Abstract
Objective
To examine neural mechanisms of action in behavioral weight loss treatment (BWL) and explore neural and genetic predictors of BWL.
Methods
Neural activation to palatable food receipt and genetics were compared in 17 women with obesity who received 12-weeks of BWL and 17 women who received no intervention. Participants were scanned twice using functional magnetic resonance imaging at baseline and 12 weeks. Weight was assessed at baseline, 12, 36, and 60 weeks.
Results
BWL participants lost more weight than controls at 12 weeks (−4.82 vs. −0.70 %). After 12 weeks, BWL had greater reduction in right caudate activation response to milkshake receipt than did controls. Among BWL participants, baseline to 12 weeks reduction in frontostriatal activation to milkshake predicted greater weight loss at 12, 36 and 60 weeks and possessing the A/A or T/A genotype of the fat mass and obesity–associated (FTO) variant rs9939609 predicted greater weight loss at 12 and 36 weeks.
Conclusions
These preliminary data reveal that reduction in right caudate activation may be a neural mechanism of weight loss in BWL and baseline FTO variant and reduction in frontostriatal activation during BWL predict short- and long-term weight loss. These findings require replication in larger samples.
Keywords: fMRI, taste, obesity, behavioral weight loss treatment, FTO
Introduction
There are very few neural and genetic predictive biomarkers of weight loss. We explored two potential biomarkers of response to behavioral weight loss (BWL): neural response to palatable food receipt and a genotype found to predict better outcome in BWL interventions1.
A weight loss of >5% body weight is associated with clinically significant improvements in cardiovascular and metabolic functioning and adiposity in individuals with overweight and obesity2–4. BWL results in clinically significant weight loss of up to 10% of initial weight5. Despite being the most commonly used intervention for obesity, little is known about the neural changes associated with BWL or whether these neural changes and baseline genetic factors predict BWL response.
The three fMRI studies examining the effects of BWL, have examined anticipatory neural responses using food picture paradigms (excluding cued learning tasks)6–8(Table 1). Greater baseline activation in the putamen, insula, and hippocampus and in visual regions in response to high-calorie food pictures predicted less weight loss at 9-months6. To our knowledge there are no studies examining neural responses to palatable food receipt. This is important as food receipt and food anticipation picture paradigms may be associated with different patterns of neural activation9,10. This study is focused on understanding palatable taste receipt, without anticipatory visual responses to food pictures. In addition, this study controls for the potential confound of sex differences11–15 by only using female participants.
Table 1.
Behavioral weight loss intervention studies using pre-post fMRI and food stimulia
| Studies | Participants | Design | fMRI Task | Results |
|---|---|---|---|---|
| Murdaugh et al. (2012) | 25 males and females with obesity and 13 normal-weight controls |
Controlled trial of 12 weeks BWL program or no-BWL control. 9 months follow- up weight-loss assessment |
High-calorie vs non-food neutral pictures |
At baseline, the participants with obesity showed enhanced activation in several brain regions, including the insula, ACCb, and amygdala. From pre to post, the participants with obesity had reduced activation in the mPFCb, IPLb, precuneus, posterior cingular cortex, premotor cortex and angular gyrus. At baseline, greater activation in the participants with obesity in areas associated with reward (ACC and insula), and visual and attentional processes were associated with less weight loss at 12 weeks At post-treatment, greater activation in participants with obesity in regions including the putamen, insula and hippocampus and visual regions predicted less weight loss at 9- months. |
| Deckersbach et al. (2014) | 13 males and females with obesity |
Randomized to waitlist control or 6 month BWL |
High- vs low- calorie food pictures |
Greater activation in right ventral putamen to low calorie foods and less activation in the left dorsal putamen to high calorie foods at 6-month compared to baseline in BWL group compared to controls |
| Bruce et al. (2014) | 15 males and females with obesity who received gastric band and 16 males and females with obesity who received 12 week BWL |
Controlled trial where groups were matched on demographics and amount of weight lost |
Food vs non-food (animals) pictures |
Diet participants vs. gastric band from pre- to post- showed significantly greater changes in right medial prefrontal cortex and left precuneus |
Whole brain analyses only
ACC = anterior cingulate cortex, mPFC = medial prefrontal cortex, and IPL = inferior parietal lobule
Prospective, observational studies examining neural response to palatable food receipt suggest that frontostriatal circuit activation16,17 is associated with weight gain over time. The frontostriatal circuit includes the dorsal and ventral striatum, midbrain, amygdala, and orbitofrontal cortex, putamen, insula, precuneus, and thalamus, which are involved in reward processing. The frontostriatal circuit is also comprised of regions implicated in cognitive control, including the ventrolateral prefrontal cortex and inferior frontal gyrus. These studies also suggest that the relationship between weight change and caudate activation response to food pictures may be moderated by the A1 allele of the TaqIA single nucleotide polymorphism (SNP) near the DRD2 gene16,18,19. However, how changes in neural response to food stimuli and genotype predict weight loss in BWL are unclear.
The fat mass and obesity–associated (FTO) gene is among the most robustly associated with BMI, across different ancestries8,20–23. FTO may regulate obesity risk through macronutrient intake22 and attenuated satiety response4. A recent meta-analysis of 10 BWL trials (total n=6,951) found that those possessing the obesity-predisposing A allele (A/A or T/A genotype) of the FTO single nucleotide polymorphism (SNP) rs9939609 show almost twice the amount of weight loss as those with the T/T genotype1. Although fMRI studies assessing FTO are limited, in European males with a range of BMIs, the A/A vs. T/T genotype at rs9939609 was associated with increased frontostriatal circuit activation to high- vs. low- calorie food images24. Together, these data suggest the importance of considering FTO genotype within both fMRI studies assessing response to food stimuli and studies of BWL.
This pilot trial examines changes in frontostriatal circuit response to palatable food receipt among women with obesity before and after 12 weeks of BWL compared to a control group who did not receive BWL. All participants were scanned at baseline and at 12 weeks. We anticipated greater weight loss in the BWL group at 12 weeks and that BWL participants would show greater reductions in frontostriatal activation in response to palatable food receipt at 12 weeks compared to controls. Finally, we explored the contribution of both neural response to palatable food receipt and possession of the A allele of the FTO SNP rs9939609 within BWL participants on weight loss from baseline to 12, 36, and 60 weeks; 12 to 60 weeks, 12 to 36 weeks and 36 to 60 weeks.
