocytosis prey-binding domain (PPBD). This tubby domain has previously been shown to bind to PtdIns(4,5)P2, but not PtdSer (114). Similarly, the C-terminal PPBD of Gal-3 is preferentially bound by N-glycans of glycoproteins (115). Consequently, lactose addition partially reduced GAL-3 mediated phagocytosis of ACs and RPE cells (112). Although Gal-3, tubby and Tulp1 were shown to interact with MERTK, induce its autophosphorylation, and bridge its interaction with ACs to stimulate phagocytosis, crystal structures showing their direct interaction with the receptor have not been obtained to date.
Together, these studies demonstrate that MERTK is a critical component of the efferocytosis machinery and that the inhibition of pathways involved in the clearance of ACs might enhance anti-tumorigenic responses. Yet, there are reports of tumor clearance and prolonged survival following blockade of the “don’t eat me” signal, CD47. How can these seemingly conflicting results be reconciled? CD47 expression on live tumor cells allows them to engage SIRP-α on MΦs and avoid phagocytosis. Therefore antibodies against CD47 promote phagocytosis of tumor cells. Interestingly, the therapeutic effects of anti-CD47 mAbs aren’t strictly derived from increasing MΦ-mediated efferocytosis of dying tumor cells. Anti-CD47 response is dependent on CD8+ T cells (116). CD47 blockade enhances STING-dependent sensing of tumor-derived cytosolic DNA in dendritic cells that are able to efficiently cross-prime CTLs (116). This raises the distinct possibility that a similar mechanism may be at work downstream of the TAM receptors. This alternate mechanism of TAM checkpoint function is discussed in the following section.
B. Negative Regulation of DC function
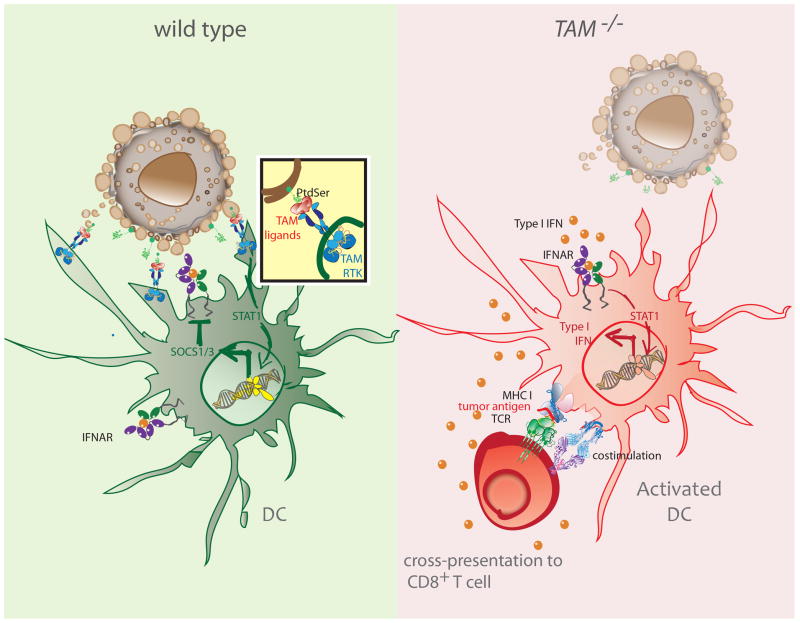
TLR engagement triggers proinflammatory cytokine production and significantly upregulates TAM RTK expression on BMDCs, in vitro. Addition of recombinant TAM ligands PROS1 and GAS6 was also shown to suppress activation of DCs and shut down cytokine production (67). Specifically, signals downstream of TLR engagement led to the production of type I IFNs, which in turn induced Axl mRNA upregulation (67). Once activated, TAM signaling triggered expression of the suppressor of cytokine signaling proteins, SOCS1 & SOCS3 (67). In the absence of TAM signaling in Tyro3−/−Axl−/−Mertk−/− DCs, the induction of SOCS1 was substantially impaired. The SOCS proteins function by degrading adaptor molecules involved in the TLR & type I IFN signaling cascades, including MAL, TRAF3/6, and molecules of the JAK-STAT pathways (67). AXL usurped the initially proinflammatory IFNAR-STAT1 signaling pathway to initiate activation of the SOCS-mediated negative regulatory axis. Consistent with this, type I IFNR and STAT1 were required for AXL-dependent upregulation of the Socs genes (67). While this rapid mechanism of co-opting the type I IFN receptor is a critical safeguard against the aberrant consequences of uncontrolled inflammation, application of such a brake in the TME can foster a tolerogenic function of DCs that stunts tumoricidal responses (Fig. 2).
Figure 2. TAM signaling inhibits activation of intratumoral DCs.
Type I IFN signaling leads to an IFNAR/STAT1 dependent induction of the TAM receptor AXL. Once engaged, the TAM receptor signaling pathway co-opts IFNAR and STAT1 to induce expression of SOCS1 and SOCS3, which suppress the type I IFN cascade and foster tolerogenic function of DCs (left). In the absence of TAM signaling, the induction of SOCS proteins is impaired. Enhanced production of type I IFNs is expected to promote cross-presentation of tumor antigens and upregulation of costimulatory molecules (right).