Materials and methods
Participants
Participants were recruited using flyers and local newspaper advertisements. Exclusion criteria were: tobacco or drug use in the last year, current Axis I or II disorder25; diabetes, blood pressure>140/9026, kidney or liver disease, history of serious neurological event; use of psychotropic or asthma medication or appetite suppressant or weight loss medication within the last year; pregnancy, breastfeeding or specific dietary needs; or contraindications to the fMRI protocol such as claustrophobia and metal implants. No participants met criteria for any obesity-related endocrine disorders. Control participants were required to agree to not participate in any weight loss program during the study. Initially 22 women with obesity or overweight were recruited for BWL and 19 female controls. Three BWL participants dropped out of the study and two BWL participants and two control participants were excluded from analysis due to excessive head motion. Thus, 17 BWL and 17 control participants completed two scans. The average age of the sample was 41 years. The majority of participants were African-American, single, professional with at least a college education (Table 2). There was a significant difference in BMI between the BWL and the control group (m=32.2 vs. m=28.5, p=0.04). There was no difference between groups in race and ethnicity status or age.
Table 2.
Demographic and clinical characteristics of participants (N = 34) at baseline.
| BWL | Control group | Total Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n=17) | (n=17) | (N=34) | χb | df | p | ||||
| n | % | n | % | N | % | ||||
| Race and Ethnicitya | 1.09 | 3 | 0.78 | ||||||
| European | 5 | 29.41% | 6 | 35.29% | 11 | 32.35% | |||
| African-American | 10 | 58.82% | 10 | 58.82% | 20 | 58.82% | |||
| Indian Asians | 1 | 5.88% | 1 | 5.88% | 2 | 5.88% | |||
| Hispanic | 1 | 5.88% | 0 | 0% | 1 | 2.94% | |||
| Marital | 2.03 | 4 | 0.73 | ||||||
| Single | 8 | 47.06% | 10 | 58.82% | 18 | 52.94% | |||
| Employment | 8.20 | 7 | 0.32 | ||||||
| Professional e.g., lawyer | 9 | 52.94% | 3 | 17.65% | 12 | 35.29% | |||
| Manager, Administrator | 2 | 11.76% | 2 | 11.76% | 4 | 11.76% | |||
| Clerical e.g., retail | 2 | 11.76% | 4 | 23.53% | 6 | 17.65% | |||
| Service worker e.g., waitress |
0 | 0.00% | 2 | 11.76% | 2 | 5.88% | |||
| Private Household Worker |
0 | 0.00% | 1 | 5.88% | 1 | 2.94% | |||
| Full-time Homemaker | 1 | 5.88% | 0 | 0.00% | 1 | 2.94% | |||
| Full-time Student | 2 | 11.76% | 3 | 17.65% | 5 | 14.71% | |||
| Other | 1 | 5.88% | 2 | 11.76% | 3 | 8.82% | |||
| Gross Annual Income | 8.75 | 7 | 0.27 | ||||||
| < $5000 | 2 | 11.76% | 2 | 11.76% | 4 | 11.76% | |||
| $5,000–9,999 | 1 | 5.88% | 1 | 5.88% | 2 | 5.88% | |||
| $10,000–14,999 | 1 | 5.88% | 0 | 0.00% | 1 | 2.94% | |||
| $15,000–19,999 | 1 | 5.88% | 3 | 17.65% | 4 | 11.76% | |||
| $20,000–24,999 | 0 | 0.00% | 1 | 5.88% | 1 | 2.94% | |||
| $25,000–29,999 | 1 | 5.88% | 4 | 23.53% | 5 | 14.71% | |||
| $30,000–49,999 | 5 | 29.41% | 5 | 29.41% | 10 | 29.41% | |||
| $50,000 or more | 6 | 35.29% | 1 | 5.88% | 7 | 20.59% | |||
| Handedness (left) | 3 | 17.65% | 1 | 5.88% | 4 | 11.76% | 1.13 | 1 | 0.29 |
| Current major depression | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | - | - | - |
| Past major depression | 1 | 5.88% | 2 | 11.76% | 3 | 8.82% | 0.37 | 1 | 0.55 |
| Current hypertensionb | 5 | 29.41% | 1 | 5.88% | 6 | 17.65% | 3.24 | 1 | 0.07 |
| Current hypothyroidismb | 1 | 5.88% | 3 | 17.65% | 4 | 11.76% | 1.13 | 1 | 0.29 |
| M | SD | M | SD | M | SD | T | df | p | |
| Age in years | 41.82 | 12.31 | 39.53 | 11.23 | 40.68 | 11.77 | −0.57 | 32 | 0.57 |
| Body Mass Index | 32.23 | 3.27 | 28.53 | 6.58 | 30.37 | 4.93 | −2.08 | 32 | 0.049 |
| Weight in lbs | 190.94 | 28.28 | 167.27 | 36.70 | 179.11 | 34.42 | −2.11 | 32 | 0.04 |
| Raven’s Progressive Matrices41, c |
33.06 | 19.12 | 27.88 | 27.81 | 30.47 | 23.46 | −0.63 | 32 | 0.53 |
Note that no participant self-identified as American-Indian / Alaska Native, Native Hawaiian / Other Pacific Islander or other.
Controlled by medication for at least one year
Percentile Rank
Procedure
Participants were phone-screened for eligibility. After giving informed consent participants completed a clinical diagnostic interview, a medical health screen review and their weight and height was measured. At a second session, participants completed further interviews, underwent a mock scan and donated saliva for DNA testing. Participants were scheduled for an fMRI session within the two weeks before starting BWL and within two weeks after ending BWL. There were no differences in the days between scans for the BWL and control groups (t(32)=0.21, p<.83, control: m=85 sd=17; BWL m=81, sd=46). The weights of BWL and control participants were assessed at baseline, end of treatment or after 12-, 36- and 60-weeks post-baseline. All procedures were reviewed and approved by the Temple University Institutional Review Board.
Behavioral weight loss treatment (BWL)
BWL sessions were 90 minutes and led by a registered dietitian or a clinical psychologist. Groups of ten to twelve met weekly for 12 weeks. BWL involved goal setting, cognitive restructuring, self-monitoring, stimulus control, and physical activity5. Dietary intake goals were 1200–1500 kcal/day. Physical activity goals were four 20-minute sessions per week starting at week 4, progressing to four 40-minute sessions by week 12.
Genetic assessment and analysis
Saliva specimens were collected at baseline using Oragene Discover collection kits. Samples were genotyped through Oragene GenoFind Services. The SNPs were analyzed using a TaqMan single tube genotyping assay. The TaqMan assay is an allele discrimination assay using PCR amplification and a pair of fluorescent dye detectors that target the SNP. One fluorescent dye is attached to the detector that is a perfect match to the first allele (e.g. an “A” nucleotide) and a different fluorescent dye is attached to the detector that is a perfect match to the second allele (e.g. a “C” nucleotide). During PCR, the polymerase releases the fluorescent probe into solution where it is detected using endpoint analysis in an Applied Biosystems, Inc. (Foster City, CA) 7900HT Real-Time instruments. Primers and probes were also obtained through Applied Biosystems. The triallelic haplotype for rs9939609, rs1421085, and rs17817449 were determined using the TaqMan single tube genotyping assay results in conjunction with “Haploview” and additional haplotyping bioinformatics software.
fMRI assessment
All participants fasted 8 hours prior to their scans at baseline and 12 weeks. Immediately prior to the scan, participants were screened for pregnancy (HCG Pregnancy Midstream Test) and breathalyzed for recent use of alcohol (BACtrack Special S70), and saliva-tested for use of cocaine, cannabis, opioids and amphetamines (Discover 4 Panel Oral Fluid Cassette). Fasting blood glucose levels were assessed to document fasting.
Stimuli Presentation
During the scan, participants were presented with a food receipt task. Chocolate milkshake, tasteless solution, and wash were administered via programmable syringe pumps (Braintree Scientific BS-8000) to participants while in the scanner using teflon tubing inserted through a wave guide into the scanning room. Stimuli18 consisted of the presentation of two runs of six conditions, each presented four times per run in a mixed event-related block design. The six conditions were (1) milkshake picture, (2) water picture, (3) milkshake picture concurrent to milkshake taste, (4) water picture concurrent to tasteless solution, (5) milkshake taste, and (6) tasteless solution. Participants received wash after each taste administration. Any liquid administration alone occurred at the same time a crosshairs was presented to avoid conflation of visual with food receipt response. During intervals between conditions, a crosshair was presented for a random period ranging from 2–10 sec.
MRI Data Acquisition
Structural and functional MRI data were collected using a Siemens 3-Tesla Verio (Siemens, A G, Erlangen, Germany) scanner using a standard 12-channel head coil, at Temple University Magnetic Resonance Imaging Center. Scanning parameters optimized the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Data was acquired using a two-shot z-shim EPI sequence1 that enables compensation of local magnetic field inhomogeneities in high susceptibility regions27,28. Calibration of the z-shim settings were determined as described previously29. Prior to functional scanning, a high-resolution T1-weighted 3D structural volume was collected from each participant (MPRAGE, TR=1,600ms, TE=2.46ms, flip angle= 9°, matrix size= 256 × 256 × 176 mm, field of view=252mm2, slice thickness=1mm, 176 slices gathered in ascending order) for accurate slice prescription. Next, BOLD functional images were acquired with a T2* weighted double-shot z-shim echo planar imaging sequence and covered 52 descending axial slices (TR=3,020ms, TE=20ms; flip angle=90°, bandwidth=2,232 Hz/pixel, matrix size=80 × 80 × 52mm, field-of-view=240mm2, slice thickness=2.5mm, distance factor=20%, 188 volumes).
fMRI Data Analysis
Data were preprocessed using SPM12 http://www.fil.ion.ucl.ac.uk/spm/software/spm12/. After using a field map, functional images were realigned and unwarped. Functional images were coregistered to the high-resolution T1-weighted images, then segmented and spatially normalized to standard Montreal Neurological Institute brain space and spatially smoothed using a three-dimensional Gaussian kernel of 6 × 6 × 5mm full-width at half-maximum, resulting in a resampled in-plane resolution of 3 × 3 × 3mm voxels. We utilized Artifact Detection Tools (ART; https://www.nitrc.org/projects/artifact_detect/) to create motion outlier and movement regressors for the first-level analyses. Any participant was removed if the data were revealed excess motion, defined as >20% of the data being outliers (i.e., global signal exceeded 9.0 and scan-to-scan movement exceeded 2mm and/or rotation exceeded 1 radians).
Participant-level analyses were performed using general linear modelling implemented in SPM12. For each participant, the regression model consisted of regressors representing the stimulus condition, convolved with the canonical hemodynamic response function six motion parameters to account for motion and regressors to account for outliers detected with ART.
This preliminary study tested if changes in frontostriatal activation to palatable food receipt from baseline to 12 weeks differed between the BWL vs. control group. Given this is the first fMRI study assessing neural changes in response to palatable taste receipt before and after BWL, we first assessed changes in all the regions of the brain (whole-brain analyses) rather than in specific brain regions (region-of-interest analyses). For the group-level analyses, whole-brain effects were examined using a 2 × 2 ANCOVA model controlling for baseline BMI to reveal any main effects for Treatment (BWL / control), Time (baseline / 12-weeks) and a Treatment group × Time interaction. To understand any interaction effects yielded, a mask was generated including the clusters where the interaction effect was significant. Post-hoc contrasts were estimated using this mask as a region of interest for evaluating specific contrasts for each Treatment group per Time point.
Given that this was a preliminary study (see Supplementary for the power yielded and the sample estimate for future trials), we used an uncorrected p<.001, with a cluster threshold of 3 for the 2 × 2 ANCOVA controlling for baseline BMI. For follow-up post-hoc contrasts, we used a family-wise error (FWE) rate corrected p< .01 to correct for multiple comparisons. Our contrast of interest was the milkshake taste vs. crosshairs contrast, which does not conflate the effects of anticipatory visual responses to food and was the most basic contrast assessing palatable food receipt available. Secondary contrasts examined were milkshake taste vs. tasteless; milkshake picture and milkshake taste vs crosshairs; and milkshake picture and milkshake taste vs tasteless.