Similar to how most viral infections lead to downstream secretion of type I IFNs, detection of tumor-derived DNA by the cytosolic sensor STING stimulates type I IFN production in tumor-infiltrating DCs (TIDCs) (21, 28). In fact, type I IFN signaling is required in Batf3+ CD8α+/CD103+ DCs for immune rejection of tumors (25, 27). Mouse allograft tumor models were used to demonstrate that only STING and IRF3 were indispensible to host IFN-β production and subsequent control of tumor growth (28). In contrast, loss of signaling through TLRs and the cytosolic RNA-sensing proteins (MAVS) did not affect spontaneous priming of T cells. STING activation thus promotes cross-priming of tumor antigen-specific CTLs [extensively reviewed in (117)].
In patient samples, the presence of T cell-associated transcripts positively correlates with the expression of genes involved in type I IFN signaling (11). Therefore, mechanisms that interfere with robust type I IFN signaling pose a threat against the optimal activation of DCs and ultimately priming and maintaining T cells. As mentioned above, the TAMs have the ability to couple with and redirect type I IFN receptor signaling for maintenance of a tolerogenic environment. However, TAM RTK function in this capacity within the TME remains to be demonstrated. If true, increased TAM expression would be expected in patient cohorts with non-T cell inflamed tumors that have a low type I IFN signature. Of note, AXL was significantly enriched in anti-PD1-resistant melanoma tumors that were sampled before therapy (118). While this study does not allow for direct and simultaneous evaluation of innate immune TAM RTK function, type I IFN signaling and T cell activity in the TME, it certainly encourages such an investigation.
It is of course possible that the loss of the TAM RTKs promotes accumulation of immunogenic apoptotic tumor cells and debris that are then picked up by cross-presenting DCs. Another phagocytic receptor on TIDCs, TIM-3, negatively regulates innate immune response to tumor-derived nucleic acids, in vivo (119). Both mouse and patient TIM-3+ TIDCs expressed lower IFN-β1 & IL-12 mRNA. Treatment of these cells with an anti-TIM3 antibody significantly upregulated IFN-β and IL-12 production. The authors also reported that TIM-3 did not inhibit IFN-β production in HEK293 cells that were transfected with vectors expressing STING. This suggested that the TIM-3 nucleic acid detection system was upstream of the cytosolic sensor. TIM-3-mediated sensing of DNA was also independent of its recognition of AC-derived PtdSer (119). Instead, TIM-3 competitively binds to the alarmin HMGB1 protein with an affinity similar to that of its other known receptors (119). Nucleic acid entrance into endosomal vesicles requires complex formation with HMGB1. Therefore, TIM-3’s interaction with HMGB1 interferes with the proper trafficking of the nucleic acid cargo into DC endosomes (119). Hence, TIM-3 can restrain anti-tumor responses by hindering HMGB1-mediated stimulation of nucleic acid sensing. A similar TAM-driven mechanism could also be functioning to limit innate immune response to tumor-derived nucleic acids.
C. Reprogramming of Migratory Cues
Successful recruitment and retention of naïve and effector T cells in any tissue, including the TME, requires production of various chemokine molecules by innate and stromal cells. Not surprisingly, melanoma patient samples that have T cells present also express transcripts of chemokines related to T cell recruitment. In sharp contrast, samples with limited T cell infiltration lacked expression of many chemokines including CCL2, CCL3, CCL4, CCL5, CXCL9 and CXCL10 (11, 120). Inability to produce these chemokines can be a barrier to effective T cell-driven killing of tumor cells. Indeed, in mouse models, transplantation of melanoma cells expressing these T cell attracting chemokines resulted in CD8+ T cell recruitment into tumors (11). Nonetheless, indiscriminate upregulation of T cell attractant chemokines may actually aid tumor progression. In cervical cancer and endometroid adenocarcinoma patients, high concentration of CXCL12 has been correlated with the detrimental accumulation of Foxp3+ Treg cells (121, 122). In fact, the level of CXCL12 has been used as a predictive determinant of survival in patients with primary ovarian cancer (123).
Failed T cell recruitment into tumors may be due to a diminished presence of chemokine secreting cells and/or activation of molecular pathways that inhibit the production and secretion of T cell recruiting chemokines. Alternatively, the inhibitory axis can also function indirectly by limiting production of a chemokine that attracts other innate cells, which in turn are themselves the producers of T cell guiding molecules. Although observed in a Th2 context, our group has found that ablation of TYRO3 RTK signaling on PDL2+ DCs led to a potent induction of factors that are chemotactic for T cells (68). Specifically, mice that were previously sensitized with OVA/papain were injected with either OVA-loaded WT or Tyro3−/− DCs. Four days later, when the T cell response was analyzed, the transfer of TYRO3-deficient DCs resulted in a significantly enhanced accumulation of T cells and increased local concentration of the T cell-recruiting chemokines CCL17 and CCL5 (68). Currently, an analogous TAM-based regulation of chemokine production has not been reported in innate immune cells of the TME.