To explore the prediction of weight loss within the BWL group, we first entered eigenvariates yielded from the 2 × 2 ANCOVA interaction into a principal components analysis (PCA) to derive fewer components to enter as predictors. Component scores were used as neural predictors of weight loss. For the FTO genotype predictor, A/A and T/A of the FTO rs9939609 were coded 0 while the T/T genotype was coded 1 because a meta-analysis of behavioral weight loss interventions showed that carriers of the FTO A/A and T/A genotypes have greater weight loss than those with the T/T1. The neural and genotype predictors were simultaneously entered into separate hierarchical linear regressions predicting weight loss in BWL from baseline to: 12-, 36- and 60-weeks post-baseline, and from 12 to 36, 12 to 60 weeks and 36 to 60 weeks. Percentage weight loss was chosen as the dependent variable to allow for control of baseline weight.
Results
Weight loss at 12-, 36-, and 60-weeks
There was significantly greater weight loss in the BWL group compared to the control group after 12 weeks (Table 3). Among the BWL participants, 59% lost > 5% or more of their body weight by 12 weeks compared to 12% among controls (χ2(1)=8.24, p= .004). Although BWL participants had weight losses that exceeded 5% and were double that of controls at 36 and 60 weeks, the differences were not statistically significant possibly due to the small sample size (Table 3).
Table 3.
Changes in weight from baseline to 12, 36, and 60 weeks in behavioral weight loss (BWL) and control groups
| Baseline | 12 weeks | 36 weeksa | 60 weeksa | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| BWL (n=17) | ||||
| Weight loss(kg) | 86.61 (12.83) | −4.36 (5.11) | −6.30 (4.92) | −4.74 (6.76) |
| Weight loss (%) | −4.82 (5.52) | −6.92 (5.58) | −5.28 (7.98) | |
| Control (n=17) | ||||
| Weight loss (kg) | 75.87 (16.64) | −0.48 (2.66) | −3.10 (7.60) | −1.76 (6.18) |
| Weight loss(%) | −0.70 (3.41) | −3.52 (8.95) | −1.95 (7.45) | |
|
Comparison of BWL and Control: T(1,df), p |
t(1,32) = 2.62, p = 0.01) |
t(1,28) = 1.25, p = 0.22) |
t(1,32) = 1.26, p = 0.22) |
|
Four participants weights could not be assessed in person at 36 weeks and one participant’s weight could not be assessed at 60 weeks.
Neural response to milkshake taste from baseline to 12-weeks in BWL vs. control (N=34)
Controlling for baseline BMI, whole brain analyses of the milkshake taste vs crosshairs contrast showed a significant main effect of Time and a significant Treatment × Time interactions in the 2 × 2 ANCOVA (Table 4). When BMI was not controlled for in the same analysis, results were similar.
Table 4.
fMRI BOLD response to milkshake taste vs crosshairs. Main effects and interaction effect of the 2 (Treatment Group) × 2 (Time) ANCOVA controlling for baseline body mass index (N=34)
| Hemi | Peak | Peak | Peak | |||||
|---|---|---|---|---|---|---|---|---|
| sphere | k | F | Z | p(unc) | Xa | y | z | |
| Treatment main effectsb | ||||||||
| Time main effects | ||||||||
| Anterior Cingulate | L | 9 | 21.99 | 4.17 | 1.56E-05 | −18 | 38 | 15 |
| Thalamus | L | 39 | 19.34 | 3.93 | 4.29E-05 | −3 | −25 | 7.5 |
| Precuneus | L | 5 | 19.11 | 3.91 | 4.72E-05 | −12 | −37 | 2.5 |
| Hippocampus | L | 5 | 15.50 | 3.53 | 0.000209 | −18 | −31 | −2.5 |
| Medial Frontal Gyrus - Rectus | L | 20 | 18.77 | 3.87 | 5.4E-05 | −9 | 26 | −12.5 |
| Anterior intraparietal sulcus | R | 17 | 16.82 | 3.67 | 0.00012 | 33 | −37 | 22.5 |
| Superior/Medial frontal | 4 | 16.76 | 3.67 | 0.000123 | 27 | 14 | 17.5 | |
| Supp Motor Area | L | 5 | 16.70 | 3.66 | 0.000126 | 0 | 17 | 67.5 |
| Angular Supramarginal Gyrus | R | 8 | 16.57 | 3.65 | 0.000133 | 42 | −49 | 32.5 |
| Precuneus | L | 10 | 15.54 | 3.53 | 0.000205 | −3 | −64 | 50 |
| Medial Frontal Gyrus Supp Motor Area | R | 4 | 15.29 | 3.51 | 0.000229 | 6 | −22 | 60 |
| Medial Frontal Gyrus Frontal Sup Medial | R | 4 | 13.12 | 3.25 | 0.000585 | 3 | 35 | 47.5 |
| Interaction Effect | ||||||||
| BWL / control × baseline / 12 week scan | ||||||||
| Orbitofrontal Cortex | R | 6 | 22.30 | 4.20 | 1.34E-05 | 24 | 35 | 5 |
| Uvula | L | 8 | 19.63 | 3.96 | 3.83E-05 | −3 | −64 | −40 |
| Medial Prefrontal Cortex | R | 8 | 17.29 | 3.72 | 9.86E-05 | 21 | 17 | 17.5 |
| Caudate | R | 4 | 15.50 | 3.53 | 0.000208 | 21 | 8 | 20 |
| Central sulcus | R | 12 | 15.27 | 3.50 | 0.00023 | 30 | −19 | 27.5 |
| Middle Temporal Gyrus | R | 4 | 13.07 | 3.24 | 0.000596 | 36 | −61 | 12.5 |
| Insula | L | 4 | 12.71 | 3.20 | 0.0007 | −33 | 8 | 12.5 |
Threshold: p < .001, kE = 3
R = right, L = left;
k = cluster size in voxels
Note that 5 voxels of the L precuneus and 5 voxels of the L hippocampus were contained within the cluster of 39 voxels of the L thalamus. Four voxels of the R caudate were contained within the cluster of 8 voxels of the R Medial Prefrontal Cortex. We used WFUPickAtlas within Xjview (http://www.alivelearn.net/xjview) for neuroanatomical labelling.
Peak F= F value of the peak of the cluster
Peak Z = Z value of the peak of the cluster
Peak p (unc) = Peak p (uncorrected)
x, y, z coordinates are in Montreal Neurological Institute space.
No effect met the threshold
Post-hoc comparisons were then conducted to assess the direction of the significant interaction effects found in the 2 × 2 ANCOVA (Table 4). These comparisons showed that there was greater activation in the right caudate, right medial prefrontal cortex and right central sulcus and right orbitofrontal cortex in the BWL group at baseline compared to the control group. From the baseline fMRI scan to the 12-week scan, all these regions showed a reduction in activation in the BWL group relative to the control group, a reduction that occurred during significant weight loss in the BWL group relative to the control group. However, for these four regions, only the results for the right caudate met a more stringent thresholding criteria of p<.01 FWE-corrected. There was greater right caudate activation in the BWL group compared to the control group at baseline in response to milkshake receipt and reduced activation in this region in the BWL group from baseline to 12 weeks where p<.01 FWE-corrected (Figure 1, Table 5). While right medial prefrontal cortex, right central sulcus and right orbitofrontal cortex activation showed similar differences and changes, these were not significant at this threshold, which may have resulted from limited power.
Figure 1.
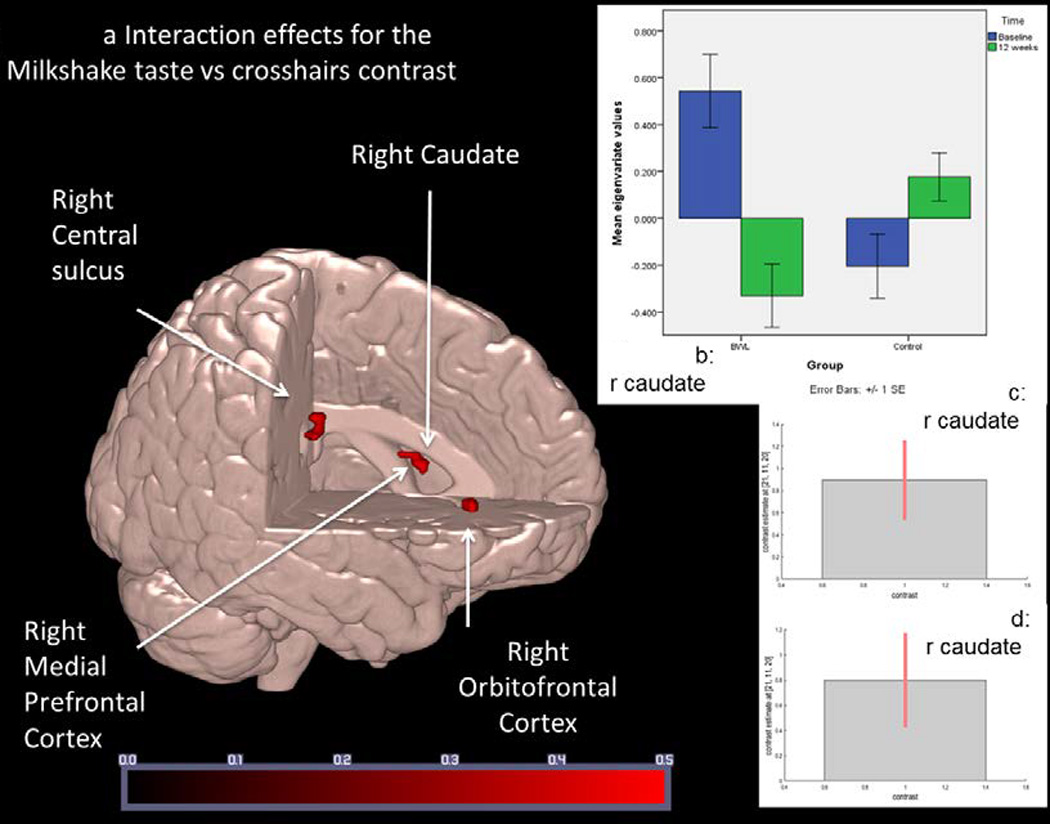
Regions associated with differential fMRI BOLD response to milkshake taste in Behavior Weight Loss (BWL) compared to control from baseline to 12-weeks post-baseline (N = 34).
a: Regions associated with differential change between the BWL and Control groups over time where p < .01 FWE-corrected. Colored bar represents Z score values. Areas are as described in Table 5.
b: Right caudate response comparing BWL and control groups at baseline and 12 weeks. Eigenvariate values show greater activation at baseline in BWL at baseline compared to control and greater activation in BWL at baseline compared to BWL at 12 weeks. Error bars represent +/− 1 standard error.
c and d: Cohen’s d and 90th percentile CI of the size of the effect of right caudate activation in BWL at baseline compared to control (Figure 1c) and between baseline BWL and 12 weeks BWL (Figure 1d).
Table 5.
Regions associated with differential fMRI BOLD response to milkshake taste in Behavioral weight loss compared to control from baseline to 12-weeks (N = 34)
| Hemi | Peak | Peak | Peak | Peak | |||||
|---|---|---|---|---|---|---|---|---|---|
| sphere e |
k | Z | T | p(FWE- corr) |
p(unc) | xa | y | z | |
| BWL baseline > Control at baselineb | |||||||||
| Caudate | R | 4 | 3.8 | 4.04 | 0.001 | 7.36E-05 | 21 | 11 | 20 |
| BWL baseline > BWL at 12 weeksb | |||||||||
| Medial prefrontal cortex | R | 6 | 4.49 | 4.90 | 0.0001 | 3.61E-06 | 24 | 14 | 18 |
| Caudate | R | 1 | 3.34 | 3.51 | <0.01 | 0.0004 | 21 | 11 | 20 |
| Central sulcus | R | 6 | 3.89 | 4.16 | 0.001 | 4.99E-05 | 33 | −19 | 28 |
| Control at 12 weeks > BWL at 12 weeksb | |||||||||
| Orbitofrontal Cortex | R | 6 | 3.95 | 4.23 | 0.001 | 3.92E-05 | 24 | 35 | 5 |
Threshold: where p < .01 FWE-corrected.