Of interest, a similar function of reprogramming migratory cues has indeed been described for the TAMs in a non-immune context. MERTK knockdown in breast cancer cells non-cell-autonomously inhibited metastatic endothelial cell migration (124). Loss of expression of the microRNA, miR-126, in breast cancer cells increased MERTK expression (124). The subsequent increase in MERTK signaling on tumor cells resulted in cleavage of its extracellular domain. The soluble form of the receptor then bound GAS6 and acted as a ligand “sink” (124). This GAS6 sequestration prevented MERTK signaling on endothelial cells. In the absence of miR-126, HUVEC cells pre-treated with MER-Fc antibody had substantially higher migration rates than control cells, in vitro (124). Additionally, incubation of cells in the presence of GAS6 significantly impaired the migration capacity of the endothelial cells. However, to date, the identity of the chemotactic factors produced in the absence of MERTK signaling remains elusive.
Overall, TAM RTKs act at key rate-limiting steps of innate immune cell function, thus rendering APCs tolerogenic and ultimately thwarting tumoricidal CTL function. TAM-driven initiation of pro-oncogenic programs downstream of efferocytic MΦs, inhibition of DC activation, and prevention of the production of T cell-attractant chemokines all together favor the escape of malignant cells. Hence, disabling TAM signaling may promote engagement of adaptive immunity in the TME (Fig. 3).
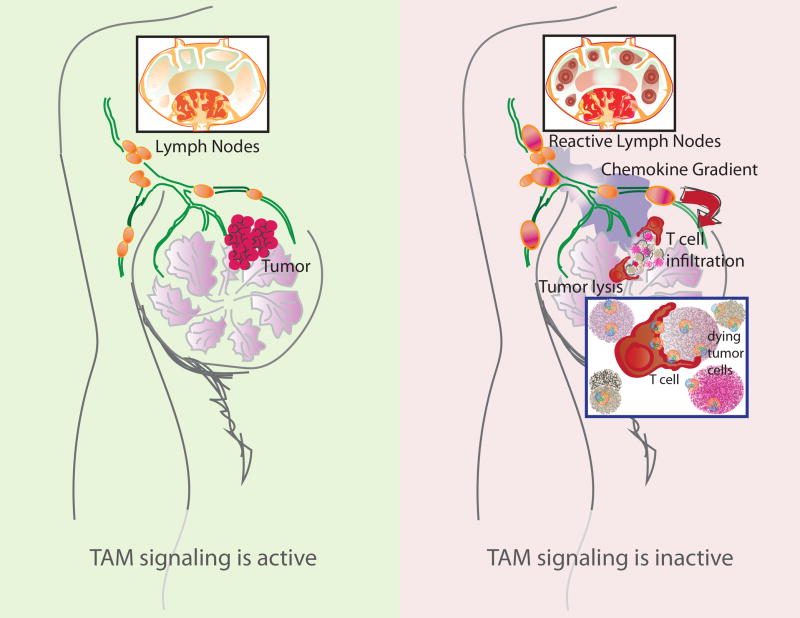
Figure 3. Loss of TAM RTK signaling favors cytolytic killing of tumor cells.
TAM signaling on TIDCs is thought to limit the generation of chemokines that are needed for the recruitment of T cells into the TME (left). In the absence of TAM signaling, TIDCs are expected to produce increased amounts of T cell-attractant chemokines. This results in the enhanced recruitment of T cells from tissue draining LNs. Concomitantly, the accumulation of apoptotic tumor cells (Fig. 1), production of pro-inflammatory factors (Fig. 1), and increased activation of DCs (Fig. 2) are hypothesized to favor the engagement of TILs. These, together with an increased recruitment of TILs to the TME promote the effective killing of tumor cells (right).
TAMs as Therapeutic Targets
Current checkpoint inhibitors show great promise in delivering long-lasting anti-tumoral immunity (3, 125, 126). Narrow objective response rates remain to be the primary limitation of these treatments. In order to circumvent this problem, concurrent treatments with multiple agents have been implemented. For instance, patients with follicular lymphoma that were simultaneously treated with rituximab and anti-PD1 mAb showed an objective response rate of 66% (127). While this is a remarkable option for a subset of patients, there remain a large fraction of patients whose effective options are limited by the unresponsiveness of their tumors to existing immunotherapies. As mentioned earlier, this failure to respond to checkpoint inhibitors is most commonly associated with a lack of an intratumoral T cell response. Therefore, targeting novel inhibitory pathways that will overcome the failure to engage T cells is critically important. Ideally, these candidate pathways would be easily targetable and disabling them would result in little or no collateral damage to the host.