R = right, L = left;
k = cluster size in voxels
We used WFUPickAtlas within Xjview (http://www.alivelearn.net/xjview) for neuroanatomical labelling.
Peak Z = Z value of the peak of the cluster
Peak T = T value of the cluster peak
Peak p (FWE-corr) = Family-wise error rate corrected p value
Peak p (unc) = Peak p (uncorrected)
x, y, z coordinates are in Montreal Neurological Institute space.
The direction of change from baseline to 12 weeks in fMRI BOLD response to milkshake taste vs crosshairs in the regions associated with differential change between the BWL and control groups.
We also examined if there were main effects for a milkshake picture and milkshake taste vs. crosshairs, milkshake picture and taste vs. tasteless and milkshake taste vs tasteless (p <.001 uncorrected, kE=3 voxels, Supplementary Tables 1–3). Follow-up contrasts for these analyses were not significant where p<.01, FWE-corrected.
Exploration of genetic and neural moderators of weight loss outcome in BWL (N=17)
The Treatment by Time interaction was associated with neural activation in seven regions. Bivariate correlations between these regions are presented in Table 6. The PCA identified two components: one which maps onto the frontostriatal circuit30–32 comprised of the right orbitofrontal cortex, the right caudate, right insula and the left insula, and the other with regions less understood but currently not associated with the frontostriatal circuit (left uvula, right central sulcus, right middle temporal gyrus, Table 6).
Table 6.
Correlations between regions that changed differentially over time in BWL compared to control and the results of the multivariate principal components analysis of these regions (N = 34).
| Intercorrelations | Multivariate principal components Pattern Matrix |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| R. Orbitofro ntal Cortex. |
R. Medial Prefro ntal Cortex , |
R. Caudate |
L. Insula |
L. Uvula |
R. Central sulcus |
Component 1 Frontostriatal Circuit |
Component 2 Other regions |
||
| R. Orbitofrontal Cortex. |
0.74 | ||||||||
| R. Medial Prefrontal Cortex |
.63** | 0.93 | |||||||
| R. Caudate | 0.46 | .72** | 0.89 | ||||||
| L. Insula | −0.20 | 0.21 | 0.12 | 0.34 | |||||
| L. Uvula | −0.16 | −0.24 | −0.07 | 0.12 | 0.81 | ||||
| R. Central sulcus, |
−0.05 | 0.14 | 0.35 | 0.16 | 0.38 | 0.70 | |||
| R. Middle Temporal Gyrus. |
−0.04 | −0.21 | 0.05 | 0.30 | 0.40 | 0.16 | 0.78 | ||
NB. R= right, L = left
p <.05
p<.001
Listing of regions and Montreal Neurological Institute coordinates
R. Orbitofrontal Cortex [24,35,5]
R. Medial Prefrontal Cortex [21,17,17.5]
R. Caudate [21,8,20]
L. Insula [−33 8 13]
L. Uvula [−3,−64,−40]
R. Central sulcus [30,−19,28]
R. Middle Temporal Gyrus. [36 −61 13]
Within the BWL group, greater frontostriatal and other region activation to milkshake receipt and the A/A or T/A versus the T/T genotype for FTO rs9939609 predicted greater 12-week weight loss (Table 7). Similarly, greater frontostriatal activation to milkshake receipt and A/A or T/A versus the T/T genotype for FTO rs9939609 predicted greater 36-week weight loss. Only greater frontostriatal activation to milkshake receipt predicted greater 60-week weight loss. Neither neural activation nor FTO genotype predicted weight loss between 12 to 36 weeks, 12 and 60 weeks and 36 to 60 weeks in BWL.
Table 7.
Hierarchical linear regressions using FTO genotype and right caudate activation response to milkshake taste to predict % weight loss (Kg) after Behavioral weight loss (BWL) where N=17.
| Dependent variable: % Weight loss baseline to 12 weeks (n = 15) | |||||||
|---|---|---|---|---|---|---|---|
| R2a | F(2,14) | bb | SE | t | p | ||
| 0.73 | 13.90 | ||||||
| FTO1 rs9939609 (A/A or T/A =10/15)c | 0.55 | 0.87 | 3.89 | 0.003 | |||
| Frontostriatal circuit | −0.75 | 0.85 | −5.37 | <0.0001 | |||
| Other regions | 0.38 | 1.09 | 2.66 | 0.02 | |||
| Dependent variable: % Weight loss baseline to 36 weeks (n = 13) | |||||||
| R2a | F(2,12) | bb | SE | t | p | ||
| 0.60 | 6.89 | ||||||
| FTO1 rs9939609 (A/A or T/A = 8/13)c | 0.49 | 2.03 | 2.62 | 0.03 | |||
| Frontostriatal circuit | −0.63 | 2.01 | −3.34 | 0.009 | |||
| Other regions | −0.14 | 2.76 | −0.76 | 0.47 | |||
| Dependent variable: % Weight loss from baseline to 60 weeks (n = 15) | |||||||
| R2a | F(2,14) | bb | SE | t | p | ||
| 0.52 | 6.14 | ||||||
| FTO1 rs9939609 (A/A or T/A =10/15)c | 0.31 | 3.25 | 1.62 | 0.14 | |||
| Frontostriatal circuit | −0.68 | 3.14 | −3.67 | 0.004 | |||
| Other regions | −0.22 | 4.04 | −1.17 | 0.27 | |||
| Dependent variable: % Weight loss from 12 to 36 weeks (n = 13) | |||||||
| R2a | F(2,12) | bb | SE | t | p | ||
| 0.26 | 2.39 | ||||||
| FTO1 rs9939609 (A/A or T/A = 8/13)c | 0.23 | 2.14 | 0.93 | 0.38 | |||
| Frontostriatal circuit | −0.31 | 2.12 | −1.20 | 0.26 | |||
| Other regions | −0.45 | 2.91 | −1.76 | 0.11 | |||
| Dependent variable: % Weight loss from 12 to 60 weeks (n = 15) | |||||||
| R2a | F(2,14) | bb | SE | t | p | ||
| 0.34 | 3.37 | ||||||
| FTO1 rs9939609 (A/A or T/A =10/15)c | 0.12 | 3.49 | 0.52 | 0.62 | |||
| Frontostriatal circuit | −0.48 | 3.37 | −2.20 | 0.05 | |||
| Other regions | −0.43 | 4.34 | −1.92 | 0.08 | |||
| Dependent variable: % Weight loss 36 to 60 weeks (n = 13) | |||||||
| R2a | F(2,12) | bb | SE | t | p | ||
| 0.04 | 1.20 | ||||||
| FTO1 rs9939609 (A/A or T/A = 8/13)c | 0.10 | 15.53 | 0.37 | 0.72 | |||
| Frontostriatal circuit | 0.18 | 14.72 | 0.64 | 0.54 | |||
| Other regions | −0.46 | 19.00 | −1.65 | 0.13 | |||
R= right
Adjusted R2
Standardized b
FTO rs9939609. Coded with A/A as the reference group. A/A and T/A = 0, T/T = 1. We report the number of participants with A/A or T/A out of the total number of participants analyzed.