Inhibition of these RTKs could be achieved through the use of small molecule inhibitors, ligand traps and other biologics. With rising interest in targeting the TAM receptors for cancer therapy, a number of TAM small molecule inhibitors are in development. Notably, the AXL inhibitor BGB324 (also known as R428) has been shown to significantly reduce the growth and migration capacity of various tumor cell lines, in vitro, and decrease angiogenesis and metastasis in orthotropic xenografts of breast cancer (128–133). Following these pre-clinical observations, a Phase 1a trial was completed. In mice, it has been reported that activation of AXL causes resistance to EGFR-targeted tyrosine kinase inhibitors (TKIs), such as erlotinib (134). Sensitivity to erlotinib was restored upon genetic loss or pharmacological inhibition of AXL. Furthermore, EGFR-mutant lung cancer samples obtained from patients with acquired resistance to TKIs showed increased expression of AXL (134). A Phase 1b study of BGB324 in erlotinib-sensitive and refractory patients with stage III and IV non-small cell lung cancer (NSCLC) is underway. Similarly, several MERTK-specific inhibitors are being developed (135–138). In mouse models of NSCLC, the small molecule inhibitor UNC2025 was demonstrated to significantly inhibit tumor growth. Using multiple NSCLC lines harboring different oncogenic mutations, including KRAS and EGFR mutations, it was shown that treatment with UNC2025 was sufficient to induce death of tumor cells (135). Of note, current MERTK inhibitors in development including UNC2025 and UNC1666 have other targets such as FLT3 (74). While this may not obstruct, and instead even support, the anti-tumoral activity of the inhibitor in some settings (136), use of more selective agents may be necessary to minimize therapy-associated side effects. Ligand traps and mAbs may present such an alternative. A variant of the AXL extracellular domain that was engineered to have very high affinity for GAS6 functioned as a sink for the ligand (139). This allowed exclusive binding and sequestering of GAS6 but not PROS1 (139). Similarly, when the soluble ligand-binding domain of MERTK was used as a decoy receptor for GAS6 (140), it led to defective engulfment of ACs by MΦs. Additionally, several monoclonal antibodies that specifically target each of the receptors have been described (83, 131, 141–145).
Foremost, TAM RTK expression on tumor cells can drive oncogenic survival and transformation. Additionally, their manifold activities on innate immune cells can support immune subversion. Finally, the TAMs, being RTKs, are ideal targets for pharmacological intervention and disabling their function will likely prove efficacious. As promising a target as they are though, caution should be taken in determining which settings are ideal for ablating their RTK function to derive anti-tumorigenic activity. An anti-oncogenic role for TAM signaling has been reported in models of inflammation-induced colon carcinoma. A study from our laboratory showed that following treatment with AOM-DSS, Axl−/−Mer−/− mice had larger, more numerous polyps as well as higher colonoscopic tumor scores compared to WT mice (90). Treatment with DSS alone caused Axl−/−Mer−/− mice to exhibit severe colonic inflammation, loss of body weight, ulceration, crypt hyperplasia, crypt loss, edema, and leukocyte infiltration (90). Consistent with the innate immune signal inhibiting function of the TAM RTKs, lamina propria MΦs from Axl−/−Mer−/− mice produced substantially more proinflammatory mediators and failed to generate adequate amounts of factors associated with tissue repair. Simultaneously, an independent study by a different group, also utilizing the AOM-DSS model, showed that Gas6−/− mice had increased susceptibility to colon cancer and markedly reduced survival (146). Gas6−/− mice had a higher frequency of PCNA- and c-Myc-positive polyps, increased NF-κB activation, and higher production of TNF-α. This increased tendency to succumb to intestinal adenomas in Gas6−/− mice was confirmed in an ApcMin model of colon cancer.
These observations imply that the outcome of TAM RTK inhibition can vastly differ depending on the amount of inflammation, the nature of TAM-expressing cells present and stage of tumorigenesis/tumor progression. Additional studies utilizing TAM inhibitors will be essential to better determine how interfering with TAM signaling shapes tumor killing. Comprehensive studies that evaluate the long-term effects of systemically blocking these RTKs are also needed to determine how disarming these receptors affects their homeostatic functions in extratumoral sites. Since genetic ablation of Mertk results in a progressive degeneration of photoreceptors (66), it will be important to assess if use of TAM RTK inhibitors in preclinical models will cause visual impairment. Homozygous mutation in MERTK is known to cause retinal pigmentosa (147). Two large-scale studies have shown that ~3% of cases are attributable to MERTK mutations (148, 149). However, no reports evaluating the effect of administering MERTK inhibitors on photoreceptors, either in mice or humans, have been published to date. Future studies utilizing MERTK targeted therapies will likely benefit from avoiding drug exposure in the eye. These are all important factors to consider while evaluating the blockade of TAM RTKs for cancer treatment. Evidence-based targeting of these innate immune checkpoints may enable recruitment and activation of T cells in patient cohorts that are otherwise unable to derive any benefit from existing immunotherapy regiments.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH-NIAID R01 AI089824 to C.V.R. and S.G.), Alliance for Lupus Research (C.V.R.), Yale Melanoma SPORE (Career Developmental award to C.V.R.), Yale Cancer Center (Pilot award to C.V.R.), Yale Immunobiology Department (Training grant to Y.T.A.), and Richard K. Gershon Fellowship (Y.T.A). C.V.R is a HHMI Faculty Scholar.
The authors thank Sagie Wagage and Lindsey Hughes for critical reading of this article.
Footnotes
Conflict of Interest:
The authors disclose that there is a potential conflict of interest as C.V.R. is a shareholder of Kolltan Pharmaceuticals and a member of the science advisory board at Surface Oncology.