% Weight in Kg lost = [(Weight in kg at later assessment time – Weight in Kg at baseline)/ baseline BMI] * 100%.
Discussion
Our findings suggest that BWL treatment leads to reduced activation of the right caudate in response to palatable food receipt in women with obesity. Using the decoding analysis in Neurosynth24(see Supplementary), the two latent topics most strongly linked to the right caudate are ‘reward’ and ‘eating’. The caudate has been implicated in the development and maintenance of habitual overeating21 without pleasure33. A prospective non-intervention study34 also found activation of the caudate to palatable food receipt was predictive of long-term weight change. BWL may serve to interrupt patterns of overeating marked by a lack of enjoyment.
In addition to the right caudate findings, the right medial prefrontal cortex, right central sulcus and the right orbitofrontal cortex showed reduced activation in response to milkshake receipt in BWL relative to control from baseline to 12 weeks. Using food picture paradigms, a previous study67 showed that BWL is associated with greater reductions in right medial prefrontal cortex activation. Reduced orbitofrontal cortex response has been observed in examination of palatable taste receipt after gastric bypass35 suggesting that weight loss may alter the reward value of palatable foods23.
Similar to findings with food picture paradigms6, we found differential changes in the insula between the BWL group and control group over 12 weeks in response to combined milkshake picture and milkshake taste relative to crosshairs. However, these findings were not significant when we probed the direction of activation.
Unlike previous findings, changes in activation in the putamen or hippocampus did not predict follow-up weight loss6. This may be due to methodological and sampling differences. Unlike previous studies, the current study enrolled only female participants12–15. In addition, this study examined neural responses to food receipt rather than food pictures in BWL. Notably there may be differential neural responses to food pictures compared to food receipt have been reported previously9,10.
The current study explored the predictive value of neural and FTO responses to BWL. Greater reductions in frontostriatal circuit activation (right medial prefrontal cortex, right orbitofrontal cortex, right caudate, and left insula) to palatable food receipt from baseline to 12 weeks among BWL participants predicted greater weight loss up to one year after BWL. We also found that possession of the A/A or T/A rather than the T/T genotype of the FTO rs993960 predicted greater weight loss at 12 weeks and at 36-weeks follow-up. This provides further evidence that this genotype may be important for BWL treatment success. A sizeable amount of the variance in both short- and long-term weight loss was accounted for by both baseline FTO and baseline to 12-week reduction in frontostriatal activation at 12 (73%) and 36 (60%) weeks, and by baseline to 12-week frontostriatal activation at 60 weeks (52%)(Table 7). However, the sample size was too small to test for interactions between genotype and neural response or covary for baseline variables like ancestry. Replication and testing of this interaction with more covariates is needed in larger samples.
Limitations of the current study include small sample size. The relatively small cluster sizes of the regions (e.g., right caudate), discovered to change differentially over time with treatment, may have resulted from the lack of power. The design would have been improved by randomizing participants to treatment or control to minimize any baseline differences between groups. Strengths of the study include a socio-economically diverse sample, eliminating confounding of sex, and examining long-term follow-up. The study is also notably diverse, including nearly 60% African American, a group disproportionately affected by obesity36 and for whom current interventions including BWL37,38 appear less effective.
To our knowledge, this is the first trial to examine neural response to food receipt in the context of BWL treatment and the first to examine both neural and genetic predictors of weight loss and maintenance. Additional long-term examination of the FTO gene and neural responses to palatable food receipt is needed in larger samples and across ancestries. Given the cluster sizes found in this preliminary study, there is a risk of Type 1 error and future studies may use whole-brain multiple comparisons correction based on cluster size such as AlphaSim or ClusterSim or FDR. Examination of predictors of weight loss at longer follow-up periods is also needed39.
There are few biomarkers of weight loss for BWL interventions. However, the current study suggests BWL may be particularly effective for individuals with the FTO A allele and those with greater frontostriatal reduction in response to food receipt during treatment. This preliminary finding requires replication but may add to our understanding of how precision-based medicine strategies40 may be useful in the treatment of obesity.
Supplementary Material
What is already known about this subject?
Previous studies suggest that increased frontostriatal response to food pictures predict weight loss outcome in behavioral weight loss treatment and a meta-analysis showed that possession of the A/A or T/A genotype of the FTO variant rs9939609 may be associated with better outcome in behavioral weight loss interventions.
What does your study add?
We found that greater reduction in right caudate activation in response to palatable food receipt occurred over 12-weeks of behavioral weight loss treatment compared to control.
Reduction in frontostriatal activation from baseline to 12-weeks of behavioral weight loss treatment predicted weight loss at 12, 36 and 60 weeks.
Presence of the A/A or T/A genotype of the FTO variant rs9939609 predicted weight loss in behavioral weight loss treatment at 12 and 36 weeks.
Acknowledgments
Funding: Anonymous Donor to Gary Foster, PhD and R21MH093932-01A1 from NIMH and NIDDK to Eunice Chen PhD. The funding sources had no involvement in the study design, data collection, analysis, interpretation or writing of the report or the decision to submit the manuscript.
Footnotes
The EPI pulse sequence provided by the Functional Neuroimaging Laboratory at Brigham and Women’s Hospital
Conflict of Interest: EYC discloses annual royalties from Guilford Press, and has consulted to Shire Pharmaceuticals in the last three years. GDF and SSV are employees and shareholders of Weightwatchers International. All other authors declare no potential conflict of interest with the current work.
Author Contributions: Dr Chen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Chen, Foster, Mohamed. Acquisition of data: Chen, Foster, Conklin, Mohamed, Hoge, Arlt, Eneva, Yiu, VanderVeur, Lent, Tewksbury, Analysis or interpretation of data: Chen, Olino, Foster, Conklin, Mohamed, Newberg; Drafting of the manuscript: Chen, Olino, Foster, Conklin, Mohamed, KR Kidd. JR Kidd, Hoge, Critical revision of the manuscript: Chen, Olino, Foster, Conklin, Mohamed, KR Kidd. JR Kidd, Hoge, Arlt, Murray Statistical Analysis: Chen, Olino, Obtained funding: Foster, Chen.
References
- 1.Xiang L, Wu H, Pan A, et al. FTO genotype and weight loss in diet and lifestyle interventions: a systematic review and meta-analysis. The American journal of clinical nutrition. 2016;103(4):1162–1170. doi: 10.3945/ajcn.115.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell metabolism. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Archives of internal medicine. 1987;147(10):1749–1753. [PubMed] [Google Scholar]
- 4.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. American Journal of Clinical nutrition. 2005;82(1):230S–235S. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- 6.Murdaugh DL, Cox JE, Cook EW, III, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. 2012/02// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce AS, Holsen LM, Chambers RJ, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity. 2010;34(10):1494–1500. doi: 10.1038/ijo.2010.84. 2010/10// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos RJ, Yeo GS. The bigger picture of FTO - the first GWAS-identified obesity gene. Nature Reviews Endocrinology. 2014;10(1):51–61. doi: 10.1038/nrendo.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57(5):786–797. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013 Feb 15;67:322–330. doi: 10.1016/j.neuroimage.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behavioural brain research. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang GJ, Volkow ND, Telang F, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proceedings of the National Academy of Sciences. 2009;106(4):1249. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiology & Behavior. 2010;99(4):538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geliebter A, Pantazatos SP, McOuatt H, Puma L, Gibson CD, Atalayer D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav Brain Res. 2013;243:91–96. doi: 10.1016/j.bbr.2012.12.023. 2013/04// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57(2):421–434. doi: 10.1016/j.appet.2011.06.009. 2011/10// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA Allele. The Journal of Neuroscience. 2015;35(28):10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19(9):1775–1783. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/science.1161550. PMCID: 2681095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monda KL, Chen GK, Taylor KC, et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nature genetics. 2013;45(6):690–696. doi: 10.1038/ng.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong J, Schumacher F, Lim U, et al. Fine mapping and identification of BMI loci in African Americans. The American Journal of Human Genetics. 2013;93(4):661–671. doi: 10.1016/j.ajhg.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauhan G, Tabassum R, Mahajan A, et al. Common variants of FTO and the risk of obesity and type 2 diabetes in Indians. Journal of human genetics. 2011;56(10):720–726. doi: 10.1038/jhg.2011.87. [DOI] [PubMed] [Google Scholar]
- 23.Vasan SK, Fall T, Neville MJ, et al. Associations of variants in FTO and near MC4R with obesity traits in South Asian Indians. Obesity. 2012;20(11):2268–2277. doi: 10.1038/oby.2012.64. [DOI] [PubMed] [Google Scholar]
- 24.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington DC: American Psychiatric Association; 2000. Text Revision ed. [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 27.Constable RT. Functional MR imaging using gradient-echo echo-planar imaging in the presence of large static field inhomogeneities. Journal of Magnetic Resonance Imaging. 1995;5(6):746–752. doi: 10.1002/jmri.1880050622. [DOI] [PubMed] [Google Scholar]
- 28.Song AW. Single-shot EPI with signal recovery from the susceptibility-induced losses. Magnetic resonance in medicine. 2001;46(2):407–411. doi: 10.1002/mrm.1205. [DOI] [PubMed] [Google Scholar]
- 29.Hoge W, Pan H, Tan H, Stern E, Kraft R. A Method for z-shim Compensated EPI-BOLD Imaging in a Single Shot. Paper presented at: Proc IEEE Intl Symp on Biomedical Imaging (ISBI).2013. [Google Scholar]
- 30.Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009 Dec;30(12):2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray EA, Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Annals of the New York Academy of Sciences. 2007;1121(1):273–296. doi: 10.1196/annals.1401.021. [DOI] [PubMed] [Google Scholar]
- 32.Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS one. 2014;9(5):e94640. doi: 10.1371/journal.pone.0094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010 Sep 2;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage. 2014 Jun 2; doi: 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J-L, Yang Q, Hajnal A, Rogers AM. A pilot functional MRI study in Roux-en-Y gastric bypass patients to study alteration in taste functions after surgery. Surgical endoscopy. 2015:1–7. doi: 10.1007/s00464-015-4288-5. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foster GD, Wadden TA, Swain RM, Anderson DA, Vogt RA. Changes in resting energy expenditure after weight loss in obese African American and white women. The American journal of clinical nutrition. 1999;69(1):13–17. doi: 10.1093/ajcn/69.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Tussing-Humphreys LM, Fitzgibbon ML, Kong A, Odoms-Young A. Weight loss maintenance in African American women: a systematic review of the behavioral lifestyle intervention literature. Journal of Obesity. 2013;2013 doi: 10.1155/2013/437369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2011;34(4):841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins FS, Varmus H. A new initiative on precision medicine. New England Journal of medicine. 2015;372(9):793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