References
- 1.Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–64. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 2.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. New Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. New Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. New Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. New Engl J Med. 2013;369(2):134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 8.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 10.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69(7):3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji RR, Chasalow SD, Wang LS, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immun. 2012;61(7):1019–31. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahan SM, Wherry EJ, Zajac AJ. T cell exhaustion during persistent viral infections. Virology. 2015;479–480:180–93. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature Reviews Immunology. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brichard VG, Lejeune D. GSK’s antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25(Suppl 2):B61–71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Dang N, Wang X, et al. Recognition of shared melanoma antigens in association with major HLA-A alleles by tumor infiltrating T lymphocytes from 123 patients with melanoma. J Immunother. 2000;23(1):17–27. doi: 10.1097/00002371-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins PF, Lu YC, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19(6):747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 20.Yadav M, Jhunjhunwala S, Phung QT, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 21.Fuertes MB, Kacha AK, Kline J, et al. Host type I IFN signals are required for antitumor CD8(+) T cell responses through CD8 alpha(+) dendritic cells. J Exp Med. 2011;208(10):2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascierto ML, Kmieciak M, Idowu MO, et al. A signature of immune function genes associated with recurrence-free survival in breast cancer patients. Breast Cancer Res Treat. 2012;131(3):871–80. doi: 10.1007/s10549-011-1470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertucci F, Ueno NT, Finetti P, et al. Gene expression profiles of inflammatory breast cancer: correlation with response to neoadjuvant chemotherapy and metastasis-free survival. Ann Oncol. 2014;25(2):358–65. doi: 10.1093/annonc/mdt496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callari M, Musella V, Di Buduo E, et al. Subtype-dependent prognostic relevance of an interferon-induced pathway metagene in node-negative breast cancer. Mol Oncol. 2014;8(7):1278–89. doi: 10.1016/j.molonc.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn GP, Bruce AT, Sheehan KC, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6(7):722–9. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 27.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woo SR, Fuertes MB, Corrales L, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sancho D, Joffre OP, Keller AM, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458(7240):899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sancho D, Mourao-Sa D, Joffre OP, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118(6):2098–110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahrens S, Zelenay S, Sancho D, et al. F-Actin Is an Evolutionarily Conserved Damage-Associated Molecular Pattern Recognized by DNGR-1, a Receptor for Dead Cells. Immunity. 2012;36(4):635–45. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 33.Mlecnik B, Tosolini M, Charoentong P, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138(4):1429–40. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 35.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray PJ. Amino acid auxotrophy as a system of immunological control nodes. Nature Immunology. 2016;17(2):132–9. doi: 10.1038/ni.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015;36(4):257–64. doi: 10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boissonnas A, Licata F, Poupel L, et al. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network. Neoplasia. 2013;15(1):85–94. doi: 10.1593/neo.121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelhardt JJ, Boldajipour B, Beemiller P, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21(3):402–17. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175(7):4583–92. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broz ML, Binnewies M, Boldajipour B, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26(5):638–52. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts EW, Broz ML, Binnewies M, et al. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 2016;30(2):324–36. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salmon H, Idoyaga J, Rahman A, et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity. 2016;44(4):924–38. doi: 10.1016/j.immuni.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27(45):5932–43. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 46.Guerra N, Tan YX, Joncker NT, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–80. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202(5):583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barber A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment (vol 183, pg 6939, 2009) Journal of Immunology. 2014;193(3):1513. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear P, Barber A, Rynda-Apple A, Sentman CL. NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol. 2013;91(6):435–40. doi: 10.1038/icb.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106(5):1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai C, Lemke G. An Extended Family of Protein-Tyrosine Kinase Genes Differentially Expressed in the Vertebrate Nervous-System. Neuron. 1991;6(5):691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 52.Stitt TN, Conn G, Gore M, et al. The Anticoagulation Factor Protein-S and Its Relative, Gas6, Are Ligands for the Tyro 3/Axl Family of Receptor Tyrosine Kinases. Cell. 1995;80(4):661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 53.Huang MD, Rigby AC, Morelli X, et al. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat Struct Biol. 2003;10(9):751–6. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 54.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu HJ, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nature Immunology. 2003;4(1):87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 55.Benzakour O, Kanthou C. The anticoagulant factor, protein S, is produced by cultured human vascular smooth muscle cells and its expression is up-regulated by thrombin. Blood. 2000;95(6):2008–14. [PubMed] [Google Scholar]
- 56.Hasanbasic I, Rajotte I, Blostein M. The role of gamma-carboxylation in the anti-apoptotic function of gas6. J Thromb Haemost. 2005;3(12):2790–7. doi: 10.1111/j.1538-7836.2005.01662.x. [DOI] [PubMed] [Google Scholar]
- 57.Nakano T, Kawamoto K, Kishino J, Nomura K, Higashino K, Arita H. Requirement of gamma-carboxyglutamic acid residues for the biological activity of Gas6: Contribution of endogenous Gas6 to the proliferation of vascular smooth muscle cells. Biochem J. 1997;323:387–92. doi: 10.1042/bj3230387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata K, Ohashi K, Nakano T, et al. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271(47):30022–7. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 59.Prasad D, Rothlin CV, Burrola P, et al. TAM receptor function in the retinal pigment epithelium. Mol Cell Neurosci. 2006;33(1):96–108. doi: 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM Receptor Signaling in Immune Homeostasis. Annu Rev Immunol. 2015;33:355–91. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angelillo-Scherrer A, Burnier L, Flores N, et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. Journal of Clinical Investigation. 2005;115(2):237–46. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angelillo-Scherrer A, de Frutos P, Aparicio C, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7(2):215–21. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 63.Cosemans JMEM, Van Kruchten R, Olieslagers S, et al. Potentiating role of Gas6 and Tyro3, Axl and Mer (TAM) receptors in human and murine platelet activation and thrombus stabilization. J Thromb Haemost. 2010;8(8):1797–808. doi: 10.1111/j.1538-7836.2010.03935.x. [DOI] [PubMed] [Google Scholar]
- 64.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. Journal of Immunology. 1999;162(6):3498–503. [PubMed] [Google Scholar]
- 65.Lu Q, Gore M, Zhang Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–8. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 66.Lu QX, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 67.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MBA, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–36. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 68.Chan PY, Silva EAC, De Kouchkovsky D, et al. The TAM family receptor tyrosine kinase TYRO3 is a negative regulator of type 2 immunity. Science. 2016;352(6281):99–103. doi: 10.1126/science.aaf1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silva EAC, Chan PY, Joannas L, et al. T Cell-Derived Protein S Engages TAM Receptor Signaling in Dendritic Cells to Control the Magnitude of the Immune Response. Immunity. 2013;39(1):160–70. doi: 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smiley ST, Boyer SN, Heeb MJ, Griffin JH, Grusby MJ. Protein S is inducible by interleukin 4 in T cells and inhibits lymphoid cell procoagulant activity. P Natl Acad Sci USA. 1997;94(21):11484–9. doi: 10.1073/pnas.94.21.11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caraux A, Lu QX, Fernandez N, et al. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nature Immunology. 2006;7(7):747–54. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 72.Lanier LL. NK cell recognition. Annual Review of Immunology. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 73.Paolino M, Choidas A, Wallner S, et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507(7493):508–12. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Graham DK, DeRyckere D, Davies KD, Earp HS. The TAM family: phosphatidylserine-sensing receptor tyrosine kinases gone awry in cancer. Nature Reviews Cancer. 2014;14(12):769–85. doi: 10.1038/nrc3847. [DOI] [PubMed] [Google Scholar]
- 75.Linger RMA, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Debruyne DN, Bhatnagar N, Sharma B, et al. ALK inhibitor resistance in ALK(F1174L)-driven neuroblastoma is associated with AXL activation and induction of EMT. Oncogene. 2016;35(28):3681–91. doi: 10.1038/onc.2015.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giles KM, Kalinowski FC, Candy PA, et al. Axl Mediates Acquired Resistance of Head and Neck Cancer Cells to the Epidermal Growth Factor Receptor Inhibitor Erlotinib. Mol Cancer Ther. 2013;12(11):2541–58. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- 78.Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The Receptor AXL Diversifies EGFR Signaling and Limits the Response to EGFR-Targeted Inhibitors in Triple-Negative Breast Cancer Cells. Sci Signal. 2013;6(287) doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and Messenger-Rna Expression Analysis of a Novel Human Protooncogene, C-Mer. Cell Growth Differ. 1994;5(6):647–57. [PubMed] [Google Scholar]
- 80.Janssen JWG, Schulz AS, Steenvoorden ACM, et al. A Novel Putative Tyrosine Kinase Receptor with Oncogenic Potential. Oncogene. 1991;6(11):2113–20. [PubMed] [Google Scholar]
- 81.Obryan JP, Frye RA, Cogswell PC, et al. Axl, a Transforming Gene Isolated from Primary Human Myeloid-Leukemia Cells, Encodes a Novel Receptor Tyrosine Kinase. Molecular and Cellular Biology. 1991;11(10):5016–31. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu S, Wurdak H, Wang Y, et al. A genomic screen identifies TYRO3 as a MITF regulator in melanoma. Proc Natl Acad Sci U S A. 2009;106(40):17025–30. doi: 10.1073/pnas.0909292106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chien CW, Hou PC, Wu HC, et al. Targeting TYRO3 inhibits epithelial-mesenchymal transition and increases drug sensitivity in colon cancer. Oncogene. 2016 doi: 10.1038/onc.2016.120. [DOI] [PubMed] [Google Scholar]
- 84.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15(2):243–50. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 85.Scott RS, McMahon EJ, Pop SM, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–11. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 86.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. Journal of Immunology. 2007;178(9):5635–42. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 87.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur J Immunol. 2011;41(9):2515–8. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5(11):a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandahl M, Hunter DM, Strunk KE, Earp HS, Cook RS. Epithelial cell-directed efferocytosis in the post-partum mammary gland is necessary for tissue homeostasis and future lactation. BMC Dev Biol. 2010;10:122. doi: 10.1186/1471-213X-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bosurgi L, Bernink JH, Delgado Cuevas V, et al. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci U S A. 2013;110(32):13091–6. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. Journal of Clinical Investigation. 1998;101(4):890–8. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Filardy AA, Pires DR, Nunes MP, et al. Proinflammatory Clearance of Apoptotic Neutrophils Induces an IL-12(low)IL-10(high) Regulatory Phenotype in Macrophages. Journal of Immunology. 2010;185(4):2044–50. doi: 10.4049/jimmunol.1000017. [DOI] [PubMed] [Google Scholar]
- 93.Huynh MLN, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta 1 secretion and the resolution of inflammation. Journal of Clinical Investigation. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tibrewal N, Wu Y, D’mello V, et al. Autophosphorylation docking site tyr-867 in mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappa B transcriptional activation. J Biol Chem. 2008;283(6):3618–27. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 95.Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc T. 1998;26(4):653–6. doi: 10.1042/bst0260653. [DOI] [PubMed] [Google Scholar]
- 96.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281(50):38376–84. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 97.Cook RS, Jacobsen KM, Wofford AM, et al. MerTK inhibition in tumor leukocytes decreases tumor growth and metastasis. Journal of Clinical Investigation. 2013;123(8):3231–42. doi: 10.1172/JCI67655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stanford JC, Young C, Hicks D, et al. Efferocytosis produces a prometastatic landscape during postpartum mammary gland involution. Journal of Clinical Investigation. 2014;124(11):4737–52. doi: 10.1172/JCI76375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crittenden MR, Baird J, Friedman D, et al. Mertk on tumor macrophages is a therapeutic target to prevent tumor recurrence following radiation therapy. Oncotarget. 2016 doi: 10.18632/oncotarget.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baghdadi M, Nagao H, Yoshiyama H, et al. Combined blockade of TIM-3 and TIM-4 augments cancer vaccine efficacy against established melanomas. Cancer Immunol Immun. 2013;62(4):629–37. doi: 10.1007/s00262-012-1371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishi C, Toda S, Segawa K, Nagata S. Tim4- and MerTK-mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol Cell Biol. 2014;34(8):1512–20. doi: 10.1128/MCB.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Finnemann SC, Nandrot EE. MerTK activation during RPE phagocytosis in vivo requires alpha v beta 5 integrin. Adv Exp Med Biol. 2006;572:499–503. doi: 10.1007/0-387-32442-9_69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in alpha v beta 5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118(3):539–53. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 104.Jinushi M, Sato M, Kanamoto A, et al. Milk fat globule epidermal growth factor-8 blockade triggers tumor destruction through coordinated cell-autonomous and immune-mediated mechanisms. J Exp Med. 2009;206(6):1317–26. doi: 10.1084/jem.20082614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ran S, He J, Huang XM, Soares M, Scothorn D, Thorpe PE. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin Cancer Res. 2005;11(4):1551–62. doi: 10.1158/1078-0432.CCR-04-1645. [DOI] [PubMed] [Google Scholar]
- 106.Gray MJ, Gong J, Hatch MMS, et al. Phosphatidylserine-targeting antibodies augment the anti-tumorigenic activity of anti-PD-1 therapy by enhancing immune activation and downregulating pro-oncogenic factors induced by T-cell checkpoint inhibition in murine triple-negative breast cancers. Breast Cancer Res. 2016:18. doi: 10.1186/s13058-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.He J, Yin Y, Luster TA, Watkins L, Thorpe PE. Antiphosphatidylserine Antibody Combined with Irradiation Damages Tumor Blood Vessels and Induces Tumor Immunity in a Rat Model of Glioblastoma. Clin Cancer Res. 2009;15(22):6871–80. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 108.Huang XM, Bennett M, Thorpe PE. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Research. 2005;65(10):4408–16. doi: 10.1158/0008-5472.CAN-05-0031. [DOI] [PubMed] [Google Scholar]
- 109.Yin Y, Huang XM, Lynn KD, Thorpe PE. Phosphatidylserine-Targeting Antibody Induces M1 Macrophage Polarization and Promotes Myeloid-Derived Suppressor Cell Differentiation. Cancer Immunology Research. 2013;1(4):256–68. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 110.Peregrine Pharmaceuticals. Peregrine Pharmaceuticals Provides Update on Phase III SUNRISE Trial of Bavituximab. 2016 Available from: http://ir.peregrineinc.com/releasedetail.cfm?releaseid=957281.
- 111.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology. 2012;13(11):1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caberoy NB, Alvarado G, Bigcas JL, Li W. Galectin-3 is a new MerTK-specific eat-me signal. J Cell Physiol. 2012;227(2):401–7. doi: 10.1002/jcp.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caberoy NB, Zhou Y, Li W. Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J. 2010;29(23):3898–910. doi: 10.1038/emboj.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santagata S, Boggon TJ, Baird CL, et al. G-protein signaling through tubby proteins. Science. 2001;292(5524):2041–50. doi: 10.1126/science.1061233. [DOI] [PubMed] [Google Scholar]
- 115.Stanley P. Galectins CLIC cargo inside. Nat Cell Biol. 2014;16(6):506–7. doi: 10.1038/ncb2983. [DOI] [PubMed] [Google Scholar]
- 116.Liu XJ, Pu Y, Cron K, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nature Medicine. 2015;21(10):1209. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. The host STING pathway at the interface of cancer and immunity. Journal of Clinical Investigation. 2016;126(7):2404–11. doi: 10.1172/JCI86892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiba S, Baghdadi M, Akiba H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nature Immunology. 2012;13(9):832–42. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hong M, Puaux AL, Huang C, et al. Chemotherapy Induces Intratumoral Expression of Chemokines in Cutaneous Melanoma, Favoring T-cell Infiltration and Tumor Control. Cancer Research. 2011;71(22):6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 121.Jaafar F, Righi E, Lindstrom V, et al. Correlation of CXCL12 Expression and FoxP3(+) Cell Infiltration with Human Papillomavirus Infection and Clinicopathological Progression of Cervical Cancer. Am J Pathol. 2009;175(4):1525–35. doi: 10.2353/ajpath.2009.090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maroo N, Dina RE. Correlation of CXCL12/CXCR4 Expression and FOXP3 Cell Infiltration in Normal Endometrium, Typical and Atypical Hyperplasia and Endometrioid Adenocarcinoma. Lab Invest. 2012;92:286a-a. [Google Scholar]
- 123.Popple A, Durrant LG, Spendlove I, et al. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Brit J Cancer. 2012;106(7):1306–13. doi: 10.1038/bjc.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481(7380):190. doi: 10.1038/nature10661. [DOI] [PubMed] [Google Scholar]
- 125.Gettinger S, Herbst RS. B7-H1/PD-1 Blockade Therapy in Non-Small Cell Lung Cancer Current Status and Future Direction. Cancer J. 2014;20(4):281–9. doi: 10.1097/PPO.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 126.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghosh AK, Secreto C, Boysen J, et al. The novel receptor tyrosine kinase Axl is constitutively active in B-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: implications for therapy. Blood. 2011;117(6):1928–37. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hector A, Montgomery EA, Karikari C, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10(10):1009–18. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holland SJ, Pan A, Franci C, et al. R428, a Selective Small Molecule Inhibitor of Axl Kinase, Blocks Tumor Spread and Prolongs Survival in Models of Metastatic Breast Cancer. Cancer Research. 2010;70(4):1544–54. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Ye X, Tan C, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28(39):3442–55. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 132.Rettew AN, Young ED, Lev DC, et al. Multiple receptor tyrosine kinases promote the in vitro phenotype of metastatic human osteosarcoma cell lines. Oncogenesis. 2012:1. doi: 10.1038/oncsis.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sensi M, Catani M, Castellano G, et al. Human Cutaneous Melanomas Lacking MITF and Melanocyte Differentiation Antigens Express a Functional Axl Receptor Kinase. J Invest Dermatol. 2011;131(12):2448–57. doi: 10.1038/jid.2011.218. [DOI] [PubMed] [Google Scholar]
- 134.Zhang Z, Lee JC, Lin L, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cummings CT, Zhang WH, Davies KD, et al. Small Molecule Inhibition of MERTK Is Efficacious in Non-Small Cell Lung Cancer Models Independent of Driver Oncogene Status. Mol Cancer Ther. 2015;14(9):2014–22. doi: 10.1158/1535-7163.MCT-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee-Sherick AB, Zhang WH, Menachof KK, et al. Efficacy of a Mer and Flt3 tyrosine kinase small molecule inhibitor, UNC1666, in acute myeloid leukemia. Oncotarget. 2015;6(9):6722–36. doi: 10.18632/oncotarget.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.von Massenhausen A, Sanders C, Thewes B, et al. MERTK as a novel therapeutic target in head and neck cancer. Oncotarget. 2016;7(22):32678–94. doi: 10.18632/oncotarget.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang WH, DeRyckere D, Hunter D, et al. UNC2025, a Potent and Orally Bioavailable MER/FLT3 Dual Inhibitor. J Med Chem. 2014;57(16):7031–41. doi: 10.1021/jm500749d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kariolis MS, Miao YR, DSJ, et al. An engineered Axl ‘decoy receptor’ effectively silences the Gas6-Axl signaling axis. Nat Chem Biol. 2014;10(11):977–83. doi: 10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sather S, Kenyon KD, Lefkowitz JB, et al. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109(3):1026–33. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cummings CT, Linger RMA, Cohen RA, et al. Mer590, a novel monoclonal antibody targeting MER receptor tyrosine kinase, decreases colony formation and increases chemosensitivity in non-small cell lung cancer. Oncotarget. 2014;5(21):10434–45. doi: 10.18632/oncotarget.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Demarest SJ, Gardner J, Vendel MC, et al. Evaluation of Tyro3 Expression, Gas6-Mediated Akt Phosphorylation, and the Impact of Anti-Tyro3 Antibodies in Melanoma Cell Lines. Biochemistry-Us. 2013;52(18):3102–18. doi: 10.1021/bi301588c. [DOI] [PubMed] [Google Scholar]
- 143.Leconet W, Larbouret C, Chardes T, et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33(47):5405–14. doi: 10.1038/onc.2013.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rogers AEJ, Le JP, Sather S, et al. Mer receptor tyrosine kinase inhibition impedes glioblastoma multiforme migration and alters cellular morphology. Oncogene. 2012;31(38):4171–81. doi: 10.1038/onc.2011.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ye X, Li Y, Stawicki S, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29(38):5254–64. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 146.Akitake-Kawano R, Seno H, Nakatsuji M, et al. Inhibitory role of Gas6 in intestinal tumorigenesis. Carcinogenesis. 2013;34(7):1567–74. doi: 10.1093/carcin/bgt069. [DOI] [PubMed] [Google Scholar]
- 147.Ksantini M, Lafont E, Bocquet B, Meunier I, Hamel CP. Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur J Ophthalmol. 2012;22(4):647–53. doi: 10.5301/ejo.5000096. [DOI] [PubMed] [Google Scholar]
- 148.Abu-Safieh L, Alrashed M, Anazi S, et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013;23(2):236–47. doi: 10.1101/gr.144105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Patel N, Aldahmesh MA, Alkuraya H, et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med. 2016;18(6):554–62. doi: 10.1038/gim.2015.127. [DOI] [PubMed] [Google Scholar]